Abstract
Purpose:
This aim of this study was to evaluate the clinical efficacy in individuals with recently healed venous leg ulcers (VLU) and diabetic foot ulcers (DFU) on pain reduction and physical activity improvement.
Design:
6-month longitudinal randomized controlled trial.
Subjects and Setting:
The sample comprised 140 individuals with previously healed VLU and DFU who received care in 3 outpatient wound centers in the Southeastern region of the United States. Participants were randomized to the MUSTCOOL or a placebo cooling patch intervention.
METHODS:
The cooling and placebo interventions comprised cooling or cotton-filled pack application to recently healed skin for 30 minutes, 3 times weekly plus standard of care including compression and leg elevation (participants with VLU) or therapeutic footwear and hygiene (participants with DFU) over a 6-month period. Pain severity and intensity were measured with the Brief Pain Inventory and physical activity with the International Physical Activity Questionnaire which assessed metabolic equivalent of task (METS) in minutes per week. Minutes in walking time per week were assessed with an accelerometer. Data were descriptively analyzed for difference changes in scores from baseline to 6 months post intervention.
RESULTS:
Data were analyzed for 81 participants randomized to cooling and placebo groups (VLUs n = 26/29) and DFU (n = 12/16). Slight reductions in VLU pain severity (−0.5, −0.2) and interference (−0.4, −0.5) and minimal reductions in DFU pain severity (0, −0.1) and interference (0.4/0.1) were achieved. However, pain scores were low to moderate at baseline (mean 4, 0 – 10 with ten worst pain possible) in both groups. For physical activity, the METS values showed low physical activity in both groups at baseline with slight improvements noted in VLU cooling and placebo groups (73/799) and DFU (1921/225) respectively. Walking time for the VLU groups improved by 1420/2523 minutes; the DFU groups improved 135/157 minutes respectively. Findings for outcomes were not statistically significant within or between groups.
CONCLUSIONS:
Application of the cooling pack compared to placebo was minimally efficacious in reducing post healing pain and improving function in this post-healed ulcer population. However for pain, scores were initially low thus outcomes on pain, while lower, were marginal.
Trial registration:
The study was prospectively registered with ClinicalTrials.gov on December 10, 2015 (Identifier: NCT02626156) https://clinicaltrials.gov/ct2/show/NCT02626156.
Keywords: Cooling, venous, diabetes, ulcers, pain, physical activity
Introduction
Chronic ulcers affect 6.5 million individuals, and of these, 5 million suffer with ulcers of the lower extremities.1 Often related to diabetes, vascular and/or neuropathic conditions, or infection, approximately 50% of these ulcers take longer than 4 weeks to heal.2 After healing, lower extremity ulcers have extremely high recurrence rates: 70 – 96% of VLUs reoccur within 2 to 3 months of healing3 while 40% of DFUs reoccur within 1 year.4 Leg and foot ulcers represent a substantial portion of the health care cost burden associated with treatment, conservatively estimated to be nearly $32 billion among Medicare beneficiaries receiving care in outpatient settings.5 This cost does not account for home-based care or out-of-pocket supplies purchased by individuals. Care of DFUs alone can increase costs by $9 – $13 billion in excess of typical diabetes mellitus care costs, which are estimated to increase to $2.1 - $2.4 trillion in 2030 from $1.3 trillion in 2015.6 These economic figures do not account for the costs of human suffering and negative impact on physical activity and quality of life.7,8 Health care costs and human suffering are increased when ulcers become chronic and recalcitrant to treatment. Unfortunately, incidence, prevalence and morbidity associated with chronic ulcers are expected to increase as a result of the growing aging population suffering with vascular disorders, diabetes and obesity.2 Further, impaired functional status of these patients often limits wound self-management and can hamper treatment effectiveness, reducing healing outcomes.
Research on chronic wound populations show high physical and functional morbidity. Findings from studies of physical complaints show pain affects between 48% to 94% of individuals seeking wound care in outpatient and community nursing clinics.9–11 It is a widely held belief that the sensory neuropathy that often accompanies foot ulcers leads to an “insensate” foot; paradoxically, pain is present in up to 75% of individuals with diabetes-related foot ulcers.12 Pain and other uncomfortable symptoms such as itch, tingling and achiness are also prevalent in patients with healed ulcers.13 Research also shows a high number of individuals with VLUs and DFUs are physically de-conditioned, less physically active, have poor balance and gait abnormalities, and are slower walkers.14,15 These individuals are at high risk for falling and sustaining injuries16 and have poor health related quality of life owing to a number of factors including pain, decreased physical function, social isolation, and wound severity.17,18,11
While non-pharmacologic interventions such as exercise and physical activity play a role in ulcer prevention and healing19, our research focuses on a patient population at highest of risk for developing ulcers; those with recently healed ulcers. These patients often experience other distressing symptoms such as pain and have low physical activity levels.20 The intervention framework selected for this study is based on cooling theory and its influence on pain reduction, espousing that skin over the healed ulcer site is highly susceptible to re-ulceration due to numerous factors including poor tissue remodeling and aberrant inflammation from venous and diabetic disease processes that can elevate skin temperature and subsequently induce chronic pain.21,22 Cooling has been found to decrease foot and leg temperatures in patients with chronic wounds; it also postulated to mitigate metabolic demands and abnormal cytokine production preventing tissue autolysis, along with reducing oxidative stress by increasing total antioxidant status.23,24
There are also benefits in reducing pain through affecting nociceptor function and slowing nerve transmission velocity in pain fibers.13,25 Pain, in particular, is known to negatively influence physical activity in patients with chronic venous disease.26 In an attempt to address pain and physical activity, we developed a topically-applied cooling patch as part of our MUSTCOOL intervention and posited that if pain was reduced, individuals would feel better and become more physically active. To explore this assumption, we evaluated the efficacy of the MUSTCOOL intervention to determine whether it prevents ulcer recurrence in individuals with recently healed VLUs or DFUs when compared to a placebo (non-cooling) patch.27
Methods
A longitudinal randomized-controlled trial design guided data collection and analysis. The target population comprised consented individuals with VLUs (n = 82) or DFUs (n = 58) that had healed up to six weeks prior to study enrollment. Patients were recruited through wound center referrals and advertisements placed in outpatient clinics in two U.S. Southeastern states. Inclusion criteria were 18 years of age and older, ankle brachial index (ABI) 0.8–1.3mmHg to confirm artery blood flow sufficiency, English speaking, and agreed to attend four study visits. Exclusion criteria were cognitive impairment measured with the Mini-Cog28 (score of ≤2 with an abnormal clock draw), inflammatory or vascular conditions such as cold hypersensitivity, chronic regional pain syndrome, Lupus erythematosus, multiple sclerosis, receiving chemotherapy, or required physical assistance to perform study-related procedures.
