Abstract
Background/Aims
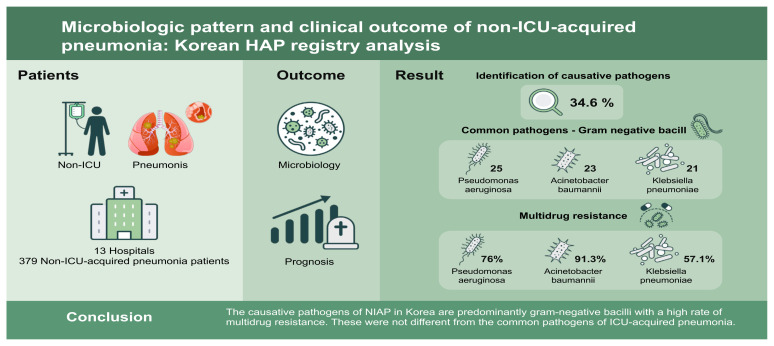
Most studies on hospital-acquired pneumonia (HAP) have been conducted in intensive care unit (ICU) settings. This study aimed to investigate the microbiological and clinical characteristics of non-ICU-acquired pneumonia (NIAP) and to identify the factors affecting clinical outcomes in Korea.
Methods
This multicenter retrospective cohort study was conducted in patients admitted to 13 tertiary hospitals between July 1, 2019 and December 31, 2019. Patients diagnosed with NIAP were included in this study. To assess the prognostic factors of NIAP, the study population was classified into treatment success and failure groups.
Results
Of 526 patients with HAP, 379 were diagnosed with NIAP. Overall, the identified causative pathogen rate was 34.6% in the study population. Among the isolated organisms (n = 113), gram-negative bacilli were common pathogens (n = 91), such as Pseudomonas aeruginosa (n = 25), Acinetobacter baumannii (n = 23), and Klebsiella pneumoniae (n = 21). The multidrug resistance rates of A. baumannii, P. aeruginosa, and K. pneumoniae were 91.3%, 76.0%, and 57.1%, respectively. Treatment failure was significantly associated with K. pneumoniae (odds ratio [OR], 3.50; 95% confidence interval [CI], 1.35 to 9.05; p = 0.010), respiratory viruses (OR, 3.81; 95% CI, 1.34 to 10.82; p = 0.012), hematological malignancies (OR, 3.54; 95% CI, 1.57 to 8.00; p = 0.002), and adjunctive corticosteroid treatment (OR, 2.40; 95% CI, 1.27 to 4.52; p = 0.007).
Conclusions
The causative pathogens of NIAP in Korea are predominantly gram-negative bacilli with a high rate of multidrug resistance. These were not different from the common pathogens of ICU-acquired pneumonia.
Keywords: Healthcare-associated pneumonia, General ward, Microbiology, Prognosis, Korea
Graphical abstract
INTRODUCTION
Hospital-acquired pneumonia (HAP) is the second most common nosocomial infection [1]. Despite improvements in supportive and preventive care, it remains an important cause of morbidity and mortality worldwide [2]. Microbiological diagnosis and an adequate choice of antibiotics are critical for the successful treatment of HAP. Nevertheless, good-quality respiratory samples are often unattainable due to technical invasiveness, and diagnostic accuracy is incomplete from the perspective of the methodology. In this context, the initial choice of empiric antibiotics is the first step in the successful treatment of HAP. An empiric antibiotic strategy has been developed based on the microbiological epidemiology of past studies. However, these data came mostly from previous HAP studies including the intensive care unit (ICU) population [3,4]. Obtaining a respiratory sample is more feasible in ICU settings than in the general ward; therefore, current guidelines for empiric antibiotics in HAP focus on ICU-acquired pneumonia or ventilator-associated pneumonia (VAP) [5]. In this context, evidence regarding non-ICU-acquired pneumonia (NIAP) is limited in terms of clinical characteristics, microbiology, and prognosis [3].
NIAP can be defined as nosocomial pneumonia acquired in the general ward outside the ICU. Currently, the microbiology and epidemiology of NIAP are not fully understood; however, its pathophysiology and etiology might differ from those of VAP, although it is categorized into the same spectrum under the current guidelines [6]. Epidemiologic and microbiological evidence is required to establish an optimal treatment strategy. Therefore, in this context, this study aimed to investigate the microbiological and clinical characteristics of NIAP and determine the factors affecting clinical outcomes in a multicenter retrospective cohort study in Korea.
METHODS
Study design and population
A multicenter retrospective cohort study was conducted in 13 tertiary or university-affiliated hospitals in Korea from July 1, 2019 to December 31, 2019. This study was conducted in the Korean HAP/VAP study group. Eligible patients included those aged 19 years or older, those with a hospitalization period of 3 days or more, and those given a pneumonia-related International Classification of Diseases-10 code (J13–J18, J85) at discharge. HAP was defined according to the 2016 American Thoracic Society (ATS)/Infectious Diseases Society of America (IDA) guidelines [6]. Patients who developed pneumonia within 48 hours of referral from other hospitals or those who had been administered antibiotics for more than 72 hours from other hospitals were excluded from this study. The exclusion criterion was pneumonia acquisition in the ICU.
