Abstract
Background
Tanzania has a high prevalence of urogenital schistosomiasis. Praziquantel is administered to school-age children on an annual basis as part of efforts to reduce transmission and morbidity associated with heavy infections. We investigated the prevalence, knowledge, and practices of urogenital schistosomiasis transmission, as well as compliance with mass drug administration (MDA) among schoolchildren in Masasi District.
Materials and methods
A cross-sectional survey was conducted in five primary schools. A pre-tested questionnaire was used to assess knowledge and practice related to the transmission of urogenital schistosomiasis, as well as compliance with MDA. Collected urine samples were examined macroscopically for macrohematuria. They were then tested for microhematuria and Schistosoma haematobium (S. haematobium) eggs with urine dipsticks and filtration technique, respectively.
Findings
The study included 389 primary school children in total. Overall, 27 (6.9%) of children had S. haematobium infection, and 37 (9.5%) had microhematuria. The mean (SD) intensity was 123.4 (247.4) eggs per 10 ml of urine. A total of 10 (2.6%) had heavy intensity of infection. The majority (94.9%) reported having complied to the previous round of MDA six months prior to this study, and 308 (79.2%) were aware that water contact is associated with an increased risk of urogenital schistosomiasis infection. Nevertheless, 182 (46.8%) of the participants engaged in swimming activities, with 92 (50.9%) of the participants being female. The prevalence of urogenital schistosomiasis was higher (10.9%) among children who participated in swimming activities versus those who did not (3.4%) (P = 0.003).
Conclusion
Despite high MDA compliance, urogenital schistosomiasis is still prevalent among primary school children in Masasi District. Children who swim in freshwater bodies such as rivers and ponds are more likely to contract urogenital schistosomiasis.
Keywords: Urogenital schistosomiasis, Schistosoma haematobium, Praziquantel, Mass drug administration, Hematuria
1. Introduction
Schistosomiasis affects nearly 240 million people worldwide. About 90% of the people at risk live in Sub-Saharan Africa (SSA) (World Health Organization, 2021a). The disease manifests itself in the gastrointestinal tract or the genital-urinary tract. Schistosoma haematobium (S. haematobium) causes urogenital schistosomiasis, which affects approximately 112 million people in SSA, 70 million of whom have hematuria (World Health Organization, 2020). Urogenital schistosomiasis is commonly characterized by hematuria (blood in the urine), bladder and ureter fibrosis, and kidney damage, which is sometimes diagnosed in advanced cases. In women, urogenital schistosomiasis can manifest as genital lesions or vaginal bleeding, a condition known as female genital schistosomiasis, which is caused by the presence of schistosome eggs and worms in the genital organs (World Health Organization, 2021a). People living in areas with poor sanitation, overcrowding, and low socioeconomic status are at a higher risk of infection (Ntonifor et al., 2015; Knopp et al., 2013; Dawaki et al., 2016; Bukindu et al., 2016). School-aged children have the highest prevalence and intensity of schistosomiasis infection (Hotez and Kamath, 2009).
Tanzania is ranked second to Nigeria for the countries with high prevalence of schistosomiasis in SSA (Mazigo et al., 2012), with a prevalence ranging from 12.7 to 87.6%. There is evidence of infection in all regions (Neglected Tropical Diseases Control Program, 2015). Urogenital schistosomiasis is highly endemic along the coastal belt of Tanzania (Brooker et al., 2009). Human exposure to urogenital schistosomiasis is primarily related to occupational activities such as fishing, farming, or recreational activities in the basin or within permanent or temporary freshwater bodies such as lakes, rivers, dams, swampy areas, or roadside ditches (Manz et al., 2020; Ndyomugyenyi and Minjas, 2001).
The National Schistosomiasis and Soil-Transmitted Helminth Control Program was established in 2004 to support schistosomiasis control initiatives by administering praziquantel and albendazole via mass drug administration (MDA) to primary school-age children (Bukindu et al., 2016; Mazigo et al., 2010). MDA aims to reduce transmission and control morbidity associated with heavy infections (Bukindu et al., 2016). However, insufficient use of preventive measures among school-age children has been reported (Munisi et al., 2017). Moreover, because chemotherapy does not prevent subsequent infection, re-infection is possible if water contact is maintained (Chaula and Tarimo, 2014). A lack of knowledge about disease transmission and preventive measures also has an influence on disease transmission (Munisi et al., 2017; Person et al., 2016; Donohue et al., 2017).
Masasi district is endemic for S. haematobium. For more than a decade, the district has been implementing a school-based deworming campaign. However, there is little data on the current state of urogenital schistosomiasis transmission among school-aged children. We assessed the prevalence, knowledge, and practices related to urogenital schistosomiasis transmission, as well as compliance with MDA, among school-aged children in the District.
