Abstract
Specific isomers of conjugated linoleic acid (CLA), a fatty acid with potentially beneficial physiological and anticarcinogenic effects, were efficiently produced from linoleic acid by washed cells of Lactobacillus acidophilus AKU 1137 under microaerobic conditions, and the metabolic pathway of CLA production from linoleic acid is explained for the first time. The CLA isomers produced were identified as cis-9, trans-11- or trans-9, cis-11-octadecadienoic acid and trans-9, trans-11-octadecadienoic acid. Preceding the production of CLA, hydroxy fatty acids identified as 10-hydroxy-cis-12-octadecaenoic acid and 10-hydroxy-trans-12-octadecaenoic acid had accumulated. The isolated 10-hydroxy-cis-12-octadecaenoic acid was transformed into CLA during incubation with washed cells of L. acidophilus, suggesting that this hydroxy fatty acid is one of the intermediates of CLA production from linoleic acid. The washed cells of L. acidophilus producing high levels of CLA were obtained by cultivation in a medium containing linoleic acid, indicating that the enzyme system for CLA production is induced by linoleic acid. After 4 days of reaction with these washed cells, more than 95% of the added linoleic acid (5 mg/ml) was transformed into CLA, and the CLA content in total fatty acids recovered exceeded 80% (wt/wt). Almost all of the CLA produced was in the cells or was associated with the cells as free fatty acid.
Conjugated linoleic acid (CLA), an octadecadienoic acid with conjugated double bonds, has a variety of positional and geometric isomers. In the last 2 decades, CLA has attracted considerable attention because of its potentially beneficial effects. Ha and colleagues identified a lipid fraction from beef fat that possessed anticarcinogenic properties and demonstrated that it was composed of CLA isomers (8). Subsequent studies with animal models showed that consumption of CLA inhibited the initiation of carcinogenesis (18) and tumorigenesis (9, 11), reduced body fat content and increased muscle mass (3), decreased atherosclerosis (17), improved hyperinsulinemia (10), controlled immune systems (4, 16), and altered the low-density lipoprotein/high-density lipoprotein cholesterol ratio (15), all of which further heightened interest in CLA. Of the individual isomers of CLA, cis-9, trans-11-octadecadienoic acid (c9, t11-18:2) has been suggested to be the most important in terms of biological activity because it is the major isomer and it is incorporated into the phospholipid fraction of tissues of animals who are fed a mixture of CLA isomers (9). However, the commercially available CLA synthesized by alkali isomerization of linoleic acid is a mixture of four (8,10-, 9,11-, 10,12-, and 11,13-18:2) cis/trans positional isomers (19). Therefore, there is interest in the development of methods for the selective production of CLA isomers.
Dairy products are among the major dietary sources of CLA, of which c9, t11-18:2 is the main isomer (3, 7). CLA has been shown to be produced from polyunsaturated fat by certain rumen microorganisms such as Butyrivibrio species (6). c9, t11-18:2 has been suggested to be the first intermediate in the biohydrogenation of linoleic acid by the anaerobic rumen bacterium Butyrivibrio fibrisolvens (14). More recently, it was reported that Propionibacterium freudenreichii, commonly used as a dairy starter culture, was able to produce CLA from free linoleic acid (13). These findings suggest that CLA is derived from linoleic acid, but little is known about the precise structures of the CLA produced or the mechanisms of CLA production, and the amounts of CLA produced under the conditions mentioned above are very low.
To establish efficient and selective methods for CLA production and to clarify the reactions involved in CLA formation from linoleic acid by microorganisms, we investigated the ability of lactic acid bacteria to produce CLA from linoleic acid and found that washed cells of Lactobacillus acidophilus AKU 1137 produce CLA under microaerobic conditions, with a preceding accumulation of hydroxy fatty acids (HY). Here, we report the chemical structures of CLA and HY produced by L. acidophilus and discuss the roles of HY as intermediates in the production of CLA.
MATERIALS AND METHODS
Chemicals.
Linoleic acid and fatty acid-free (<0.02%) bovine serum albumin (BSA) were purchased from Wako Pure Chemicals (Osaka, Japan) and Nacalai Tesque (Kyoto, Japan), respectively. All other chemicals used were of analytical grade and are commercially available.
Microorganism cultivation and preparation of washed cells.