Data collection
Data were collected and analyzed retrospectively by the coordinator of each hospital. This study was approved by the Institutional Review Board of each participating hospital, including the Pusan National University Yangsan Hospital Review Board (05-2020-067). The clinical information collected in the case report form was as follows: (1) demographic data, (2) microbiological pattern of NIAP, (3) treatment data, and (4) clinical outcomes.
Statistical analysis
All statistical analyses were performed using SPSS version 26 (IBM Corp., Armonk, NY, USA). Categorical variables are presented as numbers with percentages, and continuous variables are presented as means with standard deviations. To compare continuous variables, the Student’s t test or the Mann-Whitney U test was used, as appropriate. Categorical variables were compared using the chi-square test or Fisher’s exact test, as appropriate. Univariate logistic regression analysis was used to examine the association between each variable and treatment failure in the NIAP. Additionally, multivariate logistic regression analysis was performed to control for confounding factors. All tests were two-tailed, and significance was assessed at p < 0.05.
Definitions
Sepsis and septic shock were defined by clinical criteria according to the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) [7]. Multidrug-resistant (MDR) pathogens are microorganisms that are resistant to agents from three or more antimicrobial categories [8]. The adequacy of the initial empiric antibiotics was determined by antimicrobial susceptibility tests in patients with identified pathogens. Immunocompromised patients were defined as those with CD4 counts below 200 cells/mm3 in human immunodeficiency virus infection, those with neutrophils < 1,000/mm3, or those receiving treatment with immunosuppressants after organ transplantation. The history of taking high-dose or long-term steroids was over 20 mg/day of prednisone or equivocal for at least 2 weeks of use.
The clinical response to NIAP is classified into three main categories: cure, failure, and recurrence. Clinical cure refers to the improvement of all signs and symptoms associated with pneumonia. Clinical failure is the persistence or worsening of signs, symptoms, or both associated with pneumonia, occurring again within 3 days after the termination of treatment. Clinical recurrence is defined as the occurrence of pneumonia at 72 hours after antibiotic discontinuation. Moreover, microbiological responses can be classified into four categories: eradication (absence of the baseline pathogen in the final culture of specimens), colonization (persistence of the baseline pathogen but clinically cured), failure (persistence of the baseline pathogen and not clinically cured), and recurrence (regrowth of the baseline pathogen irrespective of the clinical outcome) [9].
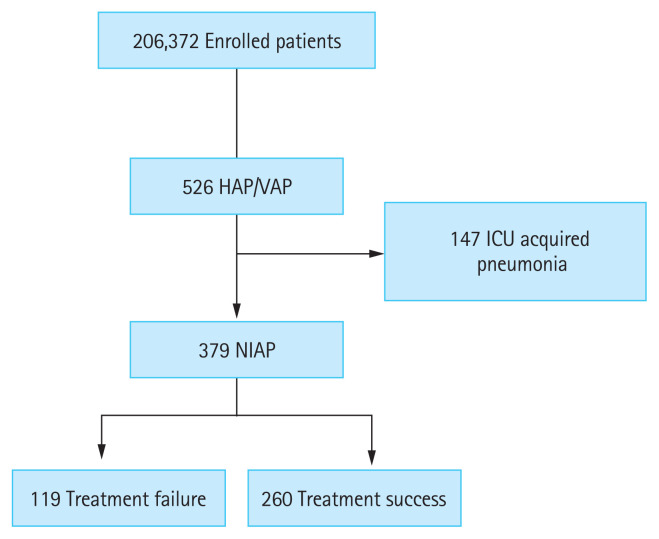
To assess the prognostic factors of NIAP, the study population was classified into treatment success and failure groups. Patients with clinical cure were classified into the treatment success group, and those with clinical failure or recurrence were classified into the treatment failure group (Fig. 1).
Figure 1.
Flow chart of patient’s enrollment. In total, 379 patients were included in the study. HAP, hospital-acquired pneumonia; VAP, ventilator-associated pneumonia; ICU, intensive care unit; NIAP, non-ICU-acquired pneumonia.
RESULTS
Baseline characteristics of NIAP and comparison of treatment failure and success groups
During the study period, a total of 206,372 patients were hospitalized and 526 of them were screened for HAP/VAP (2.54/1,000 patients). Of these, 147 were diagnosed with HAP/VAP in the ICU (‘ICU-acquired pneumonia’) and 379 in the general ward (NIAP). The baseline characteristics of the NIAP are presented in Table 1. Among the patients with NIAP, 267 (70.4%) were men, with a median age of 71.0 ± 12.6 years. The median sequential organ failure assessment (SOFA) score and Charlson comorbidity index (CCI) were 4.0 ± 3.3 and 5.0 ± 2.6, respectively. Solid malignant tumors (42.0%), diabetes (29.6%), and chronic neurological disease (25.6%) were the most common comorbidities. Overall, 52.5% of patients with NIAP had aspiration risk factors. Sepsis and septic shock were identified in 57.8% and 6.6% of patients, respectively. The proportion of prior intravenous antibiotic use within 90 days was 68.6%.
