Abstract
Background
Few prospective studies have used liquid biopsy testing in RAS-mutant metastatic colorectal cancer (mCRC), and its clinical significance remains unknown. Therefore, this study aimed to carry out a biomarker analysis by liquid biopsy using updated data of the phase II trial of FOLFOXIRI plus bevacizumab as first-line chemotherapy for RAS-mutant mCRC.
Materials and methods
A total of 64 patients who received modified FOLFOXIRI regimen (irinotecan 150 mg/m2, oxaliplatin 85 mg/m2, levofolinate 200 mg/m2, and fluorouracil 2400 mg/m2) plus bevacizumab biweekly were enrolled. The primary endpoint was the objective response rate (ORR). Plasma samples were collected at pre-treatment, 8 weeks after treatment, and progression in participants included in the biomarker study. The levels of circulating tumour DNA (ctDNA) and specific KRAS and NRAS variants were evaluated using real-time PCR assays.
Results
There were 62 patients (median age: 62.5 years, 92% performance status 0, 27% right side) who were assessable for efficacy and 51 for biomarker analysis. ORR was 75.8% (95% confidence interval 65.1% to 86.5%). The median progression-free survival was 12.1 months, and the median overall survival (OS) was 30.2 months. In 78% of patients, RAS mutations disappeared in the ctDNA at 8 weeks after treatment; these patients tended to have better outcomes than those with RAS mutations. Interestingly, RAS mutations remained undetectable during progression in 62% of patients. Survival analysis indicated that the median OS from progression was significantly longer in patients with RAS mutation clearance than in those with RAS mutation in the ctDNA at disease progression (15.1 versus 7.3 months, hazard ratio: 0.21, P = 0.0046).
Conclusions
Our biomarker study demonstrated no RAS mutations in ctDNA at disease progression in 62% of patients with RAS-mutant mCRC. Both OS and post-progression survival were better in patients with clearance of RAS mutations in ctDNA after triplet-based chemotherapy.
Key words: colorectal cancer, RAS mutation, FOLFOXIRI plus bevacizumab, liquid biopsy
Highlights
-
•
First-line FOLFOXIRI plus bevacizumab is effective for RAS-mutant mCRC with comparable efficacy in elderly patients.
-
•
RAS mutations disappeared in ctDNA after intensive chemotherapy in 62% of patients with mCRC with RAS-mutant tumours.
-
•
Survival time was longer in patients with RAS mutation clearance than in those with RAS mutations in ctDNA.
Introduction
The standard of care for metastatic colorectal cancer (mCRC) is treatment with a fluoropyrimidine-based chemotherapy in combination with either irinotecan or oxaliplatin, which is considered as the backbone therapy of first-line treatment with target agents, such as anti-vascular endothelial growth factor or anti-epidermal growth factor receptor (EGFR) antibodies. National and regional guidelines recommend regimens based on doublet combination therapy with oxaliplatin or irinotecan and triplet combination therapy, both of which are first-line treatment regimens.1 FOLFOXIRI plus bevacizumab therapy has been shown to prolong the median overall survival (OS) compared with FOLFIRI plus bevacizumab therapy in the TRIBE trial.2 Furthermore, the TRIBE-2 trial demonstrated that triplet combination therapy strategy is more effective than the sequential use of oxaliplatin and irinotecan when used with bevacizumab.3 However, triplet therapy is more toxic, and not all patients are candidates for aggressive chemotherapy.
Patients with RAS-mutant mCRC have been reported to have a worse prognosis than those with RAS wild-type mCRC, partly owing to the lack of efficacy of anti-EGFR antibody drugs4; therefore, FOLFOXIRI plus bevacizumab therapy is one of the key treatments for RAS-mutant mCRC. We have previously conducted the first phase II trial to evaluate the effectiveness of triplet combination chemotherapy in RAS-mutant mCRC and reported high response rates to FOLFOXIRI plus bevacizumab.5 However, we were unable to analyse survival data because of a short median follow-up duration.
With the improvement in genomic analysis technology and the ability to measure minute genomic alterations in the blood, liquid biopsy testing is expected to become a clinical procedure. Studies using liquid biopsy in mCRC with RAS wild-type tumours have identified changes in genomic alterations in the blood during and after progression following treatment with anti-EGFR antibody drugs.6,7 However, few prospective studies using liquid biopsy testing in RAS-mutant mCRC have been conducted. In addition, the dynamic changes in RAS mutations in the blood during and after systemic chemotherapy and their clinical significance remain unknown.
