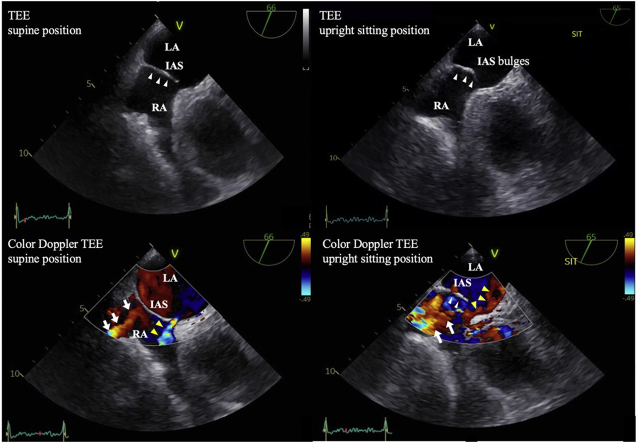
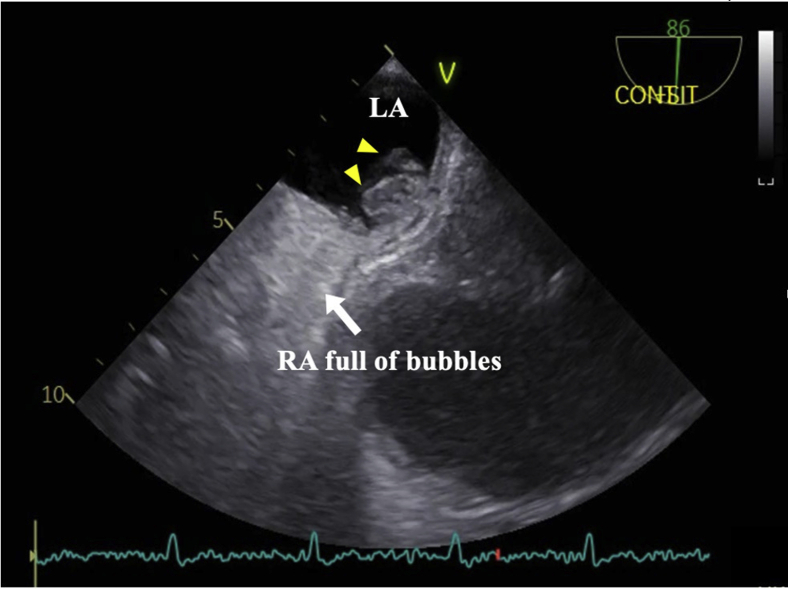
Graphical abstract
Keywords: Platypnea-orthodeoxia syndrome, Atrial septal defect, Transesophageal echocardiography, Color Doppler imaging
Highlights
-
•
TEE can demonstrate that the severity of right-to-left shunt may vary depending on posture.
-
•
Shunt reversal is likely multifactorial in most patients with POS.
-
•
Even in patients with chronic respiratory diseases, POS should be considered.
Introduction
Platypnea-orthodeoxia syndrome (POS), first reported in 1949, is a rare syndrome defined by hypoxemia that worsens in the upright position.1 Right-to-left shunting through an intracardiac communication such as a patent foramen ovale (PFO) or atrial septal defect (ASD) is the most common cause. Although previous reports have hypothesized why the right-to-left shunting is exacerbated in the upright position,2 the underlying mechanisms are not completely understood.
We describe a man with POS due to an ASD. Transesophageal echocardiography (TEE) clearly illustrated that shunting through the ASD changed from left to right in the supine position to right to left in the upright sitting position when focusing on the direction of blood flow from the inferior vena cava (IVC). In the supine position, left-to-right shunting through a small ASD was visualized. In the upright sitting position, right-to-left shunting was seen because of blood flow from the IVC redirected toward and passing through the ASD.
Case Presentation
A 65-year-old deaf man with a history of interstitial pneumonia (IP) presented with dyspnea. He had been effectively treated for IP-related dyspnea on exertion. However, he was currently experiencing dyspnea at rest; the dyspnea on exertion was not exacerbated.