2. Materials and methods
2.1. Study area
The study was carried out in Masasi District, (10.7348°S, 38.8044° E) southern-east Tanzania. The district is bordered to the south by the Ruvuma River, which serves as a fishing site. According to the National Population Census 2012, the district has a total population of 260,856 people (National Bureau of Statistics, 2012). The average temperature in the area is 25.4 °C, and the average annual rainfall is 1024 mm, with 82% humidity (Weather Atlas, n.d.). The residents primarily engage in cashew nut farming and fishing (Masasi District profile, 2021). Schistosomiasis and soil-transmitted helminths control campaigns began in 2004 with annual MDA of praziquantel and albendazole to school-aged children.
2.2. Study design and study population
A cross-sectional study was carried out in five selected primary schools: Mbaju, Chigugu, Mbemba in Chigugu ward, Lukuledi and Mkululu in Lukuledi and Mkululu wards (Fig. 1). The study population consisted of primary school students in grades one through six who attended the selected schools during the study period.
Fig. 1.
A map showing the location of Masasi district and the schools recruited for Urogenital schistosomiasis survey (Credit: Alex. J. Limwagu).
2.3. Eligibility criteria
The study included children aged 6–15 years, present during the data collection day and whose parents provided written informed consent for participation in the study. The study excluded children with a history of using anti-schistosome drugs (praziquantel) for 14 days prior to or at the time of the study.
2.4. Sample size and sampling
Pourhoseingholi's sample size estimation method was used (Pourhoseingholi et al., 2013). Based on a recent study in Lindi, the input parameters included an estimated 49% proportion of urogenital schistosomiasis (Bakuza, 2018). We also used a 95% level of confidence and a 5% absolute precision; we also adjusted for a 10% non-response rate.
A two-stage sampling technique was used. First, five primary schools were chosen from a list of 60 public schools in Masasi district council using a cluster sampling technique. To obtain the desired number of children per school, the calculated sample size of 422 was divided by five schools. The number obtained for each school was then divided by six to obtain the sample of children from class one to class six. Then, using a simple random sampling technique, we recruited the required number of children for each class. Briefly, eligible students were mobilized, informed about the study, and instructed to select one folded paper labeled “Included” or “Not Included.” Those who chose a folded piece of paper with the word “Included” on it were recruited.
2.5. Data collection
2.5.1. Questionnaire survey
A pre-tested structured questionnaire was used to collect demographic information from participants, such as age and gender. The class registers were used to determine the age of each child who took part in the study. The knowledge of urogenital schistosomiasis transmission was assessed. We also asked questions about toilet use, hand washing, shoe-wearing habits, swimming, and water contact behavior. Participation and drug uptake during the previous round of MDA, six months prior to data collection, were also evaluated. Those who did not participate/swallow the tablets were asked to explain why.
2.5.2. Urine sample collection
Each participant provided a urine sample in a clean, transparent, 50 ml dry wide-mouthed, labeled plastic container, as previously described (Ngasala et al., 2020). The urine samples were collected between 10:00 a.m. and 2:00 p.m., which is the best time for egg passage. On-site visual examinations were performed on each sample to screen for macrohematuria. Urine chemical reagent strips were also used to screen urine samples for microhematuria (Urine 10 parameters, Neotest Multistix, United Kingdom). To test for occult blood, a strip was dipped into the urine container for five seconds. To estimate the amount of blood in the urine, the color change of the strip was compared to a color chart on a strip container. According to the manufacturer's recommendations, we recorded the results as negative or positive. For the preservation of Schistosoma eggs, two to four drops of 10% formaldehyde were added to each sample. The samples were then transferred to the parasitology laboratory at Muhimbili University of Health and Allied Sciences for parasitological examination.
2.5.3. Examination for schistosoma haematobium eggs
Parasitological examination was carried out in the laboratory using the urine filtration technique. Urine samples were gently shaken before being drawn into a syringe and discharged through a nucleopore filter membrane as previously described (Ngasala et al., 2020). An experienced laboratory technologist placed a nucleopore filter membrane on the slide, stained it with Lugol's iodine, and examined it under a microscope. The number of eggs per 10 ml of urine was used to determine the intensity of the infection. To differentiate between light and heavy intensity, a threshold of 50 eggs was used (World Health Organization, 2021b).
2.6. Data analysis
The Statistical Package for Social Sciences software version 20.0 (IBM Corp., Armonk, NY, USA) was used to enter and analyze data. To estimate the overall prevalence of urogenital schistosomiasis, descriptive statistics were calculated and stratified by sex, age group, class, and school. To compare the prevalence between groups, the chi-square test was used (95% confidence interval (CI) and a P-value <0.05% considered significant). Urine filtration (microscopy) was used as the gold standard technique to determine the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of the indirect diagnostic method (microhematuria).
2.7. Ethical consideration
The ethical approval was obtained from the Muhimbili University of Health and Allied Sciences Institution Review Board (IRB Ref.No.DA.287/298/01A/). The District Executive Director (DED) granted permission to conduct the study in selected schools through the District Education Officer and the head teacher of the respective school. Finally, the parents or guardians of the selected children provided written informed consent. Participation was entirely voluntary; parents/guardians had the option of accepting or declining their children's participation in the study. Furthermore, children provided verbal assent to ensure that their participation was voluntary. If a parent or guardian refused, the child's name was removed from the list of participants. Children with urinary schistosomiasis were referred to a nearby medical facility for treatment.