Table 1.
Baseline characteristics of patients with treatment failure and success groups in NIAP
| Variable | Overall (n = 379) | Treatment failure (n = 119) | Treatment success (n = 260) |
|---|---|---|---|
| Age, yr | 71.0 ± 12.6 | 72.0 ± 13.1 | 70.5 ± 12.3 |
| Male sex | 267 (70.4) | 84 (70.6) | 183 (70.4) |
| BMI, kg/m2 | 21.9 ± 3.8 | 21.9 ± 3.7 | 21.9 ± 3.9 |
| Initial SOFA score | 4.0 ± 3.3 | 5.7 ± 3.5c | 3.2 ± 2.8 |
| Charlson comorbidity index | 5.0 ± 2.6 | 5.6 ± 2.3c | 4.7 ±2.6 |
| Comorbidities | |||
| Cardiovascular disease | 65 (17.2) | 19 (16.0) | 46 (17.7) |
| Chronic lung disease | 63 (16.6) | 19 (16.0) | 44 (16.9) |
| Chronic neurological disease | 97 (25.6) | 33 (27.7) | 64 (24.6) |
| Chronic kidney disease | 47 (12.4) | 18 (15.1) | 29 (11.2) |
| Chronic liver disease | 21 (5.5) | 6 (5.0) | 15 (5.8) |
| Diabetes mellitus | 112 (29.6) | 32 (26.9) | 80 (30.8) |
| Connective tissue disease | 6 (1.6) | 2 (1.7) | 4 (1.5) |
| Immunocompromisedd | 20 (5.3) | 12 (10.1) | 8 (3.1)b |
| Hematological malignancies | 36 (9.5) | 22 (18.5) | 14 (5.4)c |
| Solid malignant tumors | 159 (42.0) | 55 (46.2) | 104 (40.0) |
| High-dose or long-term corticosteroid usee | 22 (5.8) | 11 (9.2) | 11 (4.2) |
| Presence of artificial airwayf | 41 (10.8) | 8 (6.7) | 33 (12.7) |
| Risk of aspirationg | 199 (52.5) | 72 (60.5) | 127 (48.8)a |
| Sepsis | 219 (57.8) | 86 (72.3) | 133 (51.2)c |
| Septic shock | 25 (6.6) | 15 (12.6) | 10 (3.8)b |
| Prior IV antibiotics use within 90 days | 260 (68.6) | 92 (77.3) | 168 (65.6)b |
| Reason for admissionb | |||
| Medical for diagnostic work up | 42 (11.1) | 11 (9.2) | 31 (11.9) |
| Medical disease treatment | 223 (58.8) | 86 (72.3) | 137 (52.7) |
| Surgical for elective operation | 86 (22.7) | 13 (10.9) | 73 (28.1) |
| Surgical for emergency operation | 18 (4.7) | 5 (4.2) | 13 (5.0) |
| Surgical for other reasons than operation | 10 (2.6) | 4 (3.4) | 6 (2.3) |
Values are presented as mean±standard deviation or number (%).
BMI, body mass index; SOFA, sequential organ failure assessment; IV, intravenous.
p value was less than 0.05.
p value was less than 0.01.
p value was less than 0.001.
Human immunodeficiency virus-infected patients with CD4 counts less than 200 cells/mm3, neutrophils less than 1,000/mm3, or patients taking immunosuppressive drugs after organ transplantation.
Prednisone, or equivalent 20 mg/day taken at least 2 weeks.
Tracheostomy tube, tracheal tube, and others.
Impaired swallowing (esophageal disease, neurologic disease, recent extubation), impaired consciousness, increased chance of gastric contents reaching the lung (reflux, tubal feeding) and impaired cough reflex (medications, stroke, dementia).
Comorbidities such as immunocompromised state (10.1% vs. 3.1%, p = 0.005), hematological malignancies (18.5% vs. 5.4%, p < 0.001), and risk of aspiration (60.5% vs. 48.8%, p = 0.035) were more common in the treatment failure group than in the treatment success group. Sepsis (72.3% vs. 51.2%, p < 0.001) and septic shock (12.6% vs. 3.8%, p = 0.001) were also more common in the treatment failure group. The proportion of prior intravenous antibiotic use within 90 days (77.3% vs. 65.6%, p = 0.022) was significantly higher in the treatment failure group than in the treatment success group. The most common reasons for admission were medical disease treatment (58.8%), followed by elective surgery (22.7%).