Study Procedures
A permuted block randomization strategy developed by the study statistician was used to assign participants with newly healed VLUs and DFUs to either the cooling or placebo group. Randomization was stratified by ulcer type. The principal investigator (TJK) and external consultants were masked to treatment assignment. To minimize the likelihood that the mask could be broken (e.g., the next treatment assignment could be guessed), the block size was varied. At baseline, the research assistant consented, determined eligibility, enrolled, collected baseline data such as demographics, pain and physical activity, then instructed participants on treatment procedures and provided all study supplies (e.g., patches). The primary outcomes were pain and physical activity. For the analysis of continuous efficacy outcome measures (change from baseline) we had 85% power to detect a difference of 0.59s (Cohen’s d) between the treatment and control groups for VLU (80/group) and DFU (60/group) assuming α = 0.05 (Type I error rate), two sided independent sample t-test comparison of means and equality of variance between groups. Occurrence of VLU is twice that of DFU, hence the differences is sample size determination. Figure 1 CONSORT diagram shows enrollment, allocation, and follow-up of participants.
Figure 1.
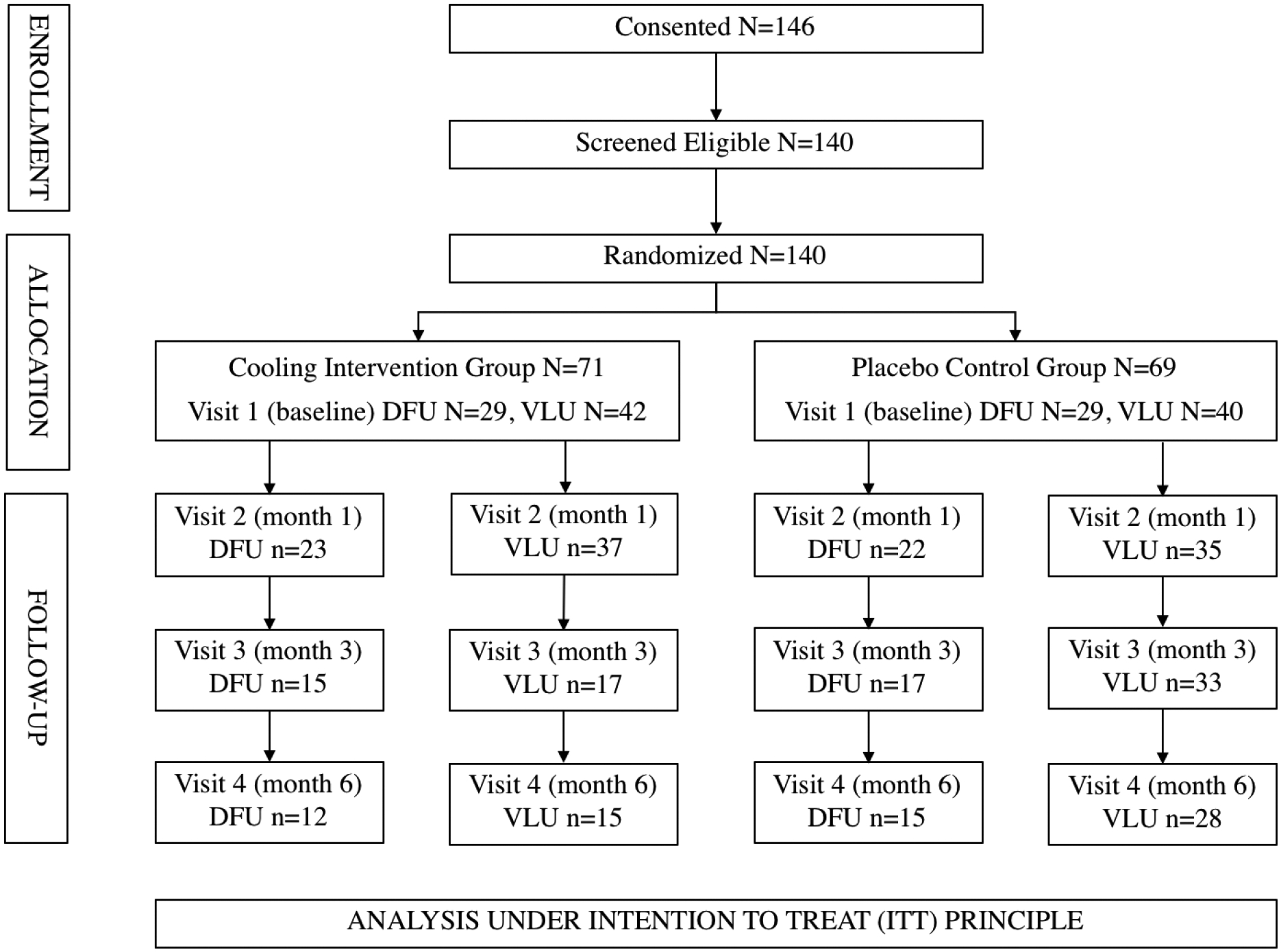
Participant flow-diagram
Abbreviations:
VLU – Venous Leg Ulcer
DFU – Diabetic Foot Ulcer
ITT – Intention-to-treat
The Medical University of South Carolina institutional review board (study #Pro00064441) approved this research conducted between July 2016 and March 2020. The study conformed to the Declaration of Helsinki and was conducted in compliance with Good Clinical Practices (GCP) of the International Conference of Harmonization (ICH). Participants provided written informed consent, received $250 (USD) in compensation and were informed that all data would remain confidential in a password protected database Research Electronic Data Capture (REDCap) (https://www.project-redcap.org/).
