Abstract
Introduction
Vaginal progesterone supplementation is frequently given to patients receiving frozen embryo transfer (FET) in the natural cycle aiming to increase the chance of pregnancy and live birth. To date, only a few studies have investigated if progesterone supplementation is beneficial in these cycles and the level of evidence for progesterone supplementation is very low.
Methods and analysis
The ProFET trial is a multicentre, open-label, randomised controlled trial powered for this investigation, including 1800 women with regular menstrual cycles (24–35 days), aged 18–43 years planned for natural cycle-FET receiving a single blastocyst for transfer. Participants are randomised (1:1:1) to either luteal phase progesterone for 3 weeks, luteal phase progesterone for 7 weeks or no luteal phase progesterone. The participating study centres consist of 12 in vitro fertilisation-clinics in Sweden and 1 in Iceland. The primary outcome is to investigate if luteal phase support (LPS) by vaginal progesterone increases the chance of a live birth per randomised patient in a natural FET cycle compared with no LPS.
Ethics and dissemination
The trial was approved by the Swedish Ethical Review Authority (ID 2020-06774, 2021-02822 and 2022-01502-02) and the Swedish Medical Products Agency (ID nr 5.1-2020-102613). All participants are required to provide written informed consent. The outcome of this study will be disseminated to the public through broadcasts, newspapers and presentations at scientific congresses as well as publications in international scientific journals.
Trial registration number
Keywords: Reproductive medicine, GYNAECOLOGY, Subfertility
Strengths and limitations of this study.
The trial has a randomised design, powered to evaluate if luteal support with vaginal progesterone will improve live birth rate (LBR) in natural cycle frozen embryo transfers (NC-FETs) when a single blastocyst is transferred.
The trial is conducted in women planning FET in NCs without exogenous ovulation trigger.
If overall superiority of progesterone is demonstrated, the sample size will allow evaluation of whether treatment duration of 7 weeks is superior to 3 weeks.
The broad inclusion criteria of women with regular menstrual cycles will ensure high generalisability of the results.
The study is open-label, blinded neither to participants nor to treating physicians, which is a limitation; however, this limitation is countered by the use of a robust primary outcome (live birth).
Introduction
In recent years, there has been a dramatic increase in the use of frozen embryo transfers (FET) cycles in in vitro fertilisation (IVF) all over the world. The FET rate in the USA has doubled since 2015, accounting for 78.8% of all embryo transfers using non-donor assisted reproductive technology (ART) in 2019.1 Similar changes are taking place in Europe2 and in Sweden, where the FET rate now accounts for 48% of all IVF-cycles.3 The main reason for this increase is the improved embryo survival and high pregnancy/LBR after transfer of vitrified/thawed blastocysts compared with the previously used technique with transfer of thawed slow-frozen cleavage stage embryos.4 5 Furthermore, high embryo survival rate facilitates the practice of single embryo transfer,6 reducing multiple pregnancy rate and thereby decreasing the risk of adverse perinatal outcomes.
Recently, the freeze-all concept has gained high popularity all over the world. Several large trials, comparing freeze-all vs fresh transfer, have shown similar live birth rates in ovulatory patients7–10 while freeze-all has been shown to be beneficial in anovulatory patients.11 The freeze-all concept is also widely used when pending risk of ovarian hyperstimulation syndrome (OHSS), and has almost eliminated the risk of OHSS, a potentially life-threatening condition.12–14
The most efficient protocol for FET is still not known. A Cochrane review, including 18 randomised controlled trial (RCTs), comparing different cycle regimens for FET, comprising a total of 3815 women did not support one treatment modality over another when investigating LBR, however, with low certainty of evidence.15
Safety aspects in ART are of great importance in treatment decision. Recently, interest has risen concerning the role of the corpus luteum (CL) in frozen cycles and studies evaluating the risks of altered vascular adaptation associated with pregnancies following FET according to the presence or absence of CL have been published.16 The CL, developing after ovulation, is known to produce oestrogen and progesterone, but also relaxin, a hormone that regulates the maternal cardiovascular and renal systems and hence mediates the haemodynamic changes occurring during pregnancy. In a prospective cohort study including almost 700 women, programmed cycles (artificial cycles using oestrogen and progesterone for endometrial preparation) in FET with no CL present were associated with an almost threefold increased risk of preeclampsia compared with modified natural cycles (natural cycles triggered by human chorionic gonadotrophin (hCG), for ovulation) with one CL present.16 Furthermore, in a recent Swedish large registry study, including almost 10 000 pregnancies/deliveries after FET, doubled rates of both hypertensive disorders of pregnancy and postpartum haemorrhage were found in programmed cycles compared with natural cycles.17 These studies thus support FET in natural cycles.
