Abstract
Polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) are ubiquitous environmental chemicals that have long half-lives. Humans are exposed to PCBs and PBDEs mainly through diet, and relative to other populations, those who consume sport-caught fish generally have elevated body burdens. Numerous studies have found associations between prenatal exposure to these chemicals and neurodevelopmental deficits, but there are few studies assessing the impact of exposure during adolescence, a period of rapid development of executive functions. We assessed executive functions in adolescents at risk for exposure to PCBs and PBDEs through consumption of fish from the Lower Fox River and other contaminated waters in northeastern Wisconsin. Between 2007 and 2012, a sample of 115 12–18-year-old children was recruited from households in the Green Bay, WI area in which at least one parent held a WI fishing license. We assessed associations of total PCBs and total PBDEs, as well as the predominant individual congeners (PBDE 47 and 153; PCB 138/163, 153 and 180) with performance on four tests from the Cambridge Neuropsychological Test Automated Battery (CANTAB): Intradimensional/Extradimensional Set-Shifting (ID/ED) which assesses cognitive flexibility, Delayed Matching to Sample (DMS) which assesses visual recognition memory, Spatial Working Memory (SWM), and Stockings of Cambridge (SOC) which assess planning and working memory. In addition to the exposure and outcome variables, multivariable regression models included the child’s age, sex and IQ score as well as a sex by exposure interaction term. All of the children were non-Hispanic whites and most parents were married, employed, and had at least some college. After adjusting for serum lipids, the GM (GSD) for total PCBs (ng/g) was 30.83 (2.46), and the GM (GSD) for the predominant PCB congeners PCB 138/163, PCB 153, and PCB 180 were 4.60 (2.39), 5.43 (2.37), and 1.01 (2.71), respectively. The GM (GSD) for total PBDEs (ng/g) was 26.82 (3.30), and the GM (GSD) for the predominant PBDE congeners PBDE 47 and PBDE 153 were 16.64 (2.94) and 3.95 (3.43), respectively. For both chemicals, the primary finding of the negative binomial regression analyses was that higher blood serum concentrations were associated with poorer cognitive flexibility as measured on the ID/ED task. In particular, the PCB x sex interaction p-value was 0.08, and stratifying by sex demonstrated that males with higher blood serum total PCB concentrations (β = 2.20, 95% CL: 1.16, 4.16, p = 0.02) took more trials to complete the ID/ED task, while both males and females with higher total PBDE concentrations (β = 1.74, 95% CL: 1.25, 2.42, p = 0.001) took more total trials to complete the task. Higher serum total PCB concentrations were also associated with more errors on the DMS task (β = 1.15, 95% CL: 1.00, 1.31, p < 0.05). These findings suggest that exposure to PCBs or PBDEs during adolescence may be associated with impaired cognitive flexibility and that adolescent PCB exposure may be associated with visual recognition memory deficits.
Keywords: Polychlorinated biphenyls, Polybrominated diphenyl ethers, Adolescence, Executive functioning
1. Introduction
Polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) are ubiquitous chemicals associated with neurodevelopmental deficits (Lam et al., 2017; Pessah et al., 2019). PCBs and PBDEs have similar molecular structures and are persistent in the environment. PCBs were used in numerous industrial capacities (Ross, 2004) and are still byproducts in the manufacture of paint pigments (Grossman, 2013). Older buildings containing PCBs in caulking and fluorescent light ballasts continue to contribute PCBs to ecosystems (Hornbuckle and Robertson, 2010). PBDEs were primarily used as flame retardants in consumer products, including furniture and electronics (de Wit, 2002; Rahman et al., 2001). Though production was phased out, many consumer products containing PBDEs are still in use.
Humans are exposed to PCBs and PBDEs mainly through diet (Agency for Toxic Substances and Disease Registry, 2017; Centers for Disease Control and Prevention, 2009), although house dust is also a source of exposure to PBDEs (Whitehead et al., 2014; Whitehead et al., 2013). Those who consume fish from contaminated waters typically have elevated PCB and PBDE blood concentrations (Anderson et al., 2008; Schaeffer et al., 2006; Turyk et al., 2012).
Developmental exposure to PCBs or PBDEs has been associated with cognitive deficits, and evidence suggests that executive functions are particularly vulnerable to disruption (Eubig et al., 2010; Pinson et al., 2016; Vuong et al., 2020). Executive functions refer to a set of abilities—including working memory, attention control, mental set shifting or cognitive flexibility, and impulse inhibition—controlled by the prefrontal cortex that allow individuals to plan steps necessary to achieve a desired goal, keep these steps in mind while acting on the goal, monitor their progress, and adjust the plan as appropriate (Aguiar et al., 2010; Berthelsen et al., 2017). Prenatal PCB exposure has been associated with deficits in executive functions including working memory, cognitive flexibility, and response inhibition (Boucher et al., 2009; Eubig et al., 2010; Jacobson and Jacobson, 2003; Stewart et al., 2003; Stewart et al., 2006).
Concerning PBDEs, evidence (Lam et al., 2017; Vuong et al., 2020) from studies examining prenatal (de Water et al., 2019; Eskenazi et al., 2013; Zhang et al., 2017) as well as postnatal exposure (Eskenazi et al., 2013; Vuong et al., 2017) suggests that PBDEs impair executive functions. Specifically, prenatal PBDE exposure has been associated with poorer attention at 5–7 years (Eskenazi et al., 2013; Roze et al., 2009) and poorer attention and working memory at 9 and 12 years (Sagiv et al., 2015).
Animals perinatally exposed to PCBs or PBDEs also show executive function deficits. Impaired cognitive flexibility and response inhibition were reported in perinatally PCB-exposed rats and monkeys (Eubig et al., 2010). Similarly, perinatal PBDE exposure was associated with deficits in memory and response inhibition in rats and mice (Pinson et al., 2016). In summary, studies in both humans and animals suggest that pre- or perinatal PCB or PBDE exposure causes persistent adverse impacts on executive functions.
Few studies have examined the impact of exposure to these contaminants during adolescence, another critical period of brain development characterized by certain developmental changes, including neural specialization and maturation of processes in the prefrontal cortex which result in more efficient and effective executive function processes (Berthelsen et al., 2017; Crews et al., 2007; Luna, 2009). As they gather experience in monitoring their performance, learning from feedback, reasoning relationally, and integrating more contextual information, adolescents increase their capacity to control attention, manipulate information in memory (i.e., working memory), and be flexible when making decisions (Berthelsen et al., 2017). Due to these significant neurodevelopmental changes, the adolescent brain may be uniquely vulnerable to the adverse effects of contaminant exposure.
Few studies have examined the relationship between PCB or PBDE concentrations in adolescents and cognitive function. Newman et al. (2006) found that serum PCB concentrations were associated with impaired long-term memory and reasoning ability in adolescents from the Akwesasne Mohawk Nation cohort which consisted of 10–17-year-olds that were exposed prenatally and concurrently to PCBs through consumption of contaminated fish and wild game (Newman et al., 2006; Newman et al., 2009) but they did not directly assess executive functions that mature during adolescence. PBDE serum levels in Flemish adolescents were negatively associated with motor function but not attention, information processing, or memory (Kiciński et al., 2012). The paucity of data on adolescent exposure to these contaminants highlights the need for research on the impact of exposure during this critical period of neurodevelopment.
We hypothesized that PCB or PBDE exposure during adolescence would be associated with impairments in executive functioning. To address this, we assessed neuropsychological function in adolescents 12–19-years-of-age at risk for elevated exposure to PCBs and PBDEs through consumption of fish from the Lower Fox River and other contaminated waters in northeastern Wisconsin. We measured blood serum concentrations of PCBs and PBDEs and assessed the association of each contaminant with performance on the following tests of executive function from the Cambridge Neuropsychological Test Automated Battery (CANTAB): Intradimensional/Extradimensional Set-Shifting (ID/ED), Delayed Matching to Sample (DMS), Spatial Working Memory (SWM), and Stockings of Cambridge (SOC).
2. Materials and methods
Between 2007 and 2012, a sample of 12–19-year-old children were recruited from households in the Green Bay, Wisconsin area. Potential participants were identified through the Wisconsin Department of Natural Resources licensed angler database as having a valid Wisconsin fishing license during the 2006–2007 fishing season. They were Wisconsin residents who purchased fishing licenses in 2006–2007 and checked a box indicating that they gave permission for the release of their identifying information to outside parties, including biomedical researchers. We identified 15,528 households located in 8 zip codes within the greater Green Bay and De Pere metropolitan areas in northeastern Wisconsin with at least one licensed angler 32–65 years of age, so as to increase the chance of finding parents of adolescent children.
Of the 15,528 households, 6930 (45%) randomly selected homes received a letter explaining the study. Letters were mailed in batches of 200–500 every few months between March 2007 and August 2011, and were followed by a phone call to determine if there were eligible children in the home and, if so, their willingness to participate. Eligible children had to be 12–19-years-of-age at the time of testing, fluent in English, and have a parent holding a current Wisconsin fishing license. Exclusion criteria included any health or physical condition (e.g., blindness, deafness, or inability to use hands) or a cognitive condition (e. g., a severe learning disability) that would significantly hinder performance on our battery of tests designed to measure learning, memory and problem-solving skills. We were able to connect via phone with 4478 (65%) of the homes that were sent the informational letter: 658 (15%) refused to talk with us; 3206 (72%) were ineligible; and 614 (17%) were eligible. Out of the eligible households, 475 (77%) declined participation and 37 (6%) asked us for more time to decide but never gave us a final answer. Of those who declined participation, only 142 (30%) households agreed to answer a brief demographics and fish consumption questionnaire that also included a question about their reason for declining participation in the study: 47% of families said they were too busy; 18% did not want to do the blood draw; 17% said they did not eat fish; 10% did not specify a reason; 5% declared lack of interest sometimes elaborating that they were not concerned about chemical exposure from consumption of fish; and 3% gave other reasons.
The final sample for the study included 116 children from 102 households. Of the 116 adolescents that enrolled in the study, one was removed from all analyses because this participant did not have exposure data, yielding a final sample of 115 adolescents from 102 households. All procedures were approved by the Institutional Review Boards (IRB) of the University of Illinois at Urbana-Champaign and Michigan State University, and all participants gave written informed consent (parents and children 18-years-of-age or older) or assent (children under 18-years-of-age).
