Abstract
Background
Antibiotic (ABX) use can reduce the efficacy of immune checkpoint inhibitors and chemotherapeutics. The effect for patients treated with targeted therapies, namely, small-molecule tyrosine kinase inhibitors (TKIs), is less known.
Patients and methods
Retrospective data were analysed for TKI-treated patients with advanced melanoma and non-small-cell lung cancer (NSCLC) between January 2015 and April 2017 at The Christie NHS Foundation Trust. Data on demographics, disease burden, lactate dehydrogenase (LDH) level, presence of brain metastases, ECOG performance status (PS) and ABX use were collected. Progression-free survival (PFS) and overall survival (OS) were compared between the ABX+ group (ABX within 2 weeks of TKI initiation-6 weeks after) and the ABX– group (no ABX during the same period).
Results
A total of 168 patients were included; 89 (53%) with NSCLC and 79 (47%) with melanoma. 55- (33%) patients received ABX. On univariable analysis, ABX+ patients demonstrated shorter PFS (208 versus 357 days; P = 0.008) and OS (294 versus 438 days; P = 0.024). Increased age, poorer PS and higher LDH were associated with shorter PFS and OS. On multivariable analysis, ABX use was independently associated with shorter PFS [hazard ratio (HR) 1.57, 95% confidence interval (CI) 1.05-2.34, P = 0.028] and OS (HR 2.19, 95% CI 1.44-3.32, P = 0.0002). The negative impact of ABX on OS was particularly pronounced for patients with PS of ≥2 (HR 3.82, 95% CI 1.18-12.36, P = 0.025).
Conclusion
For patients treated with TKIs, ABX use is independently associated with reduced PFS and OS and judicious use is warranted, particularly in patients with poorer PS.
Key words: tyrosine kinase inhibitors, antibiotics, melanoma, lung cancer
Highlights
Antibiotic use can reduce the efficacy of some systemic anticancer therapies.
The effect for patients treated with TKIs is less known.
This is a retrospective review of 168 patients with advanced melanoma and NSCLC treated with TKIs.
Patients on ABXs showed shorter progression-free (208 versus 357 days) and overall survival (294 versus 438 days).
ABX use was independently associated with shorter PFS (HR 1.57, P = 0.028) and OS (HR 2.19, P = 0.0002).
Introduction
Mutations, dysregulation and overexpression of protein kinases are recognised in many cancers. Tyrosine kinase inhibitors (TKIs) disrupt these signal transduction pathways, making them an effective therapeutic option.1 While predictive gene biomarkers have been established, responses vary among patients and it is therefore important to identify any additional prognostic factors.
It has been long recognised that some bacteria are linked to carcinogenesis.2 Emerging preclinical and clinical studies have shown that the gut microbiome plays an important role in the metabolism and efficacy of many anticancer drugs.3, 4, 5, 6 The use of antibiotics (ABX) can alter the gut microbiome and this has detrimental effects on anticancer therapy.7, 8, 9
Studies have shown that ABX use in patients receiving immune checkpoint inhibitors (ICIs) is associated with significantly worse outcomes by disrupting the gut microbiome.6 We previously showed that cumulative ABX use significantly decreases efficacy of ICIs in patients with advanced cancer.8 Specific bacterial strains have been identified that directly impact responses to ICIs and preclinical models have shown that faecal microbiota transplants can rescue and abrogate response. B. fragilis has an enhancing effect in patients treated with ipilimumab and anti-PD-1 therapies through aiding tumour-specific cytotoxic T-cell expansion.10 The negative effect of ABXs is also seen in patients receiving chemotherapy. In patients with advanced head and neck cancer treated with chemo-radiotherapy, ABX use led to worse overall survival (OS), progression-free survival (PFS) and disease-specific survival in a multivariable analysis. This negative impact was seen to be greater in those who received two or more courses.11
There are limited data regarding the effects of ABXs in patients treated with TKIs. One study of 145 patients with renal cell carcinoma treated with first-line anti-VEGF TKI showed that the use of ABXs in 17 patients improved PFS. The mechanism proposed is one related to Bacteroides species, which is prevalent in treatment-related diarrhoea and supported in preclinical chemotherapy models. The authors hypothesised that eradicating Bacteroides with ABXs would result in less reported diarrhoea, better tolerated treatment and in turn, fewer dose reductions and more ‘on-treatment’ time. Interestingly, the TKI dose and on-treatment time were comparable between the two groups, suggesting that the effect of ABXs on PFS could be an independent factor.12 In a different study, Liu et al.13 showed that in patients with non-small-cell lung cancer (NSCLC) treated with TKIs, ABX use was associated with similar overall response rate but reduced median PFS compared with the control group (median PFS 6.6 months versus 10.1 months). It is possible that ABXs can decrease the long-term effect of TKIs, rather than altering partial response. ABXs can affect the number of circulating lymphocytes and this process requires a relatively long time. Therefore the effect of ABXs on TKI may present in a chronic way, which correlates with the results of PFS.
