Abstract
Background
Cancer-related pain is one of the primary symptoms of patients with hepatocellular carcinoma (HCC). Previous studies have shown that transcutaneous electrical acupoint stimulation (TEAS) is effective in treating patients with acute or chronic pain. In China, it is indispensable to evaluate the efficacy of TEAS in combination with opioids for the treatment of moderate to severe HCC-related pain.
Methods/Design
This is a single-center clinical, prospective randomized controlled clinical trial protocol. 104 patients will be randomly divided into the observation group and the control group in a ratio of 1:1.In addition to routine cancer pain medication, the two groups of patients will receive TEAS treatment twice a day for one week. Acupoints will include bilateral Hegu(LI4), Neiguan(PC6), Zusanli(ST36), Taichong(LR3), Ganshu(BL18), Geshu(BL17), Qimen(LR14), and Zhangmen(LR13). The treatment time is from 9:00 a.m. to 11:00 p.m. and from 4:00 p.m. to 6:00 p.m. The primary outcome measures are the Numerical Rating Scale (NRS) and the secondary outcome measures include the Brief Pain Inventory(BPI), dosage and administration duration of opioid drugs, frequency of nausea, vomiting and defecation, Karnofsky Performance Status Scale (KPS), Quality of life scale (QOL), Brief Fatigue Inventory (BFI). The outcome measures will be evaluated at baseline, during treatment and 1 week after treatment.
Discussion
Results of this trial are expected to clarify the value of TEAS stimulation performed on specific points in the management of moderate to severe pain in HCC.
Trial registration
Chinese clinical trial registry, ChiCTR2100044615 (http://www.chictr.org.cn), Registered on 24 March 2021.
Keywords: protocol, hepatocellular carcinoma, TEAS, pain
Introduction
Hepatocellular carcinoma(HCC) is a leading contributor to cancer-related death worldwide, according to the World Health Organization, HCC is responsible for over 800,000 new cases and an estimated 783,000 deaths in 2018.1 Pain is one of the clinical manifestations of HCC,2,3 whose incidence is 40% in patients with early or intermediate HCC, and even up to 90% in patients with advanced HCC.4 The factors attributes to liver cancer pain are numerous and complicated. Generally, excessive tumor growth in liver cancer induces increased pressure in the hepatic veins, stimulating the sympathetic nerves and causing persistent pain in the liver area. If cancer cells infiltrate or invade adjacent tissues, giving rise to inflammation, edema, ischemia and hypoxia in the surrounding tissues, they will also stimulate the nociceptors and trigger pain.5,6 In addition, cancerous consumption and insufficient dietary intake resulting in obstruction of tissue fluid reflux and hepatic ascites are also contributory to pain. Research has shown that,7 HCC-related pain will lead to decreased quality of life, reduced survival rate, and even treatment interruptions for survivors and heavy financial burden of family medical care. Therefore, any measures that can ameliorate pain are imperative.
Over the past few decades, WHO has attached great importance to cancer pain control and developed guidelines for pain management. The WHO analgesic ladder8 refers to a step-by-step approach in which the choice of analgesia depends on the severity of pain: as the degree of pain increases, so does the intensity of recommended analgesic. According to the guidelines, opioids are highly recommended for the management of moderate to severe cancer-related pain or breakthrough pain.9,10 However, opioids can induce immunosuppression and cause symptoms such as nausea, constipation and sleepiness.11 In addition, more than 80% of HCC patients simultaneously experience liver disease or cirrhosis, and opioids must be biotransformed in the liver. Therefore, an increased dosage of opioids may also trigger further impairment of liver function and even increase the incidence of hepatic encephalopathy.12 At present, the vast majority of cancer patients are not only tortured by cancer pain, but also by the side effects of opioids.13
A systematic review showed that acupuncture could significantly reduce cancer pain and analgesic administration.14 For cancer-related pain relief, acupuncture combined with drugs is more effective than taking drugs alone.15 Transcutaneous electrical acupoint stimulation(TEAS) is an electrical stimulation technology that delivers electrical pulses to acupoints through electrodes on the skin surface. Compared with traditional acupuncture, TEAS is not only easy to operate, but also noninvasive, does not increase the risk of infection, and has a high patient acceptance. Clinical evidence reveals that this approach can be an effective adjunct in the treatment of mild to moderate musculoskeletal or neuropathic pain.16 According to studies, TEAS exhibits analgesic effects similar to those of acupuncture in the treatment of postoperative pain in patients with gynecologic malignancies.17,18 Therefore, it is hypothesized that TEAS could exert certain effects in alleviating pain in HCC patients. To assess the action and possible benefits of TEAS in the treatment of HCC pain, a rigorous design and high-quality randomized controlled study are required.
