Abstract
BACKGROUND:
Repetitive negative thinking (RNT) is a symptom dimension of depression that is associated with a poorer prognosis in terms of higher recurrence, treatment resistance, residual symptoms, and disability. This investigation examined whether RNT is associated with aberrant reward processing and fear learning.
METHODS:
Very high RNT (VH-RNT) (n = 60) and high RNT (H-RNT) (n = 60) propensity-matched individuals with depression (age, sex, race/ethnicity, income/employment, body mass index, depressive and anxiety symptom severity) participated in this study along with matched healthy comparison volunteers (n = 30). This propensity-matched sample was selected from the larger Tulsa 1000 study. Participants performed two functional magnetic resonance imaging tasks: the monetary incentive delay task probing reward processing and the fear conditioning task probing aversive learning and extinction.
RESULTS:
Both VH-RNT and H-RNT groups showed lower neural activity than healthy comparison subjects in reward circuitry, including the inferior frontal gyrus (VH-RNT: β = −1.24, H-RNT: β = −1.28) and the cerebellum (VH-RNT: β = −0.93, H-RNT: β = −1.14). However, individuals with VH-RNT exhibited lower activation than those with H-RNT in central autonomic network components during fear conditioning (β = −0.84) and continued conditioned responses during early extinction in the postcentral cortex (β = 0.71).
CONCLUSIONS:
VH-RNT showed aberrant processing in fear conditioning during both learning and extinction phases compared with H-RNT. These findings demonstrate that dysfunctions of negative valence associated with RNT may be domain specific, which should be taken into account for identifying potential specific targets of intervention.
Major depressive disorder (MDD) is a substantial public health concern, considering that it affects approximately 16% of people in their lifetime and that it is the cause for direct and indirect losses of more than $50 billion per year in the United States alone (1). About 1 in 3 individuals who receive treatment for depression fail to respond to first-line treatments, including various antidepressant medications and psychotherapies (2). Focusing on each clinical component of MDD, subserved by a well-defined brain circuit, may allow us a better understanding of MDD and potentially a more successful approach to clinical improvement based on selective modulation of the affected circuit. Ideally, this kind of single clinical manifestation (and its specific modification) should result in robust clinical effects in the full syndrome. This approach necessitates a better understanding of the underlying circuit neurobiology mechanism of a symptom dimension satisfying this criterion (3).
Repetitive negative thinking (RNT), a persistent, passive, and/or relatively uncontrollable and negative thought process (4–9), is a symptom dimension that can be explored as a targetable process important for depression. At the same time, RNT can be viewed as a transdiagnostic symptom, commonly referred to as rumination in the depression literature, worry in the anxiety literature, and obsessional thinking in the obsessive-compulsive disorder (OCD) literature. While it is a mental-behavioral construct that cuts across different diagnoses with a series of important adverse clinical and prognostic implications (6), its generation is still not well understood. RNT is associated with poorer outcomes, more negative affect, worse clinical course, suicidal ideation, poor response to treatment, and persisting disability even in individuals who respond to antidepressants (7,10,11). Therefore, it is imperative to have a better understanding of neural substrates critical to RNT generation and maintenance.
The dimensional construct of anhedonia-reward deficits has been considered of paramount importance in the depression literature (12,13). Mostly with obsessional thinking, a type of RNT, aberrant reward processing is presumed to play a role in the maintenance of RNT, as evidenced in functional neuroimaging (14) and neuromodulation studies focusing on the ventral striatum and, in particular, the nucleus accumbens (12,15,16). Alternatively, aberrant reward processing in obsessional thinking disturbances could be related to the rewarding quality of compulsive behavior and habit learning rather than obsessional thinking (17). Moreover, previous findings suggested that neural response to loss was correlated with RNT in healthy control subjects but not in individuals with depression (5). RNT has also been suggested to relate with dopamine receptor genetic variants, indicating the involvement of the neurochemistry characteristically involved in reward processing (8). Taken together, these studies suggest that the exact nature of the relationship between RNT and reward processing is still largely unsettled. Given the critical importance of mapping dysfunctional reward processing onto discrete symptoms of depression to identify modifiable disease processes at the neural level, it is vital to understand the association between RNT and reward/punishment processing in the MDD population (12).
In addition, prior studies have demonstrated that individuals with depression and anxiety also exhibit altered fear conditioning (18–21), in which a neutral stimulus is repeatedly associated with an aversive stimulus so that the neutral stimulus alone can trigger the fear response through associative learning. In this regard, it has been recently proposed that rapid-acting treatments for resistant depression might act via interference with fear learning, if RNT is conceptualized as a persisting cycle of retrieval of fearful thoughts and memories, followed by nighttime reconsolidation during sleep. In this view, disparate methods such as electroconvulsive therapy, ketamine, and sleep deprivation might owe their well-known efficacy in part to interference with different phases of this cycle of retrieval, lability, and reconsolidation of emotional memories (22).
Furthermore, significant evidence indicates fear circuit disruption in response to innately aversive stimuli, such as fearful/angry faces, in depression (23–27). Based on these findings, it is important to examine the association between RNT and fear conditioning, including both learning and extinction, to see if RNT is related to fear learning or its extinction of fear learning.
Ultimately, appropriately responding to a positive outcome and avoiding a negative outcome and emotionally negatively laden stimuli, modeled in reward- and fear-conditioning paradigms, respectively, provide the bases of normal adaptive behavior. The disruption of such approach-avoidance learning can offer insight into the mechanism accounting for several manifestations of depression. Because the severity of RNT may be clinically difficult to separate from the severity of depression (6) and individuals with depression differ in many ways from healthy comparison (HC) volunteers, case-control designs may not be suitable for examining the neural underpinnings of RNT in depression.
To investigate neural signatures specifically associated with RNT in depression, we studied two groups of individuals with depression who were propensity matched on the severity of current depression and other potential confounders but different regarding the severity of RNT. In this propensity-matched sample of patients with MDD with very high RNT (VH-RNT) and high RNT (H-RNT) and matched HC subjects, we put to test the following predictions based on previous findings. First, we hypothesized that depression, regardless of RNT levels, would be associated with attenuated reward processing, thus predicting no effect of RNT in reward processing (12). Second, we expected that RNT would play a role in fear conditioning during fear acquisition and fear extinction, in the light of previous literature showing persistent fear responses associated with a high level of RNT (20,22).
METHODS AND MATERIALS
Participants
The participants were drawn from the Tulsa 1000 cohort, a naturalistic study that aimed to longitudinally follow 1000 individuals with mood, anxiety, substance use, and/or eating disorders, and HC subjects (28). The eligibility criteria for the study are described in the Supplement. All procedures were approved by the Western Institutional Review Board. Participants provided written informed consent and received financial compensation for their participation.
