Abstract
Background. Iron deficiency anemia is a common health problem that affects children under the age of five. Children’s cognitive performance is impaired by iron deficiency, which impacts their psychomotor development. Therefore, the aim of this study was to determine the global prevalence and associated factors of iron deficiency and iron deficiency anemia among under-5 children. Methods. Relevant publications published till March 30, 2021 were identified in databases such as Medline/PubMed, Science Direct, Popline, EMBASE, African Journals Online, Scopus, and Google Scholar. The STATA version 11 software was utilized for the analysis. To determine the level of heterogeneity, I2 test statistics were used. To detect publication bias, funnel plots analysis and the Egger weighted regression test were used. Results. The global pooled prevalence of iron deficiency anemia and iron deficiency was 16.42% (95% CI: 10.82, 22.01) and 17.95% (95% CI: 13.49, 22.41), respectively. Age less than 2 years (OR = 1.26; 95% CI: 1.14, 1.38) and living in a large family size (OR = 1.38; 95% CI: 1.18, 1.58) were associated with iron deficiency anemia. Children born from anemic mother, low birth weight, and do not drink iron fortified milk (OR = 1.20; 95% CI: 1.05, 1.36), (OR = 1.15; 95% CI: 1.01, 1.36) and (OR = 1.28; 95% CI: 1.10, 1.46), respectively were associated factors of iron deficiency in under-5 children. Conclusion. The prevalence of iron deficiency anemia and iron deficiency was significant across the globe, particularly in Asia and Africa. Therefore, regular screening and treatment of iron deficiency and iron deficiency anemia are required especially in high-risk children to reduce their complication.
PROSPERO registration number: CRD42021267060
Keywords: iron deficiency anemia, iron deficiency, under-5 children, meta-analysis, systematic review
Introduction
Anemia occurs when the number of red blood cells or their capacity to carry oxygen is insufficient to meet the body’s physiological demands. Its cause might be complex and occur at the same time, but the most common cause is a quantitative and qualitative deficiency of iron in the diet. 1
Iron is an essential nutrient for human growth at all stages. It is especially important for children because it has a significant impact on their development. 2 Iron is also crucial for the growth and development of the central nervous system, especially during childhood. Iron is required for brain growth, myelination, monoamine neurotransmitter action, and neuronal and glial energy metabolism. 3
Iron deficiency (ID) is a global health problem that can be defined as plasma ferritin <12 μg/L that has not been adjusted for infection or inflammation. ID is defined as a transferrin saturation (TS) <10% or serum ferritin <15 μg/L. 4 It has an impact on children’s psychomotor development as well as their cognitive abilities. 5 It is the most common micronutrient shortage in young children around the world due to their rapid growth and high iron requirements. Globally ID affects around 2 billion people, primarily pregnant women and under-5 children residing in developing countries. 6
IDA is regarded as the proxy indicator of ID because it is believed that most of the ID is caused by poor nutritional iron intake and low iron bioavailability. 7 After the 3 successive steps of iron deficiency, iron store depletion, iron-deficient erythropoiesis, and finally end up with iron-deficiency anemia (IDA). IDA is a micronutrient deficiency that affects under-5 children in both developed and developing countries. 8 IDA is confirmed as anemia with ID. 9
When the prevalence of IDA exceeds 5% of the population, it is considered a public health problem. Anemia affects billions of people around the world, with young children and pregnant women being particularly vulnerable. IDA is responsible for half of the global prevalence of anemia. 10 IDA, the most severe type of ID, is estimated to affect 20% to 25% of pre-school children worldwide, with the highest prevalence in South Asia and Africa. 11 In Europe, the incidence of IDA in toddlers is lower than in Africa and Asia. 12
IDA in infancy has been linked to long-term poor cognitive and behavioral performance in children. 13 The brain, central nervous system, and immunological system of children under the age of 5 are all affected by IDA. 5 Low birth weight, frequent use of cow’s milk, low consumption of iron-rich supplementary foods, and low socioeconomic status are all thought to be risk factors for IDA. 14 Poor sanitation, joblessness, low wages, poor housing, low education, living in rural areas, drinking cow’s milk more frequently, and poor health conditions, are all possible causes and associated factors for anemia in people of all ages, particularly children under the age of 2. 5
Despite there are plenty of awareness about the high risk of children for ID, still they are disproportionately affected by IDA. For early detection and prevention of IDA and ID, risk factors should be recognized. Thus, the goal of this study was to determine the global prevalence of ID and IDA, as well as their associated factors, among under-5 children.
