Abstract
The prodromal phase of Parkinson’s disease is characterized by aggregation of the misfolded pathogenic protein α-synuclein in select neural centres, co-occurring with non-motor symptoms including sensory and cognitive loss, and emotional disturbances. It is unclear whether neuronal loss is significant during the prodrome. Underlying these symptoms are synaptic impairments and aberrant neural network activity. However, the relationships between synaptic defects and network-level perturbations are not established. In experimental models, pathological α-synuclein not only impacts neurotransmission at the synaptic level, but also leads to changes in brain network-level oscillatory dynamics—both of which likely contribute to non-motor deficits observed in Parkinson’s disease. Here we draw upon research from both human subjects and experimental models to propose a ‘synapse to network prodrome cascade’ wherein before overt cell death, pathological α-synuclein induces synaptic loss and contributes to aberrant network activity, which then gives rise to prodromal symptomology. As the disease progresses, abnormal patterns of neural activity ultimately lead to neuronal loss and clinical progression of disease. Finally, we outline goals and research needed to unravel the basis of functional impairments in Parkinson’s disease and other α-synucleinopathies.
Keywords: neuron, synapse, oscillation, neural network, prodrome
A common conceptualization of Parkinson’s disease is that α-syn aggregation leads to cell death, followed by symptomology. Here, Kulkarni et al. propose a novel ‘synapse to network prodrome cascade’ wherein in the absence of overt cell death, pathological α-syn induces synaptic loss and aberrant network activity, underlying prodromal non-motor symptoms.
Graphical Abstract
Graphical Abstract.
A starting framework to understand dysfunction in Parkinson’s disease
Parkinson’s disease is the second most common neurodegenerative disease, affecting nearly 1 million people in the USA and more than 6 million people worldwide.1 Death of substantia nigra (SN) neurons and intracellular inclusions composed of α-synuclein (α-syn) are the neuropathological hallmarks of Parkinson’s disease.2,3 This pathology is thought to lead to the classic motor symptoms of rest tremor, bradykinesia, rigidity and balance impairments, which make up the core diagnostic criteria of Parkinson’s disease. While motor abnormalities mark the formal onset of Parkinson’s disease, non-motor features, including olfactory perceptual deficits, sleep disturbances, cognitive impairments and autonomic dysfunction are routinely observed in the prodromal stages of the disease.4
A key driver of all complex neurologic functions including cognition, sleep and motor control is the communication of neurons between spatially distributed networks, in the form of neural oscillations.5–12 This oscillatory activity is generated in part by synaptic transmission between groups of neurons,13 and changes in synaptic dynamics can lead to alterations in neuronal firing and thus aberrant neural network activity.14–17
The onset of Parkinson’s disease pathology is considered to start at least 20 years before detectable motor abnormalities.18,19 Braak et al. proposed a staging scheme wherein Parkinson’s disease-like pathological α-Syn aggregation and dysfunction begins in Stages 1–2 in the dorsal motor nucleus of the vagus nerve and the olfactory bulb (OB), eventually spreading to the locus coeruleus and raphe nucleus. This is accompanied by olfactory deficits, dysautonomia, rapid eye movement behaviour disorder and mood disorders such as depression, apathy and anxiety. Pathology then spreads to the SN and nucleus of Meynert in early Stage 3, initially without clinically appreciable motor impairments. Stages 1–3 are commonly referred to as the pre-symptomatic or prodromal phase. As discussed in more detail later, this prodromal phase is marked by cellular and network dysfunction underlying preclinical symptomology without overt cell death. Eventually the pathology spreads throughout the midbrain and into the limbic areas in Stage 4, whereupon it is believed to result in classical motor symptoms including tremors, stiffness and slowness, and in late Parkinson’s disease, to neocortical regions leading to progressive cognitive and affective impairments. Stages 4 to 6 are commonly referred to as the symptomatic phase and, marked primarily by massive cell death, as well as cellular and network dysfunction.
In this review, we summarize insights into the cellular (synaptic) and network-level dysfunction observed in prodromal Parkinson’s disease to develop a framework for understanding the mechanisms underlying symptoms present during this prodromal stage. We propose two broad, not mutually exclusive, causal factors underlying network dysfunction in Parkinson’s disease. First, cell-autonomous mechanisms of individual neurons which when collectively operating within a neural network, result in abnormalities at the neural network level—like singers in a chorus singing out of tune. Second, cell death may remove components of the network resulting in aberrant neural network activity20—like removing several members of a chorus resulting in disharmony. Here we discuss key lines of evidence supporting these two drivers of altered network activity and synthesize a novel framework where such changes ultimately lead to impairment in day-to-day functions. This ‘synapse to network prodrome cascade’ posits that the major cause of non-motor dysfunction during prodromal Parkinson’s disease stems from the first scenario—synaptopathy driven aberrant neural network activity in the absence of overt cell death, which gives rise to clinical symptomology.
α-syn is positioned to potently influence synaptic function
α-Syn is a 140 amino acid residue protein, encoded by the SNCA gene on Chromosome 4.21 α-Syn is ubiquitous in the brain,22,23 and cycles between a soluble unfolded monomeric state in the cytosol, and a membrane-bound α-helical multimeric state (dimer, trimer and/or tetramer).24–29 Binding to lipids and membranes stabilizes the helical multimers,29 rendering them resistant to aggregation.30–34 α-syn has two domains: (i) an N-terminal lipid binding domain (residues 1–95) and (ii) a highly acidic unstructured C-terminal domain (residues 96–140).35–37 The N-terminal domain consists of a hydrophobic non-amyloidogenic core that adopts an α-helical secondary structure to associate with lipid membranes,24,35,36,38–41 whereas, the C-terminal domain acts as a chaperone capable of interacting with other proteins and metal ions.42 Physiologically, α-syn serves to regulate the synaptic vesicle pool and trafficking, by aiding the formation of soluble N-ethylmaleimide-sensitive-factor attachment protein receptor (SNARE)-complex42 (for reviews see Burré43 and Sulzer and Edwards44). Vesicle fusion is mediated by SNARE proteins located on synaptic vesicles (i.e. synaptobrevin) interacting with the proteins on the presynaptic plasma membrane. Physiological α-syn directly interacts with synaptobrevin and phosphoinositols on the plasma membrane, chaperoning SNARE complex assembly.42,45–47 Apart from regulating vesicle dynamics via protein interactions, physiological α-syn is thought to play a role in the biosynthesis and homeostasis of monoamines,48 particularly dopamine,49–55 and in the regulation of synaptic glutamate release56 and synaptic plasticity.57–59
In contrast, under pathological conditions, the non-amyloidogenic core domain of endogenous α-helical, tetrameric α-syn destabilizes,30 and can adopt an insoluble β-sheet amyloid conformation to form pathological α-syn. This β-sheet conformation is associated with α-syn aggregation forming protofibrils, amyloid fibrils and ultimately Lewy bodies.60–69 During the early stages of Parkinson’s disease, pathological α-syn fibrils are predominantly formed in the neurites, eventually maturing into Lewy body like structures which are found in axon terminals.70–72 At the synapse, pathological α-syn interacts with synaptic machinery and disrupts synaptic signalling.73 This is followed by destruction of synapses, retraction of synaptic spines and the loss of neuronal connectivity, ultimately leading to cell death in later stages of Parkinson’s disease.73–78 This sequence of events associated with synaptic dysfunction which leads to neuronal death can take years.79,80 But, it is unknown how these events in the context of α-synucleinopathy ultimately result in the symptoms observed clinically. Nevertheless, the resulting pathological α-syn engages with specific presynaptic and postsynaptic components, which renders it a potent disrupter of synaptic function (Fig. 1). While the synaptic alterations in α-synucleinopathies have been thoroughly reviewed,81 we will discuss several key aspects and recent findings that are important to tie together, before establishing insight into the causes of network dysfunction.
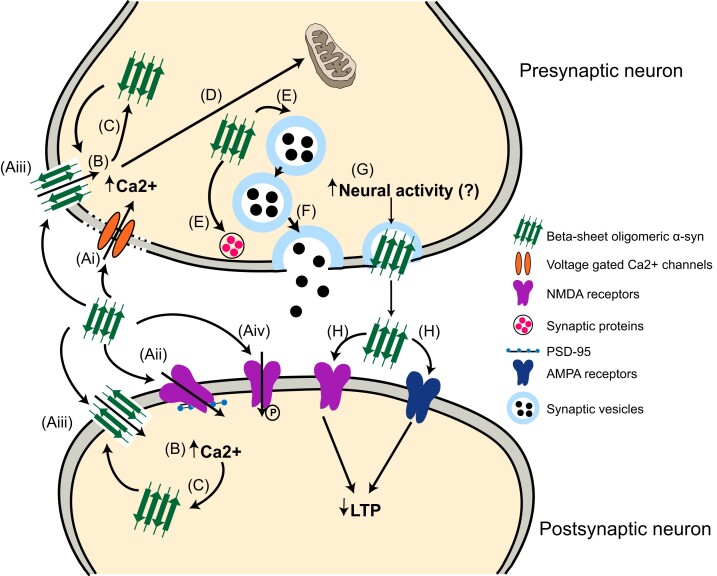
Figure 1.
Mechanisms of α-syn mediated synaptic dysfunction. (A) Oligomeric α-syn interferes with the pre- and/or postsynaptic plasma membrane integrity by dislocating the (Ai) voltage-gated Ca2+ channels, (Aii) NMDA receptors, or (Aiii) by forming pore-like structures. Additionally, (Aiv) pathological α-syn phosphorylates and activates NMDA receptors. Together, (B) these pathological α-syn interactions lead to increased intracellular Ca2+ influx. (C) Increased intracellular Ca2+ levels can further stimulate α-syn aggregation and oligomer formation. Nevertheless, (D) due to the synergistic action of elevated Ca2+ and pathological α-syn, the mitochondria undergoes oxidative stress, potentially leading to cell death. (E) Presynaptic accumulation of pathological α-syn also interferes with the synaptic pool maintenance, and synaptic proteins, entailing (F) reductions in neurotransmitter release. Neuronal activity mediates the presynaptic accumulation and extracellular release of physiological α-syn, however, it is unknown if it applies to pathological α-syn. (G) Increased neuronal activity could result in an increase in the release of extracellular oligomeric α-syn. (H) Extracellular α-syn is further proposed to impair LTP via NMDA and AMPA receptors.
α-syn dysregulates neuronal Ca2+ dynamics
α-syn oligomers82,83 and elevated levels of intracellular α-syn84 are both known to promote Ca2+ transport into neurons. While high levels of intracellular Ca2+ promote the intracellular aggregation and oligomerization of α-syn,85,86 Ca2+ removal prevents intracellular aggregation.85 Thus pathological α-syn aggregation promotes Ca2+ dyshomeostasis, which in turn promotes further aggregation.
Models of α-synucleinopathy have yielded evidence for Ca2+ dysregulation.82,84,87–94 Pathological α-syn decreases plasma membrane lipid levels, leading to lipid-raft fragmentation. This fragmentation causes plasma membrane rigidity and affects the localization of ion channels and receptors integral to synaptic transmission.95 One such example of this involves N-,92,96 and L-type voltage-dependent Ca2+ channels,97,98 specifically Cav 2.2. Changes in lipid content force these channels to move into lower-lipid containing domains, and a shift from a closed to open state, thereby, resulting in increased presynaptic Ca2+-influx.95,96
Clearance of intracellular Ca2+ is achieved primarily by sequestering it either in the endoplasmic reticulum, or in the mitochondria; or directly pumping into the extracellular space via plasma membrane Ca2+ ATPase efflux pumps. Moreover, the mitochondria are responsible for ATP production required for Ca2+ efflux pumps. Pathological α-syn interacts with the endoplasmic reticulum and mitochondria compromising Ca2+ sequestration.99–107 Elevated intracellular Ca2+ levels further promote oligomeric α-syn aggregation, suggesting a vicious cycle between elevated cytosolic Ca2+ and α-syn aggregation.107
Perturbation of cellular machinery, synaptic transmission and plasticity
Physiological α-syn is enriched in presynaptic nerve terminals,23 acting as a chaperone to maintain SNARE complex formation, which is responsible for controlling vesicle exocytosis.42,108,109 Recruitment of endogenous α-syn in the formation of pathological α-syn aggregates decreases its availability to engage in normal physiological functions.
Moreover, based upon work primarily in cultured hippocampal neurons, pathological α-syn alters the levels of several other synaptic proteins,76,94,110,111 and decreases levels of membrane lipids such as phosphoinositols,45 that regulate mechanisms of vesicle endocytosis and exocytosis. This suggests likely alterations in the number of vesicles. Physiological α-syn promotes normal clustering of vesicles at the synaptic terminal,46 thereby promoting SNARE complex formation. However, α-syn normally acts as a brake on the fusion of SNARE complexes and merging of synaptic vesicle membranes with the plasma membrane, thereby, preventing docking of the vesicles.112 Indeed, α-syn attenuates the mobility of synaptic vesicle pools between the presynaptic bouton and maintains the overall size of the clustered vesicles.113 In case of pathological α-syn inclusion formation, recruitment of α-syn away from the presynaptic terminal into aggregates releases this break and prevents vesicle clustering. This results in a decrease in the number of clustered vesicles,109,111,114–117 and conversely an increase in the number of docked vesicles.93 Alterations in neurotransmitter levels and/or the numbers of presynaptic vesicles released are reflected by changes in the frequency of miniature postsynaptic currents (mEPSCs). A recent report from Froula et al. demonstrated that pathological α-syn inclusions increase the frequency of mEPSCs, coupled with reduced Ca2+ transients in hippocampal neuronal cultures before any overt cell death. This evidence implicates an increase vesicle release probability as a likely factor contributing to altered synaptic transmission.93 On the contrary, others using similar cultures report a decrease in mEPSC frequency.76,118 This reduction is accompanied by an increase in the paired pulse ratio, which is reflective of increased presynaptic Ca2+ dynamics and therefore vesicle release probability. In this case, the dichotomous results could be primarily driven by increased presynaptic intracellular Ca2+ levels.111 Together, the effects on short-term plasticity are driven by pathological α-syn-induced disruptions in the presynaptic Ca2+ dynamics, and vesicle pool numbers and release probability. Alternative explanations for these changes in synaptic physiology could be compensatory mechanisms, based on evidence demonstrating alterations in: (i) the frequency of mEPSCs,76,93,118 (ii) numbers of dendritic spines93,119,120 or (iii) Ca2+ transient events.93 Regardless of the mechanism, these early disruptions of synaptic transmission are associated with presynaptic α-syn aggregates and perpetuates synaptic and postsynaptic dendritic loss as described below.93,111
While synaptopathy manifests in a cell intrinsic fashion, it can also act in cell extrinsic manners, including influencing postsynaptic neurons. Pathological α-syn can be released into the extracellular space in an activity-dependent fashion.121 This extracellular α-syn interacts with the postsynaptic neuron leading to a significant reduction in postsynaptic spine density and changes in morphology.122
Pathological α-syn interacts with postsynaptic neurons via several receptor mechanisms. Postsynaptic density modifications are associated with changes in N-methyl-d-aspartate (NMDA) receptor subunit composition.123 These changes in the composition can be attributed to either α-syn-induced impairment in synaptic transmission,123 or due to its direct interaction with the NMDA receptors.124 Pathological α-syn induces de-localization of postsynaptic membrane associated proteins, including PSD-95.122 PSD-95 is a scaffolding protein critical for membrane stability of NMDA receptors.125 Dislocation of PSD-95 induces movement of NMDA receptors, leading to altered receptor activity, and ultimately impairments in synaptic plasticity.123,126 Durante et al.,127 recently demonstrated that α-syn oligomers reduce the mEPSC amplitude in striatal spiny projection neurons—which indicates alterations in postsynaptic receptors. The impairments in long-term potentiation (LTP) are mediated via specific NMDA receptor subunits such as GluN2A127 and GluN2D.128 Misfolded α-syn also enhances α-amino-3-hydroxy-5-methylisoxazole-4-propionate (AMPA) receptor mediated synaptic transmission.126 In particular, impairment in LTP via inducing loss of GluA1-subunit bearing AMPA receptors in neurons overexpressing α-syn is observed.129 These impairments in LTP are indicative of decreased synaptic strength. Therefore, all these above studies support the view that synaptopathy is an early pathological consequence, before cell death in α-synucleinopathy.