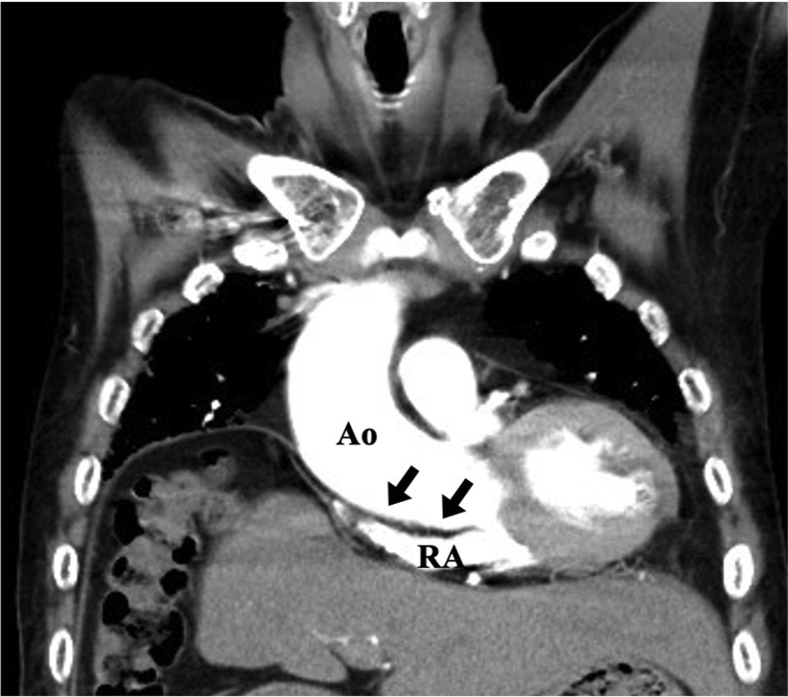
Laboratory testing showed slightly elevated concentrations of Krebs von den Lungen-6 (727 U/mL) and B-type natriuretic peptide (57.3 pg/mL). Electrocardiography showed first-degree atrioventricular block. Transthoracic echocardiography (TTE) showed normal left ventricular (LV) function, with an LV ejection fraction of 67%; normal left atrial size, with a left atrial volume index of 26 mL/m2; and normal size and function of the right ventricle (RV), right atrium (RA), and IVC, with an RV end-diastolic area of 18.9 cm2, an RV fractional area change of 47%, an RV tricuspid annular plane systolic excursion of 21 mm, a right atrial volume index of 18.6 mL/m2, and an IVC of 4 mm. Doppler evaluation showed mitral inflow with an E wave of 49 cm/sec, an A wave of 58 cm/sec, and trivial tricuspid regurgitation with a peak velocity of 2.1 m/sec. Pulmonary function testing suggested restrictive lung disease: the forced vital capacity was 57.8% of the predicted value, the forced expiratory volume in 1 second was 63% of the predicted value, and the forced expiratory volume in 1 second/forced vital capacity ratio was 0.89. Chest radiography and contrast-enhanced computed tomography showed diffuse reticular shadows, moderate loss of volume in both lower lung lobes, and elevation of the right diaphragm by lung volume loss, all of which had been previously identified. There was no evidence of exacerbation of IP, newly diagnosed pulmonary embolism, pneumonia, or upper airway stenosis. Computed tomography also showed an enlarged and horizontally positioned aortic root compressing the RA (Figure 1). In the supine position, the patient’s blood oxygen saturation was 95% on room air and he had no dyspnea. In the upright position, however, his blood oxygen saturation was 92% on 15 L/minute supplemental oxygen and dyspnea appeared. Chest auscultation revealed fine crackles in the base of both lungs, with otherwise unremarkable physical examination findings.
Figure 1.
Contrast-enhanced computed tomography imaging shows a mildly enlarged and horizontally positioned aortic root compressing the RA (black arrows) and counterclockwise rotation of the heart. The diameter of ascending aorta, sinotubular junction, sinus of Valsalva, and aortic valve annulus were 41.8, 33.9, 40.2, and 25.5 mm, respectively. Ao, Aortic root.
