Abstract
The aim was to compare the in-vitro antibacterial effectiveness of two herbal extracts (a) Saussurea-costus (S. costus) and (b) Melaleuca-alternifolia (M. alternifolia) against Porphyromonas gingivalis (P. gingivalis), Streptococcus mutans (S. mutans) and Enterococcus faecalis (E. faecalis). Aqueous extracts from M. alternifolia were prepared by adding 2 grams of S. costus and M. alternifolia, respectively to 100 ml distilled water. Bacterial strains of P. gingivalis, E. faecalis and S. mutans were treated into 3 groups. In groups 1 and 2, bacterial strains were treated with aqueous extracts of S. costus and M. alternifolia, respectively. In the control-group, bacterial strains were exposed to distilled water. Antibacterial activity of the samples and nanoparticles was determined. The minimum-inhibitory-concentration (MIC) values were determined using the microdilution method. P < 0.01 was considered statistically significant. The MIC for all bacterial strains treated with S. costus was significantly higher than that of M. alternifolia (P < 0.001). There was no significant difference in MIC for strains of P. gingivalis, E. faecalis and S. mutans treated with S. costus. For bacterial strains treated with M. alternifolia, the MIC was significantly higher for P. gingivalis compared with E. faecalis and S. mutans strains (P < 0.01). There was no difference in MIC for E. faecalis and S. mutans strains treated with M. alternifolia. The in-vitro antibacterial efficacy of M. alternifolia is higher than S. costus against P. gingivalis, E. faecalis and S. mutans.
Keywords: Saussurea costus, Melaleuca alternifolia, tea tree oil, essential oil, antibacterial
Introduction
“Phytotherapy” is a term that is used in the field of medicine in which, either herbs or their extracts are used as either health promotion agents or to treat diseases [1]. This form of therapy encompasses Ayurvedic medicine, anthroposophic medicine, and traditional Chinese medicine. Herbs and their extracts such as the Salvadora persica chewing stick or “miswak” and Azadirachta indica or “neem” are often in the form of toothpastes and oral rinses for routine oral hygiene maintenance to eradicate dental plaque from teeth surfaces [2, 3]. In addition, these natural products are also known to exhibit antibacterial action against pathogenic bacteria such as Streptococcus mutans (S. mutans), Porphyromonas gingivalis (P. gingivalis), and Enterococcus faecalis (E. faecalis) [4–9]. Although numerous studies have investigated the bactericidal efficacy of herbal extracts of neem and miswak; other natural products that have been reported to exhibit antibacterial properties include Saussurea costus (S. costus) and Melaleuca alternifolia (M. alternifolia) [10, 11].
The S. costus (India costus), is a member of the Asteraceae family and is commonly used in countries such as Saudi Arabia, and India for different medical issues such as asthma, breast and hepatic cancer and thyroid diseases [12–14]. It is also known as costus, putchuk, or kuth. Likewise, M. alternifolia, also known as “tea tree oil” exhibits antimicrobial effects against microbes including Staphylococcus aureus, S. mutans and Candida albicans [15–18]; and promotes wound healing [18]. It is well-known that pathogenic microbes play a critical part in etiopathogenesis of dental caries and periodontal and peri-implant diseases [19–22]. The authors stringently reviewed indexed literature and observed that to date, there are no clinical and/or experimental studies that have assessed the antibacterial efficacy of S. costus and M. alternifolia against oral pathogenic bacteria. We hypothesize that S. costus and M. alternifolia exhibit bactericidal effects against P. gingivalis, S. mutans and E. faecalis with no difference in efficacy between the two herbals.
With this background, the objective was to in vitro investigate the antibacterial effectiveness of S. costus and M. alternifolia against P. gingivalis, S. mutans, and E. faecalis.
Methods
Ethical Statement and Informed Consent
Ethical approval was obtained from the Research Ethics Review Committee of the Princess Nourah Bint Abdulrahman University, ArRiyadh, Riyadh, Saudi Arabia. This is a laboratory-based investigation; therefore, the study was exempted from a written informed consent requirement.