It is important to emphasize that α-syn appears to act uniquely upon different neuronal compartments within individual neurons. Further, this impact appears to differ through the stages of Parkinson’s disease. In the prodromal phase, α-syn inhibits the mitochondrial Complex I in SN dopaminergic neurons.99,130 Loss of mitochondrial Complex I function is capable of driving axon loss, and vice versa. This forces the affected neurons to remodel their physiology by suppressing pacemaking, thereby narrowing action potential waveforms and downregulating Ca2+ entry and signalling.130 Similar circuit-level changes in α-syn-induced electrophysiological properties such as an increase in whole cell conductance and reductions in firing rate are reported.131 Further, localization of α-syn aggregates in the axonal processes,132 exacerbates this phenotypic downregulation and induces deficits in synaptic transmission, for example, by loss of synaptic proteins.94,133,134 These electrophysiological alterations can be partly remediated by blocking KATP channels (ATP sensitive K+ channels), suggesting that alterations in circuit function may occur before overt changes in synaptic transmission.131,135 Nevertheless, synaptic dysfunction subsequently expands retrogradely, affecting the somatodendritic compartment. These results suggest that pathophysiology in Parkinson’s disease is initiated within synaptic terminals and progresses proximally toward neuronal cell bodies in a ‘dying back’ fashion, thus reiterating that α-syn induces synaptopathy in the early stages of Parkinson’s disease. When damage crosses a threshold in the late stage of Parkinson’s disease, it leads to eventual death of neuronal perikarya. In the context of prodromal Parkinson’s disease, we propose the resultant early pathological onset and impaired synaptic transmission in brain regions and neurons burdened with pathological α-syn, leads to network dysfunction,94 often occurring concomitantly with the manifestation of non-motor symptoms4,136–144 as discussed below.
Network dysfunction in Parkinson’s disease
It is not the dysfunction of a single synapse, which is directly consequential for cognitive, sensory or motor function. Changes in multiple synapses ultimately lead to disrupted circuits and aberrations in large functional networks, which are considered the underlying drivers of major brain functions (Fig. 2). These distributed networks generate rhythmic oscillatory activity to communicate with one another, which can be measured within a brain region using local field potentials (LFPs), and at the skull surface through an EEG.145 LFPs are generated when neurons receive multiple synaptic inputs simultaneously, leading to synchronized oscillations.146–150 Thus, several forms of disruption such as desynchronization of oscillatory activity manifesting as either hyper- or hyposynchrony, both of which may impair communication within and between networks, and perturb function.151–154 Importantly, some evidence points toward alterations in the levels of synaptic proteins as underlying this desynchronization.94 Several lines of evidence link desynchronization of oscillatory activity to a variety of motor and non-motor symptoms discussed below.
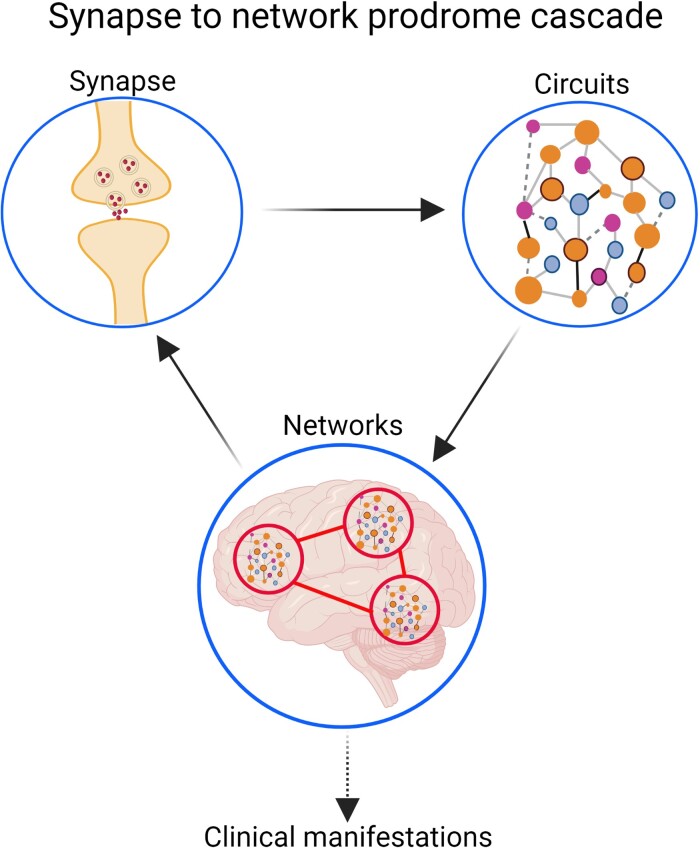
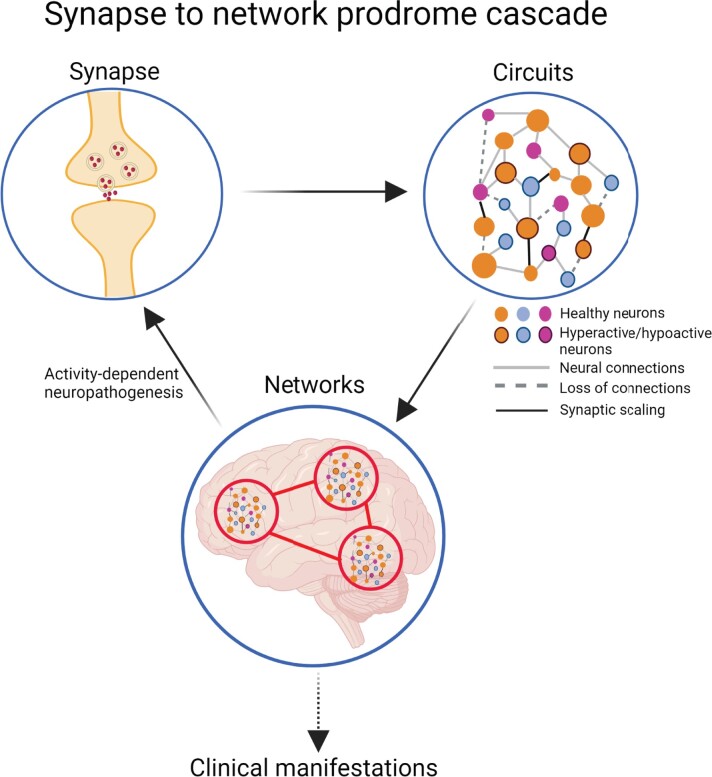
Figure 2.
Synapse to network prodrome cascade. From the top-left: in the synapse, misfolded α-syn targets membranes, receptors, and crucial components of neural signalling pathways, driving synaptopathy (see Fig. 1). Loss of synapses affects synaptic communication, causing aberrant activity in individual neurons. This perturbed activity at the single neuron level, leads to the emergence of synaptic scaling mechanisms. Thus, impaired communication between neurons manifests as impaired communication within a circuit. Faulty circuit activity results in impairments at a network level. These aberrant network dynamics can then feedback to further influence synaptic function. In this model, these outcomes synergize to give rise to prodromal clinical manifestations including motor, sensory, and cognitive decline in α-synucleinopathies.
Increases in beta-band network oscillations (∼15–35 Hz) in the basal ganglia and cortex are well documented in Parkinsonian primate and rodent models using LFPs,155–158 and Parkinson’s disease patients, commonly using electrocorticogram and electroencephalogram (for review see Brown159). While LFPs, electrocorticogram and EEG directly measure network-level electrical activity; functional MRI (fMRI) measures the indirect consequences of neural activity in the form of haemodynamic responses.160 fMRI has many strengths, including early disease detection, provides an understanding of prodromal and clinical symptoms, and predicts changes in clinical status as disease progresses.161 fMRI can also be used as a biomarker of alterations in brain physiology related to therapeutic interventions.162,163 This is important since fMRI can indicate changes in neural activity164–166 and connectivity,167–170 as well as regional differences in low frequency fMRI signals in the resting state in Parkinson’s disease patients.165 All of the above changes, whether monitored by LFPs, EEGs, electrocorticograms, or fMRI can importantly be linked to disease severity.171 Desynchronization manifesting as excessive synchronization is thought to contribute to pathological increase in beta-band activity, occurring along with motor deficits in monkey 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine models, as well as in parkinsonian individuals (for review see Refs.172–176). In line with this, normalizing beta synchronization using deep brain stimulation, or treatment with levodopa, improves motor function.177–180
Increases in beta-band activity are coupled with reductions in gamma-band oscillatory power in Parkinson’s disease, giving rise to exaggerated phase-amplitude coupling.181–184 Phase-amplitude coupling is a type of cross-frequency synchronization that represents information transmission and consolidation across multiple brain areas that are involved in pathologic and normal brain activities (e.g. Shimamoto et al.185 and Losacco et al.186). Exaggerated phase-amplitude coupling between beta and gamma oscillations is associated with Parkinsonian motor deficits.187
Mechanisms underlying early network dysfunction: what we know and do not know
The above lines of synaptic and network-level insights together lead to the prediction that α-syn aggregation itself may influence neural network function. Emerging evidence from our lab and others links similar disruption in patterns of network oscillations to prodromal symptoms, using the early anatomical target—the olfactory system.188,189 An advantage of using the olfactory system is that the sensory information is monosynaptically conveyed from the OB into down-stream cortices. α-Syn aggregate formation in the OB results in olfactory perceptual dysfunction.137,188,190–192 Several lines of recent evidence have uncovered that injecting α-syn preformed fibrils in the OB,189 or virally expressing α-syn in the OB,188 both lead to aberrant local neural dynamics. This included changes in both beta- and gamma-band LFP activity as well as hyper-excitation of OB principal neurons. Recent work by Chen et al.188 suggests that α-syn aggregation in the OB neurons may be causal to these changes in neuron firing. Notably, in these models mice did not have cell loss.188,190 Consistent with these findings, additional recent work in a biophysical model of the OB during seeding-like damage, reported that decreasing the strength of synaptic connections in the OB leads to an increase in the power of oscillatory rhythms.20,193 Like the abnormal increase in beta-band activity observed in Parkinsonian individuals (described above), an increase in oscillations occurring as a consequence to α-syn aggregates could be detrimental to normal information processing of the OB and given the OB’s strong influence over down-stream connected structures (e.g. the amygdala, hippocampus) likely would impact other regions. While other early anatomical target structures including the brainstem, locus coeruleus and the raphe nucleus are suggested to contribute in late-stage Parkinson’s disease related motor impairments,194–197 their direct involvement in the generation of prodromal aberrant beta oscillations is not yet established.
The spatial component of the active neuronal ensembles, as well as the number and timing of action potential firing, together, encodes information subserving behaviour.198–200 Even individual synapses appear important for promoting synchrony depending on the firing frequency of the neurons,201–203 intrinsic properties of the neurons203–205 and location of these synapses on the dendritic trees.205,206 Therefore, any alteration in the patterns of neuronal firing, as observed in the subthalamic neurons in both Parkinsonian monkeys and persons with Parkinson’s disease (e.g. Refs.207–209) can dramatically shape network activity. This becomes a complex problem in the context of prodromal α-synucleinopathy, especially since we do not yet fully understand which neuron populations accumulate pathological α-syn. Beyond that, local neurons, both inhibitory and excitatory, may form a variety of circuitry among each other (recurrent, feedforward, feedback). Many brain circuits are also subject to centrifugal and/or neuromodulators input from other brain areas (locus coeruleus, raphe nucleus, substania nigra, etc.). While not completely elucidated, computational studies suggest that an increase in inhibitory interneuron spiking and the resultant decrease in the firing of the glutamatergic projection neurons, drives generation of beta oscillations.210,211 That stated, the exact mechanism(s) underlying the emergence of pathological beta oscillations are unclear, but include several candidates (Table 1).
Table 1.
Candidate mechanisms linking elevated beta-band activity with excitation-inhibition imbalance
Intrinsic alterations
|
Based on the above discussed evidence, we know that α-syn-induced synaptic and network-level perturbations begin relatively soon after disease onset, however, the prodromal symptoms can extend for more than two decades before tremors and massive cell death emerges. So, what cellular or network mechanisms could account for this prolonged prodromal stage? One hypothesis, as outlined below, is for a role of synaptic scaling.
A consideration for synaptic scaling
In late stages of Parkinson’s disease, one model is that certain vulnerable neurons die, leading to either hyper- or hypo-excitability among interconnected neurons, thereby resulting in network-level changes. Yet in the early stages of Parkinson’s disease, rather than inducing cell death, pathological α-syn induces changes in plasticity through several mechanisms including interfering with NMDA receptor subunit composition,123,126 NMDA receptor trafficking,213–216 changes in spine density217 and targeting vital mitochondrial components,218 to name a few. These changes in plasticity certainly may entail altered neuronal excitability and synaptic strength. Additionally, the late-stage Parkinson’s disease conceptual model is seated largely in short-term changes of activity (for example in Parkinson’s disease, decrease in dopamine levels decrease D2 receptor signalling, thereby, increasing the excitability of indirect pathway neurons leading to hypokinetic movement). As such, the late-stage model does not fully account for the natural tendency of neurons to establish homeostatic plasticity as demonstrated in some recent Parkinson’s disease models.120,219 When neural activity is perturbed for a sustained duration, it induces homeostatic adaptations in the synapse that restores activity of a given neuron to a set point.220,221 Homeostatic plasticity is achieved by either of two mechanisms. First, changing the intrinsic excitability: neurons express a different constellation of ion channels that shifts their ability to fire in one direction or the other (more excitable versus less).222 Or two, scaling synaptic strength: for example, in case of a lower firing rate, synaptic strength becomes greater and the cells will begin to fire at a homeostatic rate. Conversely, if one raises firing rates, synaptic strength will decline.223 In the early stages of Parkinson’s disease, before significant neuronal death, it is possible that the changes in synaptic strength drive circuits to establish homeostatic plasticity, ultimately precipitating at a network level.
Regulating the firing of individual neurons via network rearrangements is another strategy to maintain circuit homeostasis. Cognitive defects observed in the prodromal stages of α-synucleinopathy,224,225 are associated with impaired brain functional connectivity.226–232 Some studies report a decrease in functional connectivity,233,234 whereas others report an increase.235 Generation of aberrant oscillations could be a result of a compensatory increase in functional connectivity. Another possibility for the generation of aberrant oscillations is that the homeostatic adaptations fail and that the functional damage to the neurons is too great to compensate. Therefore, these homeostatic forms of synaptic plasticity could be important determinates for the network-level perturbations in the early stages of α-synucleinopathies. The idea of compensatory mechanisms at play in prodromal Parkinson’s disease was recently supported by a study using human neural networks.236 Therefore, we speculate that the increase in the pathological oscillations may result from compensatory mechanisms in the early stages of the disease, which consequently delay the manifestation of clinical motor symptoms. Since loss of 50–60% of neurons is required for motor symptoms to emerge,237 synaptic scaling could increase the neurodegenerative threshold in prodromal Parkinson’s disease. Nevertheless, once neurodegeneration reaches a particular level of severity, it cannot be counteracted by compensatory mechanisms and functional deficits ensue.238
Specificity within circuits resulting in network dysfunction
Although a wide variety of neurons can accumulate pathological α-syn,239–245 not all neurons show pathological accumulation. Based upon the Braak hypothesis, all the neurons that are anatomically connected to the neurons with pathology should be vulnerable. However, there is considerable variation in the temporal pattern of α-syn accumulation and severity of neuronal damage than originally hypothesized by Braak. Several cell-autonomous factors have been proposed which could help identify at-risk neurons. Thus, it is suggested that the spreading pattern of α-syn-induced pathology and dysfunction occurs as proposed by Braak, but further that spreading to a specific population of neurons is dictated by a cellular-phenotype that renders them less able to cope with the burden of pathological α-syn.
These cell-autonomous factors can be broadly divided into two categories: neuroanatomical and/or molecular factors, and physiological factors. Molecular factors include the amount of molecular templates including endogenous α-syn available to misfold. For example, glutamatergic neurons expressing higher levels of endogenous α-syn are especially vulnerable to α-syn accumulation.246–250 Further, glutamatergic neurons express lower levels of BAG3, which is involved in autophagy.251 Lower BAG3 expression may lead to reduced α-syn degradation, in turn leading to increased accumulation.252 Additionally, in the α-syn seeding model of prodromal Parkinson’s disease, glutamatergic neurons exhibit Math2+ loss, a transcription factor underlying regulating mitochondrial homeostasis. This further increases vulnerability of these neurons to α-syn.250 Additionally, GABAergic neurons expressing parvalbumin and calbindin, playing a role in Ca2+ buffering and signalling, are also less vulnerable to inclusion development.253–255 Together, accumulation of aggregates in glutamatergic and GABAergic neurons might interfere with their physiological activity,93,127,256 leading to network-level changes. Neuroanatomical factors include hyperbranching of neurons with a large number of release sites. Indeed, hyperbranching of long projection axons is observed in almost all α-syn aggregation-prone neurons, regardless of transmitter identity.257,258 Hyperbranching of long projection axons is observed among SN dopamine neurons, dorsal motor nucleus cholinergic neurons, locus coeruleus noradrenergic neurons and raphe nucleus serotonergic neurons.259–263 Mitochondrial stress, a major driver for dysfunction and eventual neurodegeneration, is elevated in the axons of these vulnerable neurons264 due to the large axonal network that requires metabolic support and the high energy demand caused by neurotransmitter release.262 However, not all neurons that have highly branched axons are vulnerable.265 Thus, this trait alone is not sufficient to induce vulnerability.
