Abstract
Background:
Maternal obesity is one of several key factors thought to modulate neonatal immune system development. Data from murine studies demonstrate worse outcomes in models of infection, autoimmunity, and allergic sensitization in offspring of obese dams. In humans, children born to obese mothers are at increased risk for asthma. These findings suggest a dysregulation of immune function in the children of obese mothers, however, the underlying mechanisms remain poorly understood. The aim of this study was to examine the relationship between maternal body weight and the human neonatal immune system.
Methods:
Umbilical cord blood samples were collected from infants born to lean, overweight, and obese mothers. Frequency and function of major innate and adaptive immune cell populations were quantified using flow cytometry and multiplex analysis of circulating factors.
Results:
Compared to babies born to lean mothers, babies of obese mothers had fewer eosinophils and CD4 T helper cells, reduced monocyte and dendritic cell responses to toll-like receptor ligands, and increased plasma levels of IFNα2 and IL-6 in cord blood.
Conclusion:
These results support the hypothesis that maternal obesity influences programming of the neonatal immune system, providing a potential link to increased incidence of chronic inflammatory diseases such as asthma and cardiovascular disease in the offspring.
Keywords: Asthma, cytokine, dendritic cells, maternal obesity, monocytes, T-cells, Toll-like receptor
Introduction
Almost 60% of women of childbearing age are overweight (body mass index (BMI) ≥25 kg/m2) or obese (36%, BMI ≥30 kg/m2)(1). Maternal obesity is associated with several adverse health outcomes for both mother and infant (2–5). Overweight and obese women are at increased risk of developing gestational diabetes (2, 4), pre-eclampsia (2, 4), and requiring cesarean delivery (2). Complications for the fetus include restricted growth (4), stillbirth (2, 4), and increased risk of congenital anomalies including neural tube defects (2, 4). Long-term risks for the offspring include type-2 diabetes (4), cardiovascular disease, (3) and significantly higher all-cause mortality (3).
Recent studies in murine models have shown dampened immune response to ovalbumin, increased serum IgE and pro-inflammatory cytokines such as TNFα, and worse outcomes in response to bacterial infection and experimentally induced autoimmunity in pups born to dams fed a high fat diet during gestation regardless of postnatal diet (6, 7). Human children born to obese mothers have elevated levels of circulating pro-inflammatory factor C-reactive protein (CRP) (8), and are at increased risk of developing asthma and wheezing (9, 10). These observations suggest dysregulation of the immune system of children born to obese mothers, and that these defects are acquired in utero. However, the impact of maternal obesity on neonatal immune homeostasis and function has not been rigorously examined. The goal of this study was to determine the impact of maternal BMI on the neonatal immune system by characterizing the frequency and function of major innate and adaptive immune cell populations and assessing the concentration of circulating cytokines, chemokines, and growth factors in cord blood samples collected from babies born to obese, overweight and lean mothers.
Methods
Subjects
All studies were approved by the Institutional Ethics Review Board of Oregon Health and Science University (OHSU), and the University of California Riverside. All subjects provided signed consent before study. A total of 39 non-smoking mothers without diabetes who had an uncomplicated, singleton gestation at term (>37 0/7 weeks) were enrolled: 11 lean mothers with a mean age of 31.5 ± 4.95 years and pre-pregnancy BMI of 22.27 ± 1.95 kg/m2; 14 mothers with a mean age of 31.5 ± 6.5 years and pre-pregnancy BMI of 27.3±1.42 kg/m2; and 14 with mean age of 29.6 ± 5.9 years and pre-pregnancy BMI of 37.5 ± 5.0 kg/m2. The racial distribution was: 30 white, 3 Asian-American/Pacific Islander, 1 American-Indian/Alaskan native, 2 African-American, and 3 unknown.
Umbilical cord blood mononuclear cell and plasma isolation
Umbilical cord blood (UCB) samples were collected into heparinized vacutainers during delivery and processed within twelve hours. Complete blood counts were obtained by Hemavet instrument (Hemavet, Dallas, TX). Umbilical cord blood mononuclear cells (UCBMC) and plasma were obtained by standard density gradient centrifugation over Ficoll (BD Bioscience, San Jose, CA). UCBMC were suspended in 10%DMSO/FBS, frozen using Mr. Frosty Freezing Containers (Thermo Scientific Waltham, MA) and stored in liquid nitrogen while plasma was stored at −80 until analysis.
Flow cytometric analysis of UCB immune cells
UCBMC (1–2×106 cells) were stained using CD4 (OKT4, eBioscience, San Diego, CA), CD8β (2ST8.5H7, Beckman Coulter, Brea, CA), CD95 (Dx2, Biolegend, San Diego, CA), CD28 (28.2, Biolegend) and CCR7 (G043H7, Biolegend) to delineate CD4 and CD8 T cells populations as follows: naïve (CD28+CD95−); central (CM; CD28+CD95+CCR7+), transitional (TEM; CD28+CD95+CCR7−) and effector memory (EM; CD28−CD95+CCR7−) ((11), Fig. 1A). UCBMC were also stained using CD20 (2H7, eBioscience), CD27 (O323, TONBO, Temecula, CA), and IgD (C4211, Southern Biotech, Birmingham, AL) to delineate: naïve (CD20+CD27-IgD+), marginal zone-like (MZ-Like; CD20+CD27+IgD+), and memory (CD20+CD27+IgD-) populations ((12), Fig. 1D). After surface staining, UCBMC were fixed, permeabilized and intracellularly stained with Ki67 (B56, BD Bioscience).
Figure 1: Impact of maternal BMI on umbilical cord blood T and B cell populations.
