Abstract
Gliomas are invasive brain tumors characterized by high rates of recurrence and mortality. Glioblastoma (GBM), a grade IV brain tumor, is known for its heterogenicity and its resistance to the current treatment regimen. MicroRNA (miRNAs) are small non-coding sequences of RNA that regulate and influence the expression of multiple genes. The detection of certain types of micro-RNA in tissues and blood serum can be used for diagnosis and prognosis, including the response of a particular patient to therapy. The purpose of this review is to analyze studies and experimental results concerning changes in microRNA expression profiles characteristic of gliomas. Furthermore, miRNAs also contribute to autophagy at multiple stages. In this review, we summarize the functions of miRNAs in GBM pathways linked to dysregulation of cell cycle control, apoptosis and resistance to treatment, and the possible use of miRNAs in clinical settings as treatment and prediction biomarkers.
Keywords: Brain tumors, Gliomas, microRNAs, Molecular markers, Therapeutic targets
1. Introduction
Central nervous system tumors are diagnosed annually with a frequency of 3.5 per 100,000 people, which accounts for approximately 1.9% of all new cancers and 2.3% of deaths from all cancers in the world [1]. Gliomas are a type of tumor that results from malignant transformation of glial cells, and affecting the brain and spinal cord [2]. According to the WHO classification, according to clinical and pathological criteria, gliomas are divided into stages I-IV. The most dangerous is glioblastoma multiforme - a stage IV tumor. Morphologically, gliomas are subdivided into astrocytomas, oligodendrogliomas, and mixed oligoastrocytomas [3] (see Table 1).
Table 1.
Major miRs associated with gliomas/glioblastomas.
| miR | Research/detection method miR | Expression of miR in glioma | Targets | Commentary | Referenses |
|---|---|---|---|---|---|
| let-7 | qPCR, miRNA microarrays |  |
Ras, c-Myc, Stat3, cyclin D1 | The maturation of let-7 can be blocked by the LIN28 protein, an increased level of which is associated with worse survival. Let-7b may serve as a marker of cisplatin resistance. | [[57], [58], [59], [60]] |
| miR-7 | miRNA microarrays, qPCR |  |
Transcription factors PI3K and Raf-1, EGFR | Since miR-7 is characterized by a very high level of tissue specificity, it can be an ideal therapeutic target. The miR-7 level decreases significantly depending on the WHO stage. | [61,62] |
| miR-9 | qPCR (Taqman), bioinformatics calculations |  |
SPTCH1, OX2 | Potential therapeutic target of increased chemosensitivity to temozolomide. Differential expressed in glioma tissues with and without the R132H IDH1 mutation. |
[63,64], [61] |
| miR-10a,b | qPCR |  |
BCL2L11, CDKN2A | They belong to hypoxia-regulated microRNAs. | [61,65], [66,67] |
| miR-21 | Hybridization, qPCR |  |
Multiple targeted pathways including EGFR, PI3K/Akt/mTOR, PTEN | Expression profile differs in oligodendrogliomas and glioblastomas. One of the most hyperactive miRs in gliomas, it blocks apoptosis. It correlates inversely with WHO stage. Mediator of radiation resistance in gliomas. |
[6,68] |
| miR-23b | qPCR |  |
VHL, TK2B | Refers to hypoxia-regulated micro-RNA. Hyporegulation of miR-23b suppresses tumor proliferation across the VHL and inhibition of the b-catenin/Tcf-4 and HIF1a/VEGF signaling pathways. | [61,66] |
| miR-26a | qPCR |  |
PHB, PTEN | miR-26a inhibits downstream pathways by inhibiting PHB Akt and ERK. PTEN is a specific molecular marker of gliomas, is associated with lifespan, and is a tumor suppressor. |
[61,69] |
| miR-33 | RT2 miRNA PCR Array (QIAGEN) |  |
UVRAG, a negative regulator of the Notch pathway | Associated with the worst prognosis for patients. | [70] |
| miR-34a | qPCR |  |
Notch1, Notch2, cyclin-dependent protein kinase-6 (CDK6), Rictor protein (Akt/mTOR and Wnt pathways), Smad4 | Oncosuppressive micro-RNA, the expression level of which has prognostic value for patients with gliomas. | [61,71], [64,72] |
| miR-125b | qPCR |  |
Hh signaling, CDK6, LIN28, MMP9 | miR-125b is hypoactivated in gliomas with increased Gli1 expression compared to other gliomas. The levels of miR-125b and MMP9 were significantly higher in the culture of highly invasive glioma stem cells. | [61,73] |
| miR-132 | qPCR |  |
Smad7 | High expression of miR-132 is a marker of the worst prognosis for patients with gliomas. | [61] |
| miR-146b | qPCR, TCGA Bioinformatics Data Analysis |  |
EGFR, RAF6 | Tumor suppressive micro-RNA. The level of miR-146b correlates inversely with the grade of malignancy and positively with the patient's survival. | [74,75] |
| miR-155 | qPCR |  |
HBP-1, MAPK13, MAPK14 | Activation of Wnt/b-catenin signaling. Expression of miR-155 allows you to distinguish oligodendroglioma from glioblastoma. | [68,76] |
| miR-221/222 | miRNA microarrays, qPCR |  |
PTPm, MGMT, regulation of the Wnt/b-catenin pathway, RB1, WEE1 (cell cycle inhibitors), APAF1 (pro-apoptotic role), ANXA1, CTCF (transcriptional repressors). | miR-221 and -222 promote glioma growth, at least in part by controlling protein expression of PTPm. miR-221 modulates the mRNA level of 602 genes, which confirms its ability to influence many oncogenic pathways [45]. Silencing of the MGMT gene can also be caused by hypermethylation of CpG islands, which is characteristic of gliomas. | [[77], [78], [79]] |
A significant number of different miRs have been found that affect the processes of malignant transformation of cells and the progression of gliomas [4]. The possibility of using miRs as prognostic/diagnostic markers of glioma progression is being actively explored. For example, in the study [5], the profile was identified and validated of 9 micro-RNAs (miR-124a, −10b, −222, −34a, −182, −148a, −145, −370 and −9), which can effectively predict the survival of glioblastoma patients. One of the most hyperactivated miRs in glioma is the antiapoptotic miR-21, whose expression in blood and tissue samples in gliomas is characterized by high specificity and sensitivity as a diagnostic test [6]. It has been shown that those micro-RNAs that are hyporegulated in gliomas compared to normal brain tissues, function as tumor suppressors, directly acting on the oncogenes c-Met, Notch, Bmi-1, EGFR (epidermal growth factor receptor), receptor tyrosine kinases, as well as genes associated with the control of the cell cycle. For example, hyporegulation of miR-205 was found to be an independent predictor of mortality among patients with gliomas, which can serve as a significant prognostic indicator for patients with gliomas of a higher (III – IV) grade of malignancy according to the WHO classification [7]. The activation of miR-497 expression in glioma cells led to a decrease in the expression of the vascular endothelial growth factor (VEGF) and the density of blood vessels, thereby being a negative regulator of angiogenesis. This micro-RNA acts as a favorable prognostic factor: the expression of miR-497 was significantly lower in high-grade gliomas compared to low-grade gliomas [8]. It has been demonstrated that the evolutionarily conserved RNA-binding protein LIN28, capable of blocking the maturation of miR precursors of the let-7 family, is overexpressed in glioblastoma patients with a poor prognosis. A decrease in the level of LIN28 in the cell culture of U251 glioma caused the arrest of the cell cycle in the G1 phase, a slowdown in proliferation, and activation of apoptosis [9]. It was shown that miR let-7 is able to directly regulate the expression of such oncogenes as Ras, c-Myc, as well as the Stat3 transcription factor due to the presence of let-7-binding sites in the 3 ¢ -UTR of these genes; let-7 is inhibited in various types of tumors [10]. Along with this, micro-RNAs with increased expression in tumors can act as oncogenes. Thus, in glioma cells, overregulation of microRNAs such as miR-10b, −130a, −221, −125, −9, −21, −25, and −123 was established in comparison with normal brain tissue. Increased expression of miR-10b and miR-210 in gliomas is associated with unfavorable outcomes [11,12].