The second factor that is an important determinant of vulnerability is the physiological phenotype of the neurons. At-risk neurons are particularly slow autonomous pacemakers—having low frequencies of firing (∼2–5 Hz) with relatively broad action potentials (∼4 ms width). Most neurons rely on Na+ permeable channels to drive pacemaking. However, vulnerable SN dopaminergic neurons rely heavily on L-type voltage-gated calcium (Ca2+) channels, allowing large Ca2+ influx into the cytoplasm.266 Due to pathological α-syn mediated enhancement in L-type Ca2+ channel activities,95 there is a higher increase in Ca2+ influx, rendering the dopaminergic network vulnerable.107 Additionally, these neurons express low levels of Ca2+ buffering capacity.267 The sustained Ca2+ influx and Ca2+ oscillations create a bioenergetic burden, increasing oxidative stress in the mitochondria. The mitochondria, thereby, produce reactive oxygen species,268 detrimental for neuron health.102 These physiological features such as prominent transmembrane Ca2+ currents, broad action potentials and low intrinsic Ca2+ buffering capacity are shared by cholinergic neurons of the dorsal motor nucleus, noradrenergic neurons of the locus coeruleus, dopaminergic neurons in the OB and the serotonergic neurons of the raphe nucleus impacted early on in the disease.91,269–276 Taken together, depending on the neurons that are affected, where they project to, and how their responses are gated, ultimately impacts how they modulate large-scale network dynamics that they are a part of.
Beyond prodromal: the path to cell death
Axonal degeneration and loss of synaptic connections occur in parallel to cognitive impairments.277–279 Conversely, restoring synaptic dysfunction normalizes aberrant brain activity associated with cognitive decline.280–284 Together, this suggests that synaptopathy precedes neuronal loss in vivo,285 and in individuals with Parkinson’s disease.286–290 This additionally suggests that at least in the context of cognitive deficits, it is the subtle synaptic changes rather than overt cell death which underlies the network-level perturbations driven by α-syn aggregation.
How does cell death fit in the sequence of events leading to the functional deficits during the Parkinson’s disease prodrome? We propose a pathological cascade wherein synaptopathy leads to aberrant network activity, occurring concomitantly with the non-motor prodromal deficits in the absence of cell death.291 As the disease progresses over the course of several years, sustained and large-scale aberrant neural dynamics drive vulnerable neurons past their ability to maintain homeostatic plasticity and over the neurodegenerative threshold, exacerbating the preexisting impaired network dynamics, ultimately leading to motor deficits.
Specific lines of evidence support this model leading to cell death. Axonal degeneration due to accumulation of misfolded α-syn94 eventually promotes neuronal death.292,293 For example, α-syn oligomers can prolong NMDA receptor activation and increase intracellular Ca2+. Influx in Ca2+ leads to calpain activation, promoting excitotoxicity294,295 and disruptions in connectivity.94 Augmented synaptic transmission via AMPA receptors also contributes to excitotoxicity and cell death.296 Because pathological α-syn can impair mitochondrial function297,298 and induce oxidative stress,299,300 Ca2+ buffering becomes difficult, leading to cell death.253 Moreover, alterations in synaptic function can also initiate apoptotic pathways.79 Therefore, synaptic alterations manifesting as circuit and network-level perturbations may underlie cell death.
Overexpression of α-syn in vivo requires a long period of time and high level of expression to induce neuronal death.285 Recently, using a α-synucleinopathy model of prodromal Parkinson’s disease, Mahul-Miller et al. proposed that Lewy body formation, and not fibril formation, is one of the major drivers of neurodegeneration.70,72 Therefore, in prodromal Parkinson’s disease, it is possible that α-syn aggregates cause synaptic and neural activity changes, but not neurodegeneration and cell death.
Together, since pathological burden and neuronal loss are not always positively correlated,301 the subtle effects of pathological α-syn on synaptic function could explain cell death. Further, axonal degeneration co-occurring with prodromal deficits precedes neurodegeneration.277–279 This suggests that at least in the context of early α-synucleinopathy, loss of synapses, as opposed to than death of neurons, correlate much more strongly to functional deficits.
α-Synucleinopathy and neural dysfunction: future perspectives and challenges
Multiple factors can contribute to impaired neural dynamics, however, there are some gaps in the literature (Table 2) that if answered could enhance our knowledge about prodromal Parkinson’s disease-associated dysfunction.
Table 2.
Outstanding questions to understand synaptic and network causes underlying the Parkinson’s disease prodrome
|
While we have some understanding of the pathogenic roles of α-syn in neurons (as discussed above), we know very little about the functional impacts of α-syn assemblies on the immune cells. Further, their contribution to the uptake and spreading of α-syn is poorly understood. While microglia and astrocytes can contribute to phagocytic clearance of pathological α-syn,302,303 glia also appear involved in the transmission of pathology,304 in part via glial exosomes305 and possibly via tunnelling nanotubes.306 α-Syn fibrils can trigger activation of microglia, that might contribute, together with astrocytes,307 to neurotoxicity and release exosomes carrying pathological α-syn.305 Further, microglia activation can lead to dysregulated neuronal Ca2+ responses and increases the probability of neuronal death by excitotoxicity.308,309 Therefore, insights into how these non-neuronal cells are affected by α-syn is key to elucidate their role in the large-scale impaired network dynamics.
There is also a need to establish a relationship between pathological burden, network dysfunction and clinical manifestations. This has been particularly challenging due to the lack of reliable biomarkers of disease progression, however, recent advances are aiming to fill this void.310 Clearly, other mechanisms in Parkinson’s disease beyond α-syn and death of neurons and occurring either in parallel or independently, could influence the network dynamics leading to the development of clinical manifestations in prodromal α-synucleinopathies. Some of these include neuroinflammation, oxidative stress and mitochondrial respiration defect, which all can trigger neuronal dysfunction (for reviews see Refs.311–315). Most likely, cell-autonomous factors including the degree of arborization, bioenergetic requirements and their intrinsic milieu make different neuron types more or less vulnerable.100 Whether certain neurons are affected late or early in the disease process are also influenced by their connectivity, and how they are connected to the areas that are impacted first by α-syn aggregates. Trans-synaptic spread includes retrograde and anterograde transmission, and both might contribute to the pathological progression to different degrees.246,316 Further, determining if these cells are hyperactive or hypoactive will help elucidate their specific contributions to the impaired brain dynamics.
The association between neuronal activity and the spread of α-syn pathology is poorly understood. Physiological, natively unfolded α-syn is released by primary neurons317 and extracellular α-syn in the brain interstitial fluid seems to depend on neural activity.318 Recently, Wu et al.121 reported the spread of pathological α-syn due to synthetically enhanced neuronal activity. However, the contribution of activity-driven release of aggregation-prone α-syn species that can propagate to neighbouring neurons is unknown. This question is especially relevant due to the current use of deep brain stimulation to correct perturbed pathological network oscillations. Stimulation of neural network activity does not necessarily restore network activity to pre-pathological states but, rather to a third state that alleviates functional improvement relative to diseased state, but that might not necessarily be normal.319 Additionally, restorative action could increase connectivity.320 Together, one can still ask what effects does enhancing neural activity via deep brain stimulation have on the spread of pathological α-syn in networks with enhanced connectivity.
Finally, many parallels can be drawn between Parkinson’s disease and other protein aggregating neurodegenerative diseases such as Alzheimer’s disease. Alzheimer’s disease involves two major kinds of insoluble protein aggregates including extracellular aggregates comprised of amyloid-β, which shares the non-amyloidogenic core with α-syn, and intracellular aggregates of tau.321 Pathological Aβ (for review see Palop and Mucke322 and Wesson et al.323) and tau324 disrupts synaptic transmission strength and/or long- and short-term plasticity, preceding neurodegeneration. The underlying mechanisms may involve alterations in Ca2+ homeostasis, endoplasmic reticulum stress, increased levels of reactive oxygen species and activate kinases, caspases and calpains (for review see Small et al.325). Therefore, the loss of functional synapses entails profound effects on Alzheimer’s disease symptomology.325–327 Further, at the circuit and network levels, these pathological aggregates can elicit early aberrant increases in neural activity,328 which has been demonstrated in cell culture, cortical brain slices, and in vivo,329–334 suggesting both direct proexcitatory effects on glutamatergic neurons and impairments of inhibitory interneurons.322,329,335 Overexcitation of neural networks triggers compensatory responses including extensive remodelling of circuits,329 and synaptic scaling mechanisms to alter synaptic function (for review see Small et al.336). Therefore, these protein aggregating diseases appear to share a common overarching theme which involves synaptic-, circuit-, and therefore network-level impairments underlying symptomology. Further studies on the progression of these mechanisms during preclinical stages could reveal new targets for therapeutic drug development.
Conclusions
Taken together, in the stages of α-synucleinopathy that are linked to prodromal Parkinson’s disease, misfolded α-syn impacts neural function, ultimately leading to perturbed network-level activity. It is reasonable therefore, although it has not been established that this precipitates into impaired function and the emergence of debilitating non-motor symptoms—the underlying premise of the ‘synapse to network prodrome cascade’. However, it is important to emphasize that the direct relationship between network dysfunction and clinical manifestations is far from clear. Gaps in our understanding of α-syn pathogenesis are obstacles to the development of therapeutic approaches that target symptoms in the Parkinson’s disease prodrome. Information processing across distributed networks is critical for our daily functions, and understanding the mechanisms underlying impaired network dynamics will have a large impact in our conceptualization of Parkinson’s disease. The major advantage of studying the underpinnings of these functional impairments early in the disease progression cascade is the possibility to prevent irreversible cell death, especially among dopaminergic neurons which are paramount, especially to non-prodromal Parkinson’s disease. Altogether, systems neuroscience approaches when combined with a range of in vivo models—from small animal to human—will pave the way for great advances in understanding and treating Parkinson’s disease and other α-synucleinopathies.
Abbreviations
- α-syn =
α-synuclein
- Ca2+ =
calcium
- LFP =
local field potential
- LTP =
long-term potentiation
- mEPSC =
miniature postsynaptic current
- OB =
olfactory bulb
- SN =
substantia nigra
Contributor Information
Aishwarya S Kulkarni, Department of Pharmacology & Therapeutics, University of Florida, 1200 Newell Dr, Gainesville, FL 32610, USA.
Matthew R Burns, Department of Neurology, University of Florida, 1200 Newell Dr, Gainesville, FL 32610, USA; Norman Fixel Institute for Neurological Disorders, University of Florida, 1200 Newell Dr, Gainesville, FL 32610, USA.
Patrik Brundin, Pharma Research and Early Development (pRED), F. Hoffman-La Roche, Little Falls, NJ, USA.
Daniel W Wesson, Department of Pharmacology & Therapeutics, University of Florida, 1200 Newell Dr, Gainesville, FL 32610, USA; Norman Fixel Institute for Neurological Disorders, University of Florida, 1200 Newell Dr, Gainesville, FL 32610, USA.
Data availability
Data sharing is not applicable to this article since no new data were generated nor were any data analyzed in this study.
Funding
This work was supported by National Institutes of Health grants R01DC016519 to D.W.W. and P.B., and K08AG071983 to M.R.B. M.R.B. also receives salary and research support from the Claude D. Pepper Older Americans Independence Center (OAIC). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Competing interests
P.B. has consulted for Axial Therapeutics, Calico, CuraSen, Enterin Inc, Fujifilm-Cellular Dynamics Inc, Idorsia and Lundbeck A/S. He has received commercial support for research from Lundbeck A/S and Roche, and has ownership interests in Acousort AB, Axial Therapeutics, Enterin Inc., Roche and RYNE Biotechnology Inc. During the writing of this manuscript P.B. became an employee of F. Hoffman-La Roch. The other authors report no competing interests.
