Abstract
Background
The ETOP 10-16 BOOSTER trial failed to demonstrate a progression-free survival (PFS) benefit for adding bevacizumab to osimertinib in second line. An exploratory subgroup analysis, however, suggested a PFS benefit of the combination in patients with a smoking history and prompted us to do this study.
Methods
A systematic review and meta-analysis to evaluate the differential effect of smoking status on the benefit of adding an angiogenesis inhibitor to epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitor therapy was carried out. All relevant randomized controlled trials appearing in main oncology congresses or in PubMed as of 1 November 2021 were used according to the Preferred Reporting Items for Systematic Review and Meta-Analyses statement. Primarily PFS according to smoking status, and secondarily overall survival (OS) were of interest. Pooled and interaction hazard ratios (HRs) were estimated by fixed or random effects models, depending on the detected degree of heterogeneity. Bias was assessed using the revised Cochrane tool for randomized controlled trials (RoB 2).
Results
Information by smoking was available for 1291 patients for PFS (seven studies) and 678 patients for OS (four studies). The risk of bias was low for all studies. Combination treatment significantly prolonged PFS for smokers [n = 502, HR = 0.55, 95% confidence interval (CI): 0.44-0.69] but not for nonsmokers (n = 789, HR = 0.92, 95% CI: 0.66-1.27; treatment-by-smoking interaction P = 0.02). Similarly, a significant OS benefit was found for smokers (n = 271, HR = 0.66, 95% CI: 0.47-0.93) but not for nonsmokers (n = 407, HR = 1.07, 95% CI: 0.82-1.42; treatment-by-smoking interaction P = 0.03).
Conclusion
In advanced EGFR-non-small-cell lung cancer patients, the addition of an angiogenesis inhibitor to EGFR-tyrosine kinase inhibitor therapy provides a statistically significant PFS and OS benefit in smokers, but not in non-smokers. The biological basis for this observation should be pursued and could determine whether this might be due to a specific co-mutational pattern produced by tobacco exposure.
Key words: EGFR mutations, NSCLC, EGFR-TKI, randomised controlled trial, smoking status
Highlights
-
•
Target population consists of EGFR-non-small-cell lung cancer patients.
-
•
The relative effect of adding an angiogenesis inhibitor to EGFR tyrosine kinase inhibitor by smoking status was explored.
-
•
In patients with smoking history, the addition of an angiogenesis inhibitor provides significant PFS and OS benefit.
-
•
This is not the case in patients with a negative smoking history.
-
•
The biological basis for this observation should be pursued.
Introduction
First-line epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) are an effective treatment of advanced non-small-cell lung cancer (NSCLC) harboring somatic sensitizing EGFR mutations. Despite an initial response, however, the majority of these patients experience disease progression or death after first-line EGFR TKI therapy.1 Resistance to EGFR inhibition has been found to be associated with increased vascular endothelial growth factor (VEGF) levels in EGFR-mutant NSCLC patients (EGFR-NSCLC).2,3 The synergistic effect of reducing the expression of VEGF when receiving EGFR TKI therapy is explored in recent research and the combination of angiogenesis inhibitors with EGFR TKIs has shown encouraging results in EGFR-NSCLC patients.4, 5, 6, 7, 8 Before becoming approved for first-line treatment,9 osimertinib has been used for patients failing treatment with first-generation TKIs in the presence of an EGFR T790M acquired resistance mutation.1 In the randomized ETOP 10-16 BOOSTER trial on second-line osimertinib with or without bevacizumab, the primary analysis failed to show superiority of the addition of bevacizumab to osimertinib alone. In an exploratory subgroup analysis, however, an improvement in progression-free survival (PFS) was detected for smokers, current or former cigarette smokers, [hazard ratio (HR) = 0.52, 95% confidence interval (CI): 0.30-0.90, P = 0.021] with a statistically significant treatment-by-smoking interaction (P = 0.0052).10
The addition of angiogenesis inhibitors to EGFR TKIs has been examined and reported in other trials, in first line as well as in second line. A systematic review and meta-analysis was conducted to evaluate the relative effect of adding an angiogenesis inhibitor (bevacizumab or ramucirumab) to EGFR TKI therapy in advanced EGFR-NSCLC patients, according to their smoking status.
