Abstract
Multiple helminth species commonly co-occur within mammals and their interactions may negatively affect the survival and breeding success of their hosts. However, it has been difficult to prove competition or mutualism between co-infesting helminths in field studies of wild mammals. The sinus cavities of European polecats (Mustela putorius) can be parasitised by the trematode Troglotrema acutum and the nematode Skrjabingylus nasicola and both helminths can co-occur within hosts. While both parasites can damage the host's bone structure and cause severe pathologies, their impact on host body condition is unclear. It is also unknown whether both parasites interact and how this might affect cranial damage and host body condition. We examined 515 fresh polecat skulls for the presence of both helminths and measured the hosts' amount of kidney perirenal fat as a measure of body condition. Our results demonstrated that, in addition to a host-intrinsic fixed factor (sex) and random factors accounting for spatial and temporal stochasticity, the helminths influenced each other's presence. Infestation with T. acutum increased the probability of catching S. nasicola with increasing age of the host, while males already infested with S. nasicola were more likely to become infested with T. acutum than females infested with the nematode. While we speculate that both effects resulted from parasite-induced behavioural alterations (increased foot consumption), it is not clear why, in the latter case, this effect would be stronger in males than females. We showed that the abundances of both parasites had significant positive effect on the likely presence of skull damage and a significant negative effect on the predicted presence of kidney fat. Given the evolutionary arms race that both host-parasite systems have undergone, it appears unlikely that either helminth played a significant factor in the population decline of the polecat in Europe.
Keywords: Body condition, Co-infection, Cranial damage, Niche overlap, Skrjabingylus nasicola, Troglotrema acutum
Graphical abstract
Highlights
-
•
We provide evidence for interactions between co-infesting helminths in a wild mammal.
-
•
Troglotrema acutum and Skrjabingylus nasicola can co-occur in the skulls of polecats.
-
•
Infestation with one parasite can increase the probability of infestation with the other one.
-
•
Heavy infestation with either helminth may have a negative effect on host body condition.
-
•
Co-infestation does not change the severity of the parasites' clinical impact.
1. Introduction
Most free-living organisms are simultaneously infected by more than one species of parasite (Petney and Andrews, 1998; Hoarau et al., 2020). The interactions of different parasite species within a single host can have important impacts on their epidemiology as well as on host fitness (Graham, 2008). On the one hand, parasites sharing the same host may compete for resources or be impacted by cross-reactive immune defences, while, on the other hand, they may benefit from an immune system dysfunction induced by one of the parasites (Behnke, 2008). While the effects and effect size can differ substantially between systems, co-infesting parasites are likely to influence the abundance, distribution and dynamics of each other (Pedersen and Fenton, 2007; Graham, 2008). The effect of the interaction of multiple parasites is also likely to have an important impact on the severity of symptoms in the host, its body condition and fitness, thus influencing its population dynamics (Tompkins and Begon, 1999; Pedersen and Fenton, 2007).
In mammals, chronic helminth infestations can negatively affect the survival and breeding success of individual hosts (Murray et al., 1997; Albon et al., 2002; Gunn and Irvine, 2003). In addition, it is common for multiple helminth species to co-occur within a host (Poulin, 2001; Chaisiri and Morand, 2021) and their interactions may have important consequences for disease severity, especially if increasing the intensity of parasitism (Petney and Andrews, 1998; Maceda-Veiga et al., 2016). However, in contrast to laboratory studies, it has been hard to prove competition or mutualism between co-infesting helminths in field studies of wild hosts (Poulin, 2001), maybe as a result of co-occurring helminth parasites not being numerous enough to affect a competitor or of their differing resource use (Poulin, 2001; Behnke, 2008). Those field studies that did provide evidence for helminth interactions did not control for host biology and external factors that may influence species associations and/or did not analyse the impact of the interaction on the hosts’ life history traits and body condition (Lello et al., 2004; Portolés et al., 2004; Behnke et al., 2009; Chaisiri and Morand, 2021).
The nasal and frontal sinus cavities of European carnivores can be parasitised by two different genera of helminths: digenean trematodes of the genus Troglotrema and nematodes of the genus Skrjabingylus. Troglotrema acutum occurs in western, central and southern Europe. European polecats (Mustela putorius) are the definitive host of the parasite, but other carnivores can become accidental hosts (Koubek et al., 2004; Heddergott et al., 2015). After the trematode's eggs are shed via the faeces, freshwater snails of the genus Bythinella act as first intermediate hosts. Polecats and other carnivores become infested through the ingestion of anurans that contain metacercariae (Vogel and Voelker, 1978). Skrjabingylus nasicola, a common, cosmopolitan parasite of mustelid species of the genera Mustela and Neovison (Anderson, 2000; Heddergott et al., 2015), is particularly prevalent in polecats (Hansson, 1968; Shimalov and Shimalov, 2002; Torres et al., 2008; Müller and Heddergott, 2009a). The infective stages of S. nasicola develop in terrestrial gastropods, with mouse-like rodents, shrews, amphibians and reptiles acting as paratenic hosts (Anderson, 2000).
Both T. acutum and S. nasicola can damage the bone structure of their hosts’ cranium (Fig. 1; Hansson, 1968; Vogel and Voelker, 1978; Heddergott et al., 2015). The destruction caused by T. acutum to polecat skulls can be particularly severe, leading to bone perforations that can converge and lead to exposure of the frontal sinus and nasal cavity. The remaining bones can be distended and sponge-like in appearance (Lehmensick, 1942; Vogel and Voelker, 1978; Kierdorf et al., 2006; Heddergott et al., 2021). While in least weasels (M. nivalis) and stoats (M. erminea) the skull lesions caused by S. nasicola are comparably severe (Hansson, 1968; King, 1977), the pathology of S. nasicola in polecats appears to be relatively mild, with changes characterised by unevenly thinned bone tissue and in smaller lateral and/or dorsal bulges around the frontal sinus (Hansson, 1968).
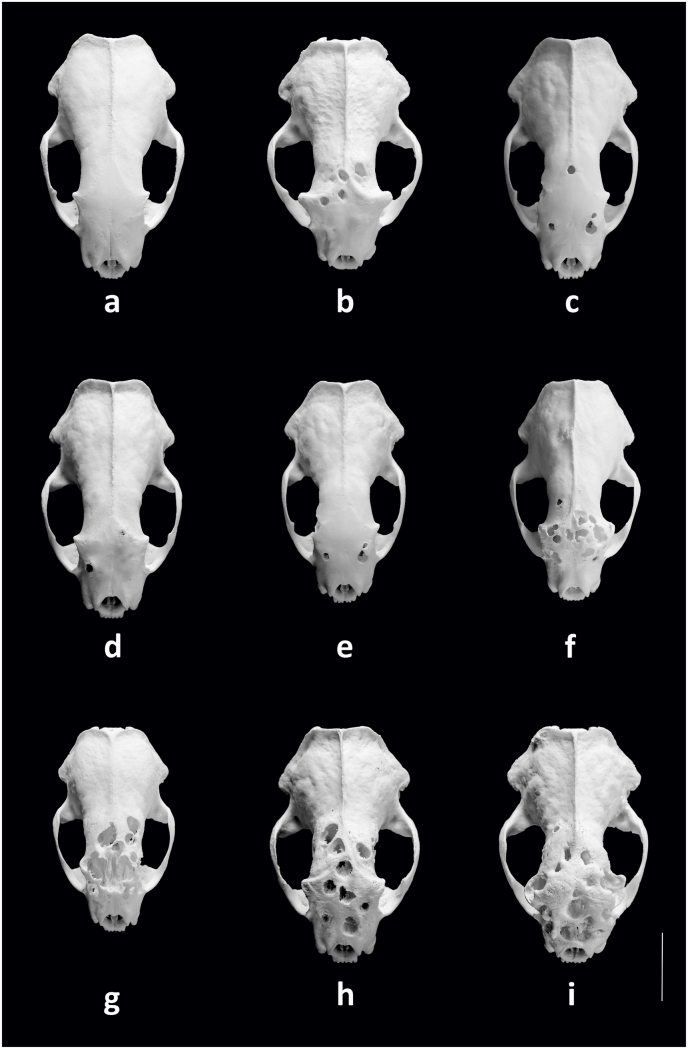
Fig. 1.
