Abstract
Background
In the BEACON CRC study (NCT02928224), encorafenib plus cetuximab with binimetinib {9.3 versus 5.9 months; hazard ratio (HR) [95% confidence interval (CI)]: 0.60 [0.47-0.75]} or without binimetinib [9.3 versus 5.9 months; HR (95% CI): 0.61 (0.48-0.77)] significantly improved overall survival (OS) compared with the previous standard of care (control) in patients with BRAF V600E metastatic colorectal cancer (mCRC). Quality of life (QoL) was a secondary endpoint, assessed using validated instruments.
Patients and methods
BEACON CRC was a randomized, open-label, phase III study comparing encorafenib plus cetuximab with or without binimetinib and the investigator’s choice of irinotecan plus cetuximab or FOLFIRI plus cetuximab (chemotherapy control) in patients with previously treated BRAF V600E mCRC. Patient-reported QoL assessments included the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC) and Functional Assessment of Cancer Therapy—Colorectal (FACT-C). The primary outcome for these tools was time to definitive 10% deterioration.
Results
Encorafenib plus cetuximab, both with and without binimetinib, was associated with longer median times to definitive 10% deterioration versus the control group in the EORTC Global Health Status scale [HR (95% CI): 0.65 (0.52-0.80) versus 0.61 (0.49-0.75), respectively] and the FACT-C functional well-being subscale [HR (95% CI): 0.62 (0.50-0.76) versus 0.58 (0.47-0.72), respectively]. Consistent results were observed across all subscales of the EORTC and FACT-C instruments. QoL was generally maintained during treatment for the global EORTC and FACT-C scales.
Conclusions
In addition to improving OS, encorafenib plus cetuximab with or without binimetinib delays QoL decline in previously treated patients with BRAF V600E-mutant mCRC.
Key words: BEACON CRC, cetuximab, colorectal cancer, encorafenib, patient-reported outcomes, quality of life
Highlights
-
•
BEACON CRC compares encorafenib + cetuximab ± binimetinib to chemotherapy in previously treated BRAF V600E mCRC.
-
•
Encorafenib + cetuximab had longer time to 10% deterioration versus control in QoL and functional well-being scales.
-
•
Encorafenib + cetuximab ± binimetinib delays QoL decline in previously treated patients with BRAF V600E mCRC.
Introduction
Globally, colorectal cancer (CRC) is the second and third most commonly diagnosed cancer for women and men, respectively, with incidence rates highest in Australia, Europe, and North America.1,2 Although CRC represents the second leading cause of cancer deaths worldwide, earlier detection and advances in screening and available treatments are contributing to a decreasing trend in new cases and death rates.2,3 In the United States, the rate of new CRC cases decreased from 56.7 per 100 000 persons in 1992 to 34.8 in 2018, with the death rate decreasing from 23.6 to 12.8 per 100 000 persons during the same time period.3
Biomarker profiling and the availability of targeted and checkpoint inhibitor therapies have resulted in an increased proportion of patients with CRC, as those surviving ≥5 years post-diagnosis are no longer uncommon.4,5 CRC survivors now represent the third largest cancer survivor group in developed countries, with a 5-year relative survival rate of 64.7% in the United States.3,6
Initially, CRC treatment strategies focused mainly on tumor response and on prolonging progression-free survival (PFS) and overall survival (OS).7,8 However, with increased survivorship and more available treatment options, each with their own unique risk/benefit profiles, quality of life (QoL) has become an important and necessary complement to increasing duration of life.9 Unfortunately, CRC treatment options can lead to QoL challenges, such as the severe and unfavorable side-effects associated with chemotherapy that impart a substantial burden on a patient’s physical and mental well-being.10, 11, 12, 13, 14 Although every effort is made by clinicians to manage treatment adverse effects,15 these adverse events (AEs) invariably have detrimental effects on patients’ psychological and emotional well-being, social interactions, and ability to carry out daily activities,4,14,16 and, by extension, directly impact survivorship.
In patients with CRC, a higher QoL has been statistically significantly associated with all-cause mortality, even when accounting for those with metastatic disease,17 and CRC disease progression is associated with a statistically significant worsening of QoL measures.18 In addition to the relationship between patient well-being and longevity, QoL is associated as an independent predictor of response to treatment.19, 20, 21 Maisey et al. investigated baseline QoL in patients with advanced CRC in four randomized clinical trials and determined that global scores were a highly significant, independent predictor of survival.20 These findings have been corroborated by numerous other studies concluding that patient-reported outcomes were better predictors of response than performance status.19 In fact, improvement in physical functioning beyond baseline assessment has been associated with increased probability of survival.21
Maintaining QoL in patients with CRC, especially in those with metastatic CRC (mCRC), is critical to survival and is recognized as one of the major endpoints to evaluate treatments.22,23 In addition, European Society of Medical Oncology Clinical Practice Guidelines for mCRC and regulatory bodies such as the European Medicines Agency recognize the value of QoL measures beyond classical efficacy endpoints by including them in their guidelines and assessments for reimbursement.8
The combination of encorafenib with cetuximab represents one of the targeted treatment options approved in the United States and Europe for previously treated patients with BRAF V600E-mutated mCRC. As the only chemotherapy-free targeted therapy for patients with BRAF-mutant mCRC, it is an important treatment option in the mCRC armamentarium for this patient population. In the phase III BEACON CRC study, encorafenib plus cetuximab regimens with and without binimetinib significantly improved OS compared with standard chemotherapy (irinotecan or FOLFIRI) plus cetuximab.15,24 Encorafenib plus cetuximab demonstrated significantly longer median OS [9.3 versus 5.9 months; hazard ratio (HR) 0.61; 95% confidence interval (CI) 0.48-0.77] and a higher objective response rate (19.5% versus 1.8%) compared with standard chemotherapy.15 The rate of AEs of grade 3 or higher was slightly greater in the control group than in the combination group; there were few treatment discontinuations (≤9%) due to AEs.15 The safety and tolerability of the encorafenib plus cetuximab treatment regimens were consistent with the known safety profiles of BRAF, EGFR, and MEK inhibitors, and AEs were manageable with standard supportive care and treatment interruptions.15
However, considering the interrelationship of efficacy measures and QoL, a more complete picture is needed to evaluate the impact of treatment with encorafenib plus cetuximab with or without binimetinib on patient’s lives. Herein, we look beyond the classic clinical trial endpoints to the functional, social, and psychological well-being of patients and the impact of treatment on patient QoL in BEACON CRC.
Patients and methods
Study design
BEACON CRC (NCT02928224) is a randomized, open-label, phase III study in patients with BRAF V600E-mutant mCRC that had progressed after one or two prior treatment regimens.15,24 Patients were randomized to encorafenib plus cetuximab with binimetinib, encorafenib plus cetuximab without binimetinib, or the investigator’s choice of irinotecan or FOLFIRI plus cetuximab. Study design details and the primary endpoints have been published.15,24 The study was conducted in accordance with the Declaration of Helsinki, approvals obtained by regional and local institutional review boards, and informed consent obtained from all participants. The data cut-off for these analyses was 11 February 2019.
