Abstract
The slow growth of mycobacteria in conventional culture methods impedes the testing of chemicals for mycobactericidal activity. An assay based on expression of the green fluorescent protein (GFP) by mycobacteria was developed as a rapid alternative. Plasmid pBEN, containing the gene encoding a red-shifted, high-intensity GFP mutant, was incorporated into Mycobacterium terrae (ATCC 15755), and GFP expression was observed by epifluorescence microscopy. Mycobactericidal activity was assessed by separately exposing a suspension of M. terrae(pBEN) to several dilutions of test germicides based on 7.5% hydrogen peroxide, 2.4% alkaline glutaraldehyde, 10% acid glutaraldehyde, and 15.5% of a phenolic agent for contact times ranging from 10 to 20 min (22°C), followed by culture of the exposed cells in broth (Middlebrook 7H9) and measurement of fluorescence every 24 h. When the fluorescence was to be compared with CFU, the samples were plated on Middlebrook 7H11 agar and incubated for 4 weeks. No increase in fluorescence or CFU occurred in cultures in which the cells had been inactivated by the germicide concentrations tested. Where the test bacterium was exposed to ineffective levels of the germicides, fluorescence increased after a lag period of 1 to 7 days, corresponding to the level of bacterial inactivation. In untreated controls, fluorescence increased rapidly to reach a peak in 2 to 4 days. A good Pearson correlation coefficient (r ≥0.85) was observed between the intensity of fluorescence and the number of CFU. The GFP-based fluorescence assay reduced the turnaround time in the screening of chemical germicides for mycobactericidal activity to ≤7 days.
Mycobacteria are important human pathogens, and their significance is on the rise with increasing use of immunosuppressive treatment, the AIDS epidemic, and multidrug resistance (11, 18). In general, mycobacteria are also more resistant to chemical germicides than most bacteria, fungi, and viruses that cause nosocomial infections (12). Hence, a germicide with proven mycobactericidal activity is deemed to be effective against most other pathogens. In particular, chemical germicides to be used for between-patient disinfection of heat-sensitive medical devices such as flexible endoscopes must have demonstrated mycobactericidal activity (14).
Currently, germicides are tested by conventional culture of the test mycobacterium in a liquid or semisolid medium after its exposure to the test formulation. Depending on the species tested, it may take 4 to >8 weeks to obtain results (13).
The use of reporter genes has enormously benefited work with mycobacteria, particularly the slow-growing species. For example, the β-galactosidase (15) and luciferase (2) reporter systems are being used in high-throughput screening of antituberculosis drugs. The luciferase system has also been applied to the screening of mycobactericidal activity of chemicals (1).
In recent years, the gfp gene, encoding the green fluorescent protein (GFP), has become established as a convenient reporter of cell viability (16). The wild-type GFP of the jellyfish Aequorea victoria absorbs light with an excitation maximum of 395 nm and emits green light with an emission maximum of 509 nm (4, 9). Collins et al. (5) reported on the improved expression of a red-shifted, higher-intensity mutant GFP and its application in a microplate-based assay for screening antituberculosis drugs. To date, the GFP system has not been reported in the rapid assessment of mycobactericidal activity of liquid chemical germicides. In this study, we describe the development of a GFP-based assay for the rapid screening of chemical germicides for mycobactericidal activity using Mycobacterium terrae expressing a high-intensity mutant GFP.
MATERIALS AND METHODS
Test organisms and growth conditions.
A seed culture of M. terrae ATCC 15755 was obtained from the American Type Culture Collection and grown at 37°C for 3 weeks in 100 ml of Middlebrook 7H9 broth (Difco, Detroit, Mich.) supplemented with 10% albumin-dextrose-catalase enrichment (Difco) and 0.05% (vol/vol) Tween 80 (M-ADC-TW broth) in 75-cm2 cell culture flasks with a canted neck and a 0.2-μm vented plug cap (Sarstedt Inc., Newton, N.C.). Unless stated otherwise, all stock cultures of the transformed M. terrae(pBEN) were grown in the presence of kanamycin (30 μg/ml).
Plasmid transformation and selection.