This study included only individuals with MDD and healthy volunteers. For diagnosing MDD, the DSM diagnosis based on the Mini-International Neuropsychiatric Interview was used, followed by a clinical case conference. The original 22-item Ruminative Response Scale was also used to quantify the intensity of RNT (29). Individuals with MDD (n = 120) were propensity matched in age, depressive symptom severity (Patient Health Questionnaire-9), anxiety symptom severity (Overall Anxiety Severity and Impairment Scale), sex, body mass index, education, race and ethnicity, employment, and income. These propensity-matched individuals with depression were median-split by their Ruminative Response Scale scores, resulting in VH-RNT and H-RNT groups, with 30 matched HC volunteers (Figure S2). All participants completed self-report measures including the Ruminative Response Scale and the World Health Organization Disability Assessment Schedule for measuring RNT and functional disability, respectively. After excluding those who did not have complete magnetic resonance imaging data (n = 14), this study was based on 136 participants (55 VH-RNT MDD, 52 H-RNT MDD, and 29 HC subjects) for the monetary incentive delay (MID) task and 130 participants (54 VH-RNT MDD, 50 H-RNT MDD, and 26 HC subjects) for the fear conditioning task (see the Supplement for task descriptions). Demographic and clinical information of participants is provided in Table 1.
Table 1.
Demographic and Clinical Characteristics
| Characteristics | HC (n = 29) | H-RNT (n = 52) | VH-RNT (n = 55) | p Value |
|---|---|---|---|---|
| Age, Years, Mean ± SD | 31.2 ± 6 9.8 | 35.3 ± 6 12.6 | 34.4 ± 6 11.3 | .313 |
| Female, n (%) | 17 (58.6%) | 38 (73.1%) | 43 (78.2%) | .161 |
| Race/Ethnicity, n (%) | ||||
| Asian | 1 (3.4%) | 0 (0%) | 0 (0%) | .208 |
| Black | 0 (0%) | 3 (5.8%) | 6 (10.9%) | |
| Hispanic | 1 (3.4%) | 2 (3.8%) | 3 (5.5%) | |
| Native American | 2 (6.9%) | 9 (17.3%) | 10 (18.2%) | |
| Other | 0 (0%) | 1 (1.9%) | 3 (5.5%) | |
| White | 25 (86.2%) | 37 (71.2%) | 33 (60%) | |
| Education, n (%) | ||||
| No High school | 0 (0%) | 1 (1.9%) | 5 (9.1%) | .446 |
| High school | 5 (17.2%) | 9 (17.3%) | 8 (14.5%) | |
| Some college | 11 (37.9%) | 22 (42.3%) | 24 (43.6%) | |
| College or higher | 13 (44.8%) | 20 (38.5%) | 18 (32.7%) | |
| Psychotropic Medication, n (%) | 4 (13.8%) | 35 (67.3%)a | 36 (65.5%)a | <.001 |
| Employed, n (%) | 22 (78.6%) | 29 (60.4%)a | 33 (62.5%)a | .236 |
| OASIS, Mean ± SD | 1.1 ± 1.6 | 10.0 ± 2.9a | 10.4 ± 3.6a | <.001 |
| PHQ-9, Mean ± SD | 0.9 ± 1.4 | 13.8 ± 3.8a | 14.6 ± 4.2a | <.001 |
| RRS, Mean ± SD | 29.03 ± 6.7 | 47.8 ± 8.0a,b | 64.8 ± 6.2a,c | <.001 |
| WHODAS, Mean ± SD | 13.8 ± 3.0 | 23.5 ± 7.1a,b | 27.3 ± 8.6a,c | <.001 |
HC, healthy comparison; H-RNT, high RNT; MDD, major depressive disorder; OASIS, Overall Anxiety Severity and Impairment Scale; PHQ-9, Patient Health Questionnaire-9; RNT, repetitive negative thinking; RRS, Ruminative Response Scale; VH-RNT, very high RNT; WHODAS, World Health Organization Disability Assessment Schedule.
Significantly different from HC.
Significantly different from VH-RNT.
Significantly different from H-RNT.
Data Analysis
Preprocessing of imaging data is described in the Supplement.
Monetary Incentive Delay.
A two-level general linear model was used to analyze functional images for neural responses to incentive cues during delay by RNT. Six events were constructed on a subject to model the response for an upcoming incentive: high gain (+$5), low gain (+$1), no gain (+$0), high loss (−$5), low loss (−$1), and no loss (−$0). The blood oxygen level–dependent response to an incentive cue was convolved with a delta function for 4 seconds spanning from the presentation of the cue. The contrasts of incentive valences were constructed by comparing high-incentive to no-incentive trials: gain (+$5 > +$0) and loss (−$5 > −$0) to examine the effect of valence clearly, as previously described (30).
At the group level, the effect of RNT in anticipatory reward processing was examined using a three-dimensional multivariate analysis of covariance model with age and sex covariates (within-subjects: gain, loss; between-subjects: RNT, age, sex). A cluster-extent threshold of α < 0.01 (k > 153) was set based on the estimated autocorrelation function parameters of the group-level error terms with a voxelwise threshold of p < .005. Significant cluster effects were further examined for probing RNT effects. Tukey honestly significant difference test was used for post hoc tests.
Fear Conditioning.
A generalized linear model was used for the analysis of neural responses to fear conditioning (conditioned stimulus [CS]+ vs. CS−) by RNT. To examine learning difference in fear conditioning in detail, acquisition and extinction phases were split into the first half and the second half of each phase. Consequently, 10 events were constructed on a subject to model responses to the CS: CS+/CS− during familiarization, CS+/CS− during the first half phase of acquisition, CS+/CS− during the second half phase of acquisition, CS+/CS− during the first half phase of extinction, and CS+/CS− during the second half phase of extinction. Blood oxygen level–dependent response to each CS image was convolved with a gamma function from the onset of the CS image. At the subject level, contrasts representing fear conditioning (CS+ vs. CS−) were constructed as within-subjects. A three-dimensional multivariate analysis of covariance model was constructed with age and sex covariates to examine fear conditioning with RNT.
A cluster-extent threshold of α < 0.01 (k > 140) was used with a voxelwise threshold of p < .005, and between-group effects from significant clusters were followed up by comparing extracted beta coefficients. Tukey honestly significant difference test was also used for post hoc tests.
RESULTS
Participant characteristics and behavioral results are described in the Supplement.
Monetary Incentive Delay
Group main effects across valence were found in the right middle/inferior frontal gyrus, left parieto-occipital sulcus to the thalamus, and middle frontal regions in decreased neural responses in VH-RNT and H-RNT groups compared with the HC group (Supplemental Results; Figure 1). In Figure 1, VH-RNT showed reduced neural responses to gain cues (+$5 > $0) compared with HC subjects in the cerebellum, midbrain, and frontal areas (Table 2). Post hoc tests on these clusters revealed that H-RNT, in addition to VH-RNT, also exhibited diminished anticipatory activity to gain: mid to right cerebellum (β = −1.03, 95% CI = −1.46 to −0.61, Cohen’s d = −1.08), ventral tegmental area (VTA) (β = −0.90, 95% CI = −1.36 to −0.45, Cohen’s d = −0.88), anterior cingulate cortex (β = −1.01, 95% CI = −1.42 to −0.60, Cohen’s d = −1.10), mid cerebellum (β = −0.76, 95% CI = −1.11 to −0.42, Cohen’s d = −0.98), and right inferior frontal gyrus (β = −0.89, 95% CI = −1.28 to −0.49, Cohen’s d = −1.0).
