Abstract
Over 50% of adolescents with chronic pain report comorbid sleep disturbances (e.g., difficulties with falling asleep), which is associated with increased pain-related disability and poorer quality of life. However, limited longitudinal data are available to understand how sleep disturbance may impact response to psychological treatment. Our primary hypothesis was that baseline sleep disturbances would significantly modify how adolescents responded to an internet-delivered psychological intervention for chronic pain in terms of outcome trajectories. The sample included 85 adolescents, 12 to 17 years, with chronic pain recruited from a multidisciplinary pain clinic and headache clinic who received access to an internet-delivered psychological intervention for chronic pain. Baseline sleep assessment included actigraphy monitoring for 7 days and survey measures. Outcomes were assessed at baseline, 8 weeks, and 3 months including core pain-related outcomes, executive functioning, fatigue, positive and negative affect. Results demonstrated that greater baseline insomnia and poorer sleep quality was associated with worse outcome trajectories for pain-related disability, depression, anxiety, fatigue, negative affect, and executive functioning. Findings extend the limited studies that examine how sleep disturbance may modify effectiveness of psychological treatments for adolescent chronic pain and emphasize the importance of treating comorbid sleep disturbance. This trial was registered at clinicaltrials.gov (NCT04043962).
Keywords: adolescent, chronic pain, psychological intervention, sleep, insomnia
Introduction
Many adolescents experience chronic pain, most commonly as head, abdominal, or musculoskeletal pain 21, 23. Unfortunately, over 50% of these youth also report symptoms of comorbid sleep disturbances (e.g., difficulties with falling asleep, poor sleep quality) 40. Numerous cross-sectional studies in adolescents and adults indicate that sleep disturbances are associated with greater concurrent pain sensitivity, disability, poorer quality of life, and greater healthcare use and costs 5, 24, 29, 37. Thus, increased attention has been directed toward assessment and management of sleep disturbances among individuals with chronic pain 8, 41, 45. However, limited longitudinal data are available to understand how sleep disturbance may impact response to pain treatment, and whether subgroups of patients with sleep disturbances may need a more personalized treatment approach.
Sleep disturbance is a multi-dimensional domain encompassing both sleep quantity and quality. It is not yet known which aspects of sleep (e.g., sleep duration, quality, insomnia) most impact pain and pain management. While sleep has been shown to directly impact subsequent pain 13, 27, sleep is also linked to factors important in engaging in psychological strategies for pain self-management including affect (positive and negative), fatigue, and higher-order executive functioning (attention, organization, planning, problem-solving, and memory)5, 11, 51, 52.
Emerging evidence suggests that sleep disturbance may modify the effectiveness of psychological treatments for chronic pain. In a pilot study of internet-delivered treatment, Fales and colleagues 12 found that shorter baseline sleep duration was associated with less improvement in pain-related disability at immediate post-treatment. Similarly, in a randomized controlled trial of internet-delivered treatment, Murray et al. 32 found that worse baseline sleep quality predicted less improvement in pain-related disability at 6-month follow-up. In youth undergoing intensive inpatient rehabilitation, better baseline self-reported sleep quality and lower insomnia symptoms were associated with improvements in pain symptoms at immediate post-treatment 3. These studies were limited in their assessments of both sleep and treatment outcomes. For example, studies did not test objective and subjective sleep measures together as modifiers of treatment response. The impact of baseline sleep on the maintenance of treatment benefits over time is also not well understood.
Building from prior research, the primary objective of this study was to characterize the relationship between baseline adolescent sleep disturbances and longitudinal outcomes following internet-delivered psychological treatment for chronic pain. We expand on prior work by using a comprehensive baseline assessment of objective sleep patterns (actigraphy measured sleep duration and wake after sleep onset) and subjective sleep quality and insomnia. We measure a range of pain-related outcomes (global health, pain, anxiety and depression, pain-related disability) guided by recommendations for core outcome domains in pediatric chronic pain 36. Because prior studies have already established the efficacy of the internet treatment program 34, 35, 38 in improving adolescent pain-related disability, emotional functioning, and parent behavior and impact, we chose a single-arm clinical trial design where all adolescents received treatment. However, we expand our outcome assessment to include variables important in the interrelationship between pain and sleep 13 including positive and negative affect, fatigue, and executive functioning.
Our primary hypothesis was that baseline sleep disturbances would significantly modify how participants responded to the internet-delivered intervention in terms of outcome trajectories. Specifically, we expected that greater baseline sleep disturbances (higher insomnia symptoms, poorer sleep quality, shorter sleep duration, and longer time awake after sleep onset) would be associated with less improvement in our primary outcome of pain-related disability. We expected to identify a similar pattern of association between baseline sleep disturbances and secondary outcomes (pain intensity, global health, and emotional functioning), as well as our new, exploratory outcomes (positive and negative affect, fatigue, and executive functioning).
Methods
Participants and Setting
The sample included 85 youth ages 12 to 17 years with chronic pain and a participating caregiver. Participants were recruited from two clinics (multidisciplinary pain clinic and multidisciplinary headache clinic) at Seattle Children’s Hospital over a 1.5-year period from November 2018 to February 2020. This study was approved by the institutional review board at Seattle Children’s Research Institute. The trial is registered at clinicaltrials.gov (Identifier NCT04043962). Data from this trial have not been previously published.
Participant Inclusion Criteria
Inclusion criteria were (a) age 12 to 17 years, (b) diagnosed with a primary pain disorder involving abdominal, headache, or musculoskeletal pain by a specialty physician in one of the participating clinics, (c) pain duration > 3 months, (d) access to the internet, and (e) if taking medication for pain or sleep, on a stable dose for the past two months. Exclusion criteria were (a) diagnosis of a comorbid serious health condition (e.g., cancer), (b) non-English speaking, (c) active psychosis or suicidal ideation, and (d) diagnosis of sleep apnea or narcolepsy.
Recruitment
Potentially eligible participants were given a study flyer by providers at the participating clinics and asked whether they would be willing to be contacted by phone by study staff to undergo additional screening. Providers then transferred referral information to the study team via secure study website, email, or fax. Potential participants could also contact study staff directly via a toll-free phone number and email listed on the study flyer. Study staff screened potential participants by phone, and if eligible, obtained electronic parent consent and child assent for study participation.
Design and Procedures
This longitudinal study included a baseline assessment, an 8-week intervention period, post-treatment assessment, and 3-month follow-up assessment. A single arm intervention design was used; during the intervention period, all participants were given access to the 8-week WebMAP program 35, which included access to separate password-protected websites for youth and parents to learn pain self-management skills. Access to the WebMAP program was in addition to standard care at the participants’ referring clinic. Standard care was not altered by study participation and included an initial interdisciplinary evaluation in the referring clinic for all participants. Participants may have also received treatment recommendations (e.g., physical therapy, psychology, medication management, acupuncture) and clinic follow-ups as needed. Recruitment into the study ended as planned in February 2020.
