Abstract
Objective:
To test a model of non-improvement in chronic fatigue syndrome (CFS) utilizing self-report activity patterns (e.g., “push-crash”), uplifts and hassles, and a biological measure of cardiac autonomic function. Activity pattern impacts on symptoms and objective measures of autonomic and physical activity were also examined.
Methods:
This prospective study in CFS collected all data remotely, including six months of weekly web diaries that recorded symptom ratings, activity patterns, and hassles and uplifts. In addition, six months of weekly heart monitoring and three months of daily waking actigraphy data were collected. Improvement or non-improvement status was assessed using semi-structured interviews at 6 months follow-up.
Results:
148 individuals (87.2% female) were enrolled and 12.2% were lost to follow-up. Participants reporting non-improvement (n=92), as compared to improvement (n=38) showed greater autonomic dysfunction (lower heart rate variability [HRV], group difference =5.93 (SE= 2.73) ms; p=.032) and lower mean intensity of behavioral uplifts (group difference=0.14 (SE=0.16); p=.043), but no significant differences in any activity pattern, including push-crash, limiting activity, and healthy pacing.
Conclusions:
This study provided evidence for linking patient-reported non-improvement to a biological variable indexing autonomic dysfunction and a behavioral measure indicating a deficit in psychological uplifts. These findings suggest a possible marker of illness trajectory that could potentially advance the biomedical underpinnings of CFS.
Keywords: chronic fatigue syndrome, heart rate variability, actigraphy, push-crash, uplifts, improvement
Introduction
Non-improvement in chronic fatigue syndrome (CFS) is a rarely studied, but commonly reported outcome, as roughly half of patients report worsened or unchanged illness in both naturalistic studies (1, 2) and behavioral intervention trials (3, 4). Patients often attribute illness non-improvement to less controllable factors such as lack of effective treatments or negative life events, e.g., job loss (5, 6). Non-improvement in CFS may reflect higher fatigue and greater limitations in activities of daily living which have both been correlated with autonomic nervous system dysregulation, as indicated by reduced heart rate variability (HRV) (7–9). These findings suggest the possibility that autonomic dysfunction in CFS may be associated with potentially identifiable activity patterns that could contribute to non-improvement. A clinical implication would be that interventions focused on HRV enhancement might yield symptomatic improvement, cf. (10), and lead to targeted research on autonomic interventions in CFS and related illnesses.
Commonly reported activity patterns in CFS that may be associated with autonomic dysfunction include “push-crash” (11, 12), a presumably unhealthy symptom-producing behavior of unsustainable high activity levels followed by steep declines into inactivity. “Push-crash” has been evaluated in studies of inter- and intra-day associations between objective physical activity levels and symptoms in CFS (13, 14, 7). Another apparently unhelpful pattern, limiting activity (13, 15), appears to be associated with very low objective activity levels (13), e.g., homebound individuals. In contrast, healthy pacing (16), a strategy of balancing activity and rest to maximize sustained functioning, may be manifested in staggering of activities at lower intensity levels to reduce symptom severity and variability on the same and next day (7). Another potentially adaptive activity pattern, practical social support seeking, has shown significant associations with greater illness control (17), improved quality of life (18), and illness improvement (19) in CFS studies. To our knowledge, helpful and unhelpful activity patterns and their associations with potential biological indicators of CFS and global outcomes have not previously been investigated.
In addition to activity patterns, the behavioral variables of uplifts (minor pleasant events, e.g., intimacy) and hassles (minor stressors, e.g., workload) may also influence outcomes in CFS. Hassles have been examined in a cross-sectional study (20) of newly diagnosed patients with CFS and fibromyalgia as compared to patients with multiple sclerosis. The authors reported that the combined CFS and fibromyalgia group showed a higher frequency of daily hassles and higher emotional impact of daily hassles. Furthermore, higher levels of pleasant activities and life events, i.e., uplifts, have been associated with significantly improved outcomes, i.e., reduced fatigue and impairment, in a one-year prospective study of 130 patients with CFS (21). A clinical model of behavioral intervention in CFS (11) suggested therapeutic prescription of uplifting activities and also the use of coping skills to diminish the impact of hassles.
To examine potential biobehavioral underpinnings of non-improvement and improvement in CFS, we hypothesized (Figure 1) that self-report non-improvers as compared to improvers at six-month follow-up assessments will show significant differences in these dimensional variables: (a) illness-exacerbating and illness-moderating activity patterns; (b) weekly hassles and uplifts; and (c) an objective indicator of autonomic dysregulation (heart rate variability, or HRV). Non-improvement and improvement reports were based on patients’ global impression of change (PGIC) ratings. The PGIC is used for determining clinically important changes in symptom severity, physical functioning and mood in chronic fatigue and chronic pain (19, 22, 23, 24).
Figure 1.
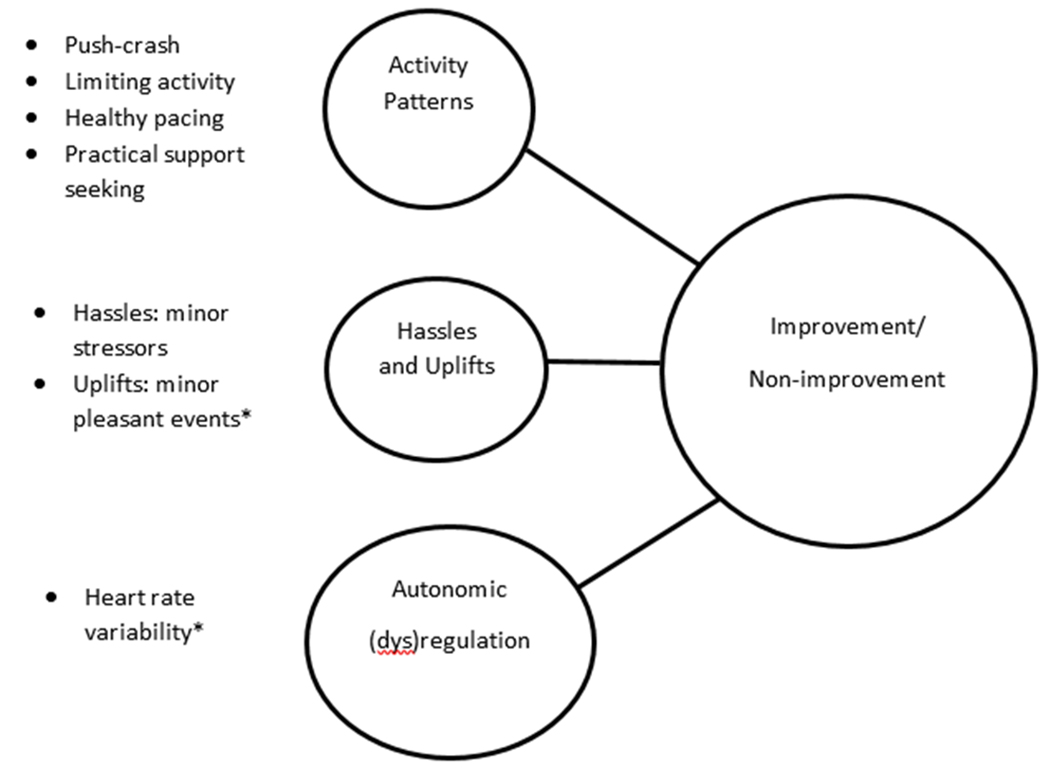
Model of biobehavioral differences between improvers and non-improvers with CFS.