Patient-reported outcomes
Patient-reported QoL was a secondary endpoint of BEACON CRC, with the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC) version 3.0 used to assess key outcomes of interest. This 30-item questionnaire consists of an overall global health status/QoL score, five functioning scores (physical, role, emotional, cognitive, and social functioning), and three composite symptom scores (fatigue, nausea, and vomiting). In addition, six single-symptom items are assessed (pain, dyspnea, insomnia, appetite loss, constipation, and diarrhea).25, 26, 27 The global health status score uses a seven-point Likert scale ranging from ‘very poor’ to ‘excellent’, and the remaining items in the EORTC use a four-point Likert scale ranging from ‘not at all’ to ‘very much’.25,27,28 Items are scored 0-100 points, with a higher score representing better QoL in accordance with the scoring manual.29
QoL was also assessed using the patient self-reporting Functional Assessment of Cancer Therapy—General (FACT-G) and Colorectal (FACT-C) quesionnaires.30,31 The FACT-G is a 27-item questionnaire with a total score and four subscale QoL domains (physical, social, emotional, and functional well-being) for all patients with cancer. The FACT-C includes an additional nine questions specific to CRC and with a total score and adds to the four domains of the FACT-G plus an additional CRC subscale domain. The questionnaires are based on a five-point Likert scale with a recall period of the past 7 days, with a higher score representing better QoL.
QoL assessments were carried out at screening/baseline on day 1 of each treatment cycle, at the end of treatment, and at 30-day follow-up visits. The number of patients completing the questionnaires and the number of missing or incomplete assessments were summarized by timepoint. Results for each instrument were scored according to their respective scoring manuals.28, 29, 30,32,33 The median scores and change from baseline for each scale at the time of each assessment were summarized using descriptive statistics.
Time to definitive deterioration in the QoL domains was assessed in the three treatment arms in the full analysis set. The time to definitive deterioration is defined as the time from the date of randomization to the date of event, which is defined as at least 10% worsening relative to baseline of the corresponding scale score with no later improvement above this threshold observed during the course of the study or death due to any cause. If a patient did not have an event before either analysis cut-off or the start of another anticancer therapy, time to deterioration was censored at the date of the last adequate QoL evaluation. The distribution was presented descriptively using Kaplan–Meier curves. Median time to definitive deterioration along with two-sided 95% CI is reported. A Cox model was fitted with treatment arm and stratification factors as covariates to obtain an HR (95% CI) estimate of the treatment effect. The stratification factors used in the test were precisely those used for randomization and were based on the actual randomization information (interactive web response system).
Results
Patient characteristics and compliance
The study recruited 665 patients who were randomized to receive encorafenib plus cetuximab with binimetinib (n = 224), encorafenib plus cetuximab (n = 220), or control (n = 221).15,24 Full details and safety and efficacy results have been published.15,24 Patient characteristics were well balanced between groups at baseline (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2022.100477). At data cut-off, treatment was ongoing in 13.4% (n = 30) of patients in the encorafenib plus cetuximab with binimetinib group, 13.6% (n = 30) of patients in the encorafenib plus cetuximab group, and 3.2% (n = 7) in the control group.15,24 The most common reason for discontinuation in all three treatment groups (56%-66% of patients) was progressive disease.
Baseline QoL assessments indicated a balance across the treatment groups for each instrument and subscale (Table 1). From baseline to cycle 8, compliance with the EORTC and FACT-C instruments was 85.7%-94.3% in the encorafenib plus cetuximab with binimetinib group, 88.8%-95.7% in the encorafenib plus cetuximab group, and 83.3%-94.8% in the control group (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2022.100477).
Table 1.
Quality of life at baseline for encorafenib plus cetuximab, with or without binimetinib, and control
| Encorafenib plus cetuximab with binimetinib n = 224 | Encorafenib plus cetuximab n = 220 | Control n = 221 | |
|---|---|---|---|
| EORTC | |||
| Global Health Status | |||
| Patients, n | 209 | 201 | 200 |
| Mean (SD) | 62.8 (22.2) | 60.7 (21.3) | 62.8 (21.8) |
| Median (range) | 67 (0-100) | 67 (0-100) | 67 (0-100) |