Methods/Design
Study Design
This single-center clinical trial, prospective randomized controlled clinical trial, will be conducted at Yueyang Hospital of Integrated Traditional Chinese and Western Medicine. This trial has been approved by the ethics committee (No.2021–021) and has been registered in clinical trials. The government approval number is ChiCTR2100044615.The trial design is summarized in Figure 1.
Figure 1.
Flow chart of the study process.
Abbreviations: HCC, hepatocellular carcinoma; n, number.
Participant Recruitment
This trial will be conducted from November 2021 to November 2023. Patients will be recruited through the WeChat official account, newspaper publicity and posters. Participants and their families will be informed about the objective, character, and potential risks of the trial. Before enrollment, participants will voluntarily sign a consent form.
Diagnostic Criteria
Hepatocellular Carcinoma
This trial referred to the diagnostic criteria of hepatocellular carcinoma formulated by the Union for International Cancer Control (UICC):19 lesions > 1cm and AFP > 20 ng /mL on ultrasound; for multiphase CT and MRI, key imaging features include size≥1 cm and highly enhanced arterial phase. If those features are not evident, a liver biopsy should be performed.
Child Pugh
It is a scoring system20 that consists of bilirubin, albumin, INR, ascites and hepatic encephalopathy. Each parameter is scored on a scale of 1 to 3. An overall score of 6 or less on the scoring system is Grade A, 7 to 9 is Grade B, and 10 or more is Grade C.
Numerical Rating Scale (NRS)
NRS will be adopted to assess pain intensity.21 The NRS pain score ranges from 0 to 10, with 0 indicating no pain, 1–3 indicating mild pain, 4–6 indicating moderate pain, and 7–10 indicating severe pain.
Inclusion Criteria
Meeting diagnostic criteria.
NRS score≥4.
The Child Pugh grade is A or B.
Aged 50–80 years (either gender).
Patients with self-assessment.
Patients who can fully understand the study content and sign the informed consent form.
Exclusion Criteria
The Child Pugh grade is C.
Pain due to other physiological or pathological causes.
Patients who have undergone tumor resection in the past 3–6 months.
Patients with serious complications, such as refractory ascites, intraperitoneal infection, gastrointestinal bleeding, liver failure and intraperitoneal bleeding.
Serious acute or chronic organic diseases or psychiatric disorders.
Patients who are pregnant or nursing.
Patients with skin damage at local acupoints.
Patients who received percutaneous electrical stimulation of acupoint.
Patients who are scheduled for surgery within 14 days.
Randomization and Blinding
Patients eligible for recruitment will be randomly in a 1:1 ratio to either the observation group or the control group. The sequence of the random assignment will be generated with the SPSS 26.0 statistical package program by an independent statistician. This random allocation sequence will be inserted into sequentially numbered, opaque, sealed envelopes. In the sequence of the patient’s admission, the operator unpacks the envelopes. Group assignment results will be concealed from the outcome assessors, supervising doctors, data analysts, and participants. Since the TEAS operator must be trained to conduct the therapy, it is not confidential to them.
Interventions
This trial will be conducted in the inpatient cancer ward of the hospital. Both groups will be given routine treatment in the oncology department according to the Clinical Practice of Guideline in Oncology (2011 Edition), and each patient will be given routine cancer pain treatment according to the WHO three-step analgesic ladder. The first step is to control pain with non-steroidal anti-inflammatory drugs (NSAIDs). The second step will utilize opioids that are considered “weak”, such as codeine, while the third step 3 advocates the application of stronger opioids. Furthermore, patients in both groups will also receive TEAS treatment twice daily for one week. The treatment time will be from 9:00 a.m. to 11:00 p.m. and from 4:00 p.m. to 6:00 p.m.