We suspected that the cause of the patient’s dyspnea was POS, and, therefore, we performed TTE with intravenous injection of agitated saline into his left arm. Transthoracic echocardiography with agitated saline injection showed the appearance of bubbles in the left atrium (LA) within the first three beats after right cavity opacification, indicating an intracardiac right-to-left shunt (Video 1). Valsalva maneuvers in the supine position resulted in more bubbles entering the LA than without the Valsalva maneuver. Furthermore, more bubbles were seen in the LA in the upright position than in the supine position (Video 2). Transesophageal echocardiography showed a small ASD (Figure 2A, Video 3). This report uses the term “small ASD” and not “patent foramen ovale (PFO)” because the appearance and location on TEE were more consistent with ASD than PFO.
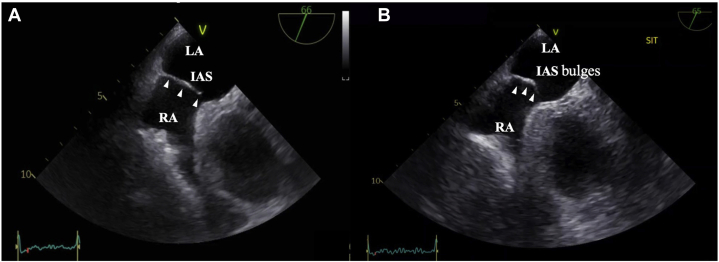
Figure 2.
Transesophageal echocardiography midesophageal short-axis view. (A) In the supine position, TEE showed a small ASD and the IAS did not bulge significantly into the LA (white arrowheads). The size of the ASD was 4.5 mm (Video 3). (B) In the upright sitting position, TEE showed that the IAS bulged significantly into the LA (white arrowheads). The size of the ASD was 4.6 mm and was not significantly larger than in the supine position; therefore stretch of the ASD was not obvious. In this case, there was no venous valve remnant such as a Eustachian valve (Video 4).
We used a reclining bed to sit the patient nearly upright (85°) to evaluate the change in intracardiac blood flow caused by postural change. In the upright sitting position, the atrial septum bulged with each beat into the LA (Figure 2B, Video 4). The TEE in the upright sitting position took several minutes to complete, and the leftward septal shift remained present while in this position. Color Doppler TEE in the supine position demonstrated the presence of a left-to-right shunt through the small ASD. Blood flowed from the IVC toward the posterior part of the interatrial septum (IAS) and not into the ASD (Figure 3A, Video 5). In the upright sitting position, right-to-left shunting through the small ASD occurred: blood flowed from the IVC toward and through the ASD (Figure 3B, Video 6). The TEE with agitated saline bubble study showed many bubbles flowing from the RA into the LA through the ASD (Figure 4, Video 7). Therefore, POS due to the small ASD was diagnosed.
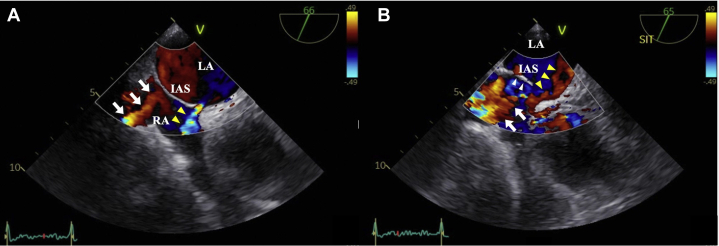
Figure 3.
Color Doppler TEE midesophageal short-axis view. (A) Color Doppler TEE in the supine position showed red-colored blood flow from the IVC directed toward the IAS (white arrows) and blue-colored blood flow passing through the ASD from the LA to the RA, indicating a left-to-right shunt (yellow arrowheads and Video 5). (B) Color Doppler TEE in the upright sitting position showed that the IAS bulged significantly into the LA (white arrowheads) and the ASD and blood flow from the IVC (white arrows) were aligned, which directed blood flow from the IVC toward and through the ASD (yellow arrowheads), indicating a right-to-left shunt (Video 6).
Figure 4.
Transesophageal echocardiography midesophageal short-axis view with agitated saline injection in the upright sitting position. Transesophageal echocardiography with agitated saline injection demonstrated many bubbles (yellow arrowheads) entering the LA via the ASD. Platypnea-orthodeoxia syndrome due to the small ASD was diagnosed (Video 7). LA, Left atrium.
We performed right heart catheterization in the supine position, without supplemental oxygen. The results were as follows: RA pressure was 2 mm Hg; RV end-diastolic pressure was 5 mm Hg; and pulmonary capillary wedge pressure was 7 mm Hg. We did not obtain catheterization data in the upright position.