L. acidophilus AKU 1137 (AKU Culture Collection, Faculty of Agriculture, Kyoto University, Kyoto, Japan) was aerobically cultivated in MRS medium comprised of 1.0% tryptone, 1.0% meat extract, 0.5% yeast extract, 2.0% glucose, 0.1% Tween 80, 0.2% K2HPO4, 0.5% sodium acetate, 0.2% diammonium citrate, 0.02% MgSO4 · 7H2O, and 0.005% MnSO4 · H2O (pH 6.5). The strain was inoculated in 15 ml of liquid medium in screw-cap tubes (16.5 by 125 mm). The liquid medium occupied approximately 80 to 90% of the volume of the tubes. Cultivations were carried out for 3 days at 28°C with shaking (120 strokes/min). The cells were harvested by centrifugation (14,000 × g, 30 min), washed twice with 0.85% NaCl, centrifuged again, and then used as the washed cells.
Reaction conditions.
Reactions were carried out at 28°C with gentle shaking (120 strokes/min) in screw-cap tubes (16.5 by 125 mm). Reactions were carried out under aerobic or microaerobic conditions with or without replacement of the air in the tubes by pure nitrogen (99.999% pure; Sumitomo-Seika Chemicals Co. Ltd., Osaka, Japan), respectively. In the microaerobic assays, the oxygen concentrations, monitored by an oxygen indicator (Mitsubishi Gas Chemical Co. Ltd., Tokyo, Japan), were kept under 1%. The reaction mixture contained, in 1 ml of 100 mM potassium phosphate buffer (pH 6.5), 5 mg of linoleic acid in a complex with BSA (0.2 mg of BSA/mg of linoleic acid) and the washed cells from 15 ml of culture broth (approximately 20 mg of cells [dry weight]).
Lipid analyses.
Lipids were extracted from the reaction mixture with chloroform-methanol (1:2, vol/vol) according to the procedure of Bligh and Dyer (2), methylated with diazomethane in diethylether for 15 min, and further methylated with 0.5 M sodium methoxide in methanol for 30 min at 50°C. The resultant fatty acid methyl esters were extracted with n-hexane and analyzed by gas-liquid chromatography (GC) using a Shimadzu (Kyoto, Japan) GC-17A gas chromatograph equipped with a flame ionization detector and a split injection system and fitted with a capillary column (HR-SS-10, 50 m by 0.25 mm inside diameter; Shinwa Kako, Kyoto, Japan). The column temperature, initially 180°C, was raised to 220°C at a rate of 2°C/min and maintained at that temperature for 5 min. The injector and detector were operated at 250°C. Helium was used as carrier gas at 225 kPa/cm2. Extraction and fractionation into lipid classes were carried out essentially as described previously (12, 20).
Isolation of reaction products.
The methyl esters of the reaction products were separated by reverse-phase high-performance liquid chromatography using a Shimadzu LC-10A system equipped with a Cosmosil column (5C18-AR, 20 by 250 mm; Nacalai Tesque). The mobile phase was acetonitrile-H2O (8:2, vol/vol) at a flow rate of 3.0 ml/min, and the effluent was monitored by UV detection (205 nm). The chemical structures of purified fatty acids were determined by mass spectroscopy (MS), infrared spectroscopy (IR), proton nuclear magnetic resonance (1H-NMR), and 1H-1H correlation spectroscopy (COSY).
Preparation of free fatty acids.
Free fatty acids were prepared by heating the fatty acid methyl esters (50 mg) in a mixture of 50 μl of 7.0 N sodium hydroxide and 50 μl of methanol in screw-cap tubes. After being heated in a boiling water bath for 1 h, the solution was acidified to pH 2.0 with 10% (wt/vol) sulfuric acid in water. The free fatty acids were extracted with diethylether. The organic extract was washed with water and dried over anhydrous Na2SO4, and the solvent was removed under vacuum in a rotary evaporator.
Preparation of pyrrolidide fatty acids.
Pyrrolidide derivatives were prepared by direct treatment of the isolated methyl esters with pyrrolidine-acetic acid (10:1, vol/vol) in screw-cap tubes for 1 h at 115°C followed by extraction according to the method of Andersson and Holman (1). The organic extract was washed with water and dried over anhydrous Na2SO4, and then the solvent was removed under vacuum in a rotary evaporator.
GC-MS analysis.