Methods
Search strategy and study selection
This systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Review and Meta-Analyses statement.11 Eligible randomized controlled trials (RCTs) that compared combination of angiogenesis inhibitor (bevacizumab or ramucirumab) with EGFR TKI therapy against EGFR TKI therapy alone were identified from the PubMed electronic database for articles published by 1 November 2021. The target population was advanced EGFR-NSCLC patients. The following MeSH terms were used (‘EGFR-TKI’ or ‘erlotinib’ or ‘osimertinib’ or ‘EGFR-mutated’ or ‘EGFR-mutation’) AND (‘bevacizumab’ or ‘ramucirumab’) AND (‘NSCLC’ or ‘non-small cell lung cancer’) AND (‘randomized’ or ‘RCT’). To identify unpublished studies, all abstracts from the most recent (2020-2021) main oncology congresses (annual congresses of American Association for Cancer Research [AACR], American Society of Clinical Oncology [ASCO] and European Society of Medical Oncology [ESMO], European Lung Cancer Congress [ELCC], and World Conference on Lung Cancer [WCLC]) were examined.
Data extraction process
For each included trial, study and patient characteristics were extracted along with the HR and 95% CI for PFS and overall survival (OS) for the subgroups defined by smoking status. Data were extracted independently by two reviewers (KV and PZ), by reviewing abstracts and full-text articles where appropriate. Disagreements were resolved by consensus, or by referral to an additional reviewer (ZT).
Bias assessment
Two independent reviewers (KV and PZ) assessed the risk of bias for each study using the recently revised Cochrane tool for randomized trials (RoB 2).12 The risk of bias was assessed for each study outcome using the five domains (D1-D5) of the tool: D1, bias arising from the randomization process; D2, bias due to deviations from intended interventions; D3, bias due to missing outcome data; D4, bias in measurement of the outcome; D5, bias in selection of the reported result. Disagreements were resolved by consensus.
Outcomes
The primary endpoint of interest was progression-free survival (PFS) by smoking status, and the secondary was the OS by smoking status.
Statistical analysis
To estimate the size of the benefit of combination treatment across studies, pooled weighted estimates (with inverse-variance weights) were derived either from fixed or random effects models,13 depending on the level of heterogeneity detected.14,15 Heterogeneity was assessed via the Cochran’s Q test (P < 10%) and the I2 measure.16 Analysis was carried out separately by smoking status (smokers [current or former smokers] and nonsmokers [never-smokers]) and a test for treatment-by-smoking interaction was used to assess differences in treatment effect across smoking subgroups. All statistical tests were two-sided and significance was tested at 5%. SAS v9.4 (SAS Institute, Cary, NC) and R v3.6.2 (R Foundation for Statistical Computing, Vienna, Austria) were used for statistical analysis.
Results
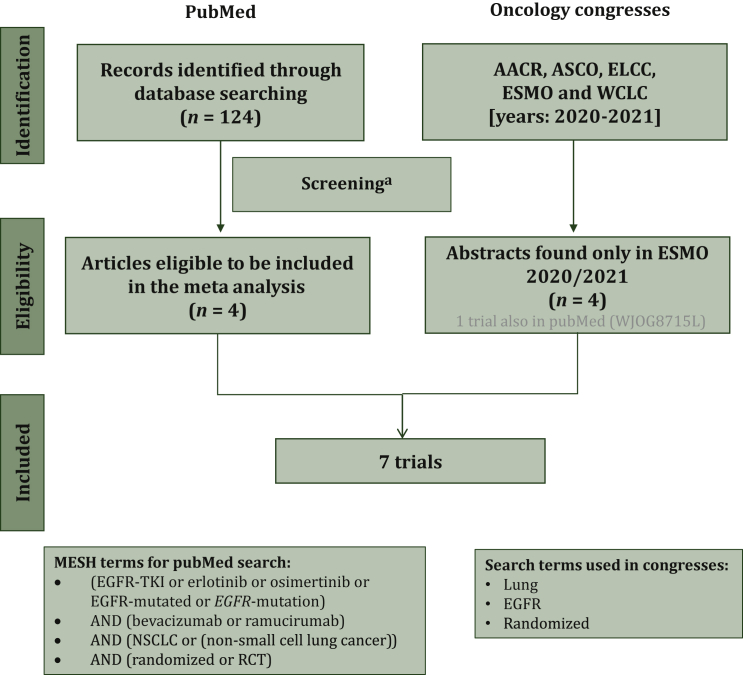
Seven eligible RCTs were identified (Figure 1; Table 1)4,6,7,10,17, 18, 19, 20, 21 with three studies only from abstracts presented in ESMO 2020/2021. A total of 1343 patients were randomized to receive either a combination of an angiogenesis inhibitor (bevacizumab or ramucirumab) with EGFR TKI therapy (osimertinib or erlotinib), or the corresponding EGFR TKI therapy alone (1 with placebo). No studies with an EGFR TKI therapy other than osimertinib or erlotinib were identified. All reasons for exclusion of studies from the initial 124 studies are detailed in Figure 1. The risk of bias of PFS and OS results was judged to be low without concerns for all included trials and in all five domains (Table 1). Five studies (71%) included patients in first line [erlotinib and bevacizumab: three studies (n = 536); erlotinib and ramucirumab: one study (n = 449); and osimertinib and bevacizumab: one study (n = 122)]. Two studies included patients in second line [osimertinib and bevacizumab (n = 236)]. Recruited patients were mainly Asian with few studies enrolling patients with non-Asian ethnicity (BEVERLY study with only Italian centers17; non-Asian participants in BOOSTER: 59%10 and RELAY: 23%4) (Table 1). The median age, per treatment arm, ranged from 64 to 70 years old, while in all studies the majority were female (from 59% up to 66% per arm) with Eastern Cooperative Oncology Group performance status (ECOG PS) 0 for first-line studies (from 52% to 65% per arm) and ECOG PS ≥1 for second-line studies. In the majority of studies most patients were nonsmokers (up to 68%), in all studies most with stage IV NSCLC (>63% per arm and up to 99%) and almost all with EGFR exon 19 deletion or exon 21 L858R mutation (Table 1). Histology was mainly non-squamous while only two studies included predominantly adenocarcinoma patients4,21 (data not shown).