Luteal phase support (LPS) in fresh IVF cycles has been proven mandatory.18 Less is known regarding the role of LPS with progesterone in natural FET cycles. A natural ovulatory cycle would suggest that no supplementation needs to be given. However, the luteinising hormone peak—used as a urine sample to detect ovulation—does not guarantee a subsequent ovulation. Furthermore, several studies have shown that CL deficiency with midluteal serum progesterone levels <10 ng/mL could be a reason to support implantation and early pregnancy with LPS, even in a cycle where ovulation has occurred.19 20 A study from 201821 showed that low but also high levels of progesterone were associated with a reduction in clinical pregnancy rate and LBR compared with normal levels. This has also been confirmed in a more recent study.22 Not only the doses, but also the duration of LPS is widely discussed and differ between studies.
A systematic review and meta-analysis from 2020, including one RCT and three retrospective studies, found no evidence of an improved clinical pregnancy rate after progesterone support in natural cycle-FET (NC-FET).23 A more recent systematic and meta-analysis showed a benefit of progesterone as LPS in NC-FET for LBR.24 However, the two meta-analyses included a mix of RCTs and observational studies and had a wide heterogeneity regarding progesterone treatment regimens. The authors concluded that further large, randomised studies are needed to improve the certainty of evidence.
In view of the limited knowledge concerning a possible advantage of progesterone as LPS in NC-FET, the aim of this large RCT is to investigate if progesterone as LPS increases LBR compared with no progesterone. In addition, assessment of perinatal and obstetric outcomes will be performed. Furthermore, the trial will investigate if the duration of progesterone support matters and assess the association between serum progesterone levels in early luteal phase and IVF outcome.20 25 26
Objectives
Primary objective
To investigate if LPS by vaginal progesterone increases the chance of a live birth after FET in a natural cycle compared with no LPS. If progesterone support is superior to no treatment, we will further investigate if 7 weeks of treatment is more effective than 3 weeks.
Secondary objectives
To compare study groups regarding secondary outcomes including biochemical, clinical and ongoing pregnancy, as well as miscarriage.
To compare perinatal and obstetrical outcomes.
To compare self-reported side effects in women receiving and not receiving LPS with vaginal progesterone.
Investigate the association between serum progesterone levels before FET and LBR .
Methods and analysis
Study design
This multicentre, open-label, randomised, controlled phase IV trial includes the participation of twelve fertility clinics in Sweden and one in Iceland. All clinics perform standardised treatment according to the public healthcare system guidelines in Sweden and Iceland. Patient enrollment began in May 2021 and is planned to continue until June 2024.
A total of 1800 women undergoing NC-FET after conventional IVF or intracytoplasmic sperm injection treatment at one of the nine participating clinics will be recruited. As a clinical routine, patients scheduled for NC-FET contact their fertility clinic on the first day of the menstrual bleeding to schedule the treatment. Subsequently, a study nurse or doctor will identify and contact patients who fulfil the inclusion criteria to ask for interest in participating. Study information is sent to the patient by regular mail or through a secured website. Signed written or digitally informed consent is returned to the clinic either by regular mail or by contact through the website (online supplemental file 1).
bmjopen-2022-062400supp001.pdf (73.8KB, pdf)
Eligibility criteria
Inclusion and exclusion criteria are specified in table 1.