This review considers the possibility of using miRNAs as prognostic markers and therapeutic targets in gliomas.
2. MICRORNAs as prognostic markers
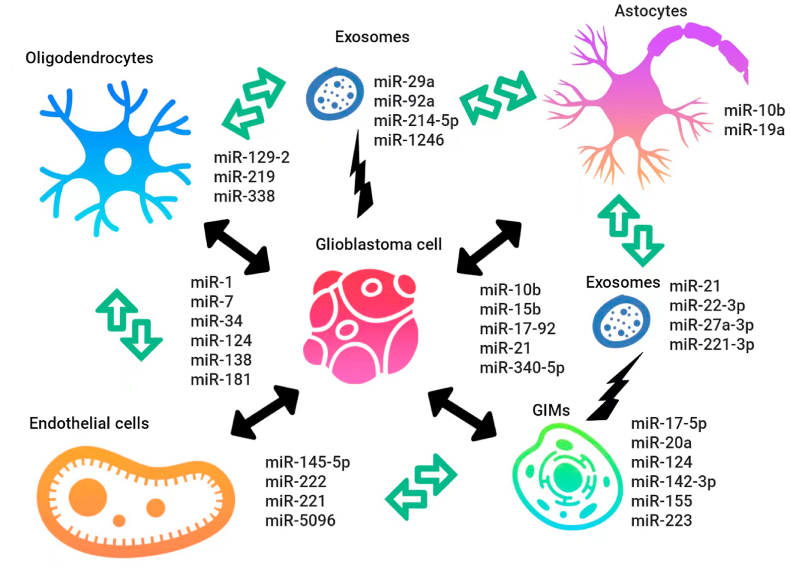
Many recently published works have confirmed the association of microRNA with chemotherapy and radiation resistance of patients' tumors (Fig. 1) [13].
Fig. 1.
Potential miRNAs that can be used as prognostic markers and therapeutic targets in gliomas.
Radiation resistance, often caused by the spread of CSCs in gliomas, remains one of the main difficulties in the treatment of glioblastoma. In the context of radiation resistance of gliomas, miR-21 is one of the most actively discussed microRNAs. Blocking this microRNA with anti-miR-21 promotes the formation of phosphorylated histone g-H2AX (an indicator of double-stranded DNA breaks) and suppression of phospho-Akt expression, activation of autophagy and apoptosis (including by inhibition of Cdc25A expression) after irradiation in cell lines gliomas [13,14]. MiR-21 also plays a significant role in the chemoresistance of glioblastoma cells: the use of the miR-21 inhibitor together with temozolomide increased glioma CSC apoptosis [15]. Knockdown of miR-135b is capable of leveling the radiation resistance of glioblastoma cells (U87R), and overexpression of miR-135b enhances the radiation resistance of U87 cells [16]. High expression of miR-34a in glioblastoma cells after irradiation with a dose of 60 Gy decreases the expression of p53; thus, the regulation of miR-34a expression can induce apoptosis even in glioblastoma cells that have acquired radiation resistance [17]. Several microRNAs of the let-7 family were overexpressed after irradiation of glioblastoma cells of the M059K line (let-7 suppresses the proliferation of glioma cells), but were overexpressed in the case of other glioblastoma cells of the M059J line. The difference between these two cell cultures is that M059J cells do not possess DNA-dependent protein kinase, which plays a central role in the restoration of double-stranded DNA breaks induced by ionizing radiation. The lack of DNA-dependent protein kinase correlates with the sensitivity of glioblastoma cells to radiation [18]. A significant increase in expression (3–4 times) was found for miR-24-1 and miR-151-5b after irradiation of glioma cell lines using doses commonly used for the treatment of brain tumors - 2 Gy [19]. An increase in the expression of miR-146b-5p in a glioma CSC culture induces apoptosis, cell differentiation and sensitivity to radiation therapy, reduces the viability of glioma CSCs, and the formation of neurospheres. One of the targets of miR-146b-5p is ELAVL1 (ELAV like RNA binding protein 1; also known as HuR), which is associated with a decrease in the level of lincRNA-p21, a regulator of cell proliferation and apoptosis [20]. The level of another microRNA, miR-590-3p, was increased in glioma tissues and radioresistant glioblastoma cells; miR-590-3p was shown to promote radioresistance by directly inhibiting the tumor suppressor LRIG1 (leucine rich repeats and immunoglobulin like domains 1) [21]. It was found that miR-221/222, an increase in the expression level of which was repeatedly noted in gliomas, mediates the restoration of DNA damage in glioblastoma cells, regardless of PTEN, which indicates its potential to be a potential therapeutic target for increasing the radiosensitivity of tumor cells [22]. Hypoxia leads to an increase in the level of HIF2A and miR-210 mRNA in glioma CSCs, while miR-210 knockdown reduced the ability of glioma CSCs to form neurospheres, expression of stem cell markers, and induced differentiation and cell cycle arrest in the G0/G1 phase [23]. In U87MG glioma cells after irradiation, the level of miR-181a decreased, while the transient overexpression of miR-181a significantly increased the response of tumor cells to radiation by inhibiting the antiapoptotic protein Bcl-2 [23]. Along with this, micro-RNAs play an important role in modulating the chemoresistance of glioma cells, including to temozolomide. Thus, it is known that the synthesis of cytochrome CYP3A4, which metabolizes most chemotherapy drugs, including those used in the treatment of gliomas, is increased in brain tumors, and can be suppressed with the participation of certain micro-RNAs, for example, miR-148a, −27b and −125b, which are therefore the targets of reducing the chemoresistance of glioblastomas. These microRNAs suppress nuclear receptors for vitamin D and PRX at the posttranscriptional level [24]. When comparing two glioblastoma CSC lines, CD133 + and CD133–, it was found that CD133 + cells have increased expression of the MDR1 gene (multiple drug resistance gene 1) and miR-9, which activates Shh signaling by decreasing the PTCH1 level. Knockdown of Gli1 and MDR1 by siRNA increases temozolomide-induced cell death [25]. Using HiSeq and bioinformatics approaches, certain long noncoding RNAs have been identified, the level of which differs in patients resistant and sensitive to temozolomide use, with lncRNA MALAT1 (metastasis associated