*p < .05
Given the potential importance of activity patterns as an outcome predictor, we also sought to validate the presumed impacts of the activity patterns of push-crash, limiting activity, healthy pacing and practical social support on symptom levels and objective measures of autonomic (HRV) and physical activity. At baseline, standard questionnaire data were collected for fatigue, physical function, depression and anxiety, and autonomic symptoms, e.g., orthostatic intolerance or secretomotor-related complaints. This six-month prospective observational study of individuals with CFS collected all data remotely from participants in their homes without any in-person visits.
Methods
Sample
Participant Recruitment
Nationwide recruitment in the United States began in September, 2016 and ended in October, 2019. Recruitment methods included study announcements posted on major CFS patient support websites (e.g., Health Rising, SolveME) and in the large private practices of CFS specialized physicians located in New York and Utah. Without a travel requirement, this home-based study was considered more likely to recruit these under-served patients, particularly those who were disabled and homebound (25).
Initial screening
The initial screening of prospective participants for CFS was conducted by the project nurses (PB, MM) utilizing a validated phone interview (1). Selection criteria were: (a) age between 21-65, the lower age limit intended to avoid cases that often resolve prior to adulthood (26) and the upper limit of 65 intended to exclude confounding comorbidities associated with older age (27); (b) Fukuda-based CFS symptoms (27) including: six months of medically unexplained, debilitating fatigue plus 4/8 secondary symptoms, i.e., impaired memory or concentration, unrefreshing sleep, sore throats, headache, muscle pain, joint pain, tender lymph nodes, post-exertional malaise; and (c) absence of exclusionary illnesses. To meet CFS symptom criteria, each of the 4 qualifying symptoms had to be endorsed by prospective participants with a frequency of “sometimes” or greater and an intensity of “moderate” or higher (28). The phone interviewer identified medical exclusions of cases of fatigue clearly attributable to self-report medical conditions, e.g., untreated hypothyroidism (27). Exclusionary psychiatric disorders included any psychosis, or alcohol/substance abuse within two years prior to illness onset and any time afterward, and current or past depression with melancholic or psychotic features within 5 years prior to onset of CFS or anytime afterward (27).
Procedure
This six-month observational study of individuals with CFS collected all data remotely from participants in their homes. There were no in-person visits. Based on previous outcome studies in CFS (1), it was expected that half of the sample would rate their illness as non-improved and half as improved on the PGIC rating. The study procedures, carried out concurrently over six months, included: (a) a participant eligibility phone screening followed by (b) mailing, completion and return of the informed consent form and standard questionnaires; (c) mailing and return of actigraphs and heart monitors; (f) six months of weekly web diary and HRV assessments and three months of daily actigraphy recordings; (g) six month follow-up interviews to administer a functional assessment (LIFE-RIFT) and PGIC rating and (h) data analysis. This study was approved by the Stony Brook University Committee on Research Involving Human Subjects and all participants provided informed consent. The study was pre-registered on ClinicalTrials.gov (NCT02948556).
Standard Questionnaires
The following validated measures provided standardized baseline data.
Fatigue Severity Scale (FSS).
This measure of the effect of fatigue on functioning is comprised of nine items rated on a seven-point Likert-type rating scale, where one indicates no impairment and seven indicates severe impairment (score range: 1.00-7.00). In the initial validation study (29), internal consistency for the FSS was excellent (Cronbach’s α =0.80) and the scale clearly distinguished between patients and controls. The scale, recommended for use in CFS (30), showed high alpha consistency in our sample (Cronbach’s α =0.87).
SF-36 Physical Function Subscale (PFS).
The PFS (31) of the SF-36 measures physical limitations of ill health on a scale of 0 to 100, where 0 indicates limited in all activities, including basic self-care and 100 indicates no limitations. The PFS is composed of ten items where each item is scored on the basis of the limitations perceived by surveyed individuals. Item scores (1, 2, or 3) are summed to obtain a total score. Normative values are available for the PFS for the US population. This subscale has shown good internal consistency (Cronbach’s α ≥ .81) in psychometric studies (31) and in our sample (α =0.88).
The Depression, Anxiety and Stress Scale (DASS-21).
This validated 21-item measure contains three seven-item subscales reflecting domains of psychological distress: anxiety, depression and stress. Patients are asked to score every item on a scale from 0 (did not apply to me at all) to 3 (applied to me very much). Domain scores are calculated by summing all items in a domain and multiplying by two (31). Sum scores for the total DASS-21 total scale range between 0 and 120, and those for each of the subscales range between 0 and 42. The DASS-21 has been used to assess stress factors in fibromyalgia (32), a symptomatically overlapping illness. Its internal consistency in our sample was excellent (Cronbach’s α = 0.92).
Composite Autonomic Symptom Score-31 (COMPASS 31) (33, 34).
This validated questionnaire of autonomic symptoms consists of six domains: orthostatic intolerance, vasomotor, secretomotor, gastrointestinal, bladder and pupillomotor. The final domain scores are generated by multiplying the raw score with a weight index. The six scores sum to a total score of 0 to 100, with higher scores indicating more severe autonomic symptoms. Internal consistency for the six domains range from 0.71 to 0.93 (33). In a recent population study (35), the COMPASS 31 indicated that 50% of individuals with CFS had symptoms consistent with autonomic dysfunction, i.e., orthostatic intolerance.
Online Web Diary
The online web diary (StudyTrax, Inc., Macon, Georgia) was scheduled at the end of each week to capture illness-related experiences for each prior 7-day period during the six-month assessment period. The end-of-week recall of symptom and activity reports are reasonably accurate representations of symptoms experienced and activities undertaken as compared to daily reports for the same measures in ecological studies (2, 36). Participants rated symptom intensity (highest, lowest and average ratings for fatigue pain, and stress) for each week.
Activity pattern subscales.
Also included on the web diary were two validated factor scales (13 items) from the Behavioral Responses to Illness Questionnaire (BRIQ)(15) that reflect specific behavioral patterns linked to non-improvement, i.e., push-crash (over-exertion followed by collapse into inactivity, e.g., lying in bed) and limiting activity (consistently low activity levels; e.g., homebound individuals). Behavioral patterns associated with patient-reported improvement were assessed with the 4-item BRIQ factor, Practical Support Seeking (e.g., asked for help from my family or friends), and the author-constructed (FF) 6-item “healthy pacing” scale that includes items about relaxation, walking, and pacing. The healthy pacing scale showed adequate internal consistency (Cronbach’s α = .70) and significant associations with PGIC ratings in pilot data. Activity pattern subscale items are available in Supplemental Digital Content 1.
Hassles and Uplifts Scale.
The weekly web diary also contained the 53-item Combined Hassles and Uplifts Scale (CHUS)(20) which measures minor daily stressors (expected link to non-improvement) and behavioral uplifts (linked to improvement) in relation to family members, work, health, etc. The CHUS yields subscales of frequency and intensity. Scores of hassles and uplifts frequency range from 0 to 53, with the total score indicating how many items were simply endorsed. When endorsing an item, participants are asked to rate how much of a hassle or uplift the specific item was. Items are rated on a 3-point Likert-based scale ranging from 1 (somewhat), 2 (quite a bit), to 3 (a great deal). The average rating of these items yields intensity scores. Participants may rate events as hassles, uplifts, or both. The CHUS has shown good reliability and validity, predicting mood and somatic health outcomes (37, 20).