A thorough literature search has not revealed any translational models, making this an interesting area of understudied research. In addition, there are no specific studies investigating pharmacokinetic effects of ABXs on TKI absorption, distribution, metabolism and elimination. The complex relationship between the gut microbiome, immune system and tumour microenvironment could be responsible for the conflicting results. Although TKI agents are not immunotherapy, they can affect systemic inflammatory cells, including a decrease in myeloid-derived suppressor cells and regulatory T cells.12
We sought to retrospectively review the effect of ABX use for patients with NSCLC and advanced melanoma who were treated with TKIs in our centre.
Materials and methods
This work was conducted under UK regulatory guidelines for audit and research. Permission was granted to collect retrospective patient data for this study by The Quality Improvement and Clinical Audit Committee of The Christie NHS Foundation Trust (The Christie), Manchester, UK on 22 January 2018 (reference SE18/2128).
Inclusion criteria included patients with advanced melanoma and NSCLC who received first-line treatment with a TKI at the Christie NHS Foundation Trust, Manchester, UK, between 1 January 2015 and 1 April 2017. The TKIs included were dabrafenib, vemurafenib, gefitinib, afatinib and erlotinib. Data were collected from patients receiving these agents from 2 weeks before until 6 weeks after commencement of TKIs. This timeframe was chosen to cover a potentially large duration of modification of gut microbiota following ABX therapy, which varies among different classes of ABXs in different studies.14
Data were collected from electronic patient records (The Christie Web Portal) and the electronic prescribing system (ePrescribing, EMIS Health, Leeds, U.K.) within the Trust. Patient demographics [age, sex, Eastern Cooperative Oncology Group (ECOG) performance status (PS), presence of comorbidities], disease characteristics [tumour type, metastatic burden, presence of brain metastases, baseline lactate dehydrogenase (LDH)] and treatment characteristics [date of commencement of TKI, TKI agent, clinical trial involvement, ABX indication, class of ABX(s), administration route and ABX duration] were collected.
The date of radiological progression was evaluated in patients using the RECIST version 1.1.15 Nontrial patients’ imaging results were recorded according to the radiologist’s report and evaluated against clinical benefit.
Patients with PFS <14 days or OS <21 days were excluded due to risk of imminent cancer-related progression or death. The effect of ABXs on these patients was not believed to be substantial. Patients were followed up until death or the time of data lock (1 January 2018). ABX use was gathered; patients who received ABXs during the specified period (whether single use of ABXs or multiple courses) were analysed in the ABX+ group and patients who did not use ABXs during the specified period were analysed in the ABX– group.
PFS was calculated as the time from the start of TKI to the diagnosis of disease progression (clinical progression or radiological progression as per RECIST version 1.1) or death, whichever came first. OS was calculated as the time from commencement of TKI to the date of death.
A detailed description on how PFS and OS were calculated can be found in Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2022.100430. A subset of patients involved in this study participated in clinical trials and this was included as a covariate in modelling.
