Abstract
Urogenital schistosomiasis is a neglected tropical disease that is endemic to Nigeria and one which continues to pose a public health problem especially among school-age children in rural communities. This study was carried out in remote areas where most people depend on natural water bodies and rainwater for their daily water needs. The present research investigates the prevalence of urogenital schistosomiasis and the significant risk factors associated with the infection among primary school children in Nigeria. From August 2019 to December 2019, a total of 5514 primary school-age children from twelve sites were diagnosed with the presence of Schistosoma haematobium eggs in their urine. Socio-demographic, sociocultural, and socioeconomic indices and data on behaviors (e.g contact frequency with freshwater bodies) were also collected for each diagnosed individual through the use of a questionnaire. Associations between each of these variables and disease infection were tested using a multivariate logistic regression. A total of 392 of the 5514-urine samples were positive for the infection, the overall prevalence reached 7.1% and ranged from 4.6% (East Nigeria) to 15,9% (West Nigeria). Multivariate logistic regression analyses showed that the significant risk factors associated with S. haematobium infection are frequent contact with freshwater bodies (rivers/steams), with an adjusted odds ratio (AOR) of 4.92; 3.34–7.24, washing/swimming, AOR: 46.49; 27.64–78.19, and fishing, AOR: 11.57; 8.74–15.32. For socioeconomic factors, primary education of fathers which resulted in an AOR of 1.63; 1.01–2.45 was significantly associated with the infection. The socio-demographic factor for the 12–14 year age group had an AOR of 1.68; 1.21–2.33, and was also significantly associated with the disease. Nigeria remains endemic for urogenital schistosomiasis as indicated by the data obtained from all the studied sites, and it is clear that efforts need to be intensified in order to control and eradicate the disease throughout the country.
Keywords: Nigeria, Urogenital schistosomiasis, School-aged children, Prevalence, Risk factors
1. Introduction
Globally, schistosomiasis is a neglected tropical disease (NTD) and is caused by dioecious blood fluke (digenetic trematodes) of the genus Schistosoma (Atalabi et al., 2018). It is ranked as the second most socioeconomically destructive parasitic disease after malaria. It is the most prevalent water-borne disease present within the rural populace. The parasite is transmitted by a specific freshwater intermediate molluscan host, while humans of all age groups act as the definitive host, (Uchendu et al., 2017). It is estimated that nearly 700 million people live in areas where the disease is endemic. Approximately 200 million people are infected across 76 countries around the world (Ossai et al., 2014), with sub-Saharan Africa and some parts of Asia reporting the highest endemicity. Sub-Saharan Africa accounts for 90% of the infected cases, and it is estimated that about 11,700 people die from this disease annually in this region (Dawet et al., 2012).
Of the six Schistosoma species that infect humans (S. haematobium, S. mansoni, S. japonicum, S. intercalatum, S. mekongi and S. malayensis) (Manson et al., 1987), three species (S. haematobium, S. mansoni and S. intercalatum) are present in Nigeria. The urogenital schistosomiasis caused by S. haematobium occurs at a higher frequency in remote areas of the country. Nigeria is one of Sub-Saharan Africa's most heavily impacted countries with respect to both urinary and intestinal schistosomiasis, accounting for almost 14% of the disease worldwide, (Hotez et al., 2012; Herrick et al., 2017). As a result, Nigeria also requires the greatest intervention to help control and eradicate the disease (Uchendu et al., 2017). The first reported case of schistosomiasis in Nigeria was in 1908, (Bishop, 2017)) and numerous studies are available on the epidemiology, prevalence and risk factors associated with this disease. It is worth noting that some of these reports conflict with one another (Emmanuel et al., 2017). For many areas in Nigeria, no reliable systematic report has been published on the epidemiological status of this disease (Houmsou et al., 2011), and it was only recently, 4th June 2015, that Nigeria's Federal Ministry of Health (FMOH) officially released the first epidemiological data on the prevalence of this disease (Global Network Neglected Tropical Diseases, 2015). The data showed that roughly 24 million people in Nigeria are at the risk of infection and that there is a prevalence of almost 9.5% (Bishop, 2017). The control program initiated by the FMOH, State Ministry of Health, and other institutions, involves the use of chemotherapy through Mass Drug Administration (MDA), and the administration of praziquantel to infected individuals or to those at risk of infection (Ejike et al., 2017). Despite Government efforts, and in spite of contributions by Research Institutions, Non-Government Organizations (NGOs) and Faith Based Organizations (FBOs), the disease remains unabated (Ejike et al., 2017). This may be the result of several hurdles encountered in the process, including, geographically remote areas, ethnic violence, religious crises as well as the marshy stream and river systems that are favorable for the multiplication of the snail intermediate host (Yauba et al., 2018). It has been suggested that integrating school-based health education with preventive chemotherapy would result in significantly lowering the prevelance of the disease. Further, it was suggested that this approach would help to prevent re-infection, reduce the resurgence in the frequency of infection after treatment and help to minimize drug resistance by the parasites (Ajakaye et al., 2017). Previous studies have provided information on the endemicity of this disease in one or several communities within the same geographic region (Opara et al., 2021). However, the data on this subject are based on results which were collated from studies that were not only conducted at different times but by different researchers (Abdulkadir et al., 2017).