GC-MS QP5050 (Shimadzu) with a GC-17A gas chromatograph was used for mass spectral analyses. The GC separation of fatty acid methyl esters was performed on an HR-SS-10 column as described above at the same temperature. The GC separation of fatty acid pyrrolidide derivatives was performed on the HR-1 column (25 m by 0.5 mm inner diameter; Shinwa Kako) at 300°C. MS was used in the electron impact mode at 70 eV with a source temperature of 250°C. Split injection was employed with the injector port at 250°C.
MS-MS analysis.
MS-MS analyses were performed on the free acids of the fatty acids with a JEOL HX110A/HX110A tandem mass spectrometer. The ionization method was fast atom bombardment (FAB) and the acceleration voltage was 3 kV. Glycerol was used for the matrix.
IR analysis.
IR analysis of fatty acid methyl esters was performed with infrared spectrophotometer IR-420 (Shimadzu) in a chloroform solution.
1H-NMR and 1H-1H COSY analyses.
All NMR experiments were performed on a JEOL EX-400 (400 MHz at 1H), and chemical shifts were assigned relative to the solvent signal. Fatty acid methyl esters were dissolved in dichloromethane-d2, and the diameter of the tube was 5 mm.
RESULTS
Transformation of linoleic acid by washed cells of L. acidophilus.
When the reaction was carried out under aerobic conditions, linoleic acid was decomposed by washed cells of L. acidophilus without generation of detectable amounts of fatty acids (Fig. 1A). On the other hand, when the reaction was carried out under microaerobic conditions, four major, newly generated fatty acids, designated CLA1, CLA2, HY1, and HY2, were found on the GC chromatograms of the methylated fatty acids (Fig. 1B). The peaks for CLA1 and CLA2, with retention times slightly greater than that of linoleic acid, were shown to have the same retention times as those from the CLA mixture purchased from Nu-Chek-Prep, Inc. (Elysian, Minn.) The peaks for HY1 and HY2 indicated that HY1 and HY2 were relatively polar fatty acids such as hydroxylated ones because of their far greater retention times.
FIG. 1.
GC chromatograms of fatty acid methyl esters produced by washed cells of L. acidophilus. (A) Reaction with linoleic acid as a substrate under aerobic conditions. (B) Reaction with linoleic acid as a substrate under microaerobic conditions. (C) Reaction with HY2 as a substrate under microaerobic conditions.
Identification of CLA1 and CLA2.
Mass spectra of the isolated methyl esters of both CLA1 and CLA2 exhibited molecular weights of m/e 294, and those of pyrrolidide derivatives showed molecular weights of m/e 333. These results suggest that CLA1 and CLA2 are C18 fatty acids containing two double bonds. FAB-MS data of the free fatty acids of both CLA1 and CLA2 exhibited molecular weights of m/e 280 ([M-H]+, 279). These peaks (m/e 279) were fragmented again by MS-MS. Typical fragments (m/e) for both CLA1 and CLA2 were 127, 141, 167, 193, 207, and 208. The m/e 141, 167 and 193 were derived from cleavage between single bonds 8–9, 10–11, and 12– 13, numbered from the carboxyl group. m/e 127 and 207, derived from the cleavage of a single bond between the α and β positions from the double bond, were clearly detected. Hence, CLA1 and CLA2 were identified as 9,11 positional isomers of octadecadienoic acid.
