Abstract
Background & Aims:
The incidence and prevalence of eosinophilic esophagitis (EoE) and inflammatory bowel disease (IBD) are rising with similar patterns. Co-occurrence of both diseases in the same patient has been increasingly reported. We sought to examine the pediatric population with both EoE and IBD to better understand the epidemiology and clinical implications of this overlap.
Methods:
We conducted a retrospective case-control study at two tertiary care children’s hospitals. Subjects with both EoE and IBD were identified and compared to randomly-selected controls with EoE and IBD alone in terms of: demographics, atopic conditions, IBD classification, location and phenotype of Crohn’s disease (CD), IBD medications, endoscopic findings, and histopathology. Descriptive statistics summarized the data.
Results:
Sixty-seven subjects with dual-diagnosis were identified across both institutions. The prevalence of IBD in the EoE population was 2.2% and EoE in IBD was 1.5%. Subjects with both diseases were more likely to have IgE-mediated food allergy compared to IBD alone (36% vs. 7%, p <0.001). Subjects with CD-EoE were less likely to have perianal disease than CD alone (2% vs 20%, p=0.004). There was no difference in fibrostenotic EoE between the dual-diagnosis group and EoE alone. Treatment with a TNF-alpha inhibitor (anti-TNF) for management of preexisting IBD was protective against development of EoE with a relative risk of 0.314 (95% CI 0.159–0.619).
Conclusions:
This is a unique population in whom the underlying pathway leading to dual-diagnosis is unclear. Concomitant atopic conditions, especially IgE-mediated food allergy, and medication exposures, particularly anti-TNFs, may help predict likelihood of developing dual-diagnosis.
Keywords: Crohn’s disease, ulcerative colitis, allergy, anti-TNF
Introduction:
Eosinophilic esophagitis (EoE) and inflammatory bowel disease (IBD) are immune-mediated diseases of the gastrointestinal tract associated with high morbidity. The incidence and prevalence of both diseases have been increasing in past decades, with a more rapid increase in industrialized countries. 1,2
EoE is characterized by eosinophilic infiltrate of the esophageal mucosa, leading to epithelial barrier defects and esophageal dysfunction.3 IBD, including Crohn’s disease (CD) and ulcerative colitis (UC), is a chronic inflammatory condition of the bowel thought to be due to dysregulation of the gut immune system and microbiota.4 Both diseases have complex pathogenesis involving helper T cell and cytokine inflammatory mechanisms, as well as abnormalities in epithelial barrier function.3,5 Given these shared pathologic mechanisms, co-occurrence of both diseases in the same patient is not uncommon.6
Recent evidence identifies a more rapid increase in the incidence of EoE and IBD in the pediatric population than in the adult population.7 Studies have begun examining the overlap of these diagnoses in the general population,6–8 however few studies have investigated this co-occurrence in children specifically.9 The aim of this study was to examine the population of patients with both EoE and IBD at two pediatric referral centers, and to compare this cohort to populations with EoE alone and IBD alone, in order to better understand the epidemiology and clinical implications of this disease overlap in pediatrics.
Methods:
We conducted a retrospective case-control study at the Children’s Hospital of Philadelphia (CHOP) and at Prisma Health Children’s Hospital in Greenville, South Carolina from January 2008 to December 2018. This time frame was selected based on availability of the electronic medical record (EMR). 2008 also represents the year the EoE diagnosis code was first introduced in the United States. Using ICD-9 and ICD-10 codes, we identified subjects with diagnoses of both EoE (530.13, K20.0) and IBD (555.x, 556.x, K50.x, K51.x). For comparison, 150 controls with EoE alone, and 150 controls with IBD alone, were identified. These controls were randomly selected via a computer program from up-to-date databases of all IBD and EoE patients at our institution.
To confirm diagnoses, EMR was reviewed in detail. EoE diagnosis had been made by the treating physician based on suggestive clinical and endoscopic signs and symptoms. All had ≥15 eosinophils per high-powered field on histology. This definition of EoE was consistent with 2018 AGREE guidelines.10 Subjects with co-existing eosinophilic gastrointestinal disorders were excluded. Subjects with esophageal granulomas or neutrophil-predominant esophagitis were classified as esophageal Crohn’s, and not as EoE. IBD diagnosis had been made by the treating physician based on clinical, laboratory, endoscopic, and histologic findings.