Statistical analysis
The association between ABX use and other clinical factors was examined using chi-square tests, where continuous variables were binarised by their median (e.g. LDH). Factors that demonstrated significant association were investigated for their interaction with ABX use in the subsequent Cox proportional hazard regression analysis. A Cox proportional hazard model was used to assess the association between ABX use and patient survival (PFS and OS). The analysis started from a univariable analysis that included each clinical factor as a sole covariate in the model, with the significance of association evaluated using a Wald test. Assumption of proportionality and linearity were verified based on Schoenfeld residuals and Martingale residues. ABX use and clinical factors with univariable P-values <0.1 were selected for subsequent multivariable analysis. Missing clinical information was imputed using a multiple imputation approach in multivariable modelling if the missing is random and the missing rate is <10%. A backward stepwise method was applied to identify the subset of clinical factors that were significantly associated with PFS and OS. Interactions between ABX use and selected clinical factors were explored in the multivariable analysis. The analysis follows the REMARK guideline.16 All P-values <.05 were considered statistically significant. All analyses were implemented using R version 4.0 (R Foundation for Statistical Computing, Vienna, Austria).17 Multiple comparison was not adjusted for due to the exploratory nature of the study.
Results
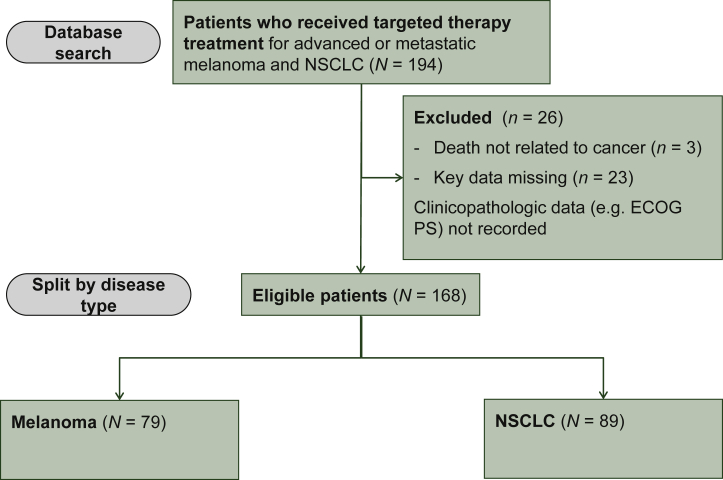
A total of 194 patients were treated with TKIs between 1 January 2015 and 1 April 2017. There were 168 evaluable patients included in the final analysis, as 26 patients were excluded (Figure 1). Reasons for exclusion included missing key data (n = 23 patients) and death due to unrelated causes (n = 3 patients). The evaluable cohort comprised 89 patients with NSCLC and 79 patients with melanoma.
Figure 1.
Patient enrolment CONSORT flow chart diagram.
Patients who had been treated with targeted therapy at The Christie NHS Foundation Trust between 1 January 2015 and 1 April 2017 were identified.
ECOG PS, Eastern Cooperative Oncology Group performance status; N, raw number of patients; NSCLC, non-small-cell lung cancer.
Patient characteristics are summarised in Table 1. A total of 55 patients received ABXs during the designed timeframe (ABX+). Female sex was more represented among the ABX– group. The types of infections can be found in Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2022.100430.
Table 1.
Characteristics of patients evaluated in the study
| Characteristics | ABX+ (n = 55) | ABX− (n = 113) |
|---|---|---|
| Age | ||
| Median | 64 | 64 |
| Range | 29-87 | 28-93 |
| Sex, n (%) | ||
| Male | 29 (53) | 43 (38) |
| Female | 26 (47) | 70 (62) |
| Tumour, n (%) | ||
| Melanoma | 25 (45) | 54 (48) |
| NSCLC | 30 (55) | 59 (52) |
| Brain metastases, n (%) | ||
| Melanoma | 4 (7) | 22 (19) |
| NSCLC | 3 (5) | 9 (8) |
| LDH >2.5 ULN, n (%) | ||
| Melanoma | 8 (15) | 8 (7) |
| NSCLC | 4 (5) | 11 (10) |
| Presence of comorbidities, n (%) | ||
| Melanoma | 11 (7) | 27 (16) |
| NSCLC | 14 (8) | 40 (24) |
| ECOG PS 0-1, n (%) | ||
| Melanoma | 19 (76) | 40 (75) |
| NSCLC | 19 (63) | 38 (64.5) |
| ECOG PS 2, n (%) | ||
| Melanoma | 5 (20) | 7 (12.5) |
| NSCLC | 6 (20) | 15 (25.5) |
| ECOG PS ≥3, n (%) | ||
| Melanoma | 1 (4) | 7 (12.5) |
| NSCLC | 5 (17) | 6 (10) |
%, percentage of total sample; ABX +, patients who received antibiotics during the period of 2 weeks before until 6 weeks after treatment with tyrosine kinase inhibitors commenced; ABX −, patients who did not receive antibiotics during the specified period; ECOG PS, Eastern Cooperative Oncology Group performance status; LDH, lactate dehydrogenase; n, raw number of patients; NSCLC, non-small-cell lung cancer; ULN, upper limit of normal.