Participant Flow
Figure 1 shows a flow diagram of participants through each phase of the study. We received a total of 129 referrals from the participating clinics. Of the families who were screened, 44 families were excluded because we could not reach them during the screening period (n = 10), they did not meet inclusion criteria (n = 15), they were not interested or did not have time to participate (n = 14), or they consented but did not complete the pre-treatment assessment (n = 5). The final sample included 85 youth-caregiver dyads. All 85 dyads who enrolled in the study were given access to the WebMAP program. Of these, 94.1% (n = 80/85) logged into the program and received the intervention. Assessment completion was high across the study period, ranging from 100% (n =85/85) at baseline to 85.9% (n=73/85) at 3-month follow-up. The full sample (n = 85) was used in analyses.
Figure 1.
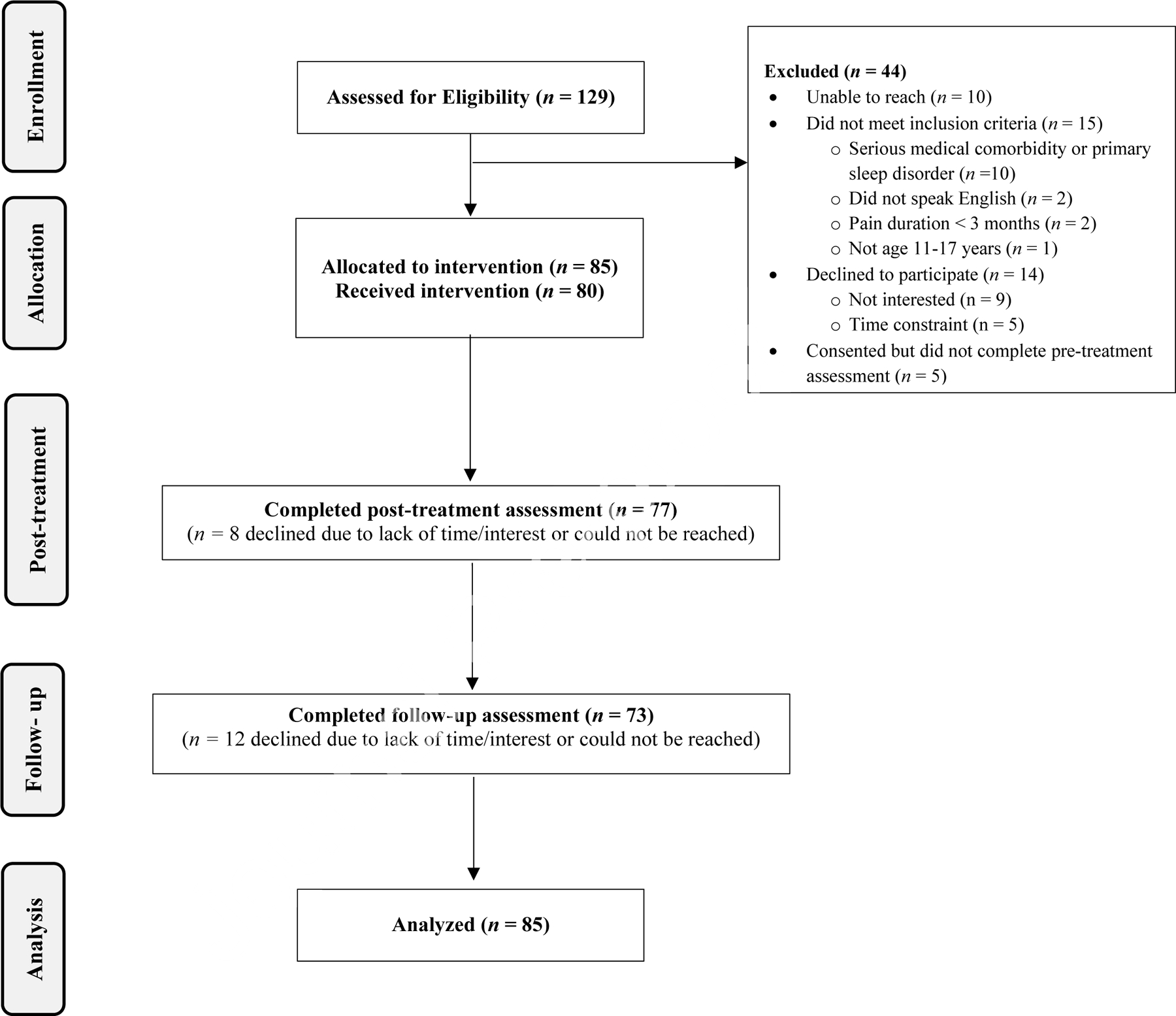
Flow Diagram
Procedures
Assessments
Standardized questionnaire measures were administered via Research Electronic Data Capture, REDCap 19, a secure web-based data acquisition system, at baseline before receiving the intervention, after completion of the 8-week intervention (immediate post-treatment), and at 3-month follow-up. At baseline, youth also completed 7-days of monitoring (daily diaries administered through REDCap) and wore an Actiwatch to measure sleep patterns. Participants were provided with gift card incentives after completion of each assessment time point.
Intervention Description
The WebMAP program teaches relaxation skills, pain coping strategies, and parent behavioral and communication techniques following cognitive-behavioral and social learning theories and has been described in previous trials 34, 35, 38. The 8 modules for adolescents include: (1) education about chronic pain and setting goals for treatment, (2) recognizing stress and negative emotions, (3) relaxation methods, (4) school, (5) cognitive skills, (6) lifestyle interventions (including brief psychoeducation about sleep needs and sleep habits), (7) staying active, and (8) relapse prevention. For parents, there is a separate program of 8 modules, including: 1) education about chronic pain and setting goals for treatment, 2) recognizing stress and negative emotions, 3) operant strategies I (using attention and praise to increase positive coping), 4) operant strategies II (using rewards to increase positive coping and reach school goals), 5) modeling, 6) lifestyle interventions, 7) communication, and 8) relapse prevention.
WebMAP is self-guided and has a travel theme. Users visit different “destinations,” such as Greece or Brazil, to learn pain self-management skills. Accompanying homework assignments are given at the end of each module to provide opportunities for skills practice. Content is metered so that users spend one week on skills practice before a homework assignment can be completed and the next destination is ‘unlocked’. This allows time for skills acquisition and consolidation. The program is built on a responsive platform and can be accessed on multiple devices (e.g., smartphones, laptops, tablets) to increase ease and flexibility of use.