Objective Measures
Accelerometry.
Our accelerometer, the actigraph model GT9X (Actigraphcorp, Fort Walton Beach, Florida) is a computerized waist worn device that records movement and provides a useful portable method for assessing activity patterns. The device captures and records high resolution raw acceleration data, which are converted into Activity Counts using publicly available algorithms. Waking actigraphy data were collected daily for the initial 90-day assessment period to objectively examine CFS activity patterns (38), i.e., mean of 90-day actigraphy counts and actigraphy variability, potentially related to push/crash, limiting activity, and healthy pacing (Figure 2).
Figure 2.
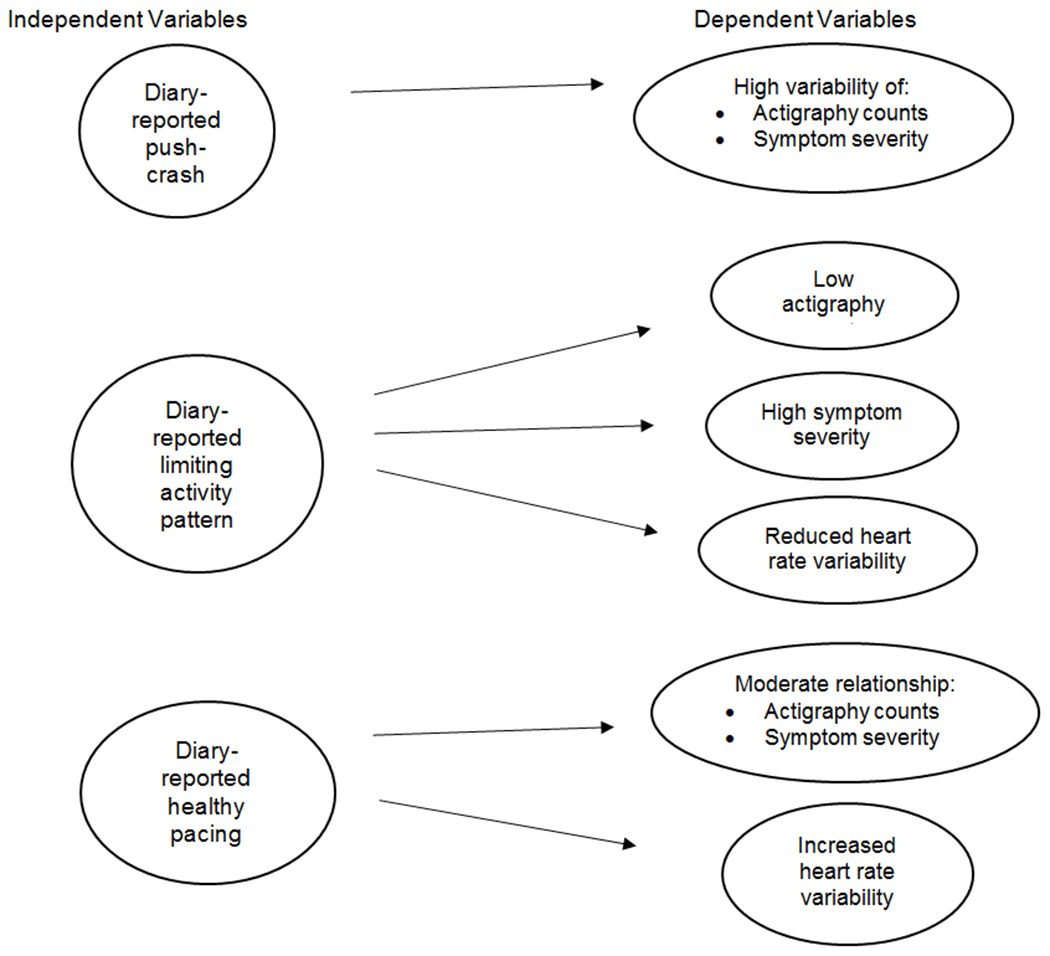
Model of activity pattern predictors of actigraphy counts, symptoms, and heart rate variability.
Heart Rate Monitor.
HRV is a non-invasive measure of cardiac autonomic function. HRV was calculated via electrocardiogram (ECG) collected with a research-grade multifunction ambulatory heart monitor (eMotion Faros 180; MegaElectronics, Kuopio, Finland). ECG data were collected at a 500Hz sampling rate using a 3-lead configuration. Data were exported for analysis in the Kubios HRV analysis suite (version 3) for R-peak detection and the visual inspection of artifacts (e.g., non-sinus beats, movement), and HRV calculation. R-peaks were detected using a modified Pan-Tompkins algorithm (39, 40). Detected artifacts were corrected by replacing artifacts with interpolated values via cubic spline interpolation.
The root mean square of successive differences (RMSSD) was then calculated to estimate HRV. RMSSD reflects beat-to-beat variance in heart rate and is the primary time-domain measure used to estimate the vagally mediated changes reflected in HRV (41). The home-worn device measured HRV at these times: (a) During sleep on the first two nights of the baseline week; and (b) 10 min. each week for 25 weeks during the six-month assessment period. To approximate a standard setting, HRV data were collected by participants at home while sitting in a comfortable chair for ten minutes in the evening between 7pm-9pm. A small beeper-size heart monitor was sent to each participant. Proper electrode placement was guided by written instructions and confirmed with an initial staff phone call to each participant. Consistent with recommendations (42), inter-beat intervals recorded for five min are sufficient to approximate parasympathetic outflow to the heart (43). The first two minutes of data were discarded to provide an acclimatization period.
Semi-structured interview (six month follow-up)
Nurse-conducted (PB, MM) phone interviews at six month follow-up included these measures:
The (Modified) Range of Impaired Functioning Tool (LIFE-RIFT).
The LIFE-RIFT (44) is a brief (10 min), reliable and valid semi-structured interview for assessing functional impairment (none to severe) in relation to current illness, as administered by a trained interviewer. Each item targets functioning in one of four domains: work, interpersonal relations, recreation and global satisfaction. The interviewer asks questions guided by the behavioral anchors provided for each item in order to obtain the information needed to make each rating on a six-point scale. The inter-rater reliability of LIFE-RIFT ratings, based on two independent raters of 15 (12%) interviews, was excellent for all four scales (Intraclass correlations: Work: 0.91; Interpersonal: 0.89; Recreation: 0.98; Global Satisfaction: 1.00)
Patient Global Impression of Change (PGIC) rating.
A PGIC rating was taken during the phone interview, based on seven levels of change ranging from very much worse to very much improved (2) as it applied to the prior six months. Participants who responded with a PGIC rating of “very much worse,” “much worse,” “somewhat worse” or “unchanged” were assigned to the “non-improvement” subgroup, and individuals who responded with a rating of “very much improved,” “much improved,” or “somewhat improved” were assigned to the “improvement” subgroup. This was the last measure in the interview to ensure that the assignment of participants to non-improvement or improvement subgroups did not influence responses to the other interview measures. The non-improvement and improvement subgroups constituted a binary dependent variable upon which our prediction model (Figure 1) was based, i.e., activity patterns, minor life events, and HRV as predictors of non-improvement or improvement. The PGIC rating, providing a generalized view of the patient’s perception of overall change (22, 24), has shown construct validity in CFS studies on standard measures of fatigue and functioning (2).