Sample Collection and Preparation of Aqueous Extracts
Samples of S. costus and M. alternifolia were purchased from a local-market in Riyadh, Saudi-Arabia. The samples were rinsed with distilled water, air-dried, and ground into a fine powder using a milling machine (IKA Werke Laboratory Equipment, Staufen, Germany). The milled materials were stored at room temperature in sealed plastic boxes until further analysis. Aqueous extracts from M. alternifolia were prepared by adding 2 grams of S. costus and M. alternifolia, respectively to 100 ml distilled water. Heat treatment was performed on the aqueous extract for 15 min at 80°C to stop the enzyme activity. The samples were filtered (Whatman candidate #1 pore size 125mm, Whatman, Maidstone, United Kingdom) and stored at 4°C till use. All samples were assessed within 24 h.
Study Groups and Evaluation of Antibacterial Activity
Bacterial strains of P. gingivalis, E. faecalis and S. mutans were therapeutically classified into 3 groups. In groups 1 and 2, bacterial strains were treated with aqueous extracts of S. costus and M. alternifolia, respectively. In the control-group, bacterial strains were exposed to distilled water. Antibacterial activity was assessed using well agar diffusion method [23]. Three types of bacterial strains, P. gingivalis (ATCC® 53978), E. faecalis (ATCC® 29212) and S. mutans (ATCC® 55676) were used. Microbial cultures were sub-cultured on Mueller-Hinton-Agar. 0.2 ml of bacteria strain (1.5 X 108 CFU/ml) was uniformly swabbed on agar plates using sterile-swabs; and 3 spaced wells (each 4 mm in diameter) were made per plate at the culture agar surface using a sterile metal-cork borer. In each well, 0.2 ml of extract was placed under aseptic conditions, and kept at room temperature for 1 h. Sterile distilled water was used as the negative reference control. Plates were incubated at 37°C for 24-h. Inhibition zones, which appeared as a clear area around the wells were evaluated.
Assessment of MIC
The minimum inhibitory concentration (MIC) values were determined via microdilution method as described by Arends et al. [24]. In summary, 10 ml of a bacterial strain containing 1.5 x 108 colony forming units per milliliter (CFU/ml) was used. Different concentrations of nanoparticles were added to the test tubes containing the bacterial strains and incubated for 24 h. After incubation, the MIC values were obtained by checking the turbidity of the bacterial growth. The MIC value corresponded to the concentration that inhibited 99% of bacterial growth.
Statistical Analysis
Statistical comparisons were done using a software (SPSS 20, Chicago, I.L. United States). Group comparisons in terms of reduction in CFU/ml in the study groups was done using the one-way analysis of variance. For multiple comparisons, the Bonferroni post-hoc correction test was also performed. P-values below 0.01 were considered statistically significant.
Results
Antibacterial Activity of S. costus and M. alternifolia
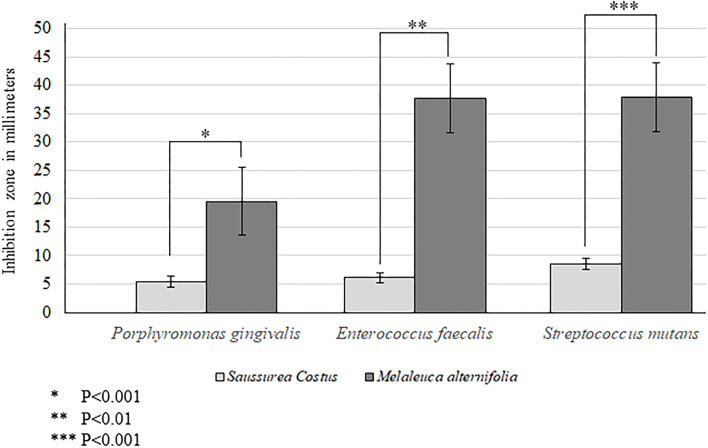
Aqueous extracts of M. alternifolia demonstrated a significantly high antimicrobial activity against strains of P. gingivalis (P < 0.001), E. faecalis (P < 0.01) and S. mutans (P < 0.001) compared with extracts of S. costus (Figure 1). The antibacterial activity of M. alternifolia was significantly higher against E. faecalis and S. mutans compared with that against P. gingivalis. There was no significant difference in the antibacterial efficacy of M. alternifolia against E. faecalis and S. mutans (Figure 1). There was no bactericidal activity observed in the control-group compared with strains that were treated with S. costus and M. alternifolia.