3. Results
3.1. Demographic characteristics of the study participants
A total of 389 of the 422 children chosen for the study met the inclusion criteria. The average age was 10.1 ± 2.1 years, and 169 (43.5%) were between the ages of 10 and 12 years. More than half of the participants, 210 (54%) were female (Table 1).
Table 1.
Demographic characteristics of study participants.
| Variable | Category | Frequency | Percentage (95%CI) |
|---|---|---|---|
| Sex | Male | 179 | 46.0 (40.6–50.9) |
| Female | 210 | 54.0 (49.1–59.4) | |
| Age group | 6–9 years | 165 | 42.4 (37.3–47.3) |
| 10–12 years | 169 | 43.5 (38.6–48.6) | |
| 13–15 years | 55 | 14.1 (10.8–18.0) | |
| School | Mbaju | 78 | 20.1 (15.9–23.7) |
| Chigugu | 78 | 20.1 (16.5–24.2) | |
| Mbemba | 77 | 19.8 (15.9–24.2) | |
| Lukuledi | 78 | 20.1 (16.2–23.9) | |
| Mkululu | 78 | 20.1 (16.2–24.2) | |
| Class | I | 65 | 16.7 (12.9–20.3) |
| II | 64 | 16.5 (12.6–20.1) | |
| III | 65 | 16.7 (13.1–20.6) | |
| IV | 65 | 16.7 (13.1–20.6) | |
| V | 65 | 16.7 (13.1–20.6) | |
| VI | 65 | 16.7 (13.1–20.8) |
3.2. Prevalence and intensity of urogenital schistosomiasis
The urine filtration method detected S. haematobium infection in 27 (6.9%) of the 389 participants. Male and female infection rates were 8.9% and 5.2%, respectively (P = 0.166). Children attending Chigugu primary school had a significantly higher prevalence of S. haematobium infection, 17.9%, while children attending Mbemba primary school had the lowest prevalence, 2.6% (P = 0.001) (Table 2).
Table 2.
Prevalence of Urogenital schistosomiasis by filtration and indirect diagnostic methods.
| Variable | Category | Macrohematuria |
Microhematuria |
Filtration |
|||
|---|---|---|---|---|---|---|---|
| Positive (%) | p-value | Positive (%) | p-value | Positive (%) | P-value | ||
| Overall (n = 389) | 5(1.3) | 37 (9.5) | 27 (6.9) | ||||
| Sex | Male | 2 (1.1) | 1.000* | 20 (11.2) | 0.302 | 16 (8.9) | 0.166 |
| Female | 3 (1.4) | 17 (8.1) | 11 (5.2) | ||||
| Age group (years) | 6–9 | 2 (1.2) | 1.000* | 10 (6.1) | 0.130 | 11 (6.7) | 0.794 |
| 10–12 | 3 (1.8) | 21 (12.4) | 11 (6.5) | ||||
| 13–15 | 0 (0.0) | 6 (10.9) | 5 (9.1) | ||||
| School class | Mbaju | 0 (0.0) | 0.207* | 9 (11.5) | 0.000 | 4 (5.1) | 0.001 |
| Chigugu | 1 (1.3) | 18 (23.1) | 14 (17.9) | ||||
| Mbemba | 1 (1.3) | 4 (5.2) | 2 (2.6) | ||||
| Lukuledi | 0 (0.0) | 2 (2.6) | 4 (5.1) | ||||
| Mkululu | 3 (3.8) | 4 (5.1) | 3 (3.8) | ||||
| I | 1 (1.5) | 0.905* | 4 (6.2) | 0.064 | 5 (7.7) | 0.862* | |
| II | 0 (0.0) | 3 (4.7) | 4 (6.2) | ||||
| III | 1 (1.5) | 4 (6.2) | 3 (4.6) | ||||
| IV | 2 (3.1) | 10 (15.4) | 4 (6.2) | ||||
| V | 0(0.0) | 11 (16.9) | 7 (10.8) | ||||
| VI | 1 (1.5) | 5 (7.7) | 4 (6.2) | ||||
The mean (SD) infection intensity (eggs per 10 ml of urine) was 123.4 (247.4). About 10/27 (37.1%) of S. haematobium infected children had high intensity, equivalent to 2.6% of all participants. Children attending Chigugu primary school had higher mean infection intensity (196.2 eggs per 10 ml of urine), whereas children attending Mbemba primary school had the lowest mean infection intensity (2 eggs per 10 ml of urine). Males had a lower mean egg count (188.6 eggs per 10 ml of urine) than females (78.6 eggs per 10 ml). Likewise, children aged 10 to 12 years had a higher mean egg count (190.5 eggs per 10 ml) (P > 0.05) (Table 3). There was no statistically significant difference in the prevalence or intensity of urogenital schistosomiasis by gender or age group.
Table 3.