Data was collected using the standardized electronic form, REDCap. We compared subjects with diagnoses of both EoE and IBD with controls in terms of: demographic information; concomitant history of atopy; age at time of each diagnosis; classification of IBD (CD, UC, or indeterminate IBD); location and phenotype of CD; IBD therapies used; endoscopic findings; and histology. This study was approved by the Institutional Review Boards at the Children’s Hospital of Philadelphia (19–016090) and the Prisma Health Children’s Hospital (Pro00018945).
Statistics
Subjects with both EoE and IBD were compared to subjects with EoE alone and IBD alone using Fisher’s Exact Test for dichotomous variables including gender, race, ethnicity and atopy. Continuous variables were compared by student’s t-test. Dual-diagnosis subjects were compared to IBD alone regarding IBD classification by Fisher’s Exact. Subjects with CD-EoE were compared to CD alone for CD location and phenotype by Fisher’s Exact. Subjects with EoE alone were compared to IBD-EoE subjects, and CD and UC specifically, for fibrostenotic EoE characteristics by Fisher’s Exact. Chi-Square test of homogeneity was performed between CD and UC among subjects with EoE first, IBD first, or both together. The odds ratio with confidence interval was determined for atopic conditions (asthma, eczema, IgE-mediated food allergy, and seasonal allergies) with presence of EoE among subjects with IBD. The relative risk of developing EoE in the IBD patients was assessed based on specific treatments. Binary logistic regression models were performed among IBD patients to determine the predictors of EoE diagnosis. Similar models were performed among EoE patients to determine predictors of IBD diagnosis. The atopic conditions were included in the models as factors: asthma, eczema, allergic rhinitis, IgE-mediated food allergy. Gender was included in each model along with age at IBD in the model to predict EoE, and age at EoE in the model to predict IBD.
Results:
Dual-Diagnosis Prevalence
Fifty-four subjects with diagnoses of both EoE and IBD were identified from CHOP and 13 were identified from Greenville, for a total of 67 (Table 1). During the time period of interest, 3,053 patients carried a diagnosis of EoE across both institutions, and 4,515 carried a diagnosis of IBD. The prevalence of IBD in the EoE population was 2.2% (67/3,053) and of EoE in the IBD population was 1.5% (67/4,515). Of those diagnosed with IBD first, 11/32 (34%) were in remission at time of EoE diagnosis. Of those diagnosed with EoE first, 10/15 (67%) were in remission at time of IBD diagnosis.
Table 1.
Clinical characteristics of dual-diagnosis subjects compared to IBD alone and to EoE alone:
| EoE and IBD (n = 67) | IBD alone (n = 150) | P | EoE alone (n = 150) | P | |
|---|---|---|---|---|---|
| Age in years at diagnosis, n (%) | |||||
| IBD | 11.3 ± 4.3 | 11.6 ± 4.0 | 0.50 | ||
| EoE | 12.2 ± 4.7 | 7.3 ± 4.9 | <0.001 | ||
|
| |||||
| Male, n (%) | 56 (84) | 83 (55) | <0.001 | 123 (82) | 0.85 |