Identified microbiologic pattern in NIAP
The microbial patterns identified are listed in Table 2. Overall, the most common pathogens were Pseudomonas aeruginosa (n = 25), followed by Acinetobacter baumannii (n = 23), Klebsiella pneumoniae (n = 21), and Staphylococcus aureus (n = 14). The identified rates of K. pneumoniae (9.2% vs. 3.8%, p = 0.033) and A. baumannii (10.9% vs. 3.8%, p = 0.007) were significantly higher in the treatment failure group than in the treatment success group. Among patients with NIAP, causative bacteria were identified in 113 patients, and MDR pathogens were identified in 69. The MDR pathogens were identified in the order of Acinetobacter species (n = 21), P. aeruginosa (n = 19), Enterobacteriaceae species (n = 18), S. aureus (n = 11), and Enterococcus species (n = 3). A total of 22 cases of seven viruses were detected in 20 patients with NIAP. The detection rate of respiratory viruses using polymerase chain reaction (PCR) was significantly higher in the treatment failure group than in the treatment success group (10.1% vs. 3.1%, p = 0.005).
Table 2.
Identified microbiologic pattern in non-ICU-acquired pneumonia
| Causative pathogens | Overall (n = 379) | Treatment failure (n = 119) | Treatment success (n = 260) |
|---|---|---|---|
| Unknown | 248 (65.4) | 61 (51.3) | 187 (71.9)c |
| Bacteria | 113 (29.8) | 49 (41.2) | 64 (24.6)c |
| Enterobacteriaceaed | 44 (11.6) | 20 (16.8) | 24 (9.2)a |
| Escherichia coli | 6 (1.6) | 3 (2.5) | 3 (1.2) |
| Klebsiella pneumoniae | 21 (5.5) | 11 (9.2) | 10 (3.8)a |
| MDR Enterobacteriaceae | 18 (4.7) | 7 (5.9) | 11 (4.2) |
| Acinetobacter baumannii | 23 (6.1) | 13 (10.9) | 10 (3.8)b |
| MDR Acinetobacter spp. | 21 (5.5) | 11 (9.2) | 10 (3.8)a |
| Pseudomonas aeruginosa | 25 (6.6) | 7 (5.9) | 18 (6.9) |
| MDR P. aeruginosa | 19 (5.0) | 6 (5.0) | 13 (5.0) |
| Staphylococcus aureus | 14 (3.7) | 5 (4.2) | 9 (3.5) |
| MRSA | 11 (2.9) | 4 (3.4) | 7 (2.7) |
| Enterococcus spp. | 4 (1.1) | 2 (1.7) | 2 (0.8) |
| MDR Enterococcus spp. | 3 (0.8) | 1 (0.8) | 2 (0.8) |
| Other bacteriae | 27 (7.1) | 8 (6.7) | 19 (7.3) |
| Polymicrobial infection | 19 (5.0) | 4 (3.4) | 15 (5.8) |
| Detection of RV by PCR | 20 (5.3) | 12 (10.1) | 8 (3.1)b |
| Parainfluenza virus | 8 (2.1) | 5 (4.2) | 3 (1.2) |
| Human coronavirus OC43 | 2 (0.5) | 1 (0.8) | 1 (0.4) |
| Influenza A virus | 3 (0.8) | 1 (0.8) | 2 (0.8) |
| Adenovirus | 3 (0.8) | 2 (1.7) | 1 (0.4) |
| Respiratory syncytial virus | 2 (0.5) | 1 (0.8) | 1 (0.4) |
| Rhinovirus | 3 (0.8) | 3 (2.5) | 0a |
| Human bocavirus | 1 (0.3) | 0 | 1 (0.4) |
| Serologic tests for atypical pathogens | 7 (1.8) | 5 (4.2) | 2 (0.8) |
| Cytomegalovirus | 1 (0.3) | 1 (0.8) | 0 |
| Aspergillus spp. | 2 (0.5) | 1 (0.8) | 1 (0.4) |
| Mycoplasma pneumoniae | 1 (0.3) | 1 (0.8) | 0 |
| Pneumocystis jirovecii | 3 (0.8) | 2 (1.7) | 1 (0.4) |
Values are presented as number (%). MDR pathogens are defined as microorganisms that are resistant to agents from three or more antimicrobial categories.
ICU, intensive care unit; MDR, multi-drug resistant; MRSA, methicillin-resistant Staphylococcus aureus; RV, respiratory virus; PCR, polymerase chain reaction.
p value was less than 0.05.
p value was less than 0.01.
p value was less than 0.001.
Such as Escherichia coli, Yersinia pestis, Klebsiella spp., Shigella spp., Proteus spp., Enterobacter spp., Serratia spp., and Citrobacter spp.
Such as Moraxella catarrhalis, Burkholderia cepacia, Listeria monocytogenes, Streptococcus pneumoniae, Streptococcus pyogenes, Streptococcus agalactiae, Streptococcus anginosus, Streptococcus mitis, coagulase-negative Staphylococcus, Stenotrophomonas maltophilia, Rothia mucilaginosa.