Figure 1.
Antibacterial efficacy of Melaleuca alternifolia and Saussurea costus.
MIC of S. costus and M. alternifolia in Relation to Their Bactericidal Efficacy
The MIC for all bacterial strains treated with S. costus was significantly than that of M. alternifolia (P < 0.001). There was no statistically significant difference in the MIC for strains of P. gingivalis, E. faecalis and S. mutans treated with S. costus. For bacterial strains treated with M. alternifolia, the MIC was significantly higher for P. gingivalis compared with E. faecalis and S. mutans strains (P < 0.01). There was no significant difference in the MIC for E. faecalis and S. mutans strains treated with M. alternifolia. The MIC for S. costus and M. alternifolia are shown in Table 1.
Table 1.
Minimum inhibitory concentrations of Saussurea costus and Melaleuca alternifolia in relation to their bactericidal efficacy.
| Bacterial strain | Porphyromonas gingivalis | Enterococcus faecalis | Streptococcus mutans |
|---|---|---|---|
| Saussurea costus | 56.2 ± 9.6 g/ml* | 56.6 ± 7.6 g/ml* | 80.5 ± 10.2 g/ml* |
| Melaleuca alternifolia | 29.5 ± 1.6 g/ml | 13.5 ± 2.5 g/ml† | 15.5 ± 3.7 g/ml† |
Compared with the same bacterial strain treated with M. alternifolia (P < 0.001).
Compared with P-gingivalis strain treated with M. alternifolia (P < 0.01).
Discussion
It has already been reported that S. costus and M. alternifolia possess antibacterial effects [9]; however, to the authors knowledge from pertinent indexed literature, the present study is the first one to compare the antibacterial efficacy of S. costus and M. alternifolia against common oral pathogenic bacteria (P. gingivalis, E. faecalis and S. mutans). The present experiment was based on the hypothesis that S. costus and M. alternifolia exhibit bactericidal effects against P. gingivalis, S. mutans and E. faecalis with no difference in efficacy between the two herbals. The results of the present experiment are partially in agreement with the proposed hypothesis as both herbal extracts (S. costus and M. alternifolia) demonstrated antibacterial efficacy against the tested bacterial strains. However, this experiment showed that antibacterial effectiveness of M. alternifolia was superior to that of S. costus in terms of MIC and identification of inhibition zone. It is challenging to determine the precise factor/s that may have contributed in this regard; however, different theories may be proposed. Firstly, tea tree oil or M. alternifolia is a well-known anti-inflammatory and antiseptic agent. Moreover, M. alternifolia inhibits bacterial respiration and disrupts permeability barrier of microbial cell membrane [17]; and at the same time increases the leakage of potassium ions in both Gram-negative and -positive bacteria [17]. These are possible factors that may be associated with the superior antibacterial effectiveness of M. alternifolia over S. costus. The current experiment showed that the MIC was significantly higher for P. gingivalis compared with E. faecalis and S. mutans strains for bacterial strains treated with M. alternifolia. One justification for this is that a standard concentration of M. alternifolia of 0.2% such as that used in the present investigation is insufficient to demonstrate antibacterial effectiveness against all types of bacteria. It is therefore hypothesized that M. alternifolia with used in concentrations higher than 0.2% demonstrates antibacterial effectiveness and creates inhibition zones that are statistically similar to those created for other bacteria such as E. faecalis and S. mutans. Further studies are needed to test this hypothesis.