Figure 1.
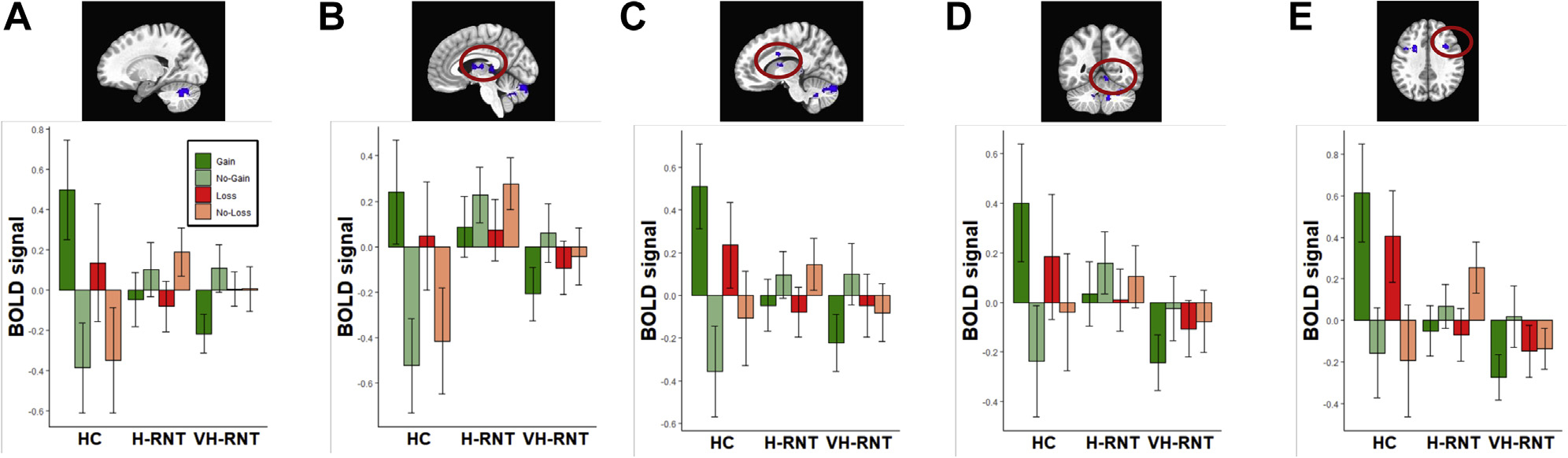
Brain regions showing significant intergroup differences in blood oxygen level–dependent (BOLD) signal response to reward (Gain: +$5, No-Gain: $0, Loss: −$5, No-Loss: $0) in individuals with RNT. Shown are the left cerebellar hemisphere (A), midbrain (B), cingulate cortex (C), cerebellar vermis (D), and dorsolateral prefrontal cortex (E). BOLD signals (arbitrary unit) were standardized (mean = 1 and SD = 1). HC, healthy comparison; H-RNT, high repetitive negative thinking; VH-RNT, very high RNT.
Table 2.
Brain Regions Showing Reward-Related Activity in Individuals With RNT Compared With HC Individuals
| Coordinates |
|||||||
|---|---|---|---|---|---|---|---|
| Test Condition | x | y | z | Number of Voxels | Region | β (CI) | Cohen’s d |
| VH-RNT vs. HC | |||||||
| Gain | −19 | −63 | −31 | 1599 | L cerebellum | −1.21 (−1.63 to −0.79) | −1.25 |
| −5 | −3 | 11 | 327 | Midbrain extending to the ventral tegmental area | −1.03 (−1.49 to −0.58) | −1.00 | |
| −11 | 5 | 31 | 229 | L anterior cingulate cortex | −1.19 (−1.60 to −0.79) | −1.28 | |
| 7 | −53 | −7 | 186 | Mid cerebellum | −0.86 (−1.20 to −0.52) | −1.09 | |
| 37 | 9 | 35 | 183 | R inferior frontal gyrus | −1.06 (−1.46 to −0.67) | −1.19 | |
| Loss | 19 | 9 | 39 | 184 | R superior frontal gyrus | −1.31 (−1.74 to −0.90) | −1.38 |
| −3 | 15 | 7 | 174 | Basal ganglia/R anterior caudate/L anterior horn | −1.06 (−1.52 to −0.61) | −1.03 | |
| H-RNT vs. HC | |||||||
| Gain | −3 | −83 | −21 | 342 | Mid cerebellum | −1.07 (−1.49 to −0.64) | −1.12 |
| 21 | −63 | −31 | 312 | R cerebellum | −0.96 (−1.31 to −0.61) | −1.21 | |
| Loss | 19 | 11 | 39 | 384 | R superior frontal gyrus | −1.43 (−1.89 to −0.98) | −1.41 |
| −21 | −47 | 21 | 228 | L inferior horn vicinity | −1.16 (−1.55 to −0.77) | −1.33 | |
| 15 | 33 | 1 | 172 | R anterior caudate | −1.01 (−1.43 to −0.59) | −1.07 | |
HC, healthy comparison; H-RNT, high RNT; L, left; R, right; RNT, repetitive negative thinking; VH-RNT, very high RNT.
For loss (−$5 > $0), VH-RNT revealed decreased activity compared with HC subjects in the right superior frontal gyrus and basal ganglia extended from the right anterior caudate to left anterior horn area. Again, follow-up tests showed that H-RNT also showed attenuated loss activity in these clusters (right superior frontal gyrus, β = −1.30, 95% CI = −1.72 to −0.87, Cohen’s d = −1.37; basal ganglia, β = −0.97, 95% CI = −1.43 to −0.51, Cohen’s d = −0.94).
Similarly, two cerebellum clusters showed reduced gain activity in H-RNT (Table 2). In these clusters, VH-RNT also exhibited attenuated neural responses to gain cues (β1 = −1.09, 95% CI = −1.51 to −0.68, Cohen’s d = −1.14; β2 = −0.85, 95% CI = −1.20 to −0.50, Cohen’s d = −1.07). Table 2 shows diminished loss activity in H-RNT in frontal regions. Again, VH-RNT exhibited attenuated loss anticipatory processing (right superior frontal gyrus: β = −1.10, 95% CI = −1.55 to −0.66, Cohen’s d = −1.08; left inferior horn: β = −0.93, 95% CI = −1.32 to −0.55, Cohen’s d = −1.06; right anterior cingulate cortex, β = −0.83, 95% CI = −1.25 to −0.42, Cohen’s d = −0.87). Nonetheless, no cluster showed significant differences between VH-RNT and H-RNT either for gains or losses.