Dorsal views of European polecat (Mustela putorius) skulls with or without infestations of the cranial helminths Skrjabingylus nasicola and Troglotrema acutum. (a) Skull of a non-infested one-year-old male. (b) Skull of a three-year-old male with lesions in the frontal bone resulting from an infestation with T. acutum. (c) Lesions in both postorbital processes and the rear of the frontal bone of a skull of a two-year-old male infested with both parasites. (d) Lesion in the right postorbital process of a skull of a two-year-old male infested with both parasites. (e) Skull of a three-year-old female with lesions in both postorbital processes resulting from an infestation with S. nasicola. (f) Lesions in both postorbital processes and the frontal bone of a skull of a four-year-old female infested with both parasites. (g) Skull of a two-year-old female infested with T. acutum that is characterised by large perforations in the frontal bone and exposure of the frontal sinus and the nasal cavity. (h) Skull of a four-year-old male with perforations in and distensions of the frontal bone resulting from an infestation with T. acutum. (i) Skull of a five-year-old male characterised by multiple perforations and distended and sponge-like appearance of the bones across the whole of the frontal dorsal cranium.
In contrast to the widespread S. nasicola, T. acutum appears to have a more discontinuous distribution, its occurrence being usually associated with the presence of the snails that act as first intermediate hosts (Vogel and Voelker, 1978; Koubek et al., 2004). Nevertheless, T. acutum and S. nasicola can co-occur in polecats. Müller and Heddergott (2009a) found a co-infestation in 26 out of 211 polecats from a study area in central Germany where Bythinella compressa probably acted as first intermediate host of T. acutum. Little is known about the nature of the cranial damage resulting from a co-infestation with both helminths, other than Müller and Heddergott (2009a) not noticing any obvious difference in cranial damage between infestations with T. acutum and a joint T. acutum/S. nasicola infestation (see also Fig. 1). Vogel and Voelker (1978) reported that T. acutum mainly, but not exclusively, parasitised the sinus frontalis of polecats, while, according to Hansson (1968), S. nasicola mainly occurred in conchae within the lateral nasal parts. It is therefore not clear whether, in case of co-infestation, both parasites have a numerical effect on each other and whether an interaction impacts cranial damage and body condition.
Despite their relatively severe pathology, the clinical impact of the cranial helminths is not entirely clear. While individuals infested with T. acutum can be in good physical condition (Müller and Heddergott, 2009a; Duscher et al., 2015), some authors state the presence of T. acutum can lead to behavioural alterations and loss of locomotory abilities (Kierdorf et al., 2006; Huck and Weber, 2015). Koubek et al. (2004) even stated that T. acutum infestation may have had a significant negative impact on the population density of polecats in the Czech Republic. Similarly, a number of authors have suggested that S. nasicola had a negative impact on the body condition of stoats in Russia and even negatively affect the species’ population dynamics in the country (King, 1977 and references therein). However, subsequent studies on the stoat and the American mink (N. vison) did not confirm a negative impact of the nematode on host body condition (King, 1977; King and Moody, 1982; Santi et al., 2006; Hawkins et al., 2010; Santi and Paker, 2012; Heddergott et al., 2015). Finally, working on fresh skulls, Müller and Heddergott (2009a) did not notice any obvious impact of infestation with T. acutum and/or S. nasicola on polecat body condition.
While the polecat is widely distributed across Europe, its distribution range and population densities declined in a number of European countries in recent decades (Croose et al., 2018). Threats to the species include direct persecution, secondary poisoning, accidental mortality from car collisions, habitat loss and competition with alien species (Croose et al., 2018). It is currently listed on Annexe V of the EC Habitats and Species Directive and Appendix III of the Bern Convention. Given the potential clinical impact of the cranial helminths, it is necessary to assess thoroughly their effect on the body condition of the polecats. This is especially relevant for cases of co-infestation with both parasites.
Working on a large number of fresh polecat skulls collected from central and western Germany, we therefore aimed to answer the following questions: (1) what is the prevalence of co-infestation with both parasites within the hosts? We hypothesise that the co-infestation is limited to geographic foci that correspond to the presence of a suitable first intermediate host for T. acutum. (2) Does sex and age of the host affect the presence/abundance of the helminths, either when occurring individually or concurrently? (3) Is there evidence for an interaction between both parasites, with the presence/abundance of T. acutum affecting the presence/abundance of S. nasicola and vice versa? We hypothesise that, because of niche specialisation, there will be little interaction between the two. (4) Does the presence of cranial helminths have an impact on the body condition of polecats and does the simultaneous presence of both parasites change the severity of the parasites’ clinical impact?
2. Materials and methods
2.1. Ethics statement
All animals were road-killed or legally harvested. No animal was killed with the aim of providing samples for this study.
2.2. Sampling of hosts and parasites
Between 1985 and 2016, we collected fresh skulls from 515 (327 males, 188 females) European polecats that had a complete body and were sufficiently well-preserved to allow to test for the presence of cranial helminth parasites (Fig. 2). After recording the sex of the animal, carcasses were stored at −20 °C until dissection. A total of 89 polecat carcasses were collected in the North German Plain ecoregion (Fig. 2). The remaining 426 animals originated from the Low Mountain Mittelgebirge ranges in central Germany, where T. acutum and S. nasicola are known to co-occur (Müller and Heddergott, 2009a). The samples included the 211 animals investigated by Müller and Heddergott (2009a). In order to be able to account for spatial autocorrelation (see Material and Methods 2.4), we also recorded the origin of each sample relative to major landscape unit (Fig. 2), subunits of the above-mentioned ecoregions that were defined on the basis of terrain, soils, climate, hydrology and vegetation (Meynen and Schmithüsen, 1953–1962).
Fig. 2.
Geographic origin of the skulls of the European polecat (Mustela putorius) analysed in this study. The size of the pie charts is indicative of the number of skulls analysed per locality and the contents of the pie charts are indicative of the infestation status of the corresponding animals. SKJ: Skrjabingylus nasicola, TRO: Troglotrema acutum. The numbers are indicative of the major landscape unit of origin of the samples.
After removing all tissue and blood in a cold-water bath, we followed the methods by Müller and Heddergott (2009b) to test for the presence of trematodes and nematodes. The skull was held against a light source, and its interior was inspected through the foramen magnum. Skulls with S. nasciola infestations were identified based on the presence of dark discolored areas in the right and left postorbital process. The parasites were removed by opening a small aperture with a 2-mm drill bit in the side of the postorbital process (Heddergott, 2009) and flushing out the underlying tissue with a water-filled syringe. The nematodes were stored in 80% ethanol. T. acutum infestations can frequently be diagnosed by the presence of lesions in the frontal bone and the postorbital process. When these lesions were present, the connective tissue was removed through the lesions using preparation needles and the trematodes were flushed out with a syringe. In the absence of bony lesions, trematodes were flushed out after removing connective tissue with long pointed preparation needles, reaching the T. acutum attachment sites on the ethmoid bone via the nasal bone or the foramen magnum.
2.3. Body measurements and organ weights
The age of the polecats in years was determined based on the number of incremental growth lines in the cementum of an upper-jaw canine (Ansorge, 1995). A precision sectioning saw (IsoMet® 1000, Buehler; Esslingen am Necker, Germany) was used to perform two longitudinal cuts of approximately 4.6 mm length from the central area of the tooth root. The growth lines were counted under a B1-220A light microscope (Motic, Wetzlar, Germany) at × 25–50 magnification. We extrapolated an animal's age in months by presuming a birth in May (Kristiansen et al., 2007).