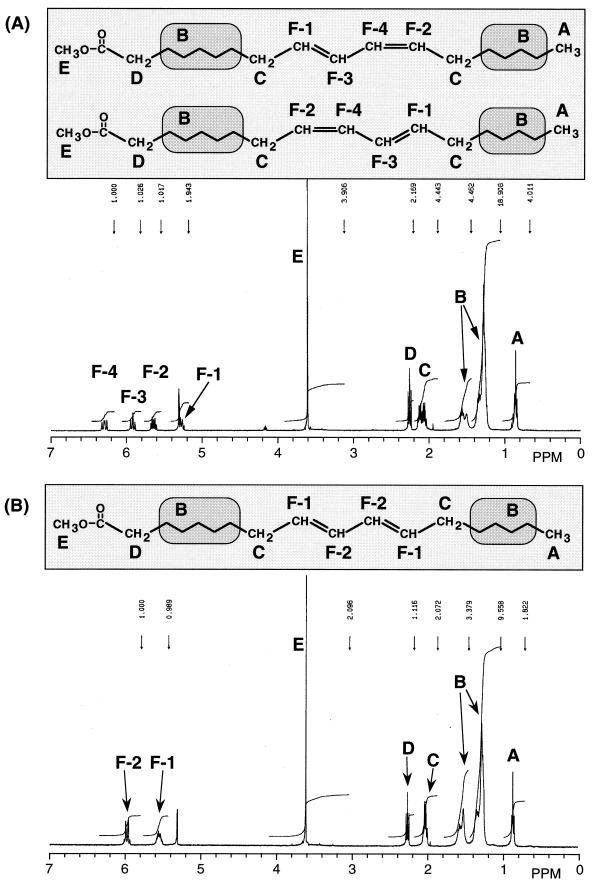
Furthermore, 1H-NMR analysis was carried out to identify the geometric configurations of CLA1 and CLA2 (Fig. 2). These deduced structures were further confirmed by 1H-1H COSY analysis (data not shown). With respect to CLA1, the signals F-1 (5.28 ppm, m, 1H), F-2 (5.64 ppm, m, 1H), F-3 (5.92 ppm, t, 1H), and F-4 (6.29 ppm, m, 1H) suggested the existence of double bonds. Other signals were identified as shown in Fig. 2A. Coupling constants were obtained based on the decoupled 1H-NMR spectra of the methyl ester of CLA1. When the methyl ester was irradiated at 2.17 ppm (m, 4H, signal C), the coupling constant between F-1 and F-3 was 10.26 Hz, which suggests that the double bond between F-1 and F-3 is in the cis configuration. When irradiated at 2.02 ppm (m, 4H, signal C), the coupling constant between F-2 and F-4 was 14.65 Hz, indicating the trans configuration. These results indicate that CLA1 is a cis/trans-conjugated octadecadienoic acid. With regard to CLA2, the signals F-1 (5.53 ppm, m, 2H) and F-2 (6.00 ppm, m, 2H), suggesting the existence of a double bond, were mixtures of two signals. It was not possible to determine the coupling constant of the double bond.
FIG. 2.
1H-NMR spectra and structures of CLA1 (A) and CLA2 (B). The letters indicate the positions of protons and their corresponding signals.
IR analysis was performed to confirm the geometric configuration. The major differences in IR spectra were in the 800 to 1,000 cm−1 range. In the spectrum of CLA1, sharp absorption peaks at 990 and 944 cm−1 were observed, indicating that it is a cis/trans isomer (21). CLA2 showed sharp absorption at 990 cm−1, indicating that it is a trans/trans-isomer (21).
On the basis of the results of spectral analyses, CLA1 and CLA2 were identified as cis-9, trans-11- or trans-9, cis-11-octadecadienoic acid and trans-9, trans-11-octadecadienoic acid, respectively.
Identification of HY1 and HY2.
FAB-MS analysis of the isolated methyl esters of HY1 and HY2 revealed molecular weights of m/e 312 ([M+H]+, 313). On MS analyses of methyl esters of both HY1 and HY2, typical fragments of m/e 169, 201, and 294 were found. The fragment m/e 294 (M-18) was thought to indicate cleavage between the hydroxyl group and carbon and the existence of one double bond in the hydrocarbon chain. Moreover, cleavage between the α and β positions from the hydroxyl group yielded m/e 201. This suggests that the hydroxyl group is located at carbon 10, numbered from the carboxyl group.