Figure 1.
Flowchart of patient disposition.
AACR, American Association for Cancer Research; ASCO, American Society of Clinical Oncology; ELCC, European Lung Cancer Congress; ESMO, European Society of Medical Oncology; WCLC, World Conference on Lung Cancer.
aReasons for exclusion: 38 review/meta-analysis, 36 non-randomized trials, 8 no epidermal growth factor receptor tyrosine kinase inhibitor (EGFR TKI) treatment, 7 no survival results, 7 no results by smoking, 4 subgroup analysis, 4 study design paper, 3 no angiogenesis inhibitor treatment, 2 clinical practice guidelines, 2 reply letter, 2 no comparison with EGFR TKI, 2 pooled analysis, 2 updated results, 1 case study, 1 highlights, 1 study on mice.
Table 1.
Baseline demographics, clinical characteristics and bias assessment for each study
| Study (N) (Line, phase) |
Bias assessmenta D1 D2 D3 D4 D5 O |
Ethnicity | Treatment | n | Age (years) |
Female |
ECOG PS 0 |
Smokers |
Nonsmokers |
Stage IVb |
EGFR Ex19 deletion |
EGFR Ex21 L858R mutation |
EGFR Other/missing |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median (range) | n (%) | ||||||||||||
| BEVERLY (N = 160) (First, III)17 |
 |
Only Italian centers | Erlo + Beva | 80 | 65.9 (58-72)c | 52 (65.0) | 52 (65.0) | 34 (42.5) | 46 (57.5) | 77 (96.3) | 44 (55.0) | 34 (42.5) | 2 (2.5) |
| Erlo | 80 | 67.7 (61-74)c | 50 (62.5) | 47 (58.8) | 43 (53.8) | 37 (46.3) | 75 (93.8) | 44 (55.0) | 32 (40.0) | 4 (5.0) | |||
| JO25567 (N = 152) (First, II)7,18 |
 |
Asian | Erlo + Beva | 75 | 67.0 (59-73)c | 45 (60.0) | 43 (57.0) | 24 (32.0) | 51 (68.0) | 60 (80.0) | 40 (53.0) | 35 (47.0) | — |
| Erlo | 77 | 67.0 (60-73)c | 51 (66.0) | 41 (53.0) | 26 (34.0) | 51 (66.0) | 62 (81.0) | 40 (52.0) | 37 (48.0) | — | |||
| NEJ026 (N = 224) (First, III)6,19 |
 |
Asian | Erlo + Beva | 112 | 67.0 (61-73)c | 71 (63.0) | 64 (57.0) | 41 (37.0) | 65 (58.0)d | 82 (73.0) | 56 (50.0) | 56 (50.0) | — |
| Erlo | 112 | 68.0 (62-73)c | 73 (65.0) | 68 (61.0) | 41 (37.0) | 64 (57.0)d | 84 (75.0) | 55 (49.0) | 57 (51.0) | — | |||
| RELAY (N = 449) (First, III)4 |
 |
77% Asian | Erlo + Ramu | 224 | 65.0 (57-71)c | 141 (63.0) | 116 (52.0) | 64 (29.0) | 134 (60.0)e | 195 (87.0) | 123 (55.0) | 99 (44.0) | 2 (1.0) |
| Erlo + placebo | 225 | 64.0 (56-70)c | 142 (63.0) | 119 (53.0) | 73 (32.0) | 139 (62.0)e | 189 (84.0) | 120 (53.0) | 105 (47.0) | — | |||
| WJOG9717L (N = 122) (First, II)20 |
 |
Asian | Osi + Beva | 61 | 67.0 (41-86) | 37 (60.7) | 32 (52.5) | 23 (37.7) | 38 (62.3) | 48 (78.7) | 35 (57.4) | 26 (42.6) | — |
| Osi | 61 | 66.0 (29-85) | 38 (62.3) | 34 (55.7) | 31 (50.8) | 30 (49.2) | 46 (75.4) | 36 (59.0) | 25 (41.0) | — | |||
| BOOSTER (N = 155) (Second, II)10 |
 |
41% Asian | Osi + Beva | 78 | 68.0 (34-85) | 47 (60.0) | 22 (28.0) | 34 (44.0) | 44 (56.0) | 76 (97.0) | 58 (74.0) | 20 (26.0) | — |
