Abstract
This double-blind, randomized controlled trial, tested fatty acid (FA) supplementation in children (ages 2−<6 years) recently diagnosed with Autism Spectrum Disorder (ASD). Participants received daily oral FA supplement containing omega-3 and omega-6 FA, or a placebo for 90 days based on participant weight. Erythrocyte FAs and the cytokines, IL-1β, IL-2, IFNγ, were measured in plasma obtained from serial blood collections. Treatment increased omega-3 and omega-6 FA levels (1.40 mol% for EPA and 1.62 mol% for DHA) and reduced IL-2 levels compared to placebo (−0.17 pg/mL, 95% CI: −0.31, −0.02, d=−0.62). Omega 3-6 treatment was tolerable and adherence was greater than 70%. Future research will assess the effects of Omega 3-6 treatment on ASD symptoms. Registered on 06/08/2018 with ClinicalTrials.gov: NCT03550209.
Keywords: Autism Spectrum Disorder, IL-2, inflammation, omega-3 fatty acids, omega-6 fatty acids, young child
Although at least 1 in 54 U.S. children has Autism Spectrum Disorder (ASD), no approved medications exist to treat the core symptoms (Maenner et al., 2020). Atypical antipsychotics are commonly prescribed for irritability with ASD, but side effects are significant, common, and of particular concern for young children (McPheeters et al., 2011). Behavioral interventions remain the most effective approaches for addressing atypical behaviors and social deficits of ASD, but they are intensive, costly, and inaccessible for some families (Dawson et al., 2010; Grosse et al., 2021; Reichow et al., 2009). Because of the dearth of effective treatments, 52-95% of families commonly try complementary therapies including omega fatty acid (FA) dietary supplements, based largely on anecdotal evidence and small trials (Whitehouse, 2013).
FA supplements are among the most popular complementary health interventions utilized by children with ASD. To date, the results of efficacy trials testing FA supplementation for ASD are mixed for reasons including small sample size, variation in the formulations tested, and design shortcomings including lack of blinding or placebo control. Prior trials using FA therapies have almost exclusively tested a combination of omega-3 (n-3) docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) derived from fish oil. However, the addition of alternative anti-inflammatory FAs such as omega-6 (n-6) gamma-linoleic acid (GLA), may enhance the anti-inflammatory effects of supplementation and may also improve behavioral outcomes. In fact, a recent meta-analysis reported larger effect sizes and more consistent improvement in Attention Deficit-Hyperactivity Disorder (ADHD) for DHA+EPA+GLA than for DHA+EPA alone (standard difference in means −0.31 favoring DHA+EPA+GLA (CI: −0.46, −0.16)) (Puri et al., 2014). The Preemie Tots trial recently reported improvements in ASD symptoms based on the Brief Infant Toddler Social and Emotional Assessment (BITSEA; (Briggs-Gowan et al., 2004) ASD scale, gesture use in communication, internalizing behaviors, and interpersonal relationship adaptive behaviors among preterm toddlers with early ASD symptoms randomized to DHA+EPA+GLA versus placebo (Boone et al., 2021; Keim et al., 2018; Sheppard KW, 2017). Only one trial focused on ASD has tested a combination of DHA and EPA with evening primrose oil, a source of GLA and arachidonic acid, but it was not placebo-controlled (Ooi et al., 2015). In addition to improvements in attention problems, parent-reported outcomes in that study indicated medium effect sized improvements related to social interaction and communication, similar to our prior reports.
Another major reason for the lack of clarity as to the effectiveness of FA supplements is the very limited extent to which studies have evaluated potential biological signatures of interest to shed light on the pathways through which FAs may exert their effect. Inflammation is one potential mechanism by which FA may affect autism-related behaviors. Inflammation is well characterized in youth with ASD – increases in inflammatory cytokines and chemokines both in the circulation and cerebral spinal fluid have been reported, and ASD symptom severity has been positively associated with levels of several cytokines (Masi et al., 2017; Theoharides et al., 2016). Only two of the prior trials testing FA supplementation examined inflammatory markers in relation to changes in behavior, despite the evidence from observational studies tying altered inflammatory profiles to ASD, and particular long-chain FAs to anti-inflammatory mechanisms (Mankad et al., 2015). Mankad et al. (2015) suggested worsened externalizing behaviors with increasing levels of IL-10 and IL-1β, but no clear path connecting supplementation to changes in cytokines and to subsequent behavior. Similarly, Mazahery et al. (2020) tested interactions between baseline IL-1β levels and treatment assignment but did not measure changes in inflammation due to treatment. The mechanisms by which FAs might improve ASD symptoms is not well understood and led us to hypothesize that the Omega 3-6 supplementation would interrupt detrimental neurological pathways by reducing inflammation, a hallmark of ASD.
The first phase of the Omega Heroes study was to evaluate the efficacy of daily supplementation with a FA therapy that included a combination of DHA+EPA+GLA versus placebo, using doses based upon the child’s weight, on inflammatory responses in young children with ASD. It also aimed to evaluate safety and bioavailability of the FA supplement.
METHODS
Study Design and Setting
Omega Heroes was a randomized, fully blind (researchers, parents, and children were blind to treatment assignment), placebo-controlled trial (NCT 03550209) reviewed and approved by the Institutional Review Board (IRB #17-00517) at the single study site, Nationwide Children’s Hospital (NCH), Columbus, OH, USA. Each child’s parent or guardian provided written informed consent. A study doctor (D.C.) and independent monitoring committee reviewed adverse events and other safety-related data. Clinicians who diagnose and care for children with ASD were involved in the study design and development of study procedures.
Participants, Sample Size, and Power
Children recently diagnosed with ASD at the Nationwide Children’s Hospital Child Development Center (Columbus, Ohio, USA), a large interdisciplinary assessment and treatment center for ASD, were assessed for eligibility using the EPIC electronic medical record system. DSM-5 criteria were applied in the diagnosis process. Inclusion criteria included age 2−<6 years, receipt of an ASD diagnosis within the prior 6 months with a score in the “autism” (severe) range on the Autism Diagnostic Observation Schedule-2nd edition (ADOS-2; (Lord et al., 2012)), and English as the primary language. .We focused on preschool aged children because of extensive evidence indicating that ASD therapies are maximally beneficial if they begin as early as possible after diagnosis (Granpeesheh et al., 2009). This is because neuroplasticity decreases significantly as children age, and also the rate of DHA accretion in the brain displays a corresponding decline (Martinez, 1994). The sample was limited to children in the severe range on the ADOS-2 to reduce heterogeneity and focus on children potentially more likely to show benefit in this relatively small cohort.
Exclusion criteria included 1) they consumed FA supplements in the past 6 months, consumed fatty fish more than 3 times per week, or were still breast or formula feeding, as these would potentially provide large amounts of the same FA being tested; 2) had been diagnosed with quadriparesis, deafness, a seizure disorder, blindness, a bleeding disorder, or an autoimmune disorder (including Type I diabetes, Fragile X, Rett, Angelman Syndromes, or Tuberous Sclerosis), as these would have effects on both inflammation and neurodevelopment, would theoretically pose safety concerns, or would mean children could not benefit from the intervention; 3) had feeding problems precluding consumption of the supplement or had a known allergy to any ingredient of the investigational products as these would preclude taking the investigational products; or 4) had planned surgeries scheduled within the timeframe of trial participation, as these would potentially pose a bleeding risk.
A parent or legal guardian of potentially eligible children was contacted via letter, phone, or email to confirm eligibility and discuss possible participation. Interested and eligible parents and children were scheduled for the baseline visit at the Center for Biobehavioral Health at Nationwide Children’s Hospital. A sample size goal of 66 (30 per group plus 10% attrition) was set based on the hypothesized effect size of at least Cohen’s d=0.75 for the change in the cytokines of interest from baseline to the end of the trial, when comparing all children randomized to DHA+EPA+GLA to all children randomized to placebo. The effect size was based on the differences observed by Masi et al. (2015) and preliminary data from our prior, unpublished, trial (d=0.75 for IL-1β, 0.77 IL-2, and 1.04 IFNγ). Additionally, we set a benchmark for what we considered a potentially clinically meaningful effect size of d=0.5 to inform decisions about proceeding to a future trial examining effects on behavior.