Carcasses were completely thawed, dried and cleaned. Body weight was measured (±10 g) using digital scales (WSE, Bosche Wägetechnik, Damme, Germany). Snout-vent length (nose tip to anus) was measured with a tape measure (±1 cm). The kidneys and the kidney perirenal fat were weighed (±0.01 g) using digital scales (WSE, Bosche Wägetechnik, Damme, Germany). We added the weight of the left and the right kidneys to obtain a single variable. The weight of the fat from the left and the right kidney we also added up.
2.4. Statistical methods
All the statistical analyses were performed in program R v.4.2.0 (R Core Team, 2022), and all the generalised linear mixed effects models (GLMMs) were fit with the glmmTMB package (Brooks et al., 2017). To check for multicollinearity, we systematically evaluated the variation inflation factors (VIFs) with the full GLMMs without interactions. We considered that there was no substantial correlation when VIF values were <5 (James et al., 2013). To avoid convergence and fitting issues, and to ease interpretation, we only included two-way interactions. We considered the year of sampling and the major landscape unit (see Material and Methods 2.2) as random effects (random intercepts) to account for inter-annual stochasticity and local spatial autocorrelation/subpopulation effects, respectively. Before conducting model selection for the fixed effects of each GLMM, we evaluated whether inclusion of random effects improved the model by comparing the Akaike information criteria (AIC) of models with zero, one, or both random effects, fit with restricted maximum likelihood (Zuur et al., 2009).
We then used the dredge() function in the MuMIn v.1.46.0 R package (Bartoń, K. 2022. MuMIn: Multi-model inference. https://cran.r-project.org/package=MuMIn) to calculate AIC for all potential models fit with non-restricted maximum likelihood, and selected the models which AIC values were within 2 of that of the model with the lowest AIC. Within that subset of models, we selected the most parsimonious model and conducted model averaging using the model.avg() function of the MuMIn package to evaluate the importance of each predictor. We also noted how many times each predictor was present in models within the subset. We tested for overdispersion in the most parsimonious models (Gelman & Hill, 2007). Some level of overdispersion (dispersion ratio: 1.927) was detected in our logistic regression identifying predictors for the presence of skull damage (see Results 3.3). The beta-binomial model that we used as an alternative failed to converge. We evaluated the explanatory power of our models using marginal and conditional coefficient of determination based on Nakagawa et al. (2017). When marginal R2 > 0.10, we plotted the marginal effects of the most parsimonious models using the plot_model() function of the sjPlot v.2.8.10 R package (Lüdecke, D., 2021. sjPlot: Data visualization for statistics in social science. https://CRAN.R-project.org/package=sjPlot). We refrained from systematically plotting models with very minor explanatory power and focused on those explaining a substantial part of the observed ecological phenomenon.
Due to the absence of T. acutum records from the North German Plain region, we used a chi-square test to test for differences in the prevalence of both parasites in these two major ecoregions (North German Plain, Mittelgebirge ranges). Then, we sought to identify predictors for the presence of both parasites by performing a logistic regression to investigate the (fixed) effects of sex, age (in months), month of sampling, as well as presence of the other parasite on the presence of either T. acutum or S. nasicola. Next, we aimed to predict parasite abundance and used the same predictors, with count instead of presence. Initial analysis showed the count data (number of parasites present in skulls) to have an excess of zero counts, to be over-dispersed and to have a variance to mean ratio much greater than 1. We also assumed that the zeros resulted from two processes, and thus, that we had structural zeros in our dataset (some animals never encountered the parasite). Based on all these considerations, we used a zero-inflated negative binomial model (ZINB; Faraway, 2016), which has two parts, a negative binomial count model and a logit model for predicting structural zeros. Due to the absence of T. acutum records from the North German Plain region, we performed the analysis identifying predictors for the presence and abundance of both parasites only with animals sampled in the Mittelgebirge ecoregion. Based on the complete dataset, we performed a logistic regression to test for the effects of sex, age, month of sampling, as well as abundance of the parasites, on the presence of damage to the skulls, defined as the presence of lesions in the frontal bone and the postorbital process.
In line with other studies on mammals (Lajeunesse and Peterson, 1993; Bonino and Bustos, 1998; Serrano et al., 2008), we used the weight of the kidney perirenal fat (kidney fat for short hereafter) of the polecats as proxy for their body condition. However, rather than using an index (e.g., weight of the kidney fat/weight of the kidneys), we followed Serrano et al. (2008) in using the weight of the kidney fat as an untransformed response variable in our modelling approach, while using snout-vent length (which better correlated with kidney fat weight than body or kidney weight) as a predictor variable. We used the same predictors as in the skull damage model. We considered the complete data set for this analysis, which, again, was characterised by a large number of zeros for kidney fat weight. Given the continuous nature of the data, we used a zero-altered gamma (ZAG) model, which is a mixture (hurdle) model with two components, one using a logistic regression to describe the probability of kidney fat being observed and another one using a gamma generalised linear model to analyse the non-zero positive continuous data (Zuur and Leno,2016). We performed a Kolmogorov–Smirnov test to confirm that the non-zero continuous data followed a gamma distribution. Due to persistent convergence problems, we omitted interactions involving the variables ‘month of sampling’, ‘snout-vent length’ and ‘age (in months)’, with the exception of the interaction of the variables ‘age (in months)’ and ‘sex’, from the full model. We plotted the logistic regression part of the ZAG model after generating predictions using the ggpredict() function of the ggeffects v. 0.16.0 R package (Lüdecke, 2018). Further information on structure of the data relative to the years of the study can be found in Table S1.
3. Results
3.1. Presence of the parasites and co-infestations
An infestation with T. acutum was diagnosed in 158 out of 515 (30.7%) of the analysed polecats, while S. nasciola nematodes were recovered from 208 animals (40.4%). All animals infested with T. acutum originated from the Mittelgebirge ranges (37.1% of all animals from this ecoregion) and there was a significant difference between the two ecoregions in T. acutum infestation rates (χ2 = 45.89, df = 1, P < 0.001). We identified S. nasicola in 41 of the 89 polecats (46.1%) sampled in the North German Plain and in 167 of 426 animals (39.2%) sampled in the Mittelgebirge ranges. There was no significant difference in nematode prevalence between the two ecoregions (χ2 = 1.17, df = 1, P = 0.279). Ninety-two of the 426 animals sampled in the Mittelgebirge ranges (21.6%) were infested with both parasites. The difference between ecoregions in the prevalence of co-infestation was significant (χ2 = 21.95, df = 1, P < 0.001).
Since VIF values were <3 in all full GLMMs (without interactions) throughout the study, our models did not have multicollinearity issues (Table S2). When trying to identify predictors for the presence of T. acutum in the Mittelgebirge ranges, we included both random effects in the analysis and our model selection procedure led to 13 equivalent models based on AIC (Table S3). According to the most parsimonious model, older polecats were significantly more likely to be infested with T. acutum and infestation with S. nasicola led to an increased probability of infestation with T. acutum (Table 1). Moreover, there was a significant interaction between the sex of the host and the presence of the nematode (Table 1), as males infested with S. nasicola had an increased probability of T. acutum infestion relative to females that were also infested by the nematode (Fig. 3). The terms of the most parsimonious model were included in the 12 equivalent models, lending support to its relevance as a representative of the subset of best models (Table S3). However, model averaging only confirmed the significance of the interaction between the sex of the host and the presence of the nematode (Table S3). The large conditional R2 of 0.823 and the relatively low marginal R2 of 0.139 of the most parsimonious model indicates that while our model accounts for a lot of variation associated with spatiotemporal stochasticity and autocorrelation, it had relatively low power to predict accurately the presence of T. acutum based on our fixed effects predictors.
Table 1.