References
- 1. Marras C, Beck JC, Bower JH, et al. Prevalence of Parkinson’s disease across North America. NPJ Park Dis. 2018;4(1):1–7. 10.1038/s41531-017-0038-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goedert M. Alpha-synuclein and neurodegenerative diseases. Nat Rev Neurosci. 2001;2(7):492–501. 10.1038/35081564 [DOI] [PubMed] [Google Scholar]
- 3. Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388(6645):839–840. 10.1038/42166 [DOI] [PubMed] [Google Scholar]
- 4. Mahlknecht P, Seppi K, Poewe W. The concept of prodromal Parkinson’s disease. J Parkinsons Dis. 2015;5(4):681–697. 10.3233/JPD-150685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fries P, Schröder J-H, Roelfsema PR, Singer W, Engel AK. Oscillatory neuronal synchronization in primary visual cortex as a correlate of stimulus selection. J Neurosci. 2002;22(9):3739–3754. 10.1523/JNEUROSCI.22-09-03739.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Engel AK, Fries P, Singer W. Dynamic predictions: Oscillations and synchrony in top-down processing. Nat Rev Neurosci. 2001;2(10):704–716. 10.1038/35094565 [DOI] [PubMed] [Google Scholar]
- 7. Fries P, Roelfsema PR, Engel AK, König P, Singer W. Synchronization of oscillatory responses in visual cortex correlates with perception in interocular rivalry. Proc Natl Acad Sci U S A. 1997;94(23):12699–12704. 10.1073/pnas.94.23.12699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fries P, Neuenschwander S, Engel AK, Goebel R, Singer W. Rapid feature selective neuronal synchronization through correlated latency shifting. Nat Neurosci. 2001;4(2):194–200. 10.1038/84032 [DOI] [PubMed] [Google Scholar]
- 9. Fries P. A mechanism for cognitive dynamics: Neuronal communication through neuronal coherence. Trends Cogn Sci. 2005; 9(10):474–480. 10.1016/j.tics.2005.08.011 [DOI] [PubMed] [Google Scholar]
- 10. Fries P. Rhythms for cognition: Communication through coherence. Neuron. 2015;88(1):220–235. 10.1016/j.neuron.2015.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grillner S, Markhram H, De Schutter E, Silberberg G, LeBeau F. Microcircuits in action – from CPGs to neocortex. Trends Neurosci. 2005;28(10):525–533. 10.1016/j.tins.2005.08.003 [DOI] [PubMed] [Google Scholar]
- 12. Chrobak JJ, Buzsáki G. Gamma oscillations in the entorhinal cortex of the freely behaving rat. J Neurosci. 1998;18(1):388–398. 10.1523/JNEUROSCI.18-01-00388.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Buzsáki G, Anastassiou CA, Koch C. The origin of extracellular fields and currents—EEG, ECoG, LFP and spikes. Nat Rev Neurosci. 2012;13(6):407–420. 10.1038/nrn3241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lavi A, Sheinin A, Shapira R, Zelmanoff D, Ashery U. DOC2B and Munc13-1 differentially regulate neuronal network activity. Cereb Cortex. 2014;24(9):2309–2323. 10.1093/cercor/bht081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cohen D, Segal M. Network bursts in hippocampal microcultures are terminated by exhaustion of vesicle pools. J Neurophysiol. 2011;106(5):2314–2321. 10.1152/jn.00969.2010 [DOI] [PubMed] [Google Scholar]
- 16. Chiappalone M, Casagrande S, Tedesco M, et al. Opposite changes in glutamatergic and GABAergic transmission underlie the diffuse hyperexcitability of synapsin i-deficient cortical networks. Cereb Cortex. 2009;19(6):1422–1439. 10.1093/cercor/bhn182 [DOI] [PubMed] [Google Scholar]
- 17. London M, Roth A, Beeren L, Häusser M, Latham PE. Sensitivity to perturbations in vivo implies high noise and suggests rate coding in cortex. Nature. 2010:466(7302):123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Braak H, Ghebremedhin E, Rüb U, Bratzke H, Del Tredici K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004;318(1):121–134. 10.1007/s00441-004-0956-9 [DOI] [PubMed] [Google Scholar]
- 19. Braak H, Del Tredici K, Bratzke H, Hamm-Clement J, Sandmann-Keil D, Rüb U. Staging of the intracerebral inclusion body pathology associated with idiopathic Parkinson’s disease (preclinical and clinical stages). J Neurol. 2002;249(Suppl 3(3)):III/1–5. 10.1007/s00415-002-1301-4 [DOI] [PubMed] [Google Scholar]
- 20. Edwards R, Beuter A, Glass L. Parkinsonian tremor and simplification in network dynamics. Bull Math Biol. 1999;61:157–177. 10.1006/bulm.1998.0086 [DOI] [PubMed] [Google Scholar]
- 21. Stefanis L. α-Synuclein in Parkinson’s disease. Cold Spring Harb Perspect Med. 2012;2(2):a009399–a009399. 10.1101/cshperspect.a009399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Iwai A, Masliah E, Yoshimoto M, et al. The precursor protein of non-aβ component of Alzheimer’s disease amyloid is a presynaptic protein of the central nervous system. Neuron. 1995;14(2):467–475. 10.1016/0896-6273(95)90302-X [DOI] [PubMed] [Google Scholar]
- 23. Jakes R, Spillantini MG, Goedert M. Identification of two distinct synucleins from human brain. FEBS Lett. 1994;345(1):27–32. 10.1016/0014-5793(94)00395-5 [DOI] [PubMed] [Google Scholar]
- 24. Chandra S, Chen X, Rizo J, Jahn R, Südhof TC. A broken α-helix in folded α-synuclein. J Biol Chem. 2003;278(17):15313–15318. 10.1074/jbc.M213128200 [DOI] [PubMed] [Google Scholar]
- 25. Weinreb PH, Zhen W, Poon AW, Conway KA, Lansbury PT. NACP, a protein implicated in Alzheimer’s disease and learning, is natively unfolded. Biochemistry. 1996;35(43):13709–13715. 10.1021/bi961799n [DOI] [PubMed] [Google Scholar]
- 26. Kim J. Evidence that the precursor protein of non-A beta component of Alzheimer’s disease amyloid (NACP) has an extended structure primarily composed of random-coil. Mol Cells. 1997;7(1):78–83. [PubMed] [Google Scholar]
- 27. Fauvet B, Mbefo MK, Fares MB, et al. α-Synuclein in central nervous system and from erythrocytes, mammalian cells, and Escherichia coli exists predominantly as disordered monomer. J Biol Chem. 2012;287(19):15345–15364. 10.1074/jbc.M111.318949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Burré J, Vivona S, Diao J, Sharma M, Brunger AT, Südhof TC. Properties of native brain α-synuclein. Nature. 2013;498(7453):E4–E6. 10.1038/nature12125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Burré J, Sharma M, Südhof TC. α-Synuclein assembles into higher-order multimers upon membrane binding to promote SNARE complex formation. Proc Natl Acad Sci U S A. 2014;111(40):E4274–E4283. 10.1073/pnas.1416598111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bartels T, Choi JG, Selkoe DJ. α-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature. 2011;477(7362):107–111. 10.1038/nature10324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhu M, Fink AL. Lipid binding inhibits α-synuclein fibril formation. J Biol Chem. 2003;278(19):16873–16877. 10.1074/jbc.M210136200 [DOI] [PubMed] [Google Scholar]
- 32. Burré J, Sharma M, Südhof TC. Definition of a molecular pathway mediating α-synuclein neurotoxicity. J Neurosci. 2015;35(13):5221–5232. 10.1523/JNEUROSCI.4650-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jo E, Darabie AA, Han K, Tandon A, Fraser PE, McLaurin J. Α-synuclein–synaptosomal membrane interactions: Implications for fibrillogenesis. Eur J Biochem. 2004;271(15):3180–3189. 10.1111/j.1432-1033.2004.04250.x [DOI] [PubMed] [Google Scholar]
- 34. Narayanan V, Scarlata S. Membrane binding and self-association of α-synucleins. Biochemistry. 2001;40(33):9927–9934. 10.1021/bi002952n [DOI] [PubMed] [Google Scholar]
- 35. Bussell R, Eliezer D. A structural and functional role for 11-mer repeats in α-synuclein and other exchangeable lipid binding proteins. J Mol Biol. 2003;329(4):763–778. 10.1016/S0022-2836(03)00520-5 [DOI] [PubMed] [Google Scholar]
- 36. Davidson WS, Jonas A, Clayton DF, George JM. Stabilization of alpha-synuclein secondary structure upon binding to synthetic membranes. J Biol Chem. 1998;273:9443–9449. 10.1074/jbc.273.16.9443 [DOI] [PubMed] [Google Scholar]
- 37. Ulmer TS, Bax A, Cole NB, Nussbaum RL. Structure and dynamics of micelle-bound human α-synuclein. J Biol Chem. 2005;280(10):9595–9603. 10.1074/jbc.M411805200 [DOI] [PubMed] [Google Scholar]
- 38. Eliezer D, Kutluay E, Bussell R, Browne G. Conformational properties of α-synuclein in its free and lipid-associated states. J Mol Biol. 2001;307(4):1061–1073. 10.1006/jmbi.2001.4538 [DOI] [PubMed] [Google Scholar]
- 39. Middleton ER, Rhoades E. Effects of curvature and composition on α-synuclein binding to lipid vesicles. Biophys J. 2010;99(7):2279–2288. 10.1016/j.bpj.2010.07.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jo E, McLaurin JA, Yip CM, St. George-Hyslop P, Fraser PE. α-Synuclein membrane interactions and lipid specificity. J Biol Chem. 2000;275(44):34328–34334. 10.1074/jbc.M004345200 [DOI] [PubMed] [Google Scholar]
- 41. Perrin RJ, Woods WS, Clayton DF, George JM. Interaction of human α-synuclein and Parkinson’s disease variants with phospholipids: Structural analysis using site-directed mutagenesis. J Biol Chem. 2000;275(44):34393–34398. 10.1074/jbc.M004851200 [DOI] [PubMed] [Google Scholar]
- 42. Burré J, Sharma M, Tsetsenis T, Buchman V, Etherton MR, Südhof TC. α-Synuclein promotes SNARE-complex assembly in vivo and in vitro. Science. 2010;329(5999):1663–1667. 10.1126/science.1195227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Burré J. The synaptic function of α-synuclein. J Parkinsons Dis. 2015;5(4):699–713. 10.3233/JPD-150642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sulzer D, Edwards RH. The physiological role of α-synuclein and its relationship to Parkinson’s disease. J Neurochem. 2019;150(5):475–486. 10.1111/JNC.14810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schechter M, Atias M, Abd Elhadi S, Davidi D, Gitler D, Sharon R. α-Synuclein facilitates endocytosis by elevating the steady-state levels of phosphatidylinositol 4,5-bisphosphate. J Biol Chem. 2020;295(52):18076–18090. 10.1074/JBC.RA120.015319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Diao J, Burré J, Vivona S, et al. Native α-synuclein induces clustering of synaptic-vesicle mimics via binding to phospholipids and synaptobrevin-2/VAMP2. Elife. 2013;2:e00592. 10.7554/ELIFE.00592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Burré J, Sharma M, Südhof TC. Systematic mutagenesis of α-synuclein reveals distinct sequence requirements for physiological and pathological activities. J Neurosci. 2012;32(43):15227. 10.1523/JNEUROSCI.3545-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wersinger C, Rusnak M, Sidhu A. Modulation of the trafficking of the human serotonin transporter by human alpha-synuclein. Eur J Neurosci. 2006;24(1):55–64. 10.1111/j.1460-9568.2006.04900.x [DOI] [PubMed] [Google Scholar]
- 49. Masliah E, Rockenstein E, Veinbergs I, et al. Dopaminergic loss and inclusion body formation in α-synuclein mice: Implications for neurodegenerative disorders. Science. 2000;287(5456):1265–1269. [DOI] [PubMed] [Google Scholar]
- 50. Perez RG, Waymire JC, Lin E, Liu JJ, Guo F, Zigmond MJ. A role for α-synuclein in the regulation of dopamine biosynthesis. J Neurosci. 2002;22(8):3090–3099. 10.1523/JNEUROSCI.22-08-03090.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Baptista MJ, O’Farrell C, Daya S, et al. Co-ordinate transcriptional regulation of dopamine synthesis genes by α-synuclein in human neuroblastoma cell lines. J Neurochem. 2003;85(4):957–968. 10.1046/j.1471-4159.2003.01742.x [DOI] [PubMed] [Google Scholar]
- 52. Yu S, Zuo X, Li Y, et al. Inhibition of tyrosine hydroxylase expression in α-synuclein-transfected dopaminergic neuronal cells. Neurosci Lett. 2004;367(1):34–39. 10.1016/j.neulet.2004.05.118 [DOI] [PubMed] [Google Scholar]
- 53. Li Y-H, Gao N, Ye Y-W, et al. Alpha-synuclein functions as a negative regulator for expression of tyrosine hydroxylase. Acta Neurol Belg. 2011;111(2):130–135. [PubMed] [Google Scholar]
- 54. Chadchankar H, Ihalainen J, Tanila H, Yavich L. Decreased reuptake of dopamine in the dorsal striatum in the absence of alpha-synuclein. Brain Res. 2011;1382:37–44. 10.1016/j.brainres.2011.01.064 [DOI] [PubMed] [Google Scholar]
- 55. Guo JT, Chen AQ, Kong QI, Zhu H, Ma CM, Qin C. Inhibition of vesicular monoamine transporter-2 activity in α-synuclein stably transfected SH-SY5Y cells. Cell Mol Neurobiol. 2008;28(1):35–47. 10.1007/s10571-007-9227-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gureviciene I, Gurevicius K, Tanila H. Role of α-synuclein in synaptic glutamate release. Neurobiol Dis. 2007;28(1):83–89. 10.1016/j.nbd.2007.06.016 [DOI] [PubMed] [Google Scholar]
- 57. Gureviciene I, Gurevicius K, Tanila H. Aging and α-synuclein affect synaptic plasticity in the dentate gyrus. J Neural Transm. 2009;116(1):13. 10.1007/s00702-008-0149-x [DOI] [PubMed] [Google Scholar]
- 58. Steidl JV, Gomez-Isla T, Mariash A, Ashe KH, Boland LM. Altered short-term hippocampal synaptic plasticity in mutant α-synuclein transgenic mice. Neuroreport. 2003;14(2):219–223. 10.1097/00001756-200302100-00012 [DOI] [PubMed] [Google Scholar]
- 59. Watson JB, Hatami A, David H, et al. Alterations in corticostriatal synaptic plasticity in mice overexpressing human alpha-synuclein. Neuroscience. 2009;159(2):501–513. 10.1016/J.NEUROSCIENCE.2009.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Conway KA, Lee S, Rochet JC, et al. Accelerated oligomerization by Parkinson’s disease linked α-synuclein mutants. Ann N Y Acad Sci. 2000;920(1):42–45. 10.1111/j.1749-6632.2000.tb06903.x [DOI] [PubMed] [Google Scholar]
- 61. Conway KA, Harper JD, Lansbury PT. Accelerated in vitro fibril formation by a mutant α-synuclein linked to early-onset Parkinson disease. Nat Med. 1998;4(11):1318–1320. 10.1038/3311 [DOI] [PubMed] [Google Scholar]
- 62. Ding TT, Lee S-J, Rochet J-C, Lansbury PT. Annular α-synuclein protofibrils are produced when spherical protofibrils are incubated in solution or bound to brain-derived membranes. Biochemistry. 2002;41(32):10209–10217. 10.1021/bi020139h [DOI] [PubMed] [Google Scholar]
- 63. Fredenburg RA, Rospigliosi C, Meray RK, et al. The impact of the E46K mutation on the properties of α-synuclein in its monomeric and oligomeric states. Biochemistry. 2007;46(24):7107–7118. 10.1021/bi7000246 [DOI] [PubMed] [Google Scholar]
- 64. Greenbaum EA, Graves CL, Mishizen-Eberz AJ, Lupoli MA, Lynch DR, Englander SW. The E46K mutation in alpha-synuclein increases amyloid fibril formation. J Biol Chem. 2005;280:7800–7807. 10.1074/jbc.M411638200 [DOI] [PubMed] [Google Scholar]
- 65. Lashuel HA, Petre BM, Wall J, et al. α-Synuclein, especially the Parkinson’s disease-associated mutants, forms pore-like annular and tubular protofibrils. J Mol Biol. 2002;322(5):1089–1102. 10.1016/S0022-2836(02)00735-0 [DOI] [PubMed] [Google Scholar]
- 66. Narhi L, Wood SJ, Steavenson S, et al. Both familial Parkinson’s disease mutations accelerate α-synuclein aggregation. J Biol Chem. 1999;274(14):9843–9846. 10.1074/jbc.274.14.9843 [DOI] [PubMed] [Google Scholar]
- 67. Rochet J-C, Conway KA, Lansbury PT. Inhibition of fibrillization and accumulation of prefibrillar oligomers in mixtures of human and mouse α-synuclein. Biochemistry. 2000;39(35):10619–10626. 10.1021/bi001315u [DOI] [PubMed] [Google Scholar]
- 68. Uversky VN. Neuropathology, biochemistry, and biophysics of α-synuclein aggregation. J Neurochem. 2007;103(1):17–37. 10.1111/j.1471-4159.2007.04764.x [DOI] [PubMed] [Google Scholar]
- 69. Yonetani M, Nonaka T, Masuda M, et al. Conversion of wild-type α-synuclein into mutant-type fibrils and its propagation in the presence of A30P mutant. J Biol Chem. 2009;284(12):7940–7950. 10.1074/jbc.M807482200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Fares MB, Jagannath S, Lashuel HA. Reverse engineering Lewy bodies: How far have we come and how far can we go? Nat Rev Neurosci. 2021;22(2):111–131. 10.1038/s41583-020-00416-6 [DOI] [PubMed] [Google Scholar]