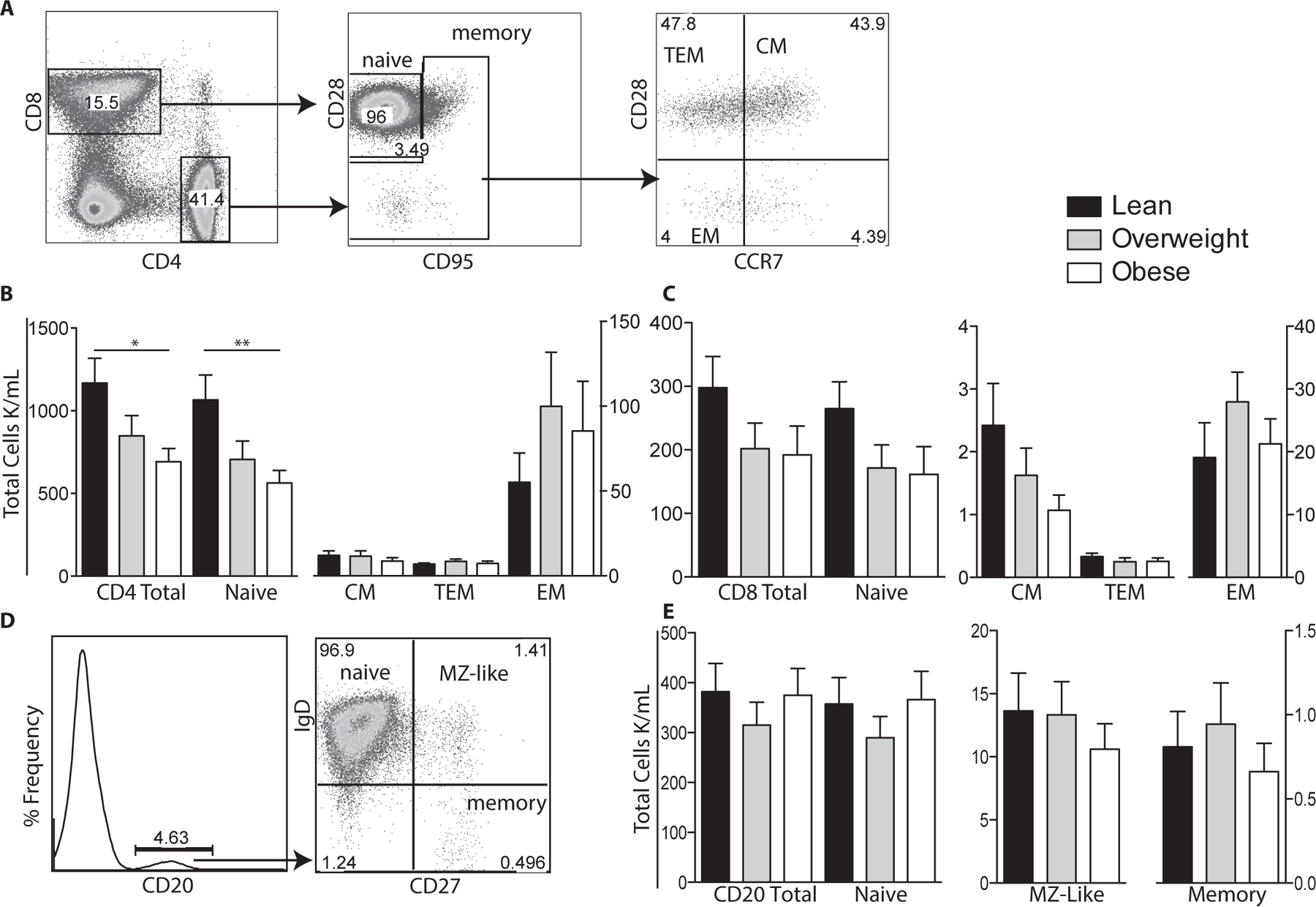
(A) Flow cytometry was used to delineate CD4 and CD8 T cells subsets: naïve, central (CM), effector (EM), and transitional effector memory (TEM) based on expression of CD28, CD95 and CCR7. (B) Numbers of total, naïve, CM, TEM and EM CD4 T cells per μl of cord blood were determined by multiplying subset frequency by the number of lymphocytes obtained by the hematology analyzer. (C) Number of total and CD8 T cell subsets were determined as described for CD4 T cells. (D) Flow cytometry was used to delineate total CD20+ B cells as well as naïve, marginal-zone like (MZ-like), and memory subsets. (E) Number of total CD20 B cells and subsets were determined as described for T cells. +/− SEM (n=11–14 UCB samples per group) *p≤0.05 **p≤0.01
A second tube of UCBMC (1–2×106 cells) was stained as follows: CD3 (UCHT1, ebioscience), CD20 (B9E9, Beckman Coulter), HLA-DR (LN3, Biolegend), CD14 (M5E2, Biolegend), CD11c (3.9, Biolegend), CD123 (6H6, Biolegend), CD56 (RPA-T8, BD Biosciences) and CD16 (3G8, Biolegend) to delineate monocytes (CD3−CD20−HLA-DR+/−CD14+) (Fig. 2A), myeloid dendritic cells (mDC; CD3−CD20−CD14−HLA−DR+CD11c+); plasmacytoid dendritic cells (pDC; CD3−CD20− CD14−HLA-DR+CD123+), and natural killer cells (NK; CD3−CD20−56bright/dim) (Fig. 2F). Monocytes were further divided into classical and non-classical based on CD16 expression, and natural killer cells were subdivided by both CD56 bright/dim expression and CD16 into four distinct subsets (Fig. 2A, F). All flow cytometry samples were acquired with LSRII instrument (Becton Dickinson, Franklin Lanes, NJ) and analyzed using FlowJo (TreeStar, Ashland, OR).