Intensity of S. haematobium infection presented by sex, age group, school and class.
| Variable | Category | No. of positive cases | Mean (SD) eggs/10 ml | Light, n (%) | Heavy, n (%) | p-value |
|---|---|---|---|---|---|---|
| Overall | – | 27 | 123.4 (247.4) | 17 (62.9) | 10 (37.1) | 0.988 |
| Sex | Male | 16 | 78.6 (117.7) | 10 (62.5) | 6 (37.5) | 1.000* |
| Female | 11 | 188.6 (361.1) | 7 (63.6) | 4 (36.4) | ||
| Age group in years | 6–9 | 11 | 90.6 (130.8) | 6 (54.5) | 5 (45.5) | 0.871* |
| 10–12 | 11 | 190.5 (360.1) | 7 (63.6) | 4 (36.4) | ||
| 13–15 | 5 | 47.8 (91.3) | 4 (80.0) | 1 (20.0) | ||
| School | Mbaju | 4 | 60.5 (100.7) | 3 (75.0) | 1 (25.0) | 0.238* |
| Chigugu | 14 | 196.2 (324.2) | 7 (50.0) | 7 (50.0) | ||
| Mbemba | 2 | 2.0 (1.4) | 2 (100.0) | 0 (0.0) | ||
| Lukuledi | 4 | 10.8 (11.4) | 4 (100.0) | 0 (0.0) | ||
| Mkululu | 3 | 98.3 (102.7) | 1 (33.3) | 2 (66.7) | ||
| Class | I | 5 | 141.0 (171.7) | 2 (40.0) | 3 (60.0) | 0.041* |
| II | 4 | 7.5 (9.2) | 4 (100.0) | 0 (0.0) | ||
| III | 3 | 136.7 (68.7) | 0 (0.0) | 3 (100.0) | ||
| IV | 4 | 292.8 (505.9) | 2 (50.0) | 2 (50.0) | ||
| V | 7 | 141.0 (279.3) | 5 (71.4) | 2 (29.6) | ||
| VI | 4 | 7.0 (4.5) | 4 (100.0) | 0 (0.0) |
3.3. Diagnostic accuracy of urogenital schistosomiasis using urine dipstick screening method
Macrohematuria and microhematuria were found in 1.3% and 9.5% of participants, respectively. The prevalence of microhematuria varied significantly between schools (P = 0.0001) (23.1% in Chigugu vs. 2.6% in Lukuledi). Children aged 10–12 years had a higher prevalence of microhematuria (12.4%) (P = 0.13) (Table 2). There were no significant differences in the prevalence of microhematuria by age, gender, or class (P > 0.05). The Fisher exact test revealed an association between the prevalence of urogenital schistosomiasis and microhematuria. The urine reagent strips had a high sensitivity (92.6%) and specificity (96.7%). The NPV and PPV were also high, at 99.4% and 67.6%, respectively (Table 4).
Table 4.
Diagnostic accuracy of urogenital schistosomiasis using urine dipstick screening method.
| Urine dipstick | Urine filtration |
Total | |
|---|---|---|---|
| Egg(s) present | Egg(s) absent | ||
| Positive | 25 | 12 | 37 |
| Negative | 2 | 350 | 352 |
| Total | 27 | 362 | 389 |
(Fisher exact test, P = 0.000)
3.4. Knowledge on transmission of urogenital schistosomiasis transmission among primary school children
Overall, 308 people (79.2%) agreed that water contact behaviors like swimming, bathing, and washing clothes in lakes or rivers are linked to urogenital schistosomiasis infection. The majority of participants (324, or 83.3%) agreed that urinating in freshwater bodies causes urogenital schistosomiasis transmission. Walking barefoot is associated with urogenital schistosomiasis infection, according to 298 (76.6%) participants. Also, 299 (76.9%) of the children reported that urogenital schistosomiasis is associated with eating without washing hands. However, knowledge of transmission was not associated with urogenital schistosomiasis infection (P > 0.05).
3.5. Practices related to transmission of urogenital schistosomiasis among primary school children
Overall, 182 (46.8%) of the participants engaged in swimming activities, with 92 (50.9%) of the participants being female. In addition, 201 (51.7%) had a history of water contact, such as bathing or washing clothes in rivers or ponds. About 386 (99.2%) used the toilets while at school; 315 (80.9%) washed their hands after using the toilet; and 374 (96.1%) washed their hands before eating. A minority (15.4%) of children reported urinating in water, and 136 (34%) wore shoes only to school. Infection rates were higher (10.9%) among children who participated in swimming activities compared to 3.4% among those who did not (P = 0.003) (Table 5). In addition, the proportion of infected children who participated in swimming activities was higher; 12 (13.3%) in boys compared to 8(8.7%) in girls. Similarly, children with water contact behavior had a higher infection rate (8.9%) than children with no reported water contact history (4.8%) (P = 0.01) (Table 5).
Table 5.