The association between ABX use and other clinical factors is listed in Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2022.100430. Patients with previous cancer treatment, high ECOG PS and high LDH were more likely to receive ABXs.
In univariable survival analysis, PFS was significantly associated with a number of variables, including older age [hazard ratio (HR) 0.6, P = 0.010], poorer ECOG PS (PS 0 versus PS 1: HR 1.7, P = 0.029; PS 0 versus PS 2: HR 2.23, P = 0.002), high LDH (HR 1.43, P < 0.001), and ABX use (HR 1.66, P = 0.009; Table 2).
Table 2.
Univariate analysis of the association between clinical factors and patient survival
| Variable | Categories | PFS |
OS |
||
|---|---|---|---|---|---|
| HR | P-value | HR | P-value | ||
| Sex | Male versus female | 1.315 | 0.148 | 1.443 | 0.048 |
| Age | Above median | 0.608 | 0.010 | 0.668 | 0.032 |
| Tumour type | NSCLC versus melanoma | 0.861 | 0.432 | 0.691 | 0.046 |
| Clinical trial participation | Yes versus no | 1.113 | 0.614 | 0.735 | 0.148 |
| Number of metastatic sites | 2 versus 0/1 | 0.948 | 0.843 | 0.983 | 0.947 |
| 3+ versus 0/1 | 1.431 | 0.175 | 1.275 | 0.332 | |
| Brain metastases | Yes versus no | 1.428 | 0.105 | 1.757 | 0.007 |
| Previous treatment | 1 versus 0 | 0.770 | 0.291 | 1.071 | 0.766 |
| 2+ versus 0 | 1.276 | 0.395 | 1.364 | 0.272 | |
| ECOG performance status | 1 versus 0 | 1.701 | 0.029 | 1.429 | 0.152 |
| 2+ versus 0 | 2.253 | 0.002 | 2.524 | <0.001 | |
| Comorbidities | Yes versus no | 0.717 | 0.079 | 0.981 | 0.918 |
| LDH | Continuous, log2 | 1.430 | <0.001 | 1.373 | <0.001 |
| Use of antibiotics | yes versus no | 1.661 | 0.009 | 1.533 | 0.025 |
| Cumulative use | Single versus no | 1.894 | 0.011 | 1.969 | 0.006 |
| cumulative versus no | 1.495 | 0.103 | 1.454 | 0.124 | |
Variables listed in bold and underlined had P-values <0.1 and were selected for subsequent multivariate analysis.
ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; LDH, lactate dehydrogenase; NSCLC, non-small-cell lung cancer; OS, overall survival; PFS, progression-free survival.
All variables significant for PFS were significant for OS. In addition, being male (HR 1.44, P = 0.048), melanoma cancer type (HR 0.69, P = 0.046) and the presence of brain metastases (HR 1.75, P = 0.007) were associated with reduced OS.