Plasmid pBEN, containing a red-shifted, high-intensity mutant gfp (gfpmut3), was a gift from R. H. Valdivia, Stanford University School of Medicine, Palo Alto, Calif. The plasmid is identical to pFPV2 (17) except that the gfp used to make pBEN was gfpmut3 (6) instead of wild-type gfp. Briefly, pBEN was constructed by inserting a PCR-amplified fragment of gfpmut3 into the mycobacterial expression vector pMV261 as a BamHI-HindIII fragment (R. H. Valdivia, personal communication). In addition to gfp, pBEN contained the origin of replication for mycobacteria and Escherichia coli, the mycobacterial hsp60 promoter, and a kanamycin resistance gene.
The plasmid was electroporated into M. terrae in a Gene Pulsar (Bio-Rad, Richmond, Calif.) set at 2,500 V, 25 μF, and 1,000 Ω. Transformants were selected by culture in Middlebrook 7H11 agar (Difco) supplemented with 10% oleic acid-albumin-dextrose-catalase (OADC; Difco) and kanamycin (30 μg/ml).
Detection of GFP expression.
A loop of cells from a colony of transformed M. terrae was suspended in a drop of normal saline on a microscope slide and examined under an epifluorescence microscope (Leitz, Wetzlar, Germany) using a 100× oil immersion objective and excitation-emission filters in the fluorescein isothiocyanate range.
Preparation of bacterial suspensions.
Log-phase cultures of the transformed and parent M. terrae strains were centrifuged at 2,000 × g for 15 min using a horizontal rotor. The sediment was resuspended in sterile water and recentrifuged. The washing and centrifugation were repeated, and the final sediment was mixed with a small volume of sterile water and homogenized by vortexing in a Bijoux bottle (Wheaton, Milville, N.J.) containing 10 glass beads. This gave 108 to 109 CFU of viable organisms/ml of the suspension.
GFP fluorescence assay.
Ten-fold serial dilutions of suspensions (109 CFU/ml) of M. terrae(pBEN) and untransformed M. terrae (control) were separately pipetted into square disposable cuvettes (Bio-Rad, Hercules, Calif.) and round, screw-cap, clear glass vials (4 ml; 45.5 mm long by 15 mm wide; J. G. Finneran Assoc., Inc., Vineland, N.J.). Fluorescence was measured as relative fluorescence units (RFU) in a cuvette-type VersaFluor fluorometer (Bio-Rad) using a set of excitation (490/10 nm) and emission (520/10 nm) filters in the fluorescein isothiocyanate range and with the sensitivity set to medium gain. M. terrae(pBEN) was grown in M-ADC-TW broth with and without kanamycin (30 μg/ml) in screw-cap glass vials at 37°C, and fluorescence was measured every 24 h.
Test germicides.
Four commercially available liquid germicides were tested in this study. The use dilutions and contact times of the products tested were (i) 7.5% (undiluted), 3.75%, 2.5%, and 1.5% stabilized hydrogen peroxide for 10 min, (ii) 2.4% (undiluted), 0.8%, 0.48%, 0.27%, 0.14%, and 0.07% alkaline glutaraldehyde for 20 min, (iii) 10% (undiluted), 2%, 1.11%, and 0.59% acid glutaraldehyde for 20 min, and (iv) 0.31% (1:50), 0.155% (1:100), 0.0775% (1:200), and 0.0387% (1:400) of a phenolic agent for 10 min. The first three products claimed tuberculocidal activity when used undiluted. The phenolic agent claim tuberculocidal activity when diluted 1:200, or 0.0775% of the active ingredient. Dilutions of the test products were made with sterile water with a standard hardness of 200 PPM as CaCO3.
GFP germicidal assay.