Power estimation.
With 7 hypothesized predictors for non-improvement or improvement (push-crash, limiting activity, healthy pacing and practical social support scores, hassles and uplifts scores, and HRV), a conservative type I error was set at 0.008 (=0.05/6) using Bonferroni adjustment. A logistic regression of a binary response variable (non-improvement vs. improvement) on a continuous, normally distributed variable (X) with a sample size of 120 observations achieves 80% power at a 0.008 significance level to detect a change in the probability of non-improvement from the value of 0.500 at the mean of X to 0.654 when X is increased to one standard deviation above the mean. This change corresponds to an odds ratio (OR) of 1.892, which is a relatively low OR (Software: PASS 12) (45). Generally, an OR of 1.5 is considered small and an OR of 2.50 is considered moderate.
Data analysis
Standard descriptive statistics characterized the study sample. Participants were classified into two groups: non-improvers and improvers based on PGIC ratings at the six month follow-up. For our improvement/non-improvement hypothesis (Figure 1), independent t-tests were used to test for differences between the binary classifier of non-improvement/improvement and these predictors, based on 25-week means: (i) illness-exacerbating (push-crash, limiting activity) and illness moderating (healthy pacing, practical social support seeking) behavioral patterns; (ii) hassles and uplifts; and (iii) autonomic dysfunction as measured with weekly HRV. In order to verify that group differences in HRV were not simply due to symptomatic differences, Wilcoxon rank sum tests were used to compare improvers and non-improvers at baseline week and follow-up week for HRV and fatigue, pain and stress ratings. Finally, our post-hoc trend analysis used one-way ANOVA. For improver and non-improver groups separately, weekly fatigue means were coded into 3 groups, weeks 1-8, 9-16, and 17-25 with the linear trend based on fatigue change across the 3 time-coded groups.
In our model of activity pattern predictors of objective and self-report measures, based on 13-week means (Figure 2), the Spearman rank correlation was used to measure the relationship of self-report activity patterns to actigraphy counts, symptom severity (fatigue and pain intensity), and HRV. Statistical analysis was performed using SAS 9.4 (SAS Institute Inc., Cary, NC) and significance level was set at 0.05.
Results
Participant Characteristics
Of the 284 phone-screened individuals, 113 (39.8%) were excluded because of not meeting entry criteria and 23 (8.1%) declined to participate. One hundred forty-eight individuals were enrolled and 12.2% were lost to follow-up. Most participants (Table 1) were in their 40s, female (87.2%), white (90.3%), and ill for over a decade. Baseline questionnaire scores showed high fatigue severity, impaired physical function, and elevated autonomic symptoms. The correlation matrix for baseline measures is presented in Supplemental Table S1, Supplemental Digital Content 2. COMPASS 31 (autonomic symptom) scores were significantly correlated with: the SF36 PFS (r=.32; p=.0008), DASS anxiety (r=.49; p=.0001), DASS Depression (r=.30; p=.004), and DASS stress (r=.24; p=.009) scores.
Table 1.
Baseline demographic characteristics of CFS study sample.
| Age, M(SD) | 46.11 (11.8) |
| Length of illness in years, M(SD) | 16.5 (10.3) |
| Sex, female n(%) | 129 (87.2%) |
| SF36 PFS, M(SD) | 68.58 (20.6) |
| Fatigue Severity Scale, M(SD) | 6.57 (0.74) |
| COMPASS 31, M(SD) | 31.03 (15.2) |
| DASS-21 – Anxiety, M(SD) | 4.54 (3.3) |
| DASS-21 – Depression, M(SD) | 4.31 (4.3) |
| DASS-21 – Stress, M(SD) | 7.06 (3.7) |
| Marital Status, n(%) | |
| Single/divorced/separated | 67 (47.9%) |
| Living with partner | 71 (50.8%) |
| Ethnicity, n(%) | |
| European | 130 (90.9%) |
| Latino | 7 (4.9%) |
| Black | 4 (2.8%) |
| Asian | 2 (1.4%) |
| Native American | 1 (0.7%) |
| Employment, n(%) | |
| Working full time | 25 (19.2%) |
| Working half time/part time | 14 (10.8%) |
| Unemployed | 44 (33.8%) |
| Receiving disability benefits | 44 (33.8%) |
SF36 PFS = Short-form 36 Physical Function Subscale; COMPASS = Composite Autonomic Symptom Score-31; DASS-21 = Depression, Anxiety and Stress Scale.
Activity Pattern Subscales: internal consistency and test-retest reliability
The mean internal consistency values (Cronbach’s α values) for activity pattern subscales over 25 weekly tests were as follows: push-crash=.90, limiting activity =.73; social support seeking=.66 and healthy pacing = .90. Levels => .70 are considered acceptable (46, 47). For test-retest reliability, an intraclass correlation (ICC) ≥0.70 is regarded as an acceptable level (46, 47). Only the social support seeking scale reached acceptable test-retest reliability (.70), while the ICCs for push-crash, limiting activity and healthy pacing scales, ranged from .41-.47. Although below acceptable levels, these scales raise potentially interesting questions about the short-term stability of activity patterns in CFS (see Discussion).
Improvers and non-improvers
A significant difference in 25-week average fatigue level was found between improvers and non-improvers (t=2.56 (126); p=.012; Cohen’s f=1.38). Given a visually apparent decline in fatigue ratings across the 25 weeks, particularly in the improver group, we tested for and found a significant 25-week linear trend for both the improver (F (2, 22) = 13.62; p<.001; η2= 0.65) and to a lesser extent the non-improver (F (2, 22) = 6.38; p=.003; η2 = 0.46) subgroups. Specifically, fatigue declined by 14.8% in the improver group and by 0.03% in the non-improver group. In the improver group only, a declining (F (2, 22) =.4.44; p=.024; η2 = .31) trend was found in Stress ratings. No trend in pain ratings was found. In addition, no significant difference in overall functioning (LIFE-RIFT) was found between improvement and non-improvement subgroups, although the global change rating was significantly associated with overall functioning (r=.26; p=.006).
Global ratings at six month follow-up indicated far more non-improved (n=92) than improved (n=38) participants. No significant differences were found on any baseline self-report measure when comparing non-improvers to improvers at six month follow-up (Table 2). The group classifier of improvement/non-improvement (Figure 1; Figure 3) showed significant differences on 25-week means for HRV values (p= .032) and behavioral uplift intensity (p= .043) (Table 3), indicating decreased HRV and decreased intensity of uplifts in the non-improvement subgroup. The HRV distinction between improvers and non-improvers was also found at baseline (p=.013) and follow-up (p=.038) weeks. By comparison, symptom ratings (fatigue, pain) were not significantly different (prange: .055-.993) between the 2 groups at baseline and follow-up weeks. No significant differences on the improvement/non-improvement classifier were found for any activity pattern or for the hassles scale scores. The improvement/non-improvement distinction is based on these seven categories of the PGIC, indicating the number of participants in each category: Improved [very much improved (n=2), much improved (n=10), somewhat improved (n= 26)], and non-improved [unchanged (n=43), somewhat worse (n=26), much worse (n= 16), very much worse (n= 7)].