Figure 2 illustrates the change of neural responses to the anticipated magnitude of reward for both gain and loss. HC subjects demonstrated graded brain activations by reward magnitude for both gain or loss. In sharp contrast, individuals with MDD, both VH-RNT and H-RNT, did not show such changes in neural responses as a function of the incentive magnitude.
Figure 2.
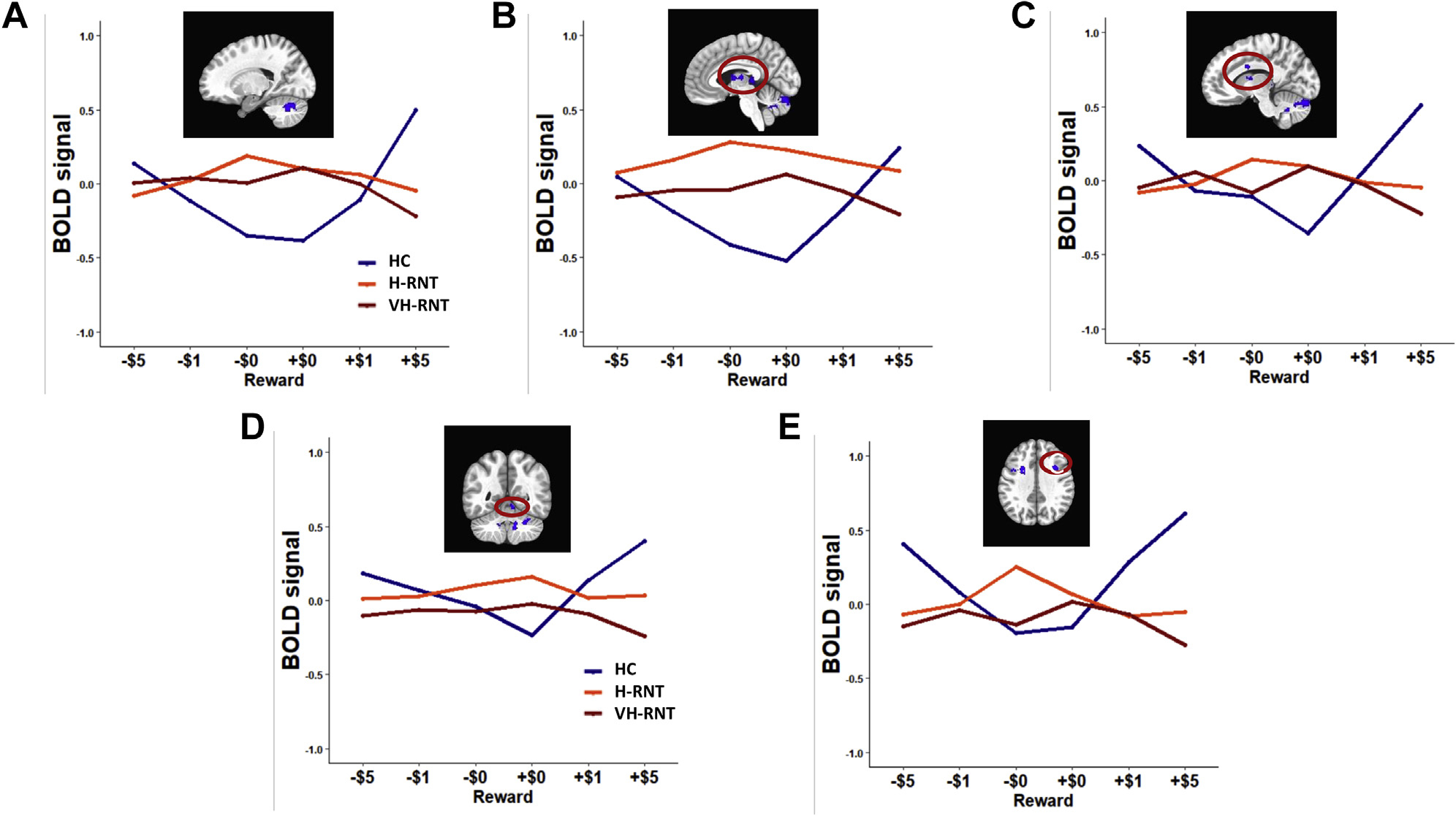
Blood oxygen level–dependent (BOLD) signal changes as a function of reward magnitude for gain (+$5, +$1, +$0) and loss (−$5,−$1,−$0) in individuals with repetitive negative thinking (RNT). $1 conditions are included for display purposes only. HC, healthy comparison; H-RNT, high RNT; VH-RNT, very high RNT.
Fear Learning and Extinction
Figure 3 shows the brain regions showing RNT differences in fear conditioning. While no RNT difference was found in the first acquisition, VH-RNT showed attenuated neural activity for fear conditioning (CS+ vs. CS−) in the left putamen extended to the caudate body and the thalamus (−21, −7, 13, 186 voxels) during the second acquisition phase compared with H-RNT (β = −0.82, 95% CI = −1.19 to −0.46, Cohen’s d = −0.88) (Figure 3A). In addition, elevated neural activity associated with fear conditioning (CS+ > CS−) was found in VH-RNT compared with H-RNT during the first extinction phase in the postcentral area (−31, −31, 33, 147 voxels) involving the somatosensory cortex extended to the posterior parietal cortex (β = 1.02, 95% CI = 0.62 to 1.43, Cohen’s d = −0.99) (Figure 3B). Post hoc tests showed that VH-RNT’s activation in response to CS+ was significantly greater than activity of H-RNT (β = 0.71, 95% CI = 0.35 to 1.07, Cohen’s d = −0.77) or HC subjects (β = 0.92, 95% CI = 0.48 to 1.35, Cohen’s d = −0.94), without a difference between H-RNT and HC subjects. During the second phase of extinction, no difference was found among groups.
Figure 3.