2.1. Neuropsychological testing
Children were administered several tests from the Cambridge Neuropsychological Test Automated Battery (CANTAB; Cambridge Cognition Ltd.), which contains a variety of tests that assesses a wide range of cognitive domains including memory, attention, and executive function (Fray and Robbins, 1996). The CANTAB tests were administered by local study staff who were trained by a developmental psychologist on the study team (coauthor, Dr. Aguiar). They used specific instructions for the children provided in the CANTAB manual. All testing was done outside of school hours at our research field office in Green Bay. Tests were administered using a computer with a touchscreen. This paper focuses on a subset of the CANTAB tests specifically designed to assess executive functions, including the Intradimensional/Extradimensional (ID/ED) set-shifting test which assesses cognitive flexibility, the Delayed Matching to Sample (DMS) and Spatial Working Memory (SWM) tests which assess working memory, and the Stockings of Cambridge (SOC) test that assesses planning and working memory (see Fig. 1). CANTAB has been used in both pharmaceutical as well as academic research in several areas including CNS, neurology, and psychiatry studies. The instrument is validated by 30 years of neuroscience research conducted around the world with diverse populations, resulting in over 2500 peer-reviewed articles (Cambridge Cognition, 2022). Other studies assessing associations of chemicals with performance on executive functioning have found the CANTAB tests to be sensitive to the effects of exposure (Canfield et al., 2004; Frndak et al., 2019).
Fig. 1.
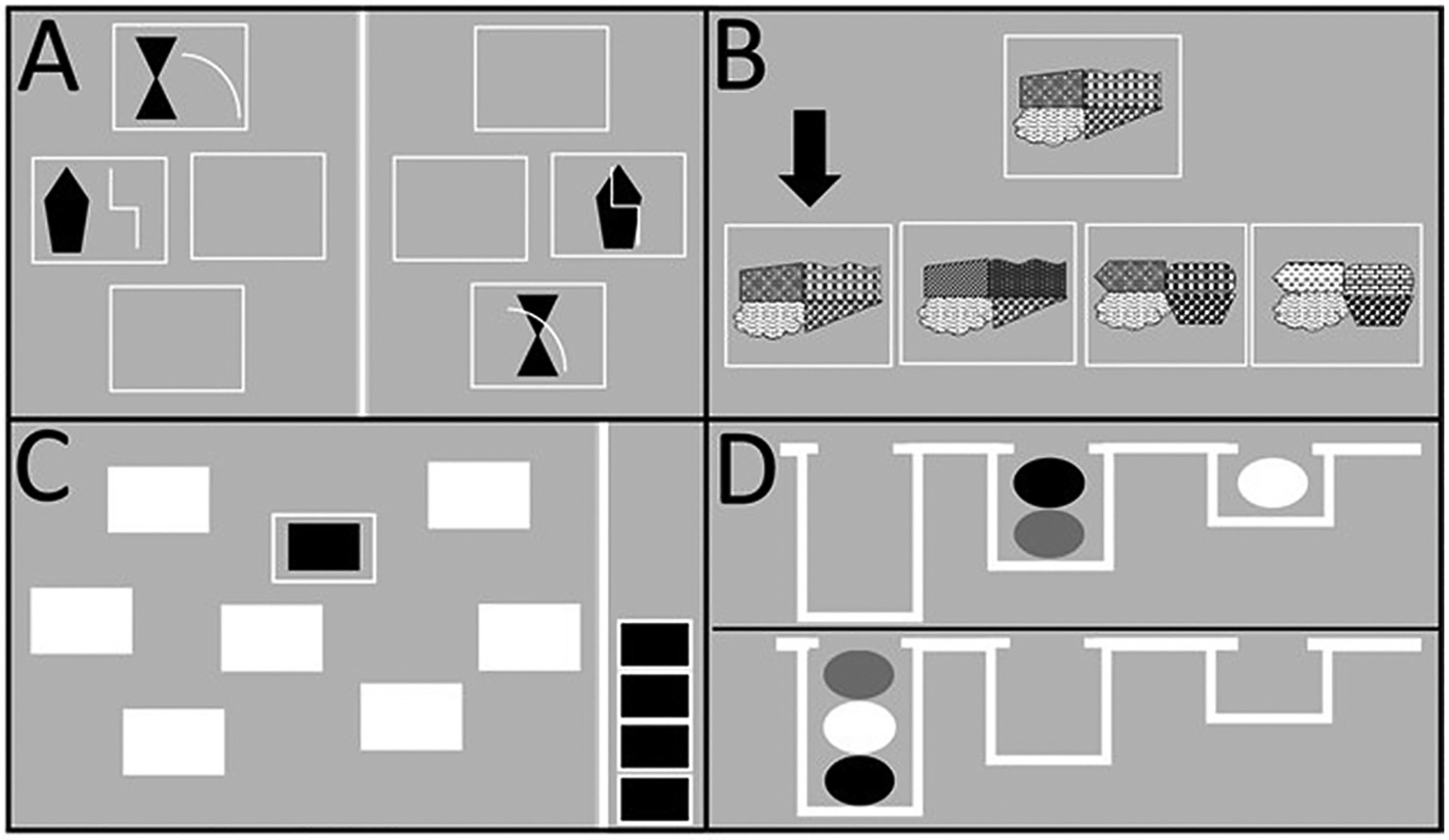
Schematic representation of 4 CANTAB tests. (A) Intra/Extradimensional (ID/ED) set-shifting, compound discrimination 1 (CD1) and compound discrimination 2 (CD2) are depicted on the left and right panel, respectively. In both stages, participants need to discriminate the shapes while ignoring the lines. (B) Delayed Matching to Sample (DMS), a no-delay trial is illustrated in which the sample pattern (top) and the 4 choices of patterns (bottom) are shown at the same time, with the arrow pointing to the correct choice matching the sample. (C) Spatial Working Memory (SWM), an 8-box search is depicted with one of the boxes “opened” revealing a hidden token. Four tokens found in previous searches are stacked in a column on the right side. (D) Stockings of Cambridge (SOC), a 3-move problem is shown. Participants need to move the balls in the lower display to match the upper display, making the least number of moves.
2.1.1. Intradimensional/Extradimensional set-shifting (ID/ED)
The ID/ED set-shifting test assessed how quickly one learns rules for identifying the correct stimulus by attending to a specific stimulus dimension and how quickly one learns new rules by shifting attention to a previously irrelevant stimulus dimension. The series began with participants learning a simple shape discrimination (SD; one of two shapes was the correct one), followed by 8 subsequent stages (see Table 1). First, there was a simple reversal (SR) in which the opposite shape became the correct one. Then an additional, but irrelevant dimension (i. e., white lines next to or superimposed on the shapes) was introduced (compound discriminations; CD1; CD2). The same shape was still correct regardless of what line it was paired with. Then, an intra-dimensional shift (ID) took place in which new shapes and lines were presented, but shape was still the relevant dimension (i.e., one of the two shapes was correct regardless of what line it was paired with). Subsequently, an intra-dimensional reversal (IR) was introduced whereby the other, previously incorrect shape was now the correct one (Fig. 1A). In the next stage, an extradimensional shift (ED) occurred in which the lines became the relevant stimulus dimension (one of the two lines was correct regardless of which shape it was paired with). In the final stage, the same shapes and lines from the previous stage were presented, but the opposite line was now correct (extradimensional reversal; ER). Participants progressed through the test by satisfying a criterion of 6 consecutive correct responses at each stage. If at any stage the participant failed to reach this criterion after 50 trials, the test terminated. The dependent variables for the ID/ED test included the number of trials to criterion at each stage and the total number of trials to complete all stages of the test.
Table 1.
Names and abbreviations of each stage of the ID/ED set-shifting test. The median for the number of trials to criterion for each stage are shown.
| Intradimensional/Extradimensional (ID/ED) Stage | Abbreviation | Median: # trials to criterion1 |
|---|---|---|
| Shape discrimination | SD | 6 |
| Simple reversal | SR | 7 |
| Compound discrimination 1 | CD1 | 8 |
| Compound discrimination 2 | CD2 | 6 |
| Compound reversal | CR | 7 |
| Intradimensional shift | ID | 7 |
| Intradimensional reversal | IR | 7 |
| Extradimensional shift | ED | 9 |
| Extradimensional reversal | ER | 7 |
Dichotomized as median or greater than the median and analyzed with logistic regression.
2.1.2. Delayed matching to sample (DMS)
The DMS test assessed immediate and short-term visual recognition memory. There were 40 trials, and in each trial, the participant was shown a complex visual pattern (the sample) and then four patterns were displayed, wherein only one correctly matched the sample. The participant was instructed to select the matching pattern. The sample and the choice patterns were shown either simultaneously or with a delay of 0, 4 or 12 s between removing the sample pattern and presenting the choice patterns (Fig. 1B). If their first choice was incorrect, the participant had to make a second choice, and so on until a correct choice was made. The test continued until the participant completed all test trials. The number of incorrect choices as well as latency (milliseconds) to make incorrect choices were assessed.
2.1.3. Spatial working memory (SWM)
The SWM test assessed spatial working memory and strategy. The test began with the presentation of 3 colored “boxes.” The number of boxes displayed on the screen increased to 4, 6, and finally 8 on later sets. The participant was instructed to touch each box until one containing a blue token was found (considered a search). When the blue token was found, the participant was instructed to begin a new search for the next blue token, which could be in any of the boxes that were empty in the previous searches within that set (Fig. 1C). A box containing the blue token in a previous search would not contain a blue token on any future search within that set. Thus, returning to that box counted as an error. The set size (3, 4, 6 or 8 boxes displayed) corresponded to the number of tokens to be found in each set. The dependent variables were the total number of errors in a set, total number of responses in a set, and the total time to complete each set.
2.1.4. Stockings of Cambridge (SOC)
The SOC assessed planning and working memory. The participant was shown 2 displays containing 3 colored balls stacked in “stockings” suspended from a beam. The participant had to use the balls in the lower display to copy the pattern shown in the upper display using as few moves as possible. In the first part of the test, planning ability was assessed by measuring the time taken to start making moves and the number of moves made to reproduce the upper display. The number of moves required to copy the pattern in each problem gradually increased in difficulty from 2 to 5 moves (Fig. 1D). In the second part of the test, the time to execute the moves was measured by having the participant copy the moves done by the computer in the upper display. This procedure controlled for motor performance. The difference between the times to initiate a move in the first part versus the second part of the test was considered an index of the “thinking time” that the participant took to plan a solution. If the participant made more than double the number of moves needed to solve the problem, the problem was terminated. If 3 problems in a row were terminated, the entire test ended. The dependent variables were thinking time (milliseconds) and the number of moves to completion.