Measures
Demographic and Clinical Characteristics
Parents reported on the adolescent’s age, sex at birth, race, and ethnicity as well as their own race, ethnicity, annual household income, and education status. Race categories included: White, Latin American, Black, South Asian, and Other. Participants who selected more than one race were coded accordingly. For the ethnicity category, participants selected either Hispanic/Latino or non-Hispanic/Latino. Youth reported on their pain location, duration of their pain condition (in years), and pain frequency over the past 3 months.
Baseline Sleep Disturbances
Adolescents completed a comprehensive baseline assessment of sleep including: 1) subjective sleep quality, 2) insomnia symptoms, and 3) total sleep time and wake after sleep onset as measured by actigraphy monitoring.
Sleep Quality.
Youth completed the Adolescent Sleep Wake Scale-Short Form (ASWS-SF; 10), a 10-item self-report measure of sleep quality over the past month. The ASWS defines sleep quality as a multifaceted domain that includes the youth’s perception of waking behaviors and nighttime sleep behaviors. Specifically, the ASWS-SF includes items assessing difficulty going to bed, difficulty falling asleep, difficulty going back to sleep after waking up in the night, and difficulty waking up in the morning. Items are scored on a 6-point Likert scale (1 = never, 6 = always) and summed to generate a total score. Higher total scores indicate better sleep quality.
Insomnia Symptoms.
Youth completed the 13-item Adolescent Insomnia Questionnaire (AIQ, 4 to evaluate difficulty with sleep onset, sleep maintenance, and sleep dissatisfaction/impairments in the past week. The AIQ has been validated for adolescents with chronic pain 4. Items are rated on a 5-point Likert scale (0 = never, 4 = almost always) with a total score ranging from 0–52. Higher scores indicate more severe insomnia symptoms.
Actigraphy Monitoring.
Adolescents wore an Actiwatch-2 (Phillips Respironics, MiniMitter Company Inc., Bend, OR) on their non-dominant wrist for 7 days/nights to assess sleep patterns. Two actigraphy variables were used in our analyses: total sleep time (TST) and wake time after sleep onset (WASO) 30. TST was the amount of sleep (in minutes) between sleep onset and sleep offset, with higher scores indicating longer sleep duration. WASO was the amount of time awake (in minutes) between initial sleep onset and final awakening, with higher scores indicating more time spent awake during the night. For analyses, average scores were computed across the 7-day assessment period.
Pain-Related Outcomes
Pain-related Disability.
Adolescents completed the daily diary version of the Child Activity Limitations Interview-9 (CALI-9) 20 for 7 days at each assessment period. The CALI-9 evaluates difficulty participating in usual daily physical, social, and recreational activities due to pain. Items are rated on a 5-point scale (0 = not very difficult, 4 = extremely difficult) and scores are linearly transformed to a 0–100 scale, with higher scores indicating greater pain-related disability. For analyses, we used the average total score across each 7-day assessment period.
Pain Intensity.
Adolescents also reported their average pain intensity for 7 days at each assessment period, once daily, using an 11-point Numerical Rating Scale with anchors of 0 = no pain and 10 = worst pain ever (NRS; 47). Average pain intensity scores across each 7-day assessment period were used in analyses, with higher scores indicating greater pain intensity.
Global Health.
Adolescents completed the PROMIS Pediatric Scale v1.0 – Global Health 7 17, to assess perceived physical, mental, and social health over the past week. This measure includes 7 items rated on a 5-point scale, which are summed to generate a total score ranging from 7–35. Items assess children’s overall well-being, one item evaluates physical health, two mental health, two social health, and one general health. Higher scores indicate better perceived global health.
Emotional Functioning.
Adolescent anxiety and depressive symptoms over the past 7 days were assessed using the Patient-Reported Outcomes Measurement Information System (PROMIS) Pediatric Anxiety - Short Form 8a (8 items) and Depressive Symptoms – Short Form 8a (8 items) 22. Items are rated on a 5-point scale (1 = never, 5 = almost always). Total scores were transformed into T-scores for analyses, with higher scores indicating more severe anxiety and depressive symptoms.
Positive and Negative Affect.
Adolescents reported on their positive and negative affect over the past few weeks using the Positive and Negative Affect Scale for Children-Short Form (PANAS-C-SF; 25. The PANAS-C-SF includes two sub-scales: a 5-item positive affect subscale (e.g., happy, cheerful, joyful), and a 5-item negative affect subscale (e.g., sad, scared, miserable). Items are rated on a 5-point Likert scale (1 = very slightly or not at all, 5 = extremely) and are summed to generate the two subscales, with higher scores indicating greater positive or negative affect (respectively).
Fatigue.
Adolescents completed the 18-item PedsQL Multidimensional Fatigue Scale (PedsQL-MFS; 46) to assess fatigue over the past four weeks. Items are rated on a 5-point scale (0 = never, 4 = almost always), and then are reverse scored and transformed to a 0–100 scale (0 = 100, 4 = 0). We used the total score in analyses, with higher scores indicating less fatigue.
Executive Functioning.
Adolescents completed the self-report version of the Behavior Rating Inventory of Executive Function-2 (BRIEF-2 Child Self-Report Form 18), to assess behavioral, cognitive, and emotional dimensions of executive functioning. The Self-Report form consists of 55 items that contribute to seven factors: Inhibit, Self-Monitor, Shift, Emotional Control, Task Completion, Working Memory, and Plan/Organize. Items are rated on a 3-point scale (1 = Never, 2 = Sometimes, 3 = Often), and T-scores are generated for subscale and total scores. We used the total score in analyses, with higher scores indicating greater difficulty with executive functioning.
Data Analysis Plan.
For this study we initially planned for a final sample size of 75 participants, after accounting for up to 10% attrition. This sample size was chosen to detect a small-to-moderate effect size d=0.3 between pre- vs. post assessments with 80% power. We enrolled and retained a larger sample size of 85 for our analytic sample. This, along with repeated assessments, should provide even better power for analyses. Demographic characteristics were summarized using descriptive statistics, and we report frequencies for categorical variables and means and SDs for continuous variables.
Positive affect, negative affect, fatigue, executive functioning, and other pain-related outcomes were assessed at 3 time points (baseline, immediate post-treatment, and 3-month follow-up). We first examined changes in these outcomes over time using linear mixed effects regression models, in which time was modelled as a categorical predictor and adolescent age, adolescent sex at birth, adolescent race (coded as 1=White, 0=all else), and adolescent ethnicity (coded as 1=Hispanic, 0=Non-Hispanic) were controlled in all models as covariates. Baseline sleep disturbance measures (insomnia symptoms, sleep quality, TST, and WASO) were modelled as predictors in separate models. All models included subject-specific random intercept to account for the within-subject correlations due to repeated assessments. Next, we examined whether changes in outcomes over time were modified by baseline sleep disturbance. This was achieved by including time-by-sleep-disturbance interaction terms in the previous set of models. The significance of effect modification was assessed by likelihood ratio test comparing models with and without the interaction terms. For most measures, >85% of participants had complete data. In linear mixed effects regression models, all available data were used in analyses and missing data were handled using maximum likelihood estimation. Statistical significance was set at p < .05. All analyses were conducted using the R statistical software version 4.1.0 44.