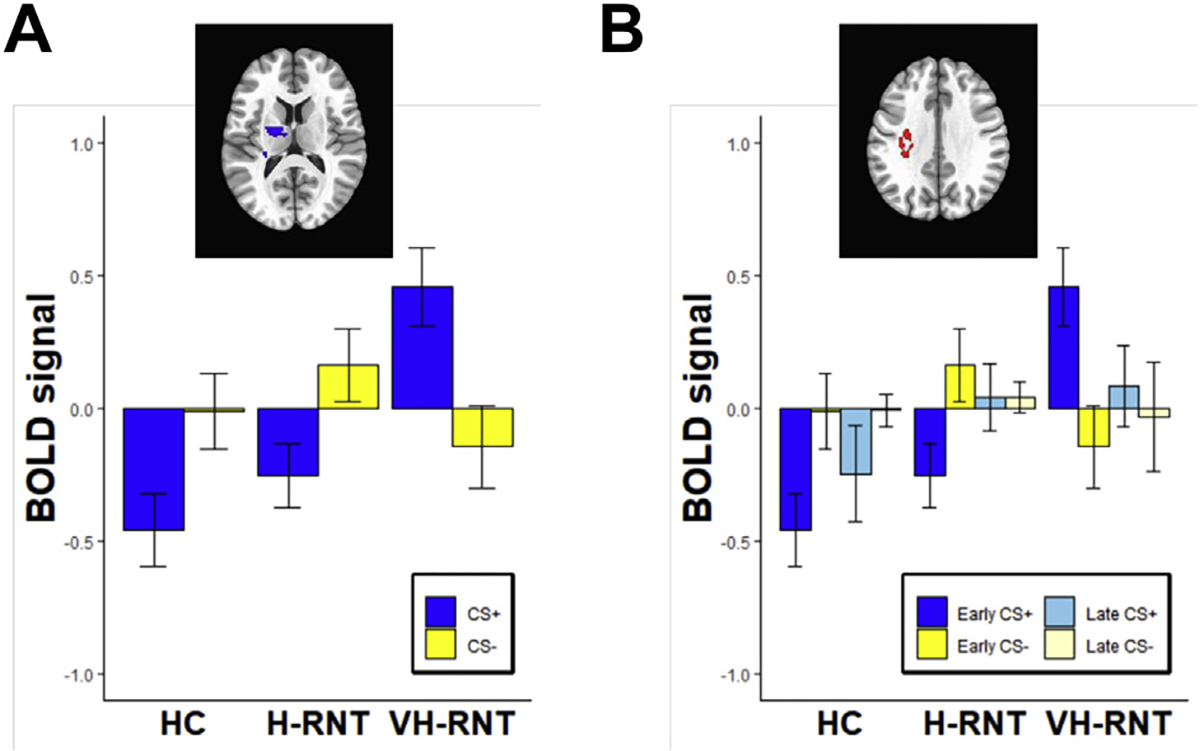
Blood oxygen level–dependent (BOLD) signals for conditioned stimuli (CS+, CS−) in individuals with repetitive negative thinking (RNT) during acquisition (A) and extinction (B). BOLD signals (arbitrary unit) were standardized (mean = 1 and SD = 1). HC, healthy comparison; H-RNT, high RNT; VH-RNT, very high RNT.
DISCUSSION
This study examined whether individuals with depression and VH-RNT relative to propensity-matched depressed individuals with H-RNT differed in reward processing and fear conditioning. There were three main findings. First, there were no differences between VH-RNT and H-RNT in reward processing. Instead, both RNT groups revealed significantly diminished activation in anticipation of monetary gains or losses in the areas known as the reward network (12), including the striatum and VTA, along with limbic structures, such as the dorsolateral prefrontal cortex, anterior thalamus, anterodorsal cingulum, and cerebellar cortex. Yet, there were no associations between RNT intensity and reward-related processing on MID. Second, individuals with VH-RNT exhibited prolonged fear conditioning activity in the left somatosensory cortex and adjacent parietal cortex during the early phase of fear extinction. These are brain areas where fear conditioning–related activity was found in normal samples (19). Third, VH-RNT was associated with longer reaction times and noticeably reduced activity in subcortical limbic structures during the acquisition of fear conditioning. The results from the MID task are consistent with deficits of gain and loss processing in depression without differential effects of RNT in gain or loss processing. In contrast, individuals with VH-RNT exhibited attenuated limbic activity for fear conditioning during fear acquisition but prolonged fear conditioning responses until the early phase of fear extinction.
RNT and Reward
Our results on MID are in agreement with previous findings that demonstrate deficits of processing reward in individuals with depression (12,31). Furthermore, in alignment with extant depression literature (32–37), these results denote the importance of the cerebellum in reward processing associated with depression (12,15). Traditionally, cerebellar physiology has been considered central to skeletal motor system functioning (38). This large portion of the encephalon has been deemed a learning machine and comparator, permitting adjustments at all levels of motor planning, output, and execution (39). In fact, the cerebellum has indirect connections with all areas of the cerebral cortex, and the areas of the cerebellum related to motor cortical regions actually account for a relatively small proportion of its surface (32,37).
Of the diverse cognitive functions of the cerebellum, a recently added one is its role as a controller of reward signal processing (37). As in motor control, reward signaling seems to rely on complementary afferent and efferent information, mostly through granule cell and climbing fiber reward signaling. A subgroup of granule cells has been termed reward anticipation neurons because they become active selectively while awaiting an expected reward; however, they do not activate when an unexpected reward is delivered (37,40). Climbing fibers are thought to be critical to cerebellar learning mechanisms because their input informs Purkinje cells, to which granule cell input is relevant (41,42). In this general framework of cerebellar functioning, burgeoning evidence suggests that climbing fibers carry critical information regarding reward prediction error, which is fundamental for reward-mediated behavioral learning (43–45). Our results suggest that cerebellar processing deficits represent a critical aspect of aberrant reward processing in MDD, in line with the recent notion of incorporating the cerebellum into the reward brain circuitry (37).
The studies of cerebellar functions in reward processing emphasize the role of dopamine neurons in the VTA and two of its major innervation targets, namely the ventral striatum and prefrontal cortical regions (46–48). While the precise connections of the cerebellar cortex with these areas are not fully understood, the connection with indirect input from the association neocortex is well documented, as well as the pathway of cerebellar output to the striatum and prefrontal cortex via thalamic relays (49,50). Cerebellum areas are known to provide monosynaptic innervation to the VTA (51,52). In these areas, we found attenuated neural activities in patients with MDD. Therefore, these findings potentially open a new avenue for studying reward processing in MDD regardless of the intensity of RNT.
Whereas we confirmed altered reward processing associated with depression in extensive reward circuits, the level of RNT was not related to reward processing. However, individuals with OCD who are also known for RNT showed treatment effects after neuromodulation targeting the ventral striatum, a structure critical to reward processing (14,53). These different results may be related to the characteristics of study populations, despite the transdiagnostic nature of RNT (17,54). For example, save for the most severe forms of the disease, obsessions in OCD have a distinctly egodystonic quality, whereas in major depression, negative thoughts subjected to perseverative repetition are usually egosyntonic. In fact, insight into illness (as implied by egodystonic obsessional thoughts) has been considered the only significant predictor of individual response to stimulation of the anterior limb of the internal capsule in OCD, which is known to modulate reward circuits (55).