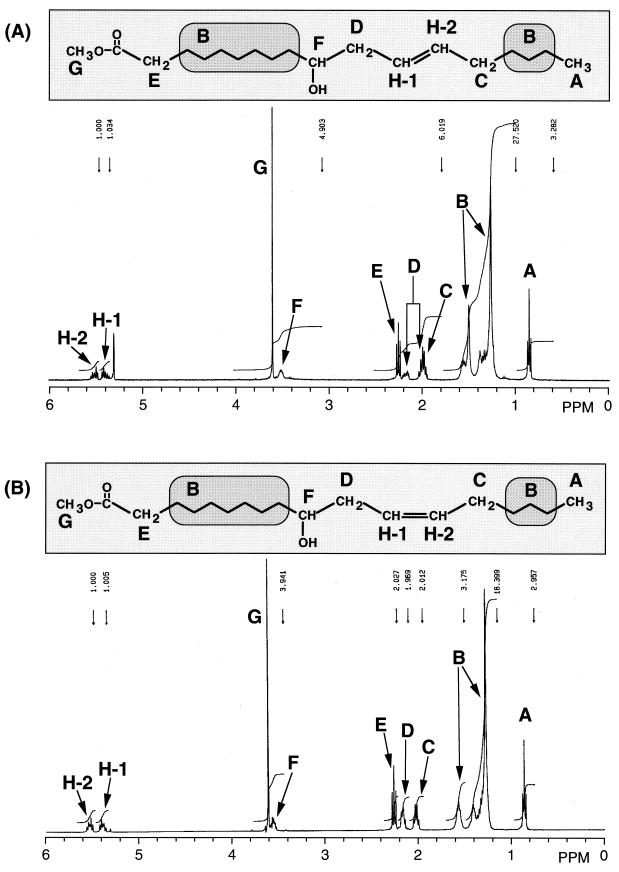
1H-NMR and 1H-1H COSY analyses were carried out to identify the positions and geometric configurations of double bonds in HY1 and HY2. 1H-NMR spectra of methyl esters of HY1 and HY2 are shown in Fig. 3. The signal at 3.5 ppm suggested the existence of a hydroxyl group, and the signals H-1 (5.4 ppm, m, 1H) and H-2 (5.5 ppm, m, 1H) were identified as the protons on the double bond. The 1H-1H COSY spectra of the methyl esters of HY1 and HY2 showed that there is one methylene group between the double bond and the carbon bonding to the hydroxyl group (data not shown). Therefore, a double bond was thought to be located at the Δ12 position. Coupling constants between H-1 and H-2 of HY1 and HY2 determined by irradiation at 2.0 ppm (m, 3H, signal C) were 15.14 and 11.23 Hz, indicating that the double bonds are in trans and cis configurations, respectively.
FIG. 3.
1H-NMR spectra and structures of HY1 (A) and HY2 (B). The letters indicate the positions of protons and their corresponding signals.
From these results, HY1 and HY2 were identified as 10-hydroxy-trans-12-octadecaenoic acid and 10-hydroxy-cis-12-octadecaenoic acid, respectively.
Time course of linoleic acid transformation by washed cells of L. acidophilus under microaerobic conditions.
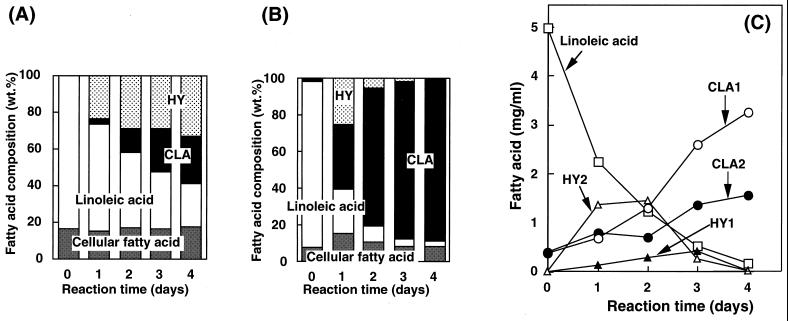
The time course of changes in fatty acid composition during linoleic acid transformation by washed cells of L. acidophilus under microaerobic conditions was studied using the washed cells obtained by cultivation in MRS medium with or without linoleic acid (0.1%, [wt/vol]). In comparison with the results shown in Fig. 4A and B, the CLA productivity of the washed cells obtained by cultivation with linoleic acid was much higher than that of the cells obtained by cultivation without linoleic acid. This may have been due to induction of the enzymes catalyzing CLA formation by linoleic acid during cultivation. The amounts of cellular fatty acids (myristic acid, palmitic acid, palmitoleic acid, oleic acid, vaccenic acid, and 2-hexyl-1-cyclopropane-octanoic acid) did not significantly change for 4 days after the reaction. With the washed cells obtained by cultivation with linoleic acid, CLA (sum of CLA1 and CLA2) levels reached 36% (wt/wt) of total fatty acids on the first day and exceeded 80% (wt/wt) on the fourth day (Fig. 4B). The ratio of HY (sum of HY1 and HY2) was 25% (wt/wt) on the first day and rapidly decreased, followed by an increase in CLA level (Fig. 4B). These results suggest that HY produced by the washed cells may be converted to CLA and that HY may be the intermediate in CLA formation. The time courses of CLA and HY production from linoleic acid are presented in Fig. 4C. The final level of CLA was 4.9 mg/ml (CLA1, 3.3 mg/ml; CLA2, 1.6 mg/ml; molar conversion yield from linoleic acid, 98%).
FIG. 4.