| Physical functioning | |||
| Patients, n | 210 | 199 | 199 |
| Mean (SD) | 76.1 (19.4) | 73.3 (20.7) | 75.7 (20.5) |
| Median (range) | 80 (13-100) | 73 (0-100) | 80 (13-100) |
| Emotional functioning | |||
| Patients, n | 209 | 199 | 200 |
| Mean (SD) | 73.7 (23.4) | 74.1 (21.7) | 73.7 (23.0) |
| Median (range) | 75 (0-100) | 75 (0-100) | 75 (0-100) |
| Social functioning | |||
| Patients, n | 206 | 199 | 200 |
| Mean (SD) | 73.1 (24.9) | 70.9 (27.1) | 73.6 (25.0) |
| Median (range) | 67 (0-100) | 67 (0-100) | 67 (0-100) |
| Role functioning | |||
| Patients, n | 209 | 201 | 200 |
| Mean (SD) | 70.2 (28.88) | 68.2 (30.24) | 72.8 (28.17) |
| Median (range) | 67 (0-100) | 67 (0-100) | 67 (0-100) |
| Cognitive functioning | |||
| Patients, n | 209 | 199 | 200 |
| Mean (SD) | 85.4 (19.16) | 84.5 (19.57) | 83.3 (19.16) |
| Median (range) | 100 (0-100) | 83 (0-100) | 83 (0-100) |
| Fatigue | |||
| Patients, n | 210 | 200 | 200 |
| Mean (SD) | 38.7 (25.12) | 40.9 (25.41) | 38.3 (25.39) |
| Median (range) | 33 (0-100) | 33 (0-100) | 33 (0-100) |
| Nausea and vomiting | |||
| Patients, n | 210 | 199 | 200 |
| Mean (SD) | 9.8 (16.25) | 8.4 (16.23) | 11.4 (20.84) |
| Median (range) | 0 (0-100) | 0 (0-100) | 0 (0-100) |
| Pain | |||
| Patients, n | 210 | 200 | 200 |
| Mean (SD) | 31.9 (29.85) | 34.1 (30.37) | 32.3 (30.54) |
| Median (range) | 33 (0-100) | 33 (0-100) | 33 (0-100) |
| Dyspnea | |||
| Patients, n | 210 | 199 | 200 |
| Mean (SD) | 17.6 (25.92) | 16.9 (25.70) | 15.5 (23.12) |
| Median (range) | 0 (0-100) | 0 (0-100) | 0 (0-100) |
| Insomnia | |||
| Patients, n | 209 | 200 | 200 |
| Mean (SD) | 27.4 (29.64) | 28.3 (31.66) | 31.8 (31.04) |
| Median (range) | 33 (0-100) | 33 (0-100) | 33 (0-100) |
| Appetite loss | |||
| Patients, n | 210 | 199 | 200 |
| Mean (SD) | 23.3 (29.72) | 25.6 (30.46) | 24.2 (30.62) |
| Median (range) | 0 (0-100) | 33 (0-100) | 0 (0-100) |
| Constipation | |||
| Patients, n | 210 | 200 | 199 |
| Mean (SD) | 16.2 (25.31) | 17.0 (27.55) | 18.1 (29.53) |
| Median (range) | 0 (0-100) | 0 (0-100) | 0 (0-100) |
| Diarrhea | |||
| Patients, n | 208 | 198 | 200 |
| Mean (SD) | 16.0 (21.97) | 17.5 (23.67) | 16.3 (21.91) |
| Median (range) | 0 (0-100) | 0 (0-100) | 0 (0-100) |
| FACT-G and FACT-C | |||
| FACT-G total score | |||
| Patients, n | 208 | 200 | 198 |
| Mean (SD) | 74.7 (16.54) | 74.8 (14.86) | 75.8 (16.13) |
| Median (range) | 77 (23-107) | 76 (21-107) | 77 (22-106) |
| FACT-C total score | |||
| Patients, n | 207 | 199 | 197 |
| Mean (SD) | 94.2 (19.82) | 93.7 (18.54) | 94.8 (19.66) |
| Median (range) | 97 (36-134) | 96 (27-135) | 98 (29-134) |
| Functional well-being | |||
| Patients, n | 211 | 200 | 200 |
| Mean (SD) | 16.3 (6.2) | 16.2 (5.9) | 16.8 (6.1) |
| Median (range) | 16 (0-28) | 16 (0-28) | 17 (4-28) |
| Physical well-being | |||
| Patients, n | 211 | 202 | 199 |
| Mean (SD) | 20.7 (5.7) | 20.3 (5.5) | 20.9 (5.4) |
| Median (range) | 22 (4-28) | 21 (3-28) | 22 (6-28) |
| Social/family well-being subscale | |||
| Patients, n | 209 | 202 | 200 |
| Mean (SD) | 21.9 (5.3) | 22.2 (5.2) | 22.2 (5.4) |
| Median (range) | 23 (0-28) | 23 (0-28) | 23 (0-28) |
| Emotional well-being | |||
| Patients, n | 210 | 200 | 199 |
| Mean (SD) | 15.9 (5.0) | 16.2 (4.4) | 16.0 (4.9) |
| Median (range) | 17 (0-24) | 17 (3-24) | 16 (0-24) |
| Colorectal cancer | |||
| Patients, n | 210 | 200 | 200 |
| Mean (SD) | 19.3 (4.7) | 18.9 (5.0) | 19.0 (5.0) |
| Median (range) | 20 (7-28) | 20 (6-28) | 20 (2-28) |
EORTC, European Organization for Research and Treatment of Cancer Quality of Life of Questionnaire Core 30; FACT-C, Functional Assessment of Cancer Therapy—Colorectal; FACT-G, Functional Assessment of Cancer Therapy—General; SD, standard deviation.
EORTC Quality of Life Questionnaire Core 30
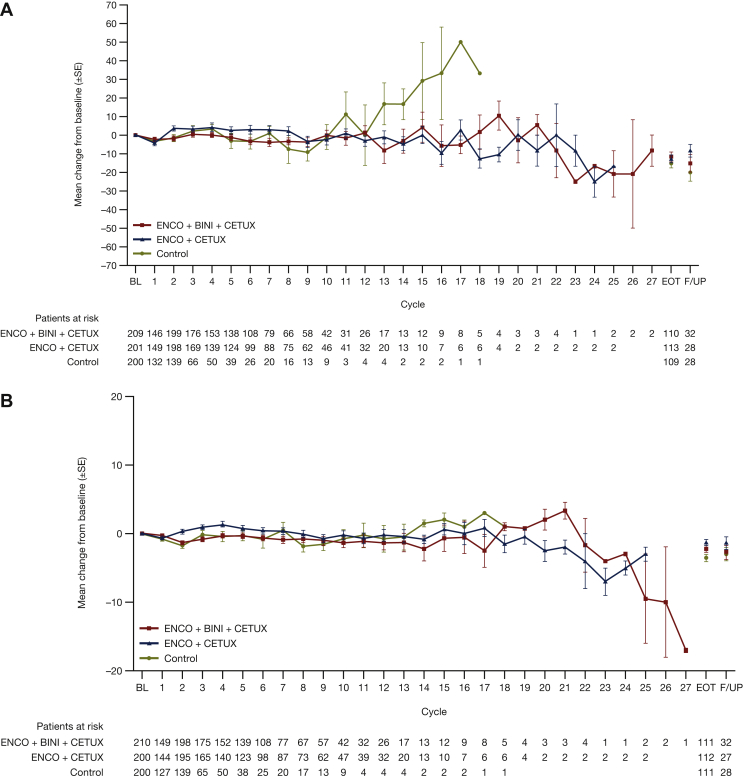
Baseline values for EORTC scores were similar across the groups (Table 1). EORTC global health status scores over time are shown in Figure 1A. QoL was generally maintained during treatment for both encorafenib plus cetuximab with or without binimetinib regimens compared with control.
Figure 1.
Scores over time for (A) EORTC global health status and (B) FACT-C colorectal cancer. Mean change of baseline (±SE) over time for (A) EORTC global health status and (B) FACT-C colorectal cancer by visit for patients treated with encorafenib plus cetuximab with or without binimetinib, or control. Baseline was defined as the last assessment carried out on or before the date of the first dose of study treatment.BINI, binimetinib; BL, baseline; CETUX, cetuximab; FACT-C, Functional Assessment of Cancer Therapy—Colorectal; ENCO, encorafenib; EORTC, European Organization for Research and Treatment of Cancer Quality of Life of Questionnaire Core 30; EOT, end of treatment; F/UP, follow-up; SE, standard error.