Table 1.
ProFET trial inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
| Natural cycle FET with blastocyst | Oocyte donor cycles |
| Regular menstrual cycle (24–35 days) | Uterine malformations: cervical anomalies, submucosal uterine fibroid or endometrial polyps requiring surgery. |
| Age 18–43 years | Hypersensitivity to vaginal progesterone |
| BMI 18.5–35 (kg/m2) | Medical contraindication to progesterone treatment |
| Understand written and spoken Swedish, English or Arabic and have signed a written informed consent. | Serious concomitant disease contraindicating ART and pregnancy |
| Preimplantation genetic testing | |
| Previously included in the ProFET study | |
| Participation in another study with an investigational product within the last 30 days |
ART, assisted reproductive technology; BMI, Body mass index; FET, Frozen embryo transfer.
Treatment and intervention
At the endogenous surge of LH (luteinising hormone ; a hormone that naturally rises to trigger ovulation), study participants are randomised 1:1:1 to one of three groups:
No vaginal progesterone.
Vaginal progesterone for 3 weeks.
Vaginal progesterone for 7 weeks.
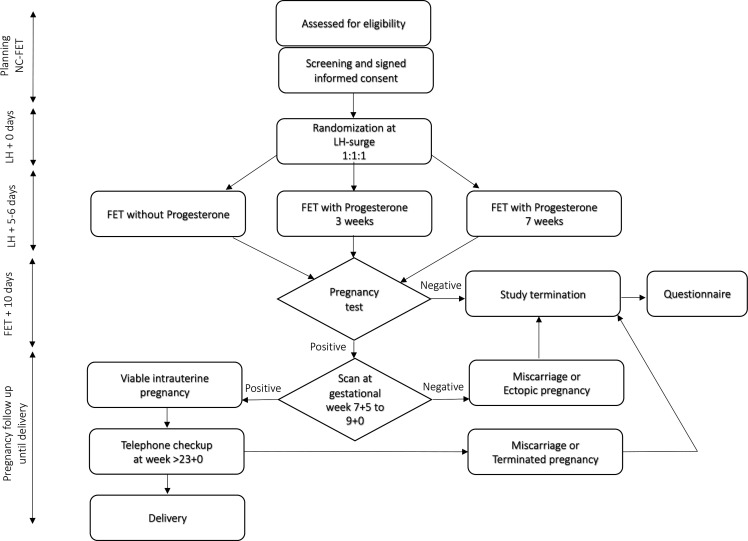
Patients randomised to luteal phase progesterone are instructed to administrate 100 mg vaginal progesterone (Lutinus; Ferring Pharmaceuticals, Saint-Prex, Switzerland) three times daily starting 3 days after the LH surge. Participants are asked to leave a blood sample for analysis of serum progesterone regardless of group allocation. A blood sample will be drawn in the morning 3 days after the LH surge, before any start of progesterone. The result will not be available to the patient, neither to the treating clinician until the end of the study. On day 5–6 after LH-surge, a blastocyst is transferred according to standard embryo transfer procedure. Patients randomised to vaginal progesterone will continue administration of progesterone until a pregnancy test. In the case of a positive pregnancy test, patients will continue with vaginal progesterone for a total of 3 or 7 weeks, respectively. In the case of a negative pregnancy test or miscarriage later on the patient will stop progesterone treatment (see figure 1).
Figure 1.
ProFET trial flow chart. FET, Frozen embryo transfer; LH, luteinising hormone; NC-FET, natural cycle frozen embryo transfer.
Randomisation
Study data are recorded in an electronic case report file (e-CRF) designed by Medicase (Sahlgrenska Science Park, Gothenburg, Sweden) which also includes a randomisation programme. Randomisation is stratified for:
Previous ET not resulting in positive pregnancy test, number (0–2, ≥3).
Parity 0/≥1.