lung adenocarcinoma transcript 1) having the highest discriminating potential [26]. Moreover, high serum MALAT1 expression is associated with an unfavorable response to chemotherapy with temozolomide and poor survival. MALAT1 inhibits miR-203, one of the targets of which is thymidylate synthase; inhibition of MALAT1 may be a further therapeutic strategy for suppressing resistance to temozolomide [26]. It has been shown that overexpression of miR-423-5p confers resistance to temozolomide to glioma cells and enhances the formation of neurospheres [27]; miR-181b has completely opposite effects [28]. It should be noted that the assessment of the expression profile of several micro-RNAs (miRNA-signature), the integration of data on the expression of micro-RNAs, genes, and proteins can reveal valuable biomarkers for the diagnosis and treatment of gliomas, characterized by statistical significance [29]. Thus, 27 differentially expressed micro-RNAs can serve as markers of initiation, progression of glioma, and prognosis; Six of these micro-RNAs were characterized by a greater predictive value (sensitivity, specificity, p) in differentiating glioma cells from non-tumor tissues. From this cluster, miR-15a, −16, −21, −23a, −9 were overexpressed in glioma tissues, while miR-124 was overexpressed. A feature of two microRNAs from this cluster, miR-124 and miR-9, is the fact that they are specifically expressed in the brain [29]. The group of Shi et al. showed that a decrease in the expression of miR-124 in tumor tissue stimulates the growth, angiogenesis, and chemoresistance of glioma; therefore, miR-124 may be a useful diagnostic marker [30]. Circulating miRNAs represent a new class of non-invasive biomarkers for the detection and prognosis of gliomas. Thus, the levels of circulating miR-128, -205, and −451a were lower in the blood serum of patients with gliomas compared to controls or patients with meningioma [[31], [32], [33]]. Analysis using microchips and qPCR, ROC curves showed that the simultaneous determination of the expression level of miR-15b and miR-21 in blood serum helps to differentiate patients with gliomas from patients with other brain lesions (metastases, etc.) with 90% sensitivity and 100% specificity [34]. A meta-analysis of 11 studies that tested the diagnostic informativeness of assessing the expression of various types of micro-RNA in blood and tissue samples in gliomas revealed high values of specificity, sensitivity and AUC (area under the ROC curve) for miR-21; however, the authors of this work note that profiles from a larger number of micro-RNAs will further improve diagnostic accuracy [6]. Indeed, the levels of miR-21, -128, and -342-3p in the blood plasma of patients with glioblastoma and healthy donors discriminated against these samples with high sensitivity and specificity: the level of miR-21 was increased, while miR-128 and miR-342-3p - decreased in glioblastoma, but not in other types of brain tumors. Moreover, the levels of miR-128 and miR-342-3p were positively correlated with the histopathological stage of gliomas [35]. ROC curves for the levels of miR-497 and miR-125b in serum showed high significance in differentiating glioblastomas from tumors of a lower malignancy - the AUC values reached 0.87 and 0.75 for miR-497 and miR-125b, respectively. Moreover, patients with glioblastoma have a significantly reduced expression level of miR-497 and miR-125b than patients with low-grade gliomas [36]. MiR-210 in serum is also a promising diagnostic and prognostic biomarker that can be detected in the peripheral blood of patients with gliomas, since the serum miR-210 level in glioblastoma is approximately 7 times higher than in healthy donors [37]. Another potential biomarker search platform for therapeutic monitoring of patients with gliomas is the analysis of cerebrospinal fluid (CSF) and extracellular vesicles isolated from blood plasma or CSF. Primary brain tumors with a tendency to disseminate can secrete microRNAs with oncogenic properties in the CSF; therefore, the study of microRNAs circulating in the CSF can help in the diagnosis of brain tumors [38]. In extracellular vesicles, CSF is present in high concentrations, which has already been repeatedly noted in connection with glioblastoma, miR-21; in general, CSF exosomes are the main extracellular compartments containing microRNAs [39]. Simultaneous testing of miR-15b and miR-21 expression levels in CSF can differentiate patients with gliomas from healthy people and patients with CNS lymphomas with 90% sensitivity and 100% specificity [40]. SVM (support vector machine, one of the machine learning algorithms) has shown that determining the expression levels of 7 microRNAs in the CSF (miR-10b, −21, −141, −200a, −200b, −200c and −125b) allows to differentiate glioblastomas and brain metastases with high accuracy (91–99%) on the test dataset. Nevertheless, the authors note that the search for additional microRNA biomarkers and subsequent analysis of large cohorts of patients will help to increase the accuracy of the approach [41].