Time to deterioration: EORTC
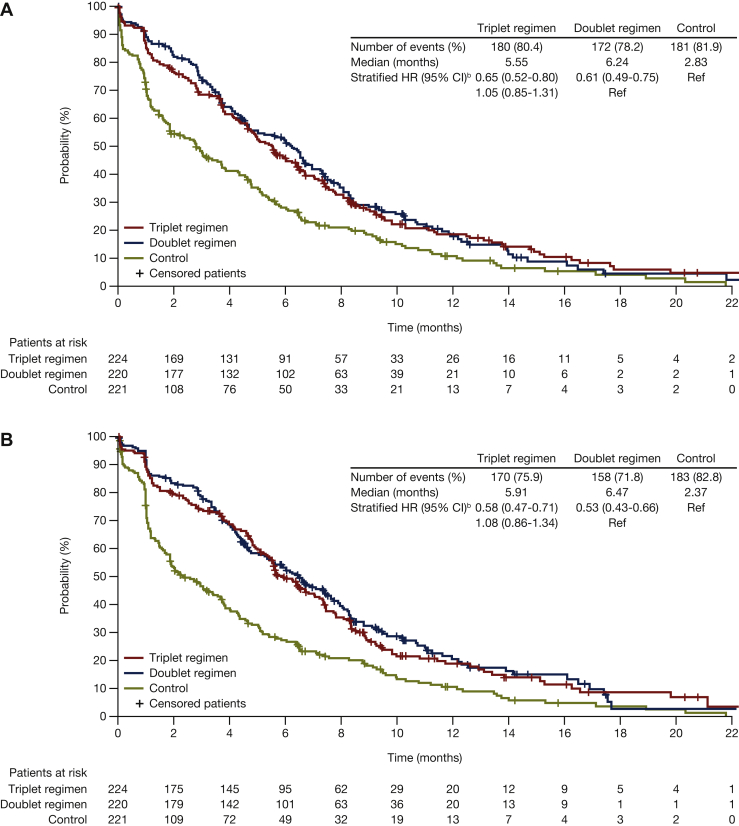
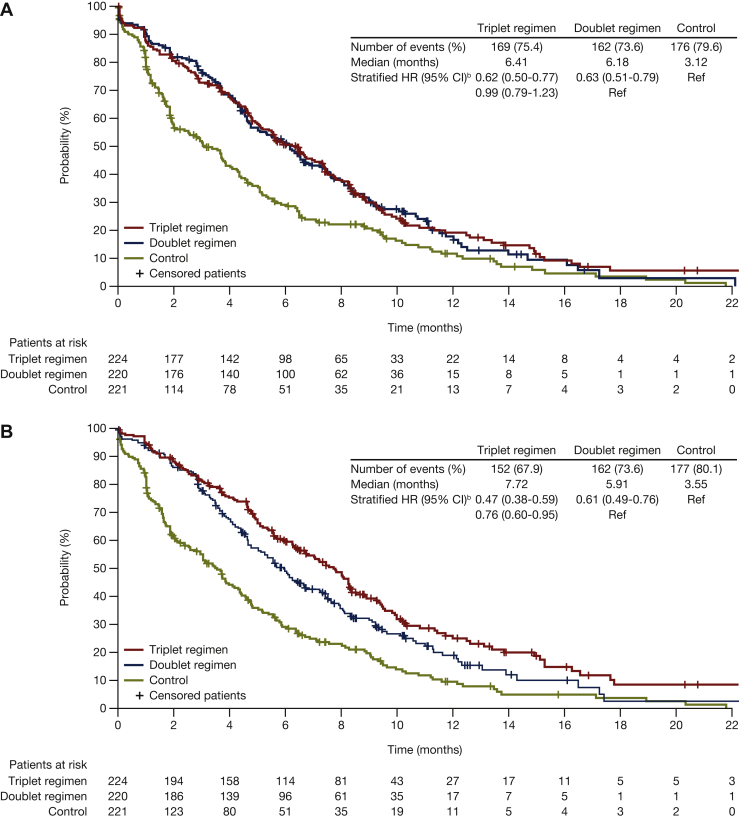
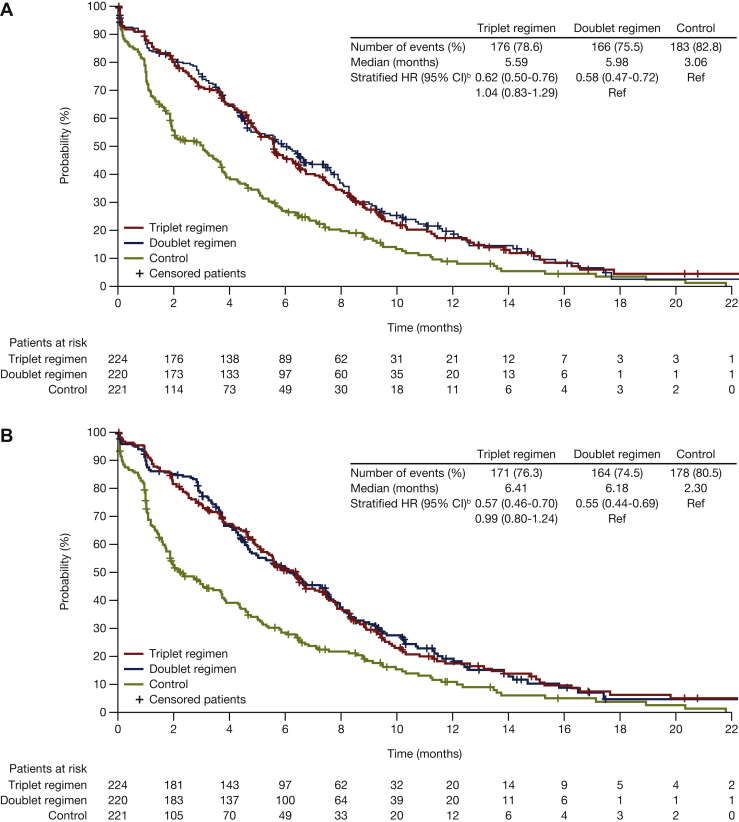
Encorafenib plus cetuximab, with or without binimetinib, was associated with longer median times to definitive 10% deterioration in the EORTC Global Health Status scale relative to the control group [5.6 and 6.2 versus 2.8 months, respectively; HR (95% CI): 0.65 (0.52-0.80) versus 0.61 (0.49-0.75), respectively; Figure 2A]. Similar findings were observed for the EORTC physical functioning, emotional functioning, and social functioning subscales (Figure 3). For the emotional functioning subscale, the encorafenib plus cetuximab with binimetinib group was associated with a longer median time to deterioration compared with the encorafenib plus cetuximab group: 7.7 versus 5.9 months [HR (95% CI): 0.76 (0.60-0.95)] (Figure 3B).
Figure 2.
Median time to definitive deteriorationafor encorafenib plus cetuximab with or without binimetinib compared with control:(A) EORTC global health status; (B) FACT-C colorectal cancer. Probability (%) of median time to definitive 10% deterioration in (A) EORTC global health status or (B) FACT-C colorectal cancer for patients treated with encorafenib plus cetuximab with or without binimetinib (triplet and doublet, respectively) compared with the control. CI, confidence interval; FACT-C, Functional Assessment of Cancer Therapy—Colorectal; HR, hazard ratio; EORTC, European Organization for Research and Treatment of Cancer Quality of Life of Questionnaire Core 30.aDefinitive deterioration is at least 10% worsening relative to baseline with no later improvement above this threshold, or death due to any cause. bStratified by Eastern Cooperative Oncology Group performance status, source of cetuximab, and prior irinotecan use at randomization.
Figure 3.