From a clinical perspective, it is well-known that periodontopathogenic bacteria such as P. gingivalis play a role in the etiopathogenesis and progression of oral inflammatory conditions such as periodontitis [25–27]. Traditionally, mechanical instrumentation of periodontal sulci and related teeth/root surfaces is performed for the management of periodontitis [25]; and postoperative oral rinses such as 0.12% Chlorhexidine gluconate (CG) are often prescribed to patients in order to facilitate healing. However, this protocol is difficult to adopt particularly in patients with chlorhexidine allergy (CA). Although CA is a rare condition, it may be a source of distress to patients as it induces type-IV hypersensitivity reactions such as erythema in gingival tissues, burning sensation in the mouth and stomatitis [28, 29]. In a recent randomized controlled trial (RCT), Al-Zawawi et al. [30] investigated the postoperative anti-inflammatory efficacy of herbal-based oral rinses after non-surgical instrumentation for the management of periodontal inflammation in patients with chlorhexidine allergy. The results showed that herbal-based oral rinses are suitable substitutes to 0.12% CG as post-operative prescriptions following periodontal treatment [30]. The present in-vitro results applaud the results reported by Al-Zawawi et al. [30]; and suggest that S. costus based mouthwashes can be used for the management of periodontal inflammatory conditions as a substitute to 0.12% CHX. However, this needs additional well-designed and power adjusted RCTs.
A major limitation of the present study is that the results were entirely based on in-vitro evaluation of aqueous extracts from both herbs. Several clinical studies have shown that habits such as tobacco smoking and systemic diseases including poorly-controlled diabetes mellitus are risk factors of periodontitis and dental caries [31–38]. Such risk-factors are known to compromise outcomes of oral therapeutic interventions [39]; and promotion of microbial colonization in the supra- and subgingival oral biofilm [40, 41]. In this context, it remains debatable whether or not periodontal therapy (surgical or non-surgical) and restoration of carious teeth with adjunct use of oral rinses derived from S. costus and M. alternifolia is effective in the restoration of a clinically acceptable oral health status. Further studies, predominantly RCTs are needed.
Conclusion
In-vitro antibacterial efficacy of M. alternifolia is higher than S. costus against P. gingivalis, E. faecalis and S. mutans. From a clinical perspective, it is speculated that oral dentifrices based on M. alternifolia extracts can play a role in the maintenance of oral health and treatment of oral diseases such as periodontitis.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author/s.
Author Contributions
MB and SA: study design and manuscript writing and revisions. BH: methodology and manuscript writing and editing. MA: statistical analysis, methodology, and manuscript writing and editing. AM: methodology and manuscript writing and editing. KA: supervision, methodology, and manuscript writing and revisions. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Falzon CC, Balabanova A. Phytotherapy: an introduction to herbal medicine. Prim Care. (2017) 44:217–27. 10.1016/j.pop.2017.02.001 [DOI] [PubMed] [Google Scholar]
- 2.Almas AK, Almas K. Miswak (Salvadora persica chewing stick): the natural toothbrush revisited. Odontostomatol Trop. (2014) 37:27–39. [PubMed] [Google Scholar]