Table 2.
Improvement and non-improvement differences in baseline demographic characteristics of CFS study sample.
| Improvement Subgroup n=38 | Non-improvement Subgroup n=92 | Effect Size | P | |
|---|---|---|---|---|
| Age, M(SD) | 46.0 (13.5) | 46.2 (12.0) | −0.02* | 0.94 |
| Length of illness in years, M(SD) | 12.8 (9.5) | 15.5 (9.7) | −0.28* | 0.18 |
| Sex, female n(%) | 36 (94.7) | 80 (86.9) | 0.23** | 0.08 |
| SF36 PFS, M(SD) | 68.2 (23.4) | 70.4 (19.9) | −0.1* | 0.62 |
| Fatigue Severity Scale, M(SD) | 6.6 (0.49) | 6.5 (0.91) | 0.07* | 0.73 |
| COMPASS 31, M(SD) | 31.3 (14.1) | 31.2 (15.2) | 0.01* | 0.96 |
| DASS-21 – Anxiety, M(SD) | 4.3 (3.7) | 4.2 (2.7) | 0.06* | 0.76 |
| DASS-21 – Depression, M(SD) | 4.0 (4.1) | 4.3 (3.9) | −0.08* | 0.68 |
| DASS-21 – Stress, M(SD) | 6.4 (2.9) | 6.7 (2.9) | −0.01* | 0.63 |
| Marital Status, n(%) | 0.25** | 0.06 | ||
| Single/divorced/separated | 23(52.2) | 39 (43.3) | ||
| Living with partner | 21 (47.7) | 51 (56.7) | ||
| Employment, n(%) | 0.20** | 0.22 | ||
| Working full time | 7 (18.9) | 8 (8.6) | ||
| Working half time/part time | 6 (16.2) | 5 (5.4) | ||
| Unemployed | 15 (13.5) | 34 (37.0) | ||
| Receiving disability benefits | 9 (24.3) | 45 (48.9) |
SF36 PFS = Short-form 36 Physical Function Subscale; COMPASS = Composite Autonomic Symptom Score-31;
DASS-21 = Depression, Anxiety and Stress Scale.
Cohen’s d
Cramer’s V
Figure 3.
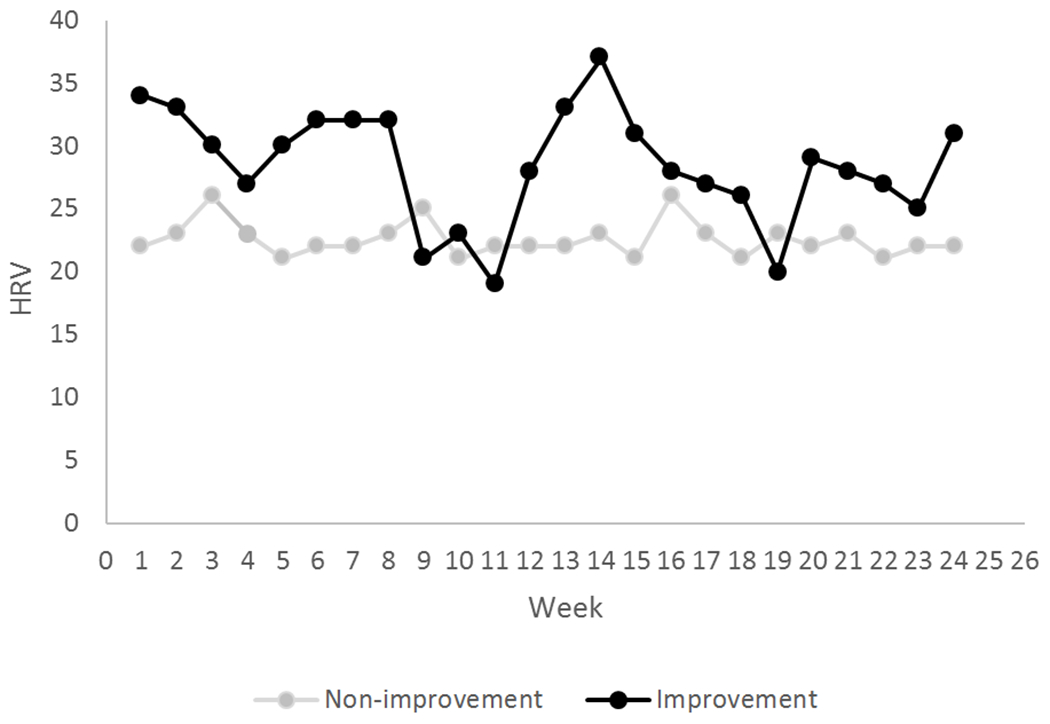
Weekly mean heart rate variability (RMSSD; ms) for self-report improvers and non-improvers over 25 weeks.
Table 3.
Twenty-five week mean scores reflecting biobehavioral differences in CFS between participants who self-report improvers and non-improvers.
| M | SE | 95% CI for Difference | n | Delta | SE Delta | t for Delta | p for Delta | D | |
|---|---|---|---|---|---|---|---|---|---|
| Push-crash | |||||||||
| Non-improvers | 4.03 | 0.09 | 91 | ||||||
| Improvers | 3.98 | 0.17 | −0.46- 0.31 | 37 | 0.05 | 0.18 | −0.28 | 0.78 | 0.04 |
| Limiting activity | |||||||||
| Non-improvers | 3.39 | 0.07 | 90 | ||||||
| Improvers | 3.25 | 0.11 | −0.12-1.39 | 37 | 0.04 | 0.13 | −1.08 | 0.28 | 0.21 |
| Healthy Pacing | |||||||||
| Non-improvers | 3.89 | 0.08 | 90 | ||||||
| Improvers | 3.81 | 0.12 | −0.20-0.37 | 37 | 0.08 | 0.14 | 0.57 | 0.57 | 0.11 |
| Practical Support | |||||||||
| Non-improvers | 4.96 | 0.12 | 90 | ||||||
| Improvers | 4.85 | 0.2 | −0.34-0.57 | 37 | 0.14 | 0.23 | 0.47 | 0.64 | 0.09 |
| Hassles Frequency | |||||||||
| Non-improvers | 21.48 | 0.72 | 90 | ||||||
| Improvers | 21.63 | 1.31 | −2.93-2.61 | 37 | 0.15 | 1.4 | −0.14 | 0.91 | 0.02 |
| Hassles Intensity | |||||||||
| Non-improvers | 1.62 | 0.04 | 90 | ||||||
| Improvers | 1.59 | 0.04 | −0.19-0.15 | 37 | 0.03 | 0.06 | 0.51 | 0.61 | 0.1 |
| Uplift Frequency | |||||||||
| Non-improvers | 18.83 | 0.8 | 90 | ||||||
| Improvers | 20.68 | 1.35 | −4.85-1.15 | 37 | 1.85 | 1.52 | −1.22 | 0.22 | 0.23 |
| Uplift Intensity | |||||||||
| Non-improvers | 1.51 | 0.04 | 90 | ||||||
| Improvers | 1.65 | 0.05 | −0.26- −0.01 | 37 | 0.14 | 0.06 | −2.08 | 0.043 | 0.4 |
| Heart Rate Variability# | |||||||||
| Non-improvers | 21.47 | 1.15 | 90 | ||||||
| Improvers | 27.4 | 3.25 | −11.33- −0.52 | 35 | 5.93 | 2.73 | −2.17 | 0.032 | 0.38 |
Root mean square standard deviation (RMSSD) (ms)
Delta = difference between participants who improved vs. participants who did not improve.