Treatment of NIAP
The initial empirical antibiotics used for the treatment of NIAP are shown in Table 3. Overall, the initial antibiotic strategy varied with monotherapy (n = 192, 50.7%), dual combination therapy (n = 161, 42.5%), and triple combination therapy (n = 21, 5.5%). The proportion of monotherapy was not significantly different between the two groups (44.5% vs. 53.5%, p = 0.107), and beta-lactams were the most preferred agents (92.2%). The use of dual combination therapy was also not significantly different between the two groups (43.7% vs. 41.9%, p = 0.746). Among the dual combination therapies, a combination of beta-lactams and fluoroquinolones was most often used (70.8%). Triple combination therapy was used significantly more often in the treatment failure group than in the treatment success group (9.2% vs. 3.8%, p = 0.033). Whether initial empiric antibiotics were appropriate could be determined only in 102 patients with NIAP. The use of initial empiric antibiotics was appropriate in 57 of 102 patients (55.9%). There was no correlation between the appropriateness of initial antibiotics and clinical outcomes. The antibiotic change rate according to the antimicrobial susceptibility test was significantly higher in the treatment failure group than in the treatment success group (42.9% vs. 31.2%, p = 0.026). Although not statistically significant, the treatment failure group had a higher rate of antibiotic escalation than the treatment success group (63.5% vs. 51.9%, p = 0.188). The number of patients who used adjunctive steroids for the treatment of NIAP in both groups was 63 (16.6%). The use of adjunctive corticosteroids was significantly higher in the treatment failure group than in the treatment success group (24.4% vs. 13.1%, p = 0.006).
Table 3.
Comparison of treatment between two groups in NIAP
| Initial empiric antibiotics for NIAP | Overall (n = 379) | Treatment failure (n = 119) | Treatment success (n = 260) |
|---|---|---|---|
| Monotherapy | 192 (50.7) | 53 (44.5) | 139 (53.5) |
| B-lactam antibiotics | 177 (46.7) | 50 (42.0) | 127 (48.8) |
| Fluoroquinolones | 6 (1.6) | 0 | 6 (1.6) |
| Glycopeptides | 1 (0.3) | 0 | 1 (0.4) |
| Aminoglycosides | 2 (0.5) | 0 | 2 (0.8) |
| Miscellaneous | 6 (1.6) | 3 (2.5) | 3 (1.2) |
| Dual combination therapy | 161 (42.5) | 52 (43.7) | 109 (41.9) |
| B-lactam + Fluoroquinolones | 114 (30.1) | 29 (24.4) | 85 (32.7) |
| B-lactam + Glycopeptides | 28 (7.4) | 13 (10.9) | 15 (5.8) |
| B-lactam + Aminoglycosides | 5 (1.3) | 3 (2.5) | 2 (0.8) |
| B-lactam + Nitroimidazoles | 5 (1.3) | 3 (2.5) | 2 (0.8) |
| B-lactam + Macrolides | 7 (1.8) | 2 (1.7) | 5 (1.9) |
| Miscellaneous | 4 (1.1) | 2 (1.7) | 2 (0.8) |
| Triple combination therapy | 21 (5.5) | 11 (9.2)a | 10 (3.8) |
| B-lactam + Fluoroquinolones + Glycopeptides | 10 (2.6) | 6 (5.0) | 4 (1.5) |
| B-lactam + Fluoroquinolones + Nitroimidazoles | 2 (0.5) | 1 (0.8) | 1 (0.4) |
| B-lactam + Aminoglycosides + Glycopeptides | 1 (0.3) | 1 (0.8) | 0 |
| B-lactam + Aminoglycosides + Nitroimidazoles | 1 (0.3) | 0 | 1 (0.4) |
| Miscellaneous | 7 (1.8) | 3 (2.5) | 4 (1.5) |
| Duration of antibiotics, day | 12 (7–24) | 10 (5–25) | 12 (7–22) |
| Adequacy of initial antibioticsc | 57 (55.9) | 25 (55.6) | 32 (56.1) |
| Change of antibiotics after pathogen confirm | 132 (34.8) | 51 (42.9)a | 81 (31.2) |
| Escalation of antibiotics | 75 (56.4) | 33 (63.5) | 42 (51.9) |
| De-escalation of antibiotics | 24 (18.0) | 6 (11.5) | 18 (22.2) |
| Adjunctive corticosteroid treatment | 63 (16.6) | 29 (24.4) | 34 (13.1)b |
Values are presented as number (%) or median (interquartile range).
NIAP, non-ICU-acquired pneumonia.
p value was less than 0.05.
p value was less than 0.01.
Only 102 patients of all NIAP patients (treatment failure 45; treatment success 57) could determine whether initial empiric antibiotics were adequate.
Clinical outcome of NIAP
The microbiological response to NIAP treatment was observed in 93 patients (Table 4). Among these, microbiological eradication, colonization, failure, and recurrence were observed in 56 (60.2%), seven (7.5%), 29 (31.2%), and one (1.1%) patients, respectively. Of all patients with NIAP, 276 (72.8%) were discharged from the hospital. Among them, only 170 (61.6%) were discharged to return home, and 106 (38.4%) were transferred to other hospitals or facilities. During hospitalization, 107 (28.2%) patients required addi tional intensive care for HAP.