2.2. Serum PCB and PBDE concentrations
A blood sample was collected from each participant by a trained phlebotomist at our research field office in Green Bay within two weeks after the neuropsychological assessment. Samples were analyzed for PCBs and PBDEs using a modification of the method described by Greizerstein et al. (1997) and also described in Kostyniak et al. (2005) and Woods et al. (2012). Briefly, PCB and PBDE congeners were extracted from the serum using pressurized liquid (n-hexane) extraction. The extract was evaporated to a final volume of 0.2 mL for PCB analysis (PCB congeners 30 and 204 added as internal standards) and further concentrated to 0.1 mL for PBDE analysis (BDE-118 added as internal standard) prior to chromatographic analysis.
A total of 97 PCB congeners and 7 PBDE congeners were measured. Selection of PCB congeners to analyze was based on knowledge about which congeners were used in commercial PCB mixtures and were most likely to be detected in environmental or human samples. For PBDEs, not as many congeners were used commercially. The 7 PBDE congeners that were detected in >60% of National Health and Nutrition Examination Survey participants (PBDE 28, 47, 99, 100, 153, 154, and 183) (Sjödin et al., 2008) were analyzed in the current study.
Total PCBs or PBDEs in serum were normalized for lipid content in sample matrices. Total PCBs or total PBDEs (ng/g) in each sample were calculated by summing individual congener concentrations detected in the serum sample. Lipid adjusted PCB or PBDE values (ng/g lipids) were obtained by dividing the total concentration by the concentration of lipid in the serum. PCB and PBDE concentrations in serum followed a log-normal distribution. Data were analyzed using both lipid adjusted and unadjusted values, as the use of lipid adjusted values only has been questioned (Schisterman et al., 2005); however, the findings were similar, and thus only results using lipid adjusted values are reported here.
In addition to total PCBs and total PBDEs, we examined congeners that were detected in at least 90% of the children. These were: PCB 138/163, PCB 153, PCB 180, PBDE 47, and PBDE 153. There were no machine read values for congener concentrations below the level of detection (LOD). For measurements below the LOD, the concentrations were replaced with LOD/√2 (Hornung and Reed, 1990). As with the total PCBs and total PBDEs, congener concentrations were adjusted for lipid serum.
2.3. Covariates
Information about possible confounders was gathered through interviews and questionnaires administered to participants and their parents. We obtained information about participants’ fish consumption, family demographic characteristics, lifestyle factors, and medical history (including conditions that may affect neuropsychological function). Additionally, the Vocabulary and Matrix Reasoning subtests of the Wechsler Abbreviated Scale of Intelligence (WASI) were administered to control for general intellectual functioning (Wechsler, 1999). When administered together, these subtests provide an estimate of overall intellectual functioning that is highly correlated with the WASI full scale IQ score (r = 0.93–0.94) (Wechsler, 1999).
2.4. Statistical analysis
All statistical analyses were performed using SAS 9.3 software (SAS Institute Inc., Cary, NC). A priori knowledge and univariate analyses were used to select covariates to include in the models. Family income and parental education were associated with some of the outcome variables, but when included in the models did not significantly contribute. Critical covariates identified for inclusion in the models were age, sex, and the WASI IQ score. A sex by exposure interaction term was also included in the analyses to explore potential sex-specific effects of exposure. If the interaction was significant (at p < 0.10), the analysis was then stratified by sex.
Multivariable linear regression was used for analysis of associations between normally distributed continuous outcomes and PCB or PBDE serum concentrations. Because the distributions of both total PCB and PBDE concentrations and their individual congeners were right-skewed, exposure values of all chemical concentrations were log-transformed. For continuous variables, checks for outliers and influential observations were based on leverage statistics and standardized residuals (Cook and Weisberg, 1982). Collinearity was assessed by variance inflation factors and condition numbers (Cook and Weisberg, 1982). Because the distributions of SWM outcomes lacked normality, long-tailed distributions for the dependent variables were assessed. After examining the fit statistics to determine the model of best fit to the data, the Weibull distribution was chosen for analysis of errors and the Burr distribution was chosen for number of responses and time to complete each set. Negative binomial regression was used for count variables (total trials on the ID/ED test and total wrong on the DMS). Outcomes with markedly skewed distributions (trials to criterion at each ID/ED stage and mean moves to completion for 5-move problems in SOC) were dichotomized and analyzed with logistic regression.
Of the 115 children with exposure data, 3 had missing WASI IQ scores and were excluded from the analyses. Data from one child with an IQ score of <70 was also excluded. The final dataset consisted of 111 children (60 males, 51 females) for whom complete exposure, outcome, and covariate data were available.
For analysis of ID/ED outcomes, 5 of the 111 children (2 males, 3 females) were missing outcome data. Based on large standardized residuals and leverage statistics, 1 male was excluded from the final model for the PCB analyses (n = 105 in final model), while 2 males were excluded from the final model for the PBDE analyses (n = 104 in final model). For trials to criterion at each ID/ED stage, data were markedly skewed; thus, this variable was dichotomized as the median or greater than the median and analyzed with logistic regression (see Table 1).
For analysis of DMS outcomes, only trials with a 12 s delay were analyzed, as all participants performed well and made few to no errors on trials with shorter delays. For this test, 3 participants had missing outcome data. Based on large standardized residuals and leverage statistics, 3 males and 2 females were removed from the final model for the PCB analyses (n = 103 in final model), while 1 male and 1 female were removed from the PBDE analyses (n = 106 in final model).
SWM data were analyzed only for sets with 4 or more boxes, because all participants performed well and made few or no errors on the set with fewer than 4 boxes. For analysis of SWM outcomes, 2 males and 1 female had missing response data (n = 108 for the final models for both PCBs and PBDEs). Outcomes were total errors, total number of responses, and total time (minutes) to complete sets ≥4 boxes (all dependent variables were calculated by summing across sets with 4, 6, and 8 boxes).
SOC outcomes were analyzed only for 5-move problems, as participants performed well on problems with 4 or fewer moves such that there was not much variability in performance. For analysis of SOC thinking time, 1 female participant was dropped due to illogical values (n = 110 for both PCB and PBDE models). Regarding the analysis of the mean number of moves to complete a problem, 3 participants had missing outcome data (n = 107 for both PCB and PBDE models). The distribution for the mean number of moves to completion was skewed. This variable was dichotomized as 5 or > 5 and analyzed with logistic regression, as approximately 34% of participants had the minimum score of 5 (range: 5–10).
3. Results
3.1. Sample characteristics and serum PCB and PBDE concentrations
Table 2 presents the demographic characteristics of participating households and enrolled adolescents for whom exposure data were available. Mean participant age was 16.1 years (range = 12–19), and 74% reported consumption of local sport-caught fish for six or more years. Nearly 100% of parents were white. Over 70% of families reported total household incomes above $60,000, while only 6% had a household income of less than $40,000. Most households were supported by two incomes, with 94% of fathers and 82% of mothers employed. Over 70% of mothers and fathers held at least an Associate’s degree. Very few mothers reported consuming alcohol or smoking during pregnancy. Out of 102 households, 7 mothers (6.9%) reporting smoking and 10 (9.8%) reported consuming alcohol during pregnancy.
Table 2.
Characteristics of households and adolescent participants.
| Parental Characteristics | Participating households1 (n = 102 households) N (%) or Mean ± SD |
|---|---|
| Maternal race | |
| White | 101 (99.0) |
| Other | 0 (0.0) |
| Missing | 1 (1.0) |
| Paternal race | |
| White | 100 (98.0) |
| Other | 2 (2.0) |
| Missing | 0 (0.0) |
| Maternal education | |
| Less than associate’s degree | 20 (19.6) |
| Associate’s degree or higher | 79 (77.5) |
| Missing | 3 (2.9) |
| Paternal education | |
| Less than associate’s degree | 23 (22.6) |
| Associate’s degree or higher | 75 (73.5) |
| Missing | 4 (3.9) |
| Household income | |
| <$60,000 | 14 (13.7) |
| ≥$60,000 | 73 (71.6) |
| Missing | 15 (14.7) |
| Maternal employment status | |
| Employed | 84 (82.3) |
| Unemployed | 17 (16.7) |
| Missing | 1 (1.0) |
| Paternal employment status | |
| Employed | 96 (94.1) |
| Unemployed | 6 (5.9) |
| Missing | 0 (0.0) |
| Maternal age (years) at child’s birth | 29.5 ± 5.0 |
| Paternal age (years) at child’s birth | 31.5 ± 5.2 |
| Child Characteristics | Enrolled adolescents with exposure data (n = 115) N (%) or Mean ± SD |
| Child sex | |
| Male | 62 (53.9) |
| Female | 53 (46.1) |
| Years consuming sports-caught fish | |
| 0 | 27 (23.5) |
| 1–5 | 9 (7.8) |
| 6–11 | 40 (34.8) |
| 12–18 | 35 (30.4) |
| Missing | 4 (3.5) |
| Child age (years) | 16.1 ± 1.4 |
Reported values represent 102 households that had one to three adolescent participants. Twelve of the 102 households had more than one child participate in the study.
There were no significant differences in serum PCB or PBDE concentrations among household income categories. Regarding parent education, there were no significant exposure differences in children whose parents did not attend college compared to those whose parents had some college. Child age was inversely correlated with total PCBs (r = −0.21, p = 0.02) but it was not significantly associated with total PBDEs (r = −0.15, p = 0.122). In addition, the concentration of total PBDEs was higher in children who never ate sport-caught fish (p = 0.03; mean ± SD: 71.61 ng/g ± 61.36 ng/g) compared to those who reported that they ate sport-caught fish (39.60 ± 43.46 ng/g), but there was no such difference for total PCBs (p = 0.38).
In the 142 families who declined participation but agreed to respond to a brief demographic and fish consumption survey over the phone, there were 154 adolescents. Sex and age information was available for 91% of these adolescents indicating that sex was evenly represented (51% female, 49% male), and mean age was 15.7 years (range = 13–19). Total household income was reported by only 51% of the families and indicated that 72% of these households had a total annual income above $60,000, while only 8% had a household income of less than $40,000. Thus, participants and non-participants were very similar in terms of their sex breakdown, their age, and total household income. No other demographic information was collected from the non-participants. In terms of consumption of local sport-caught fish, data were available for 85% of the non-participant teens with 42% reporting consumption of local sport-caught fish for six or more years, and 60% reporting consumption of local sport-caught fish for two or more years. Thus, there was a considerable difference between participants and non-participants in terms of their consumption of local sports-caught fish with non-participants reporting fewer years of fish consumption. This difference is to be expected as a common reason for declining participation was fact that the teens were not eating local sports-caught fish, and/or that the family was not interested in chemical exposure from the consumption of local sports-caught fish (possibly because the family felt they did not consume enough sports-caught fish to be concerned about this issue).