Knowledge and practice for urogenital schistosomiasis transmission among primary school children.
| Question | Options | Response | No. (%) of responses | Schistosomiasis infection |
P - value | |
|---|---|---|---|---|---|---|
| Positive | Negative | |||||
| Do the following could results to urogenital schistosomiasis transmission? | Walking with bare foot | Yes | 298 (76.6) | 22 (7.4) | 276 | 0.535 |
| No | 91 (23.4) | 5 (5.5) | 86 | |||
| Urinating outdoor | Yes | 298 (76.6) | 21 (7.0) | 277 | 0.555 | |
| No | 91 (23.4) | 6 (6.6) | 85 | |||
| Eating without hand washing | Yes | 299 (76.9) | 22 (7.3) | 277 | 0.535 | |
| No | 90 (23) | 5 (5.6) | 85 | |||
| Not washing hands after toilet | Yes | 298 (76.6) | 22 (7.4) | 276 | 0.555 | |
| No | 91 (23.4) | 5 (5.5) | 86 | |||
| Urinating in water | Yes | 324 (83.3) | 22 (6.8) | 302 | 0.790 | |
| No | 65 (16.7) | 5 (7.7) | 60 | |||
| Contact with river and pond water (swimming, bathing and washing clothes) | Yes | 308 (79.2) | 21 (6.8) | 287 | 0.853 | |
| No | 83 (21.3) | 8 (1) | 75 | |||
| Do you practice the following? | Urinate in toilet when at school | Yes | 386 (99.2) | 27 (6.9) | 359 | 1.000* |
| No | 3(0.8) | 0 (0.0) | 3 | |||
| Wash hands after toilet | Yes | 315(80.9) | 22 (6.9) | 293 | 0.945 | |
| No | 74 (19.1) | 5 (6.8) | 69 | |||
| Wash hands before eating | Yes | 374(96.1) | 27 (7.2) | 347 | 0.612* | |
| No | 15(3.9) | 0 (0.0) | 15 | |||
| Swimming | Yes | 182(46.8) | 20 (10.9) | 162 | 0.003 | |
| No | 207(53.2) | 7 (3.4) | 200 | |||
| Bath/wash clothes in river/pond | Yes | 201(51.7) | 18 (8.9) | 183 | 0.105 | |
| No | 188(48.3) | 9 (4.8) | 179 | |||
| Urinate in water | Yes | 60(15.4) | 3 (5) | 57 | 0.782* | |
| No | 329(84.6) | 24 (7.3) | 305 | |||
| How often do you wear shoes | All the time | 253(65) | 19 (7.5) | 234 | 0.547 | |
| At school | 136 (34.9) | 8 (5.9) | 128 | |||
3.6. Compliance to MDA among school children
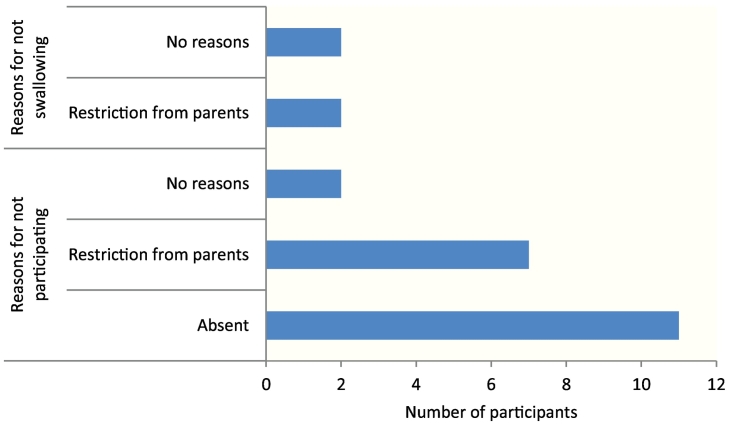
According to self-reported data, 369 (94.9%) of schoolchildren participated in the previous round of MDA by receiving the tablets; only four (1.1%) did not swallow the provided tablets. The prevalence of infection was 15% among children who did not participate in MDA vs. 6.5% among those who did (P > 0.05) (Table 6). The main reasons for not participating were being absent during MDA, 11 (57.9%), and parental restrictions, 7 (35%) (See Fig. 2).
Table 6.
Compliance to MDA among school age children and schistosomiasis infection.
| Question | Response | No. (%) | Schistosomiasis infection |
p - value | |
|---|---|---|---|---|---|
| Positive (%) | Negative (%) | ||||
| Did you take part in the last school MDA? (n = 389) | Yes | 369 (94.9) | 24 (6.5) | 345 (93.5) | > 0.05* |
| No | 20 (5.1) | 3 (15.0) | 17 (85.0) | ||
| Did you swallow the praziquantel tablet(s) (n = 369) | Yes | 365(98.9) | 24 (6.6) | 341 (93.4) | > 0.05* |
| No | 4 (1.1) | 0 (0.0) | 4 (100.0) | ||
Fig. 2.
The reasons for not participating during MDA campaigns (n = 12) and the reasons for not swallowing the tablets during MDA (n = 4).