|
| |||||
| Race/Ethnicity, n (%) | |||||
| White | 54 (81) | 107 (71) | 0.18 | 114 (76) | 0.49 |
| Black | 5 (8) | 17 (11) | 0.47 | 21 (14) | 0.26 |
| Asian | 0 (0) | 3 (2) | 0.55 | 1 (1) | 1.00 |
| Latino | 2 (3) | 1 (1) | 0.23 | 5 (3) | 1.00 |
| Indian | 1 (2) | 6 (4) | 0.68 | 0 (0) | 0.31 |
|
| |||||
| Atopy, n (%) | |||||
| Asthma | 18 (27) | 27 (18) | 0.15 | 96 (64) | <0.001 |
| Eczema | 6 (9) | 26 (17) | 0.15 | 57 (38) | <0.001 |
| Allergic rhinitis | 24 (36) | 51 (34) | 0.88 | 129 (86) | <0.001 |
| IgE-mediated food allergy | 24 (36) | 10 (7) | <0.001 | 91 (61) | <0.001 |
|
| |||||
| IBD classification, n (%) | |||||
| Crohn’s disease | 45 (67) | 93 (62) | 0.54 | ||
| Ulcerative colitis | 17 (25) | 34 (22) | 0.73 | ||
| Indeterminate IBD | 5 (6) | 23 (15) | 0.13 | ||
| VEO-IBD | 13 (19) | 20 (13) | 0.31 | ||
IBD = inflammatory bowel disease; EoE = eosinophilic esophagitis; VEO-IBD = very early onset inflammatory bowel disease
EoE-IBD Compared to EoE Alone
Subjects with EoE alone were diagnosed with EoE at a younger age than those with EoE-IBD (7.3 ± 4.9 vs. 12.2 ± 4.7, p <0.001) (Table 1). There was no significant difference in terms of gender or race. Subjects with EoE alone were significantly more likely to have concomitant atopic conditions than those with EoE-IBD (Table 1). Binary logistic regression assessed risk factors for developing IBD among EoE patients. After controlling for gender, age at EoE diagnosis, and concomitant atopic conditions, seasonal allergies and asthma were associated with reduced risk of developing IBD (OR 0.140 [0.064–0.306] and OR 0.425 [0.195–0.927]). Older age at EoE diagnosis was predictive of developing IBD (OR 2.266 [1.080–1.258]) (Table, Supplemental Digital Content 1).
EoE-IBD Compared to IBD Alone
There was no significant difference in terms of race or age at diagnosis of IBD between the IBD alone group and those with EoE-IBD (Table 1). There were significantly more males in the EoE-IBD group than IBD alone (84% vs. 55%, p <0.001). There was no difference between groups in terms of IBD classification (CD, UC, or indeterminate) or prevalence of very early onset IBD, a unique subset of IBD patients defined as those diagnosed at age 6 years or younger (19% vs. 13%, p=0.31).
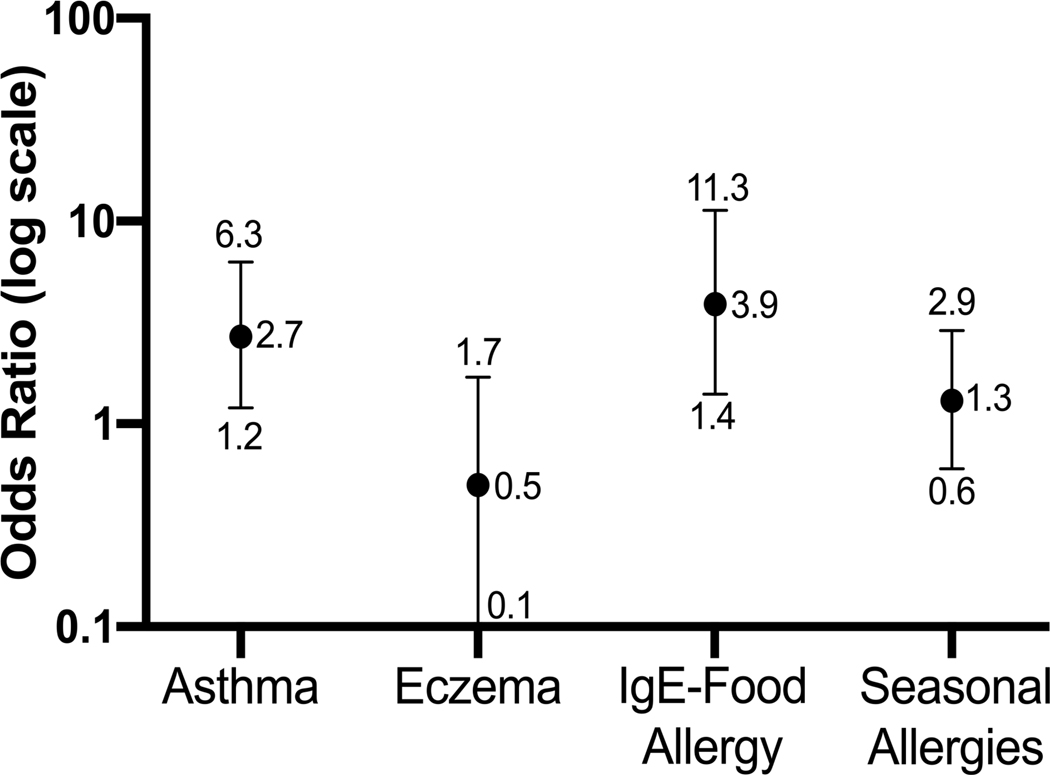
Subjects with EoE-IBD were more likely to have IgE-mediated food allergy than those with IBD alone (36% vs. 7%, p <0.001) (Table 1). For a patient with IBD, having IgE-mediated food allergy was associated with 3.9 times the odds of having EoE as well (95% CI 1.4–11.3), and having asthma was associated with 2.7 times the odds of having EoE as well (95% CI 1.2–6.3) (Figure 1). Binary logistic regression models assessed risk factors for EoE development among IBD patients. After controlling for gender, age at IBD diagnosis, and concomitant atopic conditions, IgE-mediated food allergy was a strong predictor of developing EoE (9.166 [4.051–20.740]), as was male gender (3.645 [1.780–7.463]) (Table, Supplemental Digital Content 2).