| Osi | 77 | 66.0 (41-83) | 49 (64.0) | 25 (33.0) | 28 (36.0) | 49 (64.0) | 76 (99.0) | 51 (66.0) | 26 (34.0) | — | |||
| WJOG8715L (N = 81) (Second, II)21 |
 |
Asian | Osi + Beva | 40 | 68.0 (43-82) | 24 (60.0) | 20 (50.0) | 19 (48.0) | 21 (53.0) | 33 (83.0) | 22 (55.0) | 18 (45.0) | — |
| Osi | 41 | 70.0 (41-82) | 24 (59.0) | 17 (42.0) | 21 (51.0) | 20 (49.0) | 26 (63.0) | 28 (68.0) | 13 (32.0) | — | |||
D1, bias arising from the randomization process; D2, bias due to deviations from intended interventions; D3, bias due to missing outcome data; D4, bias in measurement of the outcome; D5, bias in selection of the reported result; O, overall bias.
Beva, bevacizumab; EGFR, epidermal growth factor; EGOG PS, Eastern Cooperative Oncology Group performance status; Erlo, erlotinib; Osi, osimertinib; Ramu, ramucirumab.
The risk of bias for both endpoints (PFS and OS) was low in all domains D1-D5 and overall.
The remaining patients had other stage, missing stage, or post-surgery recurrence.
The interquartile range is presented.
A total of 6 (5.0%) and 7 (6.0%) former light smokers in Erlo + Beva and Erlo alone groups, respectively, were not included in the analysis.
A total of 26 (12.0%) and 13 (6.0%) patients with unknown/missing smoking status in Erlo + Ramu and Erlo + placebo groups, respectively, were not included in the analysis.
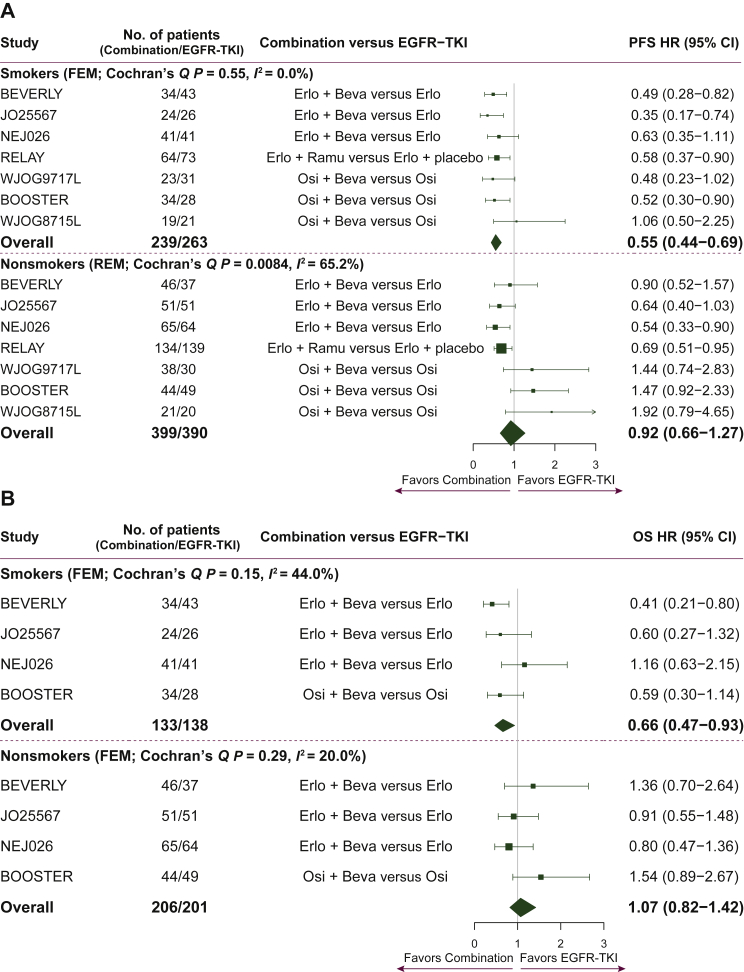
Information by smoking status in the seven studies was available for 1291 patients for PFS (39 patients with missing smoking status4 and 13 former light smokers [less than 10 pack-years in their lifetime and had stopped smoking more than 15 years before the study] were not included in the extracted survival data6,19). A total of 502 patients were former/current smokers while 789 patients were nonsmokers. Treatment with combination therapy of an angiogenesis inhibitor with EGFR TKI therapy compared with EGFR TKI therapy alone was associated with a 45% reduction in the risk of a PFS event in the subgroup of smokers (HR = 0.55, 95% CI: 0.44-0.69, P < 0.001, Cochran’s Q P = 0.55, I2 = 0%). In the nonsmokers subgroup the pooled PFS effect derived from a random-effects model was not significant (HR = 0.92, 95% CI: 0.66-1.27, P = 0.60, Cochran’s Q P = 0.0084, I2 = 65%). Only two studies showed a significant clinical benefit of combination treatment of nonsmokers in PFS (HR = 0.54, 95% CI: 0.33-0.90 and HR = 0.69, 95% CI: 0.51-0.95). Although not significant, for three studies there was a PFS trend towards benefit from osimertinib monotherapy for nonsmokers. Importantly, for the treatment effect on PFS, a statistically significant difference was found by smoking status, with a significant benefit in smokers, and no such benefit apparent in nonsmokers (interaction HR = 0.62, 95% CI: 0.41-0.93, P = 0.02) (Figure 2A).