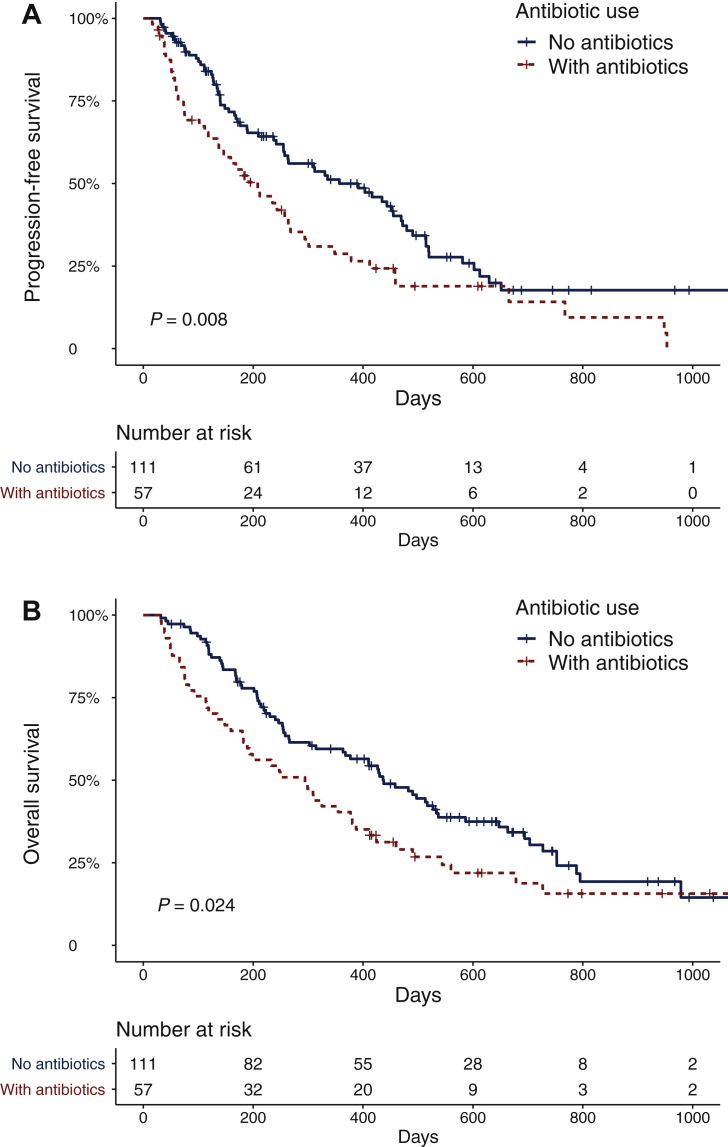
A multivariable model was developed to interrogate the impact of ABX use during TKI therapy, adjusting for significant clinical variables. Patients treated with ABXs during TKI therapy demonstrated significantly reduced PFS [Table 3 and Figure 2; HR 1.71, 95% confidence interval (CI) 1.15-2.54, P = 0.008] and OS (Table 4; HR 2.24, 95% CI 1.47-3.39, P < 0.0001). Higher age, ECOG PS and LDH level were associated with reduced PFS, while higher ECOG PS, LDH level and the presence of brain metastasis were associated with reduced OS. To better characterise the ABX treatment received, an exploratory analysis was carried out by categorising patients as either having ‘no ABXs,’ ‘single-course ABXs’, or ‘cumulative courses ABXs’, where concurrent or successive ABXs for >7 days were administered. Cumulative ABX use did not demonstrate a significantly different detrimental effect.
Table 3.
Multivariate analysis of the association between use of antibiotics and progression-free survival
| Variable | Categories | HR | P-value | 95% CI |
|---|---|---|---|---|
| Antibiotics | Yes versus no | 1.707 | 0.008 | 1.147-2.539 |
| Age | High versus low | 0.552 | 0.003 | 0.374-0.814 |
| ECOG performance status | 1 versus 0 | 1.442 | 0.140 | 0.887-2.346 |
| 2+ versus 0 | 1.924 | 0.014 | 1.142-3.242 | |
| LDH | Continuous, log2 | 1.447 | <0.001 | 1.204-1.740 |
CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; LDH, lactate dehydrogenase.
Figure 2.
Survival outcomes for patients in this study, based on their exposure to antibiotics.
(A) Progression-free survival for 168 patients for whom times (or censored values) could be obtained. The number of patients at risk in each category is shown below the table. (B) Overall survival for 168 patients for whom times (or censored values) could be obtained. The number of patients at risk in each category is shown below the table.
Patients were divided into two categories based on whether they had received antibiotics or not during the 2 weeks before until 6 weeks after treatment with tyrosine kinase inhibitors commenced. Kaplan–Meier estimator graphs illustrate survival outcomes for patients who did not receive antibiotics (blue line) and patients who received antibiotics (red line). OS; overall survival; PFS; progression-free survival.
Table 4.