- 71. Kramer ML, Schulz-Schaeffer WJ. Presynaptic α-synuclein aggregates, not Lewy bodies, cause neurodegeneration in dementia with Lewy bodies. J Neurosci. 2007;27(6):1405–1410. 10.1523/JNEUROSCI.4564-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mahul-Mellier A-L, Burtscher J, Maharjan N, et al. The process of Lewy body formation, rather than simply α-synuclein fibrillization, is one of the major drivers of neurodegeneration. Proc Natl Acad Sci U S A. 2020;117(9):4971–4982. 10.1073/pnas.1913904117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Calo L, Wegrzynowicz M, Santivañez-Perez J, Grazia Spillantini M. Synaptic failure and α-synuclein. Mov Disord. 2016;31(2):169–177. 10.1002/mds.26479 [DOI] [PubMed] [Google Scholar]
- 74. Hornykiewicz O. Biochemical aspects of Parkinson’s disease. Neurology. 1998:51(2 Suppl 2):S2–S9. 10.1212/wnl.51.2_suppl_2.s2 [DOI] [PubMed] [Google Scholar]
- 75. Caminiti SP, Presotto L, Baroncini D, et al. Axonal damage and loss of connectivity in nigrostriatal and mesolimbic dopamine pathways in early Parkinson’s disease. Neuroimage Clin. 2017;14:734–740. 10.1016/j.nicl.2017.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Scott DA, Tabarean I, Tang Y, Cartier A, Masliah E, Roy S. A pathologic cascade leading to synaptic dysfunction in {alpha}-synuclein-induced neurodegeneration. J Neurosci. 2010;30(24):8083–8095. 10.1523/jneurosci.1091-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Morales I, Sanchez A, Rodriguez-Sabate C, Rodriguez M. The degeneration of dopaminergic synapses in Parkinson’s disease: A selective animal model. Behav Brain Res. 2015;289:19–28. 10.1016/j.bbr.2015.04.019 [DOI] [PubMed] [Google Scholar]
- 78. Schulz-Schaeffer WJ. Is cell death primary or secondary in the pathophysiology of idiopathic Parkinson’s disease? Biomolecules. 2015;5(3):1467–1479. 10.3390/biom5031467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Brady S, Morfini G. A perspective on neuronal cell death signaling and neurodegeneration. Mol Neurobiol. 2010;42(1):25–31. 10.1007/s12035-010-8128-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Jellinger KA. Challenges in neuronal apoptosis. Curr Alzheimer Res. 2006;3(4):377–391. 10.2174/156720506778249434 [DOI] [PubMed] [Google Scholar]
- 81. Ghiglieri V, Calabrese V, Calabresi P. Alpha-synuclein: From early synaptic dysfunction to neurodegeneration. Front Neurol. 2018;9:295. 10.3389/fneur.2018.00295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Danzer KM, Haasen D, Karow AR, et al. Different species of α-synuclein oligomers induce calcium influx and seeding. J Neurosci. 2007;27(34):9220–9232. 10.1523/JNEUROSCI.2617-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Luth ES, Stavrovskaya IG, Bartels T, Kristal BS, Selkoe DJ. Soluble, prefibrillar α-synuclein oligomers promote complex I-dependent, Ca2+-induced mitochondrial dysfunction. J Biol Chem. 2014;289(31):21490–21507. 10.1074/jbc.M113.545749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hettiarachchi NT, Parker A, Dallas ML, et al. α-Synuclein modulation of Ca2+ signaling in human neuroblastoma (SH-SY5Y) cells. J Neurochem. 2009;111(5):1192–1201. 10.1111/j.1471-4159.2009.06411.x [DOI] [PubMed] [Google Scholar]
- 85. Follett J, Darlow B, Wong MB, Goodwin J, Pountney DL. Potassium depolarization and raised calcium induces α-synuclein aggregates. Neurotox Res. 2013;23(4):378–392. 10.1007/s12640-012-9366-z [DOI] [PubMed] [Google Scholar]
- 86. Nath S, Goodwin J, Engelborghs Y, Pountney DL. Raised calcium promotes α-synuclein aggregate formation. Mol Cell Neurosci. 2011;46(2):516–526. 10.1016/j.mcn.2010.12.004 [DOI] [PubMed] [Google Scholar]
- 87. Dryanovski DI, Guzman JN, Xie Z, et al. Calcium entry and α-synuclein inclusions elevate dendritic mitochondrial oxidant stress in dopaminergic neurons. J Neurosci. 2013;33(24):10154–10164. 10.1523/JNEUROSCI.5311-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lasser-Katz E, Simchovitz A, Chiu WH, et al. Mutant α-synuclein overexpression induces stressless pacemaking in vagal motoneurons at risk in Parkinson’s disease. J Neurosci. 2017;37(1):47–57. 10.1523/JNEUROSCI.1079-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Chiu W-H, Kovacheva L, Musgrove RE, et al. α-Synuclein-induced Kv4 channelopathy in mouse vagal motoneurons drives nonmotor parkinsonian symptoms. Sci Adv. 2021;7(11):eabd3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Cooper G, Lasser-Katz E, Simchovitz A, et al. Functional segregation of voltage-activated calcium channels in motoneurons of the dorsal motor nucleus of the vagus. J Neurophysiol. 2015;114(3):1513–1520. 10.1152/jn.00432.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Goldberg JA, Guzman JN, Estep CM, et al. Calcium entry induces mitochondrial oxidant stress in vagal neurons at risk in Parkinson’s disease. Nat Neurosci. 2012;15(10):1414–1421. 10.1038/nn.3209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Adamczyk A, Strosznajder JB. Alpha-synuclein potentiates Ca2 + influx through voltage-dependent Ca2+ channels. Neuroreport. 2006;17(18):1883–1886. 10.1097/WNR.0b013e3280115185 [DOI] [PubMed] [Google Scholar]
- 93. Froula JM, Henderson BW, Gonzalez JC, et al. α-Synuclein fibril-induced paradoxical structural and functional defects in hippocampal neurons. Acta Neuropathol Commun. 2018;6(1):35. 10.1186/s40478-018-0537-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Volpicelli-Daley LA, Luk KC, Patel TP, et al. Exogenous α-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron. 2011;72(1):57–71. 10.1016/j.neuron.2011.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ronzitti G, Bucci G, Stephens G, Chieregatti E. Raft-partitioning of calcium channels regulates their function. Channels. 2015;9(4):169–170. 10.1080/19336950.2015.1063285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ronzitti G, Bucci G, Emanuele M, et al. Exogenous α-synuclein decreases raft partitioning of Cav2.2 channels inducing dopamine release. J Neurosci. 2014;34(32):10603–10615. 10.1523/JNEUROSCI.0608-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Yamamoto K, Izumi Y, Arifuku M, Kume T, Sawada H. α-Synuclein oligomers mediate the aberrant form of spike-induced calcium release from IP3 receptor. Sci Rep. 2019;9(1):15977. 10.1038/s41598-019-52135-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Lieberman OJ, Choi SJ, Kanter E, et al. α-Synuclein-dependent calcium entry underlies differential sensitivity of cultured SN and VTA dopaminergic neurons to a parkinsonian neurotoxin. eNeuro. 2017;4(6):ENEURO.0167-17.2017. 10.1523/ENEURO.0167-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Faustini G, Bono F, Valerio A, Pizzi M, Spano P, Bellucci A. Mitochondria and α-synuclein: Friends or foes in the pathogenesis of Parkinson’s disease? Genes. 2017;8(12):377. 10.3390/genes8120377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Surmeier DJ, Schumacker PT. Calcium, bioenergetics, and neuronal vulnerability in Parkinson’s disease. J Biol Chem. 2013;288(15):10736–10741. 10.1074/jbc.R112.410530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Sanchez-Padilla J, Guzman JN, Ilijic E, et al. Mitochondrial oxidant stress in locus coeruleus is regulated by activity and nitric oxide synthase. Nat Neurosci. 2014;17(6):832–840. 10.1038/nn.3717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Guzman JN, Sanchez-Padilla J, Wokosin D, et al. Oxidant stress evoked by pacemaking in dopaminergic neurons is attenuated by DJ-1. Nature. 2010;468(7324):696–700. 10.1038/nature09536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Ryu EJ, Harding HP, Angelastro JM, Vitolo OV, Ron D, Greene LA. Endoplasmic reticulum stress and the unfolded protein response in cellular models of Parkinson’s disease. J Neurosci. 2002;22(24):10690–10698. 10.1523/JNEUROSCI.22-24-10690.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Keeney PM, Xie J, Capaldi RA, Bennett JP Jr. Parkinson’s disease brain mitochondrial complex I has oxidatively damaged subunits and is functionally impaired and misassembled. J Neurosci. 2006;26(19):5256–5264. 10.1523/JNEUROSCI.0984-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Adam-Vizi V. Production of reactive oxygen species in brain mitochondria: Contribution by electron transport chain and non-electron transport chain sources. Antioxid Redox Signal. 2005;7(9–10):1140–1149. 10.1089/ars.2005.7.1140 [DOI] [PubMed] [Google Scholar]
- 106. Devi L, Raghavendran V, Prabhu BM, Avadhani NG, Anandatheerthavarada HK. Mitochondrial import and accumulation of α-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J Biol Chem. 2008;283(14):9089–9100. 10.1074/jbc.M710012200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Goodwin J, Nath S, Engelborghs Y, Pountney DL. Raised calcium and oxidative stress cooperatively promote alpha-synuclein aggregate formation. Neurochem Int. 2013;62(5):703–711. 10.1016/j.neuint.2012.11.004 [DOI] [PubMed] [Google Scholar]
- 108. Südhof TC. The presynaptic active zone. Neuron. 2012;75(1):11–25. 10.1016/J.NEURON.2012.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Chandra S, Gallardo G, Fernández-Chacón R, Schlüter OM, Südhof TC. α-Synuclein cooperates with CSPα in preventing neurodegeneration. Cell. 2005;123(3):383–396. 10.1016/j.cell.2005.09.028 [DOI] [PubMed] [Google Scholar]
- 110. Longhena F, Faustini G, Varanita T, et al. Synapsin III is a key component of α-synuclein fibrils in Lewy bodies of PD brains. Brain Pathol. 2018;28(6):875–888. 10.1111/bpa.12587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Nemani VM, Lu W, Berge V, et al. Increased expression of α-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis. Neuron. 2010;65(1):66–79. 10.1016/j.neuron.2009.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Wang L, Das U, Scott DA, Tang Y, McLean PJ, Roy S. α-Synuclein multimers cluster synaptic-vesicles and attenuate recycling. Curr Biol. 2014;24(19):2319. 10.1016/J.CUB.2014.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Scott D, Roy S. α-Synuclein inhibits intersynaptic vesicle mobility and maintains recycling-pool homeostasis. J Neurosci. 2012;32(30):10129–10135. 10.1523/JNEUROSCI.0535-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Abeliovich A, Schmitz Y, Fariñas I, et al. Mice lacking α-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25(1):239–252. 10.1016/S0896-6273(00)80886-7 [DOI] [PubMed] [Google Scholar]
- 115. Chandra S, Fornai F, Kwon HB, et al. Double-knockout mice for α- and β-synucleins: Effect on synaptic functions. Proc Natl Acad Sci U S A. 2004;101(41):14966–14971. 10.1073/pnas.0406283101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Choi BK, Choi MG, Kim JY, et al. Large α-synuclein oligomers inhibit neuronal SNARE-mediated vesicle docking. Proc Natl Acad Sci U S A. 2013;110(10):4087–4092. 10.1073/pnas.1218424110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Hunn BHM, Cragg SJ, Bolam JP, Spillantini MG, Wade-Martins R. Impaired intracellular trafficking defines early Parkinson’s disease. Trends Neurosci. 2015;38(3):178–188. 10.1016/j.tins.2014.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Eguchi K, Taoufiq Z, Thorn-Seshold O, Trauner D, Hasegawa M, Takahashi T. Wild-type monomeric α-synuclein can impair vesicle endocytosis and synaptic fidelity via tubulin polymerization at the calyx of held. J Neurosci. 2017;37(25):6043–6052. 10.1523/JNEUROSCI.0179-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Wu Q, Takano H, Riddle DM, Trojanowski JQ, Coulter DA, Lee VM-Y. Alpha-synuclein (αSyn) preformed fibrils induce endogenous αSyn aggregation, compromise synaptic activity and enhance synapse loss in cultured excitatory hippocampal neurons. J Neurosci. 2019;29:5080–5094. 10.1523/JNEUROSCI.0060-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Day M, Wang Z, Ding J, An X CAI. Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nat Neurosci. 2006;9:251–259. [DOI] [PubMed] [Google Scholar]
- 121. Wu Q, Shaikh MA, Meymand ES, et al. Neuronal activity modulates alpha-synuclein aggregation and spreading in organotypic brain slice cultures and in vivo. Acta Neuropathol. 2020;6:1–19. 10.1007/s00401-020-02227-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Emanuele M, Esposito A, Camerini S, et al. Exogenous alpha-synuclein alters pre- and post-synaptic activity by fragmenting lipid rafts. EBioMedicine. 2016;7:191–204. 10.1016/j.ebiom.2016.03.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Costa C, Sgobio C, Siliquini S, et al. Mechanisms underlying the impairment of hippocampal long-term potentiation and memory in experimental Parkinson’s disease. Brain. 2012;135(6):1884–1899. 10.1093/brain/aws101 [DOI] [PubMed] [Google Scholar]
- 124. Ferreira DG, Temido-Ferreira M, Miranda HV, et al. α-Synuclein interacts with PrP C to induce cognitive impairment through mGluR5 and NMDAR2B. Nat Neurosci. 2017;20(11):1569–1579. 10.1038/nn.4648 [DOI] [PubMed] [Google Scholar]
- 125. Kornau HC, Schenker LT, Kennedy MB, Seeburg PH. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science. 1995;269(5231):1737–1740. 10.1126/science.7569905 [DOI] [PubMed] [Google Scholar]
- 126. Diógenes MJ, Dias RB, Rombo DM, et al. Extracellular alpha-synuclein oligomers modulate synaptic transmission and impair LTP via NMDA-receptor activation. J Neurosci. 2012;32(34):11750–11762. 10.1523/JNEUROSCI.0234-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Durante V, de Iure A, Loffredo V, et al. Alpha-synuclein targets GluN2A NMDA receptor subunit causing striatal synaptic dysfunction and visuospatial memory alteration. Brain. 2019;142(5):1365–1385. 10.1093/brain/awz065 [DOI] [PubMed] [Google Scholar]
- 128. Tozzi A, de Iure A, Bagetta V, et al. Alpha-synuclein produces early behavioral alterations via striatal cholinergic synaptic dysfunction by interacting with GluN2D N-methyl-D-aspartate receptor subunit. Biol Psychiatry. 2016;79(5):402–414. 10.1016/j.biopsych.2015.08.013 [DOI] [PubMed] [Google Scholar]
- 129. Teravskis PJ, Covelo A, Miller EC, et al. A53t mutant alpha-synuclein induces tau-dependent postsynaptic impairment independently of neurodegenerative changes. J Neurosci. 2018;38(45):9754–9767. 10.1523/JNEUROSCI.0344-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. González-Rodríguez P, Zampese E, Stout KA, et al. Disruption of mitochondrial complex I induces progressive parkinsonism. Nature. 2021;599(7886):650–656. 10.1038/s41586-021-04059-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Hill E, Gowers R, Richardson MJE, Wall MJ. α-Synuclein aggregates increase the conductance of substantia nigra dopamine neurons, an effect partly reversed by the KATP channel inhibitor glibenclamide. eNeuro. 2021;8(1):ENEURO.0330-20.2020. 10.1523/ENEURO.0330-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Braak H, Sandmann-Keil D, Gai W, Braak E. Extensive axonal Lewy neurites in Parkinson’s disease: A novel pathological feature revealed by α-synuclein immunocytochemistry. Neurosci Lett. 1999;265(1):67–69. 10.1016/S0304-3940(99)00208-6 [DOI] [PubMed] [Google Scholar]
- 133. Dijkstra AA, Ingrassia A, de Menezes RX, et al. Evidence for immune response. Axonal dysfunction and reduced endocytosis in the substantia nigra in early stage Parkinson’s disease. PLoS One. 2015;10(6):e0128651. 10.1371/journal.pone.0128651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Bereczki E, Francis PT, Howlett D, et al. Synaptic proteins predict cognitive decline in Alzheimer’s disease and Lewy body dementia. Alzheimer’s Dement. 2016;12(11):1149–1158. 10.1016/j.jalz.2016.04.005 [DOI] [PubMed] [Google Scholar]
- 135. Thakur P, Luk K, Roeper J. Selective K-ATP channel-dependent loss of pacemaking in vulnerable nigrostriatal dopamine neurons by α-synuclein aggregates. bioRxiv. 2019.