Figure 2.
(A) Forest plot of HRs comparing PFS in the smokers and nonsmokers subgroups. Note 1: interaction effect of treatment by smoking HR = 0.62, P = 0.020. Note 2: HRs for each trial are represented by the squares with the respective 95% CI. The diamonds represent the estimated overall effect based on the meta-analysis of the trials. All HRs (95% CIs) are unadjusted except for WJOG8715L and BOOSTER trials. A total of 52 patients without results by smoking status are excluded from the analysis (13 former light smokers from the NEJ026 study and 39 patients from RELAY with unknown/missing smoking status). (B) Forest plot of HRs comparing OS in the smokers and nonsmokers subgroups. Note 1: interaction effect of treatment by smoking HR = 0.62, P = 0.030. Note 2: HRs for each trial are represented by the squares with the respective 95% CI. The diamonds represent the estimated overall effect based on the meta-analysis fixed effect of the trials. All HRs (95% CIs) are unadjusted except for the BOOSTER trial. A total of 13 former light smokers from the NEJ026 study are excluded from the analysis.
Beva, bevacizumab; CI, confidence interval; Combination, EGFR-TKI plus angiogenesis inhibitor; EGFR-TKI, epidermal growth factor receptor-tyrosine kinase inhibitor; Erlo, erlotinib; FEM, fixed effects model; HR, hazard ratio; OS, overall survival; Osi, osimertinib; PFS, progression-free survival; Ramu, ramucirumab; REM random effects model.
For OS, information by smoking status was available for 678 patients from four studies (271 smokers; 407 nonsmokers). A statistically significant OS benefit was found in only one study, and this was only in the subgroup of smokers (HR = 0.41, 95% CI: 0.21-0.80). The statistically significant benefit shown in PFS for smokers was also found in OS (HR = 0.66, 95% CI: 0.47-0.93, P = 0.017, Cochran’s Q P = 0.15, I2 = 44%). OS was not significantly better in the subgroup of nonsmokers for any of the individual studies and overall (HR = 1.07, 95% CI: 0.82-1.42, P = 0.61, Cochran’s Q P = 0.29, I2 = 20%). For OS as well, similarly to PFS, the treatment-by-smoking interaction was significant (interaction HR = 0.62, 95% CI: 0.40-0.95, P = 0.03) (Figure 2B).
Discussion
The results of this literature review and meta-analysis demonstrate that in patients with advanced EGFR-mutated NSCLC with a smoking history, the addition of an angiogenesis inhibitor to EGFR TKI therapy provides a statistically significant PFS and OS benefit. In the subgroup of current or former smokers treated with the combination therapy, the risk of a PFS event was reduced by 45% and the risk of death by 34%. Importantly, a significant interaction effect was found indicating that smoking status could be a potential predictive marker for PFS and OS in this population.
