Abstract
Background
A sleeve lobectomy is a routine operation in thoracic surgery. However, sleeve lobectomy is not only a complex operation, but also has the risk of anastomotic leakage and stenosis. We used bronchial flap to reconstruct the airway instead of sleeve lobectomy. The above disadvantages can be avoided because the bronchial flap reconstruction airway has no anastomosis. This technique has not previously been reported. This paper discusses the feasibility and safety of reconstructing the bronchus with the pedicle autogenous bronchus flap in lung cancer surgery.
Methods
During the operation, when the tumor tissue had invaded ≤1/3 of the circumference of the lobar bronchus, the bronchus wall was removed at least 5 mm away from the tumor, but the contralateral healthy bronchus wall was preserved. The healthy bronchial wall was made into a “tongue-shaped” pedicled autogenous bronchial flap, approximately the size of the bronchial defect, and the flap was turned up or down to repair the root defect of the bronchus. The patients were examined every 3 months after surgery by chest computed tomography (CT) to observe the re-expansion of lung and reconstruction of the bronchus, and analyze the incidence of bronchus stenosis and local recurrence.
Results
The lobar bronchus was successfully reconstructed with the pedicled autologous bronchial flap in 45 patients; 36 males and 9 females with an average age of 56.5 years. The diameters of the tumors ranged from 3–12 cm. The pathological examination results showed that the margin of bronchus was negative. There was no perioperative death or bronchopleural fistula. The bronchoscopy showed that the reconstructed bronchus healed well, and no atelectasis or bronchostenosis was found in the follow-up period.
Conclusions
This is the first report on the application of the pedicled autogenous bronchial flap being used to reconstruct the airway instead of a sleeve lobectomy in lung cancer surgery. In the radical resection of lung cancer, the operation can simplify the operation process, and reduce the risk of anastomotic leakage or stenosis. The operation is safe and feasible, and should be more widely used.
Keywords: Bronchial flap, sleeve lobectomy, lung cancer
Introduction
In lung cancer surgery, when the tumor invades the orifice of the lobar bronchus, a sleeve lobectomy is often used to retain as much of the normal lobes as possible and minimise the loss of lung function (1,2). A sleeve lobectomy is a routine operation in thoracic surgery. However, sleeve lobectomy may face some difficulties, such as anastomosis, mismatch of distal and proximal bronchial calibers. Additionally, the interruption of blood supply and the cilia continuity of the bronchus may affect the healing and expectoration of the bronchus after operation, and there are risks of atelectasis, pulmonary infection, anastomotic leakage, and stenosis (3,4).
The repair of the trachea and carina with the autogenous bronchial flap has been reported in the literatures (5-7). To simplify the sleeve lobectomy surgery, shorten the operation time, and reduce the incidence of post-operative anastomotic and pulmonary complications, we adapted the operation technology for some cases requiring sleeve lobectomy. Under our approach, if the tumor only invades the single side wall (the tumor wall) of the root of the lobar bronchus (rather than the peripheral bronchus wall), and the opposite side wall (the healthy wall) is a healthy bronchus without tumor tissue, we conserve the healthy bronchus wall. The remaining side of the normal bronchial wall is made into a “tongue-shaped” pedicled autogenous bronchial flap of an appropriate size, which is then turned over to cover the damaged bronchial wall after the tumor resection. Instead of a sleeve lobectomy, a pedicled autogenous bronchial flap is used to reconstruct the airway. The main advantage of bronchial flap for airway reconstruction is that there is no anastomosis, which is potentially helpful for addressing the limitations of sleeve lobectomy. However, there are limited reports of using pedicled autogenous bronchial flap reconstruction of the lobar bronchus instead of a sleeve lobectomy in the treatment of lung cancer. This article discusses the safety and feasibility of airway reconstruction with the pedicled autogenous bronchial flap instead of sleeve lobectomy, and summarizes the technical characteristics and advantages of this operation. We present the following article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-347/rc).
Methods
Clinical data
Between January 2012 and December 2018, 269 patients underwent a sleeve lobectomy with a curative intent for lung cancer, of whom 47 were eligible for initial enrolment in this study. However, 2 of these patients were converted to sleeve lobectomy because of the positive margin of the frozen section. Forty-five patients underwent a lobectomy with a pedicled autogenous bronchial flap instead of a sleeve lobectomy. The cohort comprised 36 males and 9 females, with an average age of 56.5 years [standard deviation (SD): 10.52 years, range, 38–73 years]. The diameters of the tumors ranged from 3–12 cm, and were on average 5.11 cm (SD: 2.37 cm). In terms of histology, 21 patients had squamous cell carcinoma, 19 patients had adenocarcinoma, 1 patient had small cell lung cancer, 1 patient had large cell lung cancer, and 3 patients had other histological types of lung cancer. All tumors were staged using the Union for International Cancer Control (UICC, 8th edition) tumor-node-metastasis (TNM) classification.