- 3.Dhingra K, Vandana KL. Effectiveness of azadirachta indica (neem) mouthrinse in plaque and gingivitis control: a systematic review. Int J Dent Hyg. (2017) 15:4–15. 10.1111/idh.12191 [DOI] [PubMed] [Google Scholar]
- 4.Vennila K, Elanchezhiyan S, Ilavarasu S. Efficacy of 10% whole azadirachta indica (neem) chip as an adjunct to scaling and root planning in chronic periodontitis: a clinical and microbiological study. Indian J Dent Res. (2016) 27:15–21. 10.4103/0970-9290.179808 [DOI] [PubMed] [Google Scholar]
- 5.Sekar S, Jacob S, Suthanthiran TK, Dhasthaheer S, Vikraman S, Kaliappan K. Characterization and formulation of miswak film for the treatment of chronic periodontitis: an in vitro study. J Pharm Bioallied Sci. (2020) 12:S199–203. 10.4103/jpbs.JPBS_59_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Obaida MI, Al-Essa MA, Asiri AA, Al-Rahla AA. Effectiveness of a 20% miswak extract against a mixture of Candida albicans and Enterococcus faecalis. Saudi Med J. (2010) 31:640–3. [PubMed] [Google Scholar]
- 7.Kalpavriksha AJ, Siddaiah SB, Bilichodmath S, Prabhakara S, Rao HH. Comparative evaluation of antibacterial effect of GIC containing chlorhexidine and miswak on Streptococcus mutans and Streptococcus sobrinus in early childhood caries children: a PCR study. Int J Clin Pediatr Dent. (2021) 14:229–34. 10.5005/jp-journals-10005-1942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heyman L, Houri-Haddad Y, Heyman SN, Ginsburg I, Gleitman Y, Feuerstein O. Combined antioxidant effects of neem extract, bacteria, red blood cells and Lysozyme: possible relation to periodontal disease. BMC Complement Altern Med. (2017) 17:399. 10.1186/s12906-017-1900-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laleman I, Teughels W. Novel natural product-based oral topical rinses and toothpastes to prevent periodontal diseases. Periodontol. (2000) 84:102–23. 10.1111/prd.12339 [DOI] [PubMed] [Google Scholar]
- 10.Qiu J, Wang J, Luo H, Du X, Li H, Luo M, et al. The effects of subinhibitory concentrations of costus oil on virulence factor production in Staphylococcus aureus. J Appl Microbiol. (2011) 110:333–40. 10.1111/j.1365-2010.04888.x [DOI] [PubMed] [Google Scholar]
- 11.Graziano TS, Calil CM, Sartoratto A, Franco GC, Groppo FC, Cogo-Müller K. In vitro effects of Melaleuca alternifolia essential oil on growth and production of volatile Sulphur compounds by oral bacteria. J Appl Oral Sci. (2016) 24:582–9. 10.1590/1678-775720160044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Byambaragchaa M, de la Cruz J, Yang SH, Hwang SG. Anti-metastatic potential of ethanol extract of saussurea involucrata against hepatic cancer in vitro. Asian Pac J Cancer Prev. (2013) 14:5397–402. 10.7314/APJCP.14.9.5397 [DOI] [PubMed] [Google Scholar]
- 13.Shati AA, Alkahtani MA, Alfaifi MY, Elbehairi SEI, Elsaid FG, Prasanna R, et al. Secondary metabolites of Saussurea costus leaf extract induce apoptosis in breast, liver, and colon cancer cells by caspase-3-dependent intrinsic pathway. Biomed Res Int. (2020) 2020:1–11. 10.1155/2020/1608942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mujammami M. Clinical significance of Saussurea costus in thyroid treatment. Saudi Med J. (2020) 41:1047–53. 10.15537/smj.10.25416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lam NS, Long X, Su XZ, Lu F. Melaleuca alternifolia (tea tree) oil and its monoterpene constituents in treating protozoan and helminthic infections. Biomed Pharmacother. (2020) 130:110624. 10.1016/j.biopha.2020.110624 [DOI] [PubMed] [Google Scholar]