3. MICRORNAs as potential therapeutic targets
The principle of therapeutic targeting of micro-RNAs is based, on the one hand, on increasing their expression due to the introduction of synthetic micro-RNAs (micro-RNA mimetics) to inhibit oncogenes; on the other hand, on the inactivation of endogenous micro-RNAs using inhibitors (antagomiRs or anti-micro-RNA) in order to increase the expression of tumor suppressors [42]. Effective inhibition using anti-micro-RNA requires high affinity for targeted micro-RNA, resistance to nuclease degradation, low toxicity, and efficient in vivo delivery. The most widely used anti-micro-RNAs today are modified oligonucleotides carrying the so-called LNA (locked nucleic acid) [43]. For example, the use of an antisense oligonucleotide inhibitor miR-10b led to a decrease in the target level, inhibited the growth and progression of xenograft glioblastomas, while the route of administration (intratumoral injections, intravenous administration, osmotic administration) was unimportant and systemic toxicity was not observed [44]. Nevertheless, the introduction of antisense oligonucleotides, the so-called anti-miRs, into the systemic circulation is complicated by their possible degradation and the presence of the blood-brain barrier (BBB) [45]. To penetrate the BBB, various methods of targeted drug delivery are being developed. Thus, the introduction of anti-let-7 oligonucleotide into xenograft animals with glioblastoma using stereotaxic surgery showed that the most effective methods of administration are intratumoral and intraventricular methods [45]. One of the studies showed that R3V6 peptides are capable of protecting anti-miR-21 oligonucleotides from nucleases, while anti-miR-21 is delivered to the cell much more efficiently than with other modifications [46]. The use of the drug reduced the level of miR-21 and induced apoptosis in glioblastoma cells, indicating that R3V6 can serve as a “carrier” of antisense oligonucleotides [46]. An alternative to antisense oligonucleotides is the so-called “miRNA sponge” (“sponge for micro-RNA”), which contain many binding sites specific to the so-called seed-region of targeted micro-RNAs [43]. On a glioma cell line and a laboratory mouse model, the ability of such a microRNA sponge to inhibit miR-23b, which functions as an oncogene in glioblastoma, was demonstrated, which led to a significant decrease in the level of HIF1a, b-catenin, MMP-2, -9, VEGF, inhibited angiogenesis, migration, and invasion of tumor cells [47]. Synthetic micro-RNA sponges were created on the basis of their natural counterpart - cyclic RNA (circRNA), the role of which in carcinogenesis is just beginning to be studied. For instance, RNA circ-TTBK2 is overregulated in glioma tissues and cell lines, while linear TTBK2 does not change its level [48]. Circ-TTBK2 acts as a sponge for miR-217, the level of which has been reduced in glioma, and whose target is the oncogenic protein HNF1b (HNF1 homeobox beta). Knockdown of circ-TTBK2 together with miR-217 overexpression led to tumor regression in vivo [48]. More recently, a computer pipeline algorithm “UROBORUS” has been developed for circRNA detection based on RNA-seq data [50]. Using this algorithm, more than 476 cyclic RNAs were found, the expression of which differed in the normal brain and in glioma samples [49]. Another analysis [50] revealed 1411 differentially expressed cyclic RNAs in glioblastoma, of which 206 were hyperactivated and 1205 were hypoactivated. At the same time, the level of circBRAF expression in the normal brain was significantly higher relative to the glioma tissue; was lower in patients with high grade (III-IV) gliomas. A high level of circBRAF expression was an independent biomarker of a favorable prognosis for disease-free and overall survival in patients with glioma [50]. Micro-RNA-replacement therapy is aimed at restoring lost function, especially the activity of tumor suppressors, which can be achieved by introducing synthetic micro-RNA mimetics with sequences identical to natural ones [43]. For instance, Transfection of miR-203 mimetics into U251 glioblastoma cells, which showed a decrease in miR-203 levels relative to less malignant gliomas or normal gliomas, led to inhibition of the PLD2 (phospholipase D2) oncogene, the target of miR-203. This suppressed the proliferation and invasion of U251 cells, demonstrating the ability of mimetics to correct microRNA deficiency [51]. Targeted delivery of synthetic microRNA mimetics to glioma cells in vivo and glioma CSCs in culture can be performed by mesenchymal stem cells isolated from bone marrow, adipose tissue, and placenta [52]. The introduction of miR-124 and miR-145 mimetics using mesenchymal stem cells into glioma cells that express very low levels of these microRNAs has shown successful delivery of drugs into cells, where they significantly reduced the luciferase activity of the corresponding target genes miR-124 and miR −145 - SCP-1 and Sox2, and also inhibited glioma cell migration and glioma CSC self-renewal [52]. Another attractive approach in the treatment of gliomas is the use of siRNA, short interfering double-stranded RNA molecules that are potentially capable of specifically inhibiting signaling pathways associated with the development and progression of glioblastomas, for example, the EGFR and b-catenin genes [53]. Genome-wide siRNA screening in glioblastoma showed that 2 siRNAs, which target ubiquitin C and disheveled 2, significantly increase the sensitivity of glioma CSCs to temozolomide [54]. Inhibition of the gene for the transcription factor PATZ1 (POZ/BTB and AT Inhibition of the transcription factor gene PATZ1 (POZ/BTB and AT hook containing zinc finger 1) by siRNA increased the sensitivity of the glioma cell line to apoptotic stimuli [55]. Treatment of glioblastoma cells with a haemagglutinating virus vector HAJ-E containing siRNA targeting the mitotic motor protein KIF11 (kinesin family member 11, also known as EG5) led to the formation of a monopolar division spindle, cell cycle arrest, and apoptosis. Direct injection of HVJ-E + Eg5 siRNA into xenografts subcutaneous/intracranial tumors in all xenograft mice, tumor growth was inhibited [56].
4. Conclusions
Expression profiles of certain types of microRNA in gliomas repeat the changes characteristic of other types of malignant neoplasms. Similarly, to genes proper, subdivided according to their effect on tumor pathogenesis on tumor suppressors and oncogenes, by now it is already possible to distinguish classes of microRNAs that also play opposite roles in the pathogenesis and progression of gliomas - oncogenes (for example, miR-21, -96, - 155, etc.) and tumor suppressors (miR-34a, −107, −205, etc.). Studies of microRNA expression profiles have expanded our understanding of the mechanisms of progression of gliomas/glioblastomas, providing valuable information on the pathogenesis of these tumors and potential therapeutic targets (eg, inhibition of anti-apoptotic miR-21) [[81], [82], [83], [80]]. Detection and quantification of a number of micro-RNA species in tissues and blood serum of cancer patients can be of diagnostic and prognostic significance. Micro-RNAs can also be used to predict the response of a particular patient to therapy (for example, miR-338-5p sensitizes glioblastoma cells to the effects of ionizing radiation through the regulation of genes involved in the response to DNA damage) [84,85].