In this survey, we used a simple and rapid method of screening patients that yielded results within five minutes. Furthermore, we used questionnaires in addition to in-person interviews with each subject to scrutinize and draw a tentative conclusion on the prevalence and risk factors associated with the disease. This study aims to provide an abundance of new information on the disease for a much broader geographical range. Such data is crucial in order to determine the current epidemiological status of the disease.
2. Materials and methods
2.1. Study area and study population
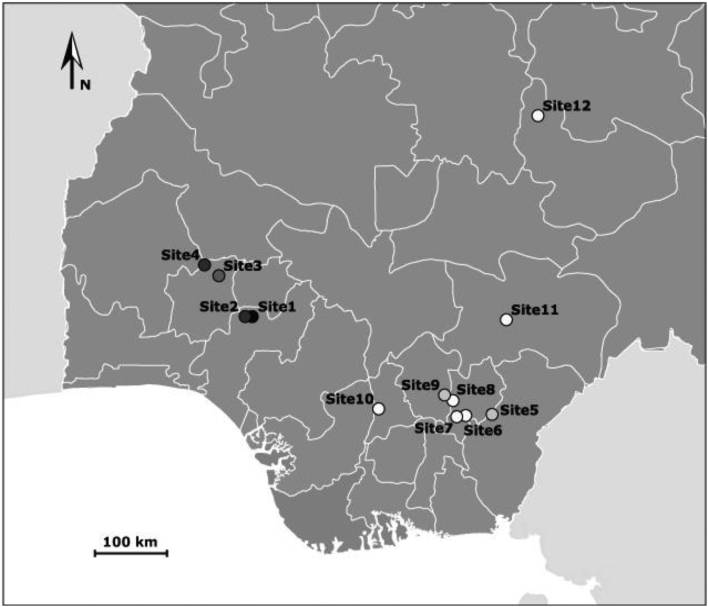
This study was carried out in 12 sites across 7 states in South Nigeria, West Africa, in order to provide a broad geographical coverage. Northern Nigeria was not included within this study due to security challenges. For each site, we examined urine samples from the local population. We targeted primary school-age children, from the 5–14 year age group, the age group known to be at the greatest risk for infection with highest intensities in endemic areas. We chose primary schools based on information obtained from previous surveys as well as those which allowed us to easily collect the required number of samples. This study was part of a standard epidemiological surveillance program and as such, was not submitted to an ethics committee. The Parent-Teacher Associations (PTA) for each of the schools were mobilized, provided with information, and each gave their approval. Informed consents for parents and pupils were obtained before samples were collected from participants. The whole school was invited to the screening. After consultations with the PTA, the head teacher directed the other teachers to organize their respective pupils for screening. Teachers helped the youngest children complete their questionnaires (Fig. 1).
Fig. 1.
Map of Nigeria (West Africa), showing the sampling sites. Sampling sites were represented according to the prevalence of S. haematobium measured among primary school pupils (see results). Lighter circles correspond to lower prevalence, while darker circles correspond to higher prevalence observed in the study sites (see Table 2 results).
2.2. Sample collection
Urine samples were collected between 10.00 h and 14.00 h, corresponding to the time of day when the excretion of S. haematobium is expected to be at its highest. Excretion generally peaks at 12.00 h, mid-day (Singh et al., 2016). Only primary school children who had lived in the study area for at least a year, and whose parents or legal guardians provided oral consent, were selected. Each patient was given an ‘identification code’ for anonymity, and a 20 ml clean labelled, plastic container for the collection of urine. We used a questionnaire to collect data on sociodemographic behaviors, such as source of drinking water, bathing, washing, farming, and fishing. Most participants visited the streams about 3–5 times per week, especially during the dry season, to collect water for domestic use. Visitation was reduced to once or twice per week during the rainy season, when rainwater became an alternative source of water. Fishing activity was predominantly carried out by males. Men and women both waded through the streams while going to and from school, to the farm or to bathe, early in the morning, before the day's activities. Other information collected included symptoms like dysuria (painful urination), haematuria (bloody urine) and the socioeconomic status of the parents or guardians, which ultimately, did not seem to influence the frequency at which participants had contact with the water bodies, but enabled us to identify the potential risk factors associated with the disease. A total of 5514 urine samples were collected for urogenital schistosomiasis screening.
2.3. Parasitological examination
On-the-spot analysis of each urine sample was performed using a rapid diagnostic technique comprised of a field microscope and a Nytrel® filter with a 40 μm mesh size. Approximately 20 ml of each urine sample was homogenized by shaking it vigorously and then filtering it through a Nytrel® filter, yielding a residue which was released into a small Petri dish using a wash bottle filled with commercial spring water (Mott et al., 1982; Colley et al., 2014). The resulting residue was examined under a binocular microscope at 20× or 40× magnifications, for the presence of a characteristic ‘terminal spine’ S. haematobium egg or of hatched miracidia. This process was performed and independently checked by two well-trained technicians. After the urine was examined, each child infected with S. haematobium was treated with a single oral dose, 40 mg/kg body weight, of praziquantel (600 mg, Biltricide, Bayer, Leverkusen, Germany), through their Primary Health Centre (PHC).
2.4. Statistical analyses
Statistical analyses were carried out for twelve parameters using STATA version 15.0 (Stata cooperation, college station, Texas, USA). Prevalence comparisons between groups (Site, Sex or Age) were tested using a univariate analysis (x2 or Fisher's exact test). Patients were said to be positive for the infection when one or more schistosome eggs/miracidia were observed during the microscopy examination. Participants were divided into three age groups (5–8, 9–11 and 12–14 years) for each sex. We performed statistical analyses to assess the relationship between parasitic infections and demographic, sociocultural and socioeconomic factors using multivariate logistic regression with random intercepts for sites and groups nested within sites. The risk factors which were investigated included domestic water source, washing, swimming, fishing, parents' occupation and parents' educational status. Associations and differences with a p-value <0.05 were considered as statistically significant.