Methods
Context and Protocol Development of the Review
The Preferred Reporting Items for Systematic Review and Meta-Analysis protocol (PRISMA-P 2020 Guidelines) 15 were used to conduct this systematic review and meta-analysis (Supplemental Table S1). The protocol was registered in PROSPERO with the CRD42021267060 registration ID.
Eligibility Criteria
This systematic review and meta-analysis include studies that were published or accepted in a peer-reviewed journal; data included articles that were conducted from all ethnicities, socioeconomic and educational backgrounds. Cross-sectional, case-control, and prospective cohort studies that include the outcome of interest in their reports were included in the study. All articles published in English language before March 30, 2021, were included. We excluded single case studies, qualitative studies, and research reports that were not accessible or were not in English.
Moreover, studies that did not look into the outcomes or looked into other types of anemia that were not related to iron consumption were excluded.
Outcome of Interest
The primary outcome of interest was to estimate the global pooled prevalence of ID and IDA among under-5 children. The second outcome of the review was to identify factors linked to ID and IDA in children under the age of 5. Hemoglobin (Hgb) <11 g/dL, ferritin <12 µg/L, and/or mean cell volume (MCV) <74 fL were used to define IDA, while ID was defined as, ferritin <12 µg/L and/or MCV < 74 fL. In many previous studies, the cutoff levels for Hb (11 g/dL) and for MCV (74 fL) were used.4,16,17 To exclude out cases with a probable rise in ferritin concentration due to infection, C-reactive protein (CRP) concentration of <5 mmol/L was utilized as a criteria. A serum concentration of soluble transferrin receptor (sTfR) more than 3.3 mg/L could be utilized to detect latent ID. 18
Search Strategy
Data were collected by searching previously published literature in databases such as PubMed, Science Direct, Google Scholar, Scopus, African journals Online, EMBASE, and Popline. There was no time restriction while searching and all articles published up to March 30, 2021, were included. Using Boolean operators like “OR” and “AND,” the search terms were utilized independently and in combination. The searching terms used in PubMed ((((iron deficiency anemia [Text Word]) OR (hematological profile[Text Word])) OR (hematological parameters[Text Word]) OR (hematological abnormalities[Text Word]) OR (iron status[Text Word]) OR (iron deficiency [MeSH Terms]) OR (hematological profile[MeSH Terms]) OR (hematological parameters[MeSH Terms]) OR (hematological abnormalities[MeSH Terms]) OR (iron deficiency anemia [MeSH Terms]) AND (under-five children[Text Word]) OR (children 6-59 months old[Text Word]) OR (pre-school children[Text Word]). Additional publications were discovered through a manual search and reference lists cited in relevant articles.
Study Selection and Quality Assessment
EndNote X7 (Thomson Reuters, Universitaet- Heidelberg, Germany) was used to collect and organize the search results, as well as to remove duplicate articles. Two authors (SG* and SG) screened articles based on their titles and abstracts. Similarly, 2 authors (SG* and SG) independently assessed the methodological quality of eligible articles using Joanna Briggs Institute (JBI) critical appraisal tools. 19 Discrepancies between authors were resolved through discussion and the involvement of the third author (MM). The tool consists of different items for each study design types, to assess the internal and external validity of studies. For analytical cross-sectional studies, the tools consist of 8 different items, to include the study the percentage of the overall “Yes” response should be greater than 50%. The tool for case-control and prospective cohort studies consists of 10 and 12 different items, respectively to assess the internal and external validity of studies. To include the study the percentage of the overall “Yes” response should be greater than 50%.20,21 Any disagreement by 2 authors during quality appraisal were resolved through discussion by the involvement of the third author.
Data Extraction
Studies that matched the eligibility criteria were subjected to data extraction using a data extraction worksheet. Items extracted for analysis were: authors’ name, year of publication, sample size, total number of cases, study design, study setting, region (continent) where the study was conducted, factors associated with IDA and ID with their odds ratio, and the prevalence of ID and IDA.