Table 3 shows the total PCB and PBDE serum concentrations (ng/g lipid; n = 115). The median serum total PCB concentration was 0.15 ng/g (lipid unadjusted) and 33.00 ng/g of serum lipid (lipid adjusted). The median serum total PBDE concentration was 0.12 ng/g (lipid unadjusted) and 29.14 ng/g of serum lipid (lipid adjusted). Supplemental Table 1 displays the distribution of lipid unadjusted and adjusted serum concentrations for PCB 138/163, PCB 153, PCB 180, PBDE 47, and PBDE 153. Correlations among total PCBs, total PBDEs, and the predominant individual congeners we examined are presented in Supplementary Table 2.
Table 3.
Distribution of chemical exposure biomarker concentrations measured in the serum of adolescent participants (n = 115).
| Chemical Biomarker Concentrations (ng/g) | Mean | Median | Geometric Mean | Geometric Standard Deviation | Minimum | Maximum |
|---|---|---|---|---|---|---|
| Lipid unadjusted | ||||||
| Total PCBs | 0.20 | 0.15 | 0.14 | 2.39 | 0.01 | 1.86 |
| Total PBDEs | 0.22 | 0.12 | 0.12 | 3.37 | 0.00 | 1.64 |
| Lipid adjusted | ||||||
| Total PCBs | 45.52 | 33.00 | 30.83 | 2.46 | 2.56 | 389.10 |
| Total PBDEs | 47.20 | 29.14 | 26.82 | 3.30 | 1.23 | 244.16 |
3.2. Associations of total PCB and PBDE serum concentrations with CANTAB measures
3.2.1. ID/ED shift
For PCBs, the negative binomial regression for total trials to complete the test revealed a sex by exposure interaction (p = 0.08). Stratum-specific analysis revealed a significant positive association of PCB exposure with total trials in males (β = 2.20, 95% CL: 1.16, 4.16, p = 0.02) but not females (β = 1.13, 95% CL: 0.73, 1.77, p = 0.58), shown as means ratio estimates and 95% confidence limits (CL) in Fig. 2A. Specifically, males with higher serum PCB concentrations required more trials to complete the entire series of tests. As expected, IQ was negatively associated with total trials on this measure (p = 0.002) whereby higher IQ was associated with fewer total trials to complete the ID/ED test. The results of logistic regressions for trials to criterion at each stage are represented as odds ratio estimates and 95% CLs (Fig. 2B). PCB concentrations were positively associated with trials to criterion in males on the first stage (simple discrimination; SD) of the ID/ED test (OR = 3.31, 95% CL: 1.12, 9.73, p = 0.03), wherein higher PCB concentrations were associated with more trials to reach criterion. Higher PCB concentrations were also associated with increased trials to criterion on the compound discrimination 2 (CD2) stage (OR = 1.97, 95% CL: 1.09, 3.56, p = 0.02) and on the extradimensional reversal (ER) stage (OR = 1.83, 95% CL: 0.98, 3.40, p = 0.06), but there was no significant interaction of PCB exposure by sex.
Fig. 2.
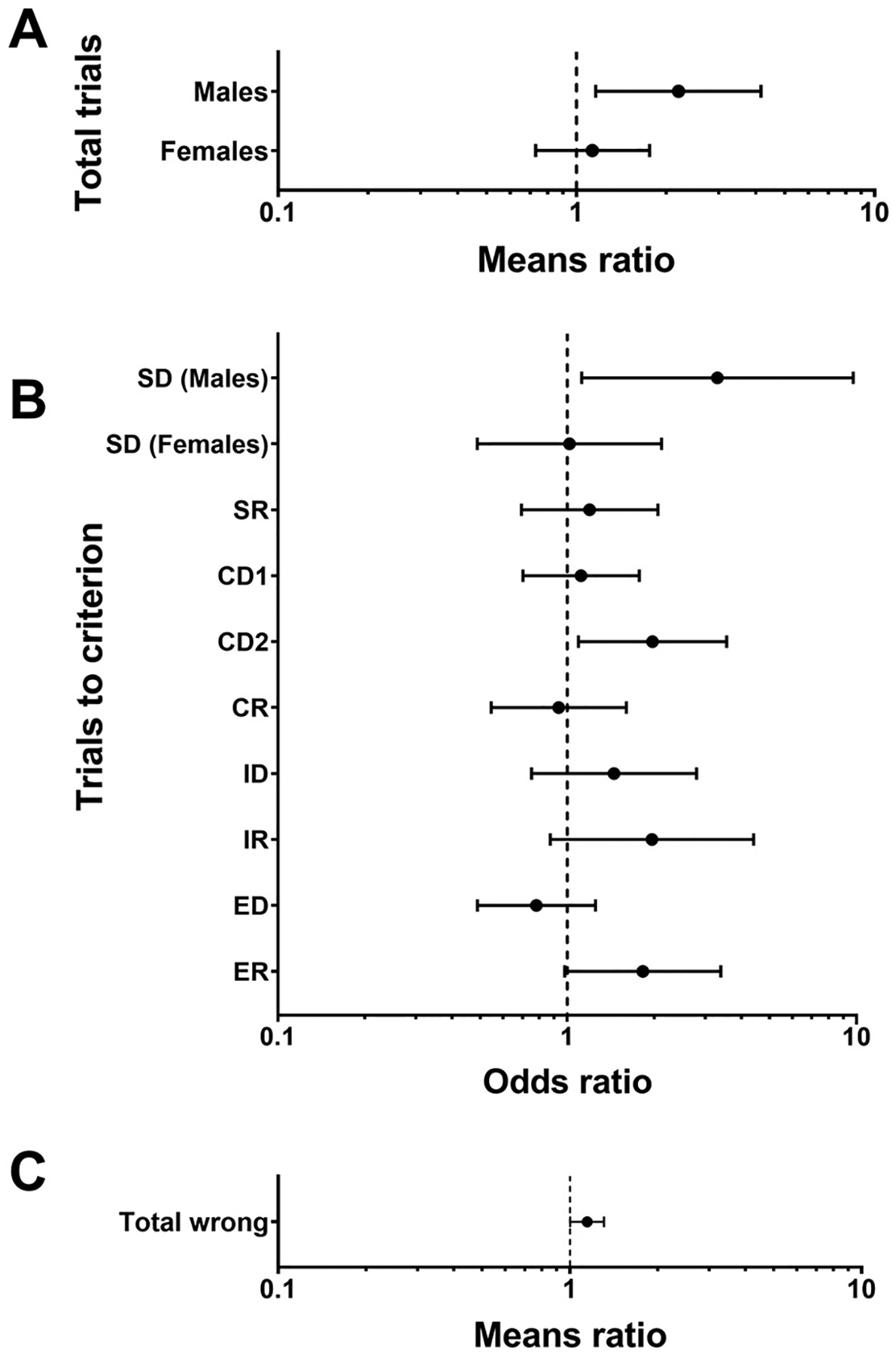
Associations between PCB serum concentrations and performance on ID/ED shift (n = 105) and DMS (n = 103). Means ratios and odds ratios are plotted on a log scale. (A) ID/ED shift: Total trials analyzed with negative binomial regression and associations reported as means ratio estimates (± 95% CL) for a 2 SD increase in PCB exposure on the log scale or a 6-fold increase in exposure on the original scale. (B) ID/ED shift: Trials to criterion for each stage analyzed with logistic regression and associations reported as odds ratio estimates (± 95% CL) for a 2 SD increase in PCB exposure on the log scale or a 6-fold increase in exposure on the original scale. (C) DMS: Total number of incorrect choices analyzed with negative binomial regression and associations reported as means ratio estimates (± 95% CL) for a 1 SD increase in PCB exposure on the log scale or a 2-fold increase in exposure on the original scale. All models were adjusted for age, sex, and IQ score. CL: confidence limit, SD: standard deviation.
Negative binomial regression for total trials to complete all nine stages of the ID/ED test revealed a significant positive association of PBDE concentrations with total trials shown as means ratio estimate and 95% CL (β = 1.74, 95% CL: 1.25, 2.42, p = 0.001; see Fig. 3A) whereby higher PBDE serum concentrations were associated with more trials to complete the entire test. The interaction of sex with exposure was not significant, but IQ was negatively associated with total trials (p = 0.04). The results of logistic regressions for trials to criterion at each stage are shown as odds ratio estimates and 95% CLs (Fig. 3B). Similar to PCBs, higher PBDE concentrations were associated with an increase in trials to criterion in males on SD (OR = 3.28, 95% CL: 1.48, 7.25, p < 0.01), but unlike PCBs, higher PBDE exposure was also associated with an increase in trials to criterion on intra-dimensional reversal (IR) in males (OR = 2.65, 95% CL: 1.06, 6.62, p = 0.04). PBDE exposure was also associated with an increase in trials to criterion on intra-dimensional shift (ID) (OR = 1.98, 95% CL: 1.10, 3.55, p = 0.02), but there was no significant interaction of PBDE exposure with sex (Fig. 3B).
Fig. 3.