Age (<35/≥35 years).
Treatment site.
Blinding procedure
The trial is not blinded, neither to patients nor to treating physicians. Analyses are done by a statistician, blinded to group allocation.
Data collection
Patient-related data are collected and variables are registered in the e-CRF programme at the following time points:
Screening before LH surge.
Randomisation at LH surge.
FET at LH +5 or 6 days.
Result of pregnancy test (urine sample).
Early pregnancy scan (7 weeks+5 days to 9 weeks+0 days) in case of positive pregnancy test.
Through a follow-up (by telephone) after gestational week 23+0 days.
From the patient’s and the newborn’s medical records after delivery.
Sample collection
Blood samples will be collected at LH +3 days, whenever possible. A blood sample of 5 mL is analysed for serum progesterone level. The blood samples will be sent to and analysed at the Swedish certified laboratory Unilabs and are then discarded.
Transvaginal ultrasound scans
If the patient conceives, an early transvaginal pregnancy scan will be made at gestational age 7 weeks+5 days to 9 weeks+0 days, for estimation of number of gestational sacs, number of fetuses, crown-rump length and viability.
Questionnaires
The participants will be asked to fill out a questionnaire regarding registration of possible study medication side effects. The form is filled out regardless of group allocation and submitted in connection with vaginal ultrasound at gestational age 7 weeks+5 days to 9 weeks+0 days—or earlier in the case of a negative pregnancy test or miscarriage. Specified reported symptoms will be recorded as adverse events (AEs) in the e-CRF. Serious AEs (SAEs) will be followed until 2 weeks after delivery.
Data management
Data are transferred to an online e-CRF; Medicase. The Medicase database is based on coded subject ID numbers used in the trial. Data are stored on a server located at Sahlgrenska University Hospital, Gothenburg, Sweden, with a daily backup. Only research staff at the Sahlgrenska University Hospital will have access to the final dataset. Ownership of data is determined by cooperation agreements as well as data processing agreements between Sahlgrenska University Hospital and the participating clinics.
Statistics
Outcome measurements
Primary outcome is live birth. Secondary outcomes include biochemical and clinical pregnancy rates, miscarriage rates and obstetric and neonatal outcomes in the study groups. For a complete list of secondary outcomes, see online supplemental file 2. Self-reported side effects will be reported as mild, moderate or severe. Progesterone levels 3 days after LH surge will be measured in units of nmol/L.
bmjopen-2022-062400supp002.pdf (33.3KB, pdf)
Sample size calculation
In order to find an effect size of a 7% increase in LBR per randomised patient, measured as a difference in proportions between no progesterone (0.33) and any progesterone group (0.40), 1800 subjects are needed if allocated 1:2. In order to find a difference between no progesterone (0.33) and progesterone for 3 weeks (0.41) and 7 weeks (0.41), respectively, 1200 subjects are needed if allocated 1:1. Also, for the comparison between the progesterone groups, 1200 subjects are needed if allocated 1:1, to detect a difference of 8%, (0.38 for 3 weeks of progesterone vs 0.46 for 7 weeks). For all comparisons above, except for the primary analysis, a difference between groups of 8% is used. If 1800 women are allocated 1:1:1, 600 to no progesterone, 600 to progesterone 3 weeks and 600 to progesterone 7 weeks, all four sample size calculations are fulfilled under the condition of a power of 0.80, a significance level 0.05 and a two-sided Fisher’s exact test.
We thus have two primary superiority analyses in this study. The first is the comparison of LBR between no progesterone and the combined group of any progesterone with Fisher’s exact test on significance level 0.05. If this test is significant the probability mass of 5% will be transferred to the second comparison of live birth between progesterone for 3 weeks compared with progesterone for 7 weeks. If the first test is significant, we have been able to show that any progesterone gives significantly higher LBR than in women without progesterone. If in the second comparison 7 weeks shows significantly higher LBR than 3 weeks, we have also confirmed superiority regarding 7 weeks over 3 weeks. If the first analysis is non-significant, we have not been able to show any confirmative results in this study. The comparisons between no progesterone and 3 weeks progesterone and between no progesterone and 7 weeks progesterone is performed to calculate mean difference with 95% CI between these groups.