Randomization, Masking, and Intervention
Children were allocated to one of six coded groups. Each child was assigned to 1 of 3 different doses of treatment (25 “low”, 50 “medium”, or 100 “high” mg/kg/day of GLA+EPA+DHA) or 1 of 3 doses of placebo (volumes were calculated to be equivalent to the corresponding treatment group low, medium, and high volumes) calculated using baseline body weight. FA supplements have traditionally been tested using a uniform dose, but recent evidence has highlighted the deficiency of this approach. Heavier children seem to require a higher dose to achieve the same response in a recent study of omega-3 supplementation and mood disorders (Arnold et al., 2017). The randomization scheme had varying block size of multiples of 6 and 12, stratified by sex and age (2-3 years, 4−<6 years), and with equal allocation to treatment and placebo (Figure 1). A pseudorandom number generator in the statistical software R was used to implement randomization. A statistician (J.R.) prepared the scheme, assigned ID numbers, and prepared opaque tamper-resistant envelopes. He had no participant contact. All investigators and staff remained masked throughout the trial. Children were assigned to either the treatment or placebo group using sealed, sequentially numbered envelopes. Each sequential envelope was pre-labeled with the next study ID number to prevent out-of-sequence assignment and was opened by a research assistant upon written informed consent.
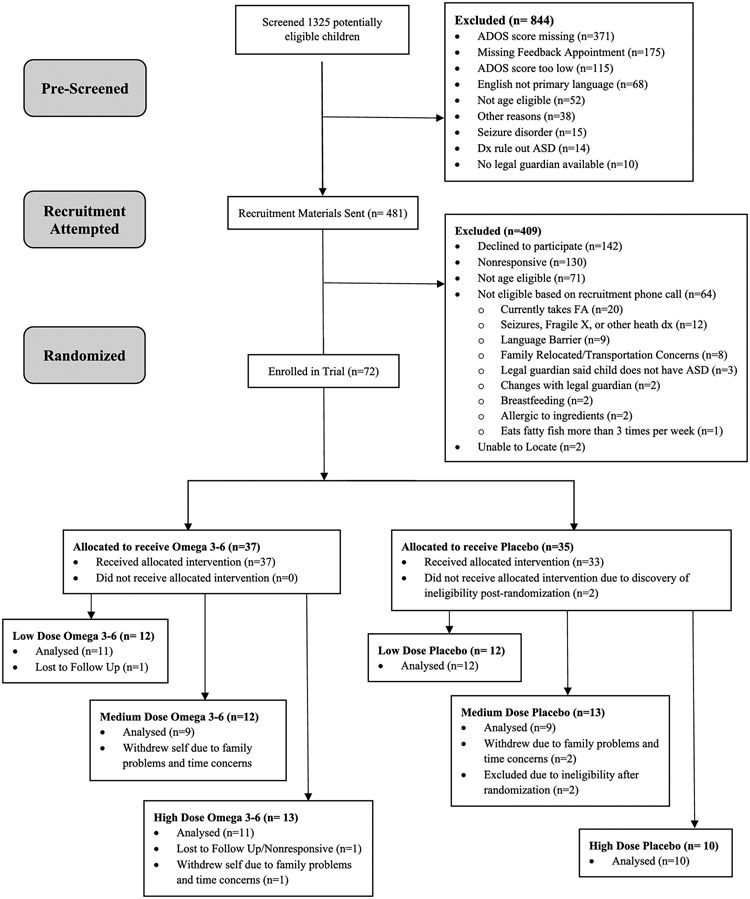
Figure 1.
CONSORT Diagram
The treatment group was assigned to 90 days of daily oral Complete Omega™ supplementation in the form of lemon oil flavored fish and borage oils (the supplement provided 185 mg total omega-3 fatty acids including 112 mg EPA, 67 mg DHA; 122 mg total omega-6 fatty acids including 32 mg GLA; and 83 mg total omega-9 fatty acids per milliliter of supplement) (Complete Omega, Nordic Naturals, Inc., Watsonville, CA). Complete Omega is considered a dietary supplement under U.S. Food and Drug Administration regulations. The placebo group was assigned to daily canola oil (the placebo provided 188 mg linoleic acid (LA, 18:2n-6), 81 mg α-linolenic acid (ALA, 18:3n-3), 590 mg oleic acid (18:1n-9) per milliliter of placebo). The placebo was prepared with lemon oil flavor by the NCH Investigational Drug Service (IDS) to match the treatment product, and both products were packaged identically. Doses were based on the child’s baseline weight and calculated to deliver 50, 100, or 150 mg/kg/day in the proportions defined by the manufacturer. Ninety days was selected because of numerous previous trials of fatty acid supplements that reported improvements in other behaviors within this timeframe (Puri et al., 2014). IDS dispensed the products directly to families and provided a medicine syringe for parents to measure and administer the product to the child by placing the liquid directly into the mouth. To promote compliance, some parents added chocolate syrup to the measured dose or mixed with food.
Data Collection
The baseline study visit (day 0) included a parent questionnaire to gather baseline child and family descriptions to characterize the sample and verify comparability across groups. In addition, parents completed a FA-specific food frequency questionnaire as an interview to measure usual dietary intake of long-chain fatty acids (Kuratko, 2013). Finally, parents were asked to report on their child’s current medications, upcoming surgeries, as well as use of complementary and behavioral therapies or programs. Child height and weight were measured using a SECA scale and stadiometer. Parents were asked about their intentions to complete the study, attend the next study visit, and give their child the assigned investigational product each day. They were counseled about how to give the investigational product and how to use study diaries to record compliance. Families were contacted by phone, email, or text message at regular intervals at least 5 times to measure adherence, assess adverse events using a modified version of the Safety Monitoring Uniform Report Form (SMURF), and address barriers to participation (Greenhill et al., 2004). Group differences (entire treatment group vs placebo group) in the total number and types of adverse events served as a prespecified primary endpoint for safety for the trial. Study visits occurred at 45 ± 14 days and 90 ± 14 days to repeat the parent questionnaire/interview, measure adherence, assess adverse events and barriers to participation. At the last study visit, the parent/guardian was asked to guess their child’s treatment assignment. Families were compensated $75 for each visit, $2 for each 2-week study diary they submitted, plus parking and a small toy, and a $20 bonus if they completed all visits.
Fatty Acid Biomarkers
Blood was collected via venipuncture to examine red blood cell (RBC) FA levels at baseline and at the last study visit. Plasma and RBCs were separated by centrifugation at the time of collection and stored at −80°C until analysis. FA were measured in patient RBCs by standard protocols using gas chromatography (Eder, 1995). Analysis was performed on a Shimadzu GC-2010 gas chromatograph equipped with an AOC-20i autosampler and with flame-ionization detection. Fatty acid methyl esters were quantified using experimentally derived standard curves. The following FA were included in the analysis: myristic (14:0), palmitic (16:0), palmitoleic (16:1n-7), steric (18:0), oleic (18:1 n-9), linoleic acid (18:2 n-6), α-linolenic acid (18:3 n-3) , γ-linolenic acid (GLA,18:3 n-6), arachidic acid (20:0), arachidonic (AA, 20:4n-6), eicosapentaenoic acid (EPA, 20:5 n-3), and docosahexaenoic acid (DHA, 22:6 n-3). Differences in the bioavailability of EPA and DHA (each FA as a percent of total RBC FA at the end of the trial) between the Omega 3-6 group and the placebo group served as a prespecified primary endpoint for the trial.
Cytokines
Three specific biological signatures indicative of inflammation were chosen a priori based on prior studies indicating increases in these markers in children with ASD (Masi et al., 2015). The three primary biological signatures included the cytokines IL-1β, IL-2, and IFNγ. The change in these cytokines from baseline to the end of the trial (entire treatment group vs placebo group) served as a prespecified primary endpoint for the trial. Six additional cytokines were selected as exploratory after the trial began but before assays were conducted in order to provide a more complete understanding of possible changes in inflammatory profiles resulting from the Omega 3-6 supplementation. These included TNFα, IL-4, IL-6, IL-8, IL-10, and IL-12p70. These cytokines were measured using an ELISA based technology offered by Meso Scale Diagnostics (Meso Scale Diagnostics, Rockville Maryland).