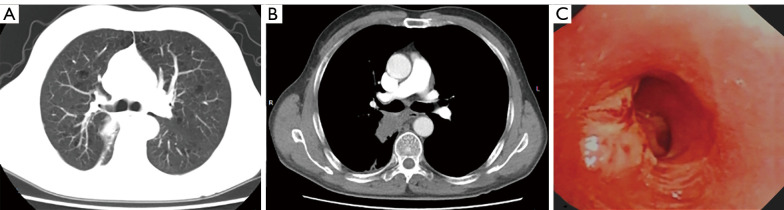
To be eligible for inclusion in this study, patients had to meet the following criteria: (I) have a chest enhanced computed tomography (CT) scan that showed that the tumor had invaded the orifice of the lobar bronchus (see Figure 1A,1B), the mediastinal lymph nodes were not enlarged, the pulmonary artery was not invaded, and a sleeve lobectomy was needed; (II) have undergone a fibreoptic bronchoscopy that showed that the tumor had invaded ≤1/3 of the circumference of the bronchial root, and the rest of the bronchial wall was healthy and had no tumor tissue (see Figure 1C); (III) have an abdominal ultrasound, brain magnetic resonance imaging (MRI), and bone emission CT (ECT) scans that showed no distant metastasis; and (IV) have heart and lung function that could tolerate the operation. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional review board of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (No. TJ-C20131011). Informed consent was taken from all the patients.
Figure 1.
Preoperative chest CT and bronchoscopy. (A,B) The chest enhanced CT showed that the tumor invaded the orifice of lobar bronchus. (C) Fiberoptic bronchoscopy showed that the tumor invaded no more than 1/3 of the circumference of the bronchial. CT, computed tomography.
Operative procedure
All patients received intravenous anesthesia and inhalation anesthesia through double-lumen endotracheal intubation. Patients were placed in the lateral decubitus position. A posterolateral incision was used to enter the chest through the fourth or fifth intercostal space. The tumor was completely resected by thoracotomy. During the operation, the vein and artery of the target pulmonary lobe were cut off, and tumor invasion to the bronchus was then explored. If the extent of tumor invasion was ≤1/3 of the circumference of the bronchi (see Figure 2A), the healthy bronchial wall was made into a “tongue-shaped” pedicled autogenous bronchial flap, approximately the size of the bronchial defect (see Figure 2B), and the flap was turned up or down to repair the root defect of the bronchus (see Figure 2C). During operation, the lateral wall of the diseased bronchi was removed at least 5 mm from the tumor margin. The bronchial excision margins were then sent for a frozen section analysis to ensure the margin was tumor-free. The remaining healthy bronchus was cut into a “tongue-shaped” pedicled bronchial flap that was approximately the same size as the defective bronchus (see Figure 3A). The pedicled autogenous bronchial flap was turned up or down to cover the bronchus defect (see Figure 3B). If the tumor tissue had invaded >1/3 of the circumference of the lobar bronchus, or the frozen section was positive, a conventional sleeve lobectomy was performed. An intraoperative frozen section examination was performed to ensure that those with negative margins were included in the study. The Surgipro II Monofilament Polypropylene 4-0 Suture (Covidien, Dublin, Ireland) was used to close the bronchial defect and reconstruct the airway integrity. The systematic dissection of the mediastinal and hilar lymph nodes was performed in all cases in a similar fashion to that in a routine sleeve lobectomy. The reconstructed bronchus was checked for air leakage in the presence of water by inflating the lung. No air leakage was detected during lung re-ventilation, and the lobes inflated normally. The pedicled aortic adventitia flap was applicable in the reinforcement of the bronchial stump in the left thoracic surgery, which we have reported previously (8), and in the pedicled azygos vein flap in the right lobectomy (see Figure 3C). The chest tube was placed, and the incisions were closed.