Activity pattern predictors of objective measures and symptoms
Our proposed model (Figure 2) of activity pattern predictors of HRV, actigraphy and symptoms received limited confirmation (Table 4). The push-crash pattern was not significantly related to actigraphy or symptom variability. However, consistent with the model, the limiting activity pattern was significantly related to fatigue, actigraphy counts and actigraphy variability, but not to HRV. Also as predicted, healthy pacing was significantly related to fatigue, pain, and actigraphy counts.
Table 4.
Model-predicted Spearman correlations between 13-week mean activity patterns and actigraphy, symptoms, and heart rate variability (HRV)+
| Objective measures and symptom ratings | Push-crash | Limiting activity | Healthy pacing | Practical social support seeking+ |
|---|---|---|---|---|
| Actigraphy count | 0.12 | −0.23** | 0.18* | −0.28** |
| Fatigue score | 0.22** | 0.21* | −0.19* | 0.03 |
| Pain score | 0.25** | 0.08 | −0.19* | −0.05 |
| Stress score | 0.41*** | 0.03 | −0.12 | 0.02 |
| HRV | 0.15 | 0.05 | 0.06 | 0.12 |
| Sleep HRV | 0.02 | −0.01 | 0.08 | 0.16 |
Practical social support seeking, a tested activity pattern, was not included in the original model (Figure 2).
p<.05;
p<.01.
p<.001
Discussion
This observational study of individuals with CFS sought to identify activity patterns and objective markers that would distinguish self-report non-improvement and improvement outcomes. Participants reporting non-improvement (as compared to improvement) showed greater autonomic dysfunction (reduced HRV) and decreased intensity of behavioral uplifts, but did not show significant differences in any activity pattern, including push-crash, limiting activity, healthy pacing, and practical social support seeking. Limiting activity was significantly related to fatigue and actigraphy counts, and healthy pacing was significantly related to fatigue, pain, and actigraphy counts. The practical support seeking pattern was significantly associated with actigraphy counts and stress ratings.
This is the first study as far as we are aware that confirmed a consistent biological difference, i.e., HRV, between self-report improvers and non-improvers with CFS. Reduced HRV in the non-improver group suggests an autonomic dysfunction that is relatively more severe than that found in the improver group. Both subgroups showed an HRV level far below that found in healthy individuals (8, 9). Apart from HRV, only self-report behavioral uplift intensity separated non-improver and improver subgroups. This is consistent with a prior observational study (21) showing that positive events over a one year period predicted improved outcomes in CFS. Although the effect sizes for HRV and uplift intensity were small, their potential clinical significance for individuals with ME/CFS awaits confirmation in a treatment trial focused on enhancing HRV and/or focusing on uplifts as intervention techniques.
Self-report activity patterns did show significant associations with symptom variables that are consistent with adaptive and maladaptive patterns, although the improvement/non-improvement model was not successful in distinguishing these patterns. These negative findings might suggest any of these explanations: (1) long-term outcomes are simply not sensitive to weekly self-report activity patterns; (2) activity patterns defined in this study do not capture other patterns that may be more predictive of outcomes; (3) the improvement/ non-improvement binary is too crude of a distinction to capture changes in illness trajectory. In addition, the PGIC as an indicator of global change folds in psychological impacts, such as stress/depression/anxiety which are all common in CFS and may be incorporated into one’s multifaceted perceptions of their own health status (22, 24). In addition, the low test-retest reliability of activity pattern scales suggest that these scales do not measure trait-like patterns, cf. (48). Alternatively, these measures may reflect a responsiveness to change over time (47, 49) that is confirmed by significant associations in the expected directions between actigraphy counts and both limiting activity and healthy pacing patterns.
The differences in HRV between non-improvers and improvers show some consistency with other published data. Reduced HRV in CFS has been associated with limitations in activities of daily living and higher fatigue (7–9). Short-term measurement norms for HRV (RMSSD) were published by Nunan et al. (50), largely based on individuals over 40 years of age, similar to the current study. The normative RMSSD mean was 42 ms (range:19-75). By comparison, our non-improvers’ mean RMSSD was 21.46 ms (range: 5-60) and the improvers’ mean was 27.40 ms (range: 11-90). These highly disparate HRV findings between CFS and healthy norms, further suggest that even in our improver subgroup, this biological correlate of health and well-being was well below healthy norms.
Given the relatively poor clinical outcomes in CFS studies (1, 2), the identification of biological patterns associated with non-improvement and improvement has implications for interventions. Our study findings may inform newer evidence-based approaches to potential illness improvement in CFS based on improving autonomic function and incorporating uplifting activities. In a 10-session pilot study of HRV biofeedback in fibromyalgia (10), clinically significant improvements in pain and functioning were found at 3 month follow-up. HRV biofeedback is available as a smartphone-delivered intervention for home use (51). In addition, noninvasive vagal nerve stimulation (nVNS) increased HRV and alters cardiovascular control towards parasympathetic predominance (52, 53). nVNS has been FDA-approved for migraine treatment (54). These preliminary studies suggest that increased HRV may be associated with clinical improvements. Also our uplifts data suggest a potentially significant role for incorporating uplifting activities in self-management-focused interventions. Perhaps highly positive uplifting activities, as found in the current study for the improver subgroup, may help to counteract the perceived stress factors that CFS participants cite in explaining their non-improvement status (5).
The findings of the present study need to be considered in the context of some limitations. The present analyses focused on the association of mean levels of activity patterns, hassles and uplifts, and autonomic dysregulation with a dichotomized retrospective self-report measure of improvement. It is possible that more fine-grained multivariable statistical models examining time-trajectories of these predictors as well as other measures of perceived CFS improvement (e.g., continuous and/or pre-post assessments) will shed additional light on the multiple factors associated with improvements in CFS. These limitations are to some extent offset by the unique and long-term ambulatory monitoring of multiple self-report, behavioral and physiological measures that are relevant to the understanding of CFS.
The relatively small sample size in the improver group in this study may have precluded more definitive comparisons with the non-improver subgroup. Perhaps our entirely home-based data collection selected for a more severely ill group. Also remote data collection methods may have resulted in missed or less accurate information as compared to supervised in-person visits. Furthermore, relatively low frequency predictors of illness change, i.e., major life events (55), were too few for analysis likely due to the brief study duration. Finally, as an observational study rather than a randomized trial, no definitive conclusions can be drawn about cause and effect with respect to illness predictors.
The main objective of this study, to assess biobehavioral status in CFS with the goal of developing an objective marker of illness change or trajectory, yielded a possible biological correlate of autonomic dysfunction, i.e., HRV, as well as a behavioral indicator of non-improvement, a deficit of high intensity uplifts. These findings might ultimately lead to improved illness management utilizing interventions such as HRV biofeedback. This study provided useful findings about the relationship of biology and behavior to outcomes in the setting that most accurately reflects the patient’s status: the home environment. The possibility of linking patient-reported change with a biological measure of cardiac autonomic function sensitive to illness trajectory would be a substantive advance in the biomedical validation of CFS.