Figure 1.
Association between atopic conditions and the development of eosinophilic esophagitis (EoE) in the inflammatory bowel disease population. IgE-mediated food allergy and asthma were associated with increased risk of developing EoE.
In terms of CD phenotype, subjects with CD-EoE were significantly less likely to have perianal disease compared to CD alone (2% vs 20%, p=0.004) (Table 2). There was no significant difference in terms of location of disease in subjects with CD-EoE compared to those with CD alone.
Table 2.
Crohn’s disease location and phenotype
| EoE and Crohn’s disease (n = 45) |
Crohn’s disease alone (n = 93) | P | |
|---|---|---|---|
| Crohn’s disease location, n (%) | |||
| Esophagus | 3 (7) | 12 (13) | 0.39 |
| Stomach | 10 (22) | 19 (20) | 0.84 |
| Duodenum | 9 (20) | 16 (17) | 0.81 |
| Jejunum | 5 (11) | 9 (10) | 0.77 |
| Ileum | 33 (73) | 78 (84) | 0.17 |
| Colon | 37 (82) | 83 (89) | 0.29 |
|
| |||
| Crohn’s disease phenotype, n (%) | |||
| Inflammatory only | 36 (80) | 61 (66) | 0.11 |
| Penetrating | 3 (7) | 5 (5) | 0.72 |
| Stricturing | 6 (13) | 14 (15) | 1.00 |
| Perianal | 1 (2) | 19 (20) | 0.004 |
Crohn’s disease location and disease phenotype in dual-diagnosis group and Crohn’s alone group. EoE = eosinophilic esophagitis.
IBD Medications
Twelve (38%) of the 32 dual-diagnosis subjects diagnosed with IBD first were on anti-TNF therapy prior to developing EoE (range 3 to 93.9 months prior to EoE diagnosis). Of these, 10 were on a single agent prior to developing EoE (nine on infliximab, one on adalimumab). One subject had been on two anti-TNFs (infliximab, adalimumab) and one had been on three (infliximab, adalimumab, golimumab) prior to developing EoE. This was compared to 101/150 (67%) subjects with IBD alone who were on anti-TNF therapy and did not go on to develop EoE. Being on an anti-TNF agent was protective against developing EoE, with a weighted relative risk of 0.314 (0.159–0.619) (Table 3). Other IBD therapies were examined in the IBD first dual-diagnosis population including oral 5-ASA, methotrexate, thiopurines, vedolizumab, ustekinumab, and tacrolimus. None of theses showed a statistically significant relative risk in terms of development of EoE, either protective or in terms of increased risk (Table 3).
Table 3.