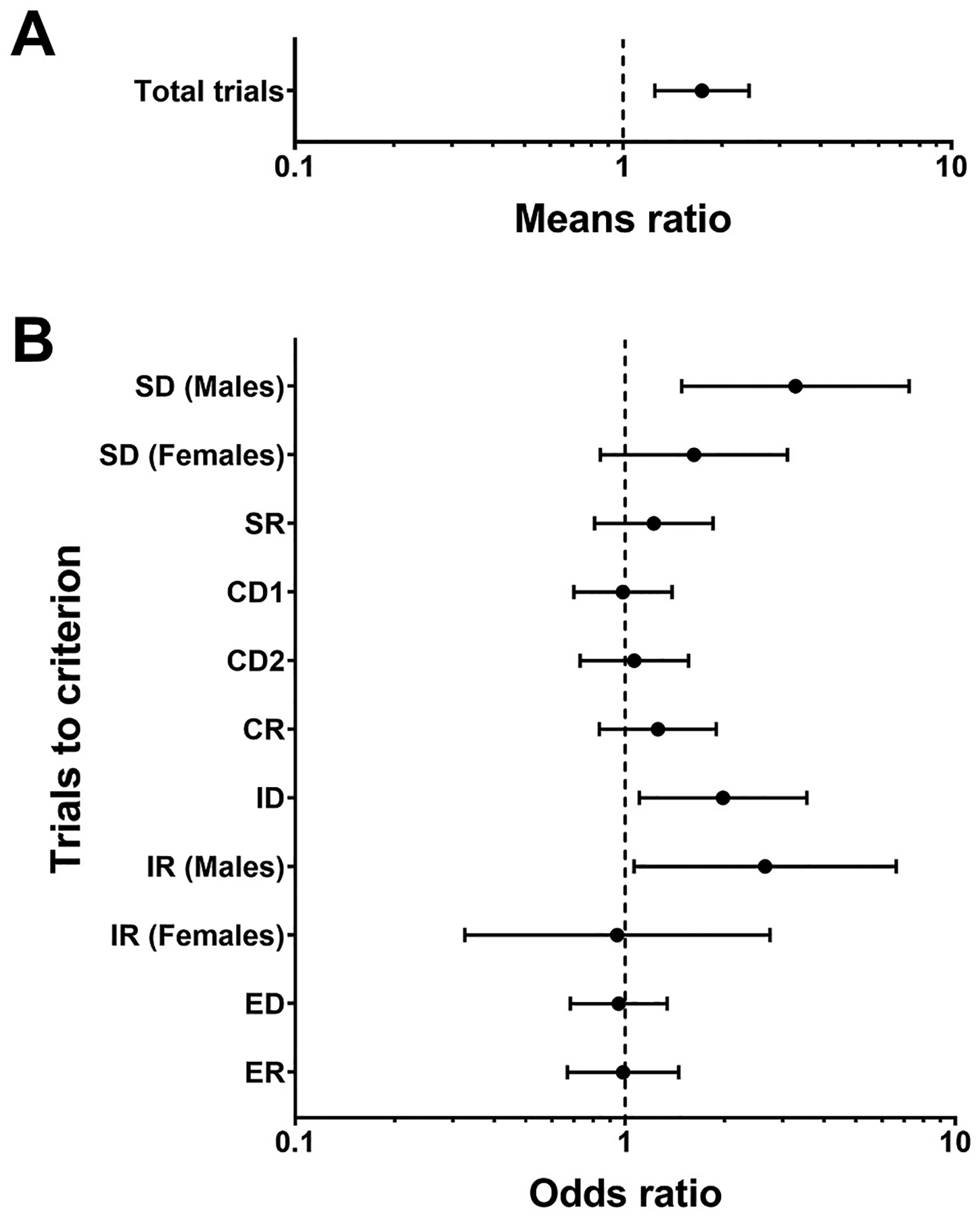
Associations between PBDE serum concentrations and performance on ID/ED shift (n = 104). Means ratios and odds ratios are plotted on a log scale. (A) ID/ED shift: Total trials analyzed with negative binomial regression and associations reported as means ratio estimates (± 95% CL) for a 2 SD increase in PBDE exposure on the log scale or an 11-fold increase on the original scale. (B) ID/ED shift: Trials to criterion for each stage analyzed with logistic regression and associations reported as odds ratio estimates (± 95% CL) for a 2 SD increase in PBDE exposure on the log scale or an 11-fold increase on the original scale. All models were adjusted for age, sex, and WASI IQ score. CL: confidence limit, SD: standard deviation.
3.2.2. DMS
Negative binomial regression for total wrong revealed a significant positive association of PCBs with total wrong trials (β = 1.15, 95% CL: 1.00, 1.31, p < 0.05) whereby participants with higher PCB serum concentrations made more errors, shown as a means ratio estimate and 95% CL (Fig. 2C). There was also a significant negative association of IQ (p = 0.04) in which higher IQ was associated with fewer total wrong trials. There was no significant sex by exposure interaction.
There was no significant main effect of PBDE exposure on total wrong (Supplemental Table 3), although as in the PCB model, there was a significant negative association of IQ (p = 0.02). There was no significant sex by exposure interaction.
There was no significant main effect of PCB or PBDE exposure on total wrong latency, although an effect of sex was apparent such that females tended to take less time to make an incorrect response than males in both PCB (p < 0.01) and PBDE (p < 0.01) statistical models (data not shown).
3.2.3. SWM
There were no significant associations of PCB or PBDE exposure (Supplemental Table 3), nor were there significant interactions of sex with exposure for any of the three outcomes assessed for the SWM test. However, there were significant negative associations of IQ with all outcomes. Higher IQ was associated with fewer errors (p < 0.001 for both PCB and PBDE models), fewer total responses to complete the set (p = 0.08 and p < 0.05 for PCB and PBDE models, respectively), and less total time to complete a set (p < 0.05 and p = 0.04 for PCB and PBDE models, respectively). There was also an effect of sex for the number of responses to complete a set in both exposure models (p = 0.05 and p = 0.03 for PCBs and PBDEs, respectively) wherein females made more responses on average.
3.2.4. SOC
As shown in Supplemental Table 3, there were no significant associations of PCB or PBDE exposure or interactions of sex with exposure for the two outcomes assessed for the SOC test (thinking time and mean moves to completion for 5-move problems). There was a significant effect of IQ for thinking time for 5-move problems such that higher IQ was associated with longer thinking time in both exposure models (p = 0.03 and p = 0.02 for PCBs and PBDEs, respectively).
3.3. Associations of PCB and PBDE congener serum concentrations with CANTAB measures
Overall, the outcomes associated with individual congeners were mostly consistent with total PCBs and total PBDEs. Associations of ID/ED outcomes with individual PCB congeners and PBDE congeners are presented in Supplementary Tables 4 and 5, respectively. Associations of outcomes for DMS, SWM, and SOC with individual PCB and PBDE congeners are presented in Supplementary Tables 6 and 7, respectively.
4. Discussion
In this study, there was a sex-specific association between PCB serum concentrations and total trials to criterion on the set-shifting test. Specifically, adolescent males with higher PCB exposure took a greater number of trials to complete the task. Similarly, there was an association between higher PBDE concentrations and total trials to criterion whereby males and females took more trials to complete the test. Higher concentrations of PCBs, but not PBDEs, were also associated with an increase in incorrect responses on the DMS test. These findings suggest that exposure to PCBs and PBDEs during adolescence may be associated with deficits in cognitive flexibility and/or visual recognition memory, two key aspects of executive function that mature during the adolescent years (Best and Miller, 2010; Theodoraki et al., 2020).
4.1. PCB & PBDE exposure
Data on adolescent PCB or PBDE exposure are sparse. To date, an Akwesasne Mohawk Nation cohort is one of the only other groups of adolescents in which PCB exposure has been assessed (Newman et al., 2006), while a cohort from Flanders, Belgium is one of the only adolescent cohorts in which PBDE exposure has been reported (Kiciński et al., 2012).
The PCB concentrations measured in the current study are lower than those reported in adult populations known to consume fish from polluted waters (Schantz et al., 2010). This was expected given that these compounds are lipophilic and gradually bioaccumulate over the lifespan. However, the PCB levels observed in our cohort are similar to those reported in the Akwesasne adolescents (median = 0.63 ppb) (Newman et al., 2006). Consistent with the results of our study, Newman et al. (2006) and Newman et al. (2009) reported significant, but subtle, associations of higher adolescent PCB serum concentrations with poorer performance on measures of cognitive function. Specifically, they found that PCBs were associated with impaired long-term memory, but not short-term memory or processing speed, as measured by the Woodcock Johnson-Revised (WJ-R), which yields overall cognitive functioning score and 7 cluster scores for specific cognitive domains. They also reported that PCBs were inversely associated with Delayed Recall, a measure similar to Long Term Retrieval, on the Test of Memory and Learning (TOMAL). Using the Ravens Progressive Matrices (RPM), a non-verbal measure of ability to discern patterns and derive meaning from complex data, they also reported an inverse association of RPM performance and dioxin-like PCBs. Though these findings show associations of PCBs with specific cognitive domains, they did not specifically assess executive functioning.
To date, adolescent PBDE data from only one other cohort that also assessed neurocognitive outcomes has been published, and that study reported a median value for total PBDEs of 7 ng/L; whether this value was lipid adjusted was not indicated (Kiciński et al., 2012). In contrast to the current findings, Kiciński et al. (2012) did not find that PBDE serum concentrations were associated with cognitive deficits, though they reported associations with poorer motor function. However, the effects of prenatal and childhood exposure to PBDEs have been assessed in the CHAMACOS birth cohort wherein the geometric mean for PBDE concentrations in 9-year-olds was 63.2 ng/g lipid (range: 6.5–430.2) (Sagiv et al., 2015), which was higher than that of our cohort. They reported associations of both prenatal and concurrent PBDE concentrations with attention and executive function. Compared to prenatal exposure, associations of cognitive outcomes with childhood PBDE concentrations were more subtle and the most robust effects were in females. There are not yet published data on PBDE concentrations in CHAMACOS adolescent participants.
4.2. ID/ED shift
Our data suggest that PCB and PBDE exposures are associated with deficits in cognitive flexibility, as adolescent males with higher PCB concentrations and adolescent males and females with higher PBDE concentrations took more trials to complete the ID/ED test. In the individual stages, PCBs were associated with more trials to criterion in the SD stage in males and the CD2 and ER stages in males and females, while PBDEs were associated with more trials to criterion in the SD and IR stages in males and the ID stage in both males and females. Overall, the ID/ED test assesses cognitive flexibility and set-shifting (Luciana, 2003). Though there are differential effects of PCBs and PBDEs on the individual stages of the ID/ED test, a notable consistency is the association of higher concentrations of both contaminants with more trials to complete the test (i.e., an increase in total errors). In general, these findings indicate potential contaminant-related deficits in cognitive flexibility which could be the result of alterations of prefrontal cortex functioning (Fray and Robbins, 1996; Luciana, 2003). Though subtle, the overall decrements in cognitive flexibility associated with both PCB and PBDE exposure, and the decrement in working memory associated with PCB exposure are consistent with what has been reported previously in children exposed prenatally and early postnatally and in laboratory animal studies of these chemicals (Eubig et al., 2010; Vuong et al., 2020).
In the current study, the association of PCB exposure with cognitive flexibility was seen primarily in males. As PCBs are known endocrine disrupters (Crinnion, 2011), differential effects in males and females are not surprising. For instance, a study by Sagiv et al. (2012) reported an association of higher prenatal PCB exposure with poorer performance on a task of attention in 8-year-old males. On a similar computerized task of attention and impulsivity, higher concurrent PCB exposure was associated with greater errors of omission, suggesting an effect on attention/vigilance in males from the Akwesasne adolescent cohort (Behforooz et al., 2017).