RNT and Fear Learning/Extinction
VH-RNT, in contrast to H-RNT, was associated with reduced activation in the thalamus and striatum, subcortical regions known for their role in the acquisition of fear conditioning (CS+ > CS−)(19). This neuroimaging finding was accompanied by a behavioral difference of longer reaction times in fear acquisition among depressed individuals with VH-RNT. A pathogenic role for abnormalities in these subcortical regions (56) has been proposed in anxiety (19), but not yet in depression. This finding of the association between RNT and aberrant processing of fear learning raises the possibility that RNT may be a mediator of aversive conditioning processing in depression. Given that RNT is a transdiagnostic manifestation of internalizing psychiatric syndromes, RNT-associated alterations in fear conditioning deserve further studies with transdiagnostic samples followed by its exploration as interventional targets. We will further discuss the implications of this finding, along with that of aberrant activity for fear extinction.
During the early extinction, fear conditioning activity was found as lingering, prolonged activation to CS+ in the left somatosensory and posterior parietal cortex during early extinction. After a recent meta-analysis (19), this area has been consistently linked to fear acquisition in humans, apart from widespread activations in the central autonomic network (19,57). Given that the posterior parietal cortex was critical in a contextual renewal of fear responses (58), it is conceivable that individuals with VH-RNT have impairments in disengaging neural fear responses after the change of contexts. Swift response modifications after the current contextual cues, both behaviorally and neurally, may signal how well contextual learning occurs. Faulty responses despite changes in the association between CS and unconditioned stimuli may reflect impaired contextual learning in individuals with VH-RNT.
RNT is a characteristic symptom dimension of depressive disorders with poor prognosis, less dependency on adverse environmental factors, and treatment resistance (59,60). In this regard, we recently proposed that RNT exerts in part its deleterious effect in the natural history of depression by inducing a repetitive cycle of negative emotional learning, persistently retrieved and reconsolidated, and that treatments that are well recognized to alleviate treatment-resistant depression might owe in part their efficacy to the interruption of this fear learning cycle, despite their disparate mechanisms of action (22). In a simplified view, if RNT in depression was conceptualized as a continuous process of disease-relevant negative emotional learning strengthened with retrieval and reconsolidation, the symptom might render the individual with depression partially refractory to the learning of disease-irrelevant stimuli, such as what was presented in this study. This is also in line with the proposal that RNT impairs executive function operations via resource depletion (61,62).
In this framework, RNT would not be limited to impairing fear conditioning, because RNT may be linked to poor operation of new negative emotion information due to persistent occupation of resources by repetitively experiencing negative emotion through retrieval and reconsolidation (22). In this view, VH-RNT does not just set the stage for fear conditioning deficits but would also overburden the emotional memory system. Such a model would explain the attenuated activation in the brain areas involved in fear acquisition and the longer reaction times in depressed individuals with VH-RNT. Alternatively, considering that RNT emerges at a similar developmental stage as some anxiety disorders, it might also represent an initially adaptive strategy during adolescence (to cope with a series of adverse social scenarios), which becomes maladaptive in later life and thus facilitates the formation of depression symptoms. A causal inference could in theory be made by examining the effect of disrupting nodes of the fear conditioning circuit (19) on the intensity of RNT in the clinical setting. In this regard, novel noninvasive methods of neuromodulation could be used to safely explore this possibility without resorting to invasive neurosurgical techniques such as deep brain stimulation (63).
This study has several limitations. First, the case-control design limits the causal inferences that can be drawn from these associative results. Second, although the cohorts were closely matched on a number of confounding variables, the differences observed between VH-RNT and H-RNT participants could still be due to unobserved confounders. Third, the study sample was obtained from a single community cohort, which may limit the generalizability of its results; moreover, the propensity-matching process used herein could have been improved by additional selection of cases to improve the matching of the different groups. Whereas we did not use this additional procedure so that observer bias was avoided as much as possible and there were no statistical differences between groups, a better matching might have yielded different results. Fourth, interpretations about the effect of RNT on the function of reward and fear learning circuits are restricted to the clinical setting of major depression. A study focusing on interindividual variability in RNT in a sample of healthy individuals would be necessary to make general inferences about this relationship in normal conditions. Moreover, while statistically nonsignificant, the proportion of females in the HC group was lower than in the clinical samples, which mandates caution in the interpretation of results in this group. Finally, while outside the purview of this study, which used a categorical treatment of RNT, the dimensional characteristics of the associations described herein remain to be determined.
In conclusion, we observed abnormal neural signatures of both fear learning (decreased activation in subcortical components of the circuit involved in fear conditioning) and fear extinction (prolonged activation in the cortical component associated with fear learning during the early phase of extinction) in association with intensity of RNT in depression. In addition, widespread activation deficits in a well-defined reward circuit were found in MDD regardless of RNT level. We also highlighted the involvement of cerebellar components in reward processing in depression, previously not described in detail in clinical settings, to the extent of our knowledge. Based on these findings, we suggest that the causal relationship between disease process and circuit abnormalities could be discerned with the application of neuromodulation techniques targeting some critical nodes described herein. In addition, the confirmation of the transdiagnostic nature of RNT-related abnormalities described herein would necessitate a similar study carried out in patients with OCD who have RNT as a defining clinical manifestation; replication of these study results in a second subsample of the Tulsa 1000 database would also be desirable. Whether neuromodulation techniques can ultimately result in specific amelioration of RNT as an important clinical dimension of MDD with high recurrence, treatment resistance, and poor prognosis remains open to further investigations.
Supplementary Material
ACKNOWLEDGMENTS AND DISCLOSURES
This work has been supported in part by the William K. Warren Foundation, the National Institutes of Health/National Institute of Mental Health (Grant No. K23MH112949 [to SSK] and Grant No. K23MH108707 [to RLA]), and the National Institute of General Medical Sciences Center (Grant No. 1P20GM121312 [to MP]).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funder had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the manuscript; or in the decision to submit the paper for publication.
Footnotes
The Tulsa 1000 Investigators include the following contributors: Robin Aupperle, Ph.D.; Jerzy Bodurka, Ph.D.; Sahib S. Khalsa, M.D., Ph.D.; Jonathan Savitz, Ph.D.; Jennifer Stewart, Ph.D.; and Teresa A. Victor, Ph.D.
MP is an adviser to Spring Care, Inc., a behavioral health startup, and he has received royalties for an article about methamphetamine in UpToDate. All other authors report no biomedical financial interests or potential conflicts of interest.
ClinicalTrials.Gov: Latent Structure of Multi-level Assessments and Predictors of Outcomes in Psychiatric Disorders; https://clinicaltrials.gov/ct2/show/NCT02450240; NCT02450240.
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.bpsc.2022.01.002.
Contributor Information
Heekyeong Park, Laureate Institute for Brain Research, Tulsa, Oklahoma; Department of Psychology (HP), University of North Texas at Dallas, Dallas, Texas.
Namik Kirlic, Laureate Institute for Brain Research, Tulsa, Oklahoma.
Rayus Kuplicki, Laureate Institute for Brain Research, Tulsa, Oklahoma.
Tulsa 1000 Investigators, Laureate Institute for Brain Research, Tulsa, Oklahoma.