Logistic regression identifying predictors for the presence of the cranial helminths in (a) Troglotrema acutum and (b) Skrjabingylus nasicola in European polecats from the Mittelgebirge ecoregion. Results are presented for the most parsimonious model identified after model selection. In the initial model, we included sex, age (in months), presence of the other parasite and month of sampling as fixed factors, while including the year of sampling and major landscape unit of origin as random effects (random intercepts). We only included two-way interactions.
|
(a)T. acutum | ||||
|---|---|---|---|---|
| Fixed effects | Estimate | s.e. | z value | p value |
| (Intercept) | −6.049 | 1.617 | −3.740 | <0.001 |
| Presence of S. nasicola | 1.211 | 0.469 | 2.581 | 0.010 |
| Age | 0.049 | 0.009 | 5.399 | <0.001 |
| Sex–Male | −0.067 | 0.391 | −0.172 | 0.863 |
| Presence of S. nasicola* Sex–Male |
1.560 |
0.658 |
2.370 |
0.018 |
| Random effects | Variance | |||
| Year | 0.396 | |||
| Major landscape unit |
12.309 |
|||
|
Residual |
3.290 |
|||
| marginal R2: 0.139 | ||||
| conditional R2: 0.823 | ||||
| Dispersion ratio: 0.614 |
||||
| (b) S. nasicola | ||||
|
Fixed effects |
Estimate |
s.e. |
z value |
p value |
| (Intercept) | −0.246 | 0.286 | −0.860 | 0.390 |
| Presence of T. acutum | 0.306 | 0.483 | 0.632 | 0.527 |
| Age | −0.017 | 0.009 | −1.861 | 0.063 |
| Sex–Male | −0.618 | 0.236 | −2.623 | 0.009 |
| Presence of T. acutum*Age |
0.046 |
0.013 |
3.434 |
<0.001 |
| Random effects | Variance | |||
| Year | 0.086 | |||
| Major landscape unit |
0.168 |
|||
|
Residual |
3.290 |
|||
| marginal R2: 0.204 | ||||
| conditional R2: 0.261 | ||||
| Dispersion ratio: 0.940 | ||||
In the case of the continuous predictor variable (Age), the logistic regression coefficient gives the change in the log odds of prevalence for a one-unit increase. In the case of the categorical variable (Sex, Presence of the other parasite), the logistic regression coefficient gives the change in the log odds of prevalence when considering males and co-infestation relative to females and infestation with a single parasite, respectively.
Fig. 3.
Marginal effects plot of a logistic regression model predicting the presence (a) of T. acutum as a function of sex of the host and the presence/absence of S. nasicola, the other parasite, (b) of S. nasciola as a function of sex of the host and (c) of S. nasciola as a function of age of the host and the presence/absence of T. acutum. The 95% confidence intervals are shown as error bars or in grey. The plots is based on the most parsimonious model identified after model selection (see Table 1).
When trying to identify predictors for the presence of S. nasicola in the Mittelgebirge ranges we included both random effects in the analysis and our model selection procedure led to five equivalent models based on AIC (Table S4). According to the most parsimonious model, females were significantly more likely to be infested with S. nasicola than males. Also, there was a significant interaction between the age of the host and the presence of the trematode (Table 1, Fig. 3). The probability of becoming infested with S. nasicola increased with age, but only if the host was already infested with T. acutum (Fig. 3). Conversely, the risk of becoming infested with S. nasicola decreased with age for those animals that were not infested with T. acutum. Model averaging confirmed the significance of both predictors and the terms of the most parsimonious model were included in the four equivalent models (Table S4). The marginal R2 of 0.204 indicated that the most parsimonious model had satisfactory power to predict the presence of S. nasicola and the conditional R2 of 0.261 suggested a limited influence of the random effects on the variation in the data.
3.2. Infestation intensities
After comparing the AIC of models with zero, one, or both random effects, we included only the major landscape unit in the ZINB model predicting the number of T. acutum and no random factor when predicting the number of S. nasicola. Both models performed poorly in predicting the abundance of either parasite based on our fixed predictors of sex, age, sampling month and the abundance of the other parasite (T. acutum marginal R2: 0.002, S. nasicola marginal R2: 0.063). In contrast, given its large conditional R2 of 0.764, our model predicting the number of T. acutum accounted for a lot of variation associated with spatial stochasticity and autocorrelation.
3.3. Skull damage
No random factor was included in the model used to predict to occurrence of skull damage. Our model selection procedure led to 11 equivalent models based on AIC (Table S5). According to the most parsimonious model, both the abundance of T. acutum and S. nasicola were a significant predictor of skull damage (Table 2). Moreover, there was a significant interaction between the abundance of S. nasicola and the sex of the host, as in females fewer nematodes were needed to increase the probability of skull damage than in males (Fig. 4). Model averaging confirmed the significance of both predictors and of the interaction and the terms of the most parsimonious model were included in the 10 equivalent models (Table S5). Relative to the models for presence and abundance, the most parsimonious model of skull damage had rather high explanatory power (marginal R2: 0.516).
Table 2.
Logistic regression identifying predictors for the presence of damage to the skull caused by the cranial helminths Troglotrema acutum and Skrjabingylus nasicola in European polecats. Results are presented for the most parsimonious model identified after model selection. In the initial model, we included sex, age (in months), abundance of T. acutum, abundance of S. nasicola and month of sampling as fixed factors. We only included two-way interactions.
| Fixed effects | Estimate | s.e. | z value | p value |
|---|---|---|---|---|
| (Intercept) | −2.848 | 0.336 | −8.483 | <0.001 |
| T. acutum abundance | 0.032 | 0.006 | 5.523 | <0.001 |
| S. nasicola abundance | 0.254 | 0.039 | 6.490 | <0.001 |
| Sex–Male | 0.461 | 0.387 | 1.190 | 0.234 |
| S. nasicola abundance* Sex–Male | −0.151 | 0.042 | −3.625 | <0.001 |
| marginal R2: 0.516 | ||||
| Dispersion ratio: 1.927 | ||||
Fig. 4.
Marginal effects plot of logistic regression model predicting the presence of skull damage as a function of sex of the host, the abundance of S. nasciola and a selection of T. acutum abundances. The colour of the 95% confidence interval corresponds to the T. acutum abundance of the same colour. The plot is based on the most parsimonious model identified after model selection (see Table 2). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.4. Body condition
Both random factors were included in the ZAG model used to predict to occurrence and abundance of kidney fat. Our model selection procedure led to 20 equivalent models based on AIC (Table S6). According to the most parsimonious model (Table 3) snout-vent length (i.e. body size) was the most significant predictor of both the presence of kidney fat (Fig. 5a) and of the amount of kidney fat (Fig. 6). While, when kidney fat was present, larger animals were likely to have larger amounts of it, larger animals were less likely to have kidney fat at all (Table 3). Increases in the abundances of both T. acutum (Fig. 5b) and S. nasicola (Fig. 5c) significantly reduced the probability of the presence of kidney fat. Males were more likely to have kidney fat (Fig. 5d). However, if kidney fat was present, females were likely to have larger amounts of it than males of similar size (Fig. 6). Model averaging confirmed the significance of all predictors and the terms of the most parsimonious model were included in the 19 equivalent models (Table S6). The marginal R2 of 0.155 indicated that the most parsimonious model had relatively low power to predict the presence of kidney fat and the conditional R2 of 0.227 suggested a limited influence of the random effects on explaining the variation in the data.
Table 3.