Observation Group
Based on previous clinical experience, the acupoints involved in the protocol will include bilateral Hegu(LI4), Neiguan(PC6), Zusanli(ST36), Taichong(LR3), Ganshu(BL18), Geshu(BL17), Qi men(LR14), and Zhangmen(LR13) (Table 1). Before treatment, the operator will carefully disinfect the local skin of the patient’s acupoints, then paste the square-shaped electrode patch(3*3cm) on the acupoints, connect the electrode patch to HANS Acupuncture Point Nerve Stimulator (HANS-100, HuaweiCo., Ltd., Beijing, China), and finally turn on the power switch to stimulate acupoints. Each TEAS has two groups of electrode pieces. In the morning, the positive pole of the two groups of electrode patches is connected to LI4 and the negative pole is connected to LR3 on the same side. In the afternoon, the positive pole of the electrode patch is connected to LR14 and the negative pole is connected to BL18 on the same side. TEAS parameters will be set as follows: alternative frequency of 2 Hz and 100 Hz alternative (pulse width: 0.6ms/0.2ms), stimulation for 30 minutes; the current intensity will be 5–10 mA so that patients experience an obvious but tolerable sensation. The locations of acupoints chosen in this trial are displayed in Table 1.
Table 1.
Location and Indication of Acupoints for Moderating Severe HCC-Related Pain
| Acupoints | Location |
|---|---|
| Hegu (LI4) | On the dorsum of the hand, radial to the midpoint of the second metacarpal bone. |
| Neiguan (PC6) | Two inches above the transverse stria of the wrist, between the palmaris longus tendon and the radial wrist flexor tendon. |
| Zusanli (ST36) | On the anterior aspect of the leg, on the line connecting ST35 with ST41, 3 inches inferior to ST35. ST36 is located next to the anterior tibial muscle with a transverse finger (middle finger). |
| Taichong (LR3) | In the depression anterior to the junction of first and second metatarsal bones. |
| Ganshu (BL18) | Two transverse fingers were opened beside the lower edge of the spinous process of the 9th thoracic vertebra. |
| Geshu (BL17) | Two transverse fingers were opened beside the lower edge of the spinous process of the 7th thoracic vertebra. |
| Qimen (LR14) | Touch 2 costal spaces vertically downward from the nipple. |
| Zhangmen (LR13) | In the lateral abdomen, the lower border of the free end of the eleventh rib. |
Control Group
In the control group, the square-shaped electrode patches will be placed on the same acupoints as in the observation group, and each TEAS manipulation step will be identical to that of the high-intensity TEAS group, but the current intensity will be set to 0.5mA, so that the patients will only have weak sensation, thus exerting no biological effects. This electrical stimulation design using low-intensity current as a placebo has been proved in several studies to be scientific, which allows better implementation of the blind method.22,23
Baseline Assessments
Before randomization, a baseline assessment will be completed, which includes demographic information (gender, age, height, weight, occupation, education level), course of disease, cancer stage, Child Pugh grade, treatment received, degree of pain, analgesic use, quality of life, biochemical indicators, blood routine, liver and kidney function.
Outcome Measurement
Primary Outcome
The primary outcome will be assessed using the Numerical rating scale(NRS). NRS will be used to evaluate the patient’s current pain level at baseline, each day during treatment, and at the one- week of follow-up after treatment.
Secondary Outcome
Brief Pain Inventory(BPI): the most severe pain level, the least pain level, and the average pain level in BPI will be assessed.
Dosage and administration duration of opioid drugs.
Frequency of nausea, vomiting and defecation.
Karnofsky Performance Status Scale (KPS), Quality of life scale(QOL), and Brief Fatigue Inventory (BFI) will be used for comprehensive quality of life assessment.
Items 1 and 4 will be assessed at baseline, at the end of treatment. The evaluation time points of items 2 and 3 are the same as the primary outcome.
Safety Evaluation
Adverse events, including shock, local infection, intolerable tingling during tea treatment, and neurotoxicity from opioid overdose, will be recorded.