Figure 2: T cell stimulation of umbilical cord blood mononuclear cells.
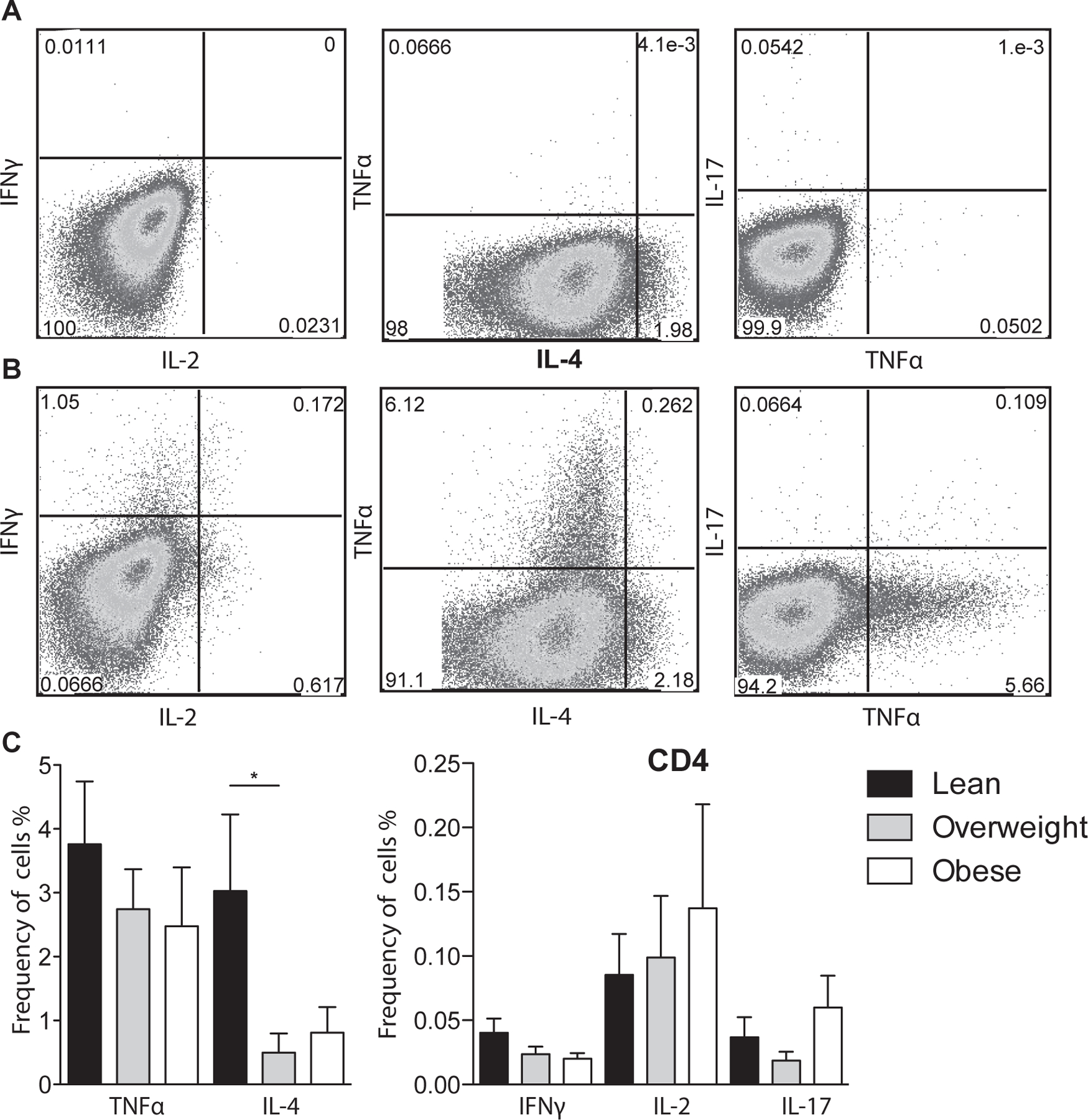
(A-B) Representative umbilical cord blood CD4+ T cell response (A) before stimulation and (B) following anti-CD3/CD28 co-stimulation determined by flow cytometry. Cells were first stained with antibodies against CD4 and CD8, permeabilized, and intracellularly stained to detect TNFa, IL-4, IFNγ, IL-2, and IL-17. (C) Average frequency of CD4 T cells producing TNFα, IL-4, IFNγ, IL-2 and IL-17 after adjusting for background using unstimulated controls. +/− SEM (n=11–14 UCB samples per group) *p≤0.05.
In vitro UCBMC stimulation
For analysis of T cell cytokine production, 1–2×106 UCBMC were stimulated for 24 hours at 37°C in supplemented RPMI (10% FBS) in the presence or absence of anti-CD3/CD28 (OKT3, TONBO; CD28.2, BD Biosciences); Brefeldin A (Sigma, St. Louis, MO) was added after 1-hour incubation. Cells were stained for CD4 and CD8, fixed, permeabilized and stained intracellularly for TNFα (MAb11, ebioscience), IFNγ (4S.B3, eBioscience), IL-4 (Biolegend), IL-2 (MQ1–17H12, Biolegend), and IL-17a (BL168, Biolegend).
To measure cytokine production by monocytes and dendritic cells (DC), a second tube of UCBMC was cultured for 14 hours at 37°C in RPMI supplemented (10%FBS) alone or in the presence of heat killed Listeria monocytogenes (HKLM, TLR1 agonist) and synthetic triacylated lipoprotein (PAM3CSK4, TLR2 agonist), or lipopolysaccharide (LPS, TLR4 agonist) (Invivogen, San Diego, CA). Brefeldin A (Sigma) was added after 1-hour incubation. Cells were stained with CD3 (ebiosciences), CD20 (Bioloegend), HLA-DR (L243, Biolegend), CD14 (Biolegend), CD11c (Biolegend), and CD123 (Biolegend), fixed, permeabilized and stained intracellularly for IL-6 (MQ2–13A5, eBioscience), and TNFα (ebioscience). All samples were acquired and analyzed as described above.