Results
Participant Characteristics
As shown in Table 1, adolescents were on average 15 years old, mostly female (77%), White (91%), and non-Hispanic (85%). The majority of adolescents had pain in more than one body region (73%) that occurred daily (67%). On average, adolescents reported that their chronic pain problem began 3.5 years prior to study enrollment. Parents were also predominantly White (92%) and non-Hispanic (11%). Most parents had completed college or vocational school (70%), and 41% had an annual household income of $100,000 or more.
Table 1.
Demographic and clinical characteristics of participants at pre-treatment (n = 85)
| Adolescent Characteristics | M (SD) or n (%) |
|
| |
| Age, M (SD) years | 15.5 (1.5) |
| Sex at birth, n (%) female | 65 (77) |
| Ethnicity, n (%) Hispanic/Latino | 13 (15) |
| Race, n (%) | |
| American Indian/AK Native | 5 (6) |
| Black | 4 (5) |
| East Asian | 4 (5) |
| Latin American | 7 (8) |
| South Asian | 3 (4) |
| White | 77 (91) |
| More than one race | 16 (18) |
| Years Since Pain Onset, M (SD) | 3.5 (2.6) |
| Pain Location, n (%) | |
| Abdomen | 42 (49) |
| Head | 57 (67) |
| Musculoskeletal | 68 (80) |
| Multi-site | 62 (73) |
| Pain Frequency, n (%) | |
| Daily | 57 (67) |
| Weekly | 22 (26) |
| Monthly | 4 (5) |
| Not reported | 2 (2) |
| Concomitant treatments, n (%)* | |
| Psychologist | 50 (59) |
| Primary or specialty physician | 73 (86) |
| Physical therapy/chiropractor | 65 (76) |
| Acupuncture/naturopath | 19 (22) |
| Prescription medications (e.g., antidepressants, anticonvulsants) | 15 (18) |
| Sleep medications (i.e., melatonin) | 1 (< 1) |
| Opioids | 0 (0) |
|
| |
| Parent Characteristics | M (SD) or n (%) |
|
| |
| Ethnicity, n (%) Hispanic/Latino | 9 (11) |
| Race, n (%) | |
| American Indian/AK Native | 3 (4) |
| Black | 2 (2) |
| East Asian | 1 (1) |
| Latin American | 5 (6) |
| South Asian | 2 (2) |
| White | 79 (92) |
| More than one race | 6 (7) |
| Education, n (%) | |
| High school or less | 4 (5) |
| College or vocational school | 60 (70) |
| Graduate or professional school | 20 (24) |
| Not reported | 1 (1) |
| Annual Household Income, n (%) | |
| $49,999 or less | 24 (28) |
| $50,000 – $99,999 | 25 (29) |
| $100,000 or more | 35 (41) |
| Not reported | 1 (1) |
categories are not mutually exclusive
All youth received a multidisciplinary evaluation in the pain or headache clinic. As shown in Table 1, concomitant therapies included psychological services, primary and specialty physician visits, physical therapy, acupuncture, and prescription medications (e.g., antidepressants, anticonvulsants). Only one participant was using sleep medication (melatonin) and no participants were using opioids.
Overall Treatment Effects
As shown in Table 2, as hypothesized, youth demonstrated improvements in several pain-related outcomes from pre-treatment to follow-up time points. At immediate post-treatment, we replicated earlier published findings of significant improvements in pain-related disability (β = −4.41, p = .049) which approached although did not reach statistical significance at follow-up (β = −3.89, p = .089). Pain intensity did not significantly change from pre- to post-treatment, and changes in pain intensity approached but did not reach statistical significance (β = −.35, p = .053) at follow-up. In addition, as hypothesized, improvements from pre-treatment to post-treatment in global health (β = 1.86, p = .013), fatigue (β = 3.91, p = .026), and negative affect (β = −2.21, p < .001) were demonstrated. At 3-month follow-up, significant improvements were maintained in global health (β = 2.83, p < .001) and negative affect (β = −1.31, p = .022). In addition, significant improvements at follow-up were also demonstrated for anxiety (β = −2.75, p = .014) and executive functioning (β = −2.12, p = .004).
Table 2.
Means and standard deviations for treatment outcomes at each time point.
| Baseline | Post-Tx | Follow-up | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| Outcomes | M | SD | M | SD | M | SD |
|
| ||||||
| Pain-related disability* | 48.33 | 21.84 | 44.48 | 22.52 | 44.45 | 23.40 |
| Pain intensity | 5.86 | 1.66 | 5.64 | 1.54 | 5.45 | 1.66 |
| Global health*,** | 34.84 | 7.38 | 36.87 | 8.45 | 38.04 | 8.67 |
| Depression | 57.60 | 11.10 | 57.91 | 10.63 | 56.81 | 11.80 |
| Anxiety** | 54.66 | 12.24 | 52.80 | 11.59 | 51.37 | 12.79 |
| Fatigue* | 44.58 | 18.24 | 48.71 | 15.70 | 47.79 | 18.94 |
| Positive affect | 14.21 | 4.44 | 14.27 | 6.01 | 14.67 | 5.36 |
| Negative affect*,** | 13.01 | 4.63 | 10.73 | 5.26 | 11.59 | 5.12 |
| Executive functioning** | 60.01 | 10.61 | 58.69 | 11.00 | 58.03 | 12.19 |
Notes.
Statistically significant change from pre-treatment to post-treatment (p < .05).
Statistically significant change from pre-treatment to follow-up (p < .05).
Baseline Sleep Disturbance is Associated with Trajectories of Treatment Outcomes
Insomnia Symptoms.
As shown in Table 3, evaluation of time X insomnia interaction effects indicated that baseline insomnia significantly modified change over time in: pain-related disability (χ2 (2) = 8.83, p = .012), pain intensity (χ2 (2) = 7.04, p = .029), global health (χ2 (2) = 10.68, p = .005), fatigue (χ2 (2) = 16.85, p < .001), and negative affect (χ2 (2) = 14.99, p < .001) where greater insomnia symptoms were associated with worse outcomes. At immediate post-treatment, effect modification was significant for: pain intensity, global health, fatigue, and negative affect. At 3-month follow up, effects were significant for: pain-related disability, fatigue, and negative affect.