Inflammatory bowel disease medications used in dual-diagnosis subjects who were diagnosed with inflammatory bowel disease first and in subjects with inflammatory bowel disease alone
| Medication | Dual IBD first n=32 [n(%)] |
IBD n=150 [n(%)] |
RR | 95% CI | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| 5-ASA | 23 (72) | 117 (78) | 0.746 | 0.361 | 1.542 |
| Methotrexate | 7 (22) | 50 (33) | 0.589 | 0.263 | 1.318 |
| Thiopurine | 6 (19) | 24 (16) | 1.188 | 0.514 | 2.748 |
| Anti-TNF | 12 (38) | 101 (67) | 0.314 | 0.159 | 0.619 |
| Vedolizumab | 1 (3) | 11 (7) | 0.433 | 0.062 | 3.033 |
| Ustekinumab | 1 (3) | 4 (3) | 1.158 | 0.177 | 7.571 |
| Tacrolimus | 1 (3) | 1 (0.01) | 3.538 | 0.690 | 18.146 |
IBD medications and weighted relative risk of developing eosinophilic esophagitis. 5-ASA = 5-aminosalicylate, CI = confidence interval, IBD = inflammatory bowel disease, RR = relative risk; TNF = tumor necrosis factor.
Fibrostenotic Disease
Fibrostenotic EoE was defined as having endoscopic or histologic suggestion of esophageal fibrosis, as evidenced by rings or trachealization on endoscopy, lamina propria fibrosis on biopsy, or history of stricture or food impaction. There was no significant difference in the rate of fibrostenotic EoE in the dual-diagnosis group overall compared to EoE alone (44/150 (19%) vs. 13/67 (29%), p = 0.14) (Table, Supplemental Digital Content 3). When the dual-diagnosis group was broken down based on IBD classification, there remained no difference (10/45 (22%) vs. 13/67 (29%), p = 0.45).
Order of Diagnoses
Of the subjects with dual-diagnosis, 32 (48%) were diagnosed with IBD first, 15 (22%) with EoE first, and 20 (30%) at the same time. The mean time between diagnoses was 28.3 months. Of those diagnosed with IBD first, a significant majority had CD compared to UC (90% vs. 10%, p <0.001) (Table, Supplemental Digital Content 4).
Discussion:
EoE and IBD are both chronic, progressive, immune-mediated diseases of the gastrointestinal tract that may overlap in clinical symptomatology. In this study, we present the largest published cohort of pediatric patients with comorbid diagnosis of both EoE and IBD. We compared the dual-diagnosis population with patients with EoE and IBD alone and noted a number of findings that allow for better understanding of the overlap population and how it differs from the single diagnosis population.
It has been reported that patients with EoE are at risk of concurrent autoimmunity. One hypothesis for this suggests possible genetic associations between EoE and genes associated with autoimmunity. Peterson et. al, in their recent population-based study looking at associations of specific autoimmune diseases in probands diagnosed with EoE and their family members, reported that multiple sclerosis, UC, and systemic sclerosis may have shared genetic etiology with EoE.6 Other recent studies have postulated an association of EoE with celiac disease.11 Capucilli et. al. reported more than a five-fold increased prevalence of pediatric celiac disease in the EoE population when compared to celiac in the general population.9 A recent case report even described co-occurrence of IBD, EoE, and celiac disease in a single patient, raising the possibility of shared underlying genetic associations.12 Larger, well powered studies evaluating genetic underpinnings of the dual-diagnosis population will be needed in the future to more precisely understand these observations.
Both EoE and IBD are increasing in incidence and prevalence in the general population. It is clear that the annual incidence of EoE in patients with IBD, and of IBD in patients with EoE, is increasing more rapidly than single disease alone. Thus, the prevalence of dual-diagnosis is rising more rapidly than single diagnosis.7 Consistent with recently published studies in the adult population, we found that the prevalence of EoE among IBD patients, and of IBD among EoE patients, was significantly higher than EoE and IBD alone in the general population. Specifically, EoE prevalence in our IBD population was 1.5%, compared to an EoE prevalence of 0.05–0.1% in the general population.1 Similarly, IBD prevalence in the EoE population was 2.2%, compared to an IBD prevalence of about 0.4% in the general population.13 In our examined population, EoE prevalence among IBD patients was actually about 2.5-fold higher than in the recently-published report by Limketkai et. al., which examined a predominantly adult population, while IBD prevalence among EoE patients was consistent with their results.7,14 One hypothesis for this finding is that in pediatrics, esophagogastroduodenoscopy (EGD) is routinely performed as part of initial IBD workup, whereas in adults this is not standard practice. Thus, EoE may be identified earlier in children than in adults diagnosed with IBD, even in the absence of symptoms.