Intervention
The self-management components of the MUSTCOOL intervention were completed by participants in both the cooling and placebo groups. Prophylactic maintenance component consisted of patch application (glycerin cooling or cotton-filled placebo) at a consistent time (e.g. 7 PM) for 30 minutes to the affected leg or foot skin with legs elevated. The dosing for prophylactic maintenance was three times each week. In addition, a preventive bolus was administered over five consecutive days when increased temperature was measured via a skin thermometer for two consecutive days above baseline was noted. The cooling patch (Therapy Pack, Elastogel, Southwest Technologies, Kansas City, MO) was a 3 × 5 flexible, half-inch thick glycerin-based sheet hydrogel that did not freeze to a solid state. We previously conducted extensive safety testing of the material without adverse events (e.g., skin injury).29 The placebo patch was identical to the glycerin patch but was cotton filled (manufactured by same company). Both patches were covered with a protective polyethylene fabric that could be laundered and stored in the freezer. Participants received freezer thermometers to make sure freezer temperature was maintained at 0°F. Once removed from the freezer, the placebo patch warmed to room temperature within 2 minutes. The patches were anchored to the site with a tubular retainer made of an expandable elastic latex-free stretch net. Participants recorded the date and times the patch was applied on a written log. The other MUSTCOOL component was self-use of standard of care; the VLU group wore compression stockings and elevated legs and the DFU group wore therapeutic footwear, per their healthcare provider recommendations.
Participants were asked to stop applying the patch and contact study personnel immediately if the ulcer returned or if a new ulcer developed elsewhere on the participant’s leg or foot. During study month 1, weekly supportive phone calls were made to participants by study personnel, and then bi-monthly thereafter. These calls included fidelity monitoring for adherence to the patch application procedure, protocol deviations, and adverse events.
Data Collection and Outcome Measures
Demographic characteristics measured were age, race, sex, education level, employment status, type of job, depression measured with the Geriatric Depression Scale30, weight, and body mass index (kg/m2). Pain was measured with the Brief Pain Inventory (BPI)31 (Cronbach’s α .88) and reported as change in worst and least pain, and pain right now from baseline to end of study at 6 months. The BPI also assesses pain severity and intensity with an average score calculated for both subscales: Pain severity – 4 items rated from 0 – no pain to 10 – pain as bad as you can imagine; and Pain Interference – 7 items rated from 0 - does not interfere to 10 - completely interferes. Higher scores indicate worse interference and severity. We also collected data on pain descriptors common in patients with venous disease and diabetes/neuropathic disorders such as ache, burning and numbness sensations.
Physical activity was assessed using the patient-reported International Physical Activity Questionnaire (IPAQ)32 (pooled Spearman R 0.81) calculated as metabolic equivalent of task (METS) minutes per week. The total METs is the sum of MET minutes per week spent walking, moderate intensity activity and vigorous intensity minutes (with the different activities weighted differently). Scoring of high, moderate or low physical activity levels are based on time and intensity of various types of activity. Objective physical activity data were captured using the Yamax Power Walker EX-510 3D accelerometer (Yamax Corp., Tokyo, Japan), a user-friendly activity tracker that recorded daily steps and time/duration. The Yamax pedometer has undergone validity studies and has also shown to be accurate in measuring physical activity among older adults.33,34 These three outcomes were assessed at baseline, and months 1, 3 and 6 during study visits at the Research Nexus, study clinics or during home visits.
Data Analysis
The primary efficacy outcomes were changes in pain and physical activity from baseline to the primary endpoint at 6 months post-baseline (continuous). In analysis of the continuous efficacy outcome measures, we compared unadjusted mean differences in pain (BPI scores) for three individual subscores “worst pain”, “least pain” and “pain right now” and composite scores (mean of subscores) via paired t-tests (or equivalently non-parametric Wilcoxon Signed-rank test) between the (cooling vs. placebo) groups for VLU and DFU participants who provided both baseline and 6-months measures. Physical activity in MET minutes per week, captured by IPAQ, was calculated as MET at 6 months minus MET at baseline and were computed as per instructions (www.ipaq.ki.se); negative numbers indicate a decrease in METs. Median MET minutes were compared via quantile regression, while Fisher’s exact tests were used to compare physical activity categories between the groups and General linear models were used for comparison of change in steps via accelerometer counts and activity time over 6 months. All statistical analyses were performed using SAS Statistical Software Version 9.4 (Copyright © 2016 by SAS Institute Inc., Cary, NC, USA). A two-sided P < .05 was considered statistically significant.
Results
The samples sizes were 15 and 28 in intervention and placebo control groups for healed VLUs and 12 and 15 for healed DFUs, respectively. Demographic data are shown in Table 1. The VLU group was slightly older (mean age 64.1 years) than the DFU group (58.4 years). The majority of the DFU group (75.9%) were males compared to the VLU group (50.7%). Most participants in both groups were married, had a least a high school education, were retired, were obese (BMI > 30) and had average scores on the GDS less than 2, indicating no depression/normal. No significant differences were noted in any of the demographic characteristics.
Table 1.
Comparison of Baseline Demographic Characteristics between Two Groups.