Statistical analyses
The main analyses will be on the full analysis set (FAS) without imputation. Complementary analyses will be performed on the per-protocol population. The primary efficacy analysis regarding live birth will be conducted with multivariable logistic regression adjusting for all stratification variables on the FAS population. The first sensitivity primary analysis will be the same analysis also adjusted for the following other predefined important predictors:
Body mass index.
Smoking status.
Duration of subfertility.
Previous miscarriage (yes/no).
Blastocyst (day 5 /day 6 at cryopreservation).
Number of embryos transferred.
The second sensitivity primary analysis will be the same analysis as the primary efficacy analysis but performed on the intention-to-treat population with multiple imputation based on 100 datasets. Both primary outcome and stratified variables will be imputed. For adjusted analyses between two groups, multivariable logistic regression will be used for dichotomous variables. If model assumption is fulfilled the corresponding model with link=log will be given to present adjusted relative risk (RR) with 95% CI. For continuous variables analysis of covariance will be used for adjusted analysis between two groups.
Explorative unadjusted mean difference between the two groups with 95% CI will be given for dichotomous variables and continuous variables together with effect sizes. For continuous variables these 95% CI will be based on t-test or Fisher’s non-parametric permutation test. For dichotomous variables, RR and OR will be given with 95% CI. Proportions will be given with exact 95% CI.
For comparison between two groups Mantel-Haenszel χ2 test will be used for ordered categorical variables and Fisher’s exact test for dichotomous variables. The distribution of continuous variables will be given as mean, SD, median, first and third quartiles (Q1, Q3), minimum and maximum. All significance tests will be two sided and conducted at the 5% significance level.
Monitoring
All study participants are monitored to meet the inclusion criteria and a check is made that voluntarily informed consent for each study participants is obtained and documented. For all study participants, the main parameters in the study are monitored (live birth, clinical pregnancy, miscarriage and ectopic pregnancy). The first two study participants at each centre will be monitored with a complete source data verification. Thereafter, a complete source data verification will be performed on every fifth randomly selected study participant.
Patient and public involvement
Development of this study protocol was done without patient or public involvement. The final study results will be disseminated to participants on request.
Ethics and dissemination
The study was approved by the Swedish Ethical Review Authority (ID 2020-06774, 2021-02822 and 2022-01502-02) and the Swedish Medical Products Agency (ID nr 5.1-2020-102613). All participants are required to sign a written informed consent form before study entry (online supplemental file 1). The safety of participants in this study is high. As the medication/treatment with vaginal progesterone is well known, SAEs or suspected unexpected serious adverse reactions (SUSARs) are unlikely. If, however, a participant should experience an SAE or a SUSAR the local investigator will contact the principal investigator with no delay and the individual treatment will be stopped immediately.
The results of this trial will be presented at national as well as international scientific congresses and published in international scientific journals. The results of the research will also be disseminated to public through broadcasts, popular science articles and newspapers.
Discussion
The rapidly increasing use of FET worldwide and the limited evidence concerning cycle regiments for FET demands further well designed large randomised trials. Progesterone supplementation in NC-FET is widely used despite scarce evidence. Two RCTs with LBR as main outcome have been published.27 28 In a Swedish study where mainly cleavage stage embryos and single—as well as double embryo transfer were used, a significantly higher LBR was found.27 Further, a small study from Israel, including only 59 patients, using a modified NC-FET protocol, also found a significantly higher LBR after LPS compared with no progesterone.28 The study included a mix of cleavage stage embryos and blastocysts and up to three embryos were transferred.