Statistical Analysis
Data was extracted and entered into Microsoft Excel before being exported to STATA version 11 for analysis. The pooled effect size and confidence interval (CI) were calculated using a random-effect model meta-analysis. The degree of heterogeneity between the included studies was quantified using Higgin’s I2 statistics. 22 Low, medium, and high heterogeneity are assumed to be represented by I2 values of 25%, 50%, and 75%, respectively. To resolve the source of high heterogeneity, sub-group analysis by study setting and continent, as well as sensitivity analysis were done. To detect publication bias, funnel plots analysis and the Egger weighted regression test were used. In Egger’s test, a P-value of <.05 was deemed evidence of statistically significant publication bias. 23 Trim and fill analysis was done for ID since there was publication bias.
Results
Identified Studies
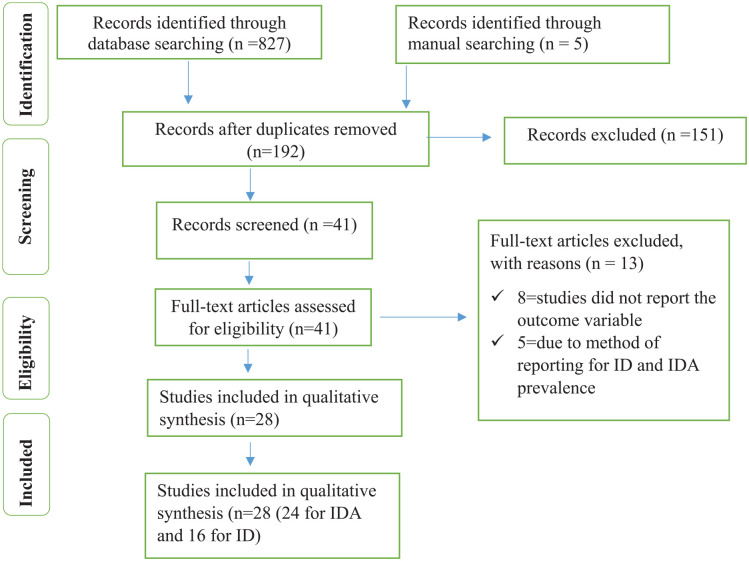
A total of 832 studies were identified through database literature searching. After removing duplicates, we got a total of 192 studies. Then 192 articles were screened. Out of which, 151 studies were excluded by reading their titles and 13 studies were excluded through reading their full-text since the outcome of interest were not reported. Finally, 28 full-text studies (24 studies report IDA, 16 studies report ID, and 16 studies report factors associated with IDA, and 10 studies report factors associated with ID) were used for the final qualitative and quantitative analysis (Figure 1).
Figure 1.
Flow chart to describe the selection of studies for the systematic review and meta-analysis on the prevalence and associated factors of IDA and ID among under-5 children.
Description of Identified Studies
In this study, 28 articles were included. Out of 28 articles, 13 were done in the Asian continent,5,24-35 7 were done in the African continent,36-42 3 in the North-America continent,43-45 1 in South-America, 46 and 4 were done in the European continent.47-50 The sample size of included study varied from 23 36 to 7138 30 under-5 children. The included studies were conducted on a total of 27,896 under-5 children, out of them 4581 had IDA. The minimum prevalence of IDA (1.1%) was reported in North America 44 and the maximum prevalence of IDA (33.2%) was reported in Pakistan, Asia. 24 Four studies5,34,35,46 only report factors that are associated with IDA and ID. The included articles’ detailed features for meta-analysis were given in detail in Table 1. The methodological quality included studies’ is provided in Supplemental Table S2.
Table 1.
Characteristics of included studies in the meta-analysis.