More specifically, some animal studies have reported male-specific effects on cognitive flexibility in PCB-exposed rats that mirror our findings on the ID/ED set-shifting test. Our group found a male-specific adverse effect of PCB exposure during adolescence on a set-shifting task that engaged cognitive flexibility (Monaikul et al., 2017). Importantly, that study exposed animals during adolescence and used an environmental mixture of PCBs designed to model PCB contamination in northeastern Wisconsin. Another study in rodents found that perinatal PCB exposure was associated with deficits in performance on a spatial reversal learning task, which also assessed cognitive flexibility, wherein exposed males exhibited increased errors on an intra-dimensional reversal similar to that presented in the IR stage of the ID/ED task (Widholm et al., 2001). Taken together, the sex-specific effect of PCBs on set-shifting in the current study is consistent with previous laboratory animal research in that males were more adversely affected and that PCB exposure was associated with deficits in cognitive flexibility.
Fewer studies have investigated whether PBDE exposure is related to deficits in cognitive flexibility, particularly in animal models. However, both prenatal PBDE and PCB exposure have been associated with poorer performance on the Wisconsin Card Sorting Test (WCST), which assesses functions similar to those assessed in the CANTAB ID/ED task (Jacobson and Jacobson, 2003; Sagiv et al., 2015). In contrast, there were no associations of concurrent childhood PBDE concentrations with WCST scores (Sagiv et al., 2015). Vuong et al. (2016) examined whether prenatal PBDE exposures were associated with executive function as measured with the Behavior Rating Inventory of Executive Function (BRIEF) in 5- and 8-year-old children. Though specific effects of PBDEs on the BRIEF shift subscale were not reported, they did find that PBDE concentrations were associated with poorer scores in behavior regulation, a composite score that included subscales for shift (ability to transition), inhibit (impulse control), and emotional control (emotional response management).
Regarding possible biological mechanisms behind sex-specific associations of PCBs and PBDEs, it is well known that both contaminants are endocrine disrupting chemicals (Dishaw et al., 2014; Hood, 2006; Schug et al., 2015). Sex-specific effects of exposure to PCBs and PBDEs are common, and various mechanisms have been proposed (Dishaw et al., 2014; Liberman et al., 2020). PCBs can disrupt estrogen and thyroid hormone actions (Schug et al., 2015), while PBDEs are primarily linked to thyroid hormone disruption (Dishaw et al., 2014). Gonadal and thyroid hormones interact with neurotransmitters, particularly dopaminergic and serotonergic systems, and act as key neural signaling molecules (Baksi and Pradhan, 2021; Barth et al., 2015; Vigil et al., 2016). During adolescence, there are significant increases in endogenous hormone activity, and hormones modulate fine-tuning of neural circuitry and structural organization, which are sexually-dimorphic (Liberman et al., 2020; Vigil et al., 2016). Though it is beyond of the scope of the current study to pinpoint specific mechanisms driving sex-specific exposure associations, we can hypothesize that through interactions with hormone-receptor complexes, PCBs and PBDEs may directly or indirectly exert endocrine-disrupting effects to alter typical sexually-dimorphic brain development, resulting in sex-specific functional differences.
4.3. DMS, SWM, & SOC
The DMS test assessed visual recognition memory and working memory (Fray and Robbins, 1996; Luciana, 2003), and we found that higher PCB exposure was associated with an increase in incorrect choices. Specifically, adolescents with higher PCB exposure made more errors before completing the test. However, contrary to our hypothesis, we did not see PCB- or PBDE-related detriments in performance on the tests that assessed spatial working memory (SWM) or planning and working memory (SOC).
Although the CANTAB DMS task has not yet been evaluated in other adolescent cohorts assessing PCB and PBDE exposure, decrements in performance on the DMS test align with previous research demonstrating associations of exposure with memory impairment. In the Akwesasne adolescent cohort, PCBs were associated with long-term memory deficits (Newman et al., 2006; Newman et al., 2009). Numerous studies in infants and children have shown that prenatal PCB exposure was associated with deficits in visual recognition memory (Darvill et al., 2000; Jacobson et al., 1992; Jacobson et al., 1985) and on measures of short term and working memory (Jacobson and Jacobson, 1996, 2003; Stewart et al., 2008; Vreugdenhil et al., 2002). Berghuis et al. (2018) also found that maternal pregnancy serum levels of PCBs were inversely associated with verbal memory in 13- to 15-year-olds. Notably, most studies have reported associations of developmental PCB exposure with adverse effects on performance on tasks of verbal working memory rather than nonverbal working memory (Eubig et al., 2010), as measured in the current study.
We did not find associations of PBDEs with memory deficits. Like-wise, Kiciński et al. (2012) found no association of adolescent PBDE serum concentrations with performance on a digit span test that assessed working memory. In the CHAMACOS cohort, there were no effects of childhood PBDE exposure, but prenatal PBDE serum concentrations were associated with working memory decrements at 7 years (Eskenazi et al., 2013) and at 9 and 12 years (Sagiv et al., 2015). Similarly, another study found that cord plasma PBDE concentrations but not childhood PBDE levels were associated with auditory working memory impairment at 9 to 14-years-of-age. They also reported associations of childhood PBDE levels with visual memory deficits (Cowell et al., 2018). Finally, prenatal PBDE exposure was inversely associated with verbal memory in 13- to 15-year-old children (Berghuis et al., 2018). It is possible that working memory may be more vulnerable to disruption from PBDE exposure during the prenatal period.
4.4. Associations of individual congeners
For total trials on ID/ED, there were no significant associations for any of the PCB congeners, indicating that none of the individual congeners drove the effect observed for total PCBs on total trials. However, associations for both PBDE congeners aligned with the results for total PBDEs, as there were significant positive associations for both PBDE 47 and PBDE 153 with total trials.
For trials to criterion at each ID/ED stage, the overall results for individual congeners were consistent with those of total PCBs and total PBDEs, though in some cases, a significant association was found only for one congener rather than all of them. For instance, for trials to criterion for CD2, there was a main effect of total PCBs; however, there was no effect for PCB 138/163 or PCB 153, though there was an exposure x sex interaction for PCB 180 wherein females took more trials to reach criterion than males, suggesting that the total PCBs association for CD2 may have been driven by PCB 180 (see Fig. 2 and Supplemental Table 4).
For DMS, there was a significant exposure x sex interaction for PCB 180 but no effects for PCB 138/163 or PCB 153, indicating that PCB 180 may be driving the observed association of total wrong with total PCBs (see Fig. 2 and Supplemental Table 6). The congener-specific findings generally agreed with the associations for total PCBs and total PBDEs. One exception was that there were several significant associations of the individual PCB congeners with SWM outcomes (see Supplemental Table 6), suggesting that examination of those individual congeners may have provided more insight into the possible effects of PCBs on spatial working memory.
4.5. Potential real-world implications
Interestingly, our findings suggesting that prenatal PCB exposure may impact cognitive flexibility in males more than females appear to be in line with research on ADHD. Cognitive flexibility deficits are one of the key defining factors in ADHD diagnosis, which is considered the most common neuropsychological disorder in children, with males being three times more likely to be diagnosed than females–9.2% vs. 3.0% respectively (Lambez et al., 2020; Roshani et al., 2020). Even more concerning, ADHD symptoms persist into adulthood in about 30–50% of cases (Lambez et al., 2020; Roshani et al., 2020), affecting social, educational, and emotional development and increasing the likelihood of delinquency, imprisonment, unemployment, marital problems and divorce (Roshani et al., 2020). Cognitive flexibility enables one to adapt strategies to cope with new and unpredictable situations and is one of the ADHD symptoms that correlates most strongly with mental health problems (Roshani et al., 2020). Although the present findings indicate only subtle impairment in cognitive flexibility due to PCB exposure, Bellinger (2007) has argued that small effect sizes in individual studies can represent a significant impact when the population is considered as a whole, especially in the case of outcomes that are seen in a large number of individuals and persist across the lifespan.
4.6. Strengths and limitations
When evaluating the current study’s findings and implications, there are many strengths as well as some limitations to consider. A major strength was the direct assessment of both cognitive function and contaminant exposure. Specifically, executive function was evaluated using an automated battery designed to interrogate distinct domains of cognition rather than relying on maternal or child self-report measures such as the BRIEF (Anderson et al., 2002; Vriezen and Pigott, 2002). Rather than obtaining measures of exposure through indirect assessment (e.g., questionnaire about contaminated fish consumption), serum PCB and PBDE concentrations provided direct biomarkers of exposure. It is important to note, however, that because our participants were children of sports anglers, serum PCB and PBDE concentrations may reflect exposure throughout the lifespan and not just exposure limited to adolescence, and PCBs and PBDEs have long half-lives. Thus, it is possible that early developmental exposure to PCBs and PBDEs may confound our results, making it difficult to associate these cognitive outcomes with exposure during the adolescent period alone. Finally, our sample size was limited and underpowered to conduct mixtures analyses or analyses examining potential joint effects of both chemicals or to examine dose-response relationships. Also, the demographics of our sample were not representative of a diverse population; thus the ability to generalize the current results is a limitation, and caution is needed when interpreting the current findings.
4.7. Conclusions
Although the findings of this study were subtle, the deficits in performance on the cognitive flexibility and working memory tests associated with adolescent PCB and/or PBDE concentrations suggest vulnerability to exposure to these contaminants during this period and that some executive functions modulated by the prefrontal cortex could be particularly sensitive (Curtis and D’Esposito, 2003; Kim et al., 2011). Furthermore, PCBs (Grossman, 2013) and PBDEs (Costa et al., 2014) will continue to persist in the environment, and there are very few studies assessing the effects of PCB and PBDE exposure in adolescents. Because these chemicals seem to affect executive functions that are developing during adolescence, future studies with larger samples sizes are needed to further investigate how contaminant exposure during this critical period of development may be associated with adverse cognitive and neurobehavioral outcomes.
Supplementary Material
Funding
This work was supported by the Agency for Toxic Substances and Disease Registry, an agency of the United States Department of Health and Human Services (R01 TS000072). Funding sources were not involved in study design, data collection, data analysis, interpretation of data, the writing of the report, or the decision to submit the paper for publication.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ntt.2022.107092.