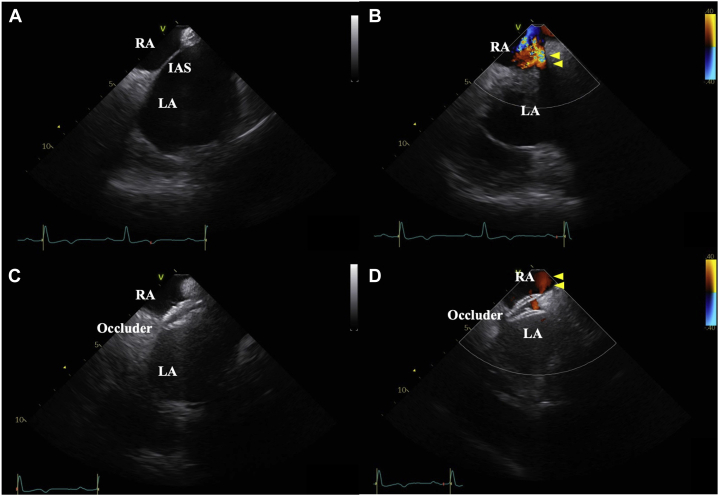
The patient had been treated with steroids for IP, and his respiratory function was poor; thus, he was considered high risk for general anesthesia and open-heart surgery. Therefore, we performed transcatheter ASD closure with a 25-mm Amplatzer Cribriform Occluder (St. Jude Medical, Saint Paul, MN) under local sedation using intracardiac echocardiography (ICE; Figure 5A–D, Videos 8 and 9).
Figure 5.
Intracardiac echocardiography septal short-axis view during ASD closure. (A) Before ASD closure, ICE showed a small ASD. (B) Before ASD closure, color Doppler ICE demonstrated red-colored flow (yellow arrowheads) passing through the ASD from the LA to the RA, indicating a left-to-right shunt, in the supine position (Video 8). (C) Intracardiac echocardiography demonstrated the ASD was closed with a 25 mm Amplatzer Cribriform Occluder. (D) After ASD closure, color Doppler ICE also demonstrated the ASD was successfully closed with an occluder device. The red-colored flow (yellow arrowheads) passed through the occluder device in the supine position, but the blood flow was considerably reduced (Video 9).
After the procedure, his symptoms improved and his blood oxygen saturation was 92% in the upright position without supplemental oxygen. The patient experienced no exacerbations of dyspnea for 1 year, but he eventually died of an acute exacerbation of IP 15 months after ASD closure.
Discussion
First reported in 1949, POS is a rare condition defined by dyspnea and oxygen desaturation induced by the upright position and relieved in the supine position.1 Diagnosis of POS may be difficult in the presence of respiratory disease; however, if the clinical course does not explain hypoxemia or dyspnea, POS should be considered. The status of our patient’s respiratory disease could not fully explain his symptoms, and we therefore suspected POS as a possible cause of his hypoxemia.
The causes of POS are classified into three categories: intracardiac shunts, pulmonary arteriovenous shunts,3 and ventilation-perfusion (V-Q) mismatch.4 The most common cause is interatrial shunting through an ASD or PFO.5,6 When the right atrial pressure is higher than the left atrial pressure in conditions such as pericardial disease, pulmonary embolism, or right heart infarction, right-to-left shunting through the defect occurs. However, when there are anatomical and functional components that direct venous blood flow toward the ASD/PFO, as in patients with a prominent Eustachian valve7 or aortic elongation or dilatation,8 right-to-left shunting can occur even in the absence of elevated right atrial pressure.
How the left-to-right shunt worsens in the upright position and improves in the supine position is not always clear. It has been proposed that in the supine position, inflow from the IVC goes toward part of the IAS, whereas in the upright position, the ASD and the IVC align and the inflow from the IVC is redirected to pass directly through the ASD, resulting in a right-to-left shunt. It has also been proposed that stretching of the interatrial communication increases the flow directed toward the ASD.9
Few case reports have actually described how postural changes alter intracardiac blood flow patterns because performing TEE in the upright position is burdensome for patients. Our patient had a physiologic mechanism of POS. His mildly enlarged and horizontally positioned aortic root compressing the RA and the counterclockwise rotation of his heart due to elevation of the diaphragm by lung volume loss may have promoted venous blood from the IVC to flow more easily through the ASD.9 Moreover, TEE revealed left-to-right shunting via a small ASD and blood flow from the IVC directed superiorly toward the posterior part of the IAS in the supine position. In the upright sitting position, stretching of the IAS was not clearly visible, but the ASD and IVC were aligned and flow from the IVC was redirected and passed through the ASD, confirming one of the above theories. In addition, the IAS bulged with each beat into the LA, which also seemed to facilitate passage of blood from the IVC through the ASD.