Median time to definitive deteriorationa,bin EORTC for encorafenib plus cetuximab with or without binimetinib compared with control: (A) Physical functioning; (B) emotional functioning; (C) social functioning. Probability (%) of median time to definitive 10% deterioration in EORTC global health status subscales (A) physical functioning, (B) emotional functioning, and (C) social functioning for patients treated with encorafenib plus cetuximab with or without binimetinib (triplet and doublet, respectively) compared with the control. CI, confidence interval; HR, hazard ratio; EORTC, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30.aDefinitive deterioration is at least 10% worsening relative to baseline with no later improvement above this threshold, or death due to any cause. bStratified by Eastern Cooperative Oncology Group performance status, source of cetuximab, and prior irinotecan use at randomization.
FACT-C
Baseline values for FACT-C scores were similar across the groups (Table 1). FACT-C CRC subscale scores over time are shown in Figure 1B. QoL was generally maintained during treatment for the encorafenib plus cetuximab with or without binimetinib regimens compared with control.
Time to deterioration: FACT-C
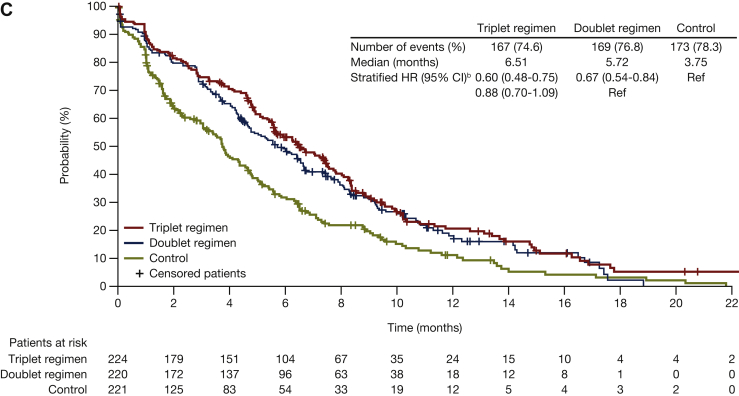
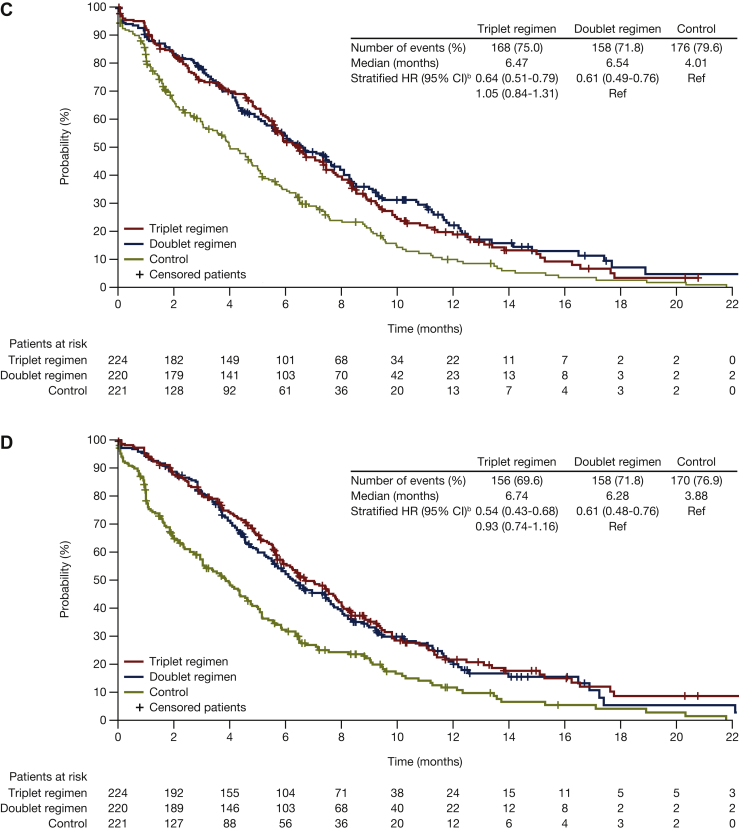
For the FACT-C CRC subscale, median times to deterioration were 5.9 months for encorafenib plus cetuximab with binimetinib, 6.5 months for encorafenib plus cetuximab, and 2.4 months for control. HR (95% CI) was 0.58 (0.47-0.71) for encorafenib plus cetuximab with binimetinib versus control, and 0.53 (0.43-0.66) for encorafenib plus cetuximab versus control (Figure 2B). With respect to the other FACT-C subscales, the median times to deterioration were longer for encorafenib plus cetuximab, with and without binimetinib, compared with control (Figure 4).
Figure 4.
Median time to definitive deteriorationain FACT-C subscales for encorafenib plus cetuximab with or without binimetinib compared with control: (A) Functional well-being; (B) physical well-being; (C) social/family well-being; (D) emotional well-being. Probability (%) of median time to definitive 10% deterioration in FACT-C colorectal cancer subscales (A) functional well-being, (B) physical well-being, (C) social/family well-being, and (D) emotional well-being for patients treated with encorafenib plus cetuximab with or without binimetinib (triplet and doublet, respectively) compared with the control. CI, confidence interval; FACT-C, Functional Assessment of Cancer Therapy—Colorectal; HR, hazard ratio.aDefinitive deterioration is at least 10% worsening relative to baseline with no later improvement above this threshold, or death due to any cause. bStratified by Eastern Cooperative Oncology Group performance status, source of cetuximab, and prior irinotecan use at randomization.
Discussion
Tumor-related endpoints, such as PFS, OS, or overall response rate, do not inevitably translate into significant improvements in the quality of survival. The US Food and Drug Administration and the American Society of Clinical Oncology have stated that QoL can be considered a co-primary endpoint besides OS if no effect of treatment is observed.34 Furthermore, in Europe, health assessments to determine reimbursement decisions require both clinical and patient-reported endpoints (such as QoL assessments); treatments are generally considered for reimbursement based on the added value that these treatments provide in terms of QoL.18,35,36
To optimize treatment approaches for mCRC, it has been suggested that treatment algorithms should be tailored according to three major themes: (1) patient characteristics, which include patient preferences/life-quality indices and acceptance of toxicities and expectations; (2) tumor features; and (3) the molecular profile of the disease.37 Differences in mechanisms of action and the distinct safety profiles of chemotherapeutic agents and treatment goals during later lines of treatment may guide treatment selection for individual patients. Management of AEs to maintain QoL is also a key consideration and is crucial to best practice in the setting of mCRC.38 The main consideration for many patients is generally to maintain QoL for as long as possible; on that basis, QoL becomes more important with successive lines of treatment.39
The findings from this study showed that encorafenib plus cetuximab with or without binimetinib consistently demonstrated longer maintenance of QoL as measured by EORTC and FACT-C as well as across the individual subscales such as physical, functional, social, and emotional functioning compared with chemotherapy in patients with previously treated BRAF V600E-mutant mCRC in the BEACON CRC study. This manuscript includes a plain language summary explaining the significance of these QoL results in a concise, easy-to-understand format as a tool to communicate to a broad audience and assist with engagement between medical professionals, patients, and others (Supplemental Figure S1, available at https://doi.org/10.1016/j.esmoop.2022.100477). Consequential changes in QoL may alert practitioners to disease progression and increased risk of mortality, but these changes can be challenging to detect.22 In the BEACON CRC study, the median time to definitive deterioration was longer for encorafenib plus cetuximab, with or without binimetinib, compared with control (EORTC and FACT-C). Time until definitive deterioration has been used to analyze changes in QoL22,40 and may provide an approach that yields meaningful, accessible results that are more easily evaluated by clinicians than patient-reported outcome scales alone, facilitating vital and timely clinical decision making.22
Possible limitations include limited data beyond the early treatment cycles due to the lower number of assessable patients. Additional data evaluating the impact of encorafenib plus cetuximab with or without binimetinib on QoL and its relationship to classical parameters such as OS in mCRC would be of value. Although the EORTC and FACT-C are widely used, they do not give a complete picture of a patient’s QoL due to limitations of memory recall and time between instrument administration. This analysis did not assess QoL in terms of financial burdens associated with mCRC as well as other mitigating life-quality factors such as socioeconomic status, access to health care, and presence or quality of a support system. As with all open-label studies, there is a risk of bias.