Available retrospective studies on LPS reveal the use of different embryo stages at embryo transfer; two-nucleus stage29 cleavage stage embryos30 and both cleavage embryo and blastocyst transfers.31 All these studies used hCG as ovulation trigger and administration of progesterone supplementation was started at different time points after LH-surge and was administrated either as intramuscular injections or as vaginal suppositories with different doses and duration of treatment.
This presented ongoing large open-label multicentre randomised clinical trial aims to investigate if vaginal LPS in NC-FET is superior to no LPS. In this set up, not only the differences in LBR and clinical pregnancy rates will be investigated, but also, the obstetrical and perinatal outcomes. This study will contribute to recommendations regarding LPS in NC-FET in the future.
The strength of this trial is the multicentre, randomised design and a large sample size of 1800 women. Broad inclusion criteria representing the patient cohort in everyday practice will ensure a high generalisability. The IVF protocols consist only of natural cycles with no ovulation trigger. The study is not blinded to participants or investigators, which is a limitation, however, the use of a robust primary outcome (live birth) makes this less likely to introduce bias.
If progesterone supplementation in natural FET cycles should be shown to significantly increase the chance of live birth, the benefit for the patients, as well as for the society, would mean (1) a shorter time to pregnancy, (2) fewer IVF cycles needed per patient, (3) reduced costs for patients and society, (4) less environmental burden due to less cycles to achieve live birth and thus less use of hormonal IVF treatment. On the other hand, if no beneficial effect of this treatment can be shown, it should be abandoned and thereby implicate less financial burden for patients as well as for society, less treatment burden for the patient and less environmental impact associated with the use of LPS.
Supplementary Material
Footnotes
Contributors: CB and ÅM were the primary initiators of the study, who designed and wrote the first study protocol. AK, AS and CS contributed to the revision and editing of the study protocol. AK, AS, CB, CS, EL, GWe, GWi, KHO, KAR-W, MB, MK, SL and ÅM will all be involved in the recruitment of patients and data collection. All authors approved the final version of the study protocol. AS and CS applied to the Swedish Ethical Review Authority. CB and ÅM applied to the Swedish Medical Products Agency. AK and CS wrote the first draft of this manuscript which was revised by AS, CB and ÅM. Finally all committed authors approved this protocol.
Funding: The project will be funded by grants from the Swedish state under the agreement between the Swedish government and the county councils, the ALF-agreement (ALFGBG-965526 and ALFGBG-720291), by an unrestricted grant from Ferring Pharmaceuticals (SU 2020-05958), by Sahlgrenska University Hospital’s Research Foundation, by Gothenburg Medical Society and by the Hjalmar Svensson foundation. We will also apply for further funding.
Disclaimer: Ferring Pharmaceuticals has not been involved in the design of the study protocol, nor will they be involved in the conduct of the study or any analysis or reporting of the results.
Competing interests: CB and ÅM declare support from Ferring Pharmaceuticals, Merck Sereno and Gedeon Richter. MB has 4 % stocks in EUGIN Sweden. None of the other authors hade conflicts of interest.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s)
References
- 1.U.S. Department of Health and Human Services, Centers for Disease Control and Prevention . Assisted reproductive Technolology, fertility clinic and national summary report 2019. Available: https://www.cdc.gov/art/reports/2019/pdf/2019-Report-ART-Fertility-Clinic-National-Summary-h.pdf [Accessed 16 Feb 2022].
- 2.European IVF-Monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE), Wyns C, De Geyter C, et al. Art in Europe, 2017: results generated from European registries by ESHRE. Hum Reprod Open 2021;2021:hoab026. 10.1093/hropen/hoab026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nationellt kvalitetsregister för assisterad befruktning . Fertility treatments in Sweden national report 2021. Available: https://www.medscinet.com/qivf/uploads/hemsida/Årsrapport%202021%20-%20ENGELSKA%20final.pdf [Accessed 16 Feb 2022].