- 136. Abbott RD, Petrovitch H, White LR, et al. Frequency of bowel movements and the future risk of Parkinson’s disease. Neurology. 2001;57(3):456–462. 10.1212/WNL.57.3.456 [DOI] [PubMed] [Google Scholar]
- 137. Ross GW, Petrovitch H, Abbott RD, et al. Association of olfactory dysfunction with risk for future Parkinson’s disease. Ann Neurol. 2008;63(2):167–173. 10.1002/ana.21291 [DOI] [PubMed] [Google Scholar]
- 138. Abbott RD, Ross GW, White LR, et al. Excessive daytime sleepiness and subsequent development of Parkinson disease. Neurology. 2005;65(9):1442–1446. 10.1212/01.wnl.0000183056.89590.0d [DOI] [PubMed] [Google Scholar]
- 139. Hawkes CH, Del Tredici K, Braak H. A timeline for Parkinson’s disease. Parkinsonism Relat Disord. 2010;16(2):79–84. 10.1016/j.parkreldis.2009.08.007 [DOI] [PubMed] [Google Scholar]
- 140. Kalia L V, Lang AE. Parkinson’s disease. Lancet. 2015;386(9996):896–912. 10.1016/S0140-6736(14)61393-3 [DOI] [PubMed] [Google Scholar]
- 141. Picconi B, Piccoli G, Calabresi P. Synaptic dysfunction in Parkinson’s disease. Adv Exp Med Biol. 2012;970:553–572. 10.1007/978-3-7091-0932-8_24 [DOI] [PubMed] [Google Scholar]
- 142. Schulz-Schaeffer WJ. The synaptic pathology of α-synuclein aggregation in dementia with Lewy bodies, Parkinson’s disease and Parkinson’s disease dementia. Acta Neuropathol. 2010;120(2):131–143. 10.1007/s00401-010-0711-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Milnerwood AJ, Raymond LA. Early synaptic pathophysiology in neurodegeneration: Insights from huntington’s disease. Trends Neurosci. 2010;33(11):513–523. 10.1016/j.tins.2010.08.002 [DOI] [PubMed] [Google Scholar]
- 144. Marui W, Iseki E, Nakai T, et al. Progression and staging of Lewy pathology in brains from patients with dementia with Lewy bodies. J Neurol Sci. 2002;195(2):153–159. 10.1016/S0022-510X(02)00006-0 [DOI] [PubMed] [Google Scholar]
- 145. Uhlhaas PJ, Singer W. Neural synchrony in brain disorders: Relevance for cognitive dysfunctions and pathophysiology. Neuron. 2006;52(1):155–168. 10.1016/j.neuron.2006.09.020 [DOI] [PubMed] [Google Scholar]
- 146. Klee MR, Offenloch K, Tigges J. Cross-correlation analysis of electroencephalographic potentials and slow membrane transients. Science. 1965;147(3657):519–521. 10.1126/science.147.3657.519 [DOI] [PubMed] [Google Scholar]
- 147. Mitzdorf U. Current source-density method and application in cat cerebral cortex: Investigation of evoked potentials and EEG phenomena. Physiol Rev. 1985;65(1):37–100. 10.1152/physrev.1985.65.1.37 [DOI] [PubMed] [Google Scholar]
- 148. Creutzfeldt OD, Watanabe S, Lux HD. Relations between EEG phenomena and potentials of single cortical cells. I. Evoked responses after thalamic and epicortical stimulation. Electroencephalogr Clin Neurophysiol. 1966;20(1):1–18. [DOI] [PubMed] [Google Scholar]
- 149. Creutzfeldt OD, Watanabe S, Lux HD. Relations between EEG phenomena and potentials of single cortical cells. II. Spontaneous and convulsoid activity. Electroencephalogr Clin Neurophysiol. 1966;20(1):19–37. 10.1016/0013-4694(66)90137-4 [DOI] [PubMed] [Google Scholar]
- 150. Destexhe A, Contreras D, Steriade M. Spatiotemporal analysis of local field potentials and unit discharges in cat cerebral cortex during natural wake and sleep states. J Neurosci. 1999;19(11):4595–4608. 10.1523/JNEUROSCI.19-11-04595.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Lepousez G, Lledo P-M. Odor discrimination requires proper olfactory fast oscillations in awake mice. Neuron. 2013;80(4):1010–1024. 10.1016/j.neuron.2013.07.025 [DOI] [PubMed] [Google Scholar]
- 152. Alekseichuk I, Pabel SC, Antal A, Paulus W. Intrahemispheric theta rhythm desynchronization impairs working memory. Restor Neurol Neurosci. 2017;35(2):147–158. 10.3233/RNN-160714 [DOI] [PubMed] [Google Scholar]
- 153. Stopfer M, Bhagavan S, Smith BH, Laurent G. Impaired odour discrimination on desynchronization of odour-encoding neural assemblies. Nature. 1997;390(6655):70–74. 10.1038/36335 [DOI] [PubMed] [Google Scholar]
- 154. Spitzer B, Haegens S. Beyond the status quo: A role for beta oscillations in endogenous content (Re)activation. Eneuro. 2017;4(4):ENEURO.0170-17.2017. 10.1523/ENEURO.0170-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Mallet N, Pogosyan A, Sharott A, et al. Disrupted dopamine transmission and the emergence of exaggerated beta oscillations in subthalamic nucleus and cerebral cortex. J Neurosci. 2008;28(18):4795–4806. 10.1523/JNEUROSCI.0123-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. de La Crompe B, Aristieta A, Leblois A, Elsherbiny S, Boraud T, Mallet NP. The globus pallidus orchestrates abnormal network dynamics in a model of parkinsonism. Nat Commun. 2020;11(1):1–14. 10.1038/s41467-020-15947-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Goldberg JA, Rokni U, Boraud T, Vaadia E, Bergman H. Spike synchronization in the cortex-basal ganglia networks of parkinsonian primates reflects global dynamics of the local field potentials. J Neurosci. 2004;24(26):6003–6010. 10.1523/JNEUROSCI.4848-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Deffains M, Bergman H. Parkinsonism-related β oscillations in the primate basal ganglia networks–recent advances and clinical implications. Parkinsonism Relat Disord. 2019;59:2–8. 10.1016/j.parkreldis.2018.12.015 [DOI] [PubMed] [Google Scholar]
- 159. Brown P. Bad oscillations in Parkinson’s disease. J Neural Transm Suppl. 2006:27–30. 10.1007/978-3-211-45295-0_6 [DOI] [PubMed] [Google Scholar]
- 160. Arthurs OJ, Boniface S. How well do we understand the neural origins of the fMRI BOLD signal? Trends Neurosci. 2002;25(1):27–31. 10.1016/S0166-2236(00)01995-0 [DOI] [PubMed] [Google Scholar]
- 161. Filippi M, Elisabetta S, Piramide N, Agosta F. Functional MRI in idiopathic Parkinson’s disease. Int Rev Neurobiol. 2018;141:439–467. 10.1016/bs.irn.2018.08.005 [DOI] [PubMed] [Google Scholar]
- 162. Al-Radaideh AM, Rababah EM. The role of magnetic resonance imaging in the diagnosis of Parkinson’s disease: A review. Clin Imaging. 2016;40(5):987–996. 10.1016/j.clinimag.2016.05.006 [DOI] [PubMed] [Google Scholar]
- 163. Berman BD, Smucny J, Wylie KP, et al. Levodopa modulates small-world architecture of functional brain networks in Parkinson’s disease. Mov Disord. 2016;31(11):1676–1684. 10.1002/mds.26713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Wu T, Long X, Zang Y, et al. Regional homogeneity changes in patients with Parkinson’s disease. Hum Brain Mapp. 2009;30(5):1502–1510. 10.1002/hbm.20622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Skidmore FM, Yang M, Baxter L, et al. Reliability analysis of the resting state can sensitively and specifically identify the presence of Parkinson disease. Neuroimage. 2013;75:249–261. 10.1016/j.neuroimage.2011.06.056 [DOI] [PubMed] [Google Scholar]
- 166. Skidmore FM, Yang M, Baxter L, et al. Apathy, depression, and motor symptoms have distinct and separable resting activity patterns in idiopathic Parkinson disease. Neuroimage. 2013;81:484–495. 10.1016/j.neuroimage.2011.07.012 [DOI] [PubMed] [Google Scholar]
- 167. Helmich RC, Janssen MJR, Oyen WJG, Bloem BR, Toni I. Pallidal dysfunction drives a cerebellothalamic circuit into Parkinson tremor. Ann Neurol. 2011;69(2):269–281. 10.1002/ana.22361 [DOI] [PubMed] [Google Scholar]
- 168. Wu T, Wang L, Chen Y, Zhao C, Li K, Chan P. Changes of functional connectivity of the motor network in the resting state in Parkinson’s disease. Neurosci Lett. 2009;460(1):6–10. 10.1016/j.neulet.2009.05.046 [DOI] [PubMed] [Google Scholar]
- 169. Wu T, Long X, Wang L, et al. Functional connectivity of cortical motor areas in the resting state in Parkinson’s disease. Hum Brain Mapp. 2011;32(9):1443–1457. 10.1002/hbm.21118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Szewczyk-Krolikowski K, Menke RAL, Rolinski M, et al. Functional connectivity in the basal ganglia network differentiates PD patients from controls. Neurology. 2014;83(3):208–214. 10.1212/WNL.0000000000000592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Hassan M, Chaton L, Benquet P, et al. Functional connectivity disruptions correlate with cognitive phenotypes in Parkinson’s disease. NeuroImage Clin. 2017;14:591–601. 10.1016/j.nicl.2017.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Hammond C, Bergman H, Brown P. Pathological synchronization in Parkinson’s disease: Networks, models and treatments. Trends Neurosci. 2007;30(7):357–364. 10.1016/j.tins.2007.05.004 [DOI] [PubMed] [Google Scholar]
- 173. Kühn AA, Williams D, Kupsch A, et al. Event-related beta desynchronization in human subthalamic nucleus correlates with motor performance. Brain. 2004;127(4):735–746. 10.1093/BRAIN/AWH106 [DOI] [PubMed] [Google Scholar]
- 174. Levy R, Hutchison W, Lozano AM, Dostrovsky JO. High-frequency synchronization of neuronal activity in the subthalamic nucleus of parkinsonian patients with limb tremor. J Neurosci. 2000;20:7766–7775. 10.1523/JNEUROSCI.20-20-07766.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Raz A, Vaadia E, Bergman H. Firing patterns and correlations of spontaneous discharge of pallidal neurons in the normal and the tremulous 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine vervet model of parkinsonism. J Neurosci. 2000;20(22):8559–8571. 10.1523/JNEUROSCI.20-22-08559.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Bergman H, Wichmann T, Karmon B, DeLong M. The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism. J Neurophysiol. 1994;72(2):507–520. 10.1152/JN.1994.72.2.507 [DOI] [PubMed] [Google Scholar]
- 177. Allers K, Kreiss D, Walters J. Multisecond oscillations in the subthalamic nucleus: Effects of apomorphine and dopamine cell lesion. Synapse. 2000;38(1):38–50. 10.1002/1098-2396(200010)38:1 [DOI] [PubMed] [Google Scholar]
- 178. Brown P, Oliviero A, Mazzone P, Insola A, Tonali P, Di Lazzaro V. Dopamine dependency of oscillations between subthalamic nucleus and pallidum in Parkinson’s disease. J Neurosci. 2001;21(3):1033–1038. 10.1523/JNEUROSCI.21-03-01033.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Levy R, Dostrovsky J, Lang A, Sime E, Hutchison W, Lozano A. Effects of apomorphine on subthalamic nucleus and globus pallidus internus neurons in patients with Parkinson’s disease. J Neurophysiol. 2001;86(1):249–260. 10.1152/JN.2001.86.1.249 [DOI] [PubMed] [Google Scholar]
- 180. Marsden J., Limousin-Dowsey P, Ashby P, Pollak P, Brown P. Subthalamic nucleus, sensorimotor cortex and muscle interrelationships in Parkinson’s disease. Brain. 2001;124(Pt 2):378–388. 10.1093/BRAIN/124.2.378 [DOI] [PubMed] [Google Scholar]
- 181. Sanabria DE, Johnson LA, Nebeck SD, et al. Biology of neuroengineering interfaces: Parkinsonism and vigilance: Alteration in neural oscillatory activity and phase-amplitude coupling in the basal ganglia and motor cortex. J Neurophysiol. 2017;118(5):2654–2669. 10.1152/jn.00388.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Connolly AT, Jensen AL, Baker KB, Vitek JL, Johnson MD. Classification of pallidal oscillations with increasing parkinsonian severity. J Neurophysiol. 2015;114(1):209–218. 10.1152/jn.00840.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Connolly AT, Jensen AL, Bello EM, et al. Modulations in oscillatory frequency and coupling in globus pallidus with increasing parkinsonian severity. J Neurosci. 2015;35(15):6231–6240. 10.1523/JNEUROSCI.4137-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. De Hemptinne C, Ryapolova-Webb ES, Air EL, et al. Exaggerated phase-amplitude coupling in the primary motor cortex in Parkinson disease. Proc Natl Acad Sci U S A. 2013;110(12):4780–4785. 10.1073/pnas.1214546110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Shimamoto SA, Ryapolova-Webb ES, Ostrem JL, Galifianakis NB, Miller KJ, Starr PA. Subthalamic nucleus neurons are synchronized to primary motor cortex local field potentials in Parkinson’s disease. J Neurosci. 2013;33(17):7220–7233. 10.1523/JNEUROSCI.4676-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Losacco J, Ramirez-Gordillo D, Gilmer J, Restrepo D. Learning improves decoding of odor identity with phase-referenced oscillations in the olfactory bulb. Elife. 2020;9:e52583. 10.7554/eLife.52583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Qasim SE, de Hemptinne C, Swann NC, Miocinovic S, Ostrem JL, Starr PA. Electrocorticography reveals beta desynchronization in the basal ganglia-cortical loop during rest tremor in Parkinson’s disease. Neurobiol Dis. 2016;86:177–186. 10.1016/j.nbd.2015.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Chen F, Liu W, Liu P, et al. α-Synuclein aggregation in the olfactory bulb induces olfactory deficits by perturbing granule cells and granular–mitral synaptic transmission. NPJ Park Dis. 2021;7(1):114. 10.1038/s41531-021-00259-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Kulkarni AS, del Mar Cortijo M, Roberts ER, et al. Perturbation of in vivo neural activity following α-synuclein seeding in the olfactory bulb. J Parkinsons Dis. 2020;10(4):1411–1427. 10.1101/2020.04.17.045013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Rey NL, Steiner JA, Maroof N, et al. Widespread transneuronal propagation of α-synucleinopathy triggered in olfactory bulb mimics prodromal Parkinson’s disease. J Exp Med. 2016;213(9):1759–1778. 10.1084/jem.20160368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Johnson ME, Bergkvist L, Mercado G, et al. Deficits in olfactory sensitivity in a mouse model of Parkinson’s disease revealed by plethysmography of odor-evoked sniffing. Sci Rep. 2020;10(1):9242. 10.1038/s41598-020-66201-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Zhang W, Sun C, Shao Y, Zhou Z, Hou Y, Li A. Partial depletion of dopaminergic neurons in the substantia nigra impairs olfaction and alters neural activity in the olfactory bulb. Sci Rep. 2019;9(1):254. 10.1038/s41598-018-36538-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Berry JK, Cox D. Increased oscillatory power in a computational model of the olfactory bulb due to synaptic degeneration. bioRxiv. 2021. 10.1101/2020.08.06.239293 [DOI] [PubMed] [Google Scholar]
- 194. Schnitzler A, Münks C, Butz M, Timmermann L, Gross J. Synchronized brain network associated with essential tremor as revealed by magnetoencephalography. Mov Disord Off J Mov Disord Soc. 2009;24(11):1629–1635. 10.1002/mds.22633 [DOI] [PubMed] [Google Scholar]
- 195. Constas C. The effects of adrenaline, noradrenaline, and isoprenaline on parkinsonian tremor. J Neurol Neurosurg Psychiatry. 1962;25(2):116–121. 10.1136/JNNP.25.2.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Isaias IU, Marzegan A, Pezzoli G, et al. A role for locus coeruleus in Parkinson tremor. Front Hum Neurosci 2012;5:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Pasquini J, Ceravolo R, Qamhawi Z, et al. Progression of tremor in early stages of Parkinson’s disease: A clinical and neuroimaging study. Brain. 2018;141(3):811–821. 10.1093/brain/awx376 [DOI] [PubMed] [Google Scholar]
- 198. Laurent G, Davidowitz H. Encoding of olfactory information with oscillating neural assemblies. Science. 1994;265(5180):1872–1875. 10.1126/science.265.5180.1872 [DOI] [PubMed] [Google Scholar]
- 199. MacLeod K, Laurent G. Distinct mechanisms for synchronization and temporal patterning of odor-encoding neural assemblies. Science. 1996;274(5289):976–979. 10.1126/science.274.5289.976 [DOI] [PubMed] [Google Scholar]
- 200. Wehr M, Laurent G. Odour encoding by temporal sequences of firing in oscillating neural assemblies. Nature. 1996;384(6605):162–166. 10.1038/384162a0 [DOI] [PubMed] [Google Scholar]
- 201. Chow CC, Kopell N. Dynamics of spiking neurons with electrical coupling. Neural Comput. 2000;12(7):1643–1678. 10.1162/089976600300015295 [DOI] [PubMed] [Google Scholar]
- 202. Lewis T, Rinzel J. Dynamics of spiking neurons connected by both inhibitory and electrical coupling. J Comput Neurosci. 2003;14(3):283–309. 10.1023/A:1023265027714 [DOI] [PubMed] [Google Scholar]