Previous clinical reports on targeted agents in patients with mCRC have shown that treatment can provide survival benefits without diminishing QoL.41, 42, 43, 44, 45, 46, 47, 48 This includes studies indicating that adding cetuximab to chemotherapy for mCRC does not negatively impact QoL relative to chemotherapy alone.41, 42, 43 Similar findings have been reported for bevacizumab, panitumumab, aflibercept, and regorafenib.44, 45, 46, 47, 48
In addition to improving OS as reported previously, encorafenib plus cetuximab with or without binimetinib delays QoL decline in previously treated patients with BRAF V600E-mutant mCRC.
Acknowledgements
We thank the patients, their families, and the sites that participated in this study. In addition, we would like to acknowledge Maria Cecilia Vieira and Arlene L. Reisman of Pfizer for their review and contributions toward the development of this manuscript. Medical writing support was provided by Joanna Hulme, PhD, and Alexus Kolb, PhD, of Caudex and funded by Pfizer.
Funding
This study was sponsored by Array BioPharma Inc, which was acquired by Pfizer, United States; National Cancer Institute, United States in July 2019. This work was also supported by the Cancer Center Core Grant [grant number P30 CA 008748] to Memorial Sloan-Kettering Cancer Center.
Disclosure
SK: Stock and other ownership interests: MolecularMatch, Navire; consulting or advisory role: Roche, Genentech, EMD Serono, Merck, Karyopharm Therapeutics, Amal Therapeutics, Navire Pharma, Symphogen, Holy Stone, Biocartis, Amgen, Novartis, Eli Lilly, Boehringer Ingelheim; research funding: Amgen (Inst), Sanofi (Inst), Biocartis (Inst), Guardant Health (Inst), Array BioPharma (Inst), Genentech (Inst), EMD Serono (Inst), MedImmune (Inst), Novartis (Inst). AG: Honoraria: Elsevier, Aptitude Health; consulting or advisory role: Genentech (Inst), Bayer (Inst), Bristol-Myers Squibb (Inst), Eli Lilly (Inst), Boston Biomedical (Inst), Amgen (Inst), Array BioPharma (Inst), Guardant Health (Inst), Daiichi Sankyo (Inst); research funding: Genentech (Inst), Bayer (Inst), Pfizer (Inst), Eisai (Inst), Eli Lilly (Inst), Boston Biomedical (Inst), Daiichi Sankyo (Inst), Array BioPharma (Inst); travel, accommodations, expenses: Genentech, Bayer, Bristol-Myers Squibb, Boston Biomedical, Amgen, Boehringer Ingelheim, Merck Sharp & Dohme. EVC: Consulting or advisory role: Array, Bayer, Biocartis, Eli Lilly, Ipsen, Roche, Servier, Bristol-Myers Squibb, Celgene, Merck Sharp & Dohme, Merck KGaA, Novartis, AstraZeneca, Sirtex, Taiho, Pierre Fabre; research funding: Amgen (Inst), Bayer (Inst), Boehringer Ingelheim (Inst), Eli Lilly (Inst), Novartis (Inst), Roche (Inst), Celgene (Inst), Ipsen (Inst), Merck (Inst), Merck KGaA (Inst), Servier (Inst), Bristol-Myers Squibb (Inst). RY: Research funding: Array BioPharma (Inst), Boehringer Ingelheim (Inst), Mirati Therapeutics (Inst); consulting: Array BioPharma, Natera, Mirati Therapeutics. HW: Honoraria: Merck KGaA, Celgene, Sirtex Medical, Servier, Array BioPharma, Shire, Genentech, ERYTECH Pharma, Amgen, Zymeworks, Pierre Fabre; consulting or advisory role: Roche Pharma AG, Sirtex Medical, ERYTECH Pharma, Shire, Incyte; speakers’ bureau: Sirtex Medical, Celgene, Merck KGaA, Servier; research funding: Sirtex Medical (Inst), Merck KGaA (Inst), Pfizer (Inst), Merck Sharp & Dohme (Inst). TY: Research funding: Chugai Pharma (Inst), Sanofi (Inst), Sumitomo Dainippon (Inst), Daiichi Sankyo (Inst), MSD (Inst), Taiho (Inst), Ono (Inst), Amgen (Inst), Parexel International (Inst). JD: Consulting or advisory role: Bionomics, Eli Lilly, Eisai, BeiGene, Ignyta (Inst); research funding: Roche (Inst), GlaxoSmithKline (Inst), Novartis (Inst), Bionomics (Inst), MedImmune (Inst), BeiGene (Inst), Eli Lilly (Inst), Bristol-Myers Squibb (Inst). FC: Consulting or advisory role: Genentech, Merck KGaA, Bayer, Amgen, Pfizer; research funding: Merck KGaA (Inst), Genentech (Inst), Servier (Inst), Symphogen (Inst), Amgen (Inst), Bayer (Inst), Merck Sharp & Dohme (Inst), Bristol-Myers Squibb (Inst), Ipsen (Inst). TKG: Honoraria: Pierre Fabre (inst). NS provided consultation or attended advisory boards for AIMM Therapeutics, Boehringer Ingelheim, Ellipses Pharma and received research grants for the institute from AB Science, Abbvie, Actuate Therapeutics, Amgen, Array, AstraZeneca/MedImmune, Bayer, Blueprint Medicines, Boehringer Ingelheim, Bristol-Myers Squibb, Cantargia, CellCentric, Cytovation, Deciphera, Genentech/Roche, GlaxoSmithKline, Incyte, Lilly, Merck Sharp & Dohme, Merus, Molecular Partners, Novartis, Pfizer, Pierre Fabre, Roche, Sanofi, Taiho, Takeda (outside the submitted work). HTA: Employment: Sarah Cannon Research Institute UK and HCAHealthcare UK; advisory boards: Roche, Servier, Biontech, Beigene, Guardant, Bayer, iOnctura, Bicycle. AB and XZ: Employment: Pfizer Inc. JT: Consulting or advisory role: Array Biopharma, AstraZeneca, Avvinity, Bayer, Boehringer Ingelheim, Chugai, DaiichiSankyo, F. Hoffmann-La Roche Ltd, Genentech Inc, HalioDX SAS, Hutchison MediPharmaInternational, Ikena Oncology, IQVIA, Lilly, Menarini, Merck Serono, Merus, MSD, Mirati, Neophore, Novartis, Ona Therapeutics, Orion Biotechnology, Peptomyc, Pfizer, Pierre Fabre, Samsung Bioepis, Sanofi, Seattle Genetics, Scandion Oncology, Servier, Sotio Biotech, Taiho, Tessa Therapeutics and TheraMyc. And also educational collaboration with Imedex, Medscape Education, MJH Life Sciences, PeerView Institute for Medical Education and Physicians Education Resource (PER). All other authors have declared no conflicts of interest.