Cytokine, chemokine, and growth factor analysis
Plasma samples were analyzed using human multiplex panels (Millipore, Temecula, CA) per the manufacturer’s instructions in duplicates using MAGPIX multiplexing platform (Luminex, Austin, TX). Values below the limit of detection were designated as not detected.
Statistical analysis
All data sets were first assessed for normal distribution. Normal data sets were then tested for variance homogeneity. Data sets not displaying homogeneity were subjected to weighted one-way analysis of variance (ANOVA). If group differences were observed, Dunnet posttest correction was applied. If data sets displayed homogeneity, they were subject to unweighted one-way ANOVA and Dunnet posttest if differences were present. Data sets that were not normally distributed, alternative transformations to normality were explored. When a suitable transformation could be found, the analysis strategy discussed above was followed. Data sets that could not be transformed were subjected to Kruskall-Wallis one-way ANOVA. If group differences were observed, a customized Dunnet posttest was applied.
Results
Maternal obesity and fetal complete blood counts
We first compared complete blood counts (CBCs; lymphocytes, monocytes, eosinophils, neutrophils, and basophils) between the three groups (Table 1). A trend towards reduced white blood cell was observed in UCB from babies born to overweight and obese compared to lean mothers. This difference was due to a significant reduction in eosinophil counts and a slight reduction in basophils as no changes in total lymphocytes, neutrophil, or monocyte counts between the three groups were observed.
Table 1:
Complete umbilical cord blood counts
| BMI Category | |||
|---|---|---|---|
| Lean | Overweight | Obese | |
| White Blood Cell (cells x 103/mL) | 16065 ± 1719 | 12497 ± 1240 | 13530 ± 996 |
| Neutrophil (cells x 103/mL) | 3281 ± 1158 | 3764 ± 1065 | 4231 ± 668 |
| Lymphocytes (cells x 103/mL) | 2470 ± 180 | 2424 ± 317 | 2506 ± 228 |
| Monocytes (cells x 103/mL) | 2011 ± 213 | 2078 ± 311 | 2124 ± 301 |
| Eosinophils (cells x 103/mL) | 7336 ± 1141 | 3852 ± 611* | 4472 ± 708* |
| Basophils (cells x 103/mL) | 514 ± 75 | 324 ± 45.1 | 292 ± 34 |
P<0.05 vs Lean.
Maternal obesity and fetal lymphocyte populations
We next investigated the impact of maternal obesity on CD4+, CD8+, and B cell subset (total, naïve and memory) frequencies in UCBMC samples using flow cytometry (Fig. 1). Analysis showed significantly lower total and naïve CD4 T cell counts in babies born to obese mothers compared to those born to lean mothers (Fig. 1B). Although there were no significant changes in CD4 CM and EM frequencies, a trend of increased CD4 EM cells occurred with increasing maternal BMI was noted (Fig. 1B). We also observed a trend towards lower total and naïve CD8 T cell counts but these changes were also not significant (Fig. 1C). In contrast to T cells, we did not detect any differences in the total or sub-populations of B cells between the three groups (Fig. 1E). Finally, we did not detect differences in the rate of homeostatic proliferation within CD4+, CD8+ or CD20+ subsets (data not shown).
To further investigate changes in T cell populations, we compared TNFα, IFNγ, IL-2 (Th1), IL-4 (Th2), and IL-17 (Th17) production following stimulation of UCBMC with anti-CD3 and anti-CD28 (Fig. 2A and B). We detected no differences in CD4+ (Fig. 2C) or CD8+ (data not shown) TNFα, IFNγ, and IL-2 production. In contrast, there was a significant decrease in CD4+ IL-4 production in the overweight group, a trend of decreased CD4+ IL-4 production and increased IL-17 production in the obese group.
Maternal obesity and fetal innate immune cell subsets
We next investigated the impact of maternal BMI on the frequency of total as well as subsets of monocytes, DCs and Natural Killer cells (Fig. 3A, F). No significant differences in total monocytes, DCs and natural killer cells (Fig. 3B–C,G) or their sub-populations (Fig. 3D–E, H) were observed between all three groups, with the exception of pDCs, which were significantly increased in babies born to obese mothers (Fig. 2E).
Figure 3: Impact of maternal obesity on circulating innate immune cells.