This report has two limitations. First, we were not able to perform catheterization in the upright position and, therefore, cannot rule out the possibility of increased RA pressure in the upright position. Second, we did not perform lung scintigraphy and, therefore, did not assess the possibility of V-Q mismatch due to loss of lung volume in the upright position. The V-Q mismatch may have contributed to the patient’s hypoxia, but considering the LA bubbles and shunting visualized on TEE and the improvement of desaturation after closure of the ASD, we think V-Q mismatch was not the main factor of desaturation in this patient.
Conclusion
Transesophageal echocardiography can clearly illustrate why right-to-left shunting occurs in POS patients in the upright position but not in the supine position. Such shunting is caused by posture-related changes in blood flow from the IVC and ASD. Transesophageal echocardiography performed in the upright position requires patient cooperation but is very useful to understand and diagnose POS.
Acknowledgments
We thank all the medical staff involved in the care of the patient in this case report. We also thank Angela Morben, DVM, ELS, and Leah Cannon, PhD, from Edanz (https://jp.edanz.com/ac), for editing a draft of this manuscript.
Footnotes
Conflicts of Interests: None.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.case.2022.03.007.
Supplementary Data
Transthoracic echocardiography apical four-chamber view with agitated saline injection in the supine position suggested the appearance of bubbles in the LA within the first three beats after right cavity opacification, indicating an intracardiac right-to-left shunt.
Transthoracic echocardiography apical four-chamber view with agitated saline injection in the upright position. More bubbles were seen in the LA in the upright position than in the supine position. The cause of the patient’s desaturation exacerbated in the upright position was strongly suspected to be POS.
Transesophageal echocardiography midesophageal short-axis view in the supine position showed a small ASD, and the IAS did not significantly bulge into the LA. The size of the ASD was 4.5 mm (Figure 2A).
Transesophageal echocardiography midesophageal short-axis view in the upright sitting position demonstrated that the IAS bulged significantly into the LA. The size of the ASD was 4.6 mm and was not significantly larger than in the supine position; therefore stretch of the ASD was not obvious (Figure 2B).
Color Doppler TEE midesophageal short-axis view in the supine position showed red-colored blood flow from the IVC directed toward part of the IAS and blue-colored blood flow passing through the ASD from the LA to the RA, indicating a left-to-right shunt (Figure 3A).
Color Doppler TEE midesophageal short-axis view in the upright sitting position showed the ASD and blood flow from the IVC were aligned and the red-colored inflow from the IVC was redirected and passed through the ASD from the RA to the LA, indicating a right-to-left shunt (Figure 3B).
Transesophageal echocardiography midesophageal short-axis view with agitated saline injection in the upright sitting position demonstrated many bubbles entering the LA via the ASD. Platypnea-orthodeoxia syndrome due to the small ASD was diagnosed (Figure 4).
Intracardiac echocardiography septal short-axis view before ASD closure in the supine position demonstrated red-colored flow passing through the ASD from the LA to the RA, indicating a left-to-right shunt (Figure 5B).
Intracardiac echocardiography septal short-axis view after ASD closure in the supine position showed the ASD was successfully closed with a 25 mm Amplatzer Cribriform Occluder. After ASD closure, a red-colored residual shunt passed through the occluder device, but the blood flow was considerably reduced (Figure 5D).