Compliance
Compliance was assessed by parent-completed daily diaries (the proportion of days the supplement/placebo was consumed divided by the total days enrolled) and measurement of the amount of remaining oil in bottles returned to IDS (the amount of missing oil divided by the expected amount).
Statistical Analysis
All analyses used SAS software (v9.4, SAS Institute) and were conducted according to intent-to-treat (all randomized children were included). The study was designed as an early phase trial to identify differences in inflammatory markers and assess safety and compliance; consequently, it was not powered to identify differences across varying doses. For our primary outcomes, we combined all doses to form one treatment group and one placebo group. No interim analyses were conducted. Sample characteristics at baseline for the treatment and placebo groups are shown in Table 1. The baseline characteristics in Table 1 were examined across the treatment and placebo groups as predictors of missingness at posttest for the outcomes of interest; none were statistically significant (p > .05).
Table 1.
Participant Baseline Characteristics, Omega Heroes Trial (Ohio, USA) (n=70)
| Characteristic | Total (n, %) (n=70) |
Omega 3-6 (n, %) (n=37) |
Placebo (n, %) (n=33) |
|---|---|---|---|
| Child sex – male | 57 (81) | 29 (78) | 28 (85) |
| Female | 13 (19) | 8 (22) | 5 (15) |
| Child age – 2-3 years | 36 (51) | 21 (57) | 15 (45) |
| 4-6 years | 34 (49) | 16 (43) | 18 (54) |
| Child race – Black or African-American | 13 (19) | 10 (27) | 3 (9) |
| White | 47 (67) | 20 (54) | 27 (82) |
| Other or multiple races | 9 (12) | 6 (17) | 3 (9) |
| Missing | 1 (1) | 1 (3) | 0 (0) |
| Child ethnicity – Hispanic | 6 (9) | 4 (11) | 2 (6) |
| Non-Hispanic | 64 (91) | 33 (89) | 31(94) |
| Caregiver marital status - Single/Never married | 12 (17) | 4 (11) | 8 (24) |
| Married or living with partner | 46 (66) | 27 (73) | 19 (58) |
| Partner not living together, Separate, Divorced, Widowed | 11 (16) | 6 (17) | 5 (15) |
| Missing | 1 (1) | 0 (0) | 1 (3) |
| Household income - <$833 per month ($10,000 per year) | 7 (10) | 1 (3) | 6 (18) |
| $833 - $1,666 per month ($10,000 - $19,999 per year) | 11 (16) | 6 (16) | 5 (15) |
| $1,667 - $2,499 per month ($20,000 - $29,999 per year) | 10 (14) | 5 (14) | 5(15) |
| $2,500 - $3,332 per month ($30,000 - $39,999 per year) | 11 (16) | 9 (24) | 2 (6) |
| $3,333 - $4,166 per month ($40,000 - $49,999 per year) | 12 (17) | 7 (19) | 5 (15) |
| $4,167 - $4,999 per month ($50,000 - $59,999 per year | 3 (4) | 2 (5) | 1 (3) |
| $5,000 - $6,666 per month ($60,000 - $79,999 per year) | 6 (9) | 2 (5) | 4 (12) |
| $6,667 - $7,499 per month ($80,000 - $89,999 per year) | 2 (3) | 2 (5) | 0 (0) |
| $7,500 - $8,332 per month ($90,000 - $99,999 per year | 2 (3) | 0 (0) | 2 (6) |
| >$8,333 per month ($100,000 per year) | 5 (7) | 2 (5) | 3 (9) |
| Missing | 1 (1) | 1 (3) | 0 (0) |
| Caregiver employment – employed | 44 (63) | 24 (65) | 20 (61) |
| Unemployed or disabled | 7 (10) | 5 (14) | 2 (6) |
| Homemaker or student | 19 (27) | 8 (22) | 11 (33) |
| Caregiver education – high school or less | 17 (24) | 9 (24) | 8 (24) |
| Some college | 27 (39) | 13 (35) | 14 (42) |
| Associate's degree | 13 (19) | 5 (14) | 8 (24) |
| Bachelor's degree + | 13 (19) | 10 (27) | 3 (9) |
| Caregiver age (years) | Mean=32.7 SD=5.4 |
Mean=32.5 SD=4.7 | Mean=32.9 SD=6.2 |
| Health insurance status – Private only | 24 (34) | 13 (35) | 11 (33) |
| Public insurance | 41 (59) | 23 (62) | 18 (55) |
| Military | 2 (3) | 0 (0) | 2 (6) |
| Uninsured | 3 (4) | 1 (3) | 2 (6) |
The cytokines and fatty acid biomarkers were analyzed as continuous outcomes. Analyses for both parameters compared the change in the particular measurement of interest between the treatment and placebo groups, controlling for baseline scores, using mixed effects regression (based on an approach analogous to analysis of covariance (Coffman et al., 2016; Winkens et al., 2007). This method leverages maximum likelihood to account for missing data. Treatment-by-time interaction terms were included as fixed effects and served as estimates of treatment effect; no participant characteristics were included as covariates in the models. Group mean differences divided by the standard deviation of the residuals from an analogous analysis of covariance model were calculated as standardized effect sizes for each outcome, i.e., an analysis of covariance version of Cohen’s d (Lai et al., 2012). Accuracy in parameter estimation for ANCOVA and ANOVA contrasts: Sample size planning via narrow confidence intervals (Lai et al., 2012). A clinically meaningful effect size was pre-defined as Cohen’s d≥0.5 or equivalent. P-values <0.05 were considered statistically significant. Analyses for the fatty acid biomarkers compared the posttest for the particular fatty acid biomarker between the treatment and placebo groups, controlling for baseline scores, using mixed effects regression (based on an approach analogous to analysis of covariance; (Coffman et al., 2016; Winkens et al., 2007)). Finally, we carried out pre-specified exploratory analyses to examine potential dose effects using the same modeling approach as above to examine change in the cytokines between the treatment and placebo groups which had the same dose.
RESULTS
Recruitment and Retention
Based on ASD clinical diagnostic information in the medical records, 1,325 children were prescreened for eligibility. Caregivers of children who were found to be potentially eligible (n=481) were mailed recruitment materials, and phone contact was attempted to complete the remaining eligibility screening and to invite enrollment if found to be eligible. One hundred forty-two refused to participate. An additional 267 were either unreachable (nonresponsive or unable to locate), determined to be ineligible after speaking with the caregiver, or were scheduled to enroll but never attended. Seventy-two children were enrolled and randomized (Omega 3-6 treatment n=37; Placebo n=35). Two participants, who were allocated to receive placebo, were immediately withdrawn by the investigators because they were found to be ineligible just after randomization. Thus, the final enrolled and randomized sample included in the analyses is 70. Six participants withdrew themselves from the trial; reasons for withdrawal were not related to study design (Table 2). Two participants, who were allocated to receive Omega 3-6 treatment, were lost to follow-up. Sixty-two children attended the last study visit. Overall, 89% of 70 who were randomized and eligible were retained to the end of the trial and included in the final analysis (Figure 1). Recruitment occurred between June 2018 and September 2019. All participant follow-up and data collection were completed in January 2020 due to reaching pre-specified recruitment goals.
Table 2.
Reasons for Withdrawal from the Trial (n=6) and Adherence Based on Measured Volumes and Study Diaries, Omega Heroes Trial (Ohio, USA)
| Reasons for Withdrawala | |||||
|---|---|---|---|---|---|
| Category | Reason | n | |||
| Family Related | Family not supportive of child participation | 2 | |||
| Family wanted child to discontinue supplement | 1 | ||||
| Personal/family problems getting in the way of involvement | 3 | ||||
| Time Related | Problems with time commitment | 4 | |||
| Too many personal/research appointments at hospital | 2 | ||||
| Requirements interfere with daily life | 2 | ||||
| Scheduling concerns | 1 | ||||
| Study Design Related | Maintaining study diary is inconvenient | 0 | |||
| Concerns with blood draw | 0 | ||||
| Preferring not to share personal information | 0 | ||||
| Study visits too stressful for child | 0 | ||||
| Inadequate compensation | 0 | ||||
| Cannot choose child’s treatment group | 0 | ||||
| Interactions with new people too stressful for child | 0 | ||||
| Study related phone calls and mail were bothersome | 0 | ||||
| Treatment Adherence | |||||
| Treatment | Omega 3-6 | Placebo | |||
| Low Dose | Medium Dose | High Dose | All | ||
| Pharmacy Volume | 125% | 89% | 65% | 92% | 75% |
| Study diaries | 77% | 62% | 61% | 68% | 77% |
Results reflect data for 6 families, caregivers could offer more than one reason
Participant Characteristics
The participant characteristics are presented in Table 1. More males were enrolled than females. ASD is diagnosed in males more frequently than females, and our enrollment population mimicked the sex-distribution commonly observed in clinical settings. Roughly equal numbers of participants were enrolled in the two age strata (2-3 years and 4−<6 years). About two-thirds of the sample identified as White, 19% reported Black or African American, 12% reported another or multiple races, and 9% reported Hispanic ethnicity. Most caregivers were married or living with a partner, employed, and had attended at least some college. A majority of participants received public health insurance, and 34% had private health insurance.