- 4.Balaban B, Urman B, Ata B, et al. A randomized controlled study of human day 3 embryo cryopreservation by slow freezing or vitrification: vitrification is associated with higher survival, metabolism and blastocyst formation. Human Reproduction 2008;23:1976–82. 10.1093/humrep/den222 [DOI] [PubMed] [Google Scholar]
- 5.Rienzi L, Gracia C, Maggiulli R, et al. Oocyte, embryo and blastocyst cryopreservation in art: systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum Reprod Update 2017;23:139–55. 10.1093/humupd/dmw038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thurin A, Hausken J, Hillensjö T, et al. Elective single-embryo transfer versus double-embryo transfer in in vitro fertilization. N Engl J Med 2004;351:2392–402. 10.1056/NEJMoa041032 [DOI] [PubMed] [Google Scholar]
- 7.Shi Y, Sun Y, Hao C, et al. Transfer of fresh versus frozen embryos in ovulatory women. N Engl J Med 2018;378:126–36. 10.1056/NEJMoa1705334 [DOI] [PubMed] [Google Scholar]
- 8.Stormlund S, Sopa N, Zedeler A, et al. Freeze-all versus fresh blastocyst transfer strategy during in vitro fertilisation in women with regular menstrual cycles: multicentre randomised controlled trial. BMJ 2020;370:m2519. 10.1136/bmj.m2519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vuong LN, Dang VQ, Ho TM, et al. IVF transfer of fresh or frozen embryos in women without polycystic ovaries. N Engl J Med 2018;378:137–47. 10.1056/NEJMoa1703768 [DOI] [PubMed] [Google Scholar]
- 10.Maheshwari A, Bell JL, Bhide P, et al. Elective freezing of embryos versus fresh embryo transfer in IVF: a multicentre randomized controlled trial in the UK (E-Freeze). Hum Reprod 2022;37:476–87. 10.1093/humrep/deab279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Z-J, Shi Y, Sun Y, et al. Fresh versus frozen embryos for infertility in the polycystic ovary syndrome. N Engl J Med 2016;375:523–33. 10.1056/NEJMoa1513873 [DOI] [PubMed] [Google Scholar]
- 12.Zaat T, Zagers M, Mol F, et al. Fresh versus frozen embryo transfers in assisted reproduction. Cochrane Database Syst Rev 2021;2:CD011184. 10.1002/14651858.CD011184.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roque M, Haahr T, Geber S, et al. Fresh versus elective frozen embryo transfer in IVF/ICSI cycles: a systematic review and meta-analysis of reproductive outcomes. Hum Reprod Update 2019;25:2–14. 10.1093/humupd/dmy033 [DOI] [PubMed] [Google Scholar]
- 14.De Boer EJ, Van Leeuwen FE, Den Tonkelaar I, et al. [Methods and results of in-vitro fertilisation in the Netherlands in the years 1983-1994]. Ned Tijdschr Geneeskd 2004;148:1448–55. [PubMed] [Google Scholar]
- 15.Ghobara T, Gelbaya TA, Ayeleke RO. Cycle regimens for frozen-thawed embryo transfer. Cochrane Database Syst Rev 2017;7:CD003414. 10.1002/14651858.CD003414.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Versen-Höynck F, Schaub AM, Chi Y-Y, et al. Increased preeclampsia risk and reduced aortic compliance with in vitro fertilization cycles in the absence of a corpus luteum. Hypertension 2019;73:640–9. 10.1161/HYPERTENSIONAHA.118.12043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ginström Ernstad E, Wennerholm U-B, Khatibi A, et al. Neonatal and maternal outcome after frozen embryo transfer: increased risks in programmed cycles. Am J Obstet Gynecol 2019;221:126.e1–126.e18. 10.1016/j.ajog.2019.03.010 [DOI] [PubMed] [Google Scholar]