One hundred microliters (107 to 108 CFU) of a suspension of the test mycobacterium was pipetted into a 50-ml conical centrifuge tube (Falcon; Becton Dickinson, Rutherford, N.J.) containing 1 ml of the germicide being tested. After the required length of exposure at 22 ± 2°C, the activity of the germicide was stopped by dilution with 50 ml of 0.85% saline followed by centrifugation at 2,000 × g for 15 min using a horizontal rotor. The supernatant was discarded, and the dilution and centrifugation steps were repeated a second time to wash away traces of the germicide. The sediment in each tube was resuspended in 9 ml of M-ADC-TW broth containing kanamycin (30 μg/ml) and dispensed equally into three screw-cap, clear glass vials (3 ml/vial). Controls were prepared by exposing the cells to 0.85% saline instead of the germicide and then proceeding in the same way as for the tests. Each glass vial containing test or control cells was vortexed for 5 s before being inserted in the cuvette holder of the VersaFluor fluorometer (Bio-Rad). RFU were measured every 24 h for at least 7 days, with incubation (37°C) and moderate shaking (100 cycles/min) in the interim period.
Comparison of RFU with CFU.
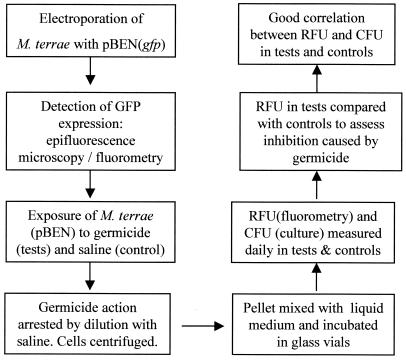
Each time RFU were measured, 100 μl of a corresponding sample was subjected to serial 10-fold dilutions, and each dilution was separately filtered through a 0.45-μm black-gridded, mixed-cellulose ester membrane (Millipore Corp., Bedford, Mass.). The membranes were placed on plates of 7H11 agar supplemented with OADC (Difco) and kanamycin (30 μg/ml) and incubated for 4 weeks at 37°C. CFU were recorded in control and test plates to determine the log10 CFU/ml as a measure of the viability of the test organism. The major steps of the GFP-based method are given in Fig. 1.
FIG. 1.
Flow chart showing the main steps in the evolution of the GFP-based method for screening chemical germicides for mycobactericidal activity.
Comparison of susceptibility.
To determine the effect of plasmid pBEN on the susceptibility of M. terrae to germicides, a culture-based quantitative carrier test was performed (3, 19). Briefly, 10 μl (106 to 107 CFU) of transformed and untransformed M. terrae was separately inoculated onto glass carriers and dried overnight in a vacuum desiccator. The dried inocula were exposed to sublethal concentrations of test germicides (3.75% hydrogen peroxide and 0.8% alkaline glutaraldehyde) to allow a proportion of the cells to survive. This was followed by dilution of the germicide-bacteria mixture with 0.85% saline, 10-fold serial dilutions of the eluate, and membrane filtration of the dilutions. The membranes were plated onto supplemented 7H11 agar and incubated for 4 weeks. Log10 reductions were calculated to compare the susceptibility of M. terrae with and without pBEN (log10 reduction = log10 CFU in controls − log10 CFU in tests). A higher log10 reduction is indicative of higher activity of the germicide and greater susceptibility of the tested mycobacteria.
Statistical analysis.
The fluorescence method was evaluated as an alternative to conventional culture to detect viable cells. In the experiments with hydrogen peroxide and alkaline glutaraldehyde, the means of three RFU and one CFU reading were used to determine the correlation between fluorescence and colony formation. The Pearson correlation coefficient (r value) was calculated using SigmaStat version 3.0 (Jandel, San Rafael, Calif.) on an IBM-compatible computer. Testing with the acid glutaraldehyde and the phenolic agent was performed twice.
RESULTS
Plasmid transformation and GFP expression.
GFP expression was evident in the transformed M. terrae, as the cells emitted green fluorescence under an epifluorescence microscope.
GFP fluorescence assay.