3. Results
3.1. Socio-demographic study (population parameter)
A total of 5514 primary school children were tested, and more males participated (3190, 57.85%) than females (2324, 42.15%). The minimum, mean and maximum age was 5 years, 9.02 years and 14 years respectively, with a standard deviation of 2.84 years. Table 1 shows the demographic characteristics of the children.
Table 2.
Infection status stratified by study site, sex and age group.
| Characteristic | Infection status |
p-value | |
|---|---|---|---|
| Total | Positive n (%) | ||
| Study site/state | <0.001 | ||
| 1 Ipogun (Ondo) (Ondo) | 308 | 49 (15.91) | |
| 2 Ilara-Mokin (Ondo) | 302 | 37 (12.27) | |
| 3 Alie Ilie (Osun) | 300 | 32 (10.67) | |
| 4 Lie Twon (Osun) | 301 | 38 (12.62) | |
| 5 Ikwo (Ebonyi) | 536 | 36 (6.72) | |
| 6 Ohaozara (Ebonyi) | 548 | 28 (5.11) | |
| 7 Onicha (Ebonyi) | 537 | 27 (5.03) | |
| 8 Ishielu (Ebonyi) | 548 | 27 (4.93) | |
| 9 Nkanu east (Enugu) | 572 | 35 (6.12) | |
| 10 Anambra west (Anambra) | 539 | 25 (4.64) | |
| 11 Gwer east (Benue) | 567 | 32 (5.64) | |
| 12 Jos north (Plateau) | 456 | 26 (5.70) | |
| Gender | 0.068 | ||
| Female | 2324 | 148 (6.37) | |
| Male | 3190 | 244 (7.65) | |
| Age (years) | <0.001 | ||
| 05–8 | 2579 | 149 (5.78) | |
| 09–11 | 1596 | 121 (7.58) | |
| 12–14 | 1339 | 122 (9.11) | |
Table 1.
Socio-demographic characteristics of study population (n = 5514).
| Variable | Number of children | Percentage of the total sample (%) |
|---|---|---|
| Study site/state | ||
| 1 Ipogun (Ondo) | 308 | 5.59 |
| 2 Ilara-Mokin (Ondo) | 302 | 5.48 |
| 3 Alie Ilie (Osun) | 300 | 5.44 |
| 4 Lie Twon (Osun) | 301 | 5.46 |
| 5 Ikwo (Ebonyi) | 536 | 9.72 |
| 6 Ohaozara (Ebonyi) | 548 | 9.94 |
| 7 Onicha (Ebonyi) | 537 | 9.74 |
| 8 Ishielu (Ebonyi) | 548 | 9.94 |
| 9 Nkanu east (Enugu) | 572 | 10.37 |
| 10 Anambra west (Anambra) | 539 | 9.78 |
| 11 Gwer east (Benue) | 567 | 10.28 |
| 12 Jos north (Plateau) | 456 | 8.27 |
| Gender | ||
| Female | 2324 | 42.15 |
| Male | 3190 | 57.85 |
| Age (years) | ||
| 5–8 | 2579 | 46.77 |
| 9–11 | 1596 | 28.95 |
| 12–14 | 1339 | 24.28 |
3.2. Prevalence of S. haematobium
A total of 392 (7.1%) of the 5514 urine samples were positive for S. haematobium. The prevalence ranged from 4.6% in East Nigeria to 15.9% in West Nigeria. The highest prevalence was recorded in West Nigeria (sites 1–4), while the lowest was recorded in East Nigeria and in the central part of the country (sites 5–12). There was a statistically significant difference among all the studied sites, p ≤ 0.001, and two distinct clusters emerged between sites. Sites from the West (sites 1–4) had a prevalence >10%, while sites from the East (sites 5–12) had a prevalence of <7%. Moreover, 244 (7.6%) males were positive, while 148 (6.4%) females were positive, but no statistically significant difference in prevalence was found between the two genders (p = 0.068). There was a statistically significant difference in the prevalence among the age groups (p ≤ 0.001), whereby the prevalence of infection increased with age.
The multivariate logistic regression analysis of variables in Table 3 shows the relationship between urogenital schistosomiasis and sociodemographic, sociocultural and socioeconomic factors. The significant risk factors associated with S. haematobium infection include frequent contact with freshwater bodies (rivers/steams), washing/swimming and fishing. As expected, these results indicate a link between water contact and infection prevalence. Very few socioeconomic factors seemed to influence the infection status and only the primary education of fathers was significantly associated with the disease. The sociodemographic factor (the 12–14 year age group) was also significantly associated with the infection.
Table 3.