Time courses of changes in fatty acid composition during the reaction with washed cells obtained by cultivation in MRS medium without (A) or with (B) linoleic acid (0.1% [wt/vol]) and of changes in levels of CLA and HY production from linoleic acid (C). The results are averages of three separate determinations that were reproducible within ±10%.
Production of CLA from hydroxy fatty acid.
HY2 was isolated by preparative high-performance liquid chromatography and was used as the substrate for the reaction instead of linoleic acid to determine whether HY is converted to CLA by washed cells of L. acidophilus. The GC chromatogram of the fatty acids obtained after reaction under microaerobic conditions is shown in Fig. 1C. No HY2 was detected after the reaction, but CLA1 and CLA2 were found. These observations suggest that HY2 may be a substrate for CLA and an intermediate in the formation of CLA from linoleic acid. Preparative isolation of HY1 resulted in contamination with a small amount of HY2, so that it was difficult to determine whether HY1 was indeed an intermediate. However, during linoleic acid transformation, HY1 accumulated, while CLA was being produced, until the third day; then the level of HY1 decreased, followed by an increase in the level of CLA (Fig. 4C), indicating that HY1 is also an intermediate of CLA formation.
Distribution and lipid classes of the fatty acids produced by washed cells of L. acidophilus.
The reaction mixture of linoleic acid transformation was centrifuged after 3 days of reaction and separated into supernatant and cells. The distribution and lipid classes of the fatty acids produced in both supernatant and cells were analyzed (Table 1). Most of the fatty acids (92.0%), were found in cells or associated with cells as free fatty acids, with CLA the most abundant. The fatty acids found in cells (or associated with cells) consisted of free fatty acids (86.4%), acylglycerols (5.6%), and trace amounts of phospholipids. Most of the CLA produced was found as free fatty acid in cells (or associated with cells).
TABLE 1.
Distribution and lipid classes of fatty acids produced from linoleic acid by washed cells of L. acidophilus under microaerobic conditionsa
| Fatty acid | Fatty acid concn (mg/ml of reaction mixture) after reaction | Distribution of fatty acids in indicated lipid class (mol%) in:
|
||||||
|---|---|---|---|---|---|---|---|---|
| Supernatant
|
Cells
|
Total | ||||||
| FAc | AGd | PLe | FA | AG | PL | |||
| Linoleic acid | 0.24 | 1.9 | 0.3 | —f | 2.7 | 0.3 | — | 5.2 |
| CLA | 3.76 | 3.5 | 0.6 | — | 74.5 | 3.9 | — | 82.5 |
| HY | 0.04 | — | — | — | 0.6 | 0.3 | — | 0.9 |
| Other fatty acidsb | 0.52 | 1.4 | 0.3 | — | 8.6 | 1.1 | trg | 11.4 |
| Total | 4.56 | 6.8 | 1.2 | — | 86.4 | 5.6 | tr | 100 |
L. acidophilus was cultivated in MRS medium with linoleic acid (0.1%) for 3 days. The reaction was carried out with 5 mg of linoleic acid per ml as the substrate for 3 days under the conditions described in Materials and Methods. The results are averages of three separate determinations that were reproducible within ±10%.
Other lipids (moles percent) were myristic acid (0.1), palmitic acid (1.5), palmitoleic acid (0.1), oleic acid (7.3), vaccenic acid (1.7), and 2-hexy-1-cyclopropane-octanoic acid (0.7).
FA, free fatty acids.
AG, acylglycerols.
PL, phospholipids.
—, not detected.
tr, trace (less than 0.05 mol%).