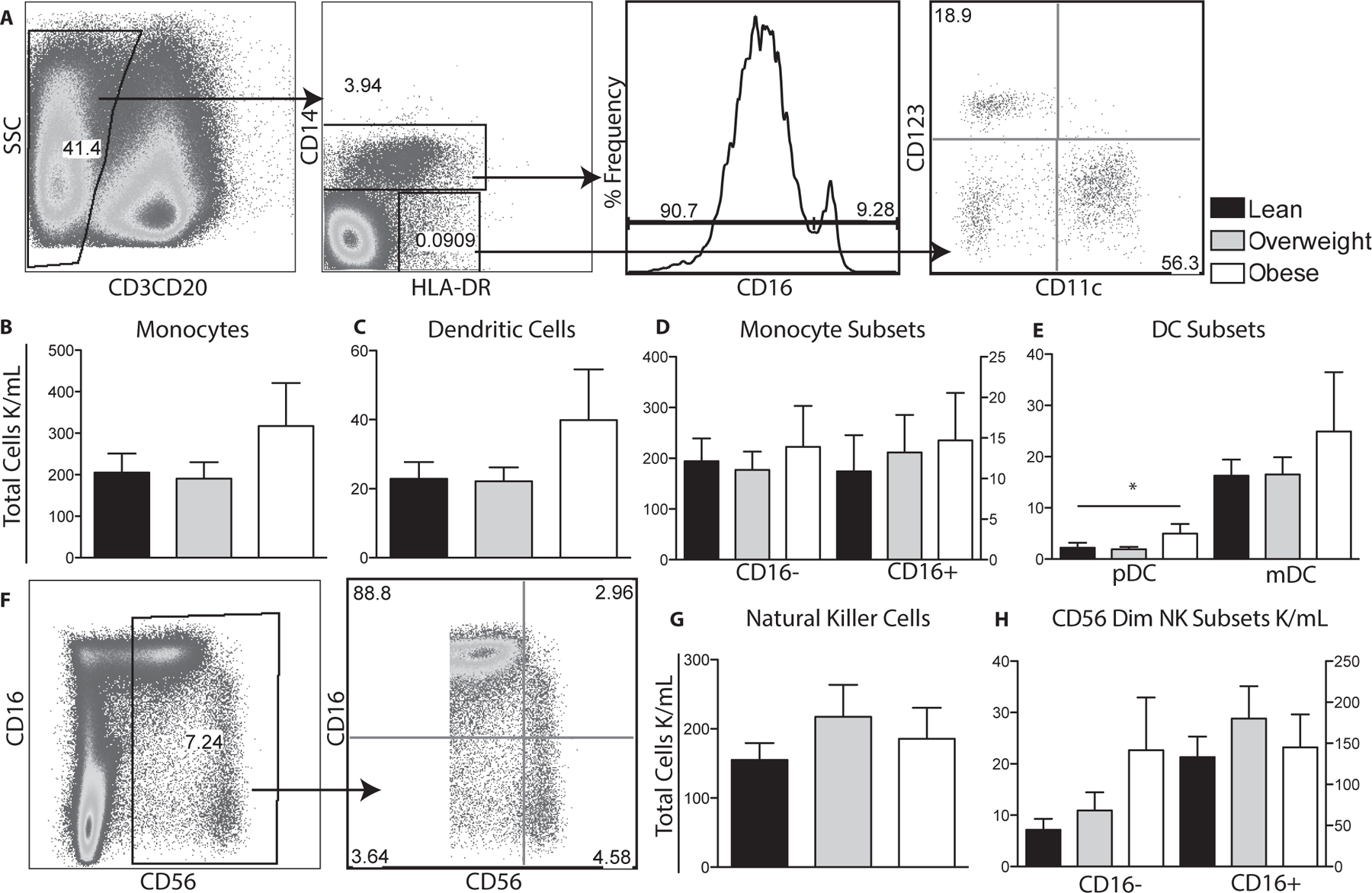
(A) Flow cytometry was used to delineate innate cell populations by first gating out CD3 and CD20 lymphocytes and then using CD14 and HLA-DR to define monocytes and dendritic cells. Monocyte subsets were further delineated by CD16 expression and DC subsets by CD11c and CD123 expression. (B-E) Total number of monocytes, dendritic cells and their subsets per μl of cord blood were determined by described above for T cells. (F) Flow cytometry was used to delineate total natural killer cells by gating on CD3 and CD20 negative cells and then based on CD56 and CD16 expression. (G, H) Numbers of total and natural killer subsets were determined as described for monocytes. +/− SEM (n=11–14 UCB samples per group) *p≤0.05
We then compared the ability of monocytes and DCs in UCBMC to respond to TLR agonists PAM3CSK4/HKLM (TLR1&2) and LPS (TLR4) (Fig. 4A and B). Our analysis revealed significant reduction in IL6 and TNFα production by monocytes in UCBMC of babies born to overweight and obese mothers in response to PAM3CSK4/ HKLM (Fig. 4C) and LPS (Fig. 4D). We also saw decreased production of IL-6 and TNFα by mDCs in the overweight babies in response to PAM3CSK4 & HKLM (Fig. 4E) and decreased TNFα in the obese babies in response to LPS (Fig. 4F) No significant changes in the response of pDCs (Fig. 4G–H) to agonists were detected.
Figure 4: Toll-like receptor stimulation of UCBMC.