4. Discussion
The current study found a low prevalence of S. haematobium infection using the urine filtration method, indicating a low transmission of urogenital schistosomiasis infection among primary school children (World Health Organization, 2010). More than half of the infected children had a light intensity of infection (<50 eggs count per 10 ml of urine). This could be attributed to the praziquantel MDA six months prior to this study. Furthermore, the low (<5%) prevalence of high heavy intensity of infection indicates that morbidity has been controlled (World Health Organization, 2010). The proportion of children with heavy intensity of infection was nearly three times higher than the <1% target for the elimination of schistosomiasis as a public health concern (World Health Organization, 2021b; World Health Organization, 2010). So, while the interventions have clearly controlled morbidity, they have yet to eliminate urogenital schistosomiasis as a public health problem.
Compliance with praziquantel and albendazole MDA was higher; surpassing the World Health Organization's (WHO) recommended minimum coverage of 75% (World Health Organization, 2010). Similarly, the majority of the children were aware of the risk factors for urogenital schistosomiasis transmission. However, nearly half of the children participated in swimming activities, and more than half had a history of water contact, such as washing clothes and utensils and fetching water from the river. Despite ongoing MDA, these findings show that urogenital schistosomiasis is still being transmitted in the district. It also suggests that re-infection could occur shortly after MDA.
The prevalence reported in this study is comparable to that reported in a recent study of schoolchildren in the Mtera Dam area (Ngasala et al., 2020). Both microhematuria and S. haematobium infection are slightly higher than in a recent study conducted in Pemba, Zanzibar (Knopp et al., 2016). This demonstrates that urogenital schistosomiasis is still prevalent in both mainland and island Tanzania. Nonetheless, the low prevalence of urogenital schistosomiasis reported in the cities of Dar es Salaam and Tanga after a decade of MDA (Mwakitalu et al., 2014) indicates heterogeneity in transmission between urban and rural settings, which is clearly linked to access to safe water.
Children at Chigugu primary school had a significantly higher prevalence of S. haematobium infection, as well as the most severe infection. According to the researchers' observations, children in this school did not have access to safe water (tap/well) near their compounds, so they relied on river water for their daily utilities, which may expose children to contaminated water, increasing their risk of infection. Similarly, the higher prevalence of S. haematobium among children aged 13–15 years and in class V could be attributed to water contact behaviors such as swimming. Furthermore, females had a higher mean intensity of S. haematobium infection than males, which could be attributed to gender roles of girls and women, such as washing clothes and fetching water for domestic purposes, which expose them to infection. However, findings from studies in Ethiopia and Senegal (Geleta et al., 2015; Senghor et al., 2014) show that males have a higher mean intensity of S. haematobium infection than females. These variations could be attributed to environmental factors and culture (Degarege et al., 2015).
The majority of the children were aware that bathing, swimming, washing, and playing in infested water sources can spread urinary schistosomiasis. This, however, was not associated with urogenital schistosomiasis infection in this study. Swimming in rivers or playing in water was, however, a common practice among the children; this was also a commonly reported practice in previous studies (Geleta et al., 2015; Pennance et al., 2016; Frigerio et al., 2016; Maseko et al., 2018). The proportion of swimming practices by girls and boys was nearly equal. However, higher prevalence of S. haematobium infection was recorded among boys that engage in swimming than girls. This suggests that boys are more susceptible to infection than girls.
Indeed, children who swam had a significantly higher prevalence of urogenital schistosomiasis than those who did not. It is certain that the continued transmission is due to continued water contact, despite the repeated rounds of MDA. Several previous studies have confirmed these findings (Geleta et al., 2015; Frigerio et al., 2016). Furthermore, urinating in open water was reported frequently among adults in Cameroon and by a minority of children in Swaziland (Maseko et al., 2018; Folefac et al., 2018). A qualitative study conducted in Zanzibar discovered that children engage in high-risk behaviors for contracting schistosomiasis due to a lack of knowledge about disease transmission (Person et al., 2016). In addition to the ongoing MDA intervention, mass education campaigns aimed at both adults and children are required.
The findings also show that MDA compliance is high among primary school children. Though self-reporting on compliance may have resulted in recall bias, non-compliance was certainly associated with a higher prevalence of infection, as previous studies have shown (Chaula and Tarimo, 2014; Knopp et al., 2016). Reasons given by students who did not participate in/swallow the tablets during MDA included parental restrictions, being absent from school on the day of MDA, and fear of the side effects. Pre-MDA education and communication with both parents and children may help to dispel these myths.
5. Conclusion
Despite a decade of MDA with praziquantel and albendazole and high compliance among the children, urogenital schistosomiasis remains prevalent among school children in Masasi district. The continued transmission of urogenital schistosomiasis among schoolchildren could be attributed to their continued water contact behavior. In addition to MDA, improving access to safe and clean water and behavioral change interventions may help to reduce urogenital schistosomiasis transmission in the district.
Authors' contributions
LN: Conceptualization, Data curation, Investigation, Methodology and Writing-original draft
EL: Conceptualization, Writing-original draft and Editing
AZ: Methodology, Data curation,Formal Analysis, Visualization, Wrting -review and Editing
BN: Conceptualization, Supervision and Writing -review
Funding
No funding received.
Declaration of Competing Interest
The authors declare that they have no competing interests.
Acknowledgments
We would like to thank all of the teachers in the participating schools for their assistance during data collection, as well as the parents who allowed their children to participate in this study. We also want to thank everyone who helped us collect specimens and data.