DISCUSSION
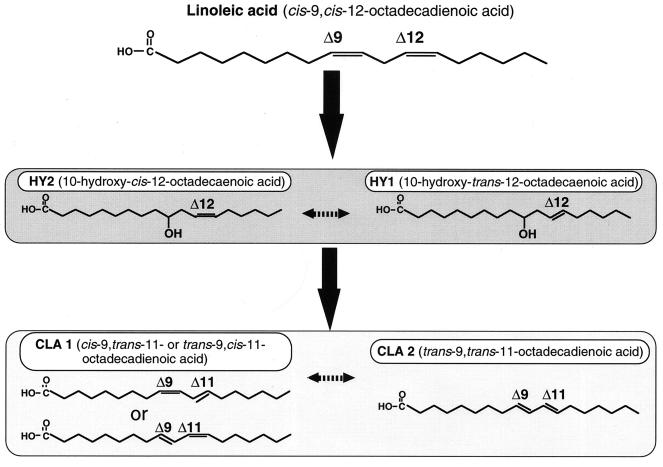
Some anaerobic bacteria have been reported to produce CLA. The rumen bacterium B. fibrisolvens produces cis-9, trans-11-octadecadienoic acid as an intermediate of the biohydrogenation of linoleic acid to oleic acid (14). The lyophilized cells of some lactobacilli have been found to contain small amounts of CLA (5). However, the mechanism of CLA formation has not been elucidated in detail. The results reported here indicate that the transformation of linoleic acid to CLA is not a one-step isomerization of a nonconjugated diene to a conjugated diene. Rather, the transformation involves the production of hydroxy fatty acids, i.e., 10-hydroxy-trans-12-octadecaenoic acid and 10-hydroxy-cis-12-octadecaenoic acid. The findings obtained here using washed cells of L. acidophilus under microaerobic conditions, i.e., (i) accumulation of HY prior to CLA formation and its decrease concomitant with increased formation of CLA and (ii) conversion of exogenously added 10-hydroxy-cis-12-octadecaenoic acid to CLA, strongly support the above suggestion. It is not yet clear whether the generation of geometric isomers of HY and CLA is a biological or chemical process occurring during analysis nor whether the trans isomer of HY is involved as an intermediate. However, the pathway involving hydroxylation at carbon 10, numbered from the carboxyl group as the first step in the reaction, could be proposed for isomerization of linoleic acid to CLA (Fig. 5). Further analyses of the enzyme systems for CLA production induced by linoleic acid are under way in our laboratory to clarify the transformation pathway and its physiological significance for lactic acid bacteria.
FIG. 5.
Proposed pathway of production of CLA from linoleic acid by washed cells of L. acidophilus under microaerobic conditions.
A study of the production of CLA was performed with P. freudenreichii, a bacterium commonly used in dairy starter cultures, and showed the extracellular production of CLA (265 μg/ml) mainly consisting of cis-9, trans-11- or trans-9, cis-11-octadecadienoic acid (13). This previous study revealed the potential ability of lactic acid bacteria to produce CLA. The transformation of linoleic acid into CLA with washed cells of L. acidophilus under microaerobic conditions is a promising system for the following reasons: (i) specific isomers of CLA, i.e., cis-9, trans-11- or trans-9, cis-11-octadecadienoic acid and trans-9, trans-11-octaecadienoic acid, are obtained as reaction products; (ii) CLA accumulates at high concentrations (nearly 5 mg/ml); (iii) CLA content in the recovered fatty acids reaches nearly 90% (wt/wt); (iv) CLA is accumulated as intracellular or cell-associated lipids in free form, making it easy to recover by centrifugation, and cells themselves could be used as sources of CLA; and (v) the reaction requires only microaerobic conditions and no energy input. Because of its beneficial physiological effects, there is a potential demand for CLA. The results presented here may lead to the development of industrial production of CLA by lactic acid bacteria.
REFERENCES
- 1.Andersson B A, Holman R T. Pyrrolidides for mass spectrometric determination of the position of the double bond in monounsaturated fatty acids. Lipids. 1974;9:185–190. doi: 10.1007/BF02532690. [DOI] [PubMed] [Google Scholar]
- 2.Bligh E G, Dyer W J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 3.Chin S F, Liu W, Storkson J M, Ha Y L, Pariza M W. Dietary sources of conjugated dienoic isomers of linoleic acid, a newly recognized class of anticarcinogens. J Food Compos Anal. 1992;5:185–197. [Google Scholar]
- 4.Cook M E, Miller C C, Park Y, Pariza M W. Immune modulation by altered nutrient metabolism: nutritional control of immune-induced growth depression. Poult Sci. 1993;72:1301–1305. doi: 10.3382/ps.0721301. [DOI] [PubMed] [Google Scholar]