Therefore, this study aimed to carry out a biomarker analysis by liquid biopsy in mCRC patients with RAS-mutant tumours using updated data of the phase II trial of FOLFOXIRI plus bevacizumab as first-line chemotherapy for RAS-mutant mCRC, JACCRO CC-11 (UMIN000015152).
Materials and methods
Patient population
The eligibility criteria of the trial were as follows: histologically confirmed adenocarcinoma of the colon or rectum; KRAS (exon 2, 3, or 4)- or NRAS (exon 2, 3, or 4)-mutant tumour with unresectable metastases; at least one measurable lesion of ≥10 mm or a residual non-measurable lesion according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1; adequate bone marrow function (haemoglobin >9.0 g/dl, neutrophil count >1500/mm3, platelet count >100 000/mm3), hepatic function, and renal function; an Eastern Cooperative Oncology Group performance status (PS) of 0-1 if patients were aged ≤70 years, or 0 if they were aged 71-75 years; previous adjuvant chemotherapy that ended >12 months before first relapse; no major surgical procedure within 28 days before treatment; no clinically significant cardiovascular disease; no evidence of proteinuria or coagulopathy; no thromboembolic or haemorrhagic events in the previous 6 months; and no current therapeutic treatment with anticoagulants. We excluded patients with the following: uncontrolled infection; massive ascites or pleural effusion; symptomatic brain metastases; other malignancies diagnosed within 5 years before enrolment, except for early carcinoma that had been treated with curative intent; a history of palliative chemotherapy for metastatic disease; previous treatment with irinotecan or bevacizumab; or peripheral neuropathy of grade 1 or higher according to the National Cancer Institute-Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 3.0.
Ethical approval and trial registration
The study was approved by the ethics committee of each participating centre and carried out in accordance with the Declaration of Helsinki. All patients provided written informed consent before enrolment. This study was completed and registered with the UMIN (number 000015152).
Treatment
The treatment consisted of modified FOLFOXIRI regimen (irinotecan 150 mg/m2, oxaliplatin 85 mg/m2, and infusion fluorouracil 2400 mg/m2) as backbone chemotherapy and bevacizumab (5 mg/kg) administered every 2 weeks. Induction treatment was administered for a maximum of 12 cycles and then maintenance therapy with fluorouracil plus bevacizumab continued until disease progression, unacceptable toxicity, or withdrawal of consent.5
Assessments of efficacy and toxicity
The primary endpoint of the study was objective response rate (ORR). Secondary endpoints included progression-free survival (PFS), OS, safety, early tumour shrinkage (ETS), and depth of response (DpR), which were evaluated every 8 weeks until disease progression. Responses were evaluated according to the RECIST version 1.1 by the investigators and validated by an external review board. Toxicity was assessed for every cycle according to the NCI-CTCAE version 3.0.5 Tumours located from the caecum to the splenic flexure were classified as right-sided, whereas tumours involving the splenic flexure, descending colon, sigmoid colon, and rectum were classified as left-sided.
Measurement of gene mutation in circulating tumour DNA
Plasma samples were prospectively collected at three points during treatment according to a pre-planned liquid biopsy study accompanying the phase II trial as follows: pre-treatment, 8 weeks after the start of treatment, and discontinuation for any reason.
Plasma samples (2 ml) were used to extract circulating cell-free DNA (cfDNA) using the Qiagen (Germantown, MD) Circulating Nucleic Acid Kit, according to the manufacturer’s instructions. The resulting circulating tumour DNA (ctDNA) samples were refrigerated at 4-8°C. Target DNA mutations were tested by quantitative PCR using competitive allele-specific TaqMan® PCR (castPCR) assays (Life Technologies, Carlsbad, CA) specific for KRAS (G12A, G12C, G12D, G12R, G12S, G12V, G13D, Q61H) and NRAS (G12D, Q61K, Q61L, Q61R). Mutant allele fractions were calculated using standard curves generated from dilutions of positive controls with known quantities of mutant over wild-type DNA using PCR cycle thresholds.
Statistical analysis
Clinical outcomes were evaluated as previously reported.5 In the biomarker analysis, the associations between RAS status in ctDNA and clinical outcomes were evaluated using the Fisher’s exact test for response, t-test for DpR, and log-rank test for survival time. The cut-off date for the updated analysis was October 2019.