| First author | Study design | Continent | Study setting | Sample size | Number of cases | IDA (%) | ID (%) |
|---|---|---|---|---|---|---|---|
| Innis et al 43 | Cross-sectional | North America | Community-based | 434 | 31 | 7.1 | 24.2 |
| Kadivar et al 33 | Case-control | Asia | Hospital-based | 583 | 101 | 17.3 | 19.7 |
| Quaderi et al 32 | Cross-sectional | Asia | Hospital-based | 331 | 60 | 18.1 | — |
| Donahue Angel et al 37 | Cross-sectional | Africa | Community-based | 577 | 174 | 30.2 | 31.0 |
| Chen et al 31 | Cross-sectional | Asia | Community-based | 509 | 21 | 4.1 | 10.0 |
| Habib et al 30 | Cross-sectional | Asia | Community-based | 7138 | 2370 | 33.2 | — |
| Keikhaei et al 29 | Cross-sectional | Asia | Hospital-based | 337 | 99 | 29.4 | — |
| Kikafunda et al 36 | Cohort study | Africa | Hospital-based | 23 | 5 | 21.7 | — |
| Harvey-Leeson et al 38 | Cross-sectional | Africa | Hospital-based | 372 | 38 | 10.2 | 12.1 |
| Gupta et al 44 | Cross-sectional | North America | Community-based | 1156 | 13 | 1.1 | 7.2 |
| Wirth et al 28 | Cross-sectional | Asia | Community-based | 1455 | 95 | 6.5 | 15.1 |
| Bahizire et al 39 | Cross-sectional | Africa | Hospital-based | 377 | 62 | 16.4 | 4.4 |
| Uijterschout et al 47 | Cross-sectional | Europe | Community-based | 400 | 34 | 8.5 | 19.0 |
| Vendt et al 48 | Cross-sectional | Europe | Hospital-based | 171 | 17 | 9.9 | 14.0 |
| Weiler et al 45 | Cross-sectional | North America | Hospital-based | 430 | 13 | 3.0 | 16.5 |
| Wirth et al 40 | Cross-sectional | Africa | Community-based | 839 | 32 | 3.8 | 5.2 |
| Sirdah et al 27 | Cross-sectional | Asia | Community-based | 735 | 261 | 33.5 | — |
| Kessy et al 41 | Cross-sectional | Africa | Hospital-based | 303 | 84 | 28.1 | 40.9 |
| Siti-Noor et al 26 | Cross-sectional | Asia | Hospital-based | 490 | 155 | 31.6 | 38.9 |
| Akodu et al 42 | Cross-sectional | Africa | Hospital-based | 87 | 9 | 10.11 | — |
| Halib et al 25 | Cross-sectional | Asia | Community-based | 249 | 20 | 7.7 | 12.6 |
| Soh et al 49 | Cross-sectional | Europe | Community-based | 323 | 14 | 4.3 | 18.6 |
| Faysal et al 24 | Cross-sectional | Asia | Hospital-based | 1595 | 798 | 50 | — |
| Tympa-Psirropoulou et al 50 | Cross-sectional | Europe | Hospital-based | 938 | 75 | 7.99 | — |
| Khan et al 34 | Cross-sectional | Asia | Community-based | 2171 | — | — | — |
| Bharati et al 5 | Case-control | Asia | Community-based | 35 591 | — | — | — |
| Ayoya et al 46 | Cross-sectional | South America | Hospital-based | 557 | — | — | — |
| Joo et al 35 | Cohort | Asia | Hospital-based | 1782 | — | — | — |
Prevalence of IDA and ID Among Under-5 Children
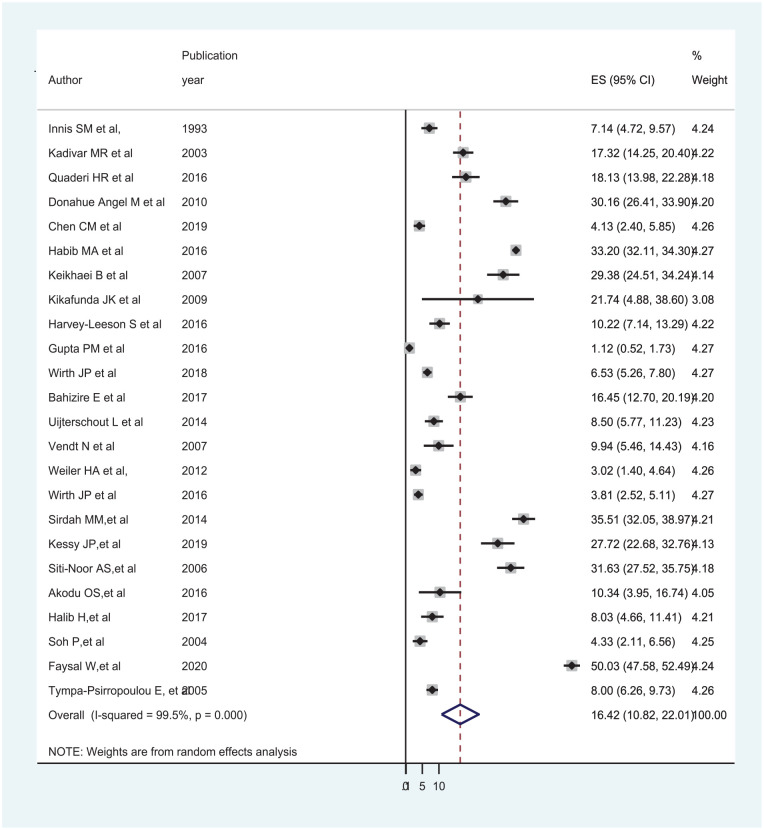
The global pooled prevalence of IDA among under-five children was 16.42% (95% CI: 10.82, 22.01) and I2 of 99.5%, P < .001 (Figure 2). Since there was significant heterogeneity, subgroup analysis was done by continent and study setting, the result showed that the pooled prevalence of IDA was 23.37% (95% CI: 12.88, 33.87) in Asia and 16.40% (95% CI: 7.07, 25.73) in Africa. Besides, the pooled prevalence of IDA was 12.91% (95% CI: 4.95, 20.85) in community-based studies and 19.49% (95% CI: 10.68, 28.30) in hospital-based studies, but still there was a significant heterogeneity (Table 2).