Table 4.
Clinical outcome of non-ICU-acquired pneumonia
| Variable | Overall (n = 379) | Treatment failure (n = 119) | Treatment success (n = 260) |
|---|---|---|---|
| Microbiologic responsec | |||
| Eradicationd | 56 (14.8) | 15 (35.7) | 41 (80.4)b |
| Colonizatione | 7 (1.8) | 0 | 7 (13.7)a |
| Failuref | 29 (7.7) | 26 (61.9) | 3 (5.9)b |
| Recurrenceg | 1 (2.6) | 1 (2.4) | 0 |
| Hospital length of stay, day | 29 (17–51) | 32 (18–63) | 28 (16–47) |
| Additional ICU admission associated with HAP | 107 (28.2) | 60 (50.4) | 47 (18.1)b |
| Survival discharge | 276 (72.8) | 25 (21.0) | 251 (96.5)b |
| Home discharge | 170 (44.9) | 6 (24.0) | 164 (65.3)b |
| Step down referral | 97 (25.6) | 16 (64.0) | 81 (32.3) |
| Step up referral | 9 (2.4) | 3 (12.0) | 6 (2.4) |
Values are presented as number (%) or median (interquartile range).
ICU, intensive care unit; HAP, hospital-acquired pneumonia.
p value was less than 0.05.
p value was less than 0.001.
Microbiologic response was revealed in only 93 of the study population (treatment failure 15; treatment success 41).
Absence of the baseline pathogen in the final culture of specimens during hospitalization.
Persistence of the baseline pathogen but clinically cured or f not clinically cured.
Regrowth of the baseline pathogen irrespective of clinical outcome.
Predictors of treatment failure in NIAP
Binary logistic regression analysis was performed to verify the predictors of treatment failure (Table 5). According to the multivariate logistic regression analysis, SOFA score (odds ratio [OR], 1.24; 95% confidence interval [CI], 1.14 to 1.34; p < 0.001), CCI (OR, 1.12; 95% CI, 1.00 to 1.24; p = 0.036), identification of K. pneumoniae (OR, 3.50; 95% CI, 1.35 to 9.05; p = 0.010), detection of respiratory viruses by PCR (OR, 3.81; 95% CI, 1.34 to 10.82; p = 0.012), hematological malignancies (OR, 3.54; 95% CI, 1.57 to 8.00; p = 0.002), and adjunctive corticosteroid treatment (OR, 2.40; 95% CI, 1.27 to 4.52; p = 0.007) were independently associated with treatment failure.
Table 5.
Binary logistic regression of predictors for treatment failure in non-ICU-acquired pneumonia
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
|
|
|
|||
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Initial SOFA score | 1.27 (1.18–1.36) | < 0.001 | 1.24 (1.14–1.34) | < 0.001 |
|
| ||||
| CCI | 1.15 (1.05–1.25) | 0.002 | 1.12 (1.00–1.24) | 0.036 |
|
| ||||
| Immunocompromiseda | 3.53 (1.40–8.89) | 0.007 | ||
|
| ||||
| Hematologic malignancies | 3.99 (1.96–8.11) | < 0.001 | 3.54 (1.57–8.00) | 0.002 |
|
| ||||
| Risk of aspirationb | 1.60 (1.03–2.49) | 0.036 | ||
|
| ||||
| Prior IV antibiotics use within 90 days | 1.79 (1.08–2.94) | 0.023 | ||
|
| ||||
| Sepsis | 2.49 (1.56–4.00) | < 0.001 | ||
|
| ||||
| Septic shock | 3.61 (1.57–8.29) | 0.003 | ||
|
| ||||
| Klebsiella pneumoniae | 2.55 (1.05–6.17) | 0.039 | 3.50 (1.35–9.05) | 0.010 |
|
| ||||
| Acinetobacter baumannii | 3.07 (1.30–7.21) | 0.010 | ||
|
| ||||
| Enterobacteiaceae spp.c | 2.00 (1.05–3.76) | 0.035 | ||
|
| ||||
| Detection of RV by PCRd | 3.53 (1.40–8.89) | 0.007 | 3.81(1.34–10.82) | 0.012 |
|
| ||||
| Triple combination therapy | 2.55 (1.05–6.17) | 0.039 | ||
|
| ||||
| Adjunctive corticosteroids treatment | 2.14 (1.23–3.72) | 0.007 | 2.40 (1.27–4.52) | 0.007 |
ICU, intensive care unit; OR, odds ratio; CI, confidence interval; SOFA, sequential organ failure assessment; CCI, Charlson comorbidity index; IV, intravenous; RV, respiratory virus; PCR, polymerase chain reaction.
Human immunodeficiency virus-infected patients with CD4 counts less than 200 cells/mm3, neutrophils less than 1,000/mm3, or patients taking immunosuppressive drugs after organ transplantation.