Funding
None.
Author contributions
Albert Sufianov and Sema Begliarzade conceptualized and designed the study. All authors participated in the acquisition, analysis and interpretation of the data. Tatiana Ilyasova and Yanchao Liang drafted the manuscript. Ozal Beylerli contributed to critical revisions of the manuscript. All authors agreed on the journal to which the article would be submitted, gave final approval for the version to be published, and agreed to be accountable for all aspects of the work.
Declaration of competing interest
The authors declare that no conflicts of interest exist.
Contributor Information
Albert Sufianov, Email: sufianov@gmail.com.
Sema Begliarzade, Email: semanagiyeva@yandex.ru.
Tatiana Ilyasova, Email: Iltanya67@yandex.ru.
Yanchao Liang, Email: liangyanchao@hrbmu.edu.cn.
Ozal Beylerli, Email: obeylerli@mail.ru.
References
- 1.International Agency for Research on Cancer (IARC) GLOBOCAN 2008. Estimated incidence, mortality and 5-year prevalence: both sexes. http://globocan.iarc.fr Available online:
- 2.Mamelak A.N., Jacoby D.B. Targeted delivery of antitumoral therapy to glioma and other malignancies with synthetic chlorotoxin (TM-601) Expet Opin. Drug Deliv. 2007;4(2):175–186. doi: 10.1517/17425247.4.2.175. [DOI] [PubMed] [Google Scholar]
- 3.Louis D.N., Ohgaki H., Wiestler O.D., et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li M., Li J., Liu L., et al. MicroRNA in human glioma. Cancers. 2013;5(4):1306–1331. doi: 10.3390/cancers5041306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayes J., Thygesen H., Tumilson C., et al. Prediction of clinical outcome in glioblastoma using a biologically relevant nine-microRNA signature. Mol. Oncol. 2015;9(3):704–714. doi: 10.1016/j.molonc.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qu S., Guan J., Liu Y. Identification of microRNAs as novel biomarkers for glioma detection: a meta-analysis based on 11 articles. J. Neurol. Sci. 2015;348(1–2):181–187. doi: 10.1016/j.jns.2014.11.036. [DOI] [PubMed] [Google Scholar]
- 7.Hou S.X., Ding B.J., Li H.Z., et al. Identification of microRNA-205 as a potential prognostic indicator for human glioma. J. Clin. Neurosci. 2013;20(7):933–937. doi: 10.1016/j.jocn.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 8.Feng F., Kuai D., Wang H., et al. Reduced expression of microRNA-497 is associated with greater angiogenesis and poor prognosis in human gliomas. Hum. Pathol. 2016;58:47–53. doi: 10.1016/j.humpath.2016.04.022. [DOI] [PubMed] [Google Scholar]
- 9.Qin R., Zhou J., Chen C., et al. LIN28 is involved in glioma carcinogenesis and predicts outcomes of glioblastoma multiforme patients. PLoS One. 2014;9(1) doi: 10.1371/journal.pone.0086446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang X., Cao L., Wang Y., et al. Regulation of let-7 and its target oncogenes (Review) Oncol. Lett. 2012;3(5):955–960. doi: 10.3892/ol.2012.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang X., Cheng J., Fu L., et al. Overexpression of tissue microRNA10b may help predict glioma prognosis. J. Clin. Neurosci. 2016;29:59–63. doi: 10.1016/j.jocn.2015.10.046. [DOI] [PubMed] [Google Scholar]
- 12.Lai N.S., Dong Q.S., Ding H., et al. MicroRNA-210 overexpression predicts poorer prognosis in glioma patients. J. Clin. Neurosci. 2014;21(5):755–760. doi: 10.1016/j.jocn.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 13.Gareev I., Beylerli O., Liang Y., Xiang H., Liu C., Xu X., Yuan C., Ahmad A., Yang G. The role of MicroRNAs in therapeutic resistance of malignant primary brain tumors. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.740303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gwak H.S., Kim T.H., Jo G.H., et al. Silencing of microRNA-21 confers radio-sensitivity through inhibition of the PI3K/AKT pathway and enhancing autophagy in malignant glioma cell lines. PLoS One. 2012;7(10) doi: 10.1371/journal.pone.0047449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang S., Wan Y., Pan T., et al. MicroRNA-21 inhibitor sensitizes human glioblastoma U251 stem cells to chemotherapeutic drug temozolomide. J. Mol. Neurosci. 2012;47(2):346–356. doi: 10.1007/s12031-012-9759-8. [DOI] [PubMed] [Google Scholar]
- 16.Xiao S., Yang Z., Lv R., et al. miR-135b contributes to the radioresistance by targeting GSK3β in human glioblastoma multiforme cells. PLoS One. 2014;9(9) doi: 10.1371/journal.pone.0108810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sasaki A., Udaka Y., Tsunoda Y., et al. Analysis of p53 and miRNA expression after irradiation of glioblastoma cell lines. Anticancer Res. 2012;32(11):4709–4713. [PubMed] [Google Scholar]
- 18.Chaudhry M.A., Sachdeva H., Omaruddin R.A. Radiation-induced micro-RNA modulation in glioblastoma cells differing in DNA-repair pathways. DNA Cell Biol. 2010;29(9):553–561. doi: 10.1089/dna.2009.0978. [DOI] [PubMed] [Google Scholar]
- 19.Niemoeller O.M., Niyazi M., Corradini S., et al. MicroRNA expression profiles in human cancer cells after ionizing radiation. Radiat. Oncol. 2011;6:29. doi: 10.1186/1748-717x-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang W., Yu H., Shen Y., et al. MiR-146b-5p overexpression attenuates stemness and radioresistance of glioma stem cells by targeting HuR/lincRNA-p21/β-catenin pathway. Oncotarget. 2016;7(27):41505–41526. doi: 10.18632/oncotarget.9214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen L., Wang W., Zhu S., et al. MicroRNA-590-3p enhances the radioresistance in glioblastoma cells by targeting LRIG1. Exp. Ther. Med. 2017;14(2):1818–1824. doi: 10.3892/etm.2017.4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li W., Guo F., Wang P., et al. miR-221/222 confers radioresistance in glioblastoma cells through activating Akt independent of PTEN status. Curr. Mol. Med. 2014;14(1):185–195. doi: 10.2174/1566524013666131203103147. [DOI] [PubMed] [Google Scholar]