Statistical analyses were carried out using JMP 9.0.3 software (SAS Institute, Cary, NC).
Results
Characteristics of study participants
A total of 64 patients from 28 Japanese institutes were enrolled in this study between October 2014 and August 2016. Treatment efficacy was assessed for all 62 patients in the full analysis set, with a median follow-up of 30 months, and 63 patients were included in the safety population. Patient characteristics are summarised in Table 1.
Table 1.
Patient characteristics
| Characteristics | n | % |
|---|---|---|
| Sex | ||
| Male | 34 | 55 |
| Female | 28 | 45 |
| Age (years) | ||
| Median (range) | 62.5 (36-75) | |
| ≤70 | 54 | 87 |
| >70 | 8 | 13 |
| Performance status | ||
| ECOG 0 | 57 | 92 |
| ECOG 1 | 5 | 8 |
| Site of primary site | ||
| Right | 17 | 27 |
| Left | 45 | 73 |
| Diagnosis | ||
| Metachronous | 14 | 23 |
| Synchronous | 48 | 77 |
| Number of metastatic sites | ||
| 0 | 2 | 3 |
| 1 | 21 | 34 |
| ≥2 | 39 | 63 |
| Metastatic sites | ||
| Liver | 48 | 77 |
| Lung | 27 | 44 |
| Para-aortic lymph node | 10 | 16 |
| Peritoneum | 11 | 18 |
| Previous adjuvant chemotherapy | ||
| Yes | 2 | 3 |
| No | 62 | 97 |
| Resection of primary tumour | ||
| Yes | 38 | 61 |
| No | 24 | 39 |
| RAS status in tissue | ||
| KRAS exon2 mt | 50 | 80 |
| KRAS exon3 mt | 1 | 2 |
| KRAS exon4 mt | 5 | 8 |
| NRAS exon2 mt | 3 | 5 |
| NRAS exon3 mt | 3 | 5 |
| NRAS exon4 mt | 0 | 0 |
ECOG, Eastern Cooperative Oncology Group; mt, mutation.
We found that 3 (4.8%) patients had a complete response and 44 (71%) had a partial response, with an ORR of 75.8% [95% confidence interval (CI) 65.1% to 86.5%]. The remaining 13 patients (21.0%) had disease stabilisation, thus achieving a disease control rate of 96.8%. All the response results were confirmed by a central review. There were 61 patients assessable for ETS and DpR, and we found that ETS was 73.8% and median DpR was 49.2%. PFS and OS events were observed in 58 (93.5%) and 45 (72.6%) patients, respectively, at the cut-off date. Median PFS and OS were 12.1 months (95% CI 9.86-14.0 months) and 30.2 months (95% CI 25.8-34.7 months), respectively. In the updated safety analysis, no unexpected toxicities were observed in the safety population (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2022.100512).
A total of 38 patients received maintenance therapy after the induction phase. The median number of cycles administered per patient for induction and maintenance treatment was 12 (range: 1-20) and 3 (range: 0-48), respectively. The average relative dose intensities for fluorouracil, irinotecan, and oxaliplatin were 97.1%, 81.0%, and 79.9%, respectively. The main causes of treatment discontinuation were disease progression in 38 patients (61%), conversion surgery in 11 patients (18%), delayed recovery from adverse events in 6 patients (10%), and withdrawal of consent in 2 patients (3%), mainly due to economic issues. Post-treatment after first-line chemotherapy was administered to 55 (89%) of the 62 patients, with 41 patients receiving second-line treatment with FOLFOXIRI plus bevacizumab (10, 24%), an irinotecan-based regimen plus bevacizumab (9, 22%), ramucirumab (6, 15%), and aflibercept (1, 2%).
Subgroup analyses for the efficacy of FOLFOXIRI plus bevacizumab
Efficacy according to tumour sidedness
A subgroup analysis by tumour sidedness indicated an ORR of 82% for the left side and 59% for the right side and median DpR values of 49.6% and 40.2% for left- and right-sided tumour patients, respectively. There was no significant difference in the PFS and OS between left-sided tumours and right-sided tumours (median PFS: 12.4 versus 10.1 months, P = 0.46; median OS: 30.5 versus 26.2 months, P = 0.66) (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2022.100512).