Quality Control
This trial will be conducted with careful reference to suggestions provided by experienced acupuncturists and oncologists. To assure the quality of this experiment, the operator will be an acupuncture clinician with at least three years of experience. All researchers will attend a series of training sessions to ensure a full understanding of the protocol and standard operating procedures. A clinical trial expert will also oversee the trial hospital and regular board meetings will be held to ensure the trial’s quality.
Sample Size
The sample size was estimated using the two proportions comparison method. To calculate the sample size based on data from the literature, the mean NRS score was decreased by 0.051 in the high-intensity observation group and increased by 0.585 in the control group. The combined standard deviation was 1.06. The sample size will be determined based on the equation below with α=0.05 (two-sided) and β=0.1 (90% power).
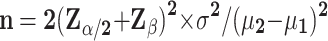 |
It was calculated that approximately 45 participants per group were required. Considering the potential case loss and attrition, the sample size was increased to 52 patients in each group in order to compensate for the 15% loss. Consequently, the sample size will be 104 patients in total.
Statistical Analysis
All data in this study will be analyzed by statisticians using SPSS26.0 and follow the intention-to-treat principle. Independent sample T-test and Chi-square test (χ2 test) will be applied for numerical variables and categorical variables, respectively. When the distribution of variables is abnormal, a non-parametric test will be selected. Thus, the resultant value of P<0.05 will be considered significant. Repeated measures analysis of variance (ANOVA) will be used to investigate the effect of treatment, time (before treatment, at the end of treatment and 1 week after treatment), and interaction conditions between treatment group and time. If differences between groups are identified, the Turkey test for multiple comparisons will be conducted.
Discussion
Traditional Chinese Medicine maintains that HCC-related pain mostly occurs in the progressive stage of the disease. At this time, the pathogenesis is complicated and different pathogenesis can interact with each other. Generally speaking, patients with HCC-related pain have the characteristics of deficiency in the disease root and excess in the disease manifestation, so acupoints that regulate qi and soothe the liver, promote blood circulation and relieve pain are mostly selected. It has been revealed that LI4, PC6, and ST36 are effective to alleviate pain;24,25 LR3, BL18, BL17, LR14, and LR13 have been employed in acupuncture clinical experiments to alleviate liver cancer pain with superior clinical effects.26
The National Comprehensive Cancer Network(NCCN) guidelines for adult cancer pain states that acupuncture-related therapies, such as TEAS, can play the same clinical role as conventional acupuncture while being non-invasive, simple and easy to operate.27 A multicenter randomized controlled trial involving 576 patients with breast cancer found that inadequate analgesia for acute intraoperative pain was strongly associated with the onset and severity of chronic postoperative pain, and the preoperative administration of TEAS significantly reduced the incidence of chronic pain after mastectomy, demonstrating the favorable acute pain relief effect of TEAS.28 Other studies have also proved that the combination of TEAS with morphine enhances the analgesic effect and reduces the side effects of morphine.29,30 Acupuncture analgesia is associated with the regulation of endogenous opioid release. Endorphins are mainly involved in acupuncture analgesia in the brain, dynorphins act in the spinal cord, and enkephalins are involved in analgesia in both the spinal cord and brain.31,32 The current frequency also affects the release of substances in the brain. Under the stimulation of a 2Hz current, the brain will produce enkephalin, endorphin, endorphin, serotonin, norepinephrine and other substances. Under the stimulation of 100Hz electroacupuncture, the brain will generate dynorphin.33 Studies have shown that TEAS, like electroacupuncture, can selectively release enkephalin and dynorphin into the cerebrospinal fluid through low frequency (2 Hz) and high frequency (100 Hz), respectively.34
Based on the above-mentioned studies, we designed this single-center, single-blind randomized controlled trial to verify the clinical role of TEAS in the control of moderate to severe HCC-related pain. In addition, several studies22 have indicated that 5mA current intensity is the threshold for perception of electrical stimulation in normal individuals, whereas cancer patients are in hyperalgesia. Therefore, the minimum current for the observation group will be set at 5mA. Considering the pain threshold varies from patient to patient, the current intensity of the instrument will be kept in the range of 5–10 mA. As for the control group, we referred to the current research design related to teas. Numerous studies have adopted non-electricity as the placebo control, but our preliminary experiments revealed that this approach still has the risk of breaking the blinding. Therefore, we will set the current intensity of the control group to 0.5 mA, which proved to be biological neutral and more conducive to the implementation of the blind method when the instrument is energized. However, there are some limitations in this study. Firstly, clinical trials conducted in the oncology ward are limited by the length of patients’ hospitalization. Therefore, the intervention time of this study is relatively short, and only short-term clinical analgesic effects of TEAS could be evaluated; Secondly, the evaluation of the results relies on the subjective sensory changes and quality of life scale without further measurement of endogenous opioids changes; Patients are recruited in a hospital with a single sample source, and there is a certain test deviation.