| Ulcer Type | Venous Leg Ulcer | Diabetic Foot Ulcer | ||
|---|---|---|---|---|
| Characteristic | Placebo (n = 40) | Cooling (n = 42) | Placebo (n = 29) | Cooling (n = 29) |
| Age (years, M
±
SD) Median [Q*1 - Q3] |
64.3 ± 12.4 66 [55–72] |
63.9 ± 14.6 63 [55–72] |
59.4 ± 16.1 60 [53–70] |
57.4 ± 11.7 60 [53–63] |
| Sex (n, %) | ||||
| Male | 23 (56.1) | 19 (45.2) | 20 (69.0) | 24 (82.8) |
| Female | 18 (43.9) | 23 (54.8) | 9 (31.0) | 5 (17.2) |
| Race (n, %) | ||||
| Black or African American |
15 (36.6) | 20 (47.6) | 5 (17.2) | 8 (27.6) |
| White | 26 (63.4) | 21 (50.0) | 24 (82.8) | 20 (69.0) |
| Ethnicity (n, %) Hispanic or Latino |
— | 3 (7.1) | 1 (3.4) | 3 (10.3) |
| Marital status (n, %) | ||||
| Not married | 19 (46.4.) | 7 (16.7) | 1 (3.4) | 4 (13.8) |
| Married | 22 (53.7) | 21 (50.0) | 18 (62.1) | 18 (62.1) |
| Education level (n, %) | ||||
| 8th grade or less | 1 (2.4) | 3 (7.1) | — | — |
| High school graduate | 11 (26.8) | 17 (40.5) | 9 (31.0) | 14 (48.3) |
| College graduate | 15 (36.6) | 5 (11.9) | 5 (17.2) | 1 (3.4) |
| Current employment status (n, %) | ||||
| Employed full-time | 10 (24.4) | 12 (28.6) | 9 (31.0) | 4 (13.8) |
| Employed part-time | 3 (7.3) | 1 (2.4) | 1 (3.4) | 2 (6.9) |
| Not employed/retired | 29 (65.8) | 27 (64.3) | 19 (65.5) | 23 (79.3) |
| Current or former job (n, %) | ||||
| Professional | 20 (48.8) | 14 (33.3) | 8 (27.6) | 8 (27.6) |
| Technical | 7 (17.1) | 6 (14.3) | 6 (20.7) | 5 (17.2) |
| Manual | 14 (34.1) | 19 (45.2) | 15 (51.7) | 15 (51.7) |
| GDS total score | 2.0 ± 2.4 | 2.0 ± 2.4 | 1.1 ± 1.2 | 2.1 ± 1.7 |
| Body mass index ((kg/m2, n, %) Median [Q1 - Q3] |
37.1 ± 10.0 37.9 [30.7 – 43.1] |
34.3 ± 10.9 33.6 [26.9 – 41.0] |
32.8 ± 6.7 33.0 [27.5 – 35.6] |
34.7 ± 9.7 33.6 [28.5 –38.4] |
| Is this a recurrent ulcer? (yes, n, %) | 19 (46.3) | 24 (57.1) | 5 (17.2) | 15 (51.7) |
Note: GDS = Geriatric Depression Scale;
Interquartile range
Pain
At baseline (Table 2), there were no significant differences between group composite scores for pain severity (VLU placebo control 0.6, and cooling intervention 0.8; DFU placebo 0.3, intervention 1.0 respectively) or pain interference (VLU placebo control 0.7, intervention 0.7; DFU 0.3, intervention 0.8 respectively). More healed VLU participants endorsed having discomfort/pain from the healed ulcer over the past month at baseline; 38.1% in the VLU placebo group and 48.8% in the intervention group compared to 24.1% with healed DFUs in the placebo group and 37.9% in the intervention group. Few individuals in both groups were taking medications or receiving treatments at baseline for pain. At baseline, the commonly reported pain descriptors reported by 5 or more individuals for 11 symptoms in either placebo or intervention groups for healed VLUs included aching, burning, numb, sharp pain, shooting pain, tenderness and throbbing. Aching, sharp shooting and throbbing symptoms were found in the healed DFU intervention or placebo groups. At study completion, no pain descriptors had more than 5 participant reports (Table 3). Table 3 shows comparisons of changes/differences in scores from baseline to 6-month end of study for the BPI worst pain, least pain and pain right now items. Difference scores showed slight reductions (scored as “a little bit” with negative scores indicating improvement) between placebo vs. intervention groups for healed VLU pain severity (−0.5) and interference −0.9) and healed DFU pain severity (−0.3) and interference (−0.2) respectively; none were statistically significantly different (all p > .16).
Table 2.
Baseline Pain and Physical Activity Between Two Groups.
| Ulcer Type | Venous Leg Ulcer | Diabetic Foot Ulcer | ||
|---|---|---|---|---|
| Characteristic | Placebo (n = 40) | Cooling (n = 42) | Placebo (n = 29) | Cooling (n = 29) |
| Brief Pain Inventory | ||||
| Discomfort or pain from healed ulcer over past month (yes n, %) | 16 (38.1) | 20 (48.8) | 7 (24.1) | 11 (37.9) |
| Worst amount of pain from healed ulcer in last 24 hours (0 = none – 10 = worst possible), (M ± SD) | 1.1 ± 2.6 | 1.4 ± 2.6 | 0.6 ± 1.6 | 1.5 ± 2.8 |
| Least amount of pain from healed ulcer in last 24 hours (0 = none – 10 = worst possible, M ± SD) | 0.3 ± 1.1 | 0.5 ± 1.3 | 0.2 ± 0.8 | 0.8 ± 1.9 |
| Pain right now (0 = none - 10 = worst possible, M ± SD) | 0.3 ± 0.9 | 0.5 ± 1.4 | 0.3 ± 1.1 | 0.8 ± 1.9 |
| Taking medications or receiving treatments for pain from healed ulcer (yes n, %) | 3 (7.7) | 0 | 0 | 2 (7.1) |
| Pain Severity composite score (M ± SD) | 0.6 ± 1.5 | 0.8 ± 1.7 | 0.3 ± 1.1 | 1.0 ± 2.1 |
| Pain Interference composite score (M ± SD) | 0.7 ± 1.9 | 0.7 ± 1.6 | 0.3 ± 1.2 | 0.8 ± 1.8 |
| International Physical Activity Questionnaire (IPAQ)* | ||||
| Total activity (min/week) – truncated (median, [25th;75th quartile] | 195 [35 – 380] | 180 [120 – 360] | 158 [93 – 315] | 60 [20 – 290] |
| Walk MET minutes per week (median, [25th;75th quartile] | 1386 [792 – 3168] | 1980 [792 – 4752] | 1114 [990 – 2970] | 1650 [116 – 3564] |
| Moderate MET minutes per week (median, [25th;75th quartile] | 1920 [240 – 6720] | 3840 [1680 – 5280] | 1000 [480 – 3000] | 300 [180 – 1620] |
| Vigorous MET minutes per week (median, [25th;75th quartile] | 960 [480 – 2880] | 8640 [3600 – 12480] | 1080 [360 – 1200] | 320 [160 – 480] |
| Total MET minutes per week (median, [25th;75th quartile] | 2514 [480 – 8160] | 2544 [1188 – 10512] | 2735 [1389 – 5460] | 699 [240 – 4480] |
| Health-enhancing physically active | 7 (17.5) | 6 (14.3) | 4 (13.8) | 2 (6.9) |
Values for all continuous IPAQ scores expressed as median, [25th;75th quartile] as per scoring instructions (www.ipaq.ki.se)
Table 3.