Survival time according to early tumour shrinkage
ETS was observed in 44 (77.3%) of 61 patients assessed. Maximal tumour shrinkage occurred at a median of 23 weeks (range: 7-118 weeks) after the start of treatment. Patients with ETS had longer PFS and OS than those without ETS, although this difference was not statistically significant (Table 2 and Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2022.100512).
Table 2.
Tumour response and survival outcomes
| Outcome | All n = 62 | ≥70 years n = 8 | <70 years n = 54 |
|---|---|---|---|
| Tumour response, n (%) | |||
| Complete response | 3 (4.8) | 1 (12.5) | 2 (3.7) |
| Partial response | 44 (71.0) | 6 (75.0) | 38 (70.4) |
| Stable disease | 13 (21.0) | 1 (12.5) | 12 (22.2) |
| Progressive disease | 1 (1.6) | 0 (0.0) | 1 (1.9) |
| Not assessable | 1 (1.6) | 0 (0.0) | 1 (1.9) |
| Objective response rate (%) | 75.8 | 87.5 | 74.1 |
| 95% CI | 65.1-86.5 | 64.6-100 | 62.4-85.8 |
| Disease control rate (%) | 96.8 | 100 | 96.3 |
| 95% CI | 92.4-100 | 100-100 | 91.3-100 |
| Early tumour shrinkagea (%) | 73.8 | 62.5 | 75.5 |
| Depth of responsea | |||
| Median (%) | 49.2 | 54.6 | 49.2 |
| Range | −28.7 to 100 | 17.3 to 100 | −28.7 to 100 |
| Median PFS (months) | 12.1 | 13.9 | 11.7 |
| 95% CI | 9.9-14.0 | 9.0-25.1 | 9.4-14.0 |
| Median OS (months) | 30.2 | 33.4 | 29.8 |
| 95% CI | 25.8-34.7 | 16.0-NR | 24.1-34.9 |
CI, confidence interval; NR, not reached; OS, overall survival; PFS, progression-free survival.
Early tumour shrinkage and depth of response in 61 patients with available data.
Clinical outcomes according to age
Of the 62 patients, 8 were aged ≥70 years, with an ORR of 87.5%, a median PFS of 13.9 months, and a median OS of 33.4 months. Efficacy outcomes were comparable between patients aged ≥70 years and those aged <70 years. However, common non-haematological toxicities, including mucositis, severe appetite loss, and severe diarrhoea, were observed in the elderly patient group. Febrile neutropenia was observed in only elderly patients (Table 2 and Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2022.100512).
Changes in the levels of gene mutations using circulating tumour DNA and their association with efficacy
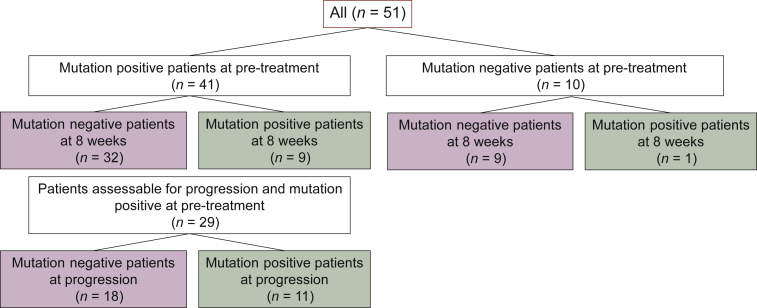
ctDNA analysis was carried out to evaluate the changes in RAS mutations during treatment in 51 patients (Figure 1). At pre-treatment, no RAS mutations were detected in the plasma of 10 patients. In 41 patients with RAS mutations in ctDNA at pre-treatment, median allele fraction was 7.46 (range: 0.21-79.186) (Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2022.100512). There were no differences in the ORR, ETS, DpR, PFS, and OS between the 41 patients with RAS mutations in ctDNA and 10 patients without RAS mutations before treatment. In 32 of the 41 (78%) patients, RAS mutations disappeared in ctDNA at 8 weeks after treatment. Patients without RAS mutations at 8 weeks post-treatment tended to have better outcomes than those with RAS mutations (Table 3). Patients with residual RAS mutations in the blood at 8 weeks had a significantly larger pre-treatment tumour burden than those without RAS mutations. In addition, the number of metastatic sites was lower in patients whose RAS mutations disappeared (Supplementary Table S4, available at https://doi.org/10.1016/j.esmoop.2022.100512).
Figure 1.