Multivariate analysis of the association between the use of antibiotics and overall survival
| Variable | Categories | HR | P-value | 95% CI |
|---|---|---|---|---|
| Antibiotics | Yes versus no | 2.236 | <0.001 | 1.473-3.394 |
| ECOG performance status | 1 versus 0 | 1.606 | 0.02 | 0.976-2.652 |
| ECOG performance status | 2+ versus 0 | 3.172 | <0.001 | 1.885-5.340 |
| Brain metastasis | Yes versus no | 1.569 | 0.041 | 1.020-2.416 |
| LDH | Continuous, log2 | 1.331 | 0.003 | 1.101-2.416 |
CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; LDH, lactate dehydrogenase.
The interaction between ABX use and ECOG PS was included in the multivariable model because the two variables are significantly associated. Patients treated with ABX had reduced OS and the detrimental impact of ABX is higher is patients with poorer PS (Supplementary Tables S3A and S3B, available at https://doi.org/10.1016/j.esmoop.2022.100430). The negative impact of ABX on OS was particularly pronounced for patients with PS ≥2 (HR 3.82, 95% CI 1.18-12.36, P = 0.025).
Discussion
This multivariable analysis demonstrates that ABX use is a negative predictor of PFS and OS in patients with advanced cancer treated with TKI. The detrimental effect of ABXs is more pronounced in patients with poorer PS. To our knowledge, this is the first study to include two distinct tumour sites, which better reflects the variation of patients receiving TKI in routine clinical practice or clinical trials. This works adds to an increasing body of evidence supporting a detrimental effect of ABXs in patients receiving anticancer treatment.
A rigorous approach was adopted for calculating PFS and OS to ensure no bias was introduced and competing risks were resolved. After adjusting for all prognostic clinical factors in multivariable analysis, ABX use emerged as an independent prognostic factor for both PFS and OS. Cumulative ABX use did not demonstrate a significantly different detrimental effect. This is an important finding, indicating no safe minimum. During the analysis, the interaction between ABX use and the significantly associated ECOG PS was investigated to ensure correct determination of the impact of ABXs on patient survival. The detrimental impact of ABX on OS was much larger in patients with PS of ≥2, compared with patients with lower PS. Further verification will be necessary because patients with ABX and PS ≥2 only accounted for 10% of our study population.
This study demonstrates an association between ABX use and poorer outcomes, but it is not possible to make a direct causal link. Further research could include increasing the sample size to other tumour sites (e.g. renal cell cancer, anaplastic lymphoma kinase positive lung adenocarcinoma), other TKIs and investigating correlative stool analysis. Evaluating the effect of ABX in patients on more novel TKIs such as encorafenib and binimetinib and on second- or third-line TKIs would bring useful insights on newer agents and on heavily pretreated and potentially more vulnerable groups. A subgroup ABX class analysis was attempted as a further explorative analysis in this study. However, due to a relatively small number of patients (55 patients received ABXs in our cohort) and the heterogeneity of ABX classes used among them, it is not possible to draw meaningful conclusions. Improvements could be made by increasing the patient sample size in other studies. In addition, other medications such as steroids or proton pump inhibitors could also play an important role and this information can be incorporated in future studies.
This study did not explore the clinical reasoning for patients starting ABXs. Questions remain regarding the potential detrimental impact of infection on outcomes for this cohort. Infections may have an unknown immunosuppressive effect, altering responses to ICIs (e.g. a decrease in lymphocyte counts has been associated with poor outcomes in CTLA-4-treated patients with melanoma).18
Additional limitations of this real-world data analysis include its retrospective approach, relying on hospital records for information. Independent database verification was undertaken to minimise inconsistencies. In addition, the timeframe analysed included an era when single-agent dabrafenib or vemurafenib were the only TKIs available for advanced melanoma, which is no longer standard practice due to the addition of MEK inhibitors. A number of patients in this study had trametinib introduced at a later stage; however, this was not regarded as significant for the scope of this study, as these patients would be responding to TKI for the addition of the MEK inhibitor to take place.
With the exception of a very small number of patients enrolled in a clinical trial, imaging was not evaluated according to RECIST criteria and may not have been performed at standardised time points across disease sites.