References
- Bakuza J. Demographic factors driving schistosomiasis and soil-transmitted helminthiases in milolaward, lindi district, Tanzania: a useful guide for launching intervention. East African Heal Res. 2018;2:156–167. doi: 10.24248/EAHRJ-D-18-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker S., Kabatereine N.B., Smith J.L., Mupfasoni D., Mwanje M.T., Ndayishimiye O., et al. An updated atlas of human helminth infections: The example of East Africa. Int. J. Health Geogr. 2009;8(42):1–11. doi: 10.1186/1476-072X-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukindu F., Morona D., Mazigo H.D. Prevalence of schistosoma mansoni and soil transmitted helminths and factors associated with uptake of preventive chemotherapy among school children in sengerema district in north-western tanzania. Tanzan J. Health Res. 2016;18(1):1–9. [Google Scholar]
- Chaula S.A., Tarimo D.S. Impact of praziquantel mass drug administration campaign on prevalence and intensity of Schistosoma haemamtobium check for this species in other resources among schoolchildren in Bahi district, Tanzania. Tanzan J. Health Res. 2014;16(1):1–10. doi: 10.4314/thrb.v16i1.1. [DOI] [PubMed] [Google Scholar]
- Dawaki S., Al-Mekhlafi H.M., Ithoi I., Ibrahim J., Abdulsalam A.M., Ahmed A., Sady H., et al. Prevalence and risk factors of schistosomiasis among Hausa communities in Kano state, Nigeria. Rev. Inst. Med. Trop. Sao Paulo. 2016;58(1):1–9. doi: 10.1590/S1678-9946201658054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degarege A., Mekonnen Z., Levecke B., Legesse M. Vol. 556. 2015. Prevalence of Schistosoma haematobium Infection among School-Age Children in Afar; pp. 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue R.E., Mashoto K.O., Mubyazi G.M., Madon S., Malecela M.N., Michael E., et al. Biosocial Determinants of Persistent Schistosomiasis among Schoolchildren in Tanzania despite Repeated Treatment. Trop. Med. Infect. Dis. 2017;2(4)):61. doi: 10.3390/tropicalmed2040061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folefac L.N., Nde-Fon P., Verla V.S., Tangye M.N., Njunda A.L., Luma H.N. Knowledge, attitudes and practices regarding urinary schistosomiasis among adults in the Ekombe Bonji Health Area, Cameroon. PAMJ. 2018;29(161):1–9. doi: 10.11604/pamj.2018.29.161.14980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigerio S., Bert F., Clari M. Knowledge, Attitudes, and Practices Related to Schistosomiasis Among Children in Northern Senegal. Ann. Glob. Heal. 2016;82(5):840–847. doi: 10.1016/j.aogh.2016.10.002. [DOI] [PubMed] [Google Scholar]
- Geleta S., Alemu A., Getie S., Mekonnen Z., Geleta B.E. Prevalence of urinary schistosomiasis and associated risk factors among abobo primary school children in gambella regional state, southwestern ethiopia: a cross sectional study. Parasit. Vectors. 2015;8(215) doi: 10.1186/s13071-015-0822-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotez P.J., Kamath A. Neglected tropical diseases in Sub-Saharan Africa: review of their prevalence, distribution, and disease burden. Cappello M, editor. PLoS Negl. Trop. Dis. 2009;3(8):e412. doi: 10.1371/journal.pntd.0000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopp S., Person B., Ame S.M., Mohammed K.A., Ali S.M., Khamis I.S., et al. Elimination of schistosomiasis transmission in Zanzibar: baseline findings before the onset of a randomized intervention trial. PLoS Negl. Trop. Dis. 2013;7(10) doi: 10.1371/journal.pntd.0002474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopp S., Person B., Ame S.M., Ali S.M., Muhsin J., Juma S., et al. Praziquantel coverage in schools and communities targeted for the elimination of urogenital schistosomiasis in Zanzibar: A cross-sectional survey. Parasit. Vectors. 2016;9(5) doi: 10.1186/s13071-015-1244-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manz K.M., Kroidl I., Clowes P., Gerhardt M., Nyembe W., Maganga L., Assisya W., et al. Schistosoma haematobium infection and environmental factors in southwestern tanzania: A cross-sectional, population-based study. PLoS Negl. Trop. Dis. 2020;14(8):e0008508. doi: 10.1371/journal.pntd.0008508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masasi District profile . 2021. Wikipedia. [Google Scholar]
- Maseko T.B., Mkhonta N.R., Masuku S.K., Dlamini S.V., Fan C. Schistosomiasis knowledge, attitude, practices, and associated factors among primary school children in the Siphofaneni area in the Lowveld of Swaziland. J. Microbiol. Immunol. 2018;51(1):103–109. doi: 10.1016/j.jmii.2015.12.003. [DOI] [PubMed] [Google Scholar]