Univariate and multivariate logistic analyses of variables associated with the infection, adjusted and non-adjusted for socio-demographic factors, socio-economic status and environmental factors. OR: Odd ratio; CI: confidence interval.
| Variable | Total | Positive (%) | Crude OR |
Adjusted OR |
|---|---|---|---|---|
| (95% CI) | (95% CI) | |||
| Socio-demographic factors | ||||
| Age (years) | ||||
| 5–8 | 2579 | 5.78 | 1 | 1 |
| 9–11 | 1596 | 7.58 | 1.34 (1.04–1.72) | 1.30 (0.94–1.81) |
| 12–14 | 1339 | 9.11 | 1.63 (1.27–2.10)⁎ | 1.68 (1.21–2.33)⁎ |
| Gender | ||||
| Female | 2324 | 6.37 | 1 | 1 |
| Male | 3190 | 7.65 | 1.23 (0.98–1.50) | 1.14 (0.86–1.50) |
| Socio-economic factors | ||||
| Father's educational status | ||||
| Illiterate | 843 | 5.34 | 1 | 1 |
| Primary school | 3346 | 7.5 | 1.44 (1.04–1.99) | 1.63 (1.01–2.45)⁎ |
| Secondary school | 1169 | 7.53 | 1.44 (1.00–2.09) | 1.28 (0.80–2.05) |
| Tertiary school | 156 | 5.13 | 0.96 (0.44–2.07) | 0.95 (0.36–2.47) |
| Mother's educational status | ||||
| Illiterate | 538 | 6.32 | 1 | 1 |
| Primary school | 2887 | 6.58 | 1.04 (0.72–1.52) | 1.14 (0.71–1.85) |
| Secondary school | 2039 | 7.95 | 1.23 (0.87–1.87) | 1.39 (0.85–2.27) |
| Tertiary school | 50 | 12 | 2.02 (0.80–5.08) | 2.01 (0.63–6.45) |
| Father's occupation | ||||
| Peasant farmer | 3925 | 6.93 | 1 | 1 |
| Petty trader | 991 | 7.47 | 1.08 (0.83–1.41) | 1.24 (0.87–1.76) |
| Fisherman | 214 | 8.41 | 1.23 (0.75–2.03) | 1.14 (0.60–2.19) |
| Civil service | 383 | 7.31 | 1.06 (0.71–1.59) | 1.13 (0.67–1.93) |
| Mother's occupation | ||||
| Peasant farmer | 3643 | 7.05 | 1 | 1 |
| Petty trader | 1647 | 6.92 | 0.98 (0.78–1.23) | 0.77 (0.73–1.04) |
| Housewife | 92 | 7.61 | 1.09 (0.50–2.34) | 1.10 (0.38–3.10) |
| Civil service | 132 | 10.61 | 1.56 (0.88–2.76) | 1.52 (0.74–3.12) |
| Sociocultural factors | ||||
| Using borehole water | ||||
| No | 3003 | 7.89 | 1 | 1 |
| Yes | 2511 | 6.17 | 0.77 (0.62–0.95) | 0.80 (0.61–1.05) |
| Using river/stream water | ||||
| No | 1917 | 2.4 | 1 | 1 |
| Yes | 3597 | 9.62 | 4.33 (3.17–5.92)⁎ | 4.92 (3.34–7.24)⁎ |
| Washing/ swimming in river/stream water | ||||
| No | 3500 | 0.6 | 1 | 1 |
| Yes | 2014 | 18.42 | 37.41 (24.0–58.29)⁎ | 46.49 (27.64–78.19)⁎ |
| Fishing in freshwater | ||||
| No | 4776 | 3.62 | 1 | 1 |
| Yes | 738 | 29.67 | 11.23 (9.02–13.98)⁎ | 11.57 (8.74–15.32)⁎ |
Significant P-value < 0.05, p-value obtained from multivariate logistic regression with fixed effects for all explanatory variables.
4. Discussion
Infection with urogenital bilharziasis was found in all studied sites, though the prevalence was heterogeneous throughout the country. Infection rates in the eastern and central parts of Nigeria fell below the national average (9.5%), while all of the western sites were slightly above the national average. A systemic review from 1994 to 2015, using a unified pooled population, revealed a pooled prevalence range from 31.0% to 38.50% for all regions (Abdulkadir et al., 2017). A 50-year review by Ezeh et al. (2019), ranked the majority of our study sites (Benue, Ebonyi, Enugu, Osun and Plateau) as hyper-endemic zones (>50% prevalence), and the others (Anambra and Ondo) as moderately endemic zones (10–50% prevalence). Results from this survey showed a decrease in the prevalence of the disease in most of the studied areas when compared with previously highlighted results (Table 4). This suggests that recent efforts to fight the disease have yielded positive results. Over the past several years, Nigeria has worked to increase control efforts, thanks to continued support from global partners to help eliminate infection. Even with this support, the risk of reinfection and transmission of the parasite remains active in many areas in Nigeria. While MDA has helped to reduce morbidity, it is not enough to stop transmission in many parts of the country. A long-term well-structured control approach could help to provide a more sustainable result, similar to results achieved in China, Japan and Brazil (Bergquist et al., 2017).
Table 4.