Supplementary Material
Acknowledgements:
We thank Daniel Gordon, Marie Codella, Sydney Zhang, and Samantha Vasquez for their invaluable assistance in carrying out the essential support tasks involved in conducting this study.
Source of Funding and Conflicts of Interest:
The project described was supported by National Institutes of Health Grant R01NR015850; (National Institute of Nursing Research; Principal Investigator: F. Friedberg). This work was also supported in part by the National Institute of Health T32 pre-doctoral training grant: T32GM108540 (trainee: J.L.A.) The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Nursing Research or the National Institutes of Health. The authors report no conflicts of interest.
Footnotes
Trial Registration: ClinicalTrials.gov ID: NCT02948556
References
- 1.Cairns R, Hotopf M. A systematic review describing the prognosis of chronic fatigue syndrome. Occupational medicine. 2005;55(1):20–31. [DOI] [PubMed] [Google Scholar]
- 2.Friedberg F, Sohl SJ. Longitudinal change in chronic fatigue syndrome: what home-based assessments reveal. Journal of behavioral medicine. 2009;32(2):209. [DOI] [PubMed] [Google Scholar]
- 3.Price JR, Mitchell E, Tidy E, Hunot V. Cognitive behaviour therapy for chronic fatigue syndrome in adults. Cochrane Database of Systematic Reviews. 2008. (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White PD, Goldsmith KA, Johnson AL, Potts L, Walwyn R, DeCesare JC, et al. Comparison of adaptive pacing therapy, cognitive behaviour therapy, graded exercise therapy, and specialist medical care for chronic fatigue syndrome (PACE): a randomised trial. The Lancet. 2011;377(9768):823–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedberg F, Coronel J, Seva V, Adamowicz JL, Napoli A. Participant attributions for global change ratings in unexplained chronic fatigue and chronic fatigue syndrome. Journal of health psychology. 2016;21(5):690–98. [DOI] [PubMed] [Google Scholar]
- 6.Adamowicz JL, Caikauskaite I, Friedberg F, Seva V. Patient change attributions in self-management of severe chronic fatigue syndrome. Fatigue: Biomedicine, Health & Behavior. 2017 2017/January/02;5(1):21–32. [Google Scholar]
- 7.Meeus M, Van Eupen I, Van Baarle E, De Boeck V, Luyckx A, Kos D, et al. Symptom fluctuations and daily physical activity in patients with chronic fatigue syndrome: a case-control study. Archives of physical medicine and rehabilitation. 2011;92(11):1820–26. [DOI] [PubMed] [Google Scholar]
- 8.Boissoneault J, Letzen J, Robinson M, Staud R. Cerebral blood flow and heart rate variability predict fatigue severity in patients with chronic fatigue syndrome. Brain imaging and behavior. 2019;13(3):789–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Escorihuela RM, Capdevila L, Castro JR, Zaragozà MC, Maurel S, Alegre J, et al. Reduced heart rate variability predicts fatigue severity in individuals with chronic fatigue syndrome/myalgic encephalomyelitis. Journal of translational medicine. 2020;18(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hassett AL, Radvanski DC, Vaschillo EG, Vaschillo B, Sigal LH, Karavidas MK, et al. A Pilot Study of the Efficacy of Heart Rate Variability (HRV) Biofeedback in Patients with Fibromyalgia. Appl Psychophysiol Biofeedback. 2007;32(1):1–10. [DOI] [PubMed] [Google Scholar]
- 11.Friedberg F Chronic fatigue syndrome, fibromyalgia, and related illnesses: a clinical model of assessment and intervention. Journal of Clinical Psychology. 2010;66(6):641–65. [DOI] [PubMed] [Google Scholar]
- 12.Goudsmit EM, Nijs J, Jason LA, Wallman KE. Pacing as a strategy to improve energy management in myalgic encephalomyelitis/chronic fatigue syndrome: a consensus document. Disability and rehabilitation. 2012;34(13):1140–47. [DOI] [PubMed] [Google Scholar]
- 13.van der Werf SP, Prins JB, Vercoulen JH, van der Meer JW, Bleijenberg G. Identifying physical activity patterns in chronic fatigue syndrome using actigraphic assessment. Journal of psychosomatic research. 2000;49(5):373–79. [DOI] [PubMed] [Google Scholar]
- 14.Kop WJ, Lyden A, Berlin AA, Ambrose K, Olsen C, Gracely RH, et al. Ambulatory monitoring of physical activity and symptoms in fibromyalgia and chronic fatigue syndrome. Arthritis & Rheumatism: Official Journal of the American College of Rheumatology. 2005;52(1):296–303. [DOI] [PubMed] [Google Scholar]
- 15.Spence M, Moss-Morris R, Chalder T. The Behavioural Responses to Illness Questionnaire (BRIQ): a new predictive measure of medically unexplained symptoms following acute infection. Psychological medicine. 2005;35(4):583. [DOI] [PubMed] [Google Scholar]
- 16.Kos D, Van Eupen I, Meirte J, Van Cauwenbergh D, Moorkens G, Meeus M, et al. Activity pacing self-management in chronic fatigue syndrome: a randomized controlled trial. American Journal of Occupational Therapy. 2015;69(5):6905290020p1-20p11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edwards CR, Thompson AR, Blair A. An ‘overwhelming illness’ women’s experiences of learning to live with chronic fatigue syndrome/myalgic encephalomyelitis. Journal of Health Psychology. 2007;12(2):203–14. [DOI] [PubMed] [Google Scholar]
- 18.Schoofs N, Bambini D, Ronning P, Bielak E, Woehl J. Death of a lifestyle: the effects of social support and healthcare support on the quality of life of persons with fibromyalgia and/or chronic fatigue syndrome. Orthopaedic Nursing. 2004;23(6):364–74. [DOI] [PubMed] [Google Scholar]
- 19.Saltzstein BJ, Wyshak G, Hubbuch JT, Perry J. A naturalistic study of the chronic fatigue syndrome among women in primary care. General Hospital Psychiatry. 1998;20(5):307–16. [DOI] [PubMed] [Google Scholar]
- 20.Van Houdenhove B, Neerinckx E, Onghena P, Vingerhoets A, Lysens R, Vertommen H. Daily hassles reported by chronic fatigue syndrome and fibromyalgia patients in tertiary care: a controlled quantitative and qualitative study. Psychotherapy and psychosomatics. 2002;71(4):207–13. [DOI] [PubMed] [Google Scholar]
- 21.Ray C, Jefferies S, Weir WR. Life-events and the course of chronic fatigue syndrome. British Journal of Medical Psychology. 1995;68(4):323–31. [DOI] [PubMed] [Google Scholar]
- 22.Farrar JT, Young JP Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149–58. [DOI] [PubMed] [Google Scholar]
- 23.Hudson JI, Arnold LM, Bradley LA, Choy EH, Mease PJ, Wang F, et al. What makes patients with fibromyalgia feel better? Correlations between Patient Global Impression of Improvement and changes in clinical symptoms and function: a pooled analysis of 4 randomized placebo-controlled trials of duloxetine. The journal of Rheumatology. 2009;36(11):2517–22. [DOI] [PubMed] [Google Scholar]