Tenfold serial dilutions of a suspension (109 CFU/ ml) of transformed M. terrae(pBEN) demonstrated a corresponding decrease in fluorescence from a maximum of 375 RFU at cell densities of 108 CFU/ml to 0 RFU at <104 CFU/ml. RFU readings were similar whether taken in disposable cuvettes or in 4-ml screw-cap glass vials. In broth culture containing kanamycin (30 μg/ml), M. terrae (pBEN) demonstrated a rapid increase in RFU from 10 to 50 to reach a peak of 300 to 350 RFU (Fig. 2 to 4) in 2 to 4 days, followed by a gradual decrease over time. In the absence of kanamycin, the fluorescence was at least 50 RFU less throughout the test period. Untransformed M. terrae exhibited low-level background fluorescence (0 to 14 RFU) irrespective of cell density, and there was no increase in the intensity of the fluorescence on culture.
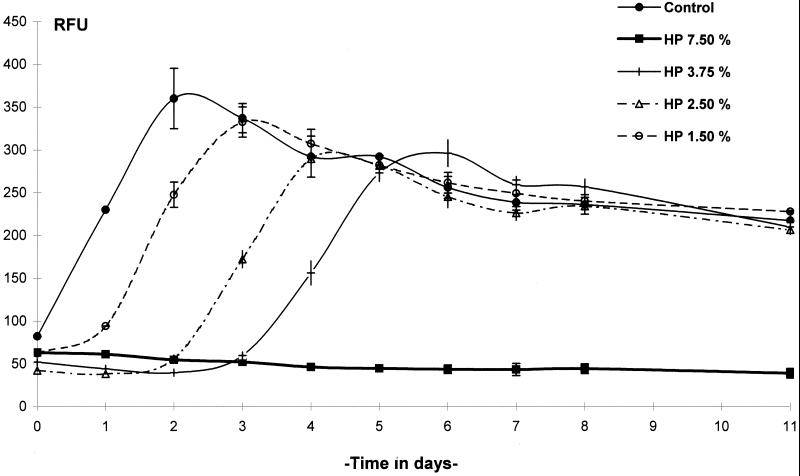
FIG. 2.
Kinetics of GFP expression by M. terrae(pBEN) after exposure to a hydrogen peroxide (HP)-based chemical germicide for 10 min. RFU were measured in controls and tests at 24-h intervals. Data are the means of three determinations ± standard deviation (SD).
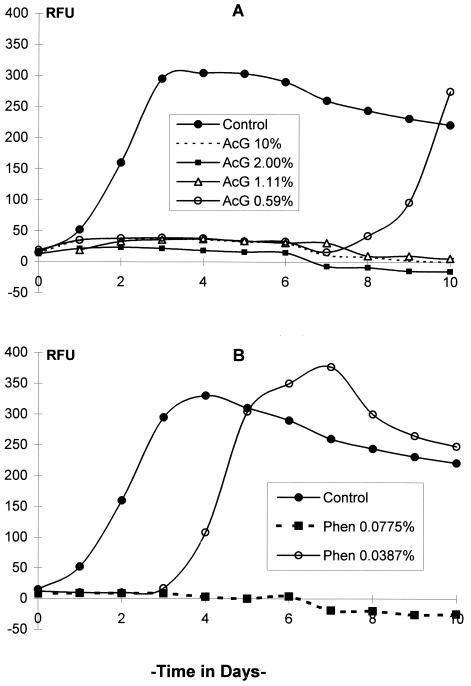
FIG. 4.
Kinetics of GFP expression by M. terrae(pBEN) after exposure to germicides based on (A) acid glutaraldehyde (AcG), and (B) a phenolic agent (Phen). Only one of three phenolic agent concentrations that caused total RFU inhibition is shown. Data are from one representative experiment of duplicate experiments.
GFP germicidal assay.