A number of findings in our study were notable. One interesting finding was that there was minimal difference phenotypically between subjects with dual-diagnosis and those with single disease. CD location did not differ between subjects with CD-EoE and those with CD alone. Subjects with CD-EoE, even stricturing CD, were no more likely to have fibrostenotic EoE than those with EoE alone. This finding is consistent with a recent report by Fan et. al. where they noted no significant difference in fibrostenotic phenotype between subjects with EoE alone and those with esophageal eosinophilia and IBD.12 This suggests perhaps there are unrelated underlying mechanisms and modifying factors leading to fibrosis in each of these conditions. The one difference phenotypically between these populations was that subjects with CD-EoE were significantly less likely to have perianal disease than CD alone, suggesting perhaps a protective effect exists in those with dual-diagnosis.
A significant difference between subjects with EoE-IBD and those with IBD alone was the increased prevalence of atopic conditions in those with dual-diagnosis. Specifically, subjects with EoE-IBD were more likely to have IgE-mediated food allergy than those with IBD alone. This becomes clinically relevant when stratifying IBD patients with regards to risk of developing EoE. In patients with IBD who have IgE-mediated food allergy, the risk of developing EoE may be significantly higher than in IBD patients without food allergy. Thus, if an IBD patient with food allergy develops symptoms of dysphagia, nausea, or vomiting, the index of suspicion should be higher for EoE. Evaluation with an EGD should be considered in this patient, perhaps even prior to a proton pump inhibitor (PPI) trial.10
Also notable was the order of diagnoses in the dual-diagnosis population. Nearly half of dual-diagnosis subjects were diagnosed with IBD first. This finding was consistent with the recently published paper by Mintz et. al. examining an adult population with EoE-IBD in which more than two-thirds of their dual-diagnosis subjects were diagnosed with IBD first.15 In our study, in subjects diagnosed with IBD first, there was no evidence suggesting the presence of EoE at the time of diagnosis of IBD. Rather, EoE clearly developed later in the disease course. It is notable that 90 percent of subjects diagnosed with IBD first had CD. A common clinical question in patients with IBD and esophageal inflammation is whether the esophagitis is a manifestation of CD or whether it is suggestive of EoE as a separate diagnosis. In this cohort of subjects in whom IBD was diagnosed prior to EoE, the complete absence of esophageal eosinophilia at the time of IBD diagnosis, even in a Crohn’s-predominant population, suggests that EoE is likely its own entity. Though two-thirds of IBD-first subjects were not in IBD remission at the time of EoE diagnosis, the fact that the esophageal inflammation was eosinophilic, and not neutrophil-predominant, suggests that esophagitis was due to EoE, and was not a manifestation of uncontrolled IBD. However, it is impossible to conclude this with certainty, and this is a limitation of this retrospective study.
This raises the question of whether IBD therapy increases the risk of subsequently developing EoE. Multiple medications such as gold, calcineurin inhibitors, and antibiotics have been implicated in the development of eosinophilia. TNF-alpha inhibitors have been associated with development of eosinophilic cellulitis and peripheral eosinophilia.16,17 We previously reported a case of severe eosinophilic gastroenteritis and peripheral eosinophilia, though not esophageal eosinophilia, in a patient with CD that was temporally correlated with the use of both infliximab and adalimumab, and resolution of eosinophilia when off anti-TNF therapy.18 On the contrary, anti-TNF agents can be useful in the treatment of eosinophilic colitis, a distinct entity.19,20 Our findings in this study suggest that anti-TNF agents may be protective against future development of EoE in IBD patients. There were no other significant associations with other medications typically used to treat IBD and subsequent development of EoE. It should be noted that there is the possibility of individuals in our IBD control population going on to develop EoE in the future, and this is a limitation of this study design that must be considered.
Another limitation to this study is that our patient population is comprised of subjects from large tertiary care health systems that often receive referrals for medically complex cases. Thus, there may be some degree of bias, which should be considered when determining the generalizability of results. However, inclusion of the population in South Carolina may allow for this data to be considered more generalizable.
The retrospective nature of the study presented a number of challenges. First, it is known that EoE and gastroesophageal reflux disease (GERD) may coexist in a single patient,10 and this retrospective analysis made it difficult to account for this disease overlap. While some subjects may have had diagnoses of both EoE and GERD, it was impossible to account for this. Those subjects with GERD alone in the absence of EoE were excluded.