Results obtained in the present study and reports by previous studies.
| Present survey |
Previous survey |
References |
|||
|---|---|---|---|---|---|
| State | Sample site | Prevalence (%) | Date | Prevalence (%) | |
| Ondo | Ifedore | 15.91 | Oct 2002-Oct 2003 | 59.0 | Oniya and Odaibo, 2006 |
| Ondo | Ifedore | 12.27 | |||
| Osun | Irepodun | 10.67 | Nov 2006-Jun 2007 Feb-July 2012 – |
62.0 55.80 51.10 |
Ugbomoiko et al., 2010 Awosolu, 2016 Bishop, 2017 |
| Osun | Irepodun | 12.62 | – 2013–2015 |
30.10 5.30 |
Igbeneghu et al., 2018 Amuga et al., 2020 |
| Ebonyi | Ikwo | 6.72 | – July-Dec 2016 |
17.50 10.0 |
Nwosu et al., 2015 Umoh et al., 2020 |
| Ebonyi | Ohaozara | 5.11 | 2012–2013 – Feb 2016-Jan 2017 |
15.30 6.30 & 79.40 3.90 |
Ivoke et al., 2014 Bishop, 2017 Nwachukwu et al., 2018 |
| Ebonyi | Onicha | 5.03 | Aug 2005-Jul 2006 | 26.80 27.0 |
Uneke et al., 2006 Elom et al., 2017 |
| Ebonyi | Ishielu | 4.93 | Jan-March 2009 – |
46.18 22.70 |
Ozowara et al., 2011 Onwe et al., 2016 |
| Enugu | Nkanu East | 6.12 | – | 34.10 13.60 |
Bishop, 2017 Aribodor et al., 2019 |
| Anambra | Anambra west | 4.64 | Oct 2007-Sept 2008 – |
12.80–19.80 2.90 |
Ugochukwu et al., 2013 Ndukwe et al., 2019 |
| Benue | Gwer east | 5.64 | Nov 2008-Sept 2009 Sept 2012 – – 2013–2015 |
38.60 36.0–64.0 41.50 20.70 13.10 |
Houmsou et al., 2012 Amuta and Houmsou, 2014 Bishop, 2017 Emmanuel et al., 2017 Amuga et al., 2020 |
| Plateau | Jos north | 5.70 | – | 6.40 | Dawet et al., 2012 |
An overall prevalence of 7.1% was obtained from a total of 5514 urine samples. Similar to results published in previous studies (Otuneme et al., 2014, Gbonhinbor and Abah, 2019), males were infected more than their female counterparts, although this result was not statistically significant. The lack of statistical significance could be as a result of a lack of differences in sociocultural behaviors between the two genders. In general, in most parts of south and central Nigeria, there are no gender-based cultural restrictions associated with the water bodies, even though Ezeh et al. (2019), reported that the females had a higher prevalence of the disease when compared to their male counterparts. Our study also revealed an increase in prevalence with respect to age among the primary school pupils (12–14 year age group). This is consistent with the findings of Gbonhinbor and Abah (2019), Amuga et al. (2020), and may be attributed to the fact that this age group engages in a number of water-related activities. In contrast, a study conducted by Angora et al. (2020) along the Ivory Coast, did not observe any difference in prevalence among the three age groups.
There was no significant association between the parents' educational status (except the father's primary education level), occupation and the infection status. This could be attributed to the fact that collecting water from the stream may not be related to the parent's profession or their societal status, but instead to daily activities in certain rural areas. This result is in contrast to the findings of Ugbomoiko et al. (2010), which showed that parents with a higher level of education could better understand the preventive strategies and explain them to their children. Nonetheless, Geleta et al. (2015), Angora et al. (2019), showed that there was a statistically significant increase in the frequency of the disease when a parent's occupation was listed as ‘farmer’.
The relationship between the prevalence of schistosomiasis and contact with freshwater bodies infested with cercariae is well established (Ugbomoiko et al., 2010; Gbonhinbor and Abah, 2019). As expected, we found that frequent contact with freshwater bodies infested with cercariae, like contact when collecting water for domestic use, recreation, swimming, washing and fishing, was associated with urogenital schistosomiasis. This is somewhat logical since bilharziasis is directly linked to exposure to contaminated freshwater.
S. haematobium and S. mansoni have been reported as endemic in Nigeria, but the latter is less prevalent and widespread (Garba et al., 2004). A survey carried out in 2015 in 19 states across Nigeria showed the proportion of S. haematobium and S. mansoni as 82% and 18%, respectively. The overlapping distribution of the two species could increase the chance of co-infection and could potentially lead to a severe effect on morbidity. As such, there is cause for concern with respect to the ongoing control challenges in these areas.
5. Conclusions
The results obtained in this study show that schistosomiasis is still endemic in the studied areas despite a significant increase in the treatment coverage index of praziquantel. This presents a significant health hazard, as the infected children may play a leading role in the spread of the parasite. However, the observed decrease in the prevalence of the disease in most of the study areas when compared to results reported by previous studies, suggests that recent efforts to fight the disease have yielded positive results. If these efforts are maintained over time, and are done in combination with a long-term well-structured control approach, elimination of the disease may be possible, at least in areas where the transmission is low.
Author contributions
A.O.M., M.N.C., M.A Conceptualization, Data curation. J.B., O.R., A.O.M., and J-F.A. Methodology, Resources. K.E.A. Formal analysis, Software. A.O.M. Writing - original draft, editing. J.B., and O.R. Writing - review. J.B. Project administration. A.O.M., J.B., and O.R. Funding acquisition
Funding
This research was funded by Campus France, France and Tertiary Education Trust Fund (TETfund), Nigeria and was supported by the program HySWARM (ANR-18-CE35-0001) from the French Research National Agency. Amos Onykwere is funded by Campus France. This study is set within the framework of the “Laboratoires d'Excellence (LABEX)” TULIP (ANR-10-LABX-41).
Declaration of Competing Interest
No conflict of interest.