RAS mutation status in circulating tumour DNA measured at three time points.
Table 3.
Treatment efficacy according to the RAS status in ctDNA at each time point
| n | ORR (%) | P valuea | ETS (%) | P valuea | Median DpR (%) | P valueb | Median PFS (months) | HR (95% CI) P valuec |
Median OS (months) | HR (95% CI) P valuec |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| At 8 weeks after treatment (n = 41) | |||||||||||
| Mutation positive | 9 | 56 | 0.22 | 67 | 0.68 | 34.1 | 0.29 | 8.8 | 0.64 (0.28-1.45) | 16.0 | 0.53 (0.23-1.21) |
| Mutation negative | 32 | 78 | 75 | 54.4 | 11.9 | 0.29 | 27.2 | 0.12 | |||
| At progression (n = 29) | |||||||||||
| Mutation positive | 11 | 64 | 0.37 | 64 | 0.43 | 38.0 | 0.11 | 9.0 | 0.65 (0.28-1.51) | 16.0 | 0.31 (0.12-0.76) |
| Mutation negative | 18 | 83 | 78 | 55.3 | 13.1 | 0.32 | 27.0 | 0.0073 |
CI, confidence interval; ctDNA, circulating tumour DNA; ETS, early tumour shrinkage; HR, hazard ratio; OS, overall survival; PFS, progression-free survival.
Fisher’s exact test.
t-Test.
Log-rank test.
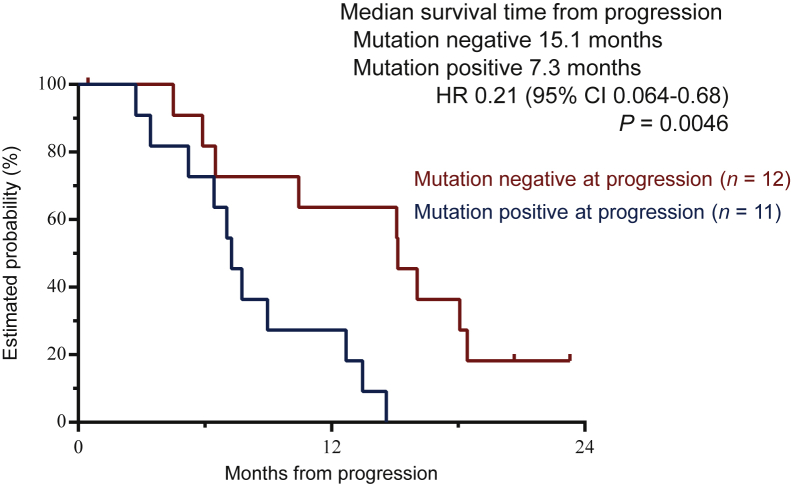
RAS mutation status in the ctDNA was evaluated in 36 patients at pre-treatment, 8 weeks after treatment, and disease progression. In 29 patients with RAS mutations in the ctDNA at pre-treatment, RAS mutations disappeared in 24 (82.8%) patients at 8 weeks after treatment. Interestingly, RAS mutations remained undetectable during disease progression in 18 (62.1%) of the 29 patients. The tumour burden at disease progression was significantly lower in patients without RAS mutations in the ctDNA than in those with RAS mutations. The target lesions became smaller after treatment in patients without RAS mutations in the ctDNA at progression (Supplementary Table S5, available at https://doi.org/10.1016/j.esmoop.2022.100512). Survival analysis of 29 patients assessable for RAS mutations in the ctDNA at the three time points showed a significantly longer OS in patients without RAS mutations in the ctDNA at disease progression (median OS: 27.0 versus 16.0 months, hazard ratio: 0.31, P = 0.0073). Moreover, the median OS from progression was significantly longer in RAS mutation-negative patients than in RAS mutation-positive patients at disease progression (median OS: 15.1 versus 7.3 months, hazard ratio: 0.21, P = 0.0046) (Table 3 and Figure 2).
Figure 2.
Survival time from progression according to RAS mutation status in circulating tumour DNA at disease progression. Median survival time from progression was 15.1 months in the mutation-negative group and 7.3 months in the mutation-positive group [hazard ratio (HR) 0.21, 95% confidence interval (CI) 0.064-0.68, P = 0.0046].