- 16.Labib RM, Ayoub IM, Michel HE, Mehanny M, Kamil V, Hany M, et al. Appraisal on the wound healing potential of Melaleuca alternifolia and Rosmarinus officinalis L. essential oil-loaded chitosan topical preparations. PLoS ONE. (2019) 14:e0219561. 10.1371/journal.pone.0219561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oliva A, Costantini S, De Angelis M, Garzoli S, Božovi M, Mascellino MT, et al. High potency of Melaleuca alternifolia essential oil against multi-drug resistant gram-negative bacteria and methicillin-resistant Staphylococcus aureus. Molecules. (2018) 23:2584. 10.3390/molecules23102584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tullio V, Roana J, Scalas D, Mandras N. Enhanced killing of Candida krusei by Polymorphonuclear leucocytes in the presence of subinhibitory concentrations of Melaleuca alternifolia and “Mentha of Pancalieri” essential oils. Molecules. (2019) 24:3824 10.3390/molecules24213824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghazal TS, Levy SM, Childers NK, Carter KD, Caplan DJ, Warren JJ, et al. Mutans streptococci and dental caries: a new statistical modeling approach. Caries Res. (2018) 52:246–52. 10.1159/000486103 [DOI] [PubMed] [Google Scholar]
- 20.Javed F, Tenenbaum HC, Nogueira-Filho G, Nooh N, Taiyeb Ali TB, Samaranayake LP, et al. Oral Candida carriage and species prevalence amongst habitual gutka-chewers and non-chewers. Int Wound J. (2014) 11:79–84. 10.1111/j.1742-481X.2012.01070.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bartold PM, Van Dyke TE. An appraisal of the role of specific bacteria in the initial pathogenesis of periodontitis. J Clin Periodontol. (2019) 46:6–11. 10.1111/jcpe.13046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belibasakis GN, Charalampakis G, Bostanci N, Stadlinger B. Peri-implant infections of oral biofilm etiology. Adv Exp Med Biol. (2015) 830:69–84. 10.1007/978-3-319-11038-7_4 [DOI] [PubMed] [Google Scholar]
- 23.Naqvi SZ, Kiran U, Ali MI, Jamal A, Hameed A, Ahmed S, et al. Combined efficacy of biologically synthesized silver nanoparticles and different antibiotics against multidrug-resistant bacteria. Int J Nanomedicine. (2013) 8:3187–95. 10.2147/IJN.S49284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arends SJR, Canino MA, Mendes R, Scangarella-Oman NE, Flamm RK. Comparison of minimum inhibitory concentration results for gepotidacin obtained using agar dilution and broth microdilution methods. Diagn Microbiol Infect Dis. (2020) 98:115107. 10.1016/j.diagmicrobio.2020.115107 [DOI] [PubMed] [Google Scholar]
- 25.Aabed K, Moubayed N, BinShabaib MS, SS AL. Is a single session of antimicrobial photodynamic therapy as an adjuvant to non-surgical scaling and root planing effective in reducing periodontal inflammation and subgingival presence of Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans in patients with periodontitis? Photodiagnosis Photodyn Ther. (2022) 38:102847. 10.1016/j.pdpdt.2022.102847 [DOI] [PubMed] [Google Scholar]
- 26.Binshabaib M, Aabed K, Alotaibi F, Alwaqid M, Alfraidy A. Alharthi S. Antimicrobial efficacy of 08% hyaluronic acid and 02% chlorhexidine against Porphyromonas gingivalis strains: an in-vitro study. Pak J Med Sci. (2020) 36:111–4. 10.12669/pjms.36.2.1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.SS AL, BinShabaib M, Saad AlMasoud N, Shawky HA, Aabed KF, Alomar TS, et al. Melaibari, myrrh mixed with silver nanoparticles demonstrates superior antimicrobial activity against Porphyromonas gingivalis compared to myrrh and silver nanoparticles alone. Saudi Dent J. (2021) 33:890–6. 10.1016/j.sdentj.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pemberton MN, Gibson J. Chlorhexidine and hypersensitivity reactions in dentistry. Br Dent J. (2012) 213:547–50. 10.1038/sj.bdj.2012.1086 [DOI] [PubMed] [Google Scholar]