Acknowledgments
We are also grateful to the teachers and head teachers, “Community Heads” and Church leaders that mobilized the children for this exercise. We want to thank the “Primary Health Centres” (PHC) for judiciously utilizing the funds provided for the purchase of medication for the infected children. We thank Jeanine Almany for proof reading services.
Contributor Information
A.M. Onyekwere, Email: onyekwereamos@gmail.com.
O. Rey, Email: Olivier.rey@univ-perp.fr.
M.C. Nwanchor, Email: mnwanchor@gmail.com.
M. Alo, Email: gianimarg@gmail.com.
E.K. Angora, Email: angorakpongbo2005@yahoo.fr.
J.F. Allienne, Email: allienne@univ-perp.fr.
J. Boissier, Email: boissier@univ-perp.fr.
References
- Abdulkadir A., Ahmed M., Abubakar B.M., Suleiman I.E., Yusuf I., Imam I.M., Sule A.A., Tela U.M., Dogo H.M., Yakasai A.M., Musa B.M. Prevalence of urinary schistosomiasis in Nigeria, 1994–2015: Systematic review and meta-analysis. Afr. J. Urol. 2017;23(3):228–239. [Google Scholar]
- Ajakaye O.G., Adedeji O.I., Ajayi P.O. Modeling the risk of transmission of schistosomiasis in Akure North Local Government Area of Ondo State, Nigeria using satellite derived environmental data. PLoS Negl. Trop. Dis. 2017;11(7):1–20. doi: 10.1371/journal.pntd.0005733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amuga G.A., Nebe O.J., Nduka F.O., Njepuome N., Dakul D.A., Isiyaku S., Ngige E., Jibrin S., Jacob S.M., Nwoye I.A., Nwankwo U., Urude R., Aliyu S.M., Garba A., Adamani W., Nwosu C.O., Anagbogu I.A., Dixon R., Clark A., Adeoye G.O. Schistosomiasis: epidemiological factors enhancing transmission in Nigeria. Glob. Res. J. Public Health Epedemiol. 2020;8(7):23–32. ISSN-2360-7920. [Google Scholar]
- Amuta E.U., Houmsou R.S. Prevalence, intensity of infection and risk factors of urinary schistosomiasis in pre-school and school aged children in Guma Local Government Area, Nigeria. Asian Pac J Trop Med. 2014;7(1):34–39. doi: 10.1016/S1995-7645(13)60188-1. [DOI] [PubMed] [Google Scholar]
- Angora E.K., Boissier J., Menan H., Rey O., Tuo K., Touré A.O., Coulibaly J.T., Méité A., Raso G., N’Goran E.K., Utzinger J., Balmer O. Prevalence and risk factors for Schistosomiasis among Schoolchildren in two settings of Côte d’Ivoire. Trop. Med. Infect. Dis. 2019;4(3):110–123. doi: 10.3390/tropicalmed4030110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angora E.K., Allienne J.F., Rey O., Menan H., Touré A.O., Coulibaly J.T., Raso G., Yavo W., N’Goran E.K., Utzinger J., Balmer O., Boissier J. High prevalence of Schistosoma haematobium × Schistosoma bovis hybrids in school children in Côte d’Ivoire. Parasitology. 2020;147(3):287–294. doi: 10.1017/S0031182019001549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aribodor D.N., Bassey S.A., Yoonuan T., Sam-Wobo S.O., Aribodor O.B. Analysis of Schistosomiasis and soil-transmitted helminths mixed infections among pupils in Enugu State, Nigeria: implications for control. Infect. Dis. Health. 2019;24(2):98–106. doi: 10.1016/j.idh.2018.12.003. [DOI] [PubMed] [Google Scholar]
- Atalabi T.E., Adoh S.D., Eze K.M. The current epidemiological status of urogenital schistosomiasis among primary school pupils in Katsina State, Nigeria: An imperative for a scale up of water and sanitation initiative and mass administration of medicines with Praziquantel. PLoS Negl. Trop. Dis. 2018;12(7):1–19. doi: 10.1371/journal.pntd.0006636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awosolu O.B. Epidemiology of urinary schistosomiasis and knowledge of health personnel in rural communities of south-werstern Nigeria. J. Parasitol. Vector Biol. 2016;8(10):99–106. doi: 10.5897/JPVB2016.0237. [DOI] [Google Scholar]
- Bergquist R., Zhou X.-N., Rollinson D., Reinhard-Rupp J., Klohe., K. Elimination of schistosomiasis: the tools required. Infect. Dis. Poverty. 2017;6(158):1–9. doi: 10.1186/s40249-017-0370-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop H.G. Menace of Schistosomiasis: its true neglected nature in Nigeria. MenCrave Online J. Pub. Health. 2017;6(5):1–7. doi: 10.15406/mojh2017.06.00186. [DOI] [Google Scholar]
- Colley D.G., Bustinduy A.L., Secor W.E., King C.H. Human schistosomiasis. Lancet. 2014;383(9936):2253–2264. doi: 10.1016/S0140-6736(13)61949-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawet A., Yakubu D.P., Longmut R., Benjamin C.B., Daburum Y.H., Nannim N. Prevalence and intensity of Schistosoma haematobium among residents of Gwong and Kabong in Jos North Local Government Area, Plateau State, Nigeria. Int. J. Biol. Chem. Sci. 2012;6(4):1557–1565. doi: 10.4314/ijbcs.v6i4.15. [DOI] [Google Scholar]