It is important to state, that the BOOSTER trial and, with the exception of the BEVERLY trial, all other trials involved in this meta-analysis were restricted to patients with the common EGFR mutations, exon 21 point mutation or exon 19 deletion. Uncommon EGFR mutations account for 10%-20% of EGFR mutations in advanced NSCLC and occur in 10%-20% of patients with NSCLC and, with the exception of EGFR exon 20 insertion mutations, generally respond to second- and third-generation EGFR TKIs.22, 23, 24, 25 Compared with common EGFR mutations, EGFR exon 20 insertion mutations are more common in patients with a smoking history.26 It would be of interest to explore the role of combination EGFR TKI and anti-angiogenic agents in such a patient population. Studies including afatinib and bevacizumab for NSCLC with uncommon EGFR point mutations (NCT05267288) and poziotinib with ramucirumab for NSCLC harboring EGFR exon 20 insertion mutation (NCT05045404) are ongoing.
The biological basis for this observation should be pursued. Tobacco exposure was shown to produce a heavy burden of genomic mutations in lung cancer, including TP53 mutations.27 Subgroup analysis in the RELAY randomised study comparing erlotinib in combination with ramucirumab versus erlotinib alone showed that TP53 mutations were associated with improved survival outcomes in patients with EGFR-mutated NSCLC, supporting a concept of improved efficacy with anti-angiogenic therapy in tumors harboring TP53 mutations.28 A possible explanation to this could be that anti-angiogenic therapy may be more effective in tumors with specific mutations, e.g. TP53 triggered by tobacco exposure. Tumors harboring TP53 mutations have been associated with improved outcomes with VEGF or VEGF receptor inhibitors.29, 30, 31
This study supports the hypothesis triggered by the significant effect found in an exploratory subgroup analysis of the ETOP 10-16 BOOSTER trial. A translational study assessing molecular alterations including TP53 in tumor tissue and plasma samples from this trial has been initiated and might shed further insight.
Acknowledgements
The authors thank Pilar Garrido (University Hospital Ramón y Cajal, Madrid, Spain), Prof. Edward Garon (UCLA Hematology/Oncology Santa Monica, CA), Prof. Hidehito Horinouchi (National Cancer Center, Tokyo, Japan), Prof. Tony Mok (Prince of Wales Hospital, Hong Kong), and Prof. Ben Solomon (Peter MacCallum Cancer Center, Melbourne, Australia) and all participants of the Q&A discussion of the ESMO Virtual Plenaries for their insightful comments that ultimately gave the idea for this meta-analysis.
Funding
This work was supported AstraZeneca (grant number: ESR-15-11666) and F. Hoffmann-La Roche (grant number: MO39447).
Disclosure
UD reports honorarium as Member of the Tumor Agnostic Evidence Generation working Group of Roche, outside the submitted work. RASo reports advisory role for Amgen, AstraZeneca, Bayer, Bristol Myers Squibb (BMS), Boehringer Ingelheim, Lily, Merck, Novartis, Pfizer, Roche, Taiho, Takeda, and Yuhan and grants from AstraZeneca, Boehringer Ingelheim, outside the submitted work. SP reports grants from Amgen, AstraZeneca, Boehringer Ingelheim, BMS, Clovis, F. Hoffman-La Roche, Illumina, Novartis, Pfizer, Merck Sharp & Dohme (MSD), personal fees from Amgen, AbbVie, AstraZeneca, Bayer, Biocartis, Boehringer Ingelheim, BMS, Clovis, Daiichi Sankyo, Debiopharm, Eli Lilly, F. Hoffman-La Roche, Foundations Medicine, Illumina, Janssen, Novartis, PharmaMar, Pfizer, Regeneron, Sanofi, Seattle Genetics, Takeda, MSD, Merck Serono, Merrimack, Medscape, Phosphoplatin Therapeutics, Beigene, Imedex, outside the submitted work. JDC reports grants and personal fees from AstraZeneca, BMS, MSD, and Hoffmann-La Roche, personal fees from Bayer, Boehringer Ingelheim, GlaxoSmithKline (GSK), Jansen-Cilag, Lilly, Novartis, Pfizer, and Takeda, outside the submitted work. LC reports advisory role for AstraZeneca, Roche, and Daichi, outside the submitted work. MF reports grants from AstraZeneca and BMS and other support AstraZeneca, BMS, Boehringer Ingelheim, Janssen, MSD, Pfizer, Roche, and Takeda, outside the submitted work. EN reports grants, personal fees, and non-financial support from Roche, personal fees and non-financial support from AstraZeneca, grants, personal fees and non-financial support from BMS, personal fees and non-financial support from MSD, grants and personal fees from Merck Serono, personal fees from Takeda, grants, personal fees, and non-financial support from Pfizer, personal fees from Lilly, personal fees from Bayer, personal fees from Amgen, personal fees from Boehringer Ingelheim, outside the submitted work. EC reports personal fees from AstraZeneca, Amgen, BMS, MSD, and Roche, outside the submitted work. MAS reports advisory role for Roche and Boehringer Ingelheim, speaker role for Pierre Fabre, and travel grants from Roche and PharmaMar, outside the submitted work. RB reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Roche, AstraZeneca, BMS, Amgen, and MSD, participation on Data Safety Monitoring Board or Advisory Board for AstraZeneca, BMS, and Roche, outside the submitted work. MP reports grants, personal fees, and non-financial support from AstraZeneca, BMS, and Roche, personal fees from MSD and Takeda, outside the submitted work. SC reports non-financial support from Pfizer, Roche, MSD, BMS, outside the submitted work. RASt reports consultant or advisory role for AstraZeneca, BMS, Boehringer Ingelheim, GSK, MSD, Pfizer, Roche, Sandoz, Seattle Genetics, Takeda, speaker honoraria from Amgen, AstraZeneca, Blueprint, BMS, Boehringer Ingelheim, GSK, MSD, Novartis, Roche, Data Monitoring Committee (DMC) role from Genentech/Roche and Takeda, and financial support for ETOP and IBCSG trials (where he is president), from AstraZeneca, BMS, Daiichi Sankyo, Celgene, Ipsen, Janssen, Mirati, MSD, Novartis, Pfizer, Pierre Fabre, Roche. All other authors have declared no conflicts of interest.