Participant Withdrawals
The six families who withdrew themselves were asked about reasons for withdrawal. All stated reasons related to family issues and time constraints, not the investigational product or study procedures. The reasons caregivers gave were typical of other trials we have conducted, with the possible exception that we had more families than usual who were experiencing divorce or separation and resulting family conflict that interfered with the child’s ongoing participation (e.g., non-resident father not willing to give the child the supplement when visiting) (Table 2).
Adherence
Based on volume dispensed and returned, children in this study took 92% of the product dispensed to them. Based on diaries, children took 73% of the product dispensed to them. We believe the former figure is somewhat overestimated because some bottles of product were returned that appeared to have been spilled or dumped (e.g., see estimate for low dose group) (Table 2). The latter figure is subject to a handful of families failing to return a complete set of diaries. No statistically significant differences between the groups were detected for either adherence measure, but the data are suggestive that adherence was higher for the lowest dose and lowest for the highest dose.
Primary Outcomes
Safety
Two hundred and ninety-five adverse events were documented (167 in the treatment group, average 4.51/child; 128 in the placebo group, average 3.88/child; no statistically significant difference, t-test value=−1.03, p=0.31). Most were upper respiratory infections, diarrhea, or appetite changes. The PIs and Study Doctor reviewed each event and found none to be serious and related to the treatment.
Fatty acid levels in RBCs
We observed an absolute change of 1.40 mol% for EPA and 1.62 mol% for DHA in the Omega 3-6 group after 90 days of supplementation, compared to baseline (Table 3). In terms of the prespecified endpoint related to bioavailability, at the end of the trial, the Omega 3-6 group displayed a 1.5 mol% (p=0.002) greater EPA level and a 1.8 mol% (p<.0001) greater DHA level than the placebo group. As expected, and similar to Keim et al. (2018), we observed no statistically significant increase in the other key FA in the supplement, GLA, because it is rapidly metabolized to longer-chain fatty acids.
Table 3.
Change in red blood cell fatty acid levels (mol%), comparing the Omega 3-6 group to the placebo group, after 90 days of supplementation, Omega Heroes Trial (Ohio, USA)
| Fatty Acid | Baseline (mean (SD), mol%) |
End of Trial (mean (SD), mol%) |
Pre-Post Change (Omega 3-6 - Placebo) (mol%, 95% CI, p-value)a |
||
|---|---|---|---|---|---|
| Omega 3-6 n=36b |
Placebo n=33 |
Omega 3-6 n=29 |
Placebo n=30 | ||
| C12:0 | 0.81 (0.14) | 0.80 (0.11) | 0.80 (0.08) | 0.81 (0.17) | −0.02 (−0.09, 0.06); p=0.65 |
| C14:0 | 1.07 (0.26) | 1.05 (0.22) | 1.09 (0.31) | 1.05 (0.21) | 0.04 (−0.10, 0.18); p=0.59 |
| C16:0 | 24.71 (1.26) | 24.60 (1.46) | 25.01 (2.13) | 24.52 (2.57) | 0.35 (−0.87, 1.56); p=0.57 |
| C16:1 | 0.98 (0.20) | 1.03 (0.21) | 0.98 (0.16) | 0.95 (0.13) | 0.04 (−0.03, 0.11); p=0.29 |
| C18:0 | 14.44 (1.13) | 14.79 (1.12) | 14.44 (1.21) | 15.06 (1.48) | −0.56 (−1.27, 0.16); p=0.12 |
| C18:1n9c | 17.41 (1.74) | 17.51 (1.62) | 16.62 (2.07) | 17.65 (2.59) | −0.92, −1.99, 0.15); p=0.09 |
| C18:2 | 19.74 (2.66) | 19.68 (2.34) | 18.73 (2.24) | 19.45 (2.24) | −0.63 (−1.78, 0.52); p=0.28 |
| C20:0 | 0.67 (0.08) | 0.68 (0.12) | 0.67 (0.07) | 0.68 (0.08) | −0.01 (−0.05, 0.03); p=0.71 |
| C18:3n6 | 0.59 (0.10) | 0.60 (0.09) | 0.59 (0.07) | 0.70 (0.63) | −0.11 (−0.35, 0.13); p=0.37 |
| C18:3n3 | 0.83 (0.17) | 0.82 (0.15) | 0.78 (0.12) | 0.88 (0.13) | −0.11 (−0.17, −0.04); p=0.002 |
| C20:4 | 15.39 (1.66) | 15.31 (1.72) | 13.72 (2.37) | 14.98 (1.75) | −1.29 (−2.27, −0.310; p=0.01 |
| C20:5 | 0.79 (0.19) | 0.80 (0.27) | 2.30 (1.86) | 0.80 (0.33) | 1.43 (0.77, 2.09); p<.0001 |
| C22:6n3 | 2.57 (0.70) | 2.33 (0.37) | 4.26 (1.53) | 2.46 (0.92) | 1.52 (0.89, 2.15); p<.0001 |
Model-based estimates.
One child in the Omega 3-6 group was missing data at baseline.
Biological Signatures
Statistical analyses per our prespecified analysis plan revealed a statistically significant decrease in IL-2, with Cohen’s d= −0.62 (the negative sign here signifies a decrease, or improvement, in inflammatory profile as desired, p=0.02) (Table 4). For IL-1β, while not reaching our pre-defined cutoff, we did observe Cohen’s d= −0.36 (p=0.18). No meaningful effect was observed for IFNγ. In terms of the post hoc selected cytokines, for TNFα, we observed a moderate suggestive improvement as well (d= −0.41, p=0.13).
Table 4.
Change in Biological Signatures Due to Omega 3-6 Supplementation, Omega Heroes Trial (Ohio, USA)
| Cytokinea | Baseline (mean (SD), pg/mL) |
End of Trial (mean (SD), pg/mL) |
Pre-Post Change (Omega 3-6 - Placebo) |
Group Change (Omega 3-6 – Placebo) (95% CI)b |
p-value | Cohen’s d | |||
|---|---|---|---|---|---|---|---|---|---|
| Omega 3-6 n=37c |
Placebo n=33 c |
Omega 3-6 n=29 c |
Placebo n=30 c |
Omega 3-6 |
Placebo | ||||
| IL-1β | 0.11 (0.15) | 0.10 (0.06) | 0.09 (0.09) | 0.11 (0.07) | −0.02 | 0.01 | −0.021 (−0.053, 0.01) | 0.18 | −0.36 |
| IL-2 | 0.36 (0.56) | 0.48 (0.47) | 0.29 (0.16) | 0.49 (0.44) | −0.09 | 0.01 | −0.17 (−0.31, −0.02) | 0.02 | −0.62 |
| IFNγ | 9.99 (43.17) | 16.72 (7.94) | 8.20 (7.43) | 8.02 (5.25) | −3.1 | −9.77 | 0.36 (−2.99, 3.72) | 0.83 | 0.06 |
| TNFα | 4.01 (0.90) | 3.78 (0.88) | 3.61 (0.82) | 3.76 (0.84) | −0.39 | 0.03 | −0.28 (−0.65, 0.08) | 0.13 | −0.41 |
| IL-6 | 0.58 (0.51) | 0.69 (0.71) | 0.49 (0.35) | 0.74 (1.22) | −0.15 | 0.04 | −0.24 (−0.71, 0.24) | 0.32 | −0.26 |
| IL-8 | 4.36 (2.30) | 4.07 (1.31) | 4.02 (1.62) | 4.20 (1.52) | −0.02 | 0.09 | −0.15 (−0.90, 0.61) | 0.70 | −0.10 |
| IL-10 | 0.79 (0.49) | 0.74 (0.60) | 0.64 (0.38) | 0.81 (0.78) | −0.19 | 0.06 | −0.19 (−0.51, 0.13) | 0.23 | −0.32 |
| IL-12p70 | 0.16 (0.07) | 0.25 (0.45) | 0.16 (0.06) | 0.22 (0.36) | 0.003 | −0.04 | 0.02 (−0.014, 0.05) | 0.27 | 0.29 |
Most values for IL-4 were below limits of detection, results not shown.