Comparison of Change from Baseline to 6-month Follow-Up in Pain and Physical Activity by Two Groups.
| Ulcer Type | Venous Leg Ulcers | |||
|---|---|---|---|---|
| Characteristic | Placebo (n = 29) | Cooling (n = 26) | Difference between groups (Δ) | P value |
| Brief Pain Inventory | ||||
| Discomfort/pain from healed ulcer over past month1 | 4 (13.8) | 6 (23.1) | −0.1 ± 0.1 (−0.3; 0.1) | .490a |
| Worst amount of pain from healed ulcer in last 24 hours (0 = none; 10 = worst possible)2 | 0.4 ± 2.5 (−0.6; 1.3) | 0.9 ± 1.8 (−0.1; 1.6) | −0.5 ± 2.2 (−1.6; 0.7) | .253b |
| Least amount of pain from healed ulcer in last 24 hours (0 = none; 10 = worst possible)2 | 0 ± 1.6 (−0.6; 0.6) | 0.3 ± 1.2 (−0.2; 0.8) | −0.3 ± 1.4 (−1.0; 0.5) | .517b |
| Pain right now (0 = none; 10 = worst possible) 2 | 0.1 ± 1.1 (−0.3; 0.5) | 0.4 ± 1.1 (−0.1; 0.9) | −0.3 ± 1.1 (−0.9; 0.3) | .659b |
| Pain severity2 | −0.2 ± 1.6 (−0.8; 0.4) | −0.5 ± 1.3 (−1.0; 0.01) | 0.3 ± 1.4 (−0.5; 1.1) | .211b |
| Pain interference2 | −0.5 ± 1.6 (−1.1; 0.1) | −0.4 ± 1.1 (−0.8; 0.1) | −0.2 ± 1.4 (−0.9; 0.6) | .900b |
| International Physical Activity Questionnaire (IPAQ) | ||||
| Total MET minutes per week3 | 3686 [594 – 7008] n = 14 | 947 [0 – 7740] n = 7 | 1788 ± 2017 (−2225; 5801) | .378c |
| Physical Activity Category (IPAQ)1 | ||||
| Remaining inactive | 18 (62.1) | 20 (76.9) | 0.2 ± 0.1 (−0.1; 0.4) | .260a |
| Improving activity level | 5 (17.2) | 3 (11.5) | −0.1 ± 0.1 (−0.2; 0.1) | .708a |
| Yamax Accelerometer2 | ||||
| Steps | 14234 ± 3750 (6874; 21593) | 17295 ± 4094 (9259; 25332) | −3062 ± 5552 (−13959; 7835) | .582d |
| Time | 2523 ± 1675 (−765; 5811) | 1420 ± 1970 (−2448; 5288) | −1103 ± 2586 (−3974; 6180) | .670d |
| Ulcer Type | Diabetic Foot Ulcers | |||
| Characteristic | Placebo (n = 16) | Cooling (n = 12) | Difference between groups Δ | p |
| Brief Pain Inventory | ||||
| Discomfort/pain from healed ulcer over past month1 | 3 (18.8) | 2 (16.7) | 0.02 ± 0.1 (−0.3; 0.3) | 1.0a |
| Worst amount of pain from healed ulcer in last 24 hours (0 = none; 10 = worst possible)2 | −1.0 ± 2.4 (−2.9; 0.9) | 0 ± 2.5 (−1.6; 1.6) | −1.0 ± 2.4 (−2.9; 0.9) | .093b |
| Least amount of pain from healed ulcer in last 24 hours (0 = none; 10 = worst possible)2 | 0 ± 0 (0; 0) | −0.1 ± 1.1 (−0.8; 0.6) | 0.1 ± 0.7 (−0.6; 0.8) | 1.0b |
| Pain right now (0 = none; 10 = worst possible)2 | −0.1 ± 0.5 (−0.4; 0.1) | 0.1 ± 0.3 (−0.1; 0.3) | −0.2 ± 0.4 (−0.5; 0.1) | .188b |
| Pain severity2 | 0.4 ± 0.9 (−0.1; 0.9) | 0 ± 0.9 (−0.5; 0.5) | 0.4 ± 0.9 (−0.3; 1.1) | .166b |
| Pain interference2 | 0.1 ± 0.5 (−0.2; 0.3) | −0.1 ± 0.2 (−0.2; 0.1) | 0.1 ± 0.4 (−0.2; 0.5) | .269b |
| Physical Activity Category (IPAQ) | ||||
| Remaining inactive | 15 (93.8)1 | 9 (75.0)1 | −0.2 ± 0.1 (−0.5; 0.1) | .285a |
| Improving activity level | 0 (0)1 | 1 (8.3)1 | 0.08 ± 0.08 (−0.07; 0.2) | .429a |
| Yamax Accelerometer2 | ||||
| Steps | 15479 ± 5019 (5614; 25343) | 13232 ± 5252 (2909; 23555) | 2246 ± 7265 (12032; 16525) | .757d |
| Time | 157 ± 52 (55; 260) | 135 ± 55 (28; 242) | 23 ± 75 (126; 171) | .670d |
Note. All values expressed as 1n (%) and risk difference ± asymptotic standard error (95% confidence interval), or 2mean ± standard deviation (95% confidence interval) and difference in means ± standard deviation (95% confidence interval) or 3median, [25th;75th quartile] as per IPAQ author instructions and difference in medians ± standard error (95% confidence interval)
p obtained from Fisher’s exact test
p obtained from Wilcoxon rank sum test
p obtained from quantile regression
p obtained from General linear models
Physical activity
Few participants completed both baseline and 6-month follow-up for the IPAQ (Table 3). Difference changes in MET minutes per week were low (several were 0) for the placebo and cooling groups for both healed VLU and DFU participants; no significant differences were noted. At baseline (Table 2), participants largely fell into the inactive/sedentary activities category with median 2544 MET minutes for VLU cooling group n = 34/42 (81.0%) versus 2514 METs in the control n = 29/40 (72.5%). Similarly, findings indicated 699 vs 2735 MET minutes for DFU n = 26/48 (89.7%) and n = 22/29 (75.9%) control groups respectively. Steps taken for both VLU and DFU placebo and intervention groups showed no statistically significant differences in average steps over 6 months; however improvements were noted in both VLU groups, although minimal. Accelerometer use was more consistent for both groups compared to completion of the IPAQ. Overall, 47 out of the VLU sample used the accelerometer between 3 and 24 weeks, 4 used it 3–5 weeks, 12 used it 11–20 weeks and 31 used it 21–24 weeks. Among controls, 6 used it 11–19 weeks, 17 used it 21–24 weeks; among the intervention group, 4 used it 3–5 weeks, 6 used it 12–20 weeks and 14 used it 21–24 weeks. Overall, 30 out of the DFU sample used the accelerometer between 3 and 24 weeks; 6 used it 3–9 weeks, 9 used it 11–16 weeks and 15 used it 23 or 24 weeks. Among controls, 2 used it 9 weeks, 4 used it 13–16 weeks and 8 used it 23–24 weeks; among the intervention group, 4 used it 3–10 weeks, 5 used it 11–14 weeks and 7 used it 24 weeks.