References
- 1.Burchell H.B., Helmholz H.F., Jr., Wood E.H. Reflex orthostatic dyspnea associated with pulmonary hypertension. Am J Physiol. 1949;159:563–564. [Google Scholar]
- 2.Rodrigues P., Palma P., Sousa-Pereira L. Platypnea-orthodeoxia syndrome in review: defining a new disease? Cardiology. 2012;123:15–23. doi: 10.1159/000339872. [DOI] [PubMed] [Google Scholar]
- 3.Robin E.D., Laman D., Horn B.R., Theodore J. Platypnea related to orthodeoxia caused by true vascular lung shunts. N Engl J Med. 1976;294:941–943. doi: 10.1056/NEJM197604222941711. [DOI] [PubMed] [Google Scholar]
- 4.Seward J.B., Hayes D.L., Smith H.C., Williams D.E., Rosenow E.C., 3rd, Reeder G.S., et al. Platypnea-orthodeoxia: clinical profile, diagnostic workup, management, and report of seven cases. Mayo Clin Proc. 1984;59:221–231. doi: 10.1016/s0025-6196(12)61253-1. [DOI] [PubMed] [Google Scholar]
- 5.Takiguchi H., Niimi K., Aoki T., Ogiya R., Ohno Y., Nakazawa G., et al. Platypnea-orthodeoxia syndrome caused by a latent atrial septal defect. Intern Med. 2013;52:1809–1811. doi: 10.2169/internalmedicine.52.0578. [DOI] [PubMed] [Google Scholar]
- 6.Agrawal A., Palkar A., Talwar A. The multiple dimensions of platypnea orthodeoxia syndrome: a review. Respir Med. 2017;129:31–38. doi: 10.1016/j.rmed.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 7.Bashour T., Kabbani S., Saalouke M., Cheng T.O. Persistent Eustachian valve causing severe cyanosis in atrial septal defect with normal right heart pressures. Angiology. 1983;34:79–83. doi: 10.1177/000331978303400201. [DOI] [PubMed] [Google Scholar]
- 8.Popp G., Melek H., Garnett A.R., Jr. Platypnea-orthodeoxia related to aortic elongation. Chest. 1997;112:1682–1684. doi: 10.1378/chest.112.6.1682. [DOI] [PubMed] [Google Scholar]
- 9.Cheng T.O. Platypnea-orthodeoxia syndrome: etiology, differential diagnosis and management. Catheter Cardiovasc Interv. 1999;47:64–66. doi: 10.1002/(SICI)1522-726X(199905)47:1<64::AID-CCD15>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transthoracic echocardiography apical four-chamber view with agitated saline injection in the supine position suggested the appearance of bubbles in the LA within the first three beats after right cavity opacification, indicating an intracardiac right-to-left shunt.
Transthoracic echocardiography apical four-chamber view with agitated saline injection in the upright position. More bubbles were seen in the LA in the upright position than in the supine position. The cause of the patient’s desaturation exacerbated in the upright position was strongly suspected to be POS.
Transesophageal echocardiography midesophageal short-axis view in the supine position showed a small ASD, and the IAS did not significantly bulge into the LA. The size of the ASD was 4.5 mm (Figure 2A).
Transesophageal echocardiography midesophageal short-axis view in the upright sitting position demonstrated that the IAS bulged significantly into the LA. The size of the ASD was 4.6 mm and was not significantly larger than in the supine position; therefore stretch of the ASD was not obvious (Figure 2B).
Color Doppler TEE midesophageal short-axis view in the supine position showed red-colored blood flow from the IVC directed toward part of the IAS and blue-colored blood flow passing through the ASD from the LA to the RA, indicating a left-to-right shunt (Figure 3A).
Color Doppler TEE midesophageal short-axis view in the upright sitting position showed the ASD and blood flow from the IVC were aligned and the red-colored inflow from the IVC was redirected and passed through the ASD from the RA to the LA, indicating a right-to-left shunt (Figure 3B).
Transesophageal echocardiography midesophageal short-axis view with agitated saline injection in the upright sitting position demonstrated many bubbles entering the LA via the ASD. Platypnea-orthodeoxia syndrome due to the small ASD was diagnosed (Figure 4).
Intracardiac echocardiography septal short-axis view before ASD closure in the supine position demonstrated red-colored flow passing through the ASD from the LA to the RA, indicating a left-to-right shunt (Figure 5B).
Intracardiac echocardiography septal short-axis view after ASD closure in the supine position showed the ASD was successfully closed with a 25 mm Amplatzer Cribriform Occluder. After ASD closure, a red-colored residual shunt passed through the occluder device, but the blood flow was considerably reduced (Figure 5D).