Funding Statement
This study is supported by Shanghai 2020 “Science and Technology Innovation Action Plan” (20Y21902800); Qihuang scholar in the National Support Program for Leading Talents of Traditional Chinese Medicine.
Trial Status
Participants are currently being recruited.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the study protocol.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries: global Cancer Statistics 2018. CA Cancer J Clin. 2020;70(4):313. [Google Scholar]
- 2.Neufeld NJ, Elnahal SM, Alvarez RH. Cancer pain: a review of epidemiology, clinical quality and value impact. Future Oncology. 2017;13(9):833–841. [DOI] [PubMed] [Google Scholar]
- 3.van den Beuken-van Everdingen M, de Rijke J. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol. 2007;18(9):1437–1449. [DOI] [PubMed] [Google Scholar]
- 4.Deandrea S, Corli O, Consonni D, et al. Prevalence of breakthrough cancer pain: a systematic review and a pooled analysis of published literature. J Pain Symptom Manag. 2014;47(1):57–76. [DOI] [PubMed] [Google Scholar]
- 5.Ibrahim NM, Abdelhameed KM, Kamal SMM, Khedr EMH, Kotb HIM. Effect of Transcranial Direct Current Stimulation of the Motor Cortex on Visceral Pain in Patients with Hepatocellular Carcinoma. Pain Med. 2018;19(3):550–560. [DOI] [PubMed] [Google Scholar]
- 6.Carr BI, Pujol L. Pain at presentation and survival in hepatocellular carcinoma. J Pain. 2010;11(10):988–993. [DOI] [PubMed] [Google Scholar]
- 7.Kaiser K, Mallick R, Butt Z, et al. Important and relevant symptoms including pain concerns in hepatocellular carcinoma (HCC): a patient interview study. Support Care Cancer. 2014;22(4):919–926. [DOI] [PubMed] [Google Scholar]
- 8.Vargas-Schaffer G. Is the WHO analgesic ladder still valid? Twenty-four years of experience. Can Fam Physician. 2010;56(6):514–e205. [PMC free article] [PubMed] [Google Scholar]
- 9.Scarborough BM, Smith CB. Optimal pain management for patients with cancer in the modern era. CA Cancer J Clin. 2018;68(3):182–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gharibo CG, Varlotta GP, Rhame EE, et al. Interlaminar versus transforaminal epidural steroids for the treatment of subacute lumbar radicular pain: a randomized, blinded, prospective outcome study. Pain Physician. 2011;14(6):499–511. [PubMed] [Google Scholar]
- 11.Wei G, Moss J, Yuan CS. Opioid-induced immunosuppression: is it centrally mediated or peripherally mediated? Biochem Pharmacol. 2003;65(11):1761–1766. [DOI] [PubMed] [Google Scholar]
- 12.Christian-Miller N, Frenette C. Hepatocellular cancer pain: impact and management challenges. J Hepatocell Carcinoma. 2018;5:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fallon M, Giusti R, Aielli F, et al. Management of cancer pain in adult patients: ESMO Clinical Practice Guidelines. Ann Oncol. 2018;29(Suppl 4):iv166–iv191. [DOI] [PubMed] [Google Scholar]
- 14.He Y, Guo X, May BH, et al. Clinical Evidence for Association of Acupuncture and Acupressure With Improved Cancer Pain: a Systematic Review and Meta-Analysis. JAMA Oncol. 2020;6(2):271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu C, Zhang H, Wu W, et al. Acupuncture for Pain Management in Cancer: a Systematic Review and Meta-Analysis. Evid Based Complement Alternat Med. 2016;2016:1720239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sykes J, Johnson R, Hanks GW. ABC of palliative care. Difficult pain problems. BMJ. 1997;315(7112):867–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meade CS, Lukas SE, McDonald LJ, et al. A randomized trial of transcutaneous electric acupoint stimulation as adjunctive treatment for opioid detoxification. J Subst Abuse Treat. 2010;38(1):12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cui H, Yu H, Huang X, et al. Electroacupuncture and Transcutaneous Electrical Nerve Stimulation Induced Sensations in Bell’s Palsy Patients: a Quantitative Current Intensity Analysis. Front Neurosci. 2021;15:692088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723–750. [DOI] [PubMed] [Google Scholar]