Additionally, because some subjects were diagnosed with EoE prior to the consistent use of EMR, diagnostic endoscopy reports were not always available for review. Thus, we relied on the clinical judgment of the treating physician when determining the clinical and endoscopic likelihood of EoE diagnosis. Pathology reports were available for all subjects, so it is certain that all subjects had ≥15 eosinophils per high-powered field on diagnostic endoscopy.
Lastly, there were challenges related to examining certain medications, specifically systemic corticosteroids and PPIs, and their impact on development of secondary diagnosis. Systemic corticosteroids are not used as long-term primary therapy for IBD or EoE at our institutions. Some IBD patients receive corticosteroids transiently as pre-medications for biologic infusions and not as IBD therapy. With PPIs, it was impossible to gauge dosing and adherence to therapy given the retrospective nature of the study. Thus, it was difficult to draw conclusions regarding these therapies and their impact on development of dual-diagnosis. Since we did not account for these medications, it is possible that corticosteroid and/or PPI use at the time of endoscopy may have masked some cases of EoE, and that corticosteroids may have masked some cases of IBD. Thus, the number of cases of dual-diagnosis may have actually been higher than observed.
In summary, we identified a large cohort of pediatric patients with co-diagnosis of EoE and IBD. This is a unique population in whom the underlying pathway leading to dual-diagnosis is not yet clear, though shared genetic and mechanistic factors likely exist. In the presence of suggestive signs or symptoms, clinical suspicion for EoE in the IBD population, and for IBD in the EoE population, should be high.
Supplementary Material
Table, Supplemental Digital Content 1. Variables predicting EoE in a patient with IBD
Table, Supplemental Digital Content 2. Variables predicting IBD in a patient with EoE
Table, Supplemental Digital Content 3. Comparison of fibrostenotic disease in the EoE alone group to fibrostenotic disease in the EoE-IBD population.
Table, Supplemental Digital Content 4. Comparison of Crohn’s disease to ulcerative colitis with respect to the order of disease diagnosis: EoE first, IBD first, or EoE and IBD diagnoses made at the same time.
What is Known/What is New.
What is known:
Co-occurrence of eosinophilic esophagitis (EoE) and inflammatory bowel disease (IBD) in the same patient is not uncommon.
Little is known about the overlap of EoE and IBD in pediatrics.
What is new:
The prevalence of IBD among pediatric EoE patients was similar to adults, but prevalence of EoE among IBD patients was 2.5-fold higher.
Children with EoE and IBD had more IgE-mediated food allergy than those with IBD alone, but there was no difference when comparing those with dual-diagnosis to those with EoE alone.
Perianal disease was less common in children with Crohn’s disease and EoE than Crohn’s alone.
Anti-TNF therapy for preexisting IBD was protective against developing EoE.
Grant Support
This study was supported by the following NIH Grants: K08DK106444 (ABM), R03DK118310–01A1 (ABM), K08DK097721 (JW)
ABM and JW are funded in part by the Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR). CEGIR (U54 AI117804) is part of the Rare Diseases Clinical Research Network (RDCRN), an initiative of the Office of Rare Diseases Research (ORDR), NCATS, and is funded through collaboration between NIAID, NIDDK, and NCATS. CEGIR is also supported by patient advocacy groups including APFED, CURED, and EFC.
Footnotes
Disclosures:
The authors have no conflicts of interest to disclose.