- 5.Fabiola D, Pierre-Alain G, Marina E, Laurent B F. Stability of cyclopropane and conjugated linoleic acid during fatty acid quantification in lactic acid bacteria. Lipids. 1999;34:1107–1115. doi: 10.1007/s11745-999-0462-8. [DOI] [PubMed] [Google Scholar]
- 6.Fogerty A C, Ford G L, Svoronos D. Octadeca-9, 11-decenoic acid in the lipids of human blood and breast milk. Nutr Rep Int. 1988;38:937–944. [Google Scholar]
- 7.Fritsche J, Steinhart H. Amounts of conjugated linoleic acid (CLA) in German foods and evaluation of daily intake. Z Lebensm-Unters-Fodrsch. 1998;206:77–82. [Google Scholar]
- 8.Ha Y L, Grimm N K, Pariza M W. Anticarcinogens from fried ground beef: heat-altered derivatives of linoleic acid. Carcinogenesis. 1987;8:1881–1887. doi: 10.1093/carcin/8.12.1881. [DOI] [PubMed] [Google Scholar]
- 9.Ha Y L, Storkson J, Pariza M W. Inhibition of benzo (a) pyrene-induced mouse forestomach neoplasia by conjugated dienoic derivatives of linoleic acid. Cancer Res. 1990;50:1097–1101. [PubMed] [Google Scholar]
- 10.Houseknecht K L, Vanden Heuvel J P, Moya-Camarena S Y, Portocarrero C P, Peck L W, Nickel K P, Belury M A. Dietary conjugated linoleic acid normalizes impaired glucose tolerance in the Zucker diabetic fatty fa/fa rat. Biochem Biophys Res Commun. 1998;244:678–682. doi: 10.1006/bbrc.1998.8303. [DOI] [PubMed] [Google Scholar]
- 11.Ip C, Chin S F, Scimeca J A, Pariza M W. Mammary cancer prevention by conjugated dienoic derivative of linoleic acid. Cancer Res. 1991;51:6118–6124. [PubMed] [Google Scholar]
- 12.Jareonkitmongkol S, Shimizu S, Yamada H. Fatty acid desaturation-defective mutants of an arachidonic-acid-producing fungus, Mortierella alpina 1S-4. J Gen Microbiol. 1992;138:997–1002. [Google Scholar]
- 13.Jiang J, Bjorck L, Fonden R. Production of conjugated linoleic acid by dairy starter cultures. J Appl Microbiol. 1998;85:95–102. doi: 10.1046/j.1365-2672.1998.00481.x. [DOI] [PubMed] [Google Scholar]
- 14.Kepler C R, Hirons K P, McNeill J J, Tove S B. Intermediates and products of the biohydrogenation of linoleic acid by Butyrivibrio fibrisolvens. J Biol Chem. 1966;241:1350–1354. [PubMed] [Google Scholar]
- 15.Lee K N, Kritchevsky D, Pariza M W. Conjugated linoleic acid and atherosclerosis in rabbits. Atherosclerosis. 1994;108:19–25. doi: 10.1016/0021-9150(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 16.Miller C C, Park Y, Pariza M W, Cook M E. Feeding conjugated linoleic acid to animals partially overcomes catabolic responses due to endotoxin injection. Biochem Biophys Res Commun. 1994;198:1107–1112. doi: 10.1006/bbrc.1994.1157. [DOI] [PubMed] [Google Scholar]
- 17.Nicolosi R J, Rogers E J, Kritchevsky D, Scimeca J A, Huth P J. Dietary conjugated linoleic acid reduces plasma lipoproteins and early aortic atherosclerosis in hypercholesterolemic hamsters. Artery. 1997;22:266–277. [PubMed] [Google Scholar]
- 18.Pariza M W, Hargraves W A. A beef-derived mutagenesis modulator inhibits initiation of mouse epidermal tumors by 7, 12-dimethylbenz[a]anthracene. Carcinogenesis. 1985;6:591–593. doi: 10.1093/carcin/6.4.591. [DOI] [PubMed] [Google Scholar]
- 19.Sehat N, Yurawecz M P, Roach J A G, Mossoba M M, Kramer J K G, Ku Y. Silver-ion high-performance liquid chromatographic separation and identification of conjugated linoleic acid isomers. Lipids. 1998;33:217–221. doi: 10.1007/s11745-998-0198-6. [DOI] [PubMed] [Google Scholar]
- 20.Shimizu S, Jareonkitmongkol S, Kawashima H, Akimoto K, Yamada H. Production of a novel ω1-eicosapentaenoic acid by Mortierella alpina 1S-4 grown on 1-hexadecene. Arch Microbiol. 1991;156:163–166. [Google Scholar]
- 21.Tolberg W E, Wheeler D H. Cis, trans isomerization of conjugated linoleates by iodin and light. J Am Oil Chem Soc. 1958;35:385–388. [Google Scholar]