- 20.Tsoris A, Marlar CA. Use of the Child Pugh Score in Liver Disease. Treasure Island (FL): StatPearls Publishing; 2021. March 22. [PubMed] [Google Scholar]
- 21.Chauny JM, Paquet J, Lavigne G, et al. Evaluating acute pain intensity relief: challenges when using an 11-point numerical rating scale. Pain. 2016;157(2):355–360. [DOI] [PubMed] [Google Scholar]
- 22.Lambert C, Berlin I, Lee TL, et al. A standardized transcutaneous electric acupoint stimulation for relieving tobacco urges in dependent smokers. Evid Based Complement Alternat Med. 2011;2011:195714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J, Xie J, Pan Z, et al. Chronic fatigue syndrome treated with transcutaneous electrical acupoint stimulation: a randomized controlled trial. Zhongguo Zhen Jiu. 2017;37(12):1276–1279. [DOI] [PubMed] [Google Scholar]
- 24.Shen YF, Younger J, Goddard G, et al. Randomized clinical trial of acupuncture for myofascial pain of the jaw muscles. J Orofac Pain. 2009;23(4):353–359. [PMC free article] [PubMed] [Google Scholar]
- 25.Lee A, Fan LT. Stimulation of the wrist acupuncture point P6 for preventing postoperative nausea and vomiting. Cochrane Database Syst Rev. 2009;2:CD003281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu L, Wan Y, Huang J, et al. analysis of electroacupuncture and multiple acupoint stimulation in relieving cancer pain in patients with advanced hepatocellular carcinoma. J Cancer Res Ther. 2018;14(1):99–102. [DOI] [PubMed] [Google Scholar]
- 27.Sanft T, Denlinger CS, Armenian S, et al. NCCN Guidelines Insights: survivorship, Version 2.2019. J Natl Compr Canc Netw. 2019;17(7):784–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu Z, Wang Q, Sun X, et al. Transcutaneous electrical acupoint stimulation before surgery reduces chronic pain after mastectomy: a randomized clinical trial. J Clin Anesth. 2021;74:110453. [DOI] [PubMed] [Google Scholar]
- 29.Yuan CS, Attele AS, Dey L, et al. Transcutaneous electrical acupoint stimulation potentiates analgesic effect of morphine. J Clin Pharmacol. 2002;42(8):899–903. [DOI] [PubMed] [Google Scholar]
- 30.Chung YC, Tsou MY, Chen HH, et al. Integrative acupoint stimulation to alleviate postoperative pain and morphine-related side effects: a sham-controlled study. Int J Nurs Stud. 2014;51(3):370–378. [DOI] [PubMed] [Google Scholar]
- 31.Han JS. Acupuncture: neuropeptide release produced by electrical stimulation of different frequencies. Trends Neurosci. 2003;26(1):17–22. [DOI] [PubMed] [Google Scholar]
- 32.Han J, Cui C, Wu L. Acupuncture-related techniques for the treatment of opiate addiction: a case of translational medicine. Front Med. 2011;5(2):141–150. [DOI] [PubMed] [Google Scholar]
- 33.Zhao ZQ. Neural mechanism underlying acupuncture analgesia. Prog Neurobiol. 2008;85(4):355–375. [DOI] [PubMed] [Google Scholar]
- 34.Huang L, Pan Y, Chen S, et al. Prevention of propofol injection-related pain using pretreatment transcutaneous electrical acupoint stimulation. Turk J Med Sci. 2017;47(4):1267–1276. [DOI] [PubMed] [Google Scholar]