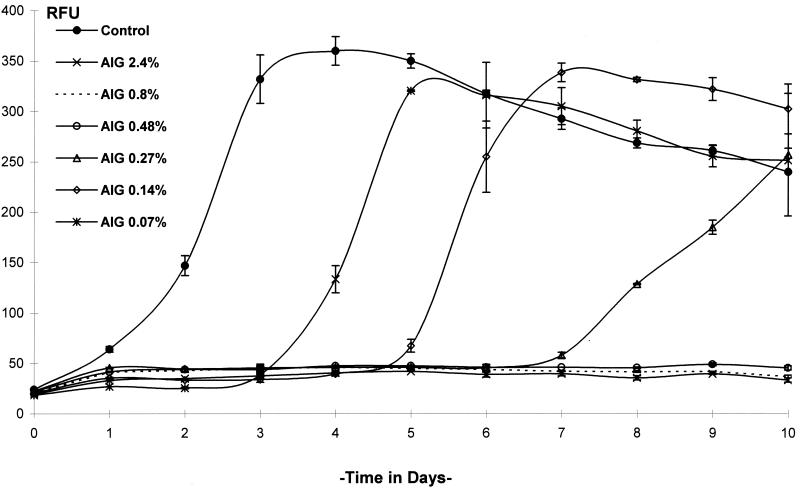
By daily measurement of RFU, changes in the levels of GFP expression in M. terrae(pBEN) in culture could be readily measured following exposure to the test germicides. Exposure to 7.5% hydrogen peroxide for 10 min was not followed by any increase in RFU, but a slight decrease in RFU occurred over time. Exposure to 1.50, 2.50, and 3.75% hydrogen peroxide allowed RFU to increase after a lag period of approximately 1, 2, and 3 days, respectively (Fig. 2). Exposure to alkaline glutaraldehyde at 0.07, 0.14, and 0.27% for 20 min was followed by a gradual increase in RFU after a lag period of 3, 5, and 7 days, respectively, whereas 0.48, 0.8, and 2.4% (undiluted) concentrations inhibited RFU completely (Fig. 3). Acid glutaraldehyde used at 1.11, 2, and 10% (undiluted) for 20 min completely inhibited RFU, and 0.59% allowed RFU to increase after 7 days (Fig. 4A). The phenolic agent tested with a contact time of 10 min at 0.0775% (1:200), 0.155% (1:100), and 0.31% (1:50) completely inhibited RFU, and 0.0387% (1:400) allowed RFU to increase from day 3 onward (Fig. 4B). In the untreated controls, RFU increased rapidly and reached a peak in 2 to 3 days, followed by a gradual fall (Fig. 2 to 4).
FIG. 3.
Kinetics of GFP expression by M. terrae(pBEN) after exposure to an alkaline glutaraldehyde (AIG)-based chemical germicide for 20 min. RFU were measured in controls and tests at 24-h intervals. Data are the means of three determinations ± SD.
Comparison of RFU and CFU.
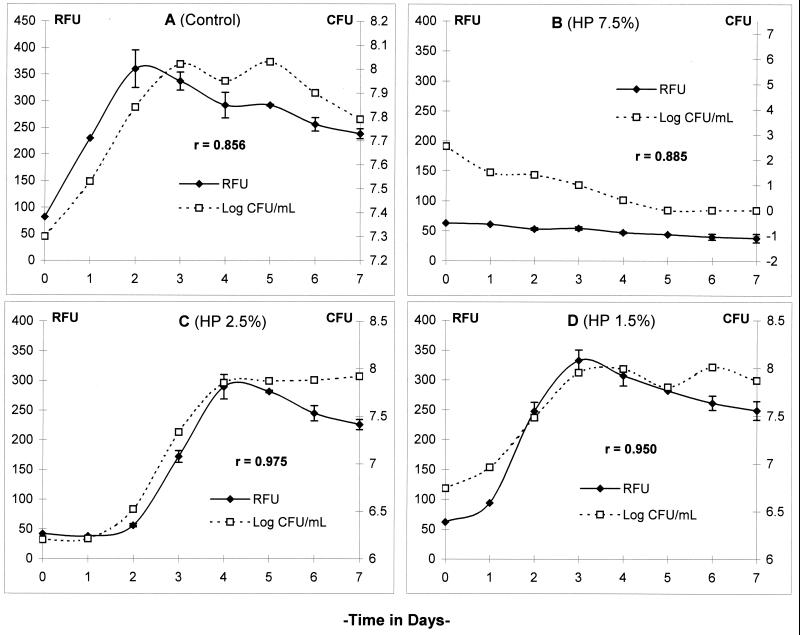
Hydrogen peroxide at 7.5% and concentrations of alkaline glutaraldehyde ranging from 0.48 to 2.4% decreased RFU below the baseline and CFU to undetectable levels. Lower concentrations of these germicides allowed RFU and CFU to increase after a lag period, which was directly proportional to the concentration of the test germicide. There was also good correspondence between RFU and CFU in the controls and all the tests (Pearson coefficient of correlation or r value of >0.85).