Martin Paulus, Laureate Institute for Brain Research, Tulsa, Oklahoma.
Salvador Guinjoan, Laureate Institute for Brain Research, Tulsa, Oklahoma.
REFERENCES
- 1.Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, et al. (2013): Global burden of disease attributable to mental and substance use disorders: Findings from the Global Burden of Disease Study 2010. Lancet 382:1575–1586. [DOI] [PubMed] [Google Scholar]
- 2.Nemeroff CB (2020): The state of our understanding of the pathophysiology and optimal treatment of depression: Glass half full or half empty? Am J Psychiatry 177:671–685. [DOI] [PubMed] [Google Scholar]
- 3.Insel TR (2009): Translating scientific opportunity into public health impact: A strategic plan for research on mental illness. Arch Gen Psychiatry 66:128–133. [DOI] [PubMed] [Google Scholar]
- 4.Ehring T, Watkins ER (2008): Repetitive negative thinking as a transdiagnostic process. Int J Cogn Ther 1:192–205. [Google Scholar]
- 5.Furman DJ, Gotlib IH (2016): Habenula responses to potential and actual loss in major depression: Preliminary evidence for lateralized dysfunction. Soc Cogn Affect Neurosci 11:843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shihata S, Johnson AR, Erceg-Hurn DM, McEvoy PM (2021): Measurement invariance of disorder-specific and transdiagnostic measures of repetitive negative thinking [published online ahead of print Jul 6]. Assessment. [DOI] [PubMed] [Google Scholar]
- 7.Wahl K, Ehring T, Kley H, Lieb R, Meyer A, Kordon A, et al. (2019): Is repetitive negative thinking a transdiagnostic process? A comparison of key processes of RNT in depression, generalized anxiety disorder, obsessive-compulsive disorder, and community controls. J Behav Ther Exp Psychiatry 64:45–53. [DOI] [PubMed] [Google Scholar]
- 8.Whitmer AJ, Gotlib IH (2012): Depressive rumination and the C957T polymorphism of the DRD2 gene. Cogn Affect Behav Neurosci 12:741–747. [DOI] [PubMed] [Google Scholar]
- 9.Whitmer AJ, Frank MJ, Gotlib IH (2012): Sensitivity to reward and punishment in major depressive disorder: Effects of rumination and of single versus multiple experiences. Cogn Emot 26:1475–1485. [DOI] [PubMed] [Google Scholar]
- 10.Knight MJ, Baune BT (2018): Executive subdomains are differentially associated with psychosocial outcomes in major depressive disorder. Front Psychiatry 9:309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spinhoven P, Drost J, van Hemert B, Penninx BW (2015): Common rather than unique aspects of repetitive negative thinking are related to depressive and anxiety disorders and symptoms. J Anxiety Disord 33:45–52. [DOI] [PubMed] [Google Scholar]
- 12.Nielson DM, Keren H, O’Callaghan G, Jackson SM, Douka I, Vidal-Ribas P, et al. (2021): Great expectations: A critical review of and suggestions for the study of reward processing as a cause and predictor of depression. Biol Psychiatry 89:134–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang S, Leri F, Rizvi SJ (2021): Anhedonia as a central factor in depression: Neural mechanisms revealed from preclinical to clinical evidence. Prog Neuropsychopharmacol Biol Psychiatry 110:110289. [DOI] [PubMed] [Google Scholar]
- 14.Alves-Pinto A, Rus OG, Reess TJ, Wohlschläger A, Wagner G, Berberich G, Koch K (2019): Altered reward-related effective connectivity in obsessive-compulsive disorder: An fMRI study. J Psychiatry Neurosci 44:395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dichter GS, Damiano CA, Allen JA (2012): Reward circuitry dysfunction in psychiatric and neurodevelopmental disorders and genetic syndromes: Animal models and clinical findings. J Neurodev Disord 4:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee DJ, Lozano CS, Dallapiazza RF, Lozano AM (2019): Current and future directions of deep brain stimulation for neurological and psychiatric disorders. J Neurosurg 131:333–342. [DOI] [PubMed] [Google Scholar]
- 17.Grover S, Nguyen JA, Viswanathan V, Reinhart RMG (2021): High-frequency neuromodulation improves obsessive-compulsive behavior. Nat Med 27:232–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lissek S, Powers AS, McClure EB, Phelps EA, Woldehawariat G, Grillon C, Pine DS (2005): Classical fear conditioning in the anxiety disorders: A meta-analysis. Behav Res Ther 43:1391–1424. [DOI] [PubMed] [Google Scholar]
- 19.Fullana MA, Harrison BJ, Soriano-Mas C, Vervliet B, Cardoner N, Àvila-Parcet A, Radua J (2016): Neural signatures of human fear conditioning: An updated and extended meta-analysis of fMRI studies. Mol Psychiatry 21:500–508. [DOI] [PubMed] [Google Scholar]
- 20.Wurst C, Schiele MA, Stonawski S, Weiß C, Nitschke F, Hommers L, et al. (2021): Impaired fear learning and extinction, but not generalization, in anxious and non-anxious depression. J Psychiatr Res 135:294–301. [DOI] [PubMed] [Google Scholar]
- 21.Young KS, Bookheimer SY, Nusslock R, Zinbarg RE, Damme KSF, Chat IKY, et al. (2021): Dysregulation of threat neurocircuitry during fear extinction: The role of anhedonia. Neuropsychopharmacology 46:1650–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guinjoan SM, Bär KJ, Camprodon JA (2021): Cognitive effects of rapid-acting treatments for resistant depression: Just adverse, or contributing to clinical efficacy? J Psychiatr Res 140:512–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drevets WC (2000): Neuroimaging studies of mood disorders. Biol Psychiatry 48:813–829. [DOI] [PubMed] [Google Scholar]
- 24.Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA (2001): Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: An fMRI study. Biol Psychiatry 50:651–658. [DOI] [PubMed] [Google Scholar]
- 25.Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS (2002): Can’t shake that feeling: Event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals [published correction appears in Biol Psychiatry 2002; 52: 771]. Biol Psychiatry 51:693–707. [DOI] [PubMed] [Google Scholar]
- 26.Dannlowski U, Ohrmann P, Bauer J, Kugel H, Arolt V, Heindel W, et al. (2007): Amygdala reactivity to masked negative faces is associated with automatic judgmental bias in major depression: A 3T fMRI study. J Psychiatry Neurosci 32:423–429. [PMC free article] [PubMed] [Google Scholar]
- 27.Koenigs M, Grafman J (2009): The functional neuroanatomy of depression: Distinct roles for ventromedial and dorsolateral prefrontal cortex. Behav Brain Res 201:239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Victor TA, Khalsa SS, Simmons WK, Feinstein JS, Savitz J, Aupperle RL, et al. (2018): Tulsa 1000: A naturalistic study protocol for multilevel assessment and outcome prediction in a large psychiatric sample. BMJ Open 8:e016620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nolen-Hoeksema S, Morrow J (1991): A prospective study of depression and posttraumatic stress symptoms after a natural disaster: The 1989 Loma Prieta earthquake. J Pers Soc Psychol 61:115–121. [DOI] [PubMed] [Google Scholar]