References
- Agency for Toxic Substances and Disease Registry, 2017. Toxicological Profile for Polybrominated Diphenyl ethers (PBDEs). https://www.atsdr.cdc.gov/toxprofiles/tp207.pdf. [PubMed]
- Aguiar A, Eubig PA, Schantz SL, 2010. Attention deficit/hyperactivity disorder: a focused overview for children’s environmental health researchers. Environ. Health Perspect 118 (12), 1646–1653. 10.1289/ehp.1002326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson VA, Anderson P, Northam E, Jacobs R, Mikiewicz O, 2002. Relationships between cognitive and behavioral measures of executive function in children with brain disease. Child Neuropsychol 8 (4), 231–240. 10.1076/chin.8.4.231.13509. [DOI] [PubMed] [Google Scholar]
- Anderson HA, Imm P, Knobeloch L, Turyk M, Mathew J, Buelow C, Persky V, 2008. Polybrominated diphenyl ethers (PBDE) in serum: findings from a US cohort of consumers of sport-caught fish. Chemosphere 73 (2), 187–194. 10.1016/j.chemosphere.2008.05.052. [DOI] [PubMed] [Google Scholar]
- Baksi S, Pradhan A, 2021. Thyroid hormone: sex-dependent role in nervous system regulation and disease. Biol. Sex Differ 12 (1), 25. 10.1186/s13293-021-00367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth C, Villringer A, Sacher J, 2015. Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Front. Neurosci 9 10.3389/fnins.2015.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behforooz B, Newman J, Gallo MV, Akwesasne Task Force on the, E, Schell LM, 2017. PCBs and measures of attention and impulsivity on a continuous performance task of young adults. Neurotoxicol. Teratol 64, 29–36. 10.1016/j.ntt.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger DC, 2007. Interpretation of small effect sizes in occupational and environmental neurotoxicology: individual versus population risk. NeuroToxicology 28 (2), 245–251. 10.1016/j.neuro.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Berghuis SA, Van Braeckel K, Sauer PJJ, Bos AF, 2018. Prenatal exposure to persistent organic pollutants and cognition and motor performance in adolescence. Environ. Int 121 (Pt 1), 13–22. 10.1016/j.envint.2018.08.030. [DOI] [PubMed] [Google Scholar]
- Berthelsen D, Hayes N, White SLJ, Williams KE, 2017. Executive function in adolescence: associations with child and family risk factors and self-regulation in early childhood. Front. Psychol 8 10.3389/fpsyg.2017.00903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best JR, Miller PH, 2010. A developmental perspective on executive function. Child Dev. 81 (6), 1641–1660. 10.1111/j.1467-8624.2010.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher O, Muckle G, Bastien CH, 2009. Prenatal exposure to polychlorinated biphenyls: a neuropsychologic analysis. Environ. Health Perspect 117 (1), 7–16. 10.1289/ehp.11294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambridge Cognition, 2022. Cognitive Research. cambridgecognition.com.
- Canfield RL, Gendle MH, Cory-Slechta DA, 2004. Impaired neuropsychological functioning in lead-exposed children. Dev. Neuropsychol 26 (1), 513–540. 10.1207/s15326942dn2601_8. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2009. Fourth National Report on Human Exposure to Environmental Chemicals. https://www.cdc.gov/exposurereport/pdf/fourthreport.pdf.
- Cook RD, Weisberg S, 1982. Residuals and Influence in Regression. Chapman and Hall, New York. [Google Scholar]
- Costa LG, de Laat R, Tagliaferri S, Pellacani C, 2014. A mechanistic view of polybrominated diphenyl ether (PBDE) developmental neurotoxicity. Toxicol. Lett 230 (2), 282–294. 10.1016/j.toxlet.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell WJ, Margolis A, Rauh VA, Sjödin A, Jones R, Wang Y, Garcia W, Perera F, Wang S, Herbstman JB, 2018. Associations between prenatal and childhood PBDE exposure and early adolescent visual, verbal and working memory. Environ. Int 118, 9–16. 10.1016/j.envint.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews F, He J, Hodge C, 2007. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacol. Biochem. Behav 86 (2), 189–199. 10.1016/j.pbb.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crinnion WJ, 2011. Polychlorinated biphenyls: persistent pollutants with immunological, neurological, and endocrinological consequences. Altern. Med. Rev 16 (1), 5–13. [PubMed] [Google Scholar]
- Curtis CE, D’Esposito M, 2003. Persistent activity in the prefrontal cortex during working memory. Trends Cogn. Sci 7 (9), 415–423. 10.1016/S1364-6613(03)00197-9. [DOI] [PubMed] [Google Scholar]
- Darvill T, Lonky E, Reihman J, Stewart P, Pagano J, 2000. Prenatal exposure to PCBs and infant performance on the Fagan test of infant intelligence. NeuroToxicology 21 (6), 1029–1038. [PubMed] [Google Scholar]
- de Water E, Curtin P, Zilverstand A, Sjödin A, Bonilla A, Herbstman JB, Ramirez J, Margolis AE, Bansal R, Whyatt RM, Peterson BS, Factor-Litvak P, Horton MK, 2019. A preliminary study on prenatal polybrominated diphenyl ether serum concentrations and intrinsic functional network organization and executive functioning in childhood. J. Child Psychol. Psychiatry 60 (9), 1010–1020. 10.1111/jcpp.13040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit CA, 2002. An overview of brominated flame retardants in the environment. Chemosphere 46 (5), 583–624. 10.1016/S0045-6535(01)00225-9. [DOI] [PubMed] [Google Scholar]
- Dishaw LV, Macaulay LJ, Roberts SC, Stapleton HM, 2014. Exposures, mechanisms, and impacts of endocrine-active flame retardants. Curr. Opin. Pharmacol 19, 125–133. 10.1016/j.coph.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Chevrier J, Rauch SA, Kogut K, Harley KG, Johnson C, Trujillo C, Sjödin A, Bradman A, 2013. In utero and childhood polybrominated diphenyl ether (PBDE) exposures and neurodevelopment in the CHAMACOS study. Environ. Health Perspect 121 (2), 257–262. 10.1289/ehp.1205597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eubig PA, Aguiar A, Schantz SL, 2010. Lead and PCBs as risk factors for attention deficit/hyperactivity disorder. Environ. Health Perspect 118 (12), 1654–1667. 10.1289/ehp.0901852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fray PJ, Robbins TW, 1996. CANTAB battery: proposed utility in neurotoxicology. Neurotoxicol. Teratol 18 (4), 499–504. 10.1016/0892-0362(96)00027-x. [DOI] [PubMed] [Google Scholar]
- Frndak S, Barg G, Canfield RL, Quierolo EI, Mañay N, Kordas K, 2019. Latent subgroups of cognitive performance in lead- and manganese-exposed Uruguayan children: examining behavioral signatures. NeuroToxicology 73, 188–198. 10.1016/j.neuro.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greizerstein HB, Gigliotti P, Vena J, Freudenheim J, Kostyniak PJ, 1997. Standardization of a method for the routine analysis of polychlorinated biphenyl congeners and selected pesticides in human serum and milk. J. Anal. Toxicol 21 (7), 558–566. 10.1093/jat/21.7.558. [DOI] [PubMed] [Google Scholar]
- Grossman E, 2013. Nonlegacy PCBs: pigment manufacturing by-products get a second look. Environ. Health Perspect 121 (3), a86–a93. 10.1289/ehp.121-a86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood E, 2006. Endocrine disruption and flame-retardant chemicals: PBDE-99 effects on rat sexual development. Environ. Health Perspect 114 (2) (A112-A3). [Google Scholar]
- Hornbuckle K, Robertson L, 2010. Polychlorinated biphenyls (PCBs): sources, exposures, toxicities. Environ. Sci. Technol 44 (8), 2749–2751. 10.1021/es100801f. [DOI] [PubMed] [Google Scholar]
- Hornung RW, Reed LD, 1990. Estimation of average concentration in the presence of nondetectable values. Appl. Occup. Environ. Hyg 5 (1), 46–51. 10.1080/1047322X.1990.10389587. [DOI] [Google Scholar]
- Jacobson JL, Jacobson SW, 1996. Intellectual impairment in children exposed to polychlorinated biphenyls in utero. N. Engl. J. Med 335 (11), 783–789. 10.1056/nejm199609123351104. [DOI] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW, 2003. Prenatal exposure to polychlorinated biphenyls and attention at school age. J. Pediatr 143 (6), 780–788. 10.1067/S0022-3476(03)00577-8. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Fein GG, Jacobson JL, Schwartz PM, Dowler JK, 1985. The effect of intrauterine PCB exposure on visual recognition memory. Child Dev. 56 (4), 853–860. 10.2307/1130097. [DOI] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW, Padgett RJ, Brumitt GA, Billings RL, 1992. Effects of prenatal PCB exposure on cognitive processing efficiency and sustained attention. Dev. Psychol 28 (2), 297–306. 10.1037/0012-1649.28.2.297. [DOI] [Google Scholar]
- Kiciński M, Viaene MK, Den Hond E, Schoeters G, Covaci A, Dirtu AC, Nelen V, Bruckers L, Croes K, Sioen I, Baeyens W, Van Larebeke N, Nawrot TS, 2012. Neurobehavioral function and low-level exposure to brominated flame retardants in adolescents: a cross-sectional study. Environ. Health 11, 86. 10.1186/1476-069x-11-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Johnson NF, Cilles SE, Gold BT, 2011. Common and distinct mechanisms of cognitive flexibility in prefrontal cortex. J. Neurosci 31 (13), 4771–4779. 10.1523/jneurosci.5923-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyniak PJ, Hansen LG, Widholm JJ, Fitzpatrick RD, Olson JR, Helferich JL, Kim KH, Sable HJK, Seegal RF, Pessah IN, Schantz SL, 2005. Formulation and characterization of an experimental PCB mixture designed to mimic human exposure from contaminated fish. Toxicol. Sci 88 (2), 400–411. 10.1093/toxsci/kfi338. [DOI] [PubMed] [Google Scholar]
- Lam J, Lanphear BP, Bellinger D, Axelrad DA, McPartland J, Sutton P, Davidson L, Daniels N, Sen S, Woodruff TJ, 2017. Developmental PBDE exposure and IQ/ADHD in childhood: a systematic review and meta-analysis. Environ. Health Perspect 125 (8) 10.1289/EHP1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambez B, Harwood-Gross A, Golumbic EZ, Rassovsky Y, 2020. Nonpharmacological interventions for cognitive difficulties in ADHD: a systematic review and meta-analysis. J. Psychiatr. Res 120, 40–55. 10.1016/j.jpsychires.2019.10.007. [DOI] [PubMed] [Google Scholar]