Figure 2.
Forest plot displaying the pooled prevalence of IDA among under-5 children.
Table 2.
Subgroup analysis on the prevalence of IDA and ID by continent and study setting among under-5 children.
| IDA sub-group analysis | |||
|---|---|---|---|
| Continent/region | Prevalence (95% CI) | I2 (%) | P-value |
| North America | 4.18 (0.99-7.38) | 90.3 | ≤.001 |
| Asia | 23.37 (12.88-33.87) | 99.3 | ≤.001 |
| Africa | 16.40 (7.07-25.73) | 98.0 | ≤.001 |
| Europe | 7.39 (5.10-9.67) | 68.4 | ≤.001 |
| Study setting | |||
| Community-based | 12.91 (4.95-20.87) | 99.7 | ≤.001 |
| Hospital-based | 19.49 (10.68-28.30) | 99.0 | ≤.001 |
| ID sub-group analysis | |||
| North America | 15.84 (5.52-26.16) | 97.3 | ≤.001 |
| Asia | 20.33 (8.77-31.90) | 97.8 | ≤.001 |
| Africa | 18.50 (7.51-29.50) | 98.7 | ≤.001 |
| Europe | 16.59 (14.18-19.00) | 46.4 | .133 |
| Study setting | |||
| Community-based | 14.44 (9.63-19.24) | 96.5 | ≤.001 |
| Hospital-based | 20.73 (12.93-28.52) | 97.8 | ≤.001 |
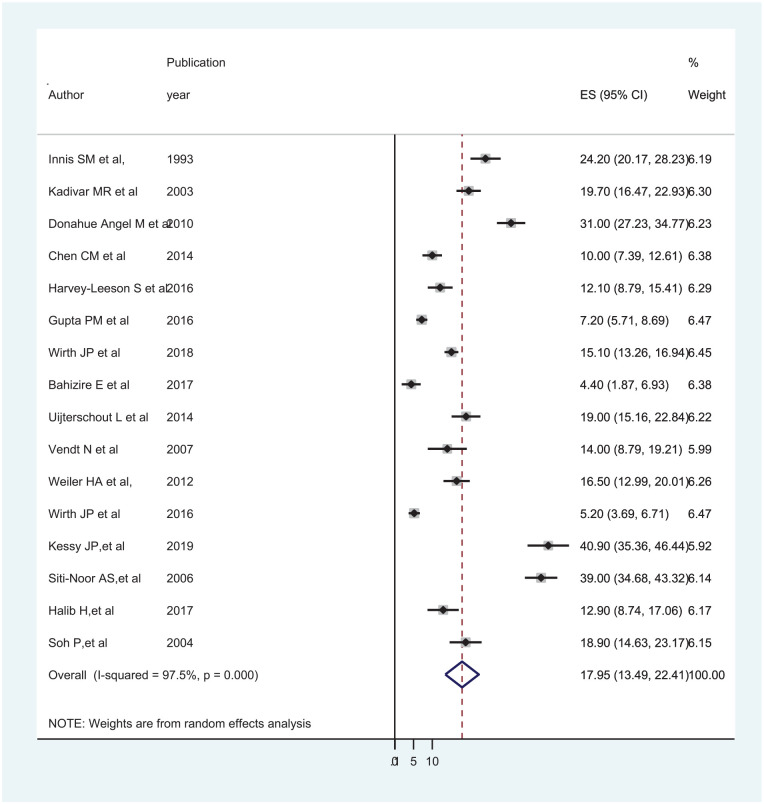
Using a random-effects model, the global pooled prevalence of ID among under-5 children was found to be 17.95% (95% CI: 13.49, 22.41) and I2 value of 97.5%, P < .001 (Figure 3). Since there was significant heterogeneity, subgroup analysis by continent showed that the pooled prevalence of ID was high in Asia (20.33% (95% CI: 8.77, 31.90)) and African continent (18.50% (95% CI: 7.51, 29.50)). In addition, the pooled prevalence of ID was 14.44% (95% CI: 9.63, 19.24) in community-based studies and 20.73% (95% CI: 12.93, 28.52) in hospital-based studies, but there was a significant heterogeneity (Table 2).