Table 3.
Linear mixed regression models examining insomnia symptoms as a predictor of change in treatment outcomes over time.
| Predictors | ||||||
|---|---|---|---|---|---|---|
| Time-by-Insomnia: Post-Tx | Time-by-Insomnia: Follow-up | |||||
|
|
||||||
| Outcomes | β | 95% CI | p | β | 95% CI | p |
|
| ||||||
| Pain-related disability | −.46 | −.93, .02 | .059 | −.76 | −1.27, −.25 | .004* |
| Pain intensity | −.05 | −.09, −.01 | .012* | −.04 | −.08, .01 | .074 |
| Global health | .27 | .11, .42 | .002* | .11 | −.05, .27 | .182 |
| Depression | −.16 | −.39, .06 | .156 | −.22 | −.45, .01 | .056 |
| Anxiety | −.16 | −.40, .08 | .196 | −.20 | −.45, .05 | .116 |
| Fatigue | .64 | .28, 1.00 | .001* | .69 | .32, 1.06 | <.001* |
| Positive affect | .09 | −.05, .23 | .207 | −.01 | −.15, .14 | .925 |
| Negative affect | −.17 | −.29, −.05 | .006* | −.19 | −.32, −.07 | .003* |
| Executive functioning | .14 | −.30, .03 | .098 | −.12 | −.28, .05 | .161 |
Notes. All models controlled for age, sex, race and ethnicity.
p < .05.
Sleep Quality.
As shown in Table 4, time X sleep quality interaction effects revealed that baseline sleep quality significantly modified change over time in global health (χ2 (2) = 7.33, p = .026), and fatigue (χ2 (2) = 8.35, p = .015). As expected, youth with poorer sleep quality at baseline achieved less improvement over time in global health and fatigue outcomes. Examination of the time x sleep quality interaction terms at post-treatment suggests that youth with poorer baseline sleep quality also achieved less improvement over time in global health, anxiety, fatigue, and executive function (all ps < .05. see Table 4). At 3-month follow-up significant time x sleep quality effect modification was found for pain intensity, fatigue, and negative affect (ps < .05).
Table 4.
Linear mixed regression models examining sleep quality as a predictor of change in treatment outcomes over time.
| Predictors | ||||||
|---|---|---|---|---|---|---|
| Time-by-Sleep Quality: Post-Tx | Time-by-Sleep Quality: Follow-up | |||||
|
|
||||||
| Outcomes | β | 95% CI | p | β | 95% CI | p |
|
| ||||||
| Pain-related disability | 4.48 | −1.14, 10.10 | .119 | 5.89 | −.09, 11.87 | .054 |
| Pain intensity | .32 | −.15, .78 | .180 | .51 | .01, 1.00 | .045* |
| Global health | −2.58 | −4.45, −.70 | .008* | −1.02 | −2.88, .85 | .286 |
| Depression | .75 | −1.83, 3.32 | .570 | .92 | −1.64, 3.47 | .481 |
| Anxiety | 2.78 | .11, 5.45 | .042* | .62 | −2.02, 3.27 | .644 |
| Fatigue | −5.23 | −9.53, −.94 | .017* | −5.49 | −9.75, −1.23 | .012* |
| Positive affect | −.84 | −2.40, .72 | .292 | .47 | −1.13, 2.06 | .564 |
| Negative affect | 1.20 | −.21, 2.61 | .096 | 1.45 | .01, 2.89 | .048* |
| Executive functioning | 1.89 | .07, 3.71 | .042* | 1.54 | −.26, 3.34 | .095 |
Notes. All models controlled for age, sex, race and ethnicity.
p < .05.
TST.
Baseline TST was not associated with change in any of the outcomes across time (all ps > .05).
WASO.
Evaluation of time X WASO interaction effects indicated that baseline WASO significantly modified change over time in pain-related disability (χ2 (2) = 7.42, p = .024), global health (χ2 (2) = 6.72, p = .034), and executive functioning (χ2 (2) = 8.25, p = .016). As hypothesized, greater baseline WASO was associated with less improvement at post-treatment in pain-related disability, global health, and executive functioning, and at 3-month follow-up in executive functioning (see Table 5). However, we did not identify significant time X WASO interaction effects for the remaining treatment outcomes.
Table 5.
Linear mixed regression models examining wake after sleep onset (WASO) as a predictor of change in treatment outcomes over time.
| Predictors | ||||||
|---|---|---|---|---|---|---|
| Time-by-WASO: Post-Tx | Time-by-WASO: Follow-up | |||||
|
|
||||||
| Outcomes | β | 95% CI | p | β | 95% CI | p |
|
| ||||||
| Pain-related disability | .30 | .09, .52 | .007* | .14 | −.08, .37 | .212 |
| Pain intensity | .02 | −.01, .03 | .075 | <.01 | −.02, .02 | .925 |
| Global health | −.09 | −.16, −.02 | .013* | .07 | −.14, .01 | .076 |
| Depression | −.01 | −.12, .09 | .792 | .02 | −.09, .12 | .731 |
| Anxiety | −.05 | −.16, .06 | .408 | −.02 | −.14, .09 | .693 |
| Fatigue | −.06 | −.23, .11 | .492 | −.10 | −.28, .08 | .276 |
| Positive affect | −.02 | −.08, .05 | .628 | <.01 | −.06, .07 | .990 |
| Negative affect | −.03 | −.08, .03 | .353 | −.01 | −.06, .05 | .847 |
| Executive functioning | .08 | .01, .15 | .028* | .10 | .03, .17 | .008* |
Notes. All models controlled for age, sex, race and ethnicity.
p < .05.
Discussion
Our findings contribute to the growing literature on the sleep-pain association in adolescents with chronic pain. By conducting a single arm trial of internet-delivered psychological therapy, we were able to examine how multiple dimensions of objective and subjective sleep measured at baseline modified the trajectory of pain-related treatment outcomes over time. While sleep has been examined in many cross-sectional studies 5, 24, 29, 37, there remains limited information on the longitudinal impact of sleep on response to pain treatment. In particular, we were interested in not only examining the impact of baseline sleep on core pain outcome domains (e.g., pain-related disability, anxiety, depression, global health) but also on affect (positive and negative), fatigue, and higher-order executive functioning. These additional outcomes have been associated with sleep in previous observational studies but are not typically measured as outcomes of CBT for chronic pain 15, 16, 36.
Overall, we found that, as expected, adolescents demonstrated improvements from baseline to follow-up time points in pain-related disability, pain intensity, global health, and anxiety. This replicates our published findings with the WebMAP intervention in adolescents with chronic pain 35. We also studied several new outcomes and found preliminary evidence suggesting that internet CBT for pain management may also lead to reductions in negative affect, fatigue, and problems with executive functioning over the course of treatment for youth as a group. These findings suggest that the skills taught in the WebMAP intervention may impact a wider range of outcomes than previously considered, and warrant further study in future, controlled trials.