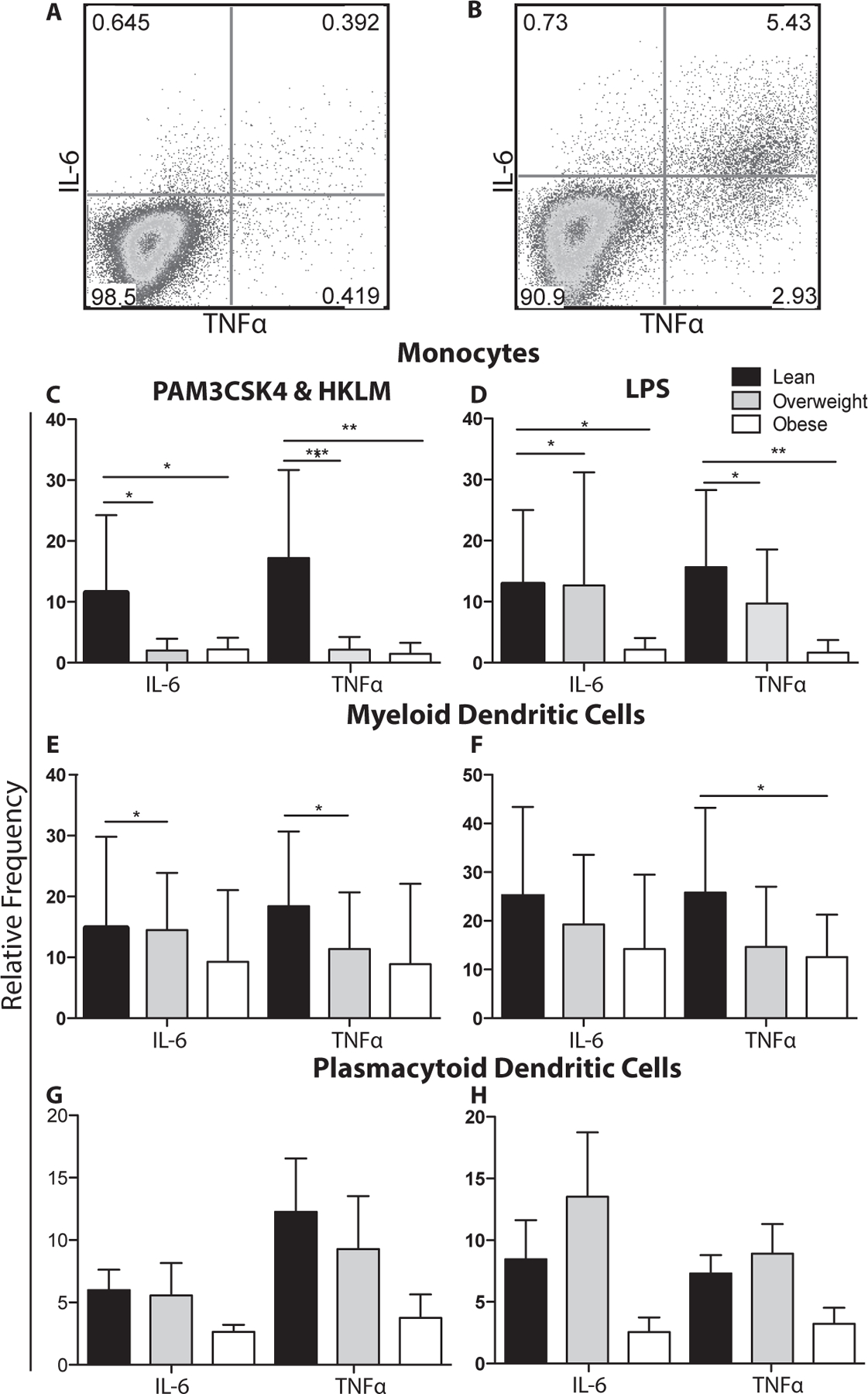
(A-B) Representative image of monocytes from UCBMC (A) before stimulation and (B) following LPS stimulation. UCBMC were stimulated with PAM3CSK4&HKLM (A,C,E) or LPS (B,D,E). Cells were then stained to define monocytes (A,B), mDCs (C,D), or pDCs (E,F) followed by intracellular staining for IL-6 and TNFα. The mean frequency of stimulation was adjusted for background using unstimulated controls.+/− SEM (n=11–14 UCB samples per group)* p ≤ 0.05. **p≤0.01 ***p≤0.001
Maternal obesity and fetal blood levels of circulating factors
Finally, we compared circulating levels of cytokines (IFNα2, IFNγ, TNFα, IL-1Ra, IL-1a, IL-1b, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IP-10, IL-12p40, IL12p70, IL-13, IL-15, Flt-3L), chemokines (MCP-1, MIP-1a, MIP-1b, GRO, MCP-3, MDC, eotaxin, and fractalkine), adipokines (insulin, leptin, total PYY, adiponectin, lipocalin-2/NGAL, adipsin, resistin, total PAI-1), and growth factors (VEGF, EGF, FGF-2.2, TGF-a, G-CSF, GM-CSF) in the UCB plasma between the three groups (Fig. 5).
Figure 5: Circulating levels of hormones, chemokines, and cytokines UCB plasma.
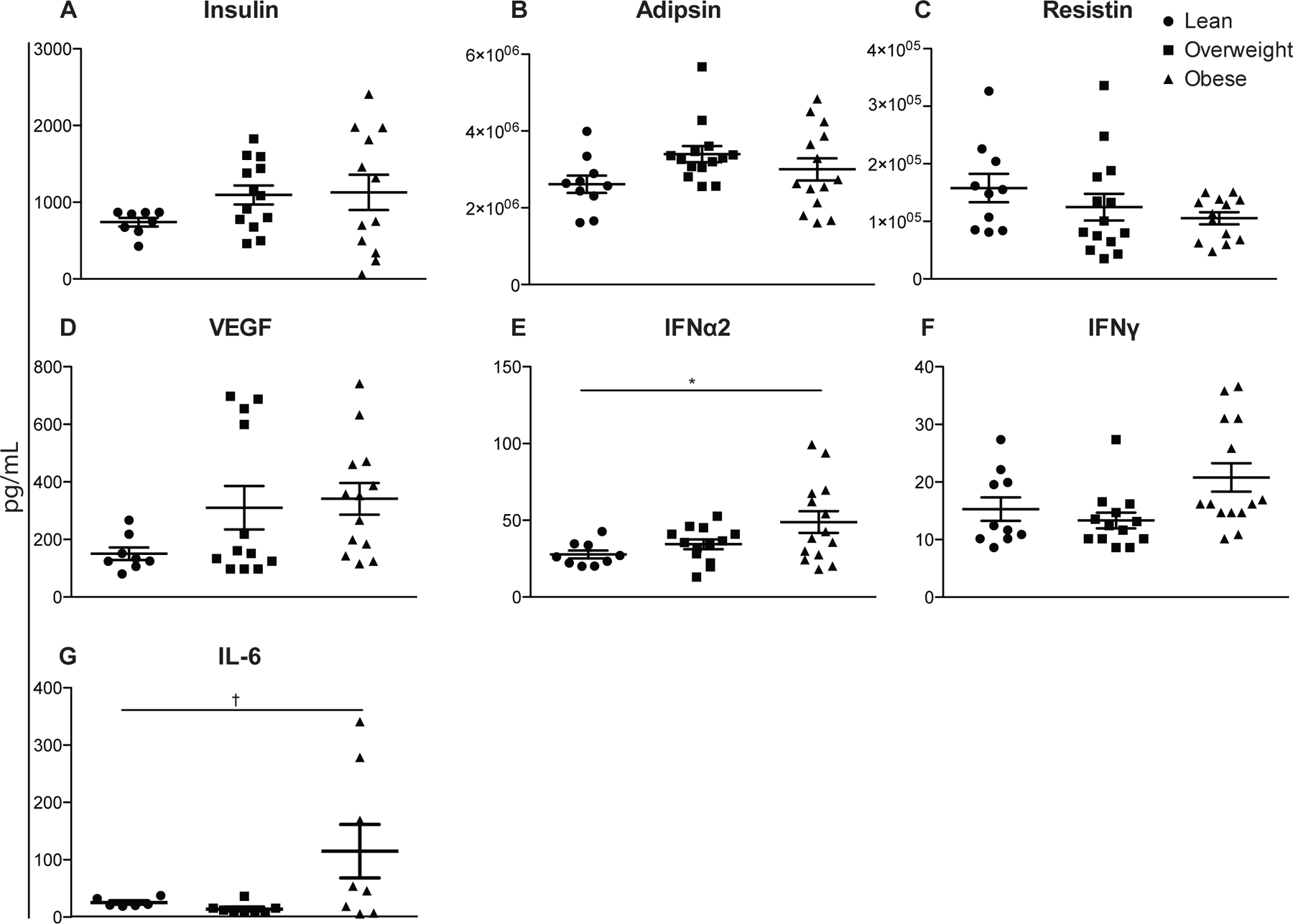
Plasma protein levels were determined by cytometric bead assay using human multiplex. (n=11–14 UCB samples per group) *p≤0.05. †p≤0.10
Although only IFNα2 and IL-6 levels were statistically increased in UBC plasma of babies born to obese mothers, there were several notable trends. We saw small increases with maternal BMI in insulin (Fig. 5A), adipsin (Fig. 5B), VEGF (Fig. 5D) and IFNγ (Fig. 5F) as well as a small decrease in resistin (Fig. 5C) in UCB plasma levels. Analytes IL-13, IL-15, IL-17a, IL-1a, IL-2, IL-3, IL-5, TNFβ were below detection and while RANTES and sCD40L were above detection, we did not see differences in the remaining analytes.
Discussion
In this study, we investigated differences in the frequency and function of key innate and adaptive immune cells in UCB samples collected from babies born to lean, overweight, and obese mothers to understand the impact of maternal obesity on the development and maturation of the human immune system. Numbers of eosinophils were significantly reduced in UCB of babies born to obese mothers. This observation was unexpected given the role that these cells play in allergic response and asthma pathogenesis and the increased incidence of these diseases in children born to obese mothers (9, 10). One potential explanation is that eosinophils and basophils might already be recruited to peripheral tissues such as the lungs where they have been implicated in airway remodeling and airway hyper-responsiveness (13, 14).