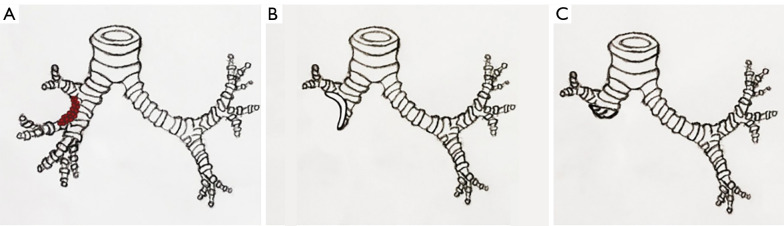
Figure 2.
Schematic diagram of airway reconstruction with a pedicled autologous bronchial flap. (A) Tumor invasion is ≤1/3 of the circumference of the bronchi. (B) The normal bronchial wall was made into a “tongue-shaped” pedicled autogenous bronchial flap. (C) The bronchial flap was turned over to cover the damaged bronchial wall.
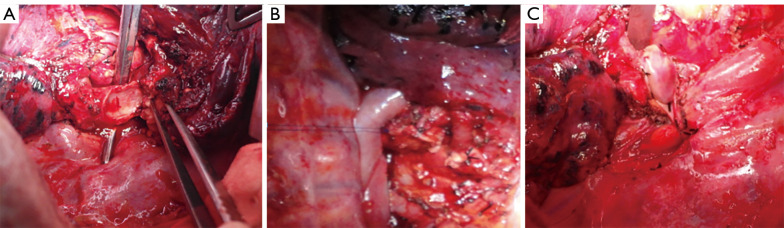
Figure 3.
Reconstruction of the airway with bronchial flaps. (A) The healthy bronchus was cut into a “tongue-shaped” pedicled bronchial flap. (B) The pedicled autogenous bronchial flap was turned up to cover the bronchus defect. (C) The pedicled azygos vein flap was used to reinforce the bronchial stump.
Post-operative management
The post-operative management of patients who underwent pedicled autologous bronchial flap surgery was similar to that of those who underwent lobectomy as we previously reported (9). Post-operative management mainly included the prophylactic use of antibiotics, post-operative analgesia, and airway management. The chest tube was removed when drainage was <200 mL/day and when no bubbles escaped. One week post-operation, chest CT was used to evaluate the recruitment of the residual lung. Every 3 months after operation, the patients were examined by chest CT to observe the re-expansion of lung and the reconstruction of the bronchus, and bronchoscopy was performed as necessary. The bronchoscopy and CT scans were used to determine the incidence of bronchial stenosis and local tumor recurrence.
Statistical analysis
All statistical tests were performed using SPSS version 23.0 (SPSS Science Inc., Chicago, IL, USA). Most of the statistical analyses are descriptive in this study. Categorical variables were presented as a count and percentage.
Results
Between January 2012 and December 2018, 269 patients underwent a sleeve lobectomy, of whom 47 were eligible for initial enrolment in this study. However, 2 of these patients were converted to sleeve lobectomy because of the positive margin of the frozen section. The airway was successfully reconstructed with the pedicled autogenous bronchial flap instead of a sleeve lobectomy in 45 patients. The reconstruction of the right upper bronchus with the right middle segment bronchial flap (instead of a sleeve resection of the right middle lower lung) was performed in 19 cases. A reconstruction of the right main bronchus with the right upper bronchial flap (instead of a sleeve resection of the right upper lung) was performed in 11 cases. A reconstruction of the left upper bronchus with the left lower bronchial flap (instead of a sleeve resection of the left lower lung) was performed in 8 cases. A reconstruction of the left lower bronchus with the left upper bronchial flap (instead of a sleeve resection of the left upper lung) was performed in 7 cases. Patients’ characteristics are set out in Table 1.
Table 1. Patients’ characteristics.
| Assessment | Value |
|---|---|
| Age (years), mean ± SD | 56.5±10.52 |
| Gender, n | |
| Male | 36 |
| Female | 9 |
| The maximum diameter of the tumor (cm), mean ± SD | 5.11±2.37 |
| FEV1 (%), mean ± SD | 69.5±10.33 |
| MVV (L), mean ± SD | 97.01±27.81 |
| Histologic type, n | |
| Squamous cell carcinoma | 21 |
| Adenocarcinoma | 19 |
| Small cell lung cancer | 1 |
| Large cell lung cancer | 1 |
| Other histologic types | 3 |
| Bronchial flap, n | |
| LLL | 8 |
| LUL | 7 |
| RML | 19 |
| RUL | 11 |
| Pathology T stage, n | |
| T1 | 45 |
| Pathology N stage, n | |
| N0 | 35 |
| N1 | 4 |
| N2 | 6 |
| Anastomosis time (min), mean ± SD | 19.5±7.6 |
| Post-operative complications, n | |
| Arrhythmia | 1 |
| Pneumonia | 5 |
| Post-operative hospital stay (days), mean ± SD | 6.52±2.66 |
| Median follow-up time (years) | 3.4 |
FEV1, forced expiratory volume in one second; MVV, maximum voluntary ventilation; LLL, left lower lung; LUL, left upper lung; RML, right middle lower lung; RUL, right upper lung; SD, standard deviation.