Our primary aim was to examine how baseline sleep modified treatment outcome trajectories. We found the strongest effects for subjective measures of sleep including insomnia symptoms and sleep quality, where having greater insomnia symptoms and poorer sleep quality at baseline modified response to treatment in multiple domains. Specifically, greater baseline insomnia and poorer sleep quality both were associated with worse outcome trajectories for pain intensity, fatigue, negative affect, and executive functioning. Greater baseline insomnia was also associated with worse outcome trajectories for pain-related disability and negative affect, whereas poorer sleep quality was associated with worse outcomes for anxiety and executive function. The similar effects of both self-reported sleep variables on treatment outcomes were likely due to the overlap between insomnia symptoms (i.e., difficulty falling asleep and staying asleep) and poor sleep quality in youth with chronic pain. In contrast, actigraphy measurements of sleep patterns including baseline sleep duration and wake after sleep onset (WASO) had more limited impact on treatment outcomes. In particular, baseline WASO modified trajectories of global health and pain-related disability only at immediate post-treatment and on executive functioning across time points while baseline TST revealed effect modification approaching significance only for pain-related disability at follow-up.
Findings extend the limited studies that examine how sleep disturbance may modify the effectiveness of psychological treatments for chronic pain. In our own earlier pilot work 12 we found that shorter pre-treatment sleep duration was associated with less improvement in pain-related disability at post-treatment and in secondary analyses from a larger RCT that worse pre-treatment sleep quality predicted less improvement in pain-related disability at long-term follow-up 32. A similar pattern has been identified in two studies of youth undergoing intensive pain rehabilitation: Boggero et al 3 found that better baseline sleep quality was associated with improvement in pain and disability over the course of treatment. Logan et al28 found that decreased night wakings over the course of intensive pain rehabilitation predicted subsequent reductions in pain intensity at follow-up. Our findings extend this prior work by allowing comparison across different aspects of sleep (subjective and objective), over multiple additional outcome domains, and a longer follow-up time period.
Differences in the pattern of findings between objective and subjective sleep assessments in this trial should be interpreted cautiously. When evaluating sleep in youth with chronic pain, careful consideration is needed to ensure the approach is efficient, psychometrically sound, and appropriate for the context8. For example, a comprehensive approach that integrates objective and subjective assessments may be needed when the goal is to expand conceptual understanding of the sleep-pain relationship, or when the research is focused on the diagnosis/treatment of a sleep disorder. In contrast, a subjective approach (e.g., self-report questionnaires) may be appropriate when the goal is to screen for the presence of sleep disturbances, or when there are resource constraints that limit feasibility of objective assessments.
One of the novel findings of the study was that baseline insomnia symptoms were a strong effect modifier across a range of pain-related outcomes. Given the high prevalence rate of insomnia found in pediatric pain populations 37, it is important to consider screening and treatment of insomnia symptoms in youth with chronic pain. Historically, screening insomnia symptoms in adolescents has been limited by lack of evidence-based assessment tools for this age group; however, more recently, new tools have been developed and validated for this purpose 4, 6. Our findings suggest that universal screening for insomnia symptoms in youth with chronic pain should be considered in clinical settings, because of the potential impact of insomnia on response to pain self-management interventions.
CBT for insomnia (CBT-I) is recommended by the American Academy of Sleep Medicine as first-line treatment for adult insomnia31; core treatment strategies include education about sleep and sleep hygiene, stimulus control, and sleep restriction. CBT-I has demonstrated efficacy in numerous RCTs for improving insomnia symptoms in adults with chronic pain 41. Emerging pilot studies in adolescent populations are promising and suggest that CBT-I is feasible and effective for reducing insomnia symptoms in youth with chronic pain and coexisting mental health and physical health conditions 26, 33. Our findings lend support for the hypothesis that synergistic benefits may occur when children and adolescents experience improvements in sleep prior to initiating pain self-management intervention. However, efficacy of CBT-I for improving sleep in youth with chronic pain and optimal sequencing of CBT-I and CBT-Pain interventions has yet to be empirically studied. Hybrid CBT programs, which deliver the core treatment components of CBT-I and CBT-Pain simultaneously43, have been used in adults with comorbid chronic pain and insomnia2. Further research is needed to determine safety and efficacy of Hybrid CBT programs for youth with chronic pain and co-occurring insomnia.
Another novel finding emerging from our study is that CBT for chronic pain may positively impact executive functioning in youth with chronic pain, and that effects on executive functioning are particularly sensitive to baseline sleep disturbances. With its focus on goal setting and building self-management skills, CBT for chronic pain does rely on higher-order and complex cognitive functions and self-regulatory skills, and with repeated practice, these skills may improve over the course of treatment. Future studies are needed to understand the impact of CBT on specific self-regulatory skills in controlled trials to elucidate these effects further. Moreover, sleep deficiency is associated with general deficits in executive functioning e.g., 1, including reduced cognitive flexibility in problem solving 48, 49, reduced integration of new learned information 50, and reduced working memory associations 9. Sleep also affects elements of self-control such as subjective effort, perceived exertion, and choice 39 and short sleep duration increases negative affect and decreases positive affect 14. Indeed, a recent conceptual model supports a potential cyclical relation between chronic pain and impaired executive functioning in adolescents, highlighting sleep as a modifier of the pathway between chronic pain, executive functioning capacity, and engagement with self-management behaviors 7.
Findings should be considered in light of several study limitations. Our sample was predominantly female and non-Hispanic White, similar to most published studies on youth with chronic pain recruited from tertiary care clinics; however findings may not generalize to more demographically diverse samples. Most participants in our sample (73%) had multi-site pain, and we were unable to examine any differences in the impact of sleep on pain outcomes by specific pain condition. Insomnia was measured at the symptom level using a screening measure rather than by establishing a clinical diagnosis of insomnia. Treatment in this trial was delivered via a self-guided, digital health technology platform, and it is unknown whether a different pattern of findings would emerge for treatments delivered face-to-face.
Finally, because we used a single arm trial without a comparator group, we were limited in our ability to conduct analysis of mediators of treatment effects. Specifically, future controlled trials are needed to uncover the cognitive, behavioral, or affective variables that may link sleep deficiency with poor treatment response. Trials that compare the efficacy of different treatment sequences (e.g., pain treatment first vs insomnia treatment first) will also enhance our conceptual understanding of the sleep-pain relationship during and after treatment.