- Ejike C.U., Oluwole A.S., Mogaji H.O., Adeniran A.A., Alabi O.M., Ekpo U.F. Development and Testing of Schisto and LaddersTM, an Innovative Health Educational Game for Control of Schistosomiasis in Schoolchildren. Development and testing of Schisto and LaddersTM, an innovative health educational game for control of schistosomiasis in schoolchildren. BMC Res. Notes. 2017;10(1):1–9. doi: 10.1186/s13104-017-2545-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elom J.E., Odikamnoro O.O., Nnachi A.U., Ikeh I., Nkwuda J.O. Variability of urine parameters in children infected with Schistosoma haematobium in Ukawu community, Onicha local government area, Ebonyi state, Nigeria. Afr. J. Infect. Dis. 2017;11(2):10–16. doi: 10.21010/ajid.v11i2.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmanuel O.I., Agbo O.E., Uche A.J., Odeh U.P., Agogo I.M. Comparative evaluation of the prevalence of urinary schistosomiasis in two contrasting communities in Benue State, Nigeria. Int. J. Infect. Dis. Therapy. 2017;2(3):48–52. doi: 10.11648/j.ijidt.20170202.15. [DOI] [Google Scholar]
- Ezeh C.O., Onyekwelu K.C., Akinwale O.P., Shan L., Wei H. Urinary schistosomiasis in Nigeria: a 50-year review of prevalence, distribution and disease burden. Parasite. 2019;26(19):1–10. doi: 10.1051/parasite/2019020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garba A., Labbo R., Tohon Z., Sidiki A., Djibrilla A. Emergence of Schistosoma mansoni in the Niger River valley, Nigeria. Trans. R. Soc. Trop. Med. Hyg. 2004;98(5):296–298. doi: 10.1016/S0035-9203(03)00070-1. [DOI] [PubMed] [Google Scholar]
- Gbonhinbor J., Abah A.E. Prevalence of Urogenital Schistosomiasis in Four Communities in Ogbia Local Government Area, Bayelsa State, Nigeria. Int. J. Trop. Dis. Health. 2019;39(3):1–9. doi: 10.9734/ijtdh/2019/v39i330206. [DOI] [Google Scholar]
- Geleta S., Alemu A., Getie S., Mekonnen Z., Erko B. Prevalence of urinary schistosomiasis and associated risk factors among Abobo primary school children in Gambella Regional State, southwestern Ethiopia: A cross sectional study. Parasit. Vectors. 2015;8(215):1–9. doi: 10.1186/s13071-015-0822-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Network Neglected Tropical Diseases . SABIN: Vaccine Institute, USA; 2015. Government of Nigeria Releases New Data on the Prevalence of Schistosomiasis and Intestinal Worms. [Google Scholar]
- Herrick J.R., Hotez P.J., Wanga V., Coffeng L.E., Haagsma J.A., BasaÂñez M.G., et al. The global burden of disease study 2013: what does it mean for the NTDs? PLoS Negl. Trop. Dis. 2017;21(8):1–21. doi: 10.1371/journal.pntd.0005424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotez P.J., Asojo O.A., Adesina A.M. Nigeria: “Ground Zero” for the high prevalence neglected tropical diseases. PLoS Negl. Trop. Dis. 2012;6(7):1–5. doi: 10.1371/journal.pntd.0001600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houmsou R., Kela S., Suleiman M. Performance of microhaematuria and proteinuria as measured by urine reagent strips in estimating intensity and prevalence of Schistosoma haematobium infection in Nigeria. Asian Pac J Trop Med. 2011;4(12):997–1000. doi: 10.1016/S1995-7645(11)60233-2. [DOI] [PubMed] [Google Scholar]
- Houmsou R.S., Amuta E.U., Sar T.T. Profile of an epidemiological study of urinary schistosomiasis in two local government areas of Benue state, Nigeria. Int. J. Med. Biomed. Res. 2012;1(1):39–48. doi: 10.14194/ijmbr.117. [DOI] [Google Scholar]
- Igbeneghu C., Adedokun S.A., Akindele A.A., Olisekodiaka J.M., Idolor D.E., Ojurongbe O. No Association between Urogenital Schistosomiasis and HIV Infection among Children in Ore Community, Southwestern Nigeria. Ann. Res. Rev. Biol. 2018;27(1):1–7. doi: 10.9734/ARRB/2018/42007. [DOI] [Google Scholar]
- Ivoke N., Ivoke O.N., Nwani C.D., Ekeh F.N., Asogwa C.N., Atama C.I., Eyo J.E. Prevalence and transmission dynamics of Schistosoma haematobium infection in a rural community of southwestern Ebonyi State, Nigeria. Trop. Biomed. 2014;31(1):77–88. https://pubmed.ncbi.nlm.nih.gov/24862047/ [PubMed] [Google Scholar]
- Manson S.P., Manson-Bahr P.E.C., Bell D.R. Manson’s tropical diseases. Baillière Tindall. 1987:448–485. [Google Scholar]
- Mott K.E., Baltes R., Bambagha J., Baldassini B. Field studies of a reusable polyamide filter for detection of Schistosoma haematobium eggs by urine filtration. Tropenmed. Parasitol. 1982;33(4):227–228. [PubMed] [Google Scholar]