- Mazigo H.D., Waihenya R., Mkoji G.M., Zinga M., Ambrose E.E., Jahanpour O.F., Bahemana E., et al. Intestinal schistosomiasis: prevalence, knowledge, attitude and practices among school children in an endemic area of North Western Tanzania. J. Rural Trop.Public Health. 2010;9:53–60. [Google Scholar]
- Mazigo H.D., Nuwaha F., Kinung’hi S.M., Morona D., de Moira A. Pinot, Wilson S., Heukelbach J., et al. Epidemiology and control of human schistosomiasis in Tanzania. Parasit. Vectors. 2012;5(1):274. doi: 10.1186/1756-3305-5-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munisi D.Z., Buza J., Mpolya E.A., Angelo T., Kinung’hi Safari M. Knowledge, attitude, and practices on intestinal schistosomiasis among primary schoolchildren in the Lake Victoria basin, Rorya District, north-western Tanzania. BMC Public Health. 2017;17(1) doi: 10.1186/s12889-017-4767-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwakitalu M.E., Mwele M.N., Mosha F.W. Urban schistosomiasis and soil transmitted helminthiases in young school children in Dar Es Salaam and Tanga, Tanzania, after a decade of anthelminthic intervention. Acta Trop. 2014;133:35–41. doi: 10.1016/j.actatropica.2014.01.012. [DOI] [PubMed] [Google Scholar]
- National Bureau of Statistics . Vol. 78. 2012. Population and Housing Census: Population Distribution by Administrative Areas; p. 2013. [Google Scholar]
- Ndyomugyenyi R., Minjas J.N. Urinary schistosomiasis in schoolchildren in Dar-es-salaam, Tanzania, and the factors influencing its transmission. Ann. Trop. Med. Parasitol. 2001;95(7):697–706. doi: 10.1080/00034980120097151. [DOI] [PubMed] [Google Scholar]
- Neglected Tropical Diseases Control Program . 2015. Schistosomiasis: Epidemiology and Burden of the Disease; p. 2015.https://www.ntdcp.go.tz/diseases/sch Accessed on 20 January 2021. [Google Scholar]
- Ngasala B., Jumaa H., Mwaiswelo R.O. The usefulness of indirect diagnostic tests for Schistosoma haematobium infection after repeated rounds of mass treatment with praziquantel in Mpwapwa and Chakechake districts in Tanzania. Int. J. Infect. Dis. 2020;90:132–137. doi: 10.1016/j.ijid.2019.10.031. [DOI] [PubMed] [Google Scholar]
- Ntonifor H.N., Green A.E., Bopda M.O., Tabot J.T. Epidemiology of urinary schistosomiasis and soil transmitted helminthiasis in a recently established focus behind Mount Cameroon. Int. J. Curr. Microbiol. App. Sci. 2015;4(3):1056–1066. [Google Scholar]
- Pennance T., Person B., Muhsin M.A., Khamis A.N., Muhsin J., Khamis I.S., et al. Urogenital schistosomiasis transmission on Unguja Island, Zanzibar: characterisation of persistent hot-spots. Parasit. Vectors. 2016;9(646) doi: 10.1186/s13071-016-1847-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Person B., Ali S.M., A’Kadir F.M., Ali J.N., Mohammed U.A., Mohammed K.A., Rollinson D. Community knowledge, perceptions, and practices associated with urogenital schistosomiasis among school-aged children in zanzibar, United Republic of Tanzania. PLoS Negl. Trop. Dis. 2016;10(7):e00. doi: 10.1371/journal.pntd.0004814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourhoseingholi M.A., Vahedi M., Rahimzadeh M. Sample size calculation in medical studies. Gastroenterol Hepatol Bed Bench. 2013;6(1):14–17. [PMC free article] [PubMed] [Google Scholar]
- Senghor B., Diallo A., Sylla S.N., Doucouré S., Ndiath M.O., Gaayeb L., Djuikwo-Teukeng F.F., et al. Prevalence and intensity of urinary schistosomiasis among school children in the district of Niakhar, region of Fatick, Senegal. Parasit. Vectors. 2014;7(5) doi: 10.1186/1756-3305-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weather Atlas Weather Forecast and Climate. 2022. https://www.weather-atlas.com/en/tanzania/masasi-climate Accessed on 6 February 2022.
- World Health Organization . Geneva; 2010. Schistosomiasis: Progress Report 2001–2011 and Strategic Plan 2012–2020.https://apps.who.int/iris/handle/10665/78074 [Google Scholar]
- World Health Organization, 2020. Schistosomiasis epidemiological situation: current estimated total number of individuals with morbidity and mortality due to schistosomiasis haematobium and S. mansoni infection in Sub-Saharan Africa. Weekly Epidemiol Rec. Accessed 13July 2021. http:www.who.int/schistosomiasis/ epidemiology/table/en.
- World Health Organization Schistosomiasis Fact sheet. 2021. https://www.who.int/health-topics/schistosomiasis Accessed on 10 May 2021.
- World Health Organization . Geneva; 2021. Ending the Neglect to Attain the Sustainable Development Goals : A Road Map for Neglected Tropical Diseases 2021–2030.https://apps.who.int/iris/handle/10665/332094 [Google Scholar]