Discussion
We prospectively evaluated the dynamics of RAS mutations in the blood of patients with RAS-mutant mCRC. In 78% of patients, RAS mutations disappeared in ctDNA 8 weeks after the induction of FOLFOXIRI plus bevacizumab treatment. Interestingly, RAS mutations remained undetectable during disease progression in 62% of patients. In patients with RAS wild-type mCRC, subclonal gene mutations of RAS were previously observed in the blood as a resistance mechanism to anti-EGFR antibody treatment.8 In contrast, in 19%-46% of patients with RAS-mutant mCRC, RAS mutations in the blood disappeared after chemotherapy.9, 10, 11 In our study, RAS mutations disappeared in 62% of the patients who progressed to intensive first-line chemotherapy with FOLFOXIRI plus bevacizumab. There are several possible explanations for the disappearance of the RAS gene in the blood. Firstly, the amount of cfDNA may have decreased owing to tumour volume reduction due to chemotherapy, and the expression of the RAS gene may have been below the detection limit of liquid biopsy testing. In our study, tumour volume was significantly lower in patients with clearance of RAS mutations. In particular, the results of ctDNA analysis at 8 weeks are likely to be undetectable because the amount of ctDNA was lower in patients with tumour shrinkage. Patients with clearance of RAS mutations during disease progression had a lower tumour burden than those before treatment, although there was no difference in the rate of formation of new lesions. In most patients with RAS mutations in ctDNA at disease progression, the allele frequency was lower compared to that at pre-treatment (Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2022.100512). Secondly, the sensitivity of liquid biopsy test assay may have been insufficient. The rate of clearance of RAS mutations depends on the performance of liquid biopsy tests. Finally, RAS-mutant colon cancer cells may have changed to different types by intensive triplet chemotherapy. In this study, we found that a few patients acquired different RAS mutations from original RAS mutation in tumours, indicating that intensive chemotherapy may change genomic status even if RAS-mutant tumours. However, these findings are only hypothetical. Therefore, prospective validation using pre-clinical studies will be necessary.
mCRC patients with RAS-mutant tumours have been reported to have favourable survival on combination chemotherapy with anti-EGFR antibody as second-line treatment in patients with clearance of RAS mutations in the blood by first-line chemotherapy.9 Our study found that patients without RAS mutations in ctDNA at 8 weeks tended to have better outcomes than those with RAS mutations. Furthermore, the median OS from progression was significantly longer in RAS mutation-negative patients than in RAS mutation-positive patients. According to this finding, RAS gene status in the blood may serve as a predictor for survival after first-line FOLFOXIRI plus bevacizumab treatment. These findings warrant additional studies that include real-time monitoring of oncogenic genes other than RAS under treatment pressure. Our data demonstrated that RAS mutations disappeared in a large percentage of patients treated with FOLFOXIRI plus bevacizumab.
Our findings on the updated analysis of the phase II study JACCRO CC-11 indicate that FOLFOXIRI plus bevacizumab as first-line treatment is effective for RAS-mutant mCRC, with a median PFS of 12.1 months and a median OS of 30.2 months, which were comparable to those of previously reported clinical trials.2,3 Subgroup analysis of the TRIBE-2 trial by RAS/BRAF mutation showed the benefit of the triplet regimen over the doublet regimen in the RAS-mutant mCRC group.3 Our clinical trial, which focused only on RAS-mutant mCRC, confirmed the high efficacy of FOLFOXIRI plus bevacizumab in mCRC patients with RAS-mutant tumours.
The prognosis of mCRC differs depending on the site of the primary tumour, with right-sided tumours having a poorer prognosis than left-sided tumours.12,13 However, the reason for the poor prognosis of right-sided RAS-mutant mCRC has not been well established. In our study, there was no significant difference in prognosis between right- and left-sided primary tumours in RAS-mutant mCRC patients treated with FOLFOXIRI plus bevacizumab. Thus, our results provide valuable data on the triplet regimen for right-sided RAS-mutant mCRC. ETS has been previously reported to correlate with prognosis. In this study, the overall ETS rate was 73.8%, and those for the left and right sides were 77.3% and 64.7%, respectively, with the left side having a higher ETS rate. Furthermore, prognostic analysis by ETS showed that the median survival of the ETS-positive group was better than that of the ETS-negative group, although the difference was not statistically significant, which might be attributed to the small sample size. Our ETS analysis findings were consistent with those of previous reports,14, 15, 16 indicating that patients treated with FOLFOXIRI plus bevacizumab may have a better prognosis if ETS is achieved in RAS-mutant mCRC. In our subgroup analysis for elderly patients aged ≥70 years, the ORR of modified FOLFOXIRI plus bevacizumab was promising at 87.5% and PFS did not significantly differ between patients aged >70 years and those aged <70 years. In the elderly patient group, febrile neutropenia and mucositis were significantly more frequent but manageable. Thus, FOLFOXIRI plus bevacizumab therapy should not be based only on the age of the patients, but also on the PS or aggressiveness of tumours. However, careful clinical monitoring is recommended in elderly patients receiving FOLFOXIRI plus bevacizumab treatment.