- Ndukwe Y.E., Obiezue R.N.N., Aguzie I.O.N., Anunobi J.T., Okafor F.C. Mapping of Urinary Schistosomiasis in Anambra State, Nigeria. Ann. Glob. Health. 2019;85(1):1–10. doi: 10.5334/aogh.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwachukwu P.C., Ohaeri C.C., Ukpai O.M., Irole-eze O.P., Amaechi E.C., E.C. Prevalence of Schistosoma haematobium infection among school aged children in Afipko North local government area, Ebonyi State, Nigeria. Sri Lankan J. Biol. 2018;3(2):1–8. doi: 10.4038/sljb.v3i2.22. [DOI] [Google Scholar]
- Nwosu D.C., Ifeanyi O.B., Ozims S.J., Ezeama M.C., Uduji H.I. Prevalence of Urinary Schistosomiasis Infection among Primary School Pupils in Ezza-North Local Government Area of Ebonyi State. Int. J. Curr. Microbiol. App. Sci. 2015;4(5):1151–1157. https://www.researchgate.net/publication/322276681 [Google Scholar]
- Oniya M.O., Odaibo A.B. Reinfection Pattern and Predictors of Urinary Schistosomiasis among School Pupils from a Southwestern Village in Nigeria. Int. J. Trop. Med. 2006;1(4):173–177. [Google Scholar]
- Onwe S.O., Ani O.C., Uhuo C.A., Onwe C.S., Odikamnoro O.O. Studies of Urinary Schistosomiasis amongst School Age Children in Ebonyi North Senatorial District of Ebonyi State, Nigeria. Int. J. Trop. Dis. Health. 2016;18(3):1–7. doi: 10.9734/IJTDH/2016/13138. [DOI] [Google Scholar]
- Opara K.N., Wilson E.U., Yaro C.A., Alkazmi L., Udoidung N.I., Chikezie F.M., Bassey B.E., Batiha G.E.L.-S. Prevalence, Risk Factors, and Coinfection of Urogenital Schistosomiasis and Soil-Transmitted Helminthiasis among Primary School Children in Biase, Southern Nigeria Prevalence, Risk Factors, and Coinfection of Urogenital Schistosomiasis and Soil-Transmitted Helminthiasis among Primary School Children in Biase, Southern Nigeria. J. Parasitol. Res. 2021;2021(3):1–12. doi: 10.1155/2021/6618394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossai O.P., Dankoli R., Nwodo C., Tukur D., Nsubuga P., Ogbuabor D., Ekwueme O., Abonyi G., Ezeanolue E., Nguku P., Nwagbo D., Idris S., Eze G. Bacteriuria and urinary schistosomiasis in primary school children in rural communities in Enugu State, Nigeria. Pan Afr. Med. J. 2014;18(suppl 1) (15):1–5. doi: 10.11694/pamj.supp.2014.18.1.4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozowara N.L., Njoku O.O., Odikamnoro O.O., Uhuo C. Study of the prevalence of Schistosoma haematobium infection and the treatment using praziquantel among school children in Ezza north local government area of Ebonyi state, Southeast Nigeria. Eur. J. Exp. Biol. 2011;1(2):103–108. ISSN-2248-9215. [Google Scholar]
- Singh K., Muddasiru D., Singh J. Current status of schistosomiasis in Sokoto, Nigeria. Parasite Epidemiol. Control. 2016;1(3):239–244. doi: 10.1016/j.parepi.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchendu O., Oladoyin V., Idowu M., Adeyera O., Olabisi O., Oluwatosin O., Leigh G. Urinary schistosomiasis among vulnerablechildren in a rehabilitation home in Ibadan, Oyo state, Nigeria. BMC Infect. Dis. 2017;17(1):1–7. doi: 10.1186/s12879-017-2591-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugbomoiko U.S., Ofoezie I.E., Okoye I.C., Heukelbach J. Factors associated with urinary schistosomiasis in two peri-urban communities in south-western Nigeria. Ann. Trop. Med. Parasitol. 2010;104(5):409–419. doi: 10.1179/136485910X12743554760469. [DOI] [PubMed] [Google Scholar]
- Ugochukwu D.O., Onwuliri C.O.E., Osuala U., Dozie I.N.S., Opara F.N., Nweny U.C. Endemicity of schistosomiasis in some parts of Anambra State, Nigeria. J. Med. Lab. Diagn. 2013;4(5):54–61. doi: 10.5897/JMLD2013.0072. [DOI] [Google Scholar]
- Umoh N.O., Nwamini C.F., Inyang N.J., Umo A.N., Usanga V.U., Nworie A., Elom M.O., Ukwah B.N. Prevalence of urinary schistosomiasis amongst primary school children in Ikwo and Ohaukwu Communities of Ebonyi State, Nigeria. Afr. J. Lab. Med. 2020;9(1):1–5. doi: 10.4102/ajlm.v9i1.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uneke C.J., Oyibo P., Ugwuoru C., Nwanokwai A., Iloegbunam R. Urinary schistosomiasis among school age children in Ebonyi State, Nigeria. Internet J. Lab. Med. 2006;2(1):1–7. doi: 10.5580/fd0. [DOI] [Google Scholar]
- Yauba S.M., Rabasa A.I., Farouk A.G., Elechi H.A., Ummate I., Ibrahim B.A., Ibrahim H.A., Baba A.S., Boda T.A., Olowu W.A. Urinary schistosomiasis in Boko Haram-related internally displaced Nigerian children. Saudi J. Kidney Dis. Transpl. 2018;29(6):1395–1402. doi: 10.4103/1319-2442.248286. [DOI] [PubMed] [Google Scholar]