Model-based estimates.
8 children in the Omega 3-6 group and 3 children in the placebo group were missing data at the end of the trial for all cytokines because of inability to collect adequate volumes of blood. Values were below the limits of detection for IL-1β for 4 children in the Omega 3-6 group at baseline and 9 at the end of the trial.
Exploratory Analysis of Biological Signatures by Dose of Omega 3-6
While this study was not powered to analyze treatment effects by dose of Omega 3-6, some differences were observed in our exploratory analyses (Table 5). The reduction in IL-2 was greater in the medium dose treatment group than the placebo group (p=0.01) and met our Cohen’s d effect size benchmark (d=1.10 > 0.5). Several cytokines showed greater reductions in one or more treatment groups than in the placebo group (e.g., IL-1β, medium and high; TNFγ, low and medium), but no others met our effect size benchmark and had a statistically-significant p-value.
Table 5.
Change in Biological Signatures Due to Omega 3-6 Supplementation: - Exploratory Analyses by Dose, Omega Heroes Trial (Ohio, USA)
| Cytokinea | Baseline (mean)(pg/mL) |
End of Trial (mean)(pg/mL) |
Group Difference in Change (Omega 3- 6 – placebo), Cohen’s d, p-value b |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Omega 3-6 | Placebo | Omega 3-6 | Placebo | ||||||||
| Low n=10c |
Med n=10c |
High n=12c |
Low n=10c |
Med n=10c |
High n=12c |
Low | Medium | High | |||
| IL-1β | 0.10 (0.05) | 0.16 (0.25) | 0.07 (0.05) | 0.10 (0.06) | 0.10 (0.05) | 0.13 (0.15) | 0.07 (0.04) | 0.11 (0.07) | −0.01 (d=−0.18, p=0.64) | −0.02 (d=−0.34, p=0.38) | −0.03 (d=−0.51, p=0.16) |
| IL-2 | 0.31 (0.12) | 0.55 (0.96) | 0.23 (0.10) | 0.48 (0.47) | 0.32 (0.13) | 0.26 (0.12) | 0.28 (0.22) | 0.49 (0.44) | −0.10 (d=−0.37, p=0.34) | −0.29 (d=−1.10, p=0.01) | −0.11 (d=−0.41, p=0.26) |
| IFNγ | 8.95 (6.40) | 11.22 (10.18) | 9.80 (7.35) | 16.72 (43.17) | 6.76 (5.01) | 6.47 (3.66) | 10.79 (10.57) | 8.02 (5.25) | −1.04 (d=−0.17, p=0.67) | −1.43 (d=−0.23, p=0.56) | 2.99 (d=0.48, p=0.19) |
| TNFα | 4.37 (0.97) | 3.55 (0.70) | 4.09 (0.88) | 3.78 (0.88) | 3.76 (1.00) | 3.27 (0.86) | 3.77 (0.60) | 3.76 (0.84) | −0.41 (d=−0.58, p=0.14) | −0.33 (d=−0.47, p=0.23) | −0.15 (d=−0.21, p=0.56) |
| IL-6 | 0.71 (0.77) | 0.45 (0.12) | 0.60 (0.44) | 0.69 (0.71) | 0.66 (0.54) | 0.39 (0.16) | 0.44 (0.24) | 0.74 (1.22) | −0.10 (d=−0.11, p=0.79) | −0.32 (d=−0.35, p=0.37) | −0.29 (d=−0.32, p=0.38) |
| IL-8 | 5.21 (3.39) | 4.50 (1.44) | 3.45 (1.30) | 4.07 (1.31) | 3.57 (1.25) | 3.67 (1.25) | 4.67 (2.01) | 4.20 (1.52) | −0.74 (d=−0.56, p=0.16) | −0.73 (d=−0.55, p=0.17) | 0.83 (d=0.63, p=0.09) |
| IL-10 | 0.71 (0.57) | 0.86 (0.49) | 0.80 (0.43) | 0.74 (0.60) | 0.62 (0.18) | 0.69 (0.56) | 0.62 (0.36) | 0.81 (0.78) | −0.21 (d=−0.34, p=0.38) | −0.17 (d=−0.28, p=0.47) | −0.19 (d=−0.32, p=0.38) |
| IL-12p70 | 0.17 (0.08) | 0.15 (0.04) | 0.14 (0.07) | 0.25 (0.45) | 0.16 (0.08) | 0.14 (0.05) | 0.17 (0.05) | 0.22 (0.36) | 0.0001 (d=0.003, p=0.99) | 0.01 (d=0.21, p=0.60) | 0.04 (d=0.61, p=0.09) |
Most values for IL-4 were below limits of detection, so results not shown.
Model-based estimates.
3 children in the low Omega 3-6 group, 3 in the medium group, 3 in the high group, and 3 children in the placebo group were missing data at the end of the trial for all cytokines because of inability to collect adequate volumes of blood. Values were below the limits of detection for IL-1β for 2 children in the medium Omega 3-6 group and 2 in the high group at baseline, and 3 children in the high Omega 3-6 group at the end of the trial.
DISCUSSION
Omega Heroes is the first randomized controlled trial to test the effects of a DHA+EPA+GLA (Omega 3-6) FA combination from fish and borage oil in children with ASD. Our hypothesis was that the Omega 3-6 combination would reduce inflammation, a common feature of ASD, thereby potentially serving as a pathway to improving the core features of ASD.(Granpeesheh et al., 2009; Martinez, 1994) This early phase trial was designed to establish safety, adherence, and changes in prescribed inflammatory markers. We observed no serious adverse outcomes and adherence was high compared to previous trials of FAs in children diagnosed with ASD (Bent et al., 2014; Mankad et al., 2015). We also observed a significant decrease in IL-2 and TNFa levels in children receiving Omega 3-6 compared children receiving placebo.
FA supplements are among the most popular complementary health interventions utilized by children with ASD. Their use is driven by anecdotal evidence of benefit and a few small RCTs that demonstrated improvements in some externalizing behaviors in this population (Amminger et al., 2007; Bent et al., 2011; Bent et al., 2014). Overall, there is little evidence for efficacy in treating the actual core symptoms of ASD, and little is known about the physiological pathways that contribute to this disorder. Our choice of specific FAs was related to the biological functions of each and their relevance to neurological health. DHA, the primary neuro-active FA, is critical to neurotransmitter function, synaptogenesis, gene expression, membrane fluidity, neurogenesis, neuroplasticity, and regulating inflammation (Bazan, 2006; Chalon, 2006; Coti Bertrand et al., 2006; Kitajka et al., 2002; Salem et al., 2001; Simopoulos, 2002). EPA is often included in omega-3 supplements and has triglyceride lowering and anti-inflammatory properties similar to DHA (Mozaffarian et al., 2012; Wei et al., 2011). GLA is an omega-6 FA commonly found in borage and evening primrose, is biologically available, and is quickly converted to dihomo-γ-linolenic acid (DGLA), which also has substantial anti-inflammatory properties (Sergeant et al., 2016).
DHA is the most widely investigated FA for treating mental health disorders; however, DHA alone has demonstrated little or no benefit in treating aberrant behavioral symptoms (Johnson et al., 2010). Adding EPA to DHA has been evaluated in 4 rigorous RCTs for ASD. One trial (n=13) showed benefit to language and maladaptive behaviors (Amminger et al., 2007). Two others reported benefits limited to hyperactivity (Bent et al., 2011; Bent et al., 2014). Another (n=36) reported worsening externalizing behaviors (Mankad et al., 2015). However, EPA+DHA was moderately efficacious in reducing symptoms of ADHD in large RCTs (meta-analysis standardized mean difference=0.31, 95% CI: 0.16, 0.47) (Bloch et al., 2011). One recent trial tested DHA, EPA, and evening primrose oil (GLA, AA) in an open-label, non-controlled design and showed improvements in parent-reported social and attention-related outcomes (Ooi et al., 2015). In this early phase trial, we did not test benefit in ASD-related behaviors but will perform extensive cognitive and behavior assessments for this purpose in the next phase of research.