Zero-altered gamma model identifying predictors for the weight of the kidney perirenal fat of the polecats. The model has two parts, one (a) using a gamma generalised linear model to analyse the non-zero positive continuous data and a second part (b) using a logistic regression to describe the probability of kidney fat being observed. Results are presented for the most parsimonious model identified after model selection. In the initial model, we included sex, age (in months), abundance of T. acutum, abundance of S. nasicola, month of sampling and snout-vent length as fixed factors, while including the year of sampling and major landscape unit of origin as random effects (random intercepts). We only included two-way interactions.
| (a) Generalised linear model coefficients (gamma with log link) | ||||
|---|---|---|---|---|
| Coefficients | Estimate | s.e. | z value | p value |
| (Intercept) | −1.609 | 0.561 | −2.870 | 0.004 |
| S. nasicola abundance | 0.005 | 0.003 | 1.662 | 0.097 |
| Sex–Male | −0.211 | 0.105 | −2.004 | 0.045 |
| Month of sampling | −0.015 | 0.009 | −1.748 | 0.080 |
| Snout-vent length |
0.009 |
0.001 |
6.494 |
<0.001 |
| (b) Zero hurdle model coefficients (binomial with log link) | ||||
|
Coefficients |
Estimate |
s.e. |
z value |
p value |
| (Intercept) | 6.702 | 1.915 | 3.501 | <0.001 |
| T. acutum abundance | −0.032 | 0.014 | −2.188 | 0.029 |
| S. nasicola abundance | −0.077 | 0.024 | −3.215 | 0.001 |
| Sex–Male | 1.367 | 0.401 | 3.412 | <0.001 |
| Snout-vent length |
−0.022 |
0.005 |
−4.350 |
<0.001 |
|
Random effects |
Variance |
|||
| Year | 0.023 | |||
| Major landscape unit |
0.017 |
|||
|
Residual |
0.430 |
|||
| marginal R2: 0.155 | ||||
| conditional R2: 0.227 | ||||
| Dispersion estimate: 0.430 | ||||
Fig. 5.
Marginal effects plot of the zero hurdle model of the Zero-altered gamma model, predicting the presence of kidney fat as a function of (a) snout-vent length, (b) abundance of T. acutum, (c) abundance of S. nasicola and (d) sex of the host. The 95% confidence intervals are shown in grey. The plot is based on the most parsimonious model identified after model selection (see Table 3).
Fig. 6.
Marginal effects plot of the gamma generalised linear model of the Zero-altered gamma model, predicting kidney fat weight as a function of snout-vent length and sex of the host. The colour of the 95% confidence interval corresponds to the sex of the same colour. The plot is based on the most parsimonious model identified after model selection (see Table 3). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
While multiple helminth species commonly co-occur within mammals, it has been hard to prove competition or mutualism between co-infesting helminths in field studies of wild mammals. Here we examine fresh skulls of 515 German polecats for the presence of the trematode Troglotrema acutum and the nematode Skrjabingylus nasicola. These helminths, which can co-occur within the skulls of European polecats, can damage the host's bone structure and cause severe pathologies. Our results demonstrated that, in addition to a host-intrinsic fixed factor (sex) and random factors accounting for spatial and temporal stochasticity, the helminths influenced each other's presence We further showed that the abundances of both parasites had significant positive effect on the likely presence of skull damage and a significant negative effect on the predicted presence of kidney fat. However, we did not find any evidence for co-infestation changing the severity of the parasites' clinical impact.
Our results clearly showed that co-infestation with T. acutum and S. nasicola in polecats is not widespread across Germany, as the presence of the trematode was limited to the Low Mountain Mittelgebierge ecoregion. This finding is in line with other studies that showed the trematode to be patchily distributed within its European range (Vogel and Voelker, 1978; Koubek et al., 2004; Müller and Heddergott, 2009a). The absence of T. acutum from the North German Plain ecoregion is in line with freshwater snails of the genus Bythinella acting as first intermediate host of the parasite. All five species of Bythinella snails known to occur in Germany (Glöer and Zettler, 2005) are cold-stenotherms that are restricted to springs and spring-fed brooks in mountain ranges at altitudes between 500 and 1500 m a.s.l. (Feldmann, 2001; Benke et al., 2009; Strätz and Kittel, 2011). The dependence of T. acutum on a patchily distributed first intermediate hosts is also likely why spatial autocorrelation explained a lot of variation in models predicting the presence and infestation intensities of T. acutum.
Our results suggested that landscape context and inter-annual stochasticity accounted for most of the variation in the model explaining the presence of T. acutum. Nevertheless, we also found evidence for some of our fixed terms influencing the presence of the parasites. When predicting the presence of T. acutum, the most parsimonious model suggested that the age of the host was a significant factor predicting the presence of the trematode, but this factor was not significant after model averaging. In the case of S. nasicola, the sex of the host had a significant impact on the likely presence of the parasite. Female polecats were more likely to be infested than males. Small rodents and amphibians, which are common dietary items of polecats (Lodé, 1997), can act as paratenic hosts of S. nasicola. Most studies based on gut contents found no significant difference between the sexes in the frequency of occurrence of large and small prey (Rzebik-Kowalska, 1972; Weber, 1989; Sainsbury et al., 2020; but see Brugge, 1977). On the other hand, to meet their substantial daily food requirement, breeding females are likely to kill a larger number of small rodents than males (Moors, 1980), thus increasing their chances of exposure to the parasite. Whatever the precise mechanism, the simplest explanation as to why females might be more susceptible to infestation with the nematode than males is that they consume a larger number of rodent (and amphibian) prey items than males.
We found evidence for the parasites affecting each other's presence, but not necessarily in a straightforward manner. When predicting the presence of S. nasicola, we showed that infestation with T. acutum increased the probability of catching S. nasicola with increasing age of the host. The presence of T. acutum per se did not significantly influence the presence of the nematode. It is thus unlikely that the interaction between the parasites resulted from an immune system dysfunction induced by T. acutum, unless the impairment built up over time. As discussed above, the probability of infestation with either parasite likely increases with increased consumption of paratenic hosts. Infection with T. acutum may thus cause the polecat to increase its food consumption. While this explanation is rather speculative, there is evidence of cranial digenean parasites affecting the behaviour of the host species (López-Rodríguez et al., 2021). Kierdorf et al. (2006) have pointed out that the skull damage caused by T. acutum (or S. nasicola) can result in abnormal pressure on the frontal region of the brain and thereby lead to behavioural alterations. Conversely, we also showed that, in the absence of a co-infection, the likely presence of S. nasicola infestations decreases with the age of the host. Given the apparent widespread distribution of the nematode, it is likely that older animals have encountered the parasite and thus developed some immune response to the parasite.
We found consistent evidence that males already infested with S. nasicola were more likely to become infested with T. acutum than females. While infection with S. nasicola may thus cause male polecats to increase their food consumption, it is not immediately obvious as to why this effect would be stronger in males than females, especially since our models predicted since skull damage to be higher in females (see below). While the most parsimonious model suggested that the presence of S. nasicola was a significant factor predicting the presence of the trematode, this was not significant after model averaging. Further research, including behavioural studies on living animals, is clearly necessary to gain a better understanding of the causal mechanisms of the interaction of the parasites.
Our models predicting the number of parasites recovered from a host performed very poorly, at least as far the fixed predictors (sex, age, sampling month, abundance of the other parasite) were concerned. Polecats are characterised by a pronounced sexual dimorphism in body size and male skulls are larger and more robust than those of females (Wolsan, 1993). Adult S. nasicola can vary in length between 6 and 32 mm (Müller and Heddergott, 2009a) and adult T. acutum between 2 and 4 mm (Vogel and Voelker, 1978; Heddergott et al., 2021). As the male skull thus offers more physical space for the parasites to spread, it is perhaps surprising that a model including ‘sex’ as a fixed factor did not perform better. Other factors that may influence infestation intensity but that we could not measure include, for example, the effectiveness of the host's immune response (McRae et al., 2015) or of the immunomodulation strategy of the parasite (Cooper and Eleftherianos, 2016) as well as the number of metacercaria or third-stage larvae that were ingested by the host. Behnke et al. (2005) similarly found that most associations between the abundances of different helminth species in wood mice disappeared after accounting for a series of confounding extrinsic and intrinsic factors. We caution against over-interpreting the results of a probabilistic model that identifies statistically significant pairwise patterns of species co-occurrence (Veech, 2013) without considering confounding factors (e.g. Dallas et al., 2019; Chaisiri and Morand, 2021) and which may not lead to an understanding of the potentially complex interactions of parasites.