Discussion
We studied individuals with recently healed venous and diabetic/neuropathic foot ulcers and compared pain and physical activity outcomes using a cooling intervention developed to prevent ulcer recurrence and mitigate negative symptoms post healing versus a sham patch. We targeted this population because rates of ulcer recurrence associated with VLUs and DFUs are high and in response developed our MUSTCOOL leg and foot ulcer prevention intervention27 as a self-management approach that was developed to reduce the incidence of re-ulceration and reduce subsequent negative physical symptoms, including pain. Conventional prevention treatments include leg compression, proper footwear, and underlying disease management35; however, there has been much less focus on self-management approaches to mitigate negative symptoms such as pain that can further impair functional outcomes in population with chronic conditions. We did not find substantial changes in pain after leg and foot ulcers healed. Pain severity and interference, while endorsed, were minimal at baseline, even less so for the DFU group.
Pain is a common physical complaint often associated with chronic wounds and has been found to be as high at 94% in a study of 73 patients seeking care in a vascular clinic11 and ranged from 48 to 81% (average 74%) in a study of symptom clusters in 318 patients with venous leg ulcers recruited from hospital outpatient and community nursing clinics.36 Paradoxically, pain has been found in up to 75% of individuals with diabetes-related foot ulcers.12 It is a widely held belief that sensory neuropathy often accompanies DFUs, leading to an “insensate” foot or lack of sensation; however, this claim is unsupported. We surmised pain would continue post healing, but this was not the case and we did not have data about pain experienced while having the ulcer. Unfortunately we were unable to find studies that reported pain specifically after wound healing to draw comparisons with our findings.
Our data suggest a large portion of the VLU and DFU groups are inactive; this finding is consistent with prior research that shows a high number of individuals with chronic ulcers are physically de-conditioned, less active, possess poor balance and gait abnormalities, and are slower walkers.14,15 In a study of 106 individuals with VLUs, 60 (44%) reported falling during the study period and 47 (78%) had a history of repeated falls.16 In a study of physical function of individuals with DFUs presenting for surgery to treat infection, 70% (n = 49/92) scored 2 standard deviations below the U.S. population mean on Patient Reported Outcomes Measurement Information System (PROMIS) for physical function.37 We measured physical activity with the IPAQ that measured MET minutes per week at baseline and end of study. Few individuals improved in their overall activity level for METs (minutes per week); however, few participants completed the questionnaire at 6-month end of study. One MET is defined as approximately 1 kcal/kg/hour and includes activities such as sleeping, lying quietly or meditating.38 Activities achieving levels of moderate-intensity physical activity typically include brisk walking, swimming leisurely, or cycling leisurely, whereas activities achieving vigorous-intensity physical activity include jogging, cycling fast, or playing a game of football. Sedentary activities typically includes lying, sitting, eating or standing quietly.39
We observed good adherence (more than 60% of completers used it through study completion) to accelerometer use for steps taken and time engaged in walking; steps taken and time did improve in the VLU group more so than the DFU group, but improvements were noted. Anecdotally during end of study interviews, participants said they liked having feedback from the device because it helped “motivate” them to walk more. Both study participant groups largely fell into the inactive/sedentary activities category. While minimal gains were achieved among some participants, we assert that even small increases such as 10 additional minutes of walking or an additional 350 to 1100 steps/day may suggest clinically important improvements in populations with chronic diseases such as COPD.40 We also acknowledge that additional research is needed to support the clinical relevance of the time and step counts in a post-healing ulcer population.
While physical activity and other non-pharmacologic interventions play a role in wound healing, our research focused on prevention of VLU and DFU recurrence using a topically-applied cooling pack to augment standard of care that included compression, leg elevation, and therapeutic footwear. Consistent inspection, skin care, and trauma prevention over the healed site are critical nursing care recommendations necessitated by the fact that during the skin closure period, the ulcer continues to remodel during the final stage of healing. Evidence suggests that aberrant inflammation may play a role in poor remodeling in individuals with venous disease and/or diabetes, placing the healed area at risk for re-ulceration.
Strengths and Limitations
The strengths of our study include the use of a well-established, self-management approach that incorporates self-report, monitoring and application of an evidence-based cooling intervention intended to augment standard of care. To enhance rigor, we selected a randomized controlled trial and implemented consistent fidelity monitoring. This study is our second RCT of the MUSTCOOL intervention. In the first, we provided standard of care devices and supplies that included compression wraps, leg elevator pillows, and skin care products as part of the study intervention. In this second trial, a more “real world” approach was used in which we instructed participants to “do what your health care provider prescribed/recommended” after the ulcer healed. Weaknesses include our inability to accurately assess adherence to standard of care which could have influenced findings, such as those who adhere less than 85% of the time to the treatment protocol would experience a less robust response. Anecdotally we observed during study visits that many of the study participants did not consistently wear compression stockings or therapeutic footwear which may have influenced their overall disease management outcomes such as edema, thus limiting the efficacy of our intervention. Our sample sizes were small and there were fewer participants with DFUs, which we anticipated (~70 – 80% of all lower extremity ulcers are VLUs while less than 30% are DFUs).
Conclusion
Health care providers play a major role in wound management which includes recommendations for improvements in post wound healing, adherence to standard of care, and meticulous self-inspection over the healed site. Despite the existence of guideline-guided care for prevention41,35, ulcer recurrence rates remain high. In this study we posited that augmenting care with our evidence-based self-management MUSTCOOL intervention, pain would be reduced and consequently physical activity, specifically walking, would be enhanced. However, we were unable to support this pain assumption chiefly because the population experienced low to no pain at study onset. We wish to emphasize MUSTCOOL is intended to augment standard of care; it was not designed to replace intervention for wound prevention. Nonetheless, our previous work supports that adherence to the MUSTCOOL intervention for at least 85% of the time decreased the risk of ulcer recurrence from 18% to 7% (p = .012), and provided clinically relevant improvements in pain, cramping, ache, itch and throb, the 5 most commonly-reported distressing symptoms associated with venous disease.42,27 Thus we suggest nurses and other health care providers recommend the use of the cooling patch in patients with venous disease or have a recently healed VLU and with these distressing symptoms consider use of MUSTCOOL for VLU prevention and/or symptom management. Recommendations for further study in DFU patients is warranted.