- 203. Pfeuty B, Mato G, Golomb D, Hansel D. Electrical synapses and synchrony: The role of intrinsic currents. J Neurosci. 2003;23(15):6280–6294. 10.1523/JNEUROSCI.23-15-06280.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Mancilla JG, Lewis TJ, Pinto DJ, Rinzel J, Connors BW. Synchronization of electrically coupled pairs of inhibitory interneurons in neocortex. J Neurosci. 2007;27(8):2058–2073. 10.1523/JNEUROSCI.2715-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Saraga F, Ng L, Skinner F. Distal gap junctions and active dendrites can tune network dynamics. J Neurophysiol. 2006;95(3):1669–1682. 10.1152/JN.00662.2005 [DOI] [PubMed] [Google Scholar]
- 206. Pfeuty B, Mato G, Golomb D, Hansel D. The combined effects of inhibitory and electrical synapses in synchrony. Neural Comput. 2005;17(3):633–670. 10.1162/0899766053019917 [DOI] [PubMed] [Google Scholar]
- 207. Miller WC, DeLong MR. Altered tonic activity of neurons in the globus pallidus and subthalamic nucleus in the primate MPTP model of Parkinsonism. Basal Ganglia II. Springer; 1987:415–427. [Google Scholar]
- 208. Little S, Brown P. The functional role of beta oscillations in Parkinson’s disease. Park Relat Disord. 2014; 20(Suppl 1):S44–S48. 10.1016/S1353-8020(13)70013-0 [DOI] [PubMed] [Google Scholar]
- 209. Kühn AA, Trottenberg T, Kivi A, Kupsch A, Schneider GH, Brown P. The relationship between local field potential and neuronal discharge in the subthalamic nucleus of patients with Parkinson’s disease. Exp Neurol. 2005;194(1):212–220. 10.1016/j.expneurol.2005.02.010 [DOI] [PubMed] [Google Scholar]
- 210. David F, Courtiol E, Buonviso N, Fourcaud-Trocmé N. Competing mechanisms of gamma and beta oscillations in the olfactory bulb based on multimodal inhibition of mitral cells over a respiratory cycle. eNeuro. 2015;2(6):ENEURO.0018-15.2015. 10.1523/ENEURO.0018-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Osinski BL, Kay LM. Granule cell excitability regulates gamma and beta oscillations in a model of the olfactory bulb dendrodendritic microcircuit. J Neurophysiol. 2016;116:522–539. https://doi.org/101152/jn009882015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Taguchi K, Watanabe Y, Tsujimura A, Tanaka M. Brain region-dependent differential expression of alpha-synuclein. J Comp Neurol. 2016;524(6):1236–1258. 10.1002/CNE.23901 [DOI] [PubMed] [Google Scholar]
- 213. Cheng F, Li X, Li Y, et al. Α-synuclein promotes clathrin-mediated NMDA receptor endocytosis and attenuates NMDA-induced dopaminergic cell death. J Neurochem. 2011;119(4):815–825. 10.1111/j.1471-4159.2011.07460.x [DOI] [PubMed] [Google Scholar]
- 214. Chen Y, Yang W, Li X, Yang H, Xu Z, Yu S. α-Synuclein-induced internalization of NMDA receptors in hippocampal neurons is associated with reduced inward current and Ca2+ influx upon NMDA stimulation. Neuroscience. 2015;300:297–306. 10.1016/j.neuroscience.2015.05.035 [DOI] [PubMed] [Google Scholar]
- 215. Navarria L, Zaltieri M, Longhena F, et al. Alpha-synuclein modulates NR2B-containing NMDA receptors and decreases their levels after rotenone exposure. Neurochem Int. 2015;85:14–23. 10.1016/j.neuint.2015.03.008 [DOI] [PubMed] [Google Scholar]
- 216. Yang J, Hertz E, Zhang X, et al. Overexpression of α-synuclein simultaneously increases glutamate NMDA receptor phosphorylation and reduces glucocerebrosidase activity. Neurosci Lett. 2016;611:51–58. 10.1016/j.neulet.2015.11.023 [DOI] [PubMed] [Google Scholar]
- 217. Blumenstock S, Rodrigues EF, Peters F, et al. Seeding and transgenic overexpression of alpha-synuclein triggers dendritic spine pathology in the neocortex. EMBO Mol Med. 2017;9(5):716–731. 10.15252/emmm.201607305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Ruggiero A, Katsenelson M, Slutsky I. Mitochondria: New players in homeostatic regulation of firing rate set points. Trends Neurosci. 2021;44(8):605–618. 10.1016/J.TINS.2021.03.002 [DOI] [PubMed] [Google Scholar]
- 219. Fieblinger T, Graves SM, Sebel LE, et al. Cell type-specific plasticity of striatal projection neurons in parkinsonism and L-DOPA-induced dyskinesia. Nat Commun. 2014;5(1):1–15. 10.1038/ncomms6316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Turrigiano GG. Homeostatic plasticity in neuronal networks: The more things change, the more they stay the same. Trends Neurosci. 1999;22:221–227. 10.1016/s0166-2236(98)01341-1 [DOI] [PubMed] [Google Scholar]
- 221. Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5(2):97–107. 10.1038/nrn1327 [DOI] [PubMed] [Google Scholar]
- 222. O’Leary T, Williams AH, Franci A, Marder E. Cell types, network homeostasis, and pathological compensation from a biologically plausible ion channel expression model. Neuron. 2014;82:809–821. 10.1016/J.NEURON.2014.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Turrigiano G. Homeostatic synaptic plasticity: Local and global mechanisms for stabilizing neuronal function. Cold Spring Harb Perspect Biol. 2012;4(1):a005736–a005736. 10.1101/CSHPERSPECT.A005736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Fereshtehnejad S-M, Yao C, Pelletier A, Montplaisir JY, Gagnon J-F, Postuma RB. Evolution of prodromal Parkinson’s disease and dementia with Lewy bodies: A prospective study. Brain. 2019;142(7):2051–2067. 10.1093/BRAIN/AWZ111 [DOI] [PubMed] [Google Scholar]
- 225. Marchand DG, Postuma RB, Escudier F, et al. How does dementia with Lewy bodies start? Prodromal cognitive changes in REM sleep behavior disorder. Ann Neurol. 2018;83(5):1016–1026. 10.1002/ANA.25239 [DOI] [PubMed] [Google Scholar]
- 226. Baggio H, Sala-Llonch R, Segura B, et al. Functional brain networks and cognitive deficits in Parkinson’s disease. Hum Brain Mapp. 2014;35(9):4620–4634. 10.1002/hbm.22499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Baggio H, Segura B, Sala-Llonch R, et al. Cognitive impairment and resting-state network connectivity in P arkinson’s disease. Hum Brain Mapp. 2015;36(1):199–212. 10.1002/hbm.22622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Bertrand J-A, McIntosh AR, Postuma RB, et al. Brain connectivity alterations are associated with the development of dementia in Parkinson’s disease. Brain Connect. 2016;6(3):216–224. 10.1089/brain.2015.0390 [DOI] [PubMed] [Google Scholar]
- 229. Bosboom JLW, Stoffers D, Wolters EC, Stam CJ, Berendse HW. MEG resting state functional connectivity in Parkinson’s disease related dementia. J Neural Transm. 2009;116(2):193–202. 10.1007/s00702-008-0132-6 [DOI] [PubMed] [Google Scholar]
- 230. Lopes R, Delmaire C, Defebvre L, et al. Cognitive phenotypes in Parkinson’s disease differ in terms of brain-network organization and connectivity. Hum Brain Mapp. 2017;38(3):1604–1621. 10.1002/hbm.23474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Olde Dubbelink KTE, Hillebrand A, Stoffers D, et al. Disrupted brain network topology in Parkinson’s disease: A longitudinal magnetoencephalography study. Brain. 2014;137(1):197–207. 10.1093/brain/awt316 [DOI] [PubMed] [Google Scholar]
- 232. Skidmore F, Korenkevych D, Liu Y, He G, Bullmore E, Pardalos PM. Connectivity brain networks based on wavelet correlation analysis in Parkinson fMRI data. Neurosci Lett. 2011;499(1):47–51. 10.1016/j.neulet.2011.05.030 [DOI] [PubMed] [Google Scholar]
- 233. Lou Y, Huang P, Li D, et al. Altered brain network centrality in depressed Parkinson’s disease patients. Mov Disord. 2015;30(13):1777–1784. 10.1002/mds.26321 [DOI] [PubMed] [Google Scholar]
- 234. Lewis SJG, Dove A, Robbins TW, Barker RA, Owen AM. Cognitive impairments in early Parkinson’s disease are accompanied by reductions in activity in frontostriatal neural circuitry. J Neurosci. 2003;23(15):6351–6356. 10.1523/JNEUROSCI.23-15-06351.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Simioni AC, Dagher A, Fellows LK. Compensatory striatal–cerebellar connectivity in mild–moderate Parkinson’s disease. NeuroImage Clin. 2016;10:54–62. 10.1016/j.nicl.2015.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Valderhaug VD, Heiney K, Ramstad OH, et al. Early functional changes associated with alpha-synuclein proteinopathy in engineered human neural networks. Am J Physiol Cell Physiol. 2021;320(6):C1141–C1152. 10.1152/AJPCELL.00413.2020/ASSET/IMAGES/LARGE/AJPCELL.00413.2020_F006.JPEG [DOI] [PubMed] [Google Scholar]
- 237. Fearnley JM, Lees AJ. Striatonigral degeneration: A clinicopathological study. Brain. 1990;113(6):1823–1842. 10.1093/brain/113.6.1823 [DOI] [PubMed] [Google Scholar]
- 238. Gregory S, Long JD, Tabrizi SJ, Rees G. Measuring compensation in neurodegeneration using MRI. Curr Opin Neurol. 2017;30(4):380. 10.1097/WCO.0000000000000469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Seidel K, Mahlke J, Siswanto S, et al. The brainstem pathologies of Parkinson’s disease and dementia with Lewy bodies. Brain Pathol. 2015;25(2):121–135. 10.1111/bpa.12168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Gómez-Tortosa E, Sanders JL, Newell K, Hyman BT. Cortical neurons expressing calcium binding proteins are spared in dementia with Lewy bodies. Acta Neuropathol. 2001;101(1):36–42. 10.1007/s004010000270 [DOI] [PubMed] [Google Scholar]
- 241. Taguchi K, Watanabe Y, Tsujimura A, Tanaka M. Expression of α-synuclein is regulated in a neuronal cell type-dependent manner. Anat Sci Int. 2019;94(1):11–22. 10.1007/s12565-018-0464-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Hall H, Reyes S, Landeck N, et al. Hippocampal Lewy pathology and cholinergic dysfunction are associated with dementia in Parkinson’s disease. Brain. 2014;137(9):2493–2508. 10.1093/brain/awu193 [DOI] [PubMed] [Google Scholar]
- 243. Wakabayashi K, Hansen LA, Masliah E. Cortical Lewy body-containing neurons are pyramidal cells: Laser confocal imaging of double-immunolabeled sections with anti-ubiquitin and SMI32. Acta Neuropathol. 1995;89(5):404–408. 10.1007/BF00307643 [DOI] [PubMed] [Google Scholar]
- 244. Del Tredici K, Braak H. Dysfunction of the locus coeruleus–norepinephrine system and related circuitry in Parkinson’s disease-related dementia. J Neurol Neurosurg Psychiatry. 2013;84(7):774–783. 10.1136/jnnp-2011-301817 [DOI] [PubMed] [Google Scholar]
- 245. Braak H, Del TK. Neuroanatomy and pathology of sporadic Parkinson’s disease. Adv Anat Embryol Cell Biol. 2009;201:1–119. [PubMed] [Google Scholar]
- 246. Henderson MX, Cornblath EJ, Darwich A, et al. Spread of α-synuclein pathology through the brain connectome is modulated by selective vulnerability and predicted by network analysis. Nat Neurosci. 2019;22(8):1248–1257. 10.1038/s41593-019-0457-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Mahul-Mellier AL, Vercruysse F, Maco B, et al. Fibril growth and seeding capacity play key roles in α-synuclein-mediated apoptotic cell death. Cell Death Differ. 2015;22(12):2107–2122. 10.1038/cdd.2015.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Luk KC, Kehm V, Carroll J, et al. Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012;338(6109):949–953. 10.1126/science.1227157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Taguchi K, Watanabe Y, Tsujimura A, et al. Differential expression of alpha-synuclein in hippocampal neurons. PLoS One. 2014;9(2):e89327. 10.1371/journal.pone.0089327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Luna E, Decker SC, Riddle DM, et al. Differential α-synuclein expression contributes to selective vulnerability of hippocampal neuron subpopulations to fibril-induced toxicity. Acta Neuropathol. 2018;135(6):855–875. 10.1007/s00401-018-1829-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Rapino F, Jung M, Fulda S. BAG3 Induction is required to mitigate proteotoxicity via selective autophagy following inhibition of constitutive protein degradation pathways. Oncogene. 2014;33(13):1713–1724. 10.1038/onc.2013.110 [DOI] [PubMed] [Google Scholar]
- 252. Cao Y-L, Yang Y-P, Mao C-J, et al. A role of BAG3 in regulating SNCA/α-synuclein clearance via selective macroautophagy. Neurobiol Aging. 2017;60:104–115. 10.1016/j.neurobiolaging.2017.08.023 [DOI] [PubMed] [Google Scholar]
- 253. Surmeier JD, Schumacker PT, Guzman JD, Ilijic E, Yang B, Zampese E. Calcium and Parkinson’s disease. Biochem Biophys Res Commun. 2017;483(4):1013–1019. 10.1016/j.bbrc.2016.08.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Flores-Cuadrado A, Ubeda-Bañon I, Saiz-Sanchez D, Martinez-Marcos A. α-Synucleinopathy in the human amygdala in Parkinson disease: Differential vulnerability of somatostatin- and parvalbumin-expressing neurons. J Neuropathol Exp Neurol. 2017;76(9):754–758. 10.1093/JNEN/NLX054 [DOI] [PubMed] [Google Scholar]
- 255. Torres ERS, Stanojlovic M, Zelikowsky M, et al. Alpha-synuclein pathology, microgliosis, and parvalbumin neuron loss in the amygdala associated with enhanced fear in the Thy1-aSyn model of Parkinson’s disease. Neurobiol Dis. 2021;158:105478. 10.1016/j.nbd.2021.105478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Caillard O, Moreno H, Schwaller B, Llano I, Celio MR, Marty A. Role of the calcium-binding protein parvalbumin in short-term synaptic plasticity. Proc Natl Acad Sci U S A. 2000;97(24):13372–13377. 10.1073/pnas.230362997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Pissadaki EK, Bolam JP. The energy cost of action potential propagation in dopamine neurons: Clues to susceptibility in Parkinson’s disease. Front Comput Neurosci. 2013;7:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Sulzer D, Surmeier DJ. Neuronal vulnerability, pathogenesis, and Parkinson’s disease. Mov Disord. 2013;28(6):715–724. 10.1002/mds.25187 [DOI] [PubMed] [Google Scholar]
- 259. Aston-Jones G, Waterhouse B. Locus coeruleus: From global projection system to adaptive regulation of behavior. Brain Res. 2016;1645:75–78. 10.1016/j.brainres.2016.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Matsuda W, Furuta T, Nakamura KC, et al. Single nigrostriatal dopaminergic neurons form widely spread and highly dense axonal arborizations in the neostriatum. J Neurosci. 2009;29(2):444–453. 10.1523/JNEUROSCI.4029-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Hornung J-P. The human raphe nuclei and the serotonergic system. J Chem Neuroanat. 2003;26(4):331–343. 10.1016/j.jchemneu.2003.10.002 [DOI] [PubMed] [Google Scholar]
- 262. Pacelli C, Giguère N, Bourque M-J, Lévesque M, Slack RS, Trudeau L-É. Elevated mitochondrial bioenergetics and axonal arborization size are key contributors to the vulnerability of dopamine neurons. Curr Biol. 2015;25(18):2349–2360. 10.1016/j.cub.2015.07.050 [DOI] [PubMed] [Google Scholar]
- 263. Ratcliffe EM, Farrar NR, Fox EA. Development of the vagal innervation of the gut: Steering the wandering nerve. Neurogastroenterol Motil. 2011;23(10):898–911. 10.1111/j.1365-2982.2011.01764.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Hunn BHM, Cragg SJ, Bolam JP, Spillantini M-G, Wade-Martins R. Impaired intracellular trafficking defines early Parkinson’s disease. Trends Neurosci. 2015;38(3):178–188. 10.1016/j.tins.2014.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Zhou F-M, Wilson CJ, Dani JA. Cholinergic interneuron characteristics and nicotinic properties in the striatum. J Neurobiol. 2002;53(4):590–605. 10.1002/neu.10150 [DOI] [PubMed] [Google Scholar]
- 266. Wilson CJ, Callaway JC. Coupled oscillator model of the dopaminergic neuron of the substantia nigra. J Neurophysiol. 2000;83(5):3084–3100. 10.1152/jn.2000.83.5.3084 [DOI] [PubMed] [Google Scholar]
- 267. Foehring RC, Zhang XF, Lee JCF, Callaway JC. Endogenous calcium buffering capacity of substantia nigral dopamine neurons. J Neurophysiol. 2009;102(4):2326–2333. 10.1152/jn.00038.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Jensen PK. Antimycin-insensitive oxidation of succinate and reduced nicotinamide-adenine dinucleotide in electron-transport particles I. pH dependency and hydrogen peroxide formation. Biochim Biophys Acta. 1966;122(2):157–166. [DOI] [PubMed] [Google Scholar]