Impaired swallowing (esophageal disease, neurologic disease, recent extubation), impaired consciousness, increased chance of gastric contents reaching the lung (reflux, tubal feeding) and impaired cough reflex (medications, stroke, dementia).
Escherichia coli, Yersinia pestis, Klebsiella spp., Shigella spp., and Proteus spp. Enterobacter spp., Serratia spp., and Citrobacter spp.
Human parainfluenza virus, human bocavirus, rhinovirus, influenza A virus, respiratory syncytial virus, adenovirus and human coronavirus OC43.
DISCUSSION
This study focused on the clinical and microbiological characteristics, treatment, and outcomes of patients with NIAP in Korea. Most of the patients were elderly, over half presented with sepsis, and approximately half had comorbidities. Overall, 72.8% of patients were discharged alive, and only 44.9% were discharged to return home. In microbiology, 34.6% of the study population identified the etiologic organisms, and MDR gram-negative bacilli were common pathogens, such as P. aeruginosa, A. baumannii, and K. pneumoniae. The treatment success rate of NIAP was 68.6% in the study population, and treatment failure was significantly associated with the identification of K. pneumoniae, respiratory viruses, hematological malignancies, and adjunctive corticosteroid treatment. The most commonly used empiric antibiotics were beta-lactam antibiotics (92.2%) for monotherapy and beta-lactam plus fluoroquinolones (70.8%) for combination therapy.
This study presents meaningful data on NIAP, which has not been fully addressed in Korea or worldwide. First, the causative pathogens of pneumonia in a non-ICU environment in Korea are quite different from those in other countries, and have a high rate of multidrug resistance [4,5,10]. Sopena et al. [4] reported that among 119 patients with NIAP in Spain, the causative pathogens were identified in the order of Streptococcus pneumoniae (13%), Enterobacteriaceae species (8%), P. aeruginosa (3%), and methicillin-resistant S. aureus (MRSA) (3%). In a retrospective study conducted in Korea, 162 neurologic patients were diagnosed with NIAP, with S. aureus (35.8%), K. pneumoniae (26.5%), A. baumannii (13.0%), P. aeruginosa (14.8%), S. pneumoniae (9.3%), and E. aerogenes (6.8%) [11]. In this study, the proportion of gram-negative bacteria (80.5%) was higher than that of gram-positive bacteria (25.7%). The most frequently identified pathogens were P. aeruginosa (22.1%), followed by A. baumannii (20.4%), K. pneumoniae (18.6%), and S. aureus (12.4%). Most of the patients were MDR. The multidrug resistance rates of A. baumanii, P. aeruginosa, S. aureus, and K. pneumoniae were 91.3%, 76.0%, 78.6%, and 57.1%, respectively. Given the exclusion of ICU-acquired pneumonia or VAP, this is an exceptional result. This implies that the causative pathogens of NIAP in Korea are not different from ICU-acquired pneumonia or VAP [12].
According to the ATS/IDA guidelines revised in 2016, patients with suspected HAP (non-VAP) were recommended empiric antibiotic regimens based on the local distribution of pathogens associated with HAP and their antimicrobial susceptibilities [6]. Antimicrobial regimens with activity against S. aureus and P. aeruginosa should always be considered in patients with clinical suspicion [13]. However, this study indicated that real-world practice in NIAP might be quite different from the guidelines. Although a high percentage of MDR pathogens were identified, monotherapy was preferred in 50.7% of patients with NIAP rather than anti-pseudomonal dual combination therapy (31.4%) or glycopeptides (10.6%) as the initial empiric antibiotics. Multivariate analysis showed that the identification of K. pneumoniae, detection of respiratory viruses by PCR, and hematological malignancies were predictors of treatment failure.
In a retrospective study conducted in Taiwan, the 28-day mortality rate of HAP caused by K. pneumoniae was 39.6% [14]. This high mortality rate was elucidated by the high percentage of MDR pathogens (49.3%) and the high number of identified hypervirulent strains (41%). In the present study, the MDR rate of K. pneumoniae was also high (57.1%), which is related to treatment failure.
The identification of respiratory viruses was also a predictor of treatment failure. Of the 20 patients from whom the viruses were identified, six were co-infected with bacteria. Treatment failure was observed in five of the six co-infected patients. In other studies of hospitalized patients with CAP, viral and bacterial co-infections were associated with prolonged hospital stay and increased mortality [15,16]. Further studies are needed to determine whether the poor prognosis of viral infection is related to co-infection or viral virulence alone.
This current study has several limitations. First, this study had a retrospective design and may have been biased. Second, due to the short study period of 6 months, long-term results, such as mortality, were not fully evaluated. Third, semi-quantitative or quantitative culture methods were not uniformly performed in all patients, so the identified pathogens were not fully confirmed as the true pathogens. However, only the results of cultures collected within 2 days before or after antibiotic treatment were recorded, unless they were deemed to be colonizers or contaminants by the managing physicians. So far, little is known about the clinical significance of NIAP and the current status of clinical practice. In this sense, this study with a relatively large number of patients collected from multiple centers provides the basis for a new concept of NIAP, and is important for providing evidence on whether the current therapeutic approach is appropriate.