Data sharing
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
Supplementary data
References
- 1.Macrae F.A. Colorectal cancer: epidemiology, risk factors, and protective factors. 2021. https://www.uptodate.com/contents/colorectal-cancer-epidemiology-risk-factors-and-protective-factors Available at.
- 2.International Agency for Research on Cancer Global Cancer Observatory. 2021. https://gco.iarc.fr/ Available at.
- 3.National Cancer Institute SEER Cancer Stat Facts: colorectal cancer. 2021. https://seer.cancer.gov/statfacts/html/colorect.html Available at.
- 4.Marventano S., Forjaz M., Grosso G., et al. Health related quality of life in colorectal cancer patients: state of the art. BMC Surg. 2013;13(suppl 2):S15. doi: 10.1186/1471-2482-13-S2-S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eyl R.E., Xie K., Koch-Gallenkamp L., Brenner H., Arndt V. Quality of life and physical activity in long-term (≥5 years post-diagnosis) colorectal cancer survivors - systematic review. Health Qual Life Outcomes. 2018;16(1):112. doi: 10.1186/s12955-018-0934-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Cancer Society Cancer treatment & survivorship facts & figures 2016–2017. 2016. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/cancer-treatment-and-survivorship-facts-and-figures/cancer-treatment-and-survivorship-facts-and-figures-2016-2017.pdf Available at.
- 7.National Comprehensive Cancer Network NCCN Guidelines. Colon cancer. Version 4.2020. 2020. https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf Available at.
- 8.Van Cutsem E., Cervantes A., Nordlinger B., Arnold D., ESMO Guidelines Working Group Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(suppl 3):iii1–9. doi: 10.1093/annonc/mdu260. [DOI] [PubMed] [Google Scholar]
- 9.Shrestha A., Martin C., Burton M., Walters S., Collins K., Wyld L. Quality of life versus length of life considerations in cancer patients: a systematic literature review. Psychooncology. 2019;28(7):1367–1380. doi: 10.1002/pon.5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heydarnejad M.S., Hassanpour D.A., Solati D.K. Factors affecting quality of life in cancer patients undergoing chemotherapy. Afr Health Sci. 2011;11(2):266–270. [PMC free article] [PubMed] [Google Scholar]
- 11.Hawkins R., Grunberg S. Chemotherapy-induced nausea and vomiting: challenges and opportunities for improved patient outcomes. Clin J Oncol Nurs. 2009;13(1):54–64. doi: 10.1188/09.CJON.54-64. [DOI] [PubMed] [Google Scholar]
- 12.Ramasubbu S.K., Pasricha R.K., Nath U.K., Rawat V.S., Das B. Quality of life and factors affecting it in adult cancer patients undergoing cancer chemotherapy in a tertiary care hospital. Cancer Rep (Hoboken) 2021;4(2):e1312. doi: 10.1002/cnr2.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewandowska A., Rudzki G., Lewandowski T., et al. Quality of life of cancer patients treated with chemotherapy. Int J Environ Res Public Health. 2020;17(19):6938. doi: 10.3390/ijerph17196938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arndt V., Merx H., Stegmaier C., Ziegler H., Brenner H. Quality of life in patients with colorectal cancer 1 year after diagnosis compared with the general population: a population-based study. J Clin Oncol. 2004;22(23):4829–4836. doi: 10.1200/JCO.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 15.Tabernero J., Valez L., Trevino T., et al. Management of adverse events from the treatment of encorafenib plus cetuximab for patients with BRAF V600E-mutant metastatic colorectal cancer: insights from the BEACON CRC study. ESMO Open. 2021;6:100328. doi: 10.1016/j.esmoop.2021.100328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kenzik K.M., Ganz P.A., Martin M.Y., et al. How much do cancer-related symptoms contribute to health-related quality of life in lung and colorectal cancer patients? A report from the Cancer Care Outcomes Research and Surveillance (CanCORS) Consortium. Cancer. 2015;121(16):2831–2839. doi: 10.1002/cncr.29415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ratjen I., Schafmayer C., Enderle J., et al. Health-related quality of life in long-term survivors of colorectal cancer and its association with all-cause mortality: a German cohort study. BMC Cancer. 2018;18(1):1156. doi: 10.1186/s12885-018-5075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marschner N., Zacharias S., Lordick F., et al. Association of disease progression with health-related quality of life among adults with breast, lung, pancreatic, and colorectal cancer. JAMA Netw Open. 2020;3(3) doi: 10.1001/jamanetworkopen.2020.0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gotay C.C., Kawamoto C.T., Bottomley A., Efficace F. The prognostic significance of patient-reported outcomes in cancer clinical trials. J Clin Oncol. 2008;26(8):1355–1363. doi: 10.1200/JCO.2007.13.3439. [DOI] [PubMed] [Google Scholar]
- 20.Maisey N.R., Norman A., Watson M., Allen M.J., Hill M.E., Cunningham D. Baseline quality of life predicts survival in patients with advanced colorectal cancer. Eur J Cancer. 2002;38(10):1351–1357. doi: 10.1016/s0959-8049(02)00098-9. [DOI] [PubMed] [Google Scholar]
- 21.Braun D.P., Gupta D., Grutsch J.F., Staren E.D. Can changes in health related quality of life scores predict survival in stages III and IV colorectal cancer? Health Qual Life Outcomes. 2011;9:62. doi: 10.1186/1477-7525-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonnetain F., Borg C., Adams R.R., et al. How health-related quality of life assessment should be used in advanced colorectal cancer clinical trials. Ann Oncol. 2017;28(9):2077–2085. doi: 10.1093/annonc/mdx191. [DOI] [PubMed] [Google Scholar]
- 23.US Department of Health and Human Services, Food and Drug Administration, Oncology Center of Excellence, et al. Guidance for industry: clinical trial endpoints for the approval of cancer drugs and biologics. 2018. https://www.fda.gov/media/71195/download Available at.