- 23.Yang W., Wei J., Guo T., et al. Knockdown of miR-210 decreases hypoxic glioma stem cells stemness and radioresistance. Exp. Cell Res. 2014;326(1):22–35. doi: 10.1016/j.yexcr.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 24.Chen G., Zhu W., Shi D., et al. MicroRNA-181a sensitizes human malignant glioma U87MG cells to radiation by targeting Bcl-2. Oncol. Rep. 2010;23(4):997–1003. doi: 10.3892/or_00000725. [DOI] [PubMed] [Google Scholar]
- 25.Munoz J.L., Rodriguez-Cruz V., Rameshwar P. High expression of miR-9 in CD133(+) glioblastoma cells in chemoresistance to temozolomide. J. Cancer Stem Cell Res. 2015;3doi doi: 10.14343/JCSCR.2015.3e1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen W., Xu X.K., Li J.L., et al. MALAT1 is a prognostic factor in glioblastoma multiforme and induces chemoresistance to temozolomide through suppressing miR-203 and promoting thymidylate synthase expression. Oncotarget. 2017;8(14):22783–22799. doi: 10.18632/oncotarget.15199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li S., Zeng A., Hu Q., et al. miR-423-5p contributes to a malignant phenotype and temozolomide chemoresistance in glioblastomas. Neuro Oncol. 2017;19(1):55–65. doi: 10.1093/neuonc/now129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li P., Lu X., Wang Y., et al. MiR-181b suppresses proliferation of and reduces chemoresistance to temozolomide in U87 glioma stem cells. J. Biomed. Res. 2010;24(6):436–443. doi: 10.1016/s1674-8301(10)60058-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ye X., Wei W., Zhang Z., et al. Identification of microRNAs associated with glioma diagnosis and prognosis. Oncotarget. 2017;8(16):26394–26403. doi: 10.18632/oncotarget.14445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi Z., Chen Q., Li C., et al. MiR-124 governs glioma growth and angiogenesis and enhances chemosensitivity by targeting R-Ras and N-Ras. Neuro Oncol. 2014;16(10):1341–1353. doi: 10.1093/neuonc/nou084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun J., Liao K., Wu X., et al. Serum microRNA-128 as a biomarker for diagnosis of glioma. Int. J. Clin. Exp. Med. 2015;8(1):456–463. [PMC free article] [PubMed] [Google Scholar]
- 32.Yue X., Lan F., Hu M., et al. Downregulation of serum microRNA-205 as a potential diagnostic and prognostic biomarker for human glioma. J. Neurosurg. 2016;124(1):122–128. doi: 10.3171/2015.1.Jns141577. [DOI] [PubMed] [Google Scholar]
- 33.Zhao S., Yue S., Yang Q., et al. Serum microRNA-451a expression and its diagnostic value in glioma. Int. J. Clin. Exp. Pathol. 2016;9(3):3678–3682. [Google Scholar]
- 34.Ivo D'Urso P., Fernando D'Urso O., Damiano Gianfreda C., et al. miR-15b and miR-21 as circulating biomarkers for diagnosis of glioma. Curr. Genom. 2015;16(5):304–311. doi: 10.2174/1389202916666150707155610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Q., Li P., Li A., et al. Plasma specific miRNAs as predictive biomarkers for diagnosis and prognosis of glioma. J. Exp. Clin. Cancer Res. 2012;31(1):97. doi: 10.1186/1756-9966-31-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Regazzo G., Terrenato I., Spagnuolo M., et al. A restricted signature of serum miRNAs distinguishes glioblastoma from lower grade gliomas. J. Exp. Clin. Cancer Res. 2016;35(1):124. doi: 10.1186/s13046-016-0393-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lai N.S., Wu D.G., Fang X.G., et al. Serum microRNA-210 as a potential noninvasive biomarker for the diagnosis and prognosis of glioma. Br. J. Cancer. 2015;112(7):1241–1246. doi: 10.1038/bjc.2015.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shalaby T., Grotzer M.A. Tumor-associated CSF MicroRNAs for the prediction and evaluation of CNS malignancies. Int. J. Mol. Sci. 2015;16(12):29103–29119. doi: 10.3390/ijms161226150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akers J.C., Ramakrishnan V., Kim R., et al. miRNA contents of cerebrospinal fluid extracellular vesicles in glioblastoma patients. J. Neuro Oncol. 2015;123(2):205–216. doi: 10.1007/s11060-015-1784-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baraniskin A., Kuhnhenn J., Schlegel U., et al. Identification of microRNAs in the cerebrospinal fluid as biomarker for the diagnosis of glioma. Neuro Oncol. 2012;14(1):29–33. doi: 10.1093/neuonc/nor169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Teplyuk N.M., Mollenhauer B., Gabriely G., et al. MicroRNAs in cerebrospinal fluid identify glioblastoma and metastatic brain cancers and reflect disease activity. Neuro Oncol. 2012;14(6):689–700. doi: 10.1093/neuonc/nos074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Short S.C. Science in focus: MicroRNA in glioma - potential as biomarkers and therapeutic targets. Clin. Oncol. 2016;28(9):543–546. doi: 10.1016/j.clon.2016.04.048. [DOI] [PubMed] [Google Scholar]
- 43.Shea A., Harish V., Afzal Z., et al. MicroRNAs in glioblastoma multiforme pathogenesis and therapeutics. Cancer Med. 2016;5(8):1917–1946. doi: 10.1002/cam4.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Teplyuk N.M., Uhlmann E.J., Gabriely G., et al. Therapeutic potential of targeting microRNA-10b in established intracranial glioblastoma: first steps toward the clinic. EMBO Mol. Med. 2016;8(3):268–287. doi: 10.15252/emmm.201505495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim D.G., Kim K.H., Seo Y.J., et al. Anti-miR delivery strategies to bypass the blood-brain barrier in glioblastoma therapy. Oncotarget. 2016;7(20):29400–29411. doi: 10.18632/oncotarget.8837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Song H., Oh B., Choi M., et al. Delivery of anti-microRNA-21 antisense-oligodeoxynucleotide using amphiphilic peptides for glioblastoma gene therapy. J. Drug Target. 2015;23(4):360–370. doi: 10.3109/1061186x.2014.1000336. [DOI] [PubMed] [Google Scholar]