Comparison of susceptibility.
Following exposure to 3.75% hydrogen peroxide and 0.8% alkaline glutaraldehyde for 10 min, reductions in CFU were 0.91 and 0.59 log10 higher, respectively, in transformed M. terrae(pBEN) than in the parent strain. A higher log10 reduction is indicative of a slightly higher susceptibility of M. terrae(pBEN) to the test germicides.
DISCUSSION
Culture-based methods for testing antimycobacterial agents are generally slow, laborious, and costly, while many rapid methods currently available for this purpose have other disadvantages, such as reliance on radiochemicals. Methods reported for testing germicides, such as the direct epifluorescence filtration technique, flow cytometry, triphenyltetrazolium reduction, and microcalorimetry (8) either involve complicated protocols or are not amenable to high-throughput screening.
Methods based on reporter genes, such as β-galactosidase (15) and luciferase (2), are rapid, sensitive, and cheap and the results correlate well with those of culture-based methods. However, antibiotics which were screened by these methods had to be incubated with the recombinant organisms throughout the test period. This is not suited to testing germicides, since arresting the effects of the test germicide after the contact period is a fundamental requirement. Although chemical neutralization of germicides was used in a luciferase-based assay (1), the availability of a suitable neutralizer for all germicides, potential toxicity to the test organisms, and interference with the expression of the reporter gene are other factors to be considered.
The GFP system was found to meet several crucial requirements for rapid assessment of the mycobactericidal activity of germicides while circumventing many of the disadvantages inherent in most other rapid methods. Unlike the luciferase system, GFP does not require the addition of exogenous substrates or cofactors. Fluorescence was detected in live replicating cells, avoiding the need to permeabilize or lyse the thick waxy cell wall of mycobacteria (9). In contrast to exogenously applied fluorochromes, GFP is synthesized continuously in replicating cells (17), has a low toxicity, and resists photobleaching well when irradiated with 450- to 490-nm light (4). GFP-expressing cells are easily detected, imaged, and monitored repeatedly and in real time using standard fluorescence equipment such as an epifluorescence microscope, and the level of expression could be measured in a fluorometer or flow cytometer (9).
In this study, M. terrae was easily transformed by electroporation with the plasmid pBEN and, like its parent, formed readily detectable colonies in 10 days. Epifluorescence microscopy revealed a higher intensity of fluorescence emission from M. terrae(pBEN) expressing a mutant GFP (5, 6) than from M. terrae containing plasmid pWES4 (10), which expresses a wild-type gfp. Since M. terrae has a germicide susceptibility profile similar to that of Mycobacterium tuberculosis, it is considered a suitable surrogate for assessing mycobactericidal activity (7).
In the presence of kanamycin in the culture broth, M. terrae(pBEN) gave a higher level of fluorescence than in its absence. Furthermore, the presence of kanamycin helped maintain the plasmid for several months, compared to only 5 to 7 days in its absence. We regard the slight increase in the germicide susceptibility of the transformed M. terrae as desirable because its use in screening tests would not present the test products with a challenge any stronger than that with the parent strain. The exact basis for the differences in the germicide susceptibility of the transformant and the parent strain is not understood at this stage. However, the difference cannot be ascribed to an increase in the kanamycin sensitivity of the transformant by prior exposure to germicides, as it was shown to grow to the same extent in the presence and absence of kanamycin in germicidal tests (data not shown). The transformed strain was also somewhat more resistant to desiccation than its parent. This is a distinct advantage in carrier tests, in which the test organism must maintain a high-enough viability titer during the initial drying of the inoculum prior to challenge with the test germicide.