One significant shortcoming to the past trials and addressed in our current study is the lack of consideration for dosing by weight. Many individuals with ASD have comorbidities that impact health and functioning: at least 34% have gastrointestinal problems (constipation), 34% are overweight, and 18% are obese (de Vinck-Baroody et al., 2015; Ibrahim et al., 2009). Theoretically, these may modify the effect of FA supplementation on ASD symptoms by inhibiting gastrointestinal absorption of the supplement or by sequestering a portion of ingested FAs in adipose tissue. In fact, differences in treatment response have been reported by weight and BMI in at least one prior omega-3 trial for mood disorder (Christian et al., 2017). We observed no adverse effects with dosing by weight.
Children with ASD often exhibit altered FA metabolism, specifically lower levels of DHA, EPA, and AA. The reasons are unclear but may be due to diet deficiencies, altered intestinal absorption, and defects in FA synthesis related to polymorphisms in FA desaturase genes (e.g., FADS2) or other metabolic alterations (Brigandi et al., 2015; Innis, 2008; Vancassel et al., 2001; Yui et al., 2016). Since all of these FAs contribute to important biological functions, and influence anti-inflammatory pathways, we speculate that decreases or deficiencies in these FAs directly contribute to increased inflammation in ASD. As observed in our previous study (Preemie Tots), no increases in RBC GLA were indicated; but we did measure increases in ALA, AA, EPA, and DHA pre- versus post-treatment (Table 3). This finding provides evidence that increases in these important FAs offer essential substrate for (anti) inflammatory responses and normal growth and neurodevelopment.
Inflammation is well characterized in youth with ASD both in the circulation and CSF (Theoharides et al., 2016). Interleukin (IL)-1β is an acute response cytokine (Xu et al., 2015), can cross the blood-brain barrier inducing increased permeability, or be produced by astrocytes and microglia in the brain. Increases in IL-1β in neurological tissues causes increased exocytosis of several neurotransmitters, resulting in increased excitability, anxiety, and activation of the HPA (Anisman et al., 2002; Vezzani et al., 2015). Interleukin (IL)-2 has been extensively studied in the context of brain development and T-cell fate (Petitto et al., 2012). IL-2 acts in a trophic manner during development and loss of IL-2 causes deficits in learning and memory processing in animal studies. Alternatively, IL-2 regulates immunological homeostasis, self-tolerance, and T cell development and function; thus implying dysregulation may play a significant role in neurological autoimmune diseases (Petitto et al., 2012; Xu et al., 2015). Interferon gamma (IFNγ) is produced primarily by epithelial cells and is the acute phase cytokine produced in response to viral infections. (Lazear et al., 2015; Sweeten et al., 2004; Tostes et al., 2012). Tumor Necrosis Factor alpha (TNFα) is an acute phase cytokine involved in innate responses. A recent meta-analysis revealed that TNFα was elevated in most studies of ASD patients versus typically developing individuals (IL-2 was not evaluated)(Masi et al., 2015). Our prior, unpublished trial observed lower levels of IL-1β, TNFα, IL-2, and IFNγ, in the Omega 3-6 group compared to the placebo group at the end of the trial and these findings were the basis for our choices and power calculations for Omega Heroes. The results of our Omega Heroes trial revealed a significant decrease (improvement) in IL-2 with a Cohen’s d= −0.62. While not reaching our pre-defined effect size benchmark, we did observe Cohen’s d= −0.36 for IL-1β and d= −0.41 for TNFα (Table 5). Decreases in these three cytokines could have important implications in suppressing inflammation in neurological tissues and thus effect behaviors. The additional cytokines (IL-4, IL-8, IL-6, IL-10, IL-12p70) have been shown to be elevated in children diagnosed with ASD in individual trials and are likely important and relevant to our outcomes with a larger sample size.
Our enrollment and retention were excellent, and despite potential oral sensitivity and feeding difficulties among young children with ASD, compliance and adherence was high (Figure 1, Table 2). The form of the investigational product (a liquid oil that has a strong taste) and the study population (young children with ASD) combine to make adherence particularly challenging. Compounding these concerns is the fact that the eligibility criteria required that children were recently diagnosed with ASD. This means that the children were not yet in therapy programs which often coach parents on how to improve feeding challenges and to address sensory issues.
Omega Heroes is unique in several ways. We are one of the first studies to test the efficacy of a combination therapy of fish and borage oil derived DHA+EPA+GLA using changes in inflammatory biomarkers as a primary outcome. Secondly, our dosing was based on weight, which can be a significant variable in the ASD population and with age in early childhood. The study population was indicative of the population in our geographical area and consisted of a similar racial and ethnic distribution (Table 1).
The greatest weaknesses were the relatively small sample size, moderate loss to follow-up or withdrawal of participants, and the single site setting. At this time, we were also unable to test a wider dose range or other relative proportions of FAs in the supplement for increases in efficacy.
Despite the limitations, the findings of the present study lend important support for a line of research whereby this particular combination of FAs and weight-based dosing may be tested for efficacy in improving the core symptoms of ASD, while simultaneously shedding light on the role of inflammatory markers as potential pathways through which the intervention may offer clinically significant benefit. This represents a logical next step. Such findings would be highly relevant for children and their families, given the limited pharmacologic tools available to treat ASD.
Acknowledgments:
We thank Investigational Drug Services staff of Nationwide Children’s Hospital, the Child Development Center of Nationwide Children’s Hospital, Beth Burkhart of Research Information Solutions and Innovation, and Yolanda Yang of Nationwide Children’s Hospital for data collection and administrative support, the Independent Monitoring Committee members Robert Voigt, Lisa Bodnar, and Kenneth Kelley, and National Center for Complementary and Integrative Health Program Officer Wendy Weber. This study was supported by grants from the National Center for Complementary and Integrative Health (R61AT009632) and the National Center for Advancing Translational Sciences (UL1TR001070) of the National Institutes of Health.
Footnotes
Conflicts of Interest
None of the authors have conflicts of interest relevant to the content of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Nordic Naturals provided the investigational product at no cost; and Welsh, Holme, & Clark Co., Inc. provided canola oil at no cost. Neither the study sponsors nor product providers had a role in the study design; the collection, analysis, and interpretation of data; writing of this report; or the decision to submit the manuscript for publication.
Compliance with Ethical Standards
All procedures performed in this study were in accordance with Good Clinical Practice (GCP) and the 1964 Declaration of Helsinki. The study was approved by the Nationwide Children’s Hospital Institutional Review Board (IRB #17-00517) and the National Institutes of Health.
Consent to Participate
Informed consent was obtained from legal guardians.