During the operation, the reconstructed bronchi were tight and firm. The median bronchial anastomosis time was 19.5±7.6 minutes (SD: 7.6 minutes; range, 10–37 minutes). The chest tube was removed on average 3 days after surgery. The routine pathological examination showed that the margin of the bronchus was negative. The incidence of major post-operative complications was 13.3% (6/45). There was 1 case of arrhythmia and 5 cases of pulmonary infection. After symptomatic treatment, all patients recovered. No perioperative death or serious complications occurred.
One week after the operation, the chest CT showed that the lung recruitment of each patient was well, and were without bronchopleural fistula, atelectasis, or bronchostenosis. The patients were discharged 5–10 days after surgery. The follow-up period ranged from 1–7 years (mean: 3.4 years), and the follow-up rate was 86.7% (39/45). During the follow-up period, the chest CT scans showed that the lungs recruitment of the patient were well (see Figure 4A,4B), bronchoscopies showed that the reconstructed bronchi healed well, and no stenosis was found (see Figure 4C). There was 1 case of local recurrence of the bronchus flap (1/45, 2.2%), and 6 cases of distant metastasis (6/45, 13.3%).
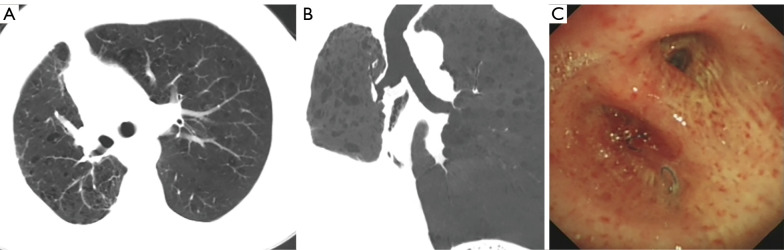
Figure 4.
Post-operative examination of a bronchial flap. (A,B) The chest CT showed that the lung recruitment was well. (C) A bronchoscopy showed that the reconstructed bronchus healed well, and no stenosis was found. CT, computed tomography.
Discussion
When a tumor invades the root of the lobular bronchus, a sleeve resection is often used for the radical resection of the lung cancer to retain the normal lobes of the lung and reduce the loss of lung function. The latest research shows that neo-adjuvant chemo-immunotherapy is effective and safe with a sleeve lobectomy for non-small cell lung cancer (NSCLC) patients (10). Sleeve lobectomy involves several complex procedures, such as bronchial disconnection, anastomosis, and a mismatch of the distance and near port diameter. Further, the interruption of bronchial blood supply and cilia continuity is not conducive to post-operative healing and expectoration, and there is a risk of anastomotic leakage and stenosis (4,11).
In some sleeve resection of the bronchus cases, if the tumor only invades one side wall of the bronchus root (the tumor wall) and not the whole circumference of the bronchial wall, the opposite side wall is a healthy bronchus without tumor tissue (the healthy wall). This report examined whether it was possible to resect the tumor wall and keep the healthy wall, to make the remaining normal bronchial wall into a bronchial flap, and to then turn this flap up or down to reconstruct the bronchus. This technique simplifies the operation process and achieves the goal of the radical operation. No previous research has examined the use of a bronchial flap (instead of a sleeve lobectomy) in the reconstruction of the lobular bronchus. However, the repair of the trachea with the bronchial flap has been reported in the literature (5-7). Bronchial flap reconstruction presents an efficient therapeutic strategy for closing massive central airway defects after non-circumferential tracheal resection (5-7). The pedicled autologous bronchial flap provides reliable material to repair and reconstruct a massive central airway defect (12). McGregor et al. described a novel method for the closure of the bronchus intermedius using a flap derived from the lower lobe of the apical segmental bronchus (13). McGregor et al. reported that they used the bronchial flap to avoid performing a right middle lung lobectomy, but made no mention of reconstructing the airway with the bronchial flap instead of a sleeve resection. Inspired by this operation, we tried to use the bronchial flap to reconstruct the airway in the lobectomy, which simplified the bronchial anastomosis process and partially preserved the continuity of the bronchus.