Screw-cap, clear glass vials made possible incubation of the bacterial culture without contamination or spillover while allowing accurate measurements of RFU and CFU of the same sample on a daily basis. The increase in RFU in the first 2 to 4 days of culture indicated the exponential increase followed by a gradual decrease over time resulting from depletion of nutrients. Readings of RFU and CFU throughout the growth cycle of M. terrae(pBEN) showed an excellent correlation. This allowed rapid assessment of the viability of slow-growing bacteria that would normally require weeks or months of incubation.
A culture-based quantitative carrier test was used to compare the susceptibilities of untransformed and transformed M. terrae, as it simulates the in-use practices of surface and equipment disinfection better than a suspension test (13, 19). However, as a preliminary screen for the germicides and for comparing the growth with fluorescence, a suspension test was considered adequate.
When a suspension of M. terrae(pBEN) was exposed to dilutions of test products based on hydrogen peroxide and alkaline glutaraldehyde, a proportion of the cells survived, as indicated by the presence of CFU in the sample. After a lag period of recovery, GFP expression, indicated by a gradual increase in RFU, resumed with the onset of replication. The higher the concentration of the test product, the longer was the lag period (Fig. 2 and 3). Effective concentrations (7.5% hydrogen peroxide and 2.4, 0.8, and 0.48% alkaline glutaraldehyde) that completely inactivated the cells were indicated by a decrease in RFU and a corresponding decrease in CFU to nondetectable values in 3 to 5 days (Fig. 5B). Based on the good correlation between RFU and CFU, products that kill a large proportion or all of the cells could be considered mycobactericidal at the end of 1 week of incubation. On the other hand, weaker germicides could be screened in less than a week because of the relatively rapid increase in RFU. This is significantly shorter than the 4 to 8 weeks or more required to test a product by the culture method. Fluorescence assays of products based on acid glutaraldehyde and a phenolic agent exhibited a similar pattern of results. In our experience, limitations of the GFP system include a lower limit of sensitivity (104 CFU/ml) and a slightly higher germicide susceptibility of the transformed strain compared to the parent strain, which could overestimate the potency of a product. While culture-based methods continue to be the standard for quantitative assessment of germicide activity, the GFP-based assay provides a convenient and rapid screening method for initial assessment of mycobactericidal activity.
FIG. 5.
Comparison of fluorescence (RFU) and growth (CFU) in M. terrae(pBEN) after exposure to hydrogen peroxide (HP)-based chemical germicide. RFU and CFU in control and tests were measured at 24-h intervals for 7 days. The r value (Pearson correlation coefficient) was >0.85 in all tests. Data are the means ± SD of three RFU and one CFU determination.
ACKNOWLEDGMENTS
We thank V.S. Springthorpe for her valuable advice on different aspects of this study. We also thank Maria Busa for her assistance in the experimental work.
REFERENCES
- 1.Andrew W P, Roberts I S. Construction of a bioluminescent mycobacterium and its use for assay of antimycobacterial agents. J Clin Microbiol. 1993;31:2251–2254. doi: 10.1128/jcm.31.9.2251-2254.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arain T M, Resconi A E, Hickey M J, Stover C K. Bioluminescence screening in vitro (Bio-Siv) assays for high-volume antimycobacterial drug discovery. Antimicrob Agents Chemother. 1996;40:1536–1541. doi: 10.1128/aac.40.6.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Best M, Springthorpe V S, Sattar S A. Feasibility of a combined test for disinfectants: studies with a mixture of five types of microorganisms. Am J Infect Control. 