Our study suggests that sleep deficiency may modify the effectiveness of psychological treatments for chronic pain, highlighting the urgent need to screen adolescents for sleep problems prior to initiating treatment, and to consider implementation of sleep-specific treatments such as cognitive-behavioral therapy for insomnia. Future studies are needed to test specific mechanisms linking comorbid sleep deficiency with poor pain-related outcomes in youth with chronic pain.
Perspective:
Our study suggests that sleep deficiency, in particular insomnia and poor sleep quality, may modify the effectiveness of psychological treatments for chronic pain, highlighting the urgent need to screen youth for sleep problems prior to initiating treatment, and to consider implementation of sleep-specific treatments such as cognitive-behavioral therapy for insomnia.
Highlights.
Limited data exist on the longitudinal impact of sleep on adolescent response to pain treatment
We provided youth with access to an internet-delivered pain self-management program
Subjective (vs. objective) sleep measures demonstrated stronger effects on treatment response
Baseline insomnia and poor sleep quality were associated with worse pain outcome trajectories
Funding:
This study was sponsored by the National Institutes of Health (R21 NR017312, PI: Palermo). RV’s work on this project was supported by the Spanish Ministry of Science and Innovation with a Ramon y Cajal contract (RYC2018-024722-I). AK’s work on this project was supported by the Medical Scholars Program.
Footnotes
Disclosures
Conflict of Interest: No conflicts exist.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Astill RG, Van der Heijden KB, Van Ijzendoorn MH, Van Someren EJ. Sleep, cognition, and behavioral problems in school-age children: a century of research meta-analyzed. Psychol. Bull. 138:1109–1138, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Babiloni AH, Beetz G, Tang NKY, Heinzer R, Nijs J, Martel MO, Lavigne GJ. Towards the endotyping of the sleep-pain interaction: a topical review on multitarget strategies based on phenotypic vulnerabilities and putative pathways. Pain. 162:1281–1288, 2021 [DOI] [PubMed] [Google Scholar]
- 3.Boggero IA, Krietsch KN, Pickerill HM, Byars KC, Homan KJ, Williams SE, King CD. Improvements in Sleep Correlate With Improvements in Clinical Outcomes Among Adolescents Undergoing Intensive Interdisciplinary Pain Treatment. Clin. J. Pain. 37:443–453, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bromberg MH, de la Vega R, Law EF, Zhou C, Palermo TM. Development and Validation of the Adolescent Insomnia Questionnaire. J. Pediatr. Psychol. 45:61–71, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butbul Aviel Y, Stremler R, Benseler SM, Cameron B, Laxer RM, Ota S, Schneider R, Spiegel L, Stinson JN, Tse SM, Feldman BM. Sleep and fatigue and the relationship to pain, disease activity and quality of life in juvenile idiopathic arthritis and juvenile dermatomyositis. Rheumatology (Oxf). 50:2051–2060, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Byars KC, Simon SL, Peugh J, Beebe DW. Validation of a Brief Insomnia Severity Measure in Youth Clinically Referred for Sleep Evaluation. J. Pediatr. Psychol. 42:466–475, 2017 [DOI] [PubMed] [Google Scholar]
- 7.Caes L, Dick B, Duncan C, Allan J. The Cyclical Relation Between Chronic Pain, Executive Functioning, Emotional Regulation, and Self-Management. J. Pediatr. Psychol. 46:286–292, 2021 [DOI] [PubMed] [Google Scholar]
- 8.de la Vega R, Miro J. The assessment of sleep in pediatric chronic pain sufferers. Sleep Med Rev. 2012 [DOI] [PubMed] [Google Scholar]
- 9.Ellenbogen JM, Hu PT, Payne JD, Titone D, Walker MP. Human relational memory requires time and sleep. Proc Natl Acad Sci U S A. 104:7723–7728, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Essner B, Noel M, Myrvik M, Palermo T. Examination of the Factor Structure of the Adolescent Sleep-Wake Scale (ASWS). Behav. Sleep. Med. 13:296–307, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans S, Djilas V, Seidman LC, Zeltzer LK, Tsao JCI. Sleep Quality, Affect, Pain, and Disability in Children With Chronic Pain: Is Affect a Mediator or Moderator? J Pain. 18:1087–1095, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fales J, Palermo TM, Law EF, Wilson AC. Sleep outcomes in youth with chronic pain participating in a randomized controlled trial of online cognitive-behavioral therapy for pain management. Behav. Sleep. Med. 13:107–123, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. Journal of Pain. 14:1539–1552, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finan PH, Quartana PJ, Remeniuk B, Garland EL, Rhudy JL, Hand M, Irwin MR, Smith MT. Partial Sleep Deprivation Attenuates the Positive Affective System: Effects Across Multiple Measurement Modalities. Sleep. 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fisher E, Law E, Dudeney J, Eccleston C, Palermo TM. Psychological therapies (remotely delivered) for the management of chronic and recurrent pain in children and adolescents. Cochrane Database Syst Rev. 4:CD011118, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fisher E, Law E, Dudeney J, Palermo TM, Stewart G, Eccleston C. Psychological therapies for the management of chronic and recurrent pain in children and adolescents. Cochrane Database Syst Rev. 9:CD003968, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forrest CB, Bevans KB, Pratiwadi R, Moon J, Teneralli RE, Minton JM, Tucker CA. Development of the PROMIS (R) pediatric global health (PGH-7) measure. Qual. Life Res. 23:1221–1231, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gioia G, Isquith PK, Guy SC, Kenworthy L: Behavior rating inventory of executive function (BRIEF-2), Psychological Assessment Resource, Lutz, FL, 2016. [Google Scholar]
- 19.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 42:377–381, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holley AL, Zhou C, Wilson AC, Hainsworth K, Palermo TM. The CALI-9: A brief measure for assessing activity limitations in children and adolescents with chronic pain. Pain. 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huguet A, Miro J. The severity of chronic pediatric pain: an epidemiological study. J Pain. 9:226–236, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Irwin DE, Stucky B, Langer MM, Thissen D, Dewitt EM, Lai JS, Varni JW, Yeatts K, DeWalt DA. An item response analysis of the pediatric PROMIS anxiety and depressive symptoms scales. Qual. Life Res. 19:595–607, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.King S, Chambers CT, Huguet A, MacNevin RC, McGrath PJ, Parker L, MacDonald AJ. The epidemiology of chronic pain in children and adolescents revisited: A systematic review. Pain. 152, 2011. [DOI] [PubMed] [Google Scholar]