- 30.Park H, Forthman KL, Kuplicki R, Victor TA, Tulsa 1000 Investigators, Yeh HW, et al. (2021): Polygenic risk for neuroticism moderates response to gains and losses in amygdala and caudate: Evidence from a clinical cohort. J Affect Disord 293:124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keren H, O’Callaghan G, Vidal-Ribas P, Buzzell GA, Brotman MA, Leibenluft E, et al. (2018): Reward processing and depression: A conceptual and meta-analytic review across fMRI and EEG studies. Am J Psychiatry 175:1111–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schutter DJLG (2016): A cerebellar framework for predictive coding and homeostatic regulation in depressive disorder. Cerebellum 15:30–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heffley W, Hull C (2019): Classical conditioning drives learned reward prediction signals in climbing fibers across the lateral cerebellum. Elife 8:e46764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larry N, Yarkoni M, Lixenberg A, Joshua M (2019): Cerebellar climbing fibers encode expected reward size. Elife 8:e46870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hull C (2020): Prediction signals in the cerebellum: Beyond supervised motor learning. Elife 9:e54073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sendhilnathan N, Semework M, Goldberg ME, Ipata AE (2020): Neural correlates of reinforcement learning in mid-lateral cerebellum [published correction appears in Neuron 2020; 106:1055]. Neuron 106:188–198.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wagner MJ, Luo L (2020): Neocortex-cerebellum circuits for cognitive processing. Trends Neurosci 43:42–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tanaka M, Kunimatsu J, Suzuki TW, Kameda M, Ohmae S, Uematsu A, Takeya R (2021): Roles of the cerebellum in motor preparation and prediction of timing. Neuroscience 462:220–234. [DOI] [PubMed] [Google Scholar]
- 39.Takahashi M, Shinoda Y (2021): Neural circuits of inputs and outputs of the cerebellar cortex and nuclei. Neuroscience 462:70–88. [DOI] [PubMed] [Google Scholar]
- 40.Wagner MJ, Kim TH, Savall J, Schnitzer MJ, Luo L (2017): Cerebellar granule cells encode the expectation of reward. Nature 544:96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marr D (1969): A theory of cerebellar cortex. J Physiol 202:437–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boyden ES, Katoh A, Raymond JL (2004): Cerebellum-dependent learning: The role of multiple plasticity mechanisms. Annu Rev Neurosci 27:581–609. [DOI] [PubMed] [Google Scholar]
- 43.Schultz W, Dayan P, Montague PR (1997): A neural substrate of prediction and reward. Science 275:1593–1599. [DOI] [PubMed] [Google Scholar]
- 44.Heffley W, Song EY, Xu Z, Taylor BN, Hughes MA, McKinney A, et al. (2018): Coordinated cerebellar climbing fiber activity signals learned sensorimotor predictions. Nat Neurosci 21:1431–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kostadinov D, Beau M, Blanco-Pozo M, Häusser M (2019): Predictive and reactive reward signals conveyed by climbing fiber inputs to cerebellar Purkinje cells [published correction appears in Nat Neurosci 2020; 23:468]. Nat Neurosci 22:950–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wise RA, Rompre PP (1989): Brain dopamine and reward. Annu Rev Psychol 40:191–225. [DOI] [PubMed] [Google Scholar]
- 47.Kelley AE (2004): Ventral striatal control of appetitive motivation: Role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev 27:765–776. [DOI] [PubMed] [Google Scholar]
- 48.Tzschentke TM (2000): The medial prefrontal cortex as a part of the brain reward system. Amino Acids 19:211–219. [DOI] [PubMed] [Google Scholar]
- 49.Hoshi E, Tremblay L, Féger J, Carras PL, Strick PL (2005): The cerebellum communicates with the basal ganglia. Nat Neurosci 8:1491–1493. [DOI] [PubMed] [Google Scholar]
- 50.Middleton FA, Strick PL (2001): Cerebellar projections to the prefrontal cortex of the primate. J Neurosci 21:700–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A, Uchida N (2012): Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron 74:858–873. [DOI] [PubMed] [Google Scholar]
- 52.Beier KT, Steinberg EE, DeLoach KE, Xie S, Miyamichi K, Schwarz L, et al. (2015): Circuit architecture of VTA dopamine neurons revealed by systematic input-output mapping. Cell 162:622–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Westen M, Rietveld E, Figee M, Denys D (2015): Clinical outcome and mechanisms of deep brain stimulation for obsessive-compulsive disorder. Curr Behav Neurosci Rep 2:41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rouhani N, Wimmer GE, Schneier FR, Fyer AJ, Shohamy D, Simpson HB (2019): Impaired generalization of reward but not loss in obsessive-compulsive disorder. Depress Anxiety 36:121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Graat I, Mocking RJT, de Koning P, Vulink N, Figee M, van den Munckhof P, et al. (2021): Predicting response to vALIC deep brain stimulation for refractory obsessive-compulsive disorder. J Clin Psychiatry 82:20m13754. [DOI] [PubMed] [Google Scholar]
- 56.Wager TD, van Ast VA, Hughes BL, Davidson ML, Lindquist MA, Ochsner KN (2009): Brain mediators of cardiovascular responses to social threat, part II: Prefrontal-subcortical pathways and relationship with anxiety. Neuroimage 47:836–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saper CB (2002): The central autonomic nervous system: Conscious visceral perception and autonomic pattern generation. Annu Rev Neurosci 25:433–469. [DOI] [PubMed] [Google Scholar]
- 58.Joo B, Koo JW, Lee S (2020): Posterior parietal cortex mediates fear renewal in a novel context. Mol Brain 13:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Post RM, Kegan R (2017): Prevention of recurrent affective episodes using extinction training in the reconsolidation window: A testable psychotherapeutic strategy. Psychiatry Res 249:327–336. [DOI] [PubMed] [Google Scholar]
- 60.Schwert C, Aschenbrenner S, Weisbrod M, Schröder A (2017): Cognitive impairments in unipolar depression: The impact of rumination. Psychopathology 50:347–354. [DOI] [PubMed] [Google Scholar]
- 61.Watkins E, Brown RG (2002): Rumination and executive function in depression: An experimental study. J Neurol Neurosurg Psychiatry 72:400–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Philippot P, Brutoux F (2008): Induced rumination dampens executive processes in dysphoric young adults. J Behav Ther Exp Psychiatry 39:219–227. [DOI] [PubMed] [Google Scholar]
- 63.Meng Y, Hynynen K, Lipsman N (2021): Applications of focused ultrasound in the brain: From thermoablation to drug delivery. Nat Rev Neurol 17:7–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