References:
- 1.Dellon ES, Hirano I. Epidemiology and Natural History of Eosinophilic Esophagitis. Gastroenterology 2018;154:319–332.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012;142:46–54.e42– quiz e30. [DOI] [PubMed] [Google Scholar]
- 3.Rothenberg ME. Molecular, genetic, and cellular bases for treating eosinophilic esophagitis. Gastroenterology 2015;148:1143–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sheehan D, Shanahan F. The Gut Microbiota in Inflammatory Bowel Disease. Gastroenterol. Clin. North Am 2017;46:143–154. [DOI] [PubMed] [Google Scholar]
- 5.Strober W, Fuss IJ. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology 2011;140:1756–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peterson K, Firszt R, Fang J, et al. Risk of Autoimmunity in EoE and Families: A Population-Based Cohort Study. Am. J. Gastroenterol 2016;111:926–932. [DOI] [PubMed] [Google Scholar]
- 7.Limketkai BN, Shah SC, Hirano I, et al. Epidemiology and implications of concurrent diagnosis of eosinophilic oesophagitis and IBD based on a prospective population-based analysis. Gut 2019;68:2152–2160. [DOI] [PubMed] [Google Scholar]
- 8.Molina-Infante J, Schoepfer AM, Lucendo AJ, et al. Eosinophilic esophagitis: What can we learn from Crohn’s disease? United European Gastroenterol J 2017;5:762–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Capucilli P, Cianferoni A, Grundmeier RW, et al. Comparison of comorbid diagnoses in children with and without eosinophilic esophagitis in a large population. Annals of Allergy, Asthma & Immunology 2018;121:711–716. Available at: https://linkinghub.elsevier.com/retrieve/pii/S1081120618307440. [DOI] [PubMed] [Google Scholar]
- 10.Dellon ES, Liacouras CA, Molina-Infante J, et al. Updated International Consensus Diagnostic Criteria for Eosinophilic Esophagitis: Proceedings of the AGREE Conference. Gastroenterology 2018;155:1022–1033.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hommeida S, Alsawas M, Murad MH, et al. The Association Between Celiac Disease and Eosinophilic Esophagitis: Mayo Experience and Meta-analysis of the Literature. J. Pediatr. Gastroenterol. Nutr 2017;65:58–63. [DOI] [PubMed] [Google Scholar]
- 12.Ahlawat R, Parikh NS, Jhaveri A. Triple Diagnosis of Crohn’s Disease, Celiac Disease, and Eosinophilic Esophagitis in a Child With Siderius-Hamel Syndrome. WMJ 2019;118:140–142. [PubMed] [Google Scholar]
- 13.Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clin. Gastroenterol. Hepatol 2007;5:1424–1429. [DOI] [PubMed] [Google Scholar]
- 14.Fan YC, Steele D, Kochar B, et al. Increased Prevalence of Esophageal Eosinophilia in Patients with Inflammatory Bowel Disease. Inflamm Intest Dis 2019;3:180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mintz MJ, Ananthakrishnan AN. Phenotype and Natural History of Inflammatory Bowel Disease in Patients With Concomitant Eosinophilic Esophagitis. Inflamm. Bowel Dis 2020;68:s1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boura P, Sarantopoulos A, Lefaki I, et al. Eosinophilic cellulitis (Wells’ syndrome) as a cutaneous reaction to the administration of adalimumab. Ann. Rheum. Dis 2006;65:839–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malisiewicz B, Murer C, Pachlopnik Schmid J, et al. Eosinophilia during psoriasis treatment with TNF antagonists. Dermatology (Basel) 2011;223:311–315. [DOI] [PubMed] [Google Scholar]
- 18.Muir A, Surrey L, Kriegermeier A, et al. Severe Eosinophilic Gastroenteritis in a Crohn’s Disease Patient Treated With Infliximab and Adalimumab. Am. J. Gastroenterol 2016;111:437–438. [DOI] [PubMed] [Google Scholar]
- 19.Koutri E, Papadopoulou A. Eosinophilic Gastrointestinal Diseases in Childhood. Ann. Nutr. Metab 2018;73 Suppl 4:18–28. [DOI] [PubMed] [Google Scholar]
- 20.Turner D, Wolters VM, Russell RK, et al. Anti-TNF, infliximab, and adalimumab can be effective in eosinophilic bowel disease. J. Pediatr. Gastroenterol. Nutr 2013;56:492–497. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table, Supplemental Digital Content 1. Variables predicting EoE in a patient with IBD
Table, Supplemental Digital Content 2. Variables predicting IBD in a patient with EoE
Table, Supplemental Digital Content 3. Comparison of fibrostenotic disease in the EoE alone group to fibrostenotic disease in the EoE-IBD population.
Table, Supplemental Digital Content 4. Comparison of Crohn’s disease to ulcerative colitis with respect to the order of disease diagnosis: EoE first, IBD first, or EoE and IBD diagnoses made at the same time.