Our study had some limitations. The observation period was short; however, the number of patients with events was sufficient for survival analysis. In addition, the number of patients analysed for liquid biopsy was very small. This study would not provide reliable evidence for dynamic changes of RAS mutations in blood after treatment of RAS-mutant mCRC. The concept of NeoRAS wild type has been recently discussed, which pertains to the observation of vanishing RAS-mutant alleles in the blood during standard-of-care chemotherapy for RAS-mutant mCRC.10 Analysis of tumour-related genes, such as APC and TP53, would allow us to assess the clearance of RAS-mutant clones more accurately. In our study, it was difficult to evaluate if RAS-mutant tumours changed to ‘NeoRAS wild type’ because we did not measure other gene alterations than RAS mutations. We carried out the subgroup analysis of clinical outcomes by sidedness and age. The data showed clinically useful results, but the number of patients was small; therefore, the results were limited. Further prospective studies are required to confirm and validate our findings.
Conclusions
Our findings demonstrated that FOLFOXIRI plus bevacizumab is one of the standards of care for first-line treatment in patients with mCRC harbouring RAS-mutant tumours, especially right-sided primary tumours. Our biomarker study demonstrated that 62% of patients showed clearance of RAS mutations in ctDNA at disease progression, which was significantly associated with patient outcome. Further studies on the benefits of subsequent anti-EGFR therapies are required.
Acknowledgements
We thank the patients, their families, and the investigators who participated in the JACCRO CC-11 trial. We also thank Editage (www.editage.com) for English language editing.
Funding
This work was supported by Yakult Honsha Co., Ltd. and JACCRO (no grant number).
Disclosure
YS has received grants and personal fees from Taiho Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Eli Lilly Japan Co., Ltd., and Sanofi; and personal fees from Bayer Yakuhin, Bristol–Myers Squibb Co., Ltd., Merck Biopharma Co., Ltd., Ono Pharmaceutical Co. Ltd., MSD Co., Ltd., Sysmex Co., Ltd., and Guardant Health Japan Corp. HS received grants and personal fees from Ono Pharmaceutical Co. Ltd., Daiichi Sankyo, Taiho Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., and Sanofi; and personal fees from Bayer Co., Ltd., Bristol–Myers Squibb Co., Ltd., Chugai Pharmaceutical Co., Ltd., Eli Lilly Japan Co., Ltd., Merck Bio Pharma Co., Ltd., MSD Co., Ltd., and Yakult Honsha Co., Ltd. MS has received personal fees from Yakult Honsha Co., Ltd., Chugai Pharmaceutical Co., Ltd., Ono Pharmaceutical Co. Ltd., Eli Lilly Japan Co., Ltd., Merck Biopharma Co., Ltd., and Taiho Pharmaceutical Co., Ltd. HJL received personal fees from Bayer, Genentech Inc, Merck KGaA, Amgen, Bristol-Myers Squibb, Fulgent Genetics, and Oncocyte Corp. All other authors have declared no conflicts of interest.