References
- 1.Mok T.S., Wu Y.-L., Ahn M.-J., et al. Osimertinib or platinum–pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2016;376:629–640. doi: 10.1056/NEJMoa1612674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hung M.S., Chen I.C., Lin P.Y., et al. Epidermal growth factor receptor mutation enhances expression of vascular endothelial growth factor in lung cancer. Oncol Lett. 2016;12:4598–4604. doi: 10.3892/ol.2016.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Viloria-Petit A., Crombet T., Jothy S., et al. Acquired resistance to the antitumor effect of epidermal growth factor receptor-blocking antibodies in vivo: a role for altered tumor angiogenesis. Cancer Res. 2001;61:5090–5101. [PubMed] [Google Scholar]
- 4.Nakagawa K., Garon E.B., Seto T., et al. Ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced non-small-cell lung cancer (RELAY): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:1655–1669. doi: 10.1016/S1470-2045(19)30634-5. [DOI] [PubMed] [Google Scholar]
- 5.Rosell R., Dafni U., Felip E., et al. Erlotinib and bevacizumab in patients with advanced non-small-cell lung cancer and activating EGFR mutations (BELIEF): an international, multicentre, single-arm, phase 2 trial. Lancet Respir Med. 2017;5:435–444. doi: 10.1016/S2213-2600(17)30129-7. [DOI] [PubMed] [Google Scholar]
- 6.Saito H., Fukuhara T., Furuya N., et al. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol. 2019;20:625–635. doi: 10.1016/S1470-2045(19)30035-X. [DOI] [PubMed] [Google Scholar]
- 7.Seto T., Kato T., Nishio M., et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol. 2014;15:1236–1244. doi: 10.1016/S1470-2045(14)70381-X. [DOI] [PubMed] [Google Scholar]
- 8.Zhou Q., Xu C.R., Cheng Y., et al. Bevacizumab plus erlotinib in Chinese patients with untreated, EGFR-mutated, advanced NSCLC (ARTEMIS-CTONG1509): a multicenter phase 3 study. Cancer Cell. 2021;39:1279–1291. doi: 10.1016/j.ccell.2021.07.005. e1273. [DOI] [PubMed] [Google Scholar]
- 9.Soria J.-C., Ohe Y., Vansteenkiste J., et al. Osimertinib in untreated EGFR-mutated advanced non–small-cell lung cancer. N Engl J Med. 2017;378:113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 10.Soo R.A., Han J.Y., Dafni U., et al. A randomised phase II study of osimertinib and bevacizumab versus osimertinib alone as second-line targeted treatment in advanced NSCLC with confirmed EGFR and acquired T790M mutations: the European Thoracic Oncology Platform (ETOP 10-16) BOOSTER trial. Ann Oncol. 2022;33:181–192. doi: 10.1016/j.annonc.2021.11.010. [DOI] [PubMed] [Google Scholar]
- 11.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med. 2021;18 doi: 10.1371/journal.pmed.1003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sterne J.A.-O., Savović J., Page M.J., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. Br Med J. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 13.Borenstein M., Hedges L.V., Higgins J.P.T., Rothstein H.R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1:97–111. doi: 10.1002/jrsm.12. [DOI] [PubMed] [Google Scholar]
- 14.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 15.DerSimonian R., Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. 2015;45:139–145. doi: 10.1016/j.cct.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. Br Med J. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gridelli C., Rossi A., Ciardiello F., et al. BEVERLY: rationale and design of a randomized open-label phase III trial comparing bevacizumab plus erlotinib versus erlotinib alone as first-line treatment of patients with EGFR-mutated advanced nonsquamous non-small-cell lung cancer. Clin Lung Cancer. 2016;17:461–465. doi: 10.1016/j.cllc.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto N., Seto T., Nishio M., et al. Erlotinib plus bevacizumab vs erlotinib monotherapy as first-line treatment for advanced EGFR mutation-positive non-squamous non-small-cell lung cancer: survival follow-up results of the randomized JO25567 study. Lung Cancer. 2021;151:20–24. doi: 10.1016/j.lungcan.2020.11.020. [DOI] [PubMed] [Google Scholar]