In summary, this is one of the few studies on the microbiological patterns and clinical outcomes of NIAP. The causative pathogens of NIAP in Korea were not different from those of ICU-acquired pneumonia or VAP, and the MDR pathogens accounted for 61.1%. The compliance with the guidelines for the selection of initial antibiotics was inadequate. The treatment failure of NIAP was related to the identification of K. pneumoniae, respiratory viruses, and comorbid conditions. The definition and epidemiologic and microbiologic data on NIAP should be clarified through further investigation.
KEY MESSAGE
1. The causative pathogens of non-intensive care unit (ICU)-acquired pneumonia in Korea were not different from those of ICU-acquired pneumonia, and the proportion of multidrug-resistant was as high as 61.1%.
2. The treatment failure of non-ICU-acquired pneumonia was related to identification of Klebsiella pneumoniae, respiratory viruses, comorbid conditions and disease severity.
Acknowledgments
This study was funded by the 2019 Research Grant (2019-E2808-00) from the Korean Disease Control and Prevention Agency and supported by a 2021 research grant from Pusan National University Yangsan Hospital.
Footnotes
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.American Thoracic Society; Infectious Diseases Society of America Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 2.Flanders SA, Collard HR, Saint S. Nosocomial pneumonia: state of the science. Am J Infect Control. 2006;34:84–93. doi: 10.1016/j.ajic.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Di Pasquale M, Aliberti S, Mantero M, Bianchini S, Blasi F. Non-intensive care unit acquired pneumonia: a new clinical entity? Int J Mol Sci. 2016;17:287. doi: 10.3390/ijms17030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sopena N, Heras E, Casas I, et al. Risk factors for hospital-acquired pneumonia outside the intensive care unit: a case-control study. Am J Infect Control. 2014;42:38–42. doi: 10.1016/j.ajic.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 5.Herer B, Fuhrman C, Gazevic Z, Cabrit R, Chouaid C. Management of nosocomial pneumonia on a medical ward: a comparative study of outcomes and costs of invasive procedures. Clin Microbiol Infect. 2009;15:165–172. doi: 10.1111/j.1469-0691.2008.02649.x. [DOI] [PubMed] [Google Scholar]
- 6.Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61–e111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basak S, Singh P, Rajurkar M. Multidrug resistant and extensively drug resistant bacteria: a study. J Pathog. 2016;2016:4065603. doi: 10.1155/2016/4065603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ko RE, Min KH, Hong SB, et al. Characteristics, management, and clinical outcomes of patients with hospital-acquired and ventilator-associated pneumonia: a multicenter cohort study in Korea. Tuberc Respir Dis (Seoul) 2021;84:317–325. doi: 10.4046/trd.2021.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weber DJ, Rutala WA, Sickbert-Bennett EE, Samsa GP, Brown V, Niederman MS. Microbiology of ventilator-associated pneumonia compared with that of hospital-acquired pneumonia. Infect Control Hosp Epidemiol. 2007;28:825–831. doi: 10.1086/518460. [DOI] [PubMed] [Google Scholar]
- 11.Lee HS, Moon J, Shin HR, et al. Pneumonia in hospitalized neurologic patients: trends in pathogen distribution and antibiotic susceptibility. Antimicrob Resist Infect Control. 2019;8:25. doi: 10.1186/s13756-019-0475-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung DR, Song JH, Kim SH, et al. High prevalence of multidrug-resistant nonfermenters in hospital-acquired pneumonia in Asia. Am J Respir Crit Care Med. 2011;184:1409–1417. doi: 10.1164/rccm.201102-0349OC. [DOI] [PubMed] [Google Scholar]
- 13.Sucher A, Whitehead S, Knutsen S. Updated IDSA/ATS guidelines on management of adults with HAP and VAP. US Pharm. 2017;42:12–21. [Google Scholar]
- 14.Juan CH, Fang SY, Chou CH, Tsai TY, Lin YT. Clinical characteristics of patients with pneumonia caused by Klebsiella pneumoniae in Taiwan and prevalence of antimicrobial-resistant and hypervirulent strains: a retrospective study. Antimicrob Resist Infect Control. 2020;9:4. doi: 10.1186/s13756-019-0660-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johansson N, Kalin M, Hedlund J. Clinical impact of combined viral and bacterial infection in patients with community-acquired pneumonia. Scand J Infect Dis. 2011;43:609–615. doi: 10.3109/00365548.2011.570785. [DOI] [PubMed] [Google Scholar]
- 16.Voiriot G, Visseaux B, Cohen J, et al. Viral-bacterial coinfection affects the presentation and alters the prognosis of severe community-acquired pneumonia. Crit Care. 2016;20:375. doi: 10.1186/s13054-016-1517-9. [DOI] [PMC free article] [PubMed] [Google Scholar]