- 269. Williams JT, North RA, Shefner SA, Nishi S, Egan TM. Membrane properties of rat locus coeruleus neurones. Neuroscience. 1984;13(1):137–156. 10.1016/0306-4522(84)90265-3 [DOI] [PubMed] [Google Scholar]
- 270. McCann MJ, Rogers RC. Oxytocin excites gastric-related neurones in rat dorsal vagal complex. J Physiol. 1990;428(1):95–108. 10.1113/jphysiol.1990.sp018202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Burlhis TM, Aghajanian GK. Pacemaker potentials of serotonergic dorsal raphe neurons: Contribution of a low-threshold Ca2+ conductance. Synapse. 1987;1(6):582–588. 10.1002/syn.890010611 [DOI] [PubMed] [Google Scholar]
- 272. Pignatelli A, Kobayashi K, Okano H, Belluzzi O. Functional properties of dopaminergic neurones in the mouse olfactory bulb. J Physiol. 2005;564(2):501–514. 10.1113/jphysiol.2005.084632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Puopolo M, Bean BP, Raviola E. Spontaneous activity of isolated dopaminergic periglomerular cells of the main olfactory bulb. J Neurophysiol. 2005;94(5):3618–3627. 10.1152/jn.00225.2005 [DOI] [PubMed] [Google Scholar]
- 274. Alreja M, Aghajanian GK. Pacemaker activity of locus coeruleus neurons: Whole-cell recordings in brain slices show dependence on cAMP and protein kinase A. Brain Res. 1991;556:339–343. 10.1016/0006-8993(91)90327-R [DOI] [PubMed] [Google Scholar]
- 275. Vandermaelen CP, Aghajanian GK. Electrophysiological and pharmacological characterization of serotonergic dorsal raphe neurons recorded extracellularly and intracellularly in rat brain slices. Brain Res. 1983;289(1–2):109–119. 10.1016/0006-8993(83)90011-2 [DOI] [PubMed] [Google Scholar]
- 276. Murchison D, Griffith WH. Calcium buffering systems and calcium signaling in aged rat basal forebrain neurons. Aging Cell. 2007;6(3):297–305. 10.1111/j.1474-9726.2007.00293.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Schulz-Schaeffer WJ. Is cell death primary or secondary in the pathophysiology of idiopathic Parkinson’s disease? Biomolecules. 2015;5(3):1467. 10.3390/BIOM5031467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Bertrand E, Lechowicz W, Lewandowska E, et al. Degenerative axonal changes in the hippocampus and amygdala in Parkinson’s disease. Folia Neuropathol. 2003;41:197–207. [PubMed] [Google Scholar]
- 279. Bridi JC, Hirth F. Mechanisms of α-synuclein induced synaptopathy in Parkinson’s disease. Front Neurosci. 2018;19:80. 10.3389/FNINS.2018.00080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Possin KL, Kang GA, Guo C, et al. Rivastigmine is associated with restoration of left frontal brain activity in Parkinson’s disease. Mov Disord. 2013;28(10):1384–1390. 10.1002/mds.25575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Adler G, Brassen S, Chwalek K, Dieter B, Teufel M. Prediction of treatment response to rivastigmine in Alzheimer’s dementia. J Neurol Neurosurg Psychiatry. 2004;75(2):292–294. [PMC free article] [PubMed] [Google Scholar]
- 282. Almkvist O, Jelic V, Amberla K, Hellström-Lindahl E, Meurling L, Nordberg A. Responder characteristics to a single oral dose of cholinesterase inhibitor: A double-blind placebo-controlled study withtacrine in Alzheimer patients. Dement Geriatr Cogn Disord. 2001;12(1):22–32. 10.1159/000051232 [DOI] [PubMed] [Google Scholar]
- 283. Lippa CF, Smith TW, Perry E. Dementia with Lewy bodies: Choline acetyltransferase parallels nucleus basalis pathology. J Neural Transm. 1999;106:525–535. 10.1007/s007020050176 [DOI] [PubMed] [Google Scholar]
- 284. Perry EK, Irving D, Kerwin JM, et al. Cholinergic transmitter and neurotrophic activities in Lewy body dementia: Similarity to Parkinson’s and distinction from Alzheimer disease. Alzheimer Dis Assoc Disord. 1993;7:69–79. [DOI] [PubMed] [Google Scholar]
- 285. Decressac M, Mattsson B, Lundblad M, Weikop P, Björklund A. Progressive neurodegenerative and behavioural changes induced by AAV-mediated overexpression of α-synuclein in midbrain dopamine neurons. Neurobiol Dis. 2012;45(3):939–953. 10.1016/j.nbd.2011.12.013 [DOI] [PubMed] [Google Scholar]
- 286. Chu Y, Morfini GA, Langhamer LB, He Y, Brady ST, Kordower JH. Alterations in axonal transport motor proteins in sporadic and experimental Parkinson’s disease. Brain. 2012;135(7):2058–2073. 10.1093/brain/aws133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Kordower JH, Olanow CW, Dodiya HB, et al. Disease duration and the integrity of the nigrostriatal system in Parkinson’s disease. Brain. 2013;136(8):2419–2431. 10.1093/brain/awt192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Kordower J, Burke R. Disease modification for Parkinson’s disease: Axonal regeneration and trophic factors. Mov Disord. 2018;33(5):678–683. 10.1002/MDS.27383 [DOI] [PubMed] [Google Scholar]
- 289. Wong Y, Luk K, Purtell K, et al. Neuronal vulnerability in Parkinson disease: Should the focus be on axons and synaptic terminals? Mov Disord. 2019;34(10):1406–1422. 10.1002/MDS.27823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Kurowska Z, Kordower JH, Stoessl AJ, et al. Is axonal degeneration a key early event in Parkinson’s disease? J Parkinsons Dis. 2016;6(4):703–707. 10.3233/JPD-160881 [DOI] [PubMed] [Google Scholar]
- 291. Stoyka L, Arrant A, Thrasher D, et al. Behavioral defects associated with amygdala and cortical dysfunction in mice with seeded α-synuclein inclusions. Neurobiol Dis. 2020;134. 10.1016/J.NBD.2019.104708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Volpicelli-Daley LA, Gamble KL, Schultheiss CE, Riddle DM, West AB, Lee VM-Y. Formation of α-synuclein Lewy neurite-like aggregates in axons impedes the transport of distinct endosomes. Mol Biol Cell. 2014;25(25):4010–4023. 10.1091/mbc.e14-02-0741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Cooper AA, Gitler AD, Cashikar A, et al. α-Synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson’s models. Science. 2006;313(5785):324–328. 10.1126/science.1129462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Fricker M, Tolkovsky AM, Borutaite V, Coleman M, Brown GC. Neuronal cell death. Physiol Rev. 2018;98:813–880. 10.1152/physrev.00011.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Brassai A, Suvanjeiev R-G, Bán E-G, Lakatos M. Role of synaptic and nonsynaptic glutamate receptors in ischaemia induced neurotoxicity. Brain Res Bull. 2015;112:1–6. 10.1016/j.brainresbull.2014.12.007 [DOI] [PubMed] [Google Scholar]
- 296. Hüls S, Högen T, Vassallo N, et al. AMPA-receptor-mediated excitatory synaptic transmission is enhanced by iron-induced α-synuclein oligomers. J Neurochem. 2011;117(5):868–878. 10.1111/j.1471-4159.2011.07254.x [DOI] [PubMed] [Google Scholar]
- 297. Ludtmann MHR, Angelova PR, Horrocks MH, et al. α-Synuclein oligomers interact with ATP synthase and open the permeability transition pore in Parkinson’s disease. Nat Commun. 2018;9(1):2293. 10.1038/s41467-017-02088-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Wang X, Becker K, Levine N, et al. Pathogenic alpha-synuclein aggregates preferentially bind to mitochondria and affect cellular respiration. Acta Neuropathol Commun. 2019;7(1):1–14. 10.1186/s40478-019-0696-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Deas E, Cremades N, Angelova PR, et al. Alpha-synuclein oligomers interact with metal ions to induce oxidative stress and neuronal death in Parkinson’s disease. Antioxid Redox Signal. 2016;24(7):376–391. 10.1089/ars.2015.6343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Büttner S, Faes L, Reichelt WN, et al. The Ca2+/Mn2+ ion-pump PMR1 links elevation of cytosolic Ca2+ levels to -synuclein toxicity in Parkinson’s disease models. Cell Death Differ. 2013;20(3):465–477. 10.1038/cdd.2012.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Braak H, Tredici K, Rüb U, de Vos RAI, Jansen Steur ENH, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24(2):197–211. 10.1016/S0197-4580(02)00065-9 [DOI] [PubMed] [Google Scholar]
- 302. Loria F, Vargas JY, Bousset L, et al. α-Synuclein transfer between neurons and astrocytes indicates that astrocytes play a role in degradation rather than in spreading. Acta Neuropathol. 2017;134(5):789–808. 10.1007/S00401-017-1746-2 [DOI] [PubMed] [Google Scholar]
- 303. Venezia S, Refolo V, Polissidis A, Stefanis L, Wenning G, Stefanova N. Toll-like receptor 4 stimulation with monophosphoryl lipid A ameliorates motor deficits and nigral neurodegeneration triggered by extraneuronal α-synucleinopathy. Mol Neurodegener. 2017;12(1):52. 10.1186/S13024-017-0195-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Uemura N, Uemura MT, Lo A, et al. Slow progressive accumulation of oligodendroglial alpha-synuclein (α-syn) pathology in synthetic α-syn fibril-induced mouse models of synucleinopathy. J Neuropathol Exp Neurol. 2019;22:877–890. 10.1093/jnen/nlz070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Guo M, Wang J, Zhao Y, et al. Microglial exosomes facilitate α-synuclein transmission in Parkinson’s disease. Brain. 2020;143(5):1476–1497. 10.1093/BRAIN/AWAA090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Scheiblich H, Dansokho C, Mercan D, et al. Microglia jointly degrade fibrillar alpha-synuclein cargo by distribution through tunneling nanotubes. Cell. 2021;184(20):5089–5106.e21. 10.1016/j.cell.2021.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Yun SP, Kam TI, Panicker N, et al. Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson’s disease. Nat Med. 2018;24(7):931–938. 10.1038/s41591-018-0051-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Szalay G, Martinecz B, Lénárt N, et al. Microglia protect against brain injury and their selective elimination dysregulates neuronal network activity after stroke. Nat Commun. 2016;7(1):1–13. 10.1038/ncomms11499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Blaylock RL. Parkinson’s disease: Microglial/macrophage-induced immunoexcitotoxicity as a central mechanism of neurodegeneration. Surg Neurol Int. 2017;8:65. 10.4103/sni.sni_441_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Mitchell T, Lehéricy S, Chiu SY, Strafella AP, Stoessl AJ, Vaillancourt DE. Emerging neuroimaging biomarkers across disease stage in Parkinson disease: A review. JAMA Neurol. 2021;78:1262–1272. 10.1001/JAMANEUROL.2021.1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Dauer W, Przedborski S. Parkinson’s disease: Mechanisms and models. Neuron. 2003;39(6):889–909. 10.1016/S0896-6273(03)00568-3 [DOI] [PubMed] [Google Scholar]
- 312. Steiner JA, Quansah E, Brundin P. The concept of alpha-synuclein as a prion-like protein: Ten years after. Cell Tissue Res. 2018;373(1):161–173. 10.1007/s00441-018-2814-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Blesa J, Trigo-Damas I, Quiroga-Varela A, Jackson-Lewis VR. Oxidative stress and Parkinson’s disease. Front Neuroanat. 2015;9:91. 10.3389/fnana.2015.00091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Wong YC, Krainc D. α-Synuclein toxicity in neurodegeneration: Mechanism and therapeutic strategies. Nat Med. 2017;23(2):1–13. 10.1038/nm.4269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Surmeier DJ, Obeso JA, Halliday GM. Parkinson’s disease is not simply a prion disorder. J Neurosci. 2017;37:9799–9807. 10.1523/JNEUROSCI.1787-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316. Mezias C, Rey N, Brundin P, Raj A. Neural connectivity predicts spreading of alpha-synuclein pathology in fibril-injected mouse models: Involvement of retrograde and anterograde axonal propagation. Neurobiol Dis. 2020;134:104623. 10.1016/j.nbd.2019.104623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Fortin DL, Nemani VM, Voglmaier SM, Anthony MD, Ryan TA, Edwards RH. Neural activity controls the synaptic accumulation of α-synuclein. J Neurosci. 2005;25(47):10913–10921. 10.1523/JNEUROSCI.2922-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. Yamada K, Iwatsubo T. Extracellular α-synuclein levels are regulated by neuronal activity. Mol Neurodegener. 2018;13(1):9. 10.1186/s13024-018-0241-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319. McIntyre CC, Hahn PJ. Network perspectives on the mechanisms of deep brain stimulation. Neurobiol Dis. 2010;38(3):329–337. 10.1016/j.nbd.2009.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320. Schweder PM, Joint C, Hansen PC, Green AL, Quaghebeur G, Aziz TZ. Chronic pedunculopontine nucleus stimulation restores functional connectivity. Neuroreport. 2010;21(17):1065–1068. 10.1097/WNR.0b013e32833ce607 [DOI] [PubMed] [Google Scholar]
- 321. Ross CA, Poirier MA. Protein aggregation and neurodegenerative disease. Nat Med. 2004;10(7):S10–S17. 10.1038/nm1066 [DOI] [PubMed] [Google Scholar]
- 322. Palop JJ, Mucke L. Amyloid-[beta]-induced neuronal dysfunction in Alzheimer’s disease: From synapses toward neural networks. Nat Neurosci. 2010;13(7):812–818. 10.1038/nn.2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. Wesson DW, Nixon RA, Levy E, Wilson DA. Mechanisms of neural and behavioral dysfunction in Alzheimer’s disease. Mol Neurobiol. 2011;43(3):163–179. 10.1007/s12035-011-8177-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Polydoro M, Acker CM, Duff K, Castillo PE, Davies P. Age-dependent impairment of cognitive and synaptic function in the htau mouse model of tau pathology. J Neurosci. 2009;29(34):10741–10749. 10.1523/JNEUROSCI.1065-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325. Small DH, Mok SS, Bornstein JC. Alzheimer’s disease and abeta toxicity: From top to bottom. Nat Rev Neurosci. 2001; 2(8):595–598. 10.1038/35086072 [DOI] [PubMed] [Google Scholar]
- 326. Palop J, Chin J, Mucke L. A network dysfunction perspective on neurodegenerative diseases. Nature. 2006;443(7113):768–773. 10.1038/nature05289 [DOI] [PubMed] [Google Scholar]
- 327. Terry RD. Cell death or synaptic loss in Alzheimer disease. J Neuropathol Exp Neurol. 2000;59(12):1118–1119. 10.1093/jnen/59.12.1118 [DOI] [PubMed] [Google Scholar]
- 328. Selkoe DJ. Early network dysfunction in Alzheimer’s disease. Science. 2019;365:540–541. [DOI] [PubMed] [Google Scholar]
- 329. Palop J, Chin J, Roberson E, et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron. 2007;55(5):697–711. 10.1016/j.neuron.2007.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330. Minkeviciene R, Rheims S, Dobszay M, et al. Amyloid beta-induced neuronal hyperexcitability triggers progressive epilepsy. J Neurosci. 2009;29(11):3453–3462. 10.1523/JNEUROSCI.5215-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331. Roberson ED, Halabisky B, Yoo JW, et al. Amyloid-β/Fyn–induced synaptic, network, and cognitive impairments depend on tau levels in multiple mouse models of Alzheimer’s disease. J Neurosci. 2011;31(2):700–711. 10.1523/JNEUROSCI.4152-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332. Sanchez-Mejia RO, Newman JW, Toh S, et al. Phospholipase A2 reduction ameliorates cognitive deficits in a mouse model of Alzheimer’s disease. Nat Neurosci 2008;11(11):1311–1318. 10.1038/nn.2213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333. Supnet C, Bezprozvanny I. The dysregulation of intracellular calcium in Alzheimer disease. Cell Calcium. 2010;47(2):183–189. 10.1016/j.ceca.2009.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334. Wesson DW, Borkowski AH, Landreth GE, Nixon RA, Levy E, Wilson DA. Sensory network dysfunction, behavioral impairments, and their reversibility in an Alzheimer’s beta-amyloidosis mouse model. J Neurosci. 2011;31(44):15962–15971. 10.1523/jneurosci.2085-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335. Busche MA, Eichhoff G, Adelsberger H, et al. Clusters of hyperactive neurons near amyloid plaques in a mouse model of Alzheimer’s disease. Science. 2008;321(5896):1686–1689. 10.1126/science.1162844 [DOI] [PubMed] [Google Scholar]
- 336. Small DH. Network dysfunction in Alzheimer’s disease: Does synaptic scaling drive disease progression? Trends Mol Med. 2008;14(3):103–108. 10.1016/j.molmed.2007.12.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article since no new data were generated nor were any data analyzed in this study.