We also report a significant reduction in total and naïve CD4 T cells numbers in UCB collected from babies born to obese mothers. These observations differ from those made in obese adults where total CD4+ counts were increased due to increased homeostatic T cell proliferation (15). The decreased numbers of circulating CD4 T cells could be indicative of dysregulated thymic output or increased peripheral CD4 T cell death and future studies will address this question. Given the important role of CD4 helper T cells, this loss might compromise responses to infection and vaccination in children born to obese mothers. Future studies will aim at evaluating the incidence of childhood diseases and response to vaccination in infants born to obese mothers. Numbers of total EM CD4 T cells were slightly increased in babies born to obese mothers, suggesting accelerated differentiation.
Changes in CD4 T subset frequency were accompanied by a significant decrease in IL-4 production in babies born to overweight mothers. Decreases in IL-4 production could be linked to the increased plasma levels of IFNα2 and IFNγ observed in the UCB plasma collected from babies born to obese mothers since IFNα2 and IFNγ are known to repress IL-4 expression (16, 17) We also detected significantly higher numbers of pDCs, which could also be linked to the increased plasma levels of IFNα2. In addition to their well-understood role in anti-viral responses, pDCs are thought to play a role in allergic asthma (18). However, these observations need to be validated in a larger cohort study.
Another major observation of our study is decreased TNFα and IL-6 production by monocytes and mDCs in response to TLR1&2 and TLR4 agonists. These observations are consistent with previous studies in adults that showed reduced responses to antigen stimulation with increasing BMI (19). Given the critical role of innate immune responses in the activation of adaptive immunity, these observations are predictive of poor responses to vaccination and infection in infants born to obese mothers. Future studies will investigate the mechanisms for reduced responses to TLR stimulation, including changes in expression of TLR and/or signaling components, and epigenetic modification of cytokine loci.
We saw small increases in UCB plasma insulin and adipsin. Higher insulin resistance in children is correlated with increased childhood diabetes, and allergic asthma (20, 21). There was also a small decrease in resistin which is thought to play a protective role in asthma (22). Finally, we also saw slight increases in VEGF which has been implicated in promoting airway remodeling and airway hyper-responsiveness (23).
In summary, our data suggest a dysregulation of immune homeostasis and function at birth in babies born to obese mothers. This is in agreement with the Barker hypothesis that adverse conditions during critical development periods in utero lead to lifelong changes in body composition and physiology during adulthood. Our data also provide a potential explanation for the increased incidence of asthma observed later in life in children born to obese mothers, and predict compromised immune responses to vaccination and/or infection. Further studies in a larger cohort are needed to extend these observations and uncover the molecular mechanisms underlying these changes and the implication for responses to vaccination and infection.