Acknowledgements:
The authors thank Dr. Dana E. King MD (independent medical monitor), Dr. Debra Miller-Cox (wound specialist advisor), MD, Dr. Mary Hanley, DO (wound specialist advisor), and Dr. Carolyn Horne, PhD, RN (nursing science advisor) for their support of this project.
Sources of funding:
This work was supported by the National Institutes of Health (NIH), National Institute of Nursing Research [grant number R01NR015647]; and the National Center for Advancing Translational Sciences of the National Institutes of Health [grant number UL1 TR001450]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflict of Interest Disclosure Statement:
The authors have no conflicts of interest to report.
Contributor Information
Teresa J. Kelechi, Medical University of South Carolina, College of Nursing, 99 Jonathan Lucas Street, MSC 160, Charleston, SC 29425, US.
Martina Mueller, Medical University of South Carolina, Charleston, SC.
Mohan Madisetti, Medical University of South Carolina, Charleston, SC.
Margaret Prentice, Medical University of South Carolina, Charleston, SC.
References
- 1.Sen CK, Gordillo GM, Roy S, et al. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17(6):763–771. doi: 10.1111/j.1524-475X.2009.00543.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schneider C, Stratman S, Kirsner RS. Lower extremity ulcers. Med Clin North Am. 2021;105(4):663–679. doi: 10.1016/j.mcna.2021.04.006 [DOI] [PubMed] [Google Scholar]
- 3.Parker CN, Finlayson KJ, Edwards HE. Predicting the likelihood of delayed venous leg ulcer healing and recurrence: Development and reliability testing of risk assessment Tools. Ostomy Wound Manage. 2017;63(10):16–33. [PubMed] [Google Scholar]
- 4.Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med. 2017;376(24):2367–2375. doi: 10.1056/NEJMra1615439 [DOI] [PubMed] [Google Scholar]
- 5.Nussbaum SR, Carter MJ, Fife CE, et al. An economic evaluation of the impact, cost, and Medicare policy implications of chronic nonhealing wounds. Value Health. 2018;21(1):27–32. doi: 10.1016/j.jval.2017.07.007 [DOI] [PubMed] [Google Scholar]
- 6.Bommer C, Sagalova V, Heesemann E, et al. Global economic burden of diabetes in adults: Projections from 2015 to 2030. Diabetes Care. 2018;41(5):963–970. doi: 10.2337/dc17-1962 [DOI] [PubMed] [Google Scholar]
- 7.Kelechi TJ, Mueller M, Madisetti M, Prentice MA, Dooley MJ. Exploring the influence of a cooling treatment on quality of life in patients with chronic venous disease. Chronic Wound Care Manage Res. 2017;4:65–76. 10.2147/CWCMR.S131917 [DOI] [Google Scholar]
- 8.Lentsck MH, Baratieri T, Trincaus MR, Mattei AP, Miyahara CTS. Quality of life related to clinical aspects in people with chronic wound. Rev Esc Enferm USP. 2018;52:e03384. doi: 10.1590/S1980-220X2017004003384 [DOI] [PubMed] [Google Scholar]
- 9.Bonham PA, Flemister BG, Droste LR, et al. Guideline for management of wounds in patients with lower-extremity arterial disease (LEAD): An Executive Summary. J Wound Ostomy Continence Nurs. 2016;43(1):23–31. doi: 10.1097/WON.0000000000000193 [DOI] [PubMed] [Google Scholar]
- 10.Edwards H, Finlayson K, Skerman H, et al. Identification of symptom clusters in patients with chronic venous leg ulcers. J Pain Symptom Manage. 2014;47(5):867–875. doi: 10.1016/j.jpainsymman.2013.06.003 [DOI] [PubMed] [Google Scholar]
- 11.Mirag A, Rahaman H, Rahaman M, Khan M. Impact of leg ulcers on quality of life: financial, social, and psychological implications among the patients attending OPD of vascular surgery. Br J Res. 2020;7(1):11–17. doi. 1 10.36648/2394-3718.7.1.51 [DOI] [Google Scholar]
- 12.Frescos N, Copnell B. Podiatrists’ views of assessment and management of pain in diabetes-related foot ulcers: A focus group study. J Foot Ankle Res. 2020;13(1):29. doi: 10.1186/s13047-020-00399-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelechi TJ, Mueller M, Madisetti M, Prentice MA, Dooley MJ. Effectiveness of cooling therapy (cryotherapy) on leg pain and self-efficacy in patients with chronic venous disease: A randomized controlled trial. Int J Nurs Stud. 2018;86:1–10. doi: 10.1016/j.ijnurstu.2018.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernando ME, Crowther RG, Lazzarini PA, et al. Gait in people With nonhealing diabetes-related plantar ulcers. Phys Ther. 2019;99(12):1602–1615. doi: 10.1093/ptj/pzz119 [DOI] [PubMed] [Google Scholar]
- 15.Francia P, Bellis A, Seghieri G, et al. Continuous movement monitoring of daily living activities for prevention of diabetic foot ulcer: A review of literature. Int J Prev Med. 2019;10:22. doi: 10.4103/ijpvm.IJPVM_410_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pieper B, Templin TN. Falls, balance confidence, and lower-body strength in patients seeking outpatient venous ulcer wound care. Adv Skin Wound Care. 2016;29(2):85–93. doi: 10.1097/01.ASW.0000476071.11690.61 [DOI] [PubMed] [Google Scholar]
- 17.Folguera-Álvarez C, Garrido-Elustondo S, Rico-Blázquez M, Verdú-Soriano J. Factors associated with the quality of life of patients with venous leg ulcers in primary care: Cross-sectional study. Int J Low Extrem Wounds. 2020;1534734620967562. doi: 10.1177/1534734620967562 [DOI] [PubMed] [Google Scholar]
- 18.Khunkaew S, Fernandez R, Sim J. Health-related quality of life among adults living with diabetic foot ulcers: A meta-analysis. Qual Life Res. 2019;28(6):1413–1427. doi: 10.1007/s11136-018-2082-2 [DOI] [PubMed] [Google Scholar]
- 19.Mutlak O, Aslam M, Standfield N. The influence of exercise on ulcer healing in patients with chronic venous insufficiency. Int Angiol. 2018;37(2):160–168. doi: 10.23736/S0392-9590.18.03950-0 [DOI] [PubMed] [Google Scholar]