Our results suggested that the likelihood of the presence of bone lesions was mainly related to the abundance of either parasites in the polecat skulls (with no evidence of interactions between the parasites). The fact that the model was somewhat overdispersed means that the statistical significance of our estimates should be considered with caution. Our results appear to contradict Heddergott et al. (2015) who, focussing on T. acutum, speculated that the presence of skull damage depended on the duration of infection with the trematode. The thickness of a skull does appear to have an impact on the likelihood of and type of skull damage by S. nasicola (Ribas et al., 2012). Working with several species of mustelids, Hansson (1968) showed that the frequency of damage increased with decreasing skull size. The fact that female skulls are less robust than those of males may thus explain that fewer nematodes were needed to increase the probability of skull damage in females. Moreover, since males’ skulls are larger than those of females, the parasites a likely to be more distributed throughout the sinuses, decreasing the parasite load and the amount of skull damage per unit area (see also Hansson, 1968).
We found some evidence for the severity of infestation with S. nasicola and T. acutum having a detrimental effect on polecat body condition. The logistic regression used within the ZAG model identified a significant negative effect of the abundance of both parasites on the predicted presence of kidney fat. The presence of T. acutum can reportedly lead to behavioural alterations and loss of locomotory abilities (Huck and Weber, 2015), presumably as a result of abnormal pressure on the frontal region of the brain resulting from skull damage (Kierdorf et al., 2006). While the pathology of S. nasicola appears to be relatively mild in polecats (Hansson, 1968), it is nevertheless conceivable that heavy infestation with the nematode can cause similar problems. Heavy parasite load could thus reduce hunting efficiency, which in turn would prevent polecats from accumulating kidney fat. On the other hand, once kidney fat was present, the abundance of both parasites did not have an effect on the predicted amount of the fat. It is possible that the behaviour and fundamental movement skills of a number of heavily infested animals do not deteriorate or improve sufficiently over time to permit them to accumulate kidney fat normally.
In animals with kidney fat, both sex and snout-vent length had an effect on the predicted amount of kidney fat. It is not surprising that males were more likely to have kidney fat. Breeding females have substantial daily food requirements (Moors, 1980). As is the case with other mammals (e.g. Cothran et al., 1987), the percentage of stored body fat is likely to decrease during pregnancy, and the rate of fat gain decreases during lactation in polecats. Therefore, it is perhaps a little surprising that we also predicted females with kidney fat to have larger amounts of kidney fat than similarly-sized males. Non-reproducing females and females outside the reproductive period must therefore gain kidney fat at a greater rate than males. Similarly, it was surprising to find that larger animals were less likely to have kidney fat, while in those animals were kidney fat was present, larger animals were more likely to have larger amounts of this fat. It is difficult to provide a parsimonious explanation for these apparently contradictory results. Perhaps larger animals of both sexes invest more in successful reproduction, while in the case of non-reproducing animals and of animals outside of the reproductive period, larger animals are more likely to have larger amounts of kidney fat. Finally, we did not find any evidence for the interaction of both parasites having an influence on either the likely presence of kidney fat or on the predicted amount of kidney fat.
Our results clearly show that both parasites influenced each other's presence, albeit in a non-straightforward manner. In contrast to the present study, most work on helminth communities has been performed on gastro-intestinal parasites, which may alter their attachment site and resource use when faced with a competitor (Holmes, 1961; Poulin, 2001; Stancampiano et al., 2010). This functional response may limit the impact of the parasites on each other, perhaps explaining the difficulties in demonstrating interactions between co-infesting helminths in field studies of wild hosts (Poulin, 2001). Our study may thus be a special case where the specificity of the attachment site (proximity to brain) and lack of niche diversification may lead to the described effects.
We found some evidence for the severity of infestation with either helminth having a detrimental effect on polecat body condition (i.e., on the predicted presence of kidney fat). We cannot exclude the possibility that another parasite (or an unmeasured factor) caused the negative effect on the body condition of the polecats. The available studies on the parasite fauna of polecats tend to find the prevalence of parasites to be quite low in this species of mustelid (Hamel et al., 2020). While a negative impact of S. nasicola on the body weight of infected stoats has been reported from Russia (King, 1977 and references therein), no other study found a detrimental effect of S. nasicola in either the polecat (Demuth et al., 2009) or other species of mustelids (King, 1977; King and Moody, 1982; Santi et al., 2006; Heddergott et al., 2016). Since these results were based on the body weight of infected animals, the conclusions cannot be directly compared with ours.
Overall, a high load of either parasite may have a negative impact on the survival of some polecats. For example, the presence of T. acutum can lead to behavioural alterations and loss of locomotory abilities in infested animals (Huck and Weber, 2015). However, it is worth bearing in mind that our model explaining the presence and abundance of kidney fat had a relatively low explanatory power, suggesting that some unmeasured factor(s) had a strong influence on body condition. In addition, we could not assess the influence of the parasite on reproductive output of the host. The host-parasite systems investigated in the present study are likely to have undergone an evolutionary arms race. Also, polecats face a plethora of other conservation threats (direct persecution, secondary poisoning, accidental mortality from car collisions, habitat loss and competition with alien species) that can negatively impact their populations (Croose et al., 2018). While Koubek et al. (2004) has suggested that T. acutum can have a significant negative effect on the population dynamics of polecats, based on the present results it appears unlikely that either helminth was a significant factor in the recent population decline of the polecat in Europe.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The analytical part of this work was funded by an internal grant from the Musée National d’Histoire Naturelle. JW was funded by the Luxembourg research fund FNR (C20/SR/14748041). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. We would like to thank Guy Colling, Laura Daco and Anna Schleimer for advice on the statistical analysis.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijppaw.2022.06.008.
Contributor Information
Alain C. Frantz, Email: alain.frantz@mnhn.lu.
Lisette Cantú Salazar, Email: lisette.cantu@list.lu.
Franz Müller, Email: franzgersfeld@web.de.
Peter Steinbach, Email: psteinbach@web.de.
Julian Wittische, Email: jwittische@gmail.com.