It has been long recognised that chemotherapies can lead to life-threatening neutropaenic sepsis in patients with cancer and this has led to the recommendation of early initiation of ABXs in this patient population.19 It is important to remember that in patients who are not at risk of neutropaenic sepsis, studies have reported negative associations between administration of ABXs and the outcome of systemic anticancer treatment.7, 8, 9,20, 21, 22, 23, 24 ABXs substantially reduce the number and diversity of gut microbiota,14 which is becoming an increasingly important factor in regulating antitumor immunity in anticancer therapeutics.25 It is therefore important to rationalise ABX prescriptions. The gut microbiome could be used as a novel method to improve outcomes in cancer.19 This reflects an increasing number of clinical trials using adjunctive therapies, for example, faecal microbiota transplantation, probiotics or prebiotics to increase the number and diversity of organisms and minimise toxicities, as well as to potentially increase response rates.
To safeguard the effective use of TKIs, pharmacokinetic studies should be performed to assess the effect of different ABX classes on absorption, distribution, metabolism and elimination of TKIs. So far, drug–drug interactions have only incorporated altered bioavailability due to differences in stomach pH, metabolism by cytochrome P450 enzymes and prolongation of the QTc interval.26 Macrolide ABXs (in particular, clarithromycin), antifungal medications (e.g. ketoconazole, itraconazole) and antiretroviral medications are strong CYP3A4 inhibitors, which could alter the metabolism of TKIs and lead to decreased absorption. By contrast, rifampicin induces CYP3A4, leading to increased absorption of TKIs.27 Little is known about other ABX classes and how these could affect TKIs in pharmacokinetic studies. Careful use of concomitant medication is warranted when treating patients with TKI.
A major challenge currently facing oncologists is to optimise the therapeutic benefits of anticancer treatments while understanding the complexity of factors that contribute to treatment responses. The processes involved in TKI responses require further investigation to generate an enhanced understanding of accurate predictive and safety biomarkers, which may include the gut microbiome.
Acknowledgements
This study was conducted at the Christie NHS Foundation Trust and supported by the NIHR Manchester Clinical Research Facility and the Manchester Experimental Cancer Medicine Centre. We thank the patients and their supporters for participating in this study.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors. No paid writing assistance was used in preparing this manuscript.
Disclosure
The authors have no conflicts of interest to declare.
Supplementary data
References
- 1.Derosa L., Routy B., Enot D., et al. Impact of antibiotics on outcome in patients with metastatic renal cell carcinoma treated with immune checkpoint inhibitors. J Clin Oncol. 2017;35 462-462. [Google Scholar]
- 2.Wroblewski L.E., Peek R.M., Wilson K.T. Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin Microbiol Rev. 2010;23(4):713–739. doi: 10.1128/CMR.00011-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fessler J.L., Gajewski T.F. The microbiota: a new variable impacting cancer treatment outcomes. Clin Cancer Res. 2017;23(13):3229–3231. doi: 10.1158/1078-0432.CCR-17-0864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matson V., Fessler J., Bao R., Chongsuwat T., Zha Y. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018;359(6371):104–108. doi: 10.1126/science.aao3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gopalakrishnan V., Spencer C., Nezi L., et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359(6371):97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Routy B., Le Chatelier E., Derosa L., et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science (80- ) 2018;359(6371):91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 7.Hakozaki T., Okuma Y., Omori M., Hosomi Y. Impact of prior antibiotic use on the efficacy of nivolumab for non-small cell lung cancer. Oncol Lett. 2019:2946–2952. doi: 10.3892/ol.2019.9899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tinsley N., Zhou C., Tan G., et al. Cumulative antibiotic use significantly decreases efficacy of checkpoint inhibitors in patients with advanced cancer. Oncologist. 2020;25:55–63. doi: 10.1634/theoncologist.2019-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuczma M.P., Ding Z.