- 24.LaPlant MM, Adams BS, Haftel HM, Chervin RD. Insomnia and quality of life in children referred for limb pain. J Rheumatol. 34:2486–2490, 2007 [PubMed] [Google Scholar]
- 25.Laurent J, Catanzaro SJ, Joiner TE, Rudolph KD, Potter KI, Lambert S, Osborne L, Gathright T. A Measure of Positive and Negative Affect for Children: Scale Development and Preliminary Validation. Psychological Assessment. 11:326–338, 1999 [Google Scholar]
- 26.Law EF, Tham SW, Aaron RV, Dudeney J, Palermo TM. Hybrid cognitive-behavioral therapy intervention for adolescents with co-occurring migraine and insomnia: A single-arm pilot trial. Headache. 58:1060–1073, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lewandowski AS, Palermo TM, Motte SDl, Fu R. Temporal daily associations between pain and sleep in adolescents with chronic pain versus healthy adolescents. Pain. 151:220–225, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Logan DE, Sieberg CB, Conroy C, Smith K, Odell S, Sethna N. Changes in sleep habits in adolescents during intensive interdisciplinary pediatric pain rehabilitation. J Youth Adolesc. 44:543–555, 2015 [DOI] [PubMed] [Google Scholar]
- 29.Meltzer LJ, Logan DE, Mindell JA. Sleep patterns in female adolescents with chronic musculoskeletal pain. Behav. Sleep. Med. 3:193–208, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Meltzer LJ, Montgomery-Downs HE, Insana SP, Walsh CM. Use of actigraphy for assessment in pediatric sleep research. Sleep Med Rev. 16:463–475, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morgenthaler T, Kramer M, Alessi C, Friedman L, Boehlecke B, Brown T, Coleman J, Kapur V, Lee-Chiong T, Owens J, Pancer J, Swick T, American Academy of Sleep M. Practice parameters for the psychological and behavioral treatment of insomnia: an update. An american academy of sleep medicine report. Sleep. 29:1415–1419, 2006 [PubMed] [Google Scholar]
- 32.Murray CB, de la Vega R, Loren DM, Palermo TM. Moderators of Internet-Delivered Cognitive-Behavioral Therapy for Adolescents With Chronic Pain: Who Benefits From Treatment at Long-Term Follow-Up? J Pain. 21:603–615, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palermo TM, Beals-Erickson S, Bromberg M, Law E, Chen M. A Single Arm Pilot Trial of Brief Cognitive Behavioral Therapy for Insomnia in Adolescents with Physical and Psychiatric Comorbidities. J Clin Sleep Med. 13:401–410, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palermo TM, de la Vega R, Murray C, Law E, Zhou C. A digital health psychological intervention (WebMAP Mobile) for children and adolescents with chronic pain: results of a hybrid effectiveness-implementation stepped-wedge cluster randomized trial. Pain. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palermo TM, Law EF, Fales J, Bromberg MH, Jessen-Fiddick T, Tai G. Internet-delivered cognitive-behavioral treatment for adolescents with chronic pain and their parents: a randomized controlled multicenter trial. Pain. 157:174–185, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palermo TM, Walco GA, Paladhi UR, Birnie KA, Crombez G, de la Vega R, Eccleston C, Kashikar-Zuck S, Stone AL. Core outcome set for pediatric chronic pain clinical trials: results from a Delphi poll and consensus meeting. Pain. 162:2539–2547, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palermo TM, Wilson AC, Lewandowski AS, Toliver-Sokol M, Murray CB. Behavioral and psychosocial factors associated with insomnia in adolescents with chronic pain. Pain. 152:89–94, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palermo TM, Wilson AC, Peters M, Lewandowski A, Somhegyi H. Randomized controlled trial of an Internet-delivered family cognitive-behavioral therapy intervention for children and adolescents with chronic pain. Pain. 146:205–213, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pilcher JJ, Morris DM, Donnelly J, Feigl HB. Interactions between sleep habits and self-control. Frontiers in human neuroscience. 9:284, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roth-Isigkeit A, Thyen U, Stoven H, Schwarzenberger J, Schmucker P. Pain among children and adolescents: Restrictions in daily living and triggering factors. Pediatrics. 115:152–162, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Selvanathan J, Pham C, Nagappa M, Peng PWH, Englesakis M, Espie CA, Morin CM, Chung F. Cognitive behavioral therapy for insomnia in patients with chronic pain - A systematic review and meta-analysis of randomized controlled trials. Sleep Med Rev. 60:101460, 2021 [DOI] [PubMed] [Google Scholar]
- 42.Smith MT, McCrae CS, Cheung J, Martin JL, Harrod CG, Heald JL, Carden KA. Use of Actigraphy for the Evaluation of Sleep Disorders and Circadian Rhythm Sleep-Wake Disorders: An American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 14:1231–1237, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang NK. Cognitive behavioural therapy in pain and psychological disorders: Towards a hybrid future. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2017 [DOI] [PubMed] [Google Scholar]
- 44.Team RC. R: A language and environment for statistical computing. Vienna, Austria, 2020 [Google Scholar]
- 45.Valrie CR, Bromberg MH, Palermo T, Schanberg LE. A systematic review of sleep in pediatric pain populations. J Dev Behav Pediatr. 34:120–128, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Varni JW, Seid M, Smith Knight T, Burwinkle T, Brown J, Szer IS. The PedsQL in pediatric rheumatology: reliability, validity, and responsiveness of the Pediatric Quality of Life Inventory Generic Core Scales and Rheumatology Module. Arthritis Rheum. 46:714–725, 2002 [DOI] [PubMed] [Google Scholar]
- 47.von Baeyer CL, Spagrud LJ, McCormick JC, Choo E, Neville K, Connelly MA. Three new datasets supporting use of the Numerical Rating Scale (NRS-11) for children’s self-reports of pain intensity. Pain. 143:223–227, 2009 [DOI] [PubMed] [Google Scholar]
- 48.Wagner U, Gais S, Haider H, Verleger R, Born J. Sleep inspires insight. Nature. 427:352–355, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Walker MP, Liston C, Hobson JA, Stickgold R. Cognitive flexibility across the sleep-wake cycle: REM-sleep enhancement of anagram problem solving. Brain Res. Cogn. Brain Res. 14:317–324, 2002 [DOI] [PubMed] [Google Scholar]
- 50.Walker MP, Stickgold R. Overnight alchemy: sleep-dependent memory evolution. Nat Rev Neurosci. 11:218; author reply 218, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ward TM, Archbold K, Lentz M, Ringold S, Wallace CA, Landis CA. Sleep disturbance, daytime sleepiness, and neurocognitive performance in children with juvenile idiopathic arthritis. Sleep. 33:252–259, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yoon IA, Sturgeon JA, Feinstein AB, Bhandari RP. The role of fatigue in functional outcomes for youth with chronic pain. Eur. J. Pain. 23:1548–1562, 2019 [DOI] [PubMed] [Google Scholar]