Mike Heddergott, Email: Mike-Heddergott@web.de.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Albon S.D., Stien A., Irvine R.J., Langvatn R., Ropstad E., Halvorsen O. The role of parasites in the dynamics of a reindeer population. Proc. Royal Soc. B. 2002;269 doi: 10.1098/rspb.2002.2064. 1652-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R.C. second ed. CABI Publishing; Wallingford: 2000. Nematode Parasites of Vertebrates: Their Development and Transmission. [Google Scholar]
- Ansorge H. In: Methoden feldökologischer Säugetierforschung, Band 1. Stubbe M., Stubbe A., Heidecke D., editors. Martin-Luther-Universität; Halle: 1995. Notizen zur Altersbestimmung nach Wachstumslinien an Säugetierschädeln; pp. 95–102. [Google Scholar]
- Behnke J.M. Structure in parasite component communities in wild rodents: predictability, stability, associations and interactions … or pure randomness? Parasitology. 2008;135:751–766. doi: 10.1017/S0031182008000334. [DOI] [PubMed] [Google Scholar]
- Behnke J.M., Gilbert F.S., Abu-Madi M.A., Lewis J.W. Do the helminth parasites of wood mice interact? J. Anim. Ecol. 2005;74:982–993. [Google Scholar]
- Behnke J.M., Eira C., Rogan M., Gilbert F.S., Torres J., Miguel J., Lewis J.W. Helminth species richness in wild wood mice, Apodemus sylvaticus, is enhanced by the presence of the intestinal nematode Heligmosomoides polygyrus. Parasitology. 2009;136:793–804. doi: 10.1017/S0031182009006039. [DOI] [PubMed] [Google Scholar]
- Benke M., Brändle M., Albrecht C., Wilke T. Pleistocene phylogeography and phylogenetic concordance in cold-adapted spring snails (Bythinella spp. Mol. Ecol. 2009;18:890–903. doi: 10.1111/j.1365-294X.2008.04073.x. [DOI] [PubMed] [Google Scholar]
- Bonino N., Bustos J.C. Kidney mass and kidney fat index in the European hare inhabiting Northwestern Patagonia. Mastozool. Neotrop. 1998;5:81–85. [Google Scholar]
- Brooks M.E., Kristensen K., van Benthem K.J., Magnusson A., Berg C.W., Nielsen A., Skaug H.J., Mächler M., Bolker B.M. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 2017;9:378–400. [Google Scholar]
- Brugge T. Prooidierkeuze van wezel, hermelijn en bunzing in relatie tot geslacht en lichaamsgmotte. Lutra. 1977;19:39–49. [Google Scholar]
- Chaisiri K., Morand S. In: Biodiversity of Southeast Asian Parasites and Vectors Causing Human Disease. Petney T.N., Saijuntha W., Mehlhorn H., editors. Vol. 14. Springer; Cham: 2021. Species richness and species co-occurrence of helminth parasites in the Rattus rattus-complex across stratified habitat landuse types in mainland Southeast Asia; pp. 17–33. (Parasitology Research Monographs). [Google Scholar]
- Cooper D., Eleftherianos I. Parasitic nematode immunomodulatory strategies: recent advances and perspectives. Pathogens. 2016;5:58. doi: 10.3390/pathogens5030058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Core Team R. R Foundation for Statistical Computing; Vienna: 2022. R: A Language and Environment for Statistical Computing. [Google Scholar]
- Cothran E.G., Chesser R.K., Smith M.H., Johns P.E. Fat levels in female white-tailed deer during the breeding season and pregnancy. J. Mammal. 1987;68:111–118. [Google Scholar]
- Croose E., Duckworth J.W., Ruette S., Skumatov D.V., Kolesnikov V.V., Saveljev A.P. A review of the status of the Western polecat Mustela putorius: a neglected and declining species? Mammalia. 2018;82:550–564. [Google Scholar]
- Dallas T.A., Laine A.L., Ovaskainen O. Detecting parasite associations within multi-species host and parasite communities. Proc. R. Soc. B. 2019;286 doi: 10.1098/rspb.2019.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demuth J., Hromada M., Krawczyk A.J., Malecha A.W., Tobolka M., Tryjanowski P. Cranial lesions caused by helminth parasites and morphological traits in the European polecat Mustela putorius. Helminthologia. 2009;46:85–89. [Google Scholar]
- Duscher G.G., Harl J., Fuehrer H.P. Evidence of Troglotrema acutum and Skrjabinhgulus sp. coinfection in a polecat from lower Austria. Helminthologica. 2015;52:63–66. [Google Scholar]
- Faraway J.J. CRC Press; Boca Raton: 2016. Extending the Linear Model with R: Generalized Linear, Mixed Effects and Nonparametric Regression Models. [Google Scholar]
- Feldmann R. Eine regionale Arealgrenze der Quellschnecke Bythinella dunkeri im Bereich der Möhne-Ruhr-Linie. Ber. Naturwiss. Verein für Bielefeld u. Umgegend. 2001;41:313–324. [Google Scholar]
- Gelman A, Hill J. Data Analysis Using Regression and Multilevel/Hierarchical Models. Cambridge University Press; Cambridge: 2007. [Google Scholar]
- Glöer P., Zettler M.L. Kommentierte Artenliste der Süßwassermollusken Deutschlands. Malakol. Abh. 2005;23:3–26. [Google Scholar]
- Graham A.L. Ecological rules governing helminth–microparasite coinfection. Proc. Natl. Acad. Sci. U.S.A. 2008;105:566–570. doi: 10.1073/pnas.0707221105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn A., Irvine R.J. Subclinical parasitism and ruminant foraging strategies – a review. Wildl. Soc. Bull. 2003;31:117–126. [Google Scholar]
- Hamel D., Kretschmar F.M., Visser M., Rehbein S. Parasites of the European polecat (Mustela putorius Linnaeus, 1758) in Europe – a review. Beitr. Jagd- und Wildforsch. 2020;45:145–163. (in German) [Google Scholar]
- Hansson I. Cranial helminth parasites in species of Mustelidae I. Frequency and damage in fresh mustelids from Sweden. Oikos. 1968;19:217–233. [Google Scholar]
- Hawkins C.J., Stuart P., Heddergott M., Lawton C. Sinus worm (Skrjabingylus nasicola (Leuckart, 1842)) infection in American mink (Mustela vision Schreber, 1777) in Ireland. Ir. Nat. J. 2010;31:108–112. [Google Scholar]
- Heddergott M. First record of Skrjabingylus petrowi (Nematoda: Metastrongyloidae) in a pine marten (Martes martes) in Germany. Eur. J. Wildl. Res. 2009;55:543–546. [Google Scholar]
- Heddergott M., Frantz A.C., Jenrich J., Müller F. Dissections of fresh skulls confirm low prevalence of Troglotrema acutum (Trematoda: Troglotrematidae) in German badgers (Meles meles) Parasitol. Res. 2015;114:789–793. doi: 10.1007/s00436-014-4297-7. [DOI] [PubMed] [Google Scholar]
- Heddergott M., Pohl D., Steinbach P., Cantú Salazar L., Müller F., Frantz A.C. Determinants and effects of sinus worm Skrjabingylus nasicola (Nematoda: Metastrongyloidae) infestation in invasive American mink Neovison vison in Germany. Parasitol. Res. 2016;115:3449–3457. doi: 10.1007/s00436-016-5107-1. [DOI] [PubMed] [Google Scholar]
- Heddergott M., Steffen C., Steinbach P., Frantz A.C. First record of Troglotrema acutum (Trematoda, Troglotrematidae) in European polecats Mustela putorius from Luxembourg. Parasitol. Res. 2021;120:2659–2663. doi: 10.1007/s00436-021-07184-x. [DOI] [PubMed] [Google Scholar]
- Hoarau A.O.G., Mavingui P., Lebarbenchon C. Coinfecions in wildlife: focus on a neglected aspect of infectious disease epidemiology. PLoS Pathog. 2020;16 doi: 10.1371/journal.ppat.1008790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes J.C. Effects of concurrent infections on Hymenolepis diminuta (Cestoda) and Moniliformis dubius (Acanthocephala). 1. General effects and comparison with crowding. J. Parasitol. 1961;47:209–216. [PubMed] [Google Scholar]
- Huck S., Weber A. Erkenntnisse zu Diagnostik, Symptomatik and Behandlungsmöglichkeiten von parasitären Infektionen u.a. durch Troglotrema acutum am Beispiel einer erkrankten Iltisfähe Mustela putorius. Säugetierkdl. Inform. 2015;50:55–60. [Google Scholar]
- James G., Witten D., Hastie T., Tibshirani R. Springer; New York: 2013. An Introduction to Statistical Learning: with Applications in R. [Google Scholar]
- Kierdorf U., Kierdorf H., Konjević D., Lazar P. Remarks on cranial lesions in the European polecat (Mustela putorius) caused by helminth parasites. Vet. Arh. 2006;76(Suppl. l):S101–S109. [Google Scholar]