-C., Li T., et al. The impact of antibiotic usage on the efficacy of chemoimmunotherapy is contingent on the source of tumor-reactive T cells. Oncotarget. 2017;8(67):111931–111942. doi: 10.18632/oncotarget.22953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peterson S.N., Bradley L.M., Ronai Z.A. The gut microbiome: an unexpected player in cancer immunity. Curr Opin Neurobiol. 2020;62:48–52. doi: 10.1016/j.conb.2019.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nenclares P., Bhide S.A., Sandoval-Insausti H., et al. Impact of antibiotic use during curative treatment of locally advanced head and neck cancers with chemotherapy and radiotherapy. Eur J Cancer. 2020;131:9–15. doi: 10.1016/j.ejca.2020.02.047. [DOI] [PubMed] [Google Scholar]
- 12.Hahn A.W., Froerer C., VanAlstine S., et al. Targeting Bacteroides in stool microbiome and response to treatment with first-line VEGF tyrosine kinase inhibitors in metastatic renal-cell carcinoma. Clin Genitourin Cancer. 2018;16(5):365–368. doi: 10.1016/j.clgc.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Liu K., Zhang W., Tan Q., Jiang G., Jia J. Antibiotic use is a negative predictor of the efficacy and toxicity of epidermal growth factor receptor-targeted therapy in advanced non-small cell lung cancer. Oncol Lett. 2019;18:2677–2683. doi: 10.3892/ol.2019.10481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Modi S.R., Collins J.J., Relman D.A. Antibiotics and the gut microbiota. J Clin Invest. 2014;124(10):4212–4218. doi: 10.1172/JCI72333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eisenhauer E.A., Therasse P., Bogaerts J., et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 16.Altman DG, McShane LM, Sauerbrei W, Taube SE. Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK): explanation and elaboration. PLoS Med. 2012 29;9(5):e1001216. [DOI] [PMC free article] [PubMed]
- 17.R Foundation for Statistical Computing. R: A Language and Environment for Statistical Computing. [Internet]. Vienna, Austria; 2020. https://www.r-project.org/ Available at.
- 18.Blank C.U., Haanen J.B., Ribas A., Schumacher T.N. The “cancer immunogram.”. Science (80- ) 2016;352(6286):658–660. doi: 10.1126/science.aaf2834. [DOI] [PubMed] [Google Scholar]
- 19.Galloway-Peña J.R., Jenq R.R., Shelburne S.A. Can consideration of the microbiome improve antimicrobial utilization and treatment outcomes in the oncology patient? Clin Cancer Res. 2017;23(13):3263–3268. doi: 10.1158/1078-0432.CCR-16-3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pflug N., Kluth S., Vehreschild J.J., et al. Efficacy of antineoplastic treatment is associated with the use of antibiotics that modulate intestinal microbiota. Oncoimmunology. 2016;5(6) doi: 10.1080/2162402X.2016.1150399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duong C., Vétizou M., Zitvogel L. The impact of microbiota on the efficacy of immune checkpoint blockade. Eur J Immunol. 2016;46:193–194. [Google Scholar]
- 22.Derosa L., Routy B., Mezquita L., et al. Antibiotics prescription to decrease progression-free survival (PFS) and overall survival (OS) in patients with advanced cancers treated with PD1/PDL1 immune checkpoint inhibitors. J Clin Oncol. 2017;35(suppl 15):3015. [Google Scholar]
- 23.Ahmed J., Kumar A., Parikh K., et al. Use of broad-spectrum antibiotics impacts outcome in patients treated with immune checkpoint inhibitors. Oncoimmunology. 2018;7(11) doi: 10.1080/2162402X.2018.1507670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Casadei C., Lolli C., Farolfi A. Immune checkpoint inhibitors and the importance of concomitant medications: focus on antibiotics. Ann Transl Med. 2019;7(suppl 8):S339. doi: 10.21037/atm.2019.09.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Derosa L., Routy B., Kroemer G., Zitvogel L. The intestinal microbiota determines the clinical efficacy of immune checkpoint blockers targeting PD-1/PD-L1. Oncoimmunology. 2018;7(6) doi: 10.1080/2162402X.2018.1434468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Leeuwen R.W.F., van Gelder T., Mathijssen R.H.J., Jansman F.G.A. Drug-drug interactions with tyrosine-kinase inhibitors: a clinical perspective. Lancet Oncol. 2014;15(8):e315–e326. doi: 10.1016/S1470-2045(13)70579-5. [DOI] [PubMed] [Google Scholar]
- 27.FDA Drug Development and Drug Interactions: Table of Substrates, Inhibitors and Inducers. https://www.fda.gov/drugs/drug-interactions-labeling/drug-development-and-drug-interactions-table-substrates-inhibitors-and-inducers Available at.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.