- 29.Kotsailidi EA, Kalogirou EM, Michelogiannakis D, Vlachodimitropoulos D, Tosios KI. Hypersensitivity reaction of the gingiva to chlorhexidine: case report and literature review. Oral Surg Oral Med Oral Pathol Oral Radiol. (2020) 130:156–60. 10.1016/j.oooo.04.814 [DOI] [PubMed] [Google Scholar]
- 30.Al-Zawawi AS, Shaheen MY, Divakar DD, Aldulaijan HA, Basudan AM. Postoperative anti-inflammatory efficacy of 2% saline rinses and a herbal—mouthwash after non-surgical periodontal therapy for the management of periodontal inflammation in young adults with chlorhexidine allergy: a randomized controlled trial. Int J Dent Hyg. (2022) 20:408–14. 10.1111/idh.12583 [DOI] [PubMed] [Google Scholar]
- 31.Golpasand Hagh L, Zakavi F, Ansarifar S, Ghasemzadeh O, Solgi G. Association of dental caries and salivary sIgA with tobacco smoking. Aust Dent J. (2013) 58:219–23. 10.1111/adj.12059 [DOI] [PubMed] [Google Scholar]
- 32.BinShabaib M, SS AL, Akram Z, Khan J, Rahman I, Romanos GE, et al. Clinical periodontal status and gingival crevicular fluid cytokine profile among cigarette-smokers, electronic-cigarette users and never-smokers. Arch Oral Biol. (2019) 102:212–7. 10.1016/j.archoralbio.05.001 [DOI] [PubMed] [Google Scholar]
- 33.Javed F, Al-Kheraif AA, Salazar-Lazo K, Yanez-Fontenla V, Aldosary KM, Alshehri M, et al. Periodontal inflammatory conditions among smokers and never-smokers with and without type 2 diabetes mellitus. J Periodontol. (2015) 86:839–46. 10.1902/jop.2015.150120 [DOI] [PubMed] [Google Scholar]
- 34.Javed F, Al-Zawawi AS, Allemailem KS, Almatroudi A, Mehmood A, Divakar DD. Periodontal conditions and whole salivary IL-17A and−23 levels among young adult cannabis sativa (Marijuana)-smokers, heavy cigarette-smokers and non-smokers. Int J Environ Res Public Health. (2020) 17:7435. 10.3390/ijerph17207435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Javed F, Bashir Ahmed H, Romanos GE. Association between environmental tobacco smoke and periodontal disease: a systematic review. Environ Res. (2014) 133:117–22. 10.1016/j.envres.05.008 [DOI] [PubMed] [Google Scholar]
- 36.Kotsakis GA, Javed F, Hinrichs JE, Karoussis IK, Romanos GE. Impact of cigarette smoking on clinical outcomes of periodontal flap surgical procedures: a systematic review and meta-analysis. J Periodontol. (2015) 86:254–63. 10.1902/jop.2014.140452 [DOI] [PubMed] [Google Scholar]
- 37.Genco RJ, Borgnakke WS. Diabetes as a potential risk for periodontitis: association studies. Periodontol. (2000) 83:40–5. 10.1111/prd.12270 [DOI] [PubMed] [Google Scholar]
- 38.Graves DT, Ding Z, Yang Y. The impact of diabetes on periodontal diseases. Periodontol. (2000) 82:214–24. 10.1111/prd.12318 [DOI] [PubMed] [Google Scholar]
- 39.Naji A, Edman K, Holmlund A. Influence of smoking on periodontal healing one year after active treatment. J Clin Periodontol. (2020) 47:343–50. 10.1111/jcpe.13228 [DOI] [PubMed] [Google Scholar]
- 40.Wade WG. The oral microbiome in health and disease. Pharmacol Res. (2013) 69:137–43. 10.1016/j.phrs.11.006 [DOI] [PubMed] [Google Scholar]
- 41.Polak D, Shapira L. An update on the evidence for pathogenic mechanisms that may link periodontitis and diabetes. J Clin Periodontol. (2018) 45:150–66. 10.1111/jcpe.12803 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author/s.