- 18.van der Linden M, Buckingham K, Farquhar C, et al. Luteal phase support for assisted reproduction cycles. Cochrane Database Syst Rev 2015;7:CD009154. 10.1002/14651858.CD009154.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hull MGR, Savage PE, Bromham DR, et al. The value of a single serum progesterone measurement in the midluteal phase as a criterion of a potentially fertile cycle (“ovulation”) derived from treated and untreated conception cycles. Fertil Steril 1982;37:355–60. 10.1016/S0015-0282(16)46095-4 [DOI] [PubMed] [Google Scholar]
- 20.Gaggiotti-Marre S, Álvarez M, González-Foruria I, et al. Low progesterone levels on the day before natural cycle frozen embryo transfer are negatively associated with live birth rates. Hum Reprod 2020;35:1623–9. 10.1093/humrep/deaa092 [DOI] [PubMed] [Google Scholar]
- 21.Thomsen LH, Kesmodel US, Erb K, et al. The impact of luteal serum progesterone levels on live birth rates—a prospective study of 602 IVF/ICSI cycles. Hum Reprod 2018;33:1506–16. 10.1093/humrep/dey226 [DOI] [PubMed] [Google Scholar]
- 22.Alsbjerg B, Thomsen L, Elbaek HO, et al. Can combining vaginal and rectal progesterone achieve the optimum progesterone range required for implantation in the HRT-FET model? Reprod Biomed Online 2020;40:805–11. 10.1016/j.rbmo.2020.02.007 [DOI] [PubMed] [Google Scholar]
- 23.Seol A, Shim YJ, Kim SW, et al. Effect of luteal phase support with vaginal progesterone on pregnancy outcomes in natural frozen embryo transfer cycles: a meta-analysis. Clin Exp Reprod Med 2020;47:147–52. 10.5653/cerm.2019.03132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mizrachi Y, Horowitz E, Ganer Herman H, et al. Should women receive luteal support following natural cycle frozen embryo transfer? a systematic review and meta-analysis. Hum Reprod Update 2021;27:643–50. 10.1093/humupd/dmab011 [DOI] [PubMed] [Google Scholar]
- 25.Filicori M, Butler JP, Crowley WF. Neuroendocrine regulation of the corpus luteum in the human. evidence for pulsatile progesterone secretion. J Clin Invest 1984;73:1638–47. 10.1172/JCI111370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jordan J, Craig K, Clifton DK, et al. Luteal phase defect: the sensitivity and specificity of diagnostic methods in common clinical use. Fertil Steril 1994;62:54–62. 10.1016/s0015-0282(16)56815-0 [DOI] [PubMed] [Google Scholar]
- 27.Bjuresten K, Landgren B-M, Hovatta O, et al. Luteal phase progesterone increases live birth rate after frozen embryo transfer. Fertil Steril 2011;95:534–7. 10.1016/j.fertnstert.2010.05.019 [DOI] [PubMed] [Google Scholar]
- 28.Horowitz E, Mizrachi Y, Finkelstein M, et al. A randomized controlled trial of vaginal progesterone for luteal phase support in modified natural cycle - frozen embryo transfer. Gynecol Endocrinol 2021;37:792–7. 10.1080/09513590.2020.1854717 [DOI] [PubMed] [Google Scholar]
- 29.Kim C-H, Lee Y-J, Lee K-H, et al. The effect of luteal phase progesterone supplementation on natural frozen-thawed embryo transfer cycles. Obstet Gynecol Sci 2014;57:291–6. 10.5468/ogs.2014.57.4.291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kyrou D, Fatemi HM, Tournaye H, et al. Luteal phase support in normo-ovulatory women stimulated with clomiphene citrate for intrauterine insemination: need or habit? Hum Reprod 2010;25:2501–6. 10.1093/humrep/deq223 [DOI] [PubMed] [Google Scholar]
- 31.Schwartz E, Bernard L, Ohl J, et al. Luteal phase progesterone supplementation following induced natural cycle frozen embryo transfer: a retrospective cohort study. J Gynecol Obstet Hum Reprod 2019;48:95–8. 10.1016/j.jogoh.2018.10.011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-062400supp001.pdf (73.8KB, pdf)
bmjopen-2022-062400supp002.pdf (33.3KB, pdf)