- 47.Chen L., Zhang K., Shi Z., et al. A lentivirus-mediated miR-23b sponge diminishes the malignant phenotype of glioma cells in vitro and in vivo. Oncol. Rep. 2014;31(4):1573–1580. doi: 10.3892/or.2014.3012. [DOI] [PubMed] [Google Scholar]
- 48.Zheng J., Liu X., Xue Y., et al. TTBK2 circular RNA promotes glioma malignancy by regulating miR-217/HNF1β/Derlin-1 pathway. J. Hematol. Oncol. 2017;10(1):52. doi: 10.1186/s13045-017-0422-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Song X., Zhang N., Han P., et al. Circular RNA profile in gliomas revealed by identification tool UROBORUS. Nucleic Acids Res. 2016;44(9):e87. doi: 10.1093/nar/gkw075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu J., Ye J., Zhang L., et al. Differential expression of circular RNAs in glioblastoma multiforme and its correlation with prognosis. Transl. Oncol. 2017;10(2):271–279. doi: 10.1016/j.tranon.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen Z., Li D., Cheng Q., et al. MicroRNA-203 inhibits the proliferation and invasion of U251 glioblastoma cells by directly targeting PLD2. Mol. Med. Rep. 2014;9(2):503–508. doi: 10.3892/mmr.2013.1814. [DOI] [PubMed] [Google Scholar]
- 52.Lee H.K., Finniss S., Cazacu S., et al. Mesenchymal stem cells deliver synthetic microRNA mimics to glioma cells and glioma stem cells and inhibit their cell migration and self-renewal. Oncotarget. 2013;4(2):346–361. doi: 10.18632/oncotarget.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang K., Park J.O., Zhang M. Treatment of glioblastoma multiforme using a combination of small interfering RNA targeting epidermal growth factor receptor and β-catenin. J. Gene Med. 2013;15(1):42–50. doi: 10.1002/jgm.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Himes B. Yale Medicine Thesis Digital Library; 2013. Sirna Therapy in Glioblastoma Stem Cells: Identification of Target Genes and Potential Therapeutic Implications. 1798. [Google Scholar]
- 55.Tritz R., Mueller B.M., Hickey M.J., et al. siRNA down-regulation of the PATZ1 gene in human glioma cells increases their sensitivity to apoptotic stimuli. Cancer Ther. 2008;6(B):865–876. [PMC free article] [PubMed] [Google Scholar]
- 56.Matsuda M., Yamamoto T., Matsumura A., et al. Highly efficient eradication of intracranial glioblastoma using Eg5 siRNA combined with HVJ envelope. Gene Ther. 2009;16(12):1465–1476. doi: 10.1038/gt.2009.99. [DOI] [PubMed] [Google Scholar]
- 57.Qin R., Zhou J., Chen C., Xu T., Yan Y., Ma Y., Zheng Z., Shen Y., Lu Y., Fu D. Lin28 is involved in glioma carcinogenesis and predicts outcomes of glioblastoma multiforme patients. PLoS One. 2014;9 doi: 10.1371/journal.pone.0086446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang X., Cao L., Wang Y., Wang X., Liu N., You Y. Regulation of let-7 and its target oncogenes (Review) Oncol. Lett. 2012;3(5):955–960. doi: 10.3892/ol.2012.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee S.T., Chu K., Oh H.J., Im W.S., Lim J.Y., Kim S.K., Park C.K., Jung K.H., Lee S.K., Kim M., Roh J.K. Let-7 microRNA inhibits the proliferation of human glioblastoma cells. J. Neuro Oncol. 2011;102(1):19–24. doi: 10.1007/s11060-010-0286-6. [DOI] [PubMed] [Google Scholar]
- 60.Guo Y., Yan K., Fang J., Qu Q., Zhou M., Chen F. Let-7b expression determines response to chemotherapy through the regulation of cyclin D1 in glioblastoma. J. Exp. Clin. Cancer Res. 2013 Jun 27;32(1):41. doi: 10.1186/1756-9966-32-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shea A., Harish V., Afzal Z., Chijioke J., Kedir H., Dusmatova S., Roy A., Ramalinga M., Harris B., Blancato J., Verma M., Kumar D. MicroRNAs in glioblastoma multiforme pathogenesis and therapeutics. Cancer Med. 2016;5(8):1917–1946. doi: 10.1002/cam4.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu Zhenlin, Jiang Zhongmin, Huang Jianyong, Huang Shuqiang, Li Yanxia, Yu Simiao, Yu Shizhu, Liu Xiaozhi. 2014. miR-7 Inhibits Glioblastoma Growth by Simultaneously Interfering with the PI3K/ATK and Raf/MEK/ERK Pathways; pp. 1571–1580. [DOI] [PubMed] [Google Scholar]
- 63.Munoz J.L., Rodriguez-Cruz V., Ramkissoon S.H., Ligon K.L., Greco S.J., Rameshwar P. Temozolomide resistance in glioblastoma occurs by miRNA-9-targeted PTCH1, independent of sonic hedgehog level. Oncotarget. 2015;6(2):1190–1201. doi: 10.18632/oncotarget.2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang Z., Bao Z., Yan W., You G., Wang Y., Li X., Zhang W. Isocitrate dehydrogenase 1 (IDH1) mutation-specific microRNA signature predicts favorable prognosis in glioblastoma patients with IDH1 wild type. J. Exp. Clin. Cancer Res. 2013;32(1):59. doi: 10.1186/1756-9966-32-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang X., Cheng J., Fu L., Li Q. Overexpression of tissue microRNA10b may help predict glioma prognosis. J. Clin. Neurosci. 2016;29:59–63. doi: 10.1016/j.jocn.2015.10.046. [DOI] [PubMed] [Google Scholar]