REFERENCES
- Amminger GP, Berger GE, Schäfer MR, Klier C, Friedrich MH, & Feucht M (2007). Omega-3 Fatty Acids Supplementation in Children with Autism: A Double-blind Randomized, Placebo-controlled Pilot Study. Biological Psychiatry, 61, 551–553. doi: 10.1016/j.biopsych.2006.05.007 [DOI] [PubMed] [Google Scholar]
- Anisman H, Hayley S, Turrin N, & Merali Z (2002). Cytokines as a stressor: implications for depressive illness. International Journal of Neuropsychopharmacology, 5(4), 357–373. doi: 10.1017/S1461145702003097 [DOI] [PubMed] [Google Scholar]
- Arnold L, Young A, Belury M, Cole R, Gracious B, Seidenfeld A, Wolfson H, & Fristad M (2017). Omega-3 Fatty Acid Plasma Levels Before and After Supplementation: Correlations with Mood and Clinical Outcomes in the Omega 3 and Therapy Studies (OATS). Journal of Child and Adolescent Psychopharmacology, 27(3), 223–233. doi: 10.1089/cap.2016.0123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan NG (2006). Cell survival matters: docosahexaenoic acid signaling, neuroprotection and photoreceptors. Trends in Neurosciences, 29(5), 263–271. doi: 10.1016/j.tins.2006.03.005 [DOI] [PubMed] [Google Scholar]
- Bent S, Bertoglio K, Ashwood P, Bostrom A, & Hendren RL (2011). A pilot randomized controlled trial of omega-3 fatty acids for autism spectrum disorder. Journal of Autism and Developmental Disorders, 41(5), 545–554. doi: 10.1007/s10803-010-1078-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bent S, Hendren RL, Zandi T, Law K, Choi JE, Widjaja F, Kalb L, Nestle J, & Law P (2014). Internet-based, randomized, controlled trial of omega-3 fatty acids for hyperactivity in autism. Journal of the American Academy of Child and Adolescent Psychiatry, 53(6), 658–666. doi: 10.1016/j.jaac.2014.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch MH, & Qawasmi A (2011). Omega-3 fatty acid supplementation for the treatment of children with attention-deficit/hyperactivity disorder symptomatology: systematic review and meta-analysis. Journal of the American Academy of Child and Adolescent Psychiatry, 50(10), 991–1000. doi: 10.1016/j.jaac.2011.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone KM, Pattison K, Pelak G, Sheppard KW, Rausch J, Yeates KO, Nelin MA, Klebanoff MA, Norris Turner A, & Rogers LK (2021). Docosahexaenoic and arachidonic acid supplementation at 1 year has mixed effects on development and behavior at age 2 for preterm children. Acta Paediatrica. doi: 10.1111/apa.15858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigandi SA, Shao H, Qian SY, Shen YP, Wu BL, & Kang JX (2015). Autistic Children Exhibit Decreased Levels of Essential Fatty Acids in Red Blood Cells. International Journal of Molecular Sciences, 16(5), 10061–10076. doi: 10.3390/ijms160510061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs-Gowan MJ, Carter AS, Irwin JR, Wachtel K, & Cicchetti DV (2004). The Brief Infant-Toddler Social and Emotional Assessment: screening for social-emotional problems and delays in competence. Journal of Pediatric Psychology, 29(2), 143–155. doi: 10.1093/jpepsy/jsh017 [DOI] [PubMed] [Google Scholar]
- Chalon S (2006). Omega-3 fatty acids and monoamine neurotransmission. Prostaglandins, Leukotrienes, and Essential Fatty Acids, 75(4-5), 259–269. doi: 10.1016/j.plefa.2006.07.005 [DOI] [PubMed] [Google Scholar]
- Christian LM, Young AS, Mitchell AM, Belury MA, Gracious BL, Arnold LE, & Fristad MA (2017). Body weight affects ω-3 polyunsaturated fatty acid (PUFA) accumulation in youth following supplementation in post-hoc analyses of a randomized controlled trial. PloS One, 12(4), e0173087. doi: 10.1371/journal.pone.0173087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman CJ, Edelman D, & Woolson RF (2016). To condition or not condition? Analysing 'change' in longitudinal randomised controlled trials. BMJ open, 6(12), e013096. doi: 10.1136/bmjopen-2016-013096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coti Bertrand P, O'Kusky JR, & Innis SM (2006). Maternal dietary (n-3) fatty acid deficiency alters neurogenesis in the embryonic rat brain. Journal of Nutrition, 136(6), 1570–1575. doi: 10.1093/jn/136.6.1570 [DOI] [PubMed] [Google Scholar]
- Dawson G, Rogers S, Munson J, Smith M, Winter J, Greenson J, Donaldson A, & Varley J (2010). Randomized, controlled trial of an intervention for toddlers with autism: the Early Start Denver Model. Pediatrics, 125(1), e17–2l3. doi: 10.1542/peds.2009-0958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vinck-Baroody O, Shui A, Macklin EA, Hyman SL, Leventhal JM, & Weitzman C (2015). Overweight and Obesity in a Sample of Children With Autism Spectrum Disorder. Academic Pediatrics, 15(4), 396–404. doi: 10.1016/j.acap.2015.03.008 [DOI] [PubMed] [Google Scholar]
- Eder K (1995). Gas chromatographic analysis of fatty acid methyl esters. Journal of Chromatography B: Biomedical Applications, 671(1-2), 113–131. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8520689 [DOI] [PubMed] [Google Scholar]
- Granpeesheh D, Dixon DR, Tarbox J, Kaplan AM, & Wilke AE (2009). The effects of age and treatment intensity on behavioral intervention outcomes for children with autism spectrum disorders. Research in Autism Spectrum Disorders, 3(4), 1014–1022. doi: 10.1016/j.rasd.2009.06.007 [DOI] [Google Scholar]
- Greenhill LL, Vitiello B, Fisher P, Levine J, Davies M, Abikoff H, Chrisman AK, Chuang S, Findling RL, March J, Scahill L, Walkup J, & Riddle MA (2004). Comparison of increasingly detailed elicitation methods for the assessment of adverse events in pediatric psychopharmacology. Journal of the American Academy of Child and Adolescent Psychiatry, 43(12), 1488–1496. doi: 10.1097/01.chi.0000142668.29191.13 [DOI] [PubMed] [Google Scholar]
- Grosse SD, Ji X, Nichols P, Zuvekas SH, Rice CE, & Yeargin-Allsopp M (2021). Spending on Young Children With Autism Spectrum Disorder in Employer-Sponsored Plans, 2011-2017. Psychiatric Services, 72(1), 16–22. doi: 10.1176/appi.ps.202000099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim SH, Voigt RG, Katusic SK, Weaver AL, & Barbaresi WJ (2009). Incidence of gastrointestinal symptoms in children with autism: a population-based study. Pediatrics, 124(2), 680–686. doi: 10.1542/peds.2008-2933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis SM (2008). Dietary omega 3 fatty acids and the developing brain. Brain Research, 1237, 35–43. doi: 10.1016/j.brainres.2008.08.078 [DOI] [PubMed] [Google Scholar]
- Johnson CR, Handen BL, Zimmer M, & Sacco K (2010). Polyunsaturated Fatty Acid Supplementation in Young Children with Autism. Journal of Developmental and Physical Disabilities, 22(1), 1–10. doi: 10.1007/s10882-009-9152-x [DOI] [Google Scholar]
- Keim SA, Gracious B, Boone KM, Klebanoff MA, Rogers LK, Rausch J, Coury DL, Sheppard KW, Husk J, & Rhoda DA (2018). ω-3 and ω-6 fatty acid supplementation may reduce autism symptoms based on parent report in preterm toddlers. The Journal of nutrition, 148(2), 227–235. doi: 10.1093/Jn/nxx047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajka K, Puskas LG, Zvara A, Hackler L Jr., Barcelo-Coblijn G, Yeo YK, & Farkas T (2002). The role of n-3 polyunsaturated fatty acids in brain: modulation of rat brain gene expression by dietary n-3 fatty acids. Proc Natl Acad Sci USA, 99(5), 2619–2624. doi: 10.1073/pnas.042698699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuratko C (2013). Food-frequency questionnaire for assessing long-chain ω-3 fatty-acid intake : Re: Assessing long-chain ω-3 polyunsaturated fatty acids: A tailored food-frequency questionnaire is better. Nutrition, 29(5), 807–808. doi: 10.1016/J.nut.2012.10.013 [DOI] [PubMed] [Google Scholar]
- Lai K, & Kelley K (2012). Accuracy in parameter estimation for ANCOVA and ANOVA contrasts: sample size planning via narrow confidence intervals. British Journal of Mathematical and Statistical Psychology, 65(2), 350–370. doi: 10.1111/J.2044-8317.2011.02029.x [DOI] [PubMed] [Google Scholar]