First, the feasibility was demonstrated in theory. According to statistics, the average diameter of the lobar bronchus is 10.42 mm (14). The diameter of the lobular bronchus is 10 mm, and the circumference is 31.4 mm according to C=πD. If the tumor invades 1/3 of the circumference, this is equal 10.4 mm. The distance from the tumor is ≥5 mm (15), so the remaining diameter of the bronchial flap is 11 mm, which is enough to reconstruct and repair the bronchial defect. In this study, the airways of 45 patients with tumors invading ≤1/3 of the circumference of the root of the bronchus were successfully reconstructed with the bronchial flap. The reconstructed bronchus was tight, firm, and elastic. There was no bronchopleural fistula in the perioperative period. The bronchus healed well and no stenosis was found by fibrobronchoscopy after surgery. Only 1 patient had a local recurrence of a tumor in the bronchus, and the overall effect was satisfactory.
This procedure can partly replace the sleeve resection of the bronchus, but it has high selectivity. Specifically, it is only suitable for lung cancer patients whose tumor is ≤1/3 the circumference of the lobar bronchus. This group of cases only accounts for 16.9% (45/269) of the cases requiring sleeve resection. This operation is not a replacement for the traditional sleeve lobectomy. Sleeve resection is needed when the tumor invades >1/3 of the circumference of the bronchi, or when the frozen pathological examination results are positive. In this study, 2 cases were converted to a conventional sleeve lobectomy because of the positive frozen pathological examination results. Thus, it is necessary to emphasize the importance of the negative margin of the frozen section during surgery to ensure that the tumor resection is thorough. The median follow-up time was 3.4 years, and the local recurrence rate was 2.2% (1/45). The local recurrence rate of bronchus after sleeve lobectomy was 4.6% (10/218) (16).
This study is the first to report the application of airway reconstruction with the pedicled autogenous bronchial flap instead of a sleeve lobectomy in lung cancer surgery, which has 5 advantages: (I) as there is no bronchial anastomosis, it is not necessary to consider the mismatch between the distal and proximal bronchi, which simplifies the procedure of anastomosis; (II) the reconstruction of the bronchus involves homogeneous autogenous bronchus material, and results in good shaping, no collapse, and no rejection; (III) the materials for operation are easy to obtain; (IV) the preservation of partial blood supply and cilia continuity in the endobronchial beneficial to the healing of the bronchus and the elimination of phlegm; and (V) as the closed suture reconstruction of the bronchus does not occur in the same plane, the likelihood of post-operative bronchus stenosis is potentially reduced. Compared to the cases of sleeve resection in the same period, the process was simplified, which is conducive to post-operative rehabilitation. However, we need to further compare our approach with the sleeve lobectomy to explore its advantages.
For the radical resection of lung cancer, our approach is as effective at reducing the loss of lung as a sleeve lobectomy, does not involve a complicated anastomosis process, and reduces the risk of leakage or stenosis of the bronchi. It is suitable for lung cancer cases in which the tumor is ≤1/3 of the circumference of the root of the lobar bronchus, and when there is enough healthy bronchus wall to reconstruct the airway. The frozen section examination during the operation ensures that the margin of the bronchus was negative. Our preliminary results show that this method has potential advantages over the sleeve lobectomy and anastomosis in selected cases. A further prospective comparative study with a sleeve lobectomy is warranted.
Supplementary
The article’s supplementary files as
Acknowledgments
The authors appreciate the academic support from the AME Thoracic Surgery Collaborative Group.
Funding: This work was supported by the National Science Foundation of China (No. 82072593).
Ethical Statement: The authors are accountable for all aspects of the work, including ensuring that any questions related to the accuracy or integrity of any part of the work have been appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional review board of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (No. TJ-C20131011). Informed consent was taken from all the patients.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-347/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-347/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-347/coif). Yongde Liao serves as an unpaid editorial board member of Translational Lung Cancer Research from October 2021 to September 2022. The other authors have no conflicts of interest to declare.