- 66.Agrawal R., Pandey P., Jha P., Dwivedi V., Sarkar C., Kulshreshtha R. Hypoxic signature of microRNAs in glioblastoma: insights from small RNA deep sequencing. BMC Genom. 2014;15:686. doi: 10.1186/1471-2164-15-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gabriely G., Yi M., Narayan R.S., Niers J.M., Wurdinger T., Imitola J., Ligon K.L., Kesari S., Esau C., Stephens R.M., Tannous B.A., Krichevsky A.M. Human glioma growth is controlled by MicroRNA-10b. Cancer Res. 2011;71(10):3563–3572. doi: 10.1158/0008-5472.CAN-10-3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lages E., Guttin A., El Atifi M., Ramus C., Ipas H., Dupré I., et al. MicroRNA and target protein patterns reveal physiopathological features of glioma subtypes. PLoS One. 2011;6(5) doi: 10.1371/journal.pone.0020600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qian X., Zhao P., Li W., Shi Z.-M., Wang L., Xu Q., Wang M., Liu N., Liu L.Z., Jiang B.H. MicroRNA‐26a promotes tumor growth and angiogenesis in glioma by directly targeting prohibitin. CNS Neurosci. Ther. 2013;19:804–812. doi: 10.1111/cns.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sun J.J., Wang Z.Y., Li L.S., Yu H.Y., Xu Y.S., Wu H.B., Luo Y., Liu B., Zheng M., Mao J.L., Lou X.H. Prevention against diffuse spinal cord astrocytoma: can the Notch pathway be a novel treatment target? Neural Regen. Res. 2015;10(2):244–251. doi: 10.4103/1673-5374.152378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li Y., Guessous F., Zhang Y., Dipierro C., Kefas B., Johnson E., Marcinkiewicz L., Jiang J., Yang Y., Schmittgen T.D., Lopes B., Schiff D., Purow B., Abounader R. MicroRNA-34a inhibits glioblastoma growth by targeting multiple oncogenes. Cancer Res. 2009;69(19):7569–7576. doi: 10.1158/0008-5472.CAN-09-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rathod S.S., Rani S.B., Khan M., Muzumdar D., Shiras A. Tumor suppressive miRNA-34a suppresses cell proliferation and tumor growth of glioma stem cells by targeting Akt and Wnt signaling pathways. FEBS Open Bio. 2014;4:485–495. doi: 10.1016/j.fob.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Genovese G., Ergun A., Shukla S.A., Campos B., Hanna J., Ghosh P., Quayle S.N., Rai K., Colla S., Ying H., et al. microRNA regulatory network inference identifies miR-34a as a novel regulator of TGF-β signaling in glioblastoma. Cancer Discov. 2012;2(8):736–749. doi: 10.1158/2159-8290.CD-12-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wan Y., Fei X.F., Wang Z.M., Jiang D.Y., Chen H.C., Yang J., Shi L., Huang Q. Expression of miR-125b in the new, highly invasive glioma stem cell and progenitor cell line SU3. Chin. J. Cancer. 2012;31(4):207–214. doi: 10.5732/cjc.011.10336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Katakowski M., Zheng X., Jiang F., Rogers T., Szalad A., Chopp M. MiR-146b-5p suppresses EGFR expression and reduces in vitro migration and invasion of glioma. Cancer Invest. 2010;28(10):1024–1030. doi: 10.3109/07357907.2010.512596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li H., Yu L., Liu J., Bian X., Shi C., Sun C., Zhou X., Wen Y., Hua D., Zhao S., Ren L., An T., Luo W., Wang Q., Yu S. miR-320a functions as a suppressor for gliomas by targeting SND1 and β-catenin, and predicts the prognosis of patients. Oncotarget. 2017;8(12):19723–19737. doi: 10.18632/oncotarget.14975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yan Z., Che S., Wang J., Jiao Y., Wang C., Meng Q. miR-155 contributes to the progression of glioma by enhancing Wnt/β-catenin pathway. Tumour Biol. 2015 Jul;36(7):5323–5331. doi: 10.1007/s13277-015-3193-9. [DOI] [PubMed] [Google Scholar]
- 78.Lupini L., Bassi C., Ferracin M., Bartonicek N., D'Abundo L., Zagatti B., Callegari E., Musa G., Moshiri F., Gramantieri L., Corrales F.J., Enright A.J., Sabbioni S., Negrini M. miR-221 affects multiple cancer pathways by modulating the level of hundreds messenger RNAs. Front. Genet. 2013;4:64. doi: 10.3389/fgene.2013.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Quintavalle C., Garofalo M., Zanca C., Romano G., Iaboni M., del Basso De Caro M., Martinez-Montero J.C., Incoronato M., Nuovo G., Croce C.M., Condorelli G. miR-221/222 overexpression in human glioblastoma increases invasiveness by targeting the protein phosphate PTPμ. Oncogene. 2012;31(7):858–868. doi: 10.1038/onc.2011.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gareev I., Gileva Y., Dzidzaria A., Beylerli O., Pavlov V., Agaverdiev M., Mazorov B., Biganyakov I., Vardikyan A., Jin M., Ahmad A. Long non-coding RNAs in oncourology. Noncoding RNA Res. 2021 Aug 26;6(3):139–145. doi: 10.1016/j.ncrna.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sun J., Sun Z., Gareev I., Yan T., Chen X., Ahmad A., Zhang D., Zhao B., Beylerli O., Yang G., Zhao S. Exosomal miR-2276-5p in plasma is a potential diagnostic and prognostic biomarker in glioma. Front. Cell Dev. Biol. 2021 Jun 1;9 doi: 10.3389/fcell.2021.671202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Beylerli O., Gareev I., Sufianov A., Ilyasova T., Zhang F. The role of microRNA in the pathogenesis of glial brain tumors. Non-coding RNA Res. 2022 Feb 25;7(2):71–76. doi: 10.1016/j.ncrna.2022.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wu J., Al-Zahrani A., Beylerli O., Sufianov R., Talybov R., Meshcheryakova S., Sufianova G., Gareev I., Sufianov A. Circulating miRNAs as diagnostic and prognostic biomarkers in high-grade gliomas. Front. Oncol. 2022 May 12;12 doi: 10.3389/fonc.2022.898537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang Z., Bao Z., Yan W., et al. Isocitrate dehydrogenase 1 (IDH1) mutation-specific microRNA signature predicts favorable prognosis in glioblastoma patients with IDH1 wild type. J. Exp. Clin. Cancer Res. 2013;32(1):59. doi: 10.1186/1756-9966-32-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Besse A., Sana J., Lakomy R., et al. MiR-338-5p sensitizes glioblastoma cells to radiation through regulation of genes involved in DNA damage response. Tumour Biol. 2016;37(6):7719–7727. doi: 10.1007/s13277-015-4654-x. [DOI] [PubMed] [Google Scholar]