Figure 3.
Forest plot displaying prevalence of ID among under-5 children.
Publication Bias
The funnel plot and Egger’s statistics were used to visually examine the included studies for potential publication bias. As shown in Figures 4 and 5, the funnel plot is asymmetric and shows an evidence of publication bias. The Egger weighted regression statistics indicated that there was no publication bias for studies included for IDA (P = .061), but for studies included for ID indicated that an evidence of publication bias (P = .001).
Figure 4.
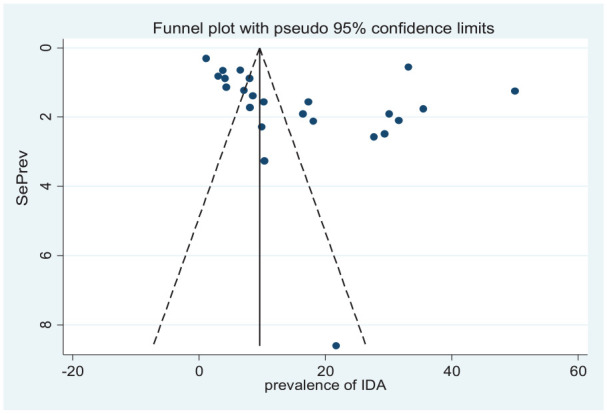
Funnel plot of studies included on the prevalence of IDA among under-5 children.
Figure 5.
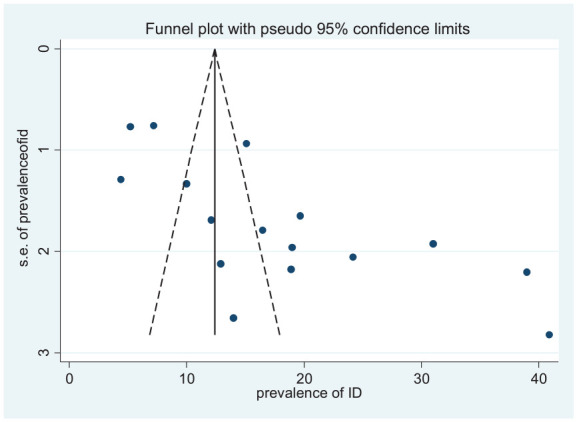
Funnel plot of studies included on the prevalence of ID among under-5 children.
Trim and fill analysis
Trim and fill analysis was done to overcome the influence of the small-study effect due to the presence of publication bias. In the random-effect model, 7 additional studies were added to estimate the pooled prevalence of ID among under-5 children, which was determined to be 9.73% (95% CI: 4.88-14.57).
Sensitivity Analysis
A random effect model was used to do a sensitivity analysis on the prevalence of IDA and ID among under-5 children. By removing each study in a stepwise manner, the effect of each study on the pooled estimated prevalence of IDA and ID was evaluated. The findings revealed that excluded studies had no significant effect on the pooled prevalence of both IDA and ID among children under the age of 5, with substantial heterogeneity between studies (Supplemental Tables S3 and S4).