- 24.Kopetz S., Grothey A., Yaeger R., et al. Encorafenib, binimetinib, and cetuximab in BRAF V600E-mutated colorectal cancer. N Engl J Med. 2019;381(17):1632–1643. doi: 10.1056/NEJMoa1908075. [DOI] [PubMed] [Google Scholar]
- 25.Aaronson N.K., Ahmedzai S., Bergman B., et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 26.Musoro Z.J., Hamel J.F., Ediebah D.E., et al. Establishing anchor-based minimally important differences (MID) with the EORTC quality-of-life measures: a meta-analysis protocol. BMJ Open. 2018;8(1) doi: 10.1136/bmjopen-2017-019117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Musoro J.Z., Sodergren S.C., Coens C., et al. Minimally important differences for interpreting the EORTC QLQ-C30 in patients with advanced colorectal cancer treated with chemotherapy. Colorectal Dis. 2020;22(12):2278–2287. doi: 10.1111/codi.15295. [DOI] [PubMed] [Google Scholar]
- 28.European Organization for Research and Treatment of Cancer Quality of life of cancer patients: EORTC QLQ-C30 (version 3) https://qol.eortc.org/questionnaire/eortc-qlq-c30/ Available at.
- 29.Fayers P., Aaronson N.K., Bjordal K., Groenvold M., Curran D., Bottomley A. 3rd ed. European Organisation for Research and Treatment of Cancer; Brussels, Belgium: 2001. EORTC QLQ-C30 Scoring Manual. [Google Scholar]
- 30.FACIT Functional Assessment of Cancer Therapy Colon Cancer (FACT C) Questionnaire. https://www.facit.org/FACITOrg/Questionnaires Available at.
- 31.Ward W.L., Hahn E.A., Mo F., Hernandez L., Tulsky D.S., Cella D. Reliability and validity of the Functional Assessment of Cancer Therapy-Colorectal (FACT-C) quality of life instrument. Qual Life Res. 1999;8(3):181–195. doi: 10.1023/a:1008821826499. [DOI] [PubMed] [Google Scholar]
- 32.National Institute of Mental Health Patient Global Impressions scale - Change, Improvement, Severity (PGI-C, PGI-I, PGI-S) 2021. https://eprovide.mapi-trust.org/instruments/patient-global-impression-of-change Available at.
- 33.European Organization for Research and Treatment of Cancer QLQ-C30 Questionnaire. https://qol.eortc.org/questionnaire/eortc-qlq-c30/ Available at.
- 34.Anota A., Hamidou Z., Paget-Bailly S., et al. Time to health-related quality of life score deterioration as a modality of longitudinal analysis for health-related quality of life studies in oncology: do we need RECIST for quality of life to achieve standardization? Qual Life Res. 2015;24(1):5–18. doi: 10.1007/s11136-013-0583-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prager G., Köhne C.H., O'Connor J.M., et al. The Screening and COnsensus Based on Practices and Evidence (SCOPE) program-results of a survey on daily practice patterns for patients with mCRC. Curr Oncol. 2021;28(3):2097–2106. doi: 10.3390/curroncol28030194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.World Health Organization. Medicines Reimbursement Policies in Europe (2018); 2018. Available at https://www.euro.who.int/en/publications/abstracts/medicines-reimbursement-policies-in-europe. Accessed February 3, 2022.
- 37.De Falco V., Napolitano S., Rosello S., et al. How we treat metastatic colorectal cancer. ESMO Open. 2020;4(suppl 2) doi: 10.1136/esmoopen-2020-000813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bekaii-Saab T., Kim R., Kim T.W., et al. Third- or later-line therapy for metastatic colorectal cancer: reviewing best practice. Clin Colorectal Cancer. 2019;18(1):e117–e129. doi: 10.1016/j.clcc.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 39.Akhlaghi E., Lehto R.H., Torabikhah M., et al. Chemotherapy use and quality of life in cancer patients at the end of life: an integrative review. Health Qual Life Outcomes. 2020;18(1):332. doi: 10.1186/s12955-020-01580-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamidou Z., Chibaudel B., Hebbar M., et al. Time to definitive health-related quality of life score deterioration in patients with resectable metastatic colorectal cancer treated with FOLFOX4 versus sequential dose-dense FOLFOX7 followed by FOLFIRI: the MIROX randomized phase III trial. PLoS One. 2016;11(6) doi: 10.1371/journal.pone.0157067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Au H.J., Karapetis C.S., O'Callaghan C.J., et al. Health-related quality of life in patients with advanced colorectal cancer treated with cetuximab: overall and KRAS-specific results of the NCIC CTG and AGITG CO.17 Trial. J Clin Oncol. 2009;27(11):1822–1828. doi: 10.1200/JCO.2008.19.6048. [DOI] [PubMed] [Google Scholar]
- 42.Yamaguchi K., Ando M., Ooki A., et al. Quality of life analysis in patients with RAS wild-type metastatic colorectal cancer treated with first-line cetuximab plus chemotherapy. Clin Colorectal Cancer. 2017;16(2):e29–e37. doi: 10.1016/j.clcc.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 43.Lang I., Kohne C.H., Folprecht G., et al. Quality of life analysis in patients with KRAS wild-type metastatic colorectal cancer treated first-line with cetuximab plus irinotecan, fluorouracil and leucovorin. Eur J Cancer. 2013;49(2):439–448. doi: 10.1016/j.ejca.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 44.Battaglin F., Puccini A., Ahcene Djaballah S., Lenz H.-J. The impact of panitumumab treatment on survival and quality of life in patients with RAS wild-type metastatic colorectal cancer. Cancer Manag Res. 2019;11:5911–5924. doi: 10.2147/CMAR.S186042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thaler J., Karthaus M., Mineur L., et al. Skin toxicity and quality of life in patients with metastatic colorectal cancer during first-line panitumumab plus FOLFIRI treatment in a single-arm phase II study. BMC Cancer. 2012;12:438. doi: 10.1186/1471-2407-12-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kabbinavar F.F., Wallace J.F., Holmgren E., et al. Health-related quality of life impact of bevacizumab when combined with irinotecan, 5-fluorouracil, and leucovorin or 5-fluorouracil and leucovorin for metastatic colorectal cancer. Oncologist. 2008;13(9):1021–1029. doi: 10.1634/theoncologist.2008-0003. [DOI] [PubMed] [Google Scholar]
- 47.Riechelmann R.P., Srimuninnimit V., Bordonaro R., et al. Aflibercept plus FOLFIRI for second-line treatment of metastatic colorectal cancer: observations from the Global Aflibercept Safety and Health-Related Quality-of-Life Program (ASQoP) Clin Colorectal Cancer. 2019;18(3):183–191.e3. doi: 10.1016/j.clcc.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 48.Shitara K., Yamanaka T., Denda T., et al. REVERCE: a randomized phase II study of regorafenib followed by cetuximab versus the reverse sequence for previously treated metastatic colorectal cancer patients. Ann Oncol. 2019;30(2):259–265. doi: 10.1093/annonc/mdy526. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.