- 24.Geisser ME, Clauw DJ, Strand V, Gendreau RM, Palmer R, Williams DA. Contributions of change in clinical status parameters to Patient Global Impression of Change (PGIC) scores among persons with fibromyalgia treated with milnacipran. PAIN®. 2010;149(2):373–78. [DOI] [PubMed] [Google Scholar]
- 25.Friedberg F, Adamowicz J, Caikauskaite I, Seva V, Napoli A. Efficacy of two delivery modes of behavioral self-management in severe chronic fatigue syndrome. Fatigue: Biomedicine, Health & Behavior. 2016;4(3):158–74. [Google Scholar]
- 26.Kendig H, Browning CJ, Young AE. Impacts of illness and disability on the well-being of older people. Disability and rehabilitation. 2000;22(1-2):15–22. [DOI] [PubMed] [Google Scholar]
- 27.Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The chronic fatigue syndrome: a comprehensive approach to its definition and study. Annals of internal medicine. 1994;121(12):953–59. [DOI] [PubMed] [Google Scholar]
- 28.King C, Jason LA. Improving the diagnostic criteria and procedures for chronic fatigue syndrome. Biological psychology. 2005;68(2):87–106. [DOI] [PubMed] [Google Scholar]
- 29.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale: application to patients with multiple sclerosis and systemic lupus erythematosus. Archives of neurology. 1989;46(10):1121–23. [DOI] [PubMed] [Google Scholar]
- 30.Taylor R, Jason LA, Torres A. Fatigue rating scales: an empirical comparison. Psychological medicine. 2000;30(4):849–56. [DOI] [PubMed] [Google Scholar]
- 31.Antony MM, Bieling PJ, Cox BJ, Enns MW, Swinson RP. Psychometric properties of the 42-item and 21-item versions of the Depression Anxiety Stress Scales in clinical groups and a community sample. Psychological assessment. 1998;10(2):176. [Google Scholar]
- 32.Alok R, Das S, Agarwal G, Salwahan L, Srivastava R. Relationship of severity of depression, anxiety and stress with severity of fibromyalgia. Clinical and Experimental Rheumatology-Incl Supplements. 2011;29(6):S70. [PubMed] [Google Scholar]
- 33.Suarez GA, Opfer-Gehrking T, Offord K, Atkinson E, O’brien P, Low PA. The Autonomic Symptom Profile: a new instrument to assess autonomic symptoms. Neurology. 1999;52(3):523–23. [DOI] [PubMed] [Google Scholar]
- 34.Sletten DM, Suarez GA, Low PA, Mandrekar J, Singer W. COMPASS 31: a refined and abbreviated Composite Autonomic Symptom Score. Mayo Clinic Proceedings: Elsevier; 2012. p. 1196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Słomko J, Newton JL, Kujawski S, Tafil-Klawe M, Klawe J, Staines D, et al. Prevalence and characteristics of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) in Poland: a cross-sectional study. BMJ Open. 2019. Mar 7;9(3):e023955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stone AA, Broderick JE, Schwartz JE. Validity of average, minimum, and maximum end-of-day recall assessments of pain and fatigue. Contemporary clinical trials. 2010;31(5):483–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dancey CP, Whitehouse A, Painter J, Backhouse S. The relationship between hassles, uplifts and irritable bowel syndrome: A preliminary study. J Psychosom Res. 1995;39(7):827–32. [DOI] [PubMed] [Google Scholar]
- 38.Tryon WW. Actigraphy: The ambulatory measurement of physical activity. Behavioral Sport Psychology. Springer; 2011. p. 25–41. [Google Scholar]
- 39.Pan J, Tompkins WJ. A real-time QRS detection algorithm. IEEE transactions on biomedical engineering. 1985. (3):230–36. [DOI] [PubMed] [Google Scholar]
- 40.Tarvainen MP, Niskanen J-P, Lipponen JA, Ranta-Aho PO, Karjalainen PA. Kubios HRV–heart rate variability analysis software. Computer methods and programs in biomedicine. 2014;113(1):210–20. [DOI] [PubMed] [Google Scholar]
- 41.Shaffer F, McCraty R, Zerr CL. A healthy heart is not a metronome: an integrative review of the heart’s anatomy and heart rate variability. Frontiers in psychology. 2014;5:1040–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger A, Cohen RJ. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. science. 1981;213(4504):220–22. [DOI] [PubMed] [Google Scholar]
- 43.Malik M, Bigger JT, Camm AJ, Kleiger RE, Malliani A, Moss AJ, et al. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. European heart journal. 1996;17(3):354–81. [PubMed] [Google Scholar]
- 44.Leon AC, Solomon DA, Mueller TI, Turvey CL, Endicott J, Keller MB. The Range of Impaired Functioning Tool (LIFE–RIFT): a brief measure of functional impairment. Psychol Med. 1999;29(4):869–78. [DOI] [PubMed] [Google Scholar]
- 45.Hintze J PASS 12. NCSS, LLC. Kaysville, Utah, USA: 2013. [Google Scholar]
- 46.McGraw KO, Wong SP. Forming Inferences About Some Intraclass Correlation Coefficients. Psychological methods. 1996;1(1):30–46. [Google Scholar]
- 47.Enkavi AZ, Eisenberg IW, Bissett PG, Mazza GL, MacKinnon DP, Marsch LA, et al. Large-scale analysis of test–retest reliabilities of self-regulation measures. Proc Natl Acad Sci U S A. 2019;116(12):5472–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guyatt G, Walter S, Norman G. Measuring change over time: Assessing the usefulness of evaluative instruments. J Chronic Dis. 1987;40(2):171–78. [DOI] [PubMed] [Google Scholar]
- 49.Harris B, Regan T, Schueler J, Fields SA. Problematic Mobile Phone and Smartphone Use Scales: A Systematic Review. Front Psychol. 2020;11:672–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nunan D, Sandercock GRH, Brodie DA. A Quantitative Systematic Review of Normal Values for Short-Term Heart Rate Variability in Healthy Adults. Pacing Clin Electrophysiol. 2010;33(11):1407–17. [DOI] [PubMed] [Google Scholar]
- 51.Economides M, Lehrer P, Ranta K, Nazander A, Hilgert O, Raevuori A, et al. Feasibility and Efficacy of the Addition of Heart Rate Variability Biofeedback to a Remote Digital Health Intervention for Depression. Appl Psychophysiol Biofeedback. 2020;45(2):75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clancy JA, Mary DA, Witte KK, Greenwood JP, Deuchars SA, Deuchars J. Non-invasive Vagus Nerve Stimulation in Healthy Humans Reduces Sympathetic Nerve Activity. Brain Stimul. 2014;7(6):871–77. [DOI] [PubMed] [Google Scholar]
- 53.Gurel NZ, Gazi AH, Scott KL, Wittbrodt MT, Shah AJ, Vaccarino V, et al. Timing Considerations for Noninvasive Vagal Nerve Stimulation in Clinical Studies. AMIA Annu Symp Proc. 2019;2019:1061–70. [PMC free article] [PubMed] [Google Scholar]
- 54.Digre KB. What’s New in the Treatment of Migraine? J Neuroophthalmol. 2019;39(3):352–59. [DOI] [PubMed] [Google Scholar]
- 55.Theorell T, Blomkvist V, Lindh G, Evengård B. Critical life events, infections, and symptoms during the year preceding chronic fatigue syndrome (CFS): an examination of CFS patients and subjects with a nonspecific life crisis. Psychosom Med. 1999;61(3):304–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