- 19.Kawashima Y., Fukuhara T., Saito H., et al. Bevacizumab plus erlotinib versus erlotinib alone in Japanese patients with advanced, metastatic, EGFR-mutant non-small-cell lung cancer (NEJ026): overall survival analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Respir Med. 2022;10:72–82. doi: 10.1016/S2213-2600(21)00166-1. [DOI] [PubMed] [Google Scholar]
- 20.Kenmotsu H., Wakuda K., Mori K., et al. LBA44 Primary results of a randomized phase II study of osimertinib plus bevacizumab versus osimertinib monotherapy for untreated patients with non-squamous non-small cell lung cancer harboring EGFR mutations: WJOG9717L study. Ann Oncol. 2021;32:S1322–S1323. doi: 10.1016/j.jtho.2022.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Akamatsu H., Toi Y., Hayashi H., et al. Efficacy of osimertinib plus bevacizumab vs osimertinib in patients with EGFR T790M–mutated non–small cell lung cancer previously treated with epidermal growth factor receptor–tyrosine kinase inhibitor: West Japan Oncology Group 8715L Phase 2 randomized clinical trial. JAMA Oncol. 2021;7:386–394. doi: 10.1001/jamaoncol.2020.6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tu H.Y., Ke E.E., Yang J.J., et al. A comprehensive review of uncommon EGFR mutations in patients with non-small cell lung cancer. Lung Cancer. 2017;114:96–102. doi: 10.1016/j.lungcan.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 23.Yang J.C., Sequist L.V., Geater S.L., et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol. 2015;16:830–838. doi: 10.1016/S1470-2045(15)00026-1. [DOI] [PubMed] [Google Scholar]
- 24.Passaro A., Mok T., Peters S., et al. Recent advances on the role of EGFR tyrosine kinase inhibitors in the management of NSCLC with uncommon, non Exon 20 insertions, EGFR mutations. J Thorac Oncol. 2021;16:764–773. doi: 10.1016/j.jtho.2020.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Janning M., Süptitz J., Albers-Leischner C., et al. Treatment outcome of atypical EGFR mutations in the German National Network Genomic Medicine Lung Cancer (nNGM) Ann Oncol. 2022;33:602–615. doi: 10.1016/j.annonc.2022.02.225. [DOI] [PubMed] [Google Scholar]
- 26.Friedlaender A., Subbiah V., Russo A., et al. EGFR and HER2 exon 20 insertions in solid tumours: from biology to treatment. Nat Rev Clin Oncol. 2022;19:51–69. doi: 10.1038/s41571-021-00558-1. [DOI] [PubMed] [Google Scholar]
- 27.Gibbons D.L., Byers L.A., Kurie J.M. Smoking, p53 mutation, and lung cancer. Mol Cancer Res. 2014;12:3–13. doi: 10.1158/1541-7786.MCR-13-0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakagawa K., Nadal E., Garon E.B., et al. RELAY subgroup analyses by EGFR Ex19del and Ex21L858R mutations for ramucirumab plus erlotinib in metastatic non–small cell lung cancer. Clin Cancer Res. 2021;27:5258–5271. doi: 10.1158/1078-0432.CCR-21-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwaederlé M., Lazar V., Validire P., et al. VEGF-A expression correlates with TP53 mutations in non–small cell lung cancer: implications for antiangiogenesis therapy. Cancer Res. 2015;75:1187–1190. doi: 10.1158/0008-5472.CAN-14-2305. [DOI] [PubMed] [Google Scholar]
- 30.Said R., Hong D.S., Warneke C.L., et al. P53 mutations in advanced cancers: clinical characteristics, outcomes, and correlation between progression-free survival and bevacizumab-containing therapy. Oncotarget. 2013;4:705–714. doi: 10.18632/oncotarget.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wheler J.J., Janku F., Naing A., et al. TP53 alterations correlate with response to VEGF/VEGFR inhibitors: implications for targeted therapeutics. Mol Cancer Ther. 2016;15:2475–2485. doi: 10.1158/1535-7163.MCT-16-0196. [DOI] [PubMed] [Google Scholar]