- Lazear HM, Nice TJ, & Diamond MS (2015). Interferon-lambda: Immune Functions at Barrier Surfaces and Beyond. Immunity, 43(1), 15–28. doi: 10.1016/J.immuni.2015.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, Dilavore PC, Risi S, Gotham K, & Bishop SL (2012). Autism Diagnostic Observation Schedule, Second Edition (ADOS-2). WPS. [Google Scholar]
- Maenner MJ, Shaw KA, Baio J, Washington A, Patrick M, DiRienzo M, Christensen DL, Wiggins LD, Pettygrove S, Andrews JG, Lopez M, Hudson A, Baroud T, Schwenk Y, White T, Rosenberg CR, Lee LC, Harrington RA, Huston M, Hewitt A, Esler A, Hall-Lande J, Poynter JN, Hallas-Muchow L, Constantino JN, Fitzgerald RT, Zahorodny W, Shenouda J, Daniels JL, Warren Z, Vehorn A, Salinas A, Durkin MS, & Dietz PM (2020). Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2016. MMWR: Surveillance Summaries, 69(4), 1–12. doi: 10.15585/mmwr.ss6904a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankad D, Dupuis A, Smile S, Roberts W, Brian J, Lui T, Genore L, Zaghloul D, Iaboni A, Marcon PM, & Anagnostou E (2015). A randomized, placebo controlled trial of omega-3 fatty acids in the treatment of young children with autism. Molecular Autism, 6, 18. doi: 10.1186/s13229-015-0010-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez M (1994). Polyunsaturated Fatty Acids in the Developing Human Brain, Red Cells and Plasma -Influence of Nutrition and Peroxisomal Disease. Fatty Acids and Lipids: Biological Aspects, 75, 70–78. doi: 10.1159/000423553 [DOI] [PubMed] [Google Scholar]
- Masi A, Glozier N, Dale R, & Guastella AJ (2017). The immune system, cytokines, and biomarkers in autism spectrum disorder. Neuroscience Bulletin, 33(2), 194–204. doi: 10.1007/s12264-017-0103-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masi A, Quintana DS, Glozier N, Lloyd AR, Hickie IB, & Guastella AJ (2015). Cytokine aberrations in autism spectrum disorder: a systematic review and meta-analysis. Molecular Psychiatry, 20(4), 440–446. doi: 10.1038/mp.2014.59 [DOI] [PubMed] [Google Scholar]
- Mazahery H, Conlon CA, Beck KL, Mugridge O, Kruger MC, Stonehouse W, Camargo CA, Meyer BJ, Tsang B, & von Hurst PR (2020). Inflammation (IL-1β) Modifies the Effect of Vitamin D and Omega-3 Long Chain Polyunsaturated Fatty Acids on Core Symptoms of Autism Spectrum Disorder—An Exploratory Pilot Study. Nutrients, 12(3), 661. doi: 10.3390/nu12030661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPheeters ML, Warren Z, Sathe N, Bruzek JL, Krishnaswami S, Jerome RN, & Veenstra-Vanderweele J (2011). A systematic review of medical treatments for children with autism spectrum disorders. Pediatrics, 127(5), e1312–1321. doi: 10.1542/peds.2011-0427 [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, & Wu JH (2012). (n-3) fatty acids and cardiovascular health: are effects of EPA and DHA shared or complementary? Journal of Nutrition, 142(3), 614S–625S. doi: 10.3945/jn.111.149633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi YP, Weng SJ, Jang LY, Low L, Seah J, Teo S, Ang RP, Lim CG, Liew A, Fung DS, & Sung M (2015). Omega-3 fatty acids in the management of autism spectrum disorders: findings from an open-label pilot study in Singapore. European Journal of Clinical Nutrition, 69(8), 969–971. doi: 10.1038/ejcn.2015.28 [DOI] [PubMed] [Google Scholar]
- Petitto JM, Meola D, & Huang Z (2012). Interleukin-2 and the brain: dissecting central versus peripheral contributions using unique mouse models. Methods in Molecular Biology, 934, 301–311. doi: 10.1007/978-1-62703-071-7_15 [DOI] [PubMed] [Google Scholar]
- Puri BK, & Martins JG (2014). Which polyunsaturated fatty acids are active in children with attention-deficit hyperactivity disorder receiving PUFA supplementation? A fatty acid validated meta-regression analysis of randomized controlled trials. Prostaglandins, Leukotrienes, and Essential Fatty Acids, 90(5), 179–189. doi: 10.1016/j.plefa.2014.01.004 [DOI] [PubMed] [Google Scholar]
- Reichow B, & Wolery M (2009). Comprehensive synthesis of early intensive behavioral interventions for young children with autism based on the UCLA young autism project model. Journal of Autism and Developmental Disorders, 39(1), 23–41. doi: 10.1007/s10803-008-0596-0 [DOI] [PubMed] [Google Scholar]
- Salem N, Litman B, Kim HY, & Gawrisch K (2001). Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids, 36(9), 945–959. doi: 10.1007/s11745-001-0805-6 [DOI] [PubMed] [Google Scholar]
- Sergeant S, Rahbar E, & Chilton FH (2016). Gamma-linolenic acid, Dihommo-gamma linolenic, Eicosanoids and Inflammatory Processes. European Journal of Pharmacology, 785, 77–86. doi: 10.1016/j.ejphar.2016.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard KW, B. K, Gracious B, Klebanoff MA, Rogers LK, Rausch J, Bartlett C, Coury DL, Keim SA. (2017). Effect of Omega-3 and −6 Supplementation on Language in Preterm Toddlers Exhibiting Autism Spectrum Disorder Symptoms. Journal of Autism and Developmental Disorders, 47(11), 3358–3369. doi: 10.1007/s10803-017-3249-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simopoulos AP (2002). Omega-3 fatty acids in inflammation and autoimmune diseases. Journal of the American College of Nutrition, 21(6), 495–505. doi: 10.1080/07315724.2002.10719248 [DOI] [PubMed] [Google Scholar]
- Sweeten TL, Posey DJ, Shankar S, & McDougle CJ (2004). High nitric oxide production in autistic disorder: a possible role for interferon-gamma. Biological Psychiatry, 55(4), 434–437. doi: 10.1016/j.biopsych.2003.09.001 [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Tsilioni I, Patel AB, & Doyle R (2016). Atopic diseases and inflammation of the brain in the pathogenesis of autism spectrum disorders. Transl Psychiatry, 6(6), e844. doi: 10.1038/tp.2016.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tostes MH, Teixeira HC, Gattaz WF, Brandao MA, & Raposo NR (2012). Altered neurotrophin, neuropeptide, cytokines and nitric oxide levels in autism. Pharmacopsychiatry, 45(6), 241–243. doi: 10.1055/s-0032-1301914 [DOI] [PubMed] [Google Scholar]
- Vancassel S, Durand G, Barthelemy C, Lejeune B, Martineau J, Guilloteau D, Andres C, & Chalon S (2001). Plasma fatty acid levels in autistic children. Prostaglandins, Leukotrienes, and Essential Fatty Acids, 65(1), 1–7. doi: 10.1054/plef.2001.0281 [DOI] [PubMed] [Google Scholar]
- Vezzani A, & Viviani B (2015). Neuromodulatory properties of inflammatory cytokines and their impact on neuronal excitability. Neuropharmacology, 96(Pt A), 70–82. doi: 10.1016/j.neuropharm.2014.10.027 [DOI] [PubMed] [Google Scholar]
- Wei MY, & Jacobson TA (2011). Effects of eicosapentaenoic acid versus docosahexaenoic acid on serum lipids: a systematic review and meta-analysis. Curr Atheroscler Rep, 13(6), 474–483. doi: 10.1007/s11883-011-0210-3 [DOI] [PubMed] [Google Scholar]
- Whitehouse AJ (2013). Complementary and alternative medicine for autism spectrum disorders: rationale, safety and efficacy. Journal of Paediatrics and Child Health, 49(9), E438–442:quiz E442. doi: 10.1111/jpc.12242 [DOI] [PubMed] [Google Scholar]
- Winkens B, van Breukelen GJ, Schouten HJ, & Berger MP (2007). Randomized clinical trials with a pre- and a post-treatment measurement: repeated measures versus ANCOVA models. Contemporary Clinical Trials, 28(6), 713–719. doi: 10.1016/j.cct.2007.04.002 [DOI] [PubMed] [Google Scholar]
- Xu N, Li X, & Zhong Y (2015). Inflammatory cytokines: potential biomarkers of immunologic dysfunction in autism spectrum disorders. Mediators of Inflammation, 2015, 531518. doi: 10.1155/2015/531518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yui K, Imataka G, Kawasak Y, & Yamada H (2016). Increased omega-3 polyunsaturated fatty acid/arachidonic acid ratios and upregulation of signaling mediator in individuals with autism spectrum disorders. Life Sciences, 145, 205–212. doi: 10.1016/j.lfs.2015.12.039 [DOI] [PubMed] [Google Scholar]