- Liberman DA, Walker KA, Gore AC, Bell MR, 2020. Sex-specific effects of developmental exposure to polychlorinated biphenyls on neuroimmune and dopaminergic endpoints in adolescent rats. Neurotoxicol. Teratol 79, 106880. 10.1016/j.ntt.2020.106880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciana M, 2003. Practitioner review: computerized assessment of neuropsychological function in children: clinical and research applications of the Cambridge neuropsychological testing automated battery (CANTAB). J. Child Psychol. Psychiatry 44 (5), 649–663. 10.1111/1469-7610.00152. [DOI] [PubMed] [Google Scholar]
- Luna B, 2009. Developmental changes in cognitive control through adolescence. In:Bauer P (Ed.), Adv. Child Dev. Behav. JAI, pp. 233–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaikul S, Eubig P, Floresco S, Schantz S, 2017. Strategy set-shifting and response inhibition in adult rats exposed to an environmental polychlorinated biphenyl mixture during adolescence. Neurotoxicol. Teratol 63, 14–23. 10.1016/j.ntt.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman J, Aucompaugh AG, Schell LM, Denham M, DeCaprio AP, Gallo MV, Ravenscroft J, Kao C-C, Hanover MR, David D, Jacobs AM, Tarbell AM, Worswick P, 2006. PCBs and cognitive functioning of Mohawk adolescents. Neurotoxicol. Teratol 28 (4), 439–445. 10.1016/j.ntt.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Newman J, Gallo MV, Schell LM, DeCaprio AP, Denham M, Deane GD, 2009. Analysis of PCB congeners related to cognitive functioning in adolescents. NeuroToxicology 30 (4), 686–696. 10.1016/j.neuro.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessah IN, Lein PJ, Seegal RF, Sagiv SK, 2019. Neurotoxicity of polychlorinated biphenyls and related organohalogens. Acta Neuropathol. 138 (3), 363–387. 10.1007/s00401-019-01978-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinson A, Bourguignon JP, Parent AS, 2016. Exposure to endocrine disrupting chemicals and neurodevelopmental alterations. Andrology 4 (4), 706–722. 10.1111/andr.12211. [DOI] [PubMed] [Google Scholar]
- Rahman F, Langford KH, Scrimshaw MD, Lester JN, 2001. Polybrominated diphenyl ether (PBDE) flame retardants. Sci. Total Environ 275 (1–3), 1–17. 10.1016/s0048-9697(01)00852-x. [DOI] [PubMed] [Google Scholar]
- Roshani F, Piri R, Malek A, Michel TM, Vafaee MS, 2020. Comparison of cognitive flexibility, appropriate risk-taking and reaction time in individuals with and without adult ADHD. Psychiatry Res. 284, 112494 10.1016/j.psychres.2019.112494. [DOI] [PubMed] [Google Scholar]
- Ross G, 2004. The public health implications of polychlorinated biphenyls (PCBs) in the environment. Ecotoxicol. Environ. Saf 59 (3), 275–291. 10.1016/j.ecoenv.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Roze E, Meijer L, Bakker A, Van Braeckel KN, Sauer PJ, Bos AF, 2009. Prenatal exposure to organohalogens, including brominated flame retardants, influences motor, cognitive, and behavioral performance at school age. Environ. Health Perspect 117 (12), 1953–1958. 10.1289/ehp.0901015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagiv SK, Thurston SW, Bellinger DC, Altshul LM, Korrick SA, 2012. Neuropsychological measures of attention and impulse control among 8-year-old children exposed prenatally to organochlorines. Environ. Health Perspect 120 (6), 904–909. 10.1289/ehp.1104372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagiv SK, Kogut K, Gaspar F, Gunier R, Harley K, Parra K, Villaseñor D, Bradman A, Holland N, Eskenazi B, 2015. Prenatal and childhood polybrominated diphenyl ether (PBDE) exposure and attention and executive function at 9–12 years of age. Neurotoxicol. Teratol 52 (Pt B), 151–161. 10.1016/j.ntt.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer DJ, Dellinger JA, Needham LL, Hansen LG, 2006. Serum PCB profiles in native Americans from Wisconsin based on region, diet, age, and gender: implications for epidemiology studies. Sci. Total Environ 357 (1–3), 74–87. 10.1016/j.scitotenv.2005.04.030. [DOI] [PubMed] [Google Scholar]
- Schantz SL, Gardiner JC, Aguiar A, Tang X, Gasior DM, Sweeney AM, Peck JD, Gillard D, Kostyniak PJ, 2010. Contaminant profiles in southeast Asian immigrants consuming fish from polluted waters in northeastern Wisconsin. Environ. Res 110 (1), 33–39. 10.1016/j.envres.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schisterman EF, Whitcomb BW, Louis GMB, Louis TA, 2005. Lipid adjustment in the analysis of environmental contaminants and human health risks. Environ. Health Perspect 113 (7), 853–857. 10.1289/ehp.7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schug TT, Blawas AM, Gray K, Heindel JJ, Lawler CP, 2015. Elucidating the links between endocrine disruptors and neurodevelopment. Endocrinology 156 (6), 1941–1951. 10.1210/en.2014-1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjödin A, Wong L-Y, Jones RS, Park A, Zhang Y, Hodge C, DiPietro E, McClure C, Turner W, Needham LL, Patterson DG, 2008. Serum concentrations of polybrominated diphenyl ethers (PBDEs) and polybrominated biphenyl (PBB) in the United States population: 2003–2004. Environ. Sci. Technol 42 (4), 1377–1384. 10.1021/es702451p. [DOI] [PubMed] [Google Scholar]
- Stewart PW, Reihman J, Lonky EI, Darvill TJ, Pagano J, 2003. Cognitive development in preschool children prenatally exposed to PCBs and MeHg. Neurotoxicol. Teratol 25 (1), 11–22. 10.1016/s0892-0362(02)00320-3. [DOI] [PubMed] [Google Scholar]
- Stewart PW, Sargent DM, Reihman J, Gump BB, Lonky E, Darvill T, Hicks H, Pagano J, 2006. Response inhibition during differential reinforcement of low rates (DRL) schedules may be sensitive to low-level polychlorinated biphenyl, methylmercury, and lead exposure in children. Environ. Health Perspect 114 (12), 1923–1929. 10.1289/ehp.9216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart PW, Lonky E, Reihman J, Pagano J, Gump BB, Darvill T, 2008. The relationship between prenatal PCB exposure and intelligence (IQ) in 9-year-old children. Environ. Health Perspect 116 (10), 1416–1422. 10.1289/ehp.11058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodoraki TE, McGeown SP, Rhodes SM, MacPherson SE, 2020. Developmental changes in executive functions during adolescence: a study of inhibition, shifting, and working memory. Br. J. Dev. Psychol 38 (1), 74–89. 10.1111/bjdp.12307. [DOI] [PubMed] [Google Scholar]
- Turyk ME, Bhavsar SP, Bowerman W, Boysen E, Clark M, Diamond M, Mergler D, Pantazopoulos P, Schantz S, Carpenter DO, 2012. Risks and benefits of consumption of Great Lakes fish. Environ. Health Perspect 120 (1), 11–18. 10.1289/ehp.1003396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigil P, Del Río JP, Carrera B, ArÁnguiz FC, Rioseco H, Cortés ME, 2016. Influence of sex steroid hormones on the adolescent brain and behavior: an update. Linacre Q 83 (3), 308–329. 10.1080/00243639.2016.1211863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreugdenhil HJ, Lanting CI, Mulder PG, Boersma ER, Weisglas-Kuperus N, 2002. Effects of prenatal PCB and dioxin background exposure on cognitive and motor abilities in Dutch children at school age. J. Pediatr 140 (1), 48–56. 10.1067/mpd.2002.119625. [DOI] [PubMed] [Google Scholar]
- Vriezen ER, Pigott SE, 2002. The relationship between parental report on the BRIEF and performance-based measures of executive function in children with moderate to severe traumatic brain injury. Child Neuropsychol 8 (4), 296–303. 10.1076/chin.8.4.296.13505. [DOI] [PubMed] [Google Scholar]
- Vuong AM, Yolton K, Webster GM, Sjödin A, Calafat AM, Braun JM, Dietrich KN, Lanphear BP, Chen A, 2016. Prenatal polybrominated diphenyl ether and perfluoroalkyl substance exposures and executive function in school-age children. Environ. Res 147, 556–564. 10.1016/j.envres.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong AM, Yolton K, Poston KL, Xie C, Webster GM, Sjödin A, Braun JM, Dietrich KN, Lanphear BP, Chen A, 2017. Prenatal and postnatal polybrominated diphenyl ether (PBDE) exposure and measures of inattention and impulsivity in children. Neurotoxicol. Teratol 64, 20–28. 10.1016/j.ntt.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong AM, Yolton K, Cecil KM, Braun JM, Lanphear BP, Chen A, 2020. Flame retardants and neurodevelopment: an updated review of epidemiological literature. Curr. Epidemiol. Rep 7 (4), 220–236. 10.1007/s40471-020-00256-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D, 1999. Wechsler Abbreviated Scale of Intelligence. PsychCorp, San Antonio, TX. [Google Scholar]
- Whitehead TP, Brown FR, Metayer C, Park JS, Does M, Petreas MX, Buffler PA, Rappaport SM, 2013. Polybrominated diphenyl ethers in residential dust: sources of variability. Environ. Int 57–58, 11–24. 10.1016/j.envint.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead TP, Brown FR, Metayer C, Park JS, Does M, Dhaliwal J, Petreas MX, Buffler PA, Rappaport SM, 2014. Polychlorinated biphenyls in residential dust: sources of variability. Environ. Sci. Technol 48 (1), 157–164. 10.1021/es403863m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widholm JJ, Clarkson GB, Strupp BJ, Crofton KM, Seegal RF, Schantz SL, 2001. Spatial reversal learning in Aroclor 1254-exposed rats: sex-specific deficits in associative ability and inhibitory control. Toxicol. Appl. Pharmacol 174 (2), 188–198. 10.1006/taap.2001.9199. [DOI] [PubMed] [Google Scholar]
- Woods R, Vallero RO, Golub MS, Suarez JK, Ta TA, Yasui DH, Chi LH,Kostyniak PJ, Pessah IN, Berman RF, LaSalle JM, 2012. Long-lived epigenetic interactions between perinatal PBDE exposure and Mecp2308 mutation. Hum. Mol. Genet 21 (11), 2399–2411. 10.1093/hmg/dds046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Yolton K, Webster GM, Sjödin A, Calafat AM, Dietrich KN, Xu Y, Xie C, Braun JM, Lanphear BP, Chen A, 2017. Prenatal PBDE and PCB exposures and reading, cognition, and externalizing behavior in children. Environ. Health Perspect 125 (4), 746–752. 10.1289/EHP478. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