Supplementary data
References
- 1.Yoshino T., Arnold D., Taniguchi H., et al. Pan-Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: a JSMO-ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS. Ann Oncol. 2018;29(1):44–70. doi: 10.1093/annonc/mdx738. [DOI] [PubMed] [Google Scholar]
- 2.Loupakis F., Cremolini C., Masi G., et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med. 2014;371(17):1609–1618. doi: 10.1056/NEJMoa1403108. [DOI] [PubMed] [Google Scholar]
- 3.Cremolini C., Antoniotti C., Rossini D., et al. Upfront FOLFOXIRI plus bevacizumab and reintroduction after progression versus mFOLFOX6 plus bevacizumab followed by FOLFIRI plus bevacizumab in the treatment of patients with metastatic colorectal cancer (TRIBE2): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2020;21(4):497–507. doi: 10.1016/S1470-2045(19)30862-9. [DOI] [PubMed] [Google Scholar]
- 4.Modest D.P., Ricard I., Heinemann V., et al. Outcome according to KRAS-, NRAS- and BRAF-mutation as well as KRAS mutation variants: pooled analysis of five randomized trials in metastatic colorectal cancer by the AIO colorectal cancer study group. Ann Oncol. 2016;27(9):1746–1753. doi: 10.1093/annonc/mdw261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Satake H., Sunakawa Y., Miyamoto Y., et al. A phase II trial of 1st-line modified-FOLFOXIRI plus bevacizumab treatment for metastatic colorectal cancer harboring RAS mutation: JACCRO CC-11. Oncotarget. 2018;9(27):18811–18820. doi: 10.18632/oncotarget.24702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mauri G., Pizzutilo E.G., Amatu A., et al. Retreatment with anti-EGFR monoclonal antibodies in metastatic colorectal cancer: systematic review of different strategies. Cancer Treat Rev. 2019;73:41–53. doi: 10.1016/j.ctrv.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Vidal J., Muinelo L., Dalmases A., et al. Plasma ctDNA RAS mutation analysis for the diagnosis and treatment monitoring of metastatic colorectal cancer patients. Ann Oncol. 2017;28(6):1325–1332. doi: 10.1093/annonc/mdx125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siravegna G., Mussolin B., Buscarino M., et al. Clonal evolution and resistance to EGFR blockade in the blood of colorectal cancer patients. Nat Med. 2015;21(7):795–82701. doi: 10.1038/nm.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gazzaniga P., Raimondi C., Urbano F., et al. EGFR inhibitor as second-line therapy in a patient with mutant RAS metastatic colorectal cancer: circulating tumor DNA to personalize treatment. JCO Precis Oncol. 2018;2:1–6. doi: 10.1200/PO.17.00277. [DOI] [PubMed] [Google Scholar]
- 10.Osumi H., Vecchione L., Keilholz U., et al. NeoRAS wild-type in metastatic colorectal cancer: myth or truth?-case series and review of the literature. Eur J Cancer. 2021;153:86–95. doi: 10.1016/j.ejca.2021.05.010. [DOI] [PubMed] [Google Scholar]
- 11.Raimondi C., Nicolazzo C., Belardinilli F., et al. Transient disappearance of RAS mutant clones in plasma: a counterintuitive clinical use of EGFR inhibitors in RAS mutant metastatic colorectal cancer. Cancers. 2019;11(1):42. doi: 10.3390/cancers11010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tejpar S., Stintzing S., Ciardiello F., et al. Prognostic and predictive relevance of primary tumor location in patients with RAS wild-type metastatic colorectal cancer: retrospective analyses of the CRYSTAL and FIRE-3 trials. JAMA Oncol. 2017;3(2):194–201. doi: 10.1001/jamaoncol.2016.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cremolini C., Antoniotti C., Lonardi S., et al. Primary tumor sidedness and benefit from FOLFOXIRI plus bevacizumab as initial therapy for metastatic colorectal cancer. Retrospective analysis of the TRIBE trial by GONO. Ann Oncol. 2018;29(7):1528–1534. doi: 10.1093/annonc/mdy140. [DOI] [PubMed] [Google Scholar]
- 14.Petrelli F., Pietrantonio F., Cremolini C., et al. Early tumour shrinkage as a prognostic factor and surrogate end-point in colorectal cancer: a systematic review and pooled-analysis. Eur J Cancer. 2015;51(7):800–807. doi: 10.1016/j.ejca.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 15.Cremolini C., Loupakis F., Antoniotti C., et al. Early tumor shrinkage and depth of response predict long-term outcome in metastatic colorectal cancer patients treated with first-line chemotherapy plus bevacizumab: results from phase III TRIBE trial by the Gruppo Oncologico del Nord Ovest. Ann Oncol. 2015;26(6):1188–1194. doi: 10.1093/annonc/mdv112. [DOI] [PubMed] [Google Scholar]
- 16.Manca P., Corallo S., Randon G., et al. Impact of early tumor shrinkage and depth of response on the outcomes of panitumumab-based maintenance in patients with RAS wild-type metastatic colorectal cancer. Eur J Cancer. 2021;144:31–40. doi: 10.1016/j.ejca.2020.11.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.