Acknowledgments
This work was supported by NIH grants KL2TR000152 (Marshall) and R03AI112808 (Messaoudi) and the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR000128. We also would like to thank Ms. Flora Engelmann and Dr. Christine Meyer for their help with sample processing.
References
- 1.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of Obesity and Trends in the Distribution of Body Mass Index Among US Adults, 1999–2010. Jama-J Am Med Assoc 2012; 307: 491–97. [DOI] [PubMed] [Google Scholar]
- 2.Ramachenderan J, Bradford J, McLean M. Maternal obesity and pregnancy complications: a review. The Australian & New Zealand journal of obstetrics & gynaecology 2008; 48: 228–35. [DOI] [PubMed] [Google Scholar]
- 3.Reynolds RM, Allan KM, Raja EA, et al. Maternal obesity during pregnancy and premature mortality from cardiovascular event in adult offspring: follow-up of 1 323 275 person years. Bmj 2013; 347: f4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leddy MA, Power ML, Schulkin J. The impact of maternal obesity on maternal and fetal health. Reviews in obstetrics and gynecology 2008; 1: 170–8. [PMC free article] [PubMed] [Google Scholar]
- 5.Gaillard R, Durmus B, Hofman A, Mackenbach JP, Steegers EA, Jaddoe VW. Risk factors and outcomes of maternal obesity and excessive weight gain during pregnancy. Obesity 2013; 21: 1046–55. [DOI] [PubMed] [Google Scholar]
- 6.Odaka Y, Nakano M, Tanaka T, et al. The influence of a high-fat dietary environment in the fetal period on postnatal metabolic and immune function. Obesity 2010; 18: 1688–94. [DOI] [PubMed] [Google Scholar]
- 7.Myles IA, Fontecilla NM, Janelsins BM, Vithayathil PJ, Segre JA, Datta SK. Parental dietary fat intake alters offspring microbiome and immunity. Journal of immunology 2013; 191: 3200–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leibowitz KL, Moore RH, Ahima RS, et al. Maternal obesity associated with inflammation in their children. World J Pediatr 2012; 8: 76–79. [DOI] [PubMed] [Google Scholar]
- 9.Forno E, Young OM, Kumar R, Simhan H, Celedon JC. Maternal obesity in pregnancy, gestational weight gain, and risk of childhood asthma. Pediatrics 2014; 134: e535–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Reilly JR, Reynolds RM. The risk of maternal obesity to the long-term health of the offspring. Clinical endocrinology 2013; 78: 9–16. [DOI] [PubMed] [Google Scholar]
- 11.Fagnoni FF, Lozza L, Zibera C, et al. T-cell dynamics after high-dose chemotherapy in adults: elucidation of the elusive CD8+ subset reveals multiple homeostatic T-cell compartments with distinct implications for immune competence. Immunology 2002; 106: 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bar-Or A, Oliveira EM, Anderson DE, et al. Immunological memory: contribution of memory B cells expressing costimulatory molecules in the resting state. Journal of immunology 2001; 167: 5669–77. [DOI] [PubMed] [Google Scholar]
- 13.Trivedi SG, Lloyd CM. Eosinophils in the pathogenesis of allergic airways disease. Cellular and molecular life sciences : CMLS 2007; 64: 1269–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barnes PJ, Drazen JM, Rennard SI, Thomson NC. Asthma and COPD Basic Mechanisms and Clinical Management Second Edition Preface to the 2nd Edition. Asthma and Copd: Basic Mechanisms and Clinical Management, 2nd Edition. 2009: Ix–Ix. [Google Scholar]
- 15.van der Weerd K, Dik WA, Schrijver B, et al. Morbidly Obese Human Subjects Have Increased Peripheral Blood CD4(+) T Cells With Skewing Toward a Treg- and Th2-Dominated Phenotype. Diabetes 2012; 61: 401–08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paludan SR. Interleukin-4 and interferon-gamma: the quintessence of a mutual antagonistic relationship. Scandinavian journal of immunology 1998; 48: 459–68. [DOI] [PubMed] [Google Scholar]
- 17.Huber JP, Gonzales-van Horn SR, Roybal KT, Gill MA, Farrar JD. IFN-alpha suppresses GATA3 transcription from a distal exon and promotes H3K27 trimethylation of the CNS-1 enhancer in human Th2 cells. Journal of immunology 2014; 192: 5687–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuda H, Suda T, Hashizume H, et al. Alteration of balance between myeloid dendritic cells and plasmacytoid dendritic cells in peripheral blood of patients with asthma. Am J Resp Crit Care 2002; 166: 1050–54. [DOI] [PubMed] [Google Scholar]
- 19.Sheridan PA, Paich HA, Handy J, et al. Obesity is associated with impaired immune response to influenza vaccination in humans. International journal of obesity 2012; 36: 1072–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Husemoen LL, Glumer C, Lau C, Pisinger C, Morch LS, Linneberg A. Association of obesity and insulin resistance with asthma and aeroallergen sensitization. Allergy 2008; 63: 575–82. [DOI] [PubMed] [Google Scholar]
- 21.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006; 444: 840–6. [DOI] [PubMed] [Google Scholar]
- 22.Leivo-Korpela S, Lehtimaki L, Vuolteenaho K, et al. Adipokine resistin predicts anti-inflammatory effect of glucocorticoids in asthma. Journal of inflammation 2011; 8: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Homer RJ, Elias JA. Airway remodeling in asthma: therapeutic implications of mechanisms. Physiology 2005; 20: 28–35. [DOI] [PubMed] [Google Scholar]