References
- 1.Pagès PB, Mordant P, Renaud S, et al. Sleeve lobectomy may provide better outcomes than pneumonectomy for non-small cell lung cancer. A decade in a nationwide study. J Thorac Cardiovasc Surg 2017;153:184-195.e3. 10.1016/j.jtcvs.2016.09.060 [DOI] [PubMed] [Google Scholar]
- 2.Li Z, Chen W, Xia M, et al. Sleeve lobectomy compared with pneumonectomy for operable centrally located non-small cell lung cancer: a meta-analysis. Transl Lung Cancer Res 2019;8:775-86. 10.21037/tlcr.2019.10.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ludwig C, Stoelben E, Olschewski M, et al. Comparison of morbidity, 30-day mortality, and long-term survival after pneumonectomy and sleeve lobectomy for non-small cell lung carcinoma. Ann Thorac Surg 2005;79:968-73. 10.1016/j.athoracsur.2004.08.062 [DOI] [PubMed] [Google Scholar]
- 4.Krüger M, Uschinsky K, Hässler K, et al. Postoperative complications after bronchoplastic procedures in the treatment of bronchial malignancies. Eur J Cardiothorac Surg 1998;14:46-52; discussion 52-3. 10.1016/S1010-7940(98)00143-2 [DOI] [PubMed] [Google Scholar]
- 5.Tomita M, Shimizu T, Ayabe T, et al. Bronchial flap closure of the lower membranous trachea. Ann Thorac Surg 2011;91:935-7. 10.1016/j.athoracsur.2010.08.014 [DOI] [PubMed] [Google Scholar]
- 6.Ren YJ, Zheng H, Shen LX, et al. A Novel Technique of Long-Segment Tracheal Repair With Extended Bronchial Flap of Right Upper and Main Bronchus Plus Tracheoplasty. Ann Thorac Surg 2015;99:2188-90. 10.1016/j.athoracsur.2014.08.052 [DOI] [PubMed] [Google Scholar]
- 7.Peng Q, Zhang L, Ren Y, et al. Reconstruction of Long Noncircumferential Tracheal or Carinal Resections With Bronchial Flaps. Ann Thorac Surg 2019;108:417-23. 10.1016/j.athoracsur.2019.02.057 [DOI] [PubMed] [Google Scholar]
- 8.Jiang WY, Liao YD, Cai YX, et al. Application of pedicled aortic adventitia flap in the reinforcement of bronchial stump or bronchial anastomotic stoma closure in left pulmonary resection. J Thorac Cardiovasc Surg 2014;148:351-3. 10.1016/j.jtcvs.2014.02.007 [DOI] [PubMed] [Google Scholar]
- 9.Zhang Z, Huang Q, Liao Y, et al. Application of the "continuous suture dividing and equal suture tightening" method in video-assisted thoracoscopic surgery sleeve lobectomy. J Thorac Dis 2018;10:5199-207. 10.21037/jtd.2018.08.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Y, Zhang L, Yan B, et al. Feasibility of sleeve lobectomy after neo-adjuvant chemo-immunotherapy in non-small cell lung cancer. Transl Lung Cancer Res 2020;9:761-7. 10.21037/tlcr-20-539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki T, Suzuki S, Kamio Y, et al. Closure with bronchial wall flap and omental pedicle of defect caused by dehiscence of tracheal suture line after extended right upper sleeve lobectomy. J Thorac Cardiovasc Surg 1996;112:1116-7. 10.1016/S0022-5223(96)70117-4 [DOI] [PubMed] [Google Scholar]
- 12.Chen QK, Jiang GN, Ding JA, et al. Reconstruction of the lower trachea using a pedicled autologous bronchial flap. Ann Thorac Surg 2010;89:e29-30. 10.1016/j.athoracsur.2010.01.059 [DOI] [PubMed] [Google Scholar]
- 13.McGregor RJ, West D, Walker WS. Bronchial flap closure of the right lower lobe bronchus. Ann Thorac Surg 2009;88:1712-3. 10.1016/j.athoracsur.2009.01.068 [DOI] [PubMed] [Google Scholar]
- 14.Montaudon M, Desbarats P, Berger P, et al. Assessment of bronchial wall thickness and lumen diameter in human adults using multi-detector computed tomography: comparison with theoretical models. J Anat 2007;211:579-88. 10.1111/j.1469-7580.2007.00811.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kayser K, Anyanwu E, Bauer HG, et al. Tumor presence at resection boundaries and lymph-node metastasis in bronchial carcinoma patients. Thorac Cardiovasc Surg 1993;41:308-11. 10.1055/s-2007-1013878 [DOI] [PubMed] [Google Scholar]
- 16.Yildizeli B, Fadel E, Mussot S, et al. Morbidity, mortality, and long-term survival after sleeve lobectomy for non-small cell lung cancer. Eur J Cardiothorac Surg 2007;31:95-102. 10.1016/j.ejcts.2006.10.031 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as