- 20.González de la Torre H, Quintana-Lorenzo ML, Perdomo-Pérez E, Verdú J. Correlation between health-related quality of life and venous leg ulcer’s severity and characteristics: A cross-sectional study. Int Wound J. 2017;14(2):360–368. doi: 10.1111/iwj.12610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moura J, Sørensen A, Leal EC, et al. microRNA-155 inhibition restores Fibroblast Growth Factor 7 expression in diabetic skin and decreases wound inflammation. Sci Rep. 2019;9(1):5836. doi: 10.1038/s41598-019-42309-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raffetto JD, Ligi D, Maniscalco R, Khalil RA, Mannello F. Why venous leg ulcers have difficulty healing: Overview on pathophysiology, clinical consequences, and treatment. J Clin Med. 2020;10(1):29. doi: 10.3390/jcm10010029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yavuz M, Ersen A, Monga A, et al. Temperature- and pressure-regulating insoles for prevention of diabetic foot ulcers. J Foot Ankle Surg. 2020;59(4):685–688. doi: 10.1053/j.jfas.2019.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia C, Karri J, Zacharias NA, Abd-Elsayed A. Use of cryotherapy for managing chronic pain: An evidence-based narrative. Pain Ther. 2021;10(1):81–100. doi: 10.1007/s40122-020-00225-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herrera E, Sandoval MC, Camargo DM, Salvini TF. Motor and sensory nerve conduction are affected differently by ice pack, ice massage, and cold water immersion. Phys Ther. 2010;90(4):581–591. doi: 10.2522/ptj.20090131 [DOI] [PubMed] [Google Scholar]
- 26.Keser İ, Özdemir K, Erer D, Onurlu İ, Bezgin S. Differences in pain, fatigue, and quality of life in patients with chronic venous insufficiency based on physical activity level. Turk Gogus Kalp Damar Cerrahisi Derg. 2020;28(1):76–83. doi: 10.5606/tgkdc.dergisi.2020.18068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelechi TJ, Madisetti M, Prentice M, Mueller M. Cooling intervention (MUSTCOOL) for prevention of lower extremity ulcer recurrence: A randomized controlled trial. J Wound Ostomy Continence Nurs. 2021;48(3):203–210. doi: 10.1097/WON.0000000000000753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borson S, Scanlan JM, Chen P, Ganguli M. The Mini-Cog as a screen for dementia: validation in a population-based sample. J Am Geriatr Soc. 2003;51(10):1451–1454. doi: 10.1046/j.1532-5415.2003.51465.x [DOI] [PubMed] [Google Scholar]
- 29.Kelechi TJ, Madisetti M, Mueller M, Dooley M, Prentice M. Self-monitoring of lower leg skin temperature: accuracy of self-reported data and adherence to a cooling protocol for the prevention of venous leg ulcers. Patient Prefer Adherence. 2015;9:1751–1761. doi: 10.2147/PPA.S91992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yesavage JA. Direct and indirect hostility and self-destructive behavior by hospitalized depressives. Acta Psychiatr Scand. 1983;68(5):345–350. doi: 10.1111/j.1600-0447.1983.tb07016.x [DOI] [PubMed] [Google Scholar]
- 31.Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the Brief Pain Inventory for chronic nonmalignant pain. J Pain. 2004;5(2):133–137. doi: 10.1016/j.jpain.2003.12.005 [DOI] [PubMed] [Google Scholar]
- 32.Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB [DOI] [PubMed] [Google Scholar]
- 33.Cruz J, Brooks D, Marques A. Accuracy of piezoelectric pedometer and accelerometer step counts. J Sports Med Phys Fitness. 2017;57(4):426–433. doi: 10.23736/S0022-4707.16.06177-X [DOI] [PubMed] [Google Scholar]
- 34.Webber SC, Magill SM, Schafer JL, Wilson KC. GT3X+ accelerometer, Yamax pedometer and SC-StepMX pedometer step count accuracy in community-dwelling older adults. J Aging Phys Act. 2014;22(3):334–341. doi: 10.1123/japa.2013-0002 [DOI] [PubMed] [Google Scholar]
- 35.Kelechi TJ, Brunette G, Bonham PA, et al. 2019 guideline for management of wounds in patients with lower-extremity venous disease (LEVD): An executive summary. J Wound Ostomy Continence Nurs. 2020;47(2):97–110. doi: 10.1097/WON.0000000000000622 [DOI] [PubMed] [Google Scholar]
- 36.Edwards H, Finlayson K, Skerman H, et al. Identification of symptom clusters in patients with chronic venous leg ulcers. J Pain Symptom Manage. 2014;47(5):867–875. doi: 10.1016/j.jpainsymman.2013.06.003 [DOI] [PubMed] [Google Scholar]
- 37.Hao SP, Houck JR, Waldman OV, Baumhauer JF, Oh I. Prediction of post-interventional physical function in diabetic foot ulcer patients using patient reported outcome measurement information system (PROMIS). Foot Ankle Surg 2021;27(2):224–230. doi: 10.1016/j.fas.2020.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee M, van Netten JJ, Sheahan H, Lazzarini PA. Moderate-to-vigorous-intensity physical activity observed in people with diabetes-related foot ulcers over a one-week period. J Diabetes Sci Technol. 2019;13(5):827–835. doi: 10.1177/1932296819848735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ainsworth BE, Haskell WL, Herrmann SD, et al. Compendium of physical activities: A second update of codes and MET values. Med Sci Sports Exerc. 2011;43(8):1575–1581. doi: 10.1249/MSS.0b013e31821ece12 [DOI] [PubMed] [Google Scholar]
- 40.Teylan M, Kantorowski A, Homsy D, Kadri R, Richardson C, Moy M. Physical activity in COPD: Minimal clinically important difference for medical events. Chron Respir Dis. 2019;16:1479973118816424. doi: 10.1177/1479973118816424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bonham PA, Brunette G, Crestonida L, et al. Guideline for the management of lower-extremity wounds due to diabetes mellitus and/or neuropathic disease. Journal of Wound, Ostomy and Continence Nurses Society; 2022; 49(2): in press. [DOI] [PubMed] [Google Scholar]
- 42.Monsen KA, Kelechi TJ, McRae ME, Mathiason MA, Martin KS. Nursing theory, terminology, and big data: Data-driven discovery of novel patterns in archival randomized clinical trial data. Nurs Res. 2018;67(2):122–132. doi: 10.1097/NNR.0000000000000269 [DOI] [PubMed] [Google Scholar]