1994;22:152–162. doi: 10.1016/0196-6553(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 4.Chalfie M, Tu Y, Euskirchen G, Ward W W, Prasher D C. Green fluorescent protein as a marker for gene expression. Science. 1994;263:802–805. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- 5.Collins L A, Torrero M N, Franzblau S G. Green fluorescent protein reporter microplate assay for high-throughput screening of compounds against Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1998;42:344–347. doi: 10.1128/aac.42.2.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cormack B P, Valdivia R H, Falkow S. FACS-optimized mutants of the green fluorescent protein (GFP) Gene. 1996;173:33–38. doi: 10.1016/0378-1119(95)00685-0. [DOI] [PubMed] [Google Scholar]
- 7.Griffiths P A, Babb J R, Fraise A P. Mycobacterium terrae: a potential surrogate for Mycobacterium tuberculosis in a standard disinfectant test. J Hosp Infect. 1998;38:183–192. doi: 10.1016/s0195-6701(98)90273-0. [DOI] [PubMed] [Google Scholar]
- 8.Hugo W B, Russell A D. Evaluation of biocidal activity by rapid methods. In: Russell A D, Hugo W B, Ayliffe G A J, editors. Principles and practice of disinfection, preservation and sterilization. 3rd ed. Osney Mead, Oxford, U.K: Blackwell Science; 1999. pp. 145–148. [Google Scholar]
- 9.Kremer L, Baulard A, Estaquier J, Poulain-Godefroy O, Locht C. Green fluorescent protein as a new expression marker in mycobacteria. Mol Microbiol. 1995;17:913–922. doi: 10.1111/j.1365-2958.1995.mmi_17050913.x. [DOI] [PubMed] [Google Scholar]
- 10.Parker A E, Bermudez L E. Expression of the green fluorescent protein (GFP) in Mycobacterium avium as a tool to study the interaction between mycobacteria and host cells. Microb Pathog. 1997;22:193–198. doi: 10.1006/mpat.1996.0106. [DOI] [PubMed] [Google Scholar]
- 11.Parry C, Davis P D O. The resurgence of tuberculosis. In: Collins C H, Grange J M, Russell A D, Andrew P W, editors. Mycobacterial disease—old problems, new solutions. Oxford, UK, Society for Applied Bacteriology Symposium Series Number 25. J. Appl. Bacteriol. 81(Suppl.):23S–26S. 1996. [PubMed] [Google Scholar]
- 12.Sattar S A, Springthorpe S. Viricidal activity of biocides: activity against human viruses. In: Russell A D, Hugo W B, Ayliffe G A J, editors. Principles and practice of disinfection, preservation and sterilization. 3rd ed. Osney Mead, Oxford, U.K: Blackwell Science; 1999. pp. 168–186. [Google Scholar]
- 13.Sattar S A, Best M, Springthorpe V S, Sanani G. Mycobactericidal testing of disinfectants: an update. J Hosp Infect. 1995;30(Suppl.):372–382. doi: 10.1016/0195-6701(95)90041-1. [DOI] [PubMed] [Google Scholar]
- 14.Spach D H, Silverstein F E, Stamm W E. Transmission of infection by gastrointestinal endoscopy and bronchoscopy. Ann Intern Med. 1993;118:117–128. doi: 10.7326/0003-4819-118-2-199301150-00008. [DOI] [PubMed] [Google Scholar]
- 15.Srivastava R, Kumar D, Srivastava B S. Recombinant Mycobacterium aurum expressing Escherichia coli β-galactosidase in high throughput screening of antituberculosis drugs. Biochem Biophys Res Commun. 1997;240:536–539. doi: 10.1006/bbrc.1997.7689. [DOI] [PubMed] [Google Scholar]
- 16.Tsien R Y. The green fluorescent protein. Annu Rev Biochem. 1998;67:509–544. doi: 10.1146/annurev.biochem.67.1.509. [DOI] [PubMed] [Google Scholar]
- 17.Valdivia R H, Hromockyj A E, Monack D, Ramakrishnan L, Falkow S. Applications for green fluorescent protein (GFP) in the study of host-pathogen interactions. Gene. 1996;173:47–52. doi: 10.1016/0378-1119(95)00706-7. [DOI] [PubMed] [Google Scholar]
- 18.Wallace R J, Jr, Brown B A, Griffith D E. Nosocomial outbreaks/pseudo-outbreaks caused by non-tuberculous mycobacteria. Annu Rev Microbiol. 1998;52:453–490. doi: 10.1146/annurev.micro.52.1.453. [DOI] [PubMed] [Google Scholar]
- 19.Zafer A A. Mycobactericidal testing of chemical germicides: comparison of conventional culture with a reporter gene assay. M.Sc. thesis. Ottawa, Ontario, Canada: University of Ottawa; 1999. [Google Scholar]