- King C.M. The effects of the nematode parasite Skrjabingulus nasicola on British weasels (Mustela nivalis) J. Zool. 1977;182:225–249. [Google Scholar]
- King C.M., Moody J.E. The biology of the stoat (Mustela erminea) in the National Parks of New Zealand VI. Infestation with Skrjabingylus nasicola. New Zeal. J. Zool. 1982;9:131–140. [Google Scholar]
- Koubek P., Beruš B., Koubková B. Troglotrema acutum (Digenea) from carnivores in the Czech Republic. Helminthologica. 2004;41:25–31. [Google Scholar]
- Kristiansen L.V., Sunde P., Nachman G., Madsen A.B. Mortality and reproductive patterns of wild European polecats Mustela putorius in Denmark. Acta Theriol. 2007;52:371–378. [Google Scholar]
- Lajeunesse T.A., Peterson R.O. Marrow and kidney far as condition indices in gray wolves. Wildl. Soc. Bull. 1993;21:87–90. [Google Scholar]
- Lehmensick R. Über die Veränderung am Iltisschädel durch den Befall mit Troglotrema acutum. Z. Parasitenk. 1942;12:659–661. [Google Scholar]
- Lello J., Boag B., Fenton A., Stevenson I.R., Hudson P.J. Competition and mutualism among the gut helminths of a mammalian host. Nature. 2004;428:840–844. doi: 10.1038/nature02490. [DOI] [PubMed] [Google Scholar]
- Lodé T. Trophic status and feeding habits of the European polecat Mustela putorius L. 1758. Mamm Rev. 1997;27:177–187. [Google Scholar]
- López-Rodríguez R., George-Nascimento M., Górski K. Effects of the cranial parasite Tylodelphys sp. on the behavior and physiology of puye Galaxias maculatus (Jenyns, 1842) PeerJ. 2021;9 doi: 10.7717/peerj.11095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüdecke D. ggeffects: tidy data frames of marginal effects from regression models. J. Open Source Softw. 2018;3:772. [Google Scholar]
- Maceda-Veiga A., Green A.J., Poulin R., Lagrue C. Body condition peaks at intermediate parasite loads in the common bully Gobiomorphius cotidianus. PLoS One. 2016;11 doi: 10.1371/journal.pone.0168992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K.M., Stear M.J., Good B., Keane O.M. The host immune response to gastrointestinal nematode infection in sheep. Parasite Immunol. 2015;37:605–613. doi: 10.1111/pim.12290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meynen E., Schmithüsen J. 1953–1962. Handbuch der naturräumlichen Gliederung Deutschlands. Bundesanstalt für Landeskunde, Remagen and Bad Godesberg. [Google Scholar]
- Moors P.J. Sexual dimorphism in the body size of mustelids (Carnivora): the roles of food habits and breeding systems. Oikos. 1980;34:147–158. [Google Scholar]
- Müller F., Heddergott M. Befall des Europäischen Iltis Mustela putorius (Mustelidae) mit Troglotrema acutum (Trematoda) und Skrjabingulus nasicola (Nematoda) in den Regionen Osthessen und in der angrenzenden Rhön (Bayern, Hessen und Thüringen) Beitr. Jagd- und Wildforsch. 2009;34:367–383. [Google Scholar]
- Müller F., Heddergott M. Nachweismethodik für einen Befall von Musteliden mit Troglotrema acutum (Trematoda) und Skrjabingylus spp. (Nematoda) Beitr. Jagd- und Wildforsch. 2009;34:385–390. [Google Scholar]
- Murray D.L., Cary J.R., Keith L.B. Interactive effects of sublethal nematodes and nutritional status on snowshoe hare vulnerability to predation. J. Anim. Ecol. 1997;66:250–264. [Google Scholar]
- Nakagawa S., Johnson P.C.D., Schielzeth H. The coefficient of determination R2 and intra-class correlation coefficient from generalized linear mixed-effects models revisited and expanded. J. R. Soc. Interface. 2017;14 doi: 10.1098/rsif.2017.0213. 2017.0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen A.B., Fenton A. Emphasizing the ecology in parasite community ecology. Trends Ecol. Evol. 2007;22:133–139. doi: 10.1016/j.tree.2006.11.005. 86:222–227. [DOI] [PubMed] [Google Scholar]
- Petney T.N., Andrews R.H. Multiparasite communities in animals and humans: frequency, structure and pathogenic significance. Int. J. Parasitol. 1998;28:377–393. doi: 10.1016/s0020-7519(97)00189-6. [DOI] [PubMed] [Google Scholar]
- Portolés E., Granel P., Esteban J.G., Cabaret J. Helminth associations in white-toothed shrews Crocidura russula (Insectivora: Soricidae) from the Albufera natural park, Spain. J. Parasitol. 2004;90:572–578. doi: 10.1645/GE-3211. [DOI] [PubMed] [Google Scholar]
- Poulin R. Interactions between species and the structure of helminth communities. Parasitology. 2001;122:S3–S11. doi: 10.1017/s0031182000016991. [DOI] [PubMed] [Google Scholar]
- Ribas A., Molina-Vacas G., Boadella M., Rodríguez-Teijeiro J.D., Fernández-Cardo R., Arrizabalaga A. First report of Troglotrema acutum (Digenea, Troglotrematidae) in the European badger Meles meles in the Iberian Peninsula and presumptive lesions caused in the host. J. Helminthol. 2012;86:222–227. doi: 10.1017/S0022149X11000289. [DOI] [PubMed] [Google Scholar]
- Rzebik-Kowalska B. Badania nad pokarmen ssaków drapieżnych w Polsce. Acta Zool. Cracov. 1972;17:415–506. [Google Scholar]
- Sainsbury C.A., Shore R.F., Schofield H., Croose E., Hantke G., Kitchener A.C., McDonald R.A. Diets of European polecat Mustela putorius in Great Britain during fifty years of population recovery. Mamm Rev. 2020;65:181–190. [Google Scholar]
- Santi S., Paker G.H. Lambert Academic Publishing; Saarbrücken: 2012. Biology of Sinus Worm (Skrjabingylus) Infections in Mustelid Species. [Google Scholar]
- Santi S., Paker G.H., Schaffner N.P., Capodagli L., Persinger M.A. Prevalence, intensity, and geographic distribution of sinus worm (Skrjabingylus nasicola) infection in mink (Mustela vison) of central Ontario. Can. J. Zool. 2006;84:1011–1018. [Google Scholar]
- Serrano E., Alpizar-Jara R., Morellet N., Hewsion A.J.M. A half century of measuring ungulate body condition using indices: is it time for a change? Eur. J. Wildl. Res. 2008;54:675–680. [Google Scholar]
- Shimalov V.V., Shimalov V.T. Helminth fauna of the European polecat (Mustela putorius Linnaeus, 1758) in Belorussian Polesie. Parasitol. Res. 2002;88:259–260. doi: 10.1007/s00436-001-0521-3. [DOI] [PubMed] [Google Scholar]
- Stancampiano L., Mughini Gras L., Poglayen G. Spatial niche competition among helminth parasites in horse's large intestine. Vet. Parasitol. 2010;170:88–95. doi: 10.1016/j.vetpar.2010.01.031. [DOI] [PubMed] [Google Scholar]
- Strätz C., Kittel K. Die Verbreitung der Rhön-Quellschnecke Bythinella compressa (Frauenfeld 1857) in Nordbayern. Mitt. Dtsch. Malakozool. Ges. 2011;84:1–10. [Google Scholar]
- Tompkins D.M., Begon M. Parasites can regulate wildlife populations. Parasitol. Today. 1999;15:311–313. doi: 10.1016/s0169-4758(99)01484-2. [DOI] [PubMed] [Google Scholar]
- Torres J., Miguek J., Fournier P., Fournier-Chambrillon C., Liberge M., Fons R., Feliu C. Helminth communities of the autochthonous mustelids Mustela lutreola and M. putorius and the introduced Mustela vison in south-western France. J. Helminthol. 2008;82:349–355. doi: 10.1017/S0022149X08046920. [DOI] [PubMed] [Google Scholar]
- Veech J.A. A probabilistic model for analysing species co-occurrence. Global Ecol. Biogeogr. 2013;22:252–260. [Google Scholar]
- Vogel H., Voelker J. Über den Lebenszyklus von Troglotrema acutum. Tropenmed. Parasitol. 1978;29:385–405. [PubMed] [Google Scholar]
- Weber D. The diet of polecats (Mustela putorius L.) in Switzerland. Z. Saugetierkd. 1989;54:157–171. [Google Scholar]
- Wolsan M. In: Handbuch der Säugetiere Europas. Raubsäuger Teill II. Stubbe M., Krapp F., editors. Aula Verlag; Wiesbaden: 1993. Mustela putorius Linnaeus, 1758 – Waldiltis, Europäischer Iltis, Iltis; pp. 699–769. [Google Scholar]
- Zuur A.F., Leno E.N. Highland Statistics Ltd; Newburgh: 2016. Beginner's Guide to Zero-Inflated Models R. [Google Scholar]
- Zuur A.F., Leno E.N., Walker N.J., Saveliev A.A., Smith G.M. Springer; New York: 2009. Mixed Effects Models and Extensions in Ecology with R. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.