Factors Associated with IDA and ID
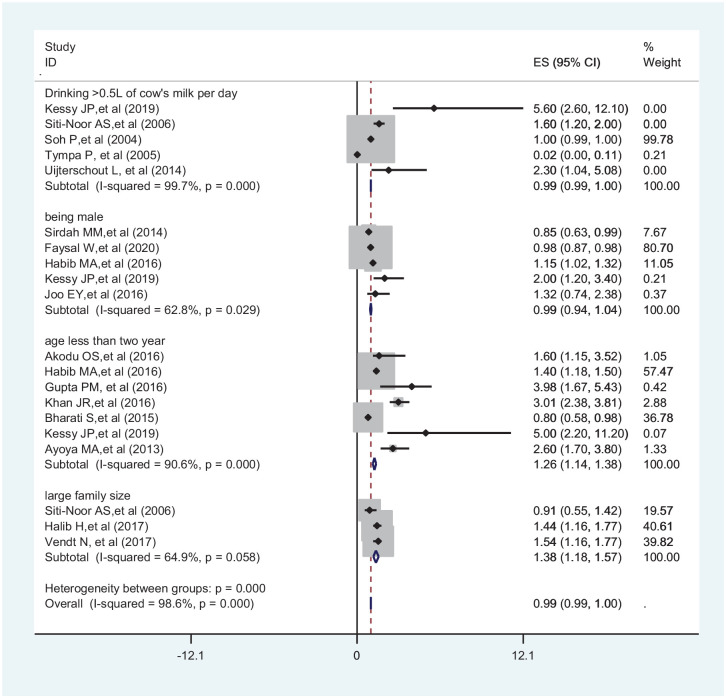
The association between drinking cow’s milk frequently, being a male child, age less than 2 years, and living in a large family size IDA was carried out. In this meta-analysis, to identify the associated factors, 5 articles were used for drinking cow’s milk frequently,26,41,47,49,50 5 for being male child,24,27,30,35,41 6 for age less than 2 years,5,30,41,42,44,46 and 3 for living in large family size.25,26,48 Accordingly, children in the age group less 2 years (OR = 1.26; 95% CI: 1.14-1.38) and living in a large family size (OR = 1.38; 95% CI: 1.18-1.57) were significantly associated with IDA (Figure 6).
Figure 6.
Forest plot showing factors associated with IDA among under-5 children.
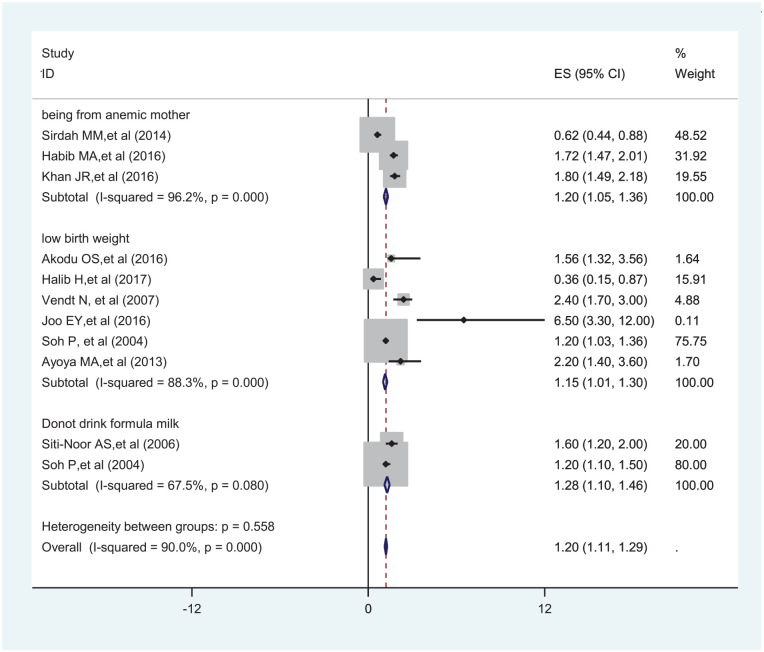
To identify factors associated with ID, a total of 3 articles were used for children from anemic mother,27,30,34 6 for having low birth weight,25,26,35,42,46,48 and 2 for do not drink formula milk26,49 were used for analysis. Children from anemic mother (OR = 1.20; 95% CI: 1.05-1.36), having low birth weight (OR = 1.15; 95% CI: 1.01-1.30), and do not drink formula milk (OR = 1.28; 95% CI: 1.10-1.46) were significantly associated with ID (Figure 7).
Figure 7.
Forest plot showing factors associated with ID among under-5 children.
Discussion
The goal of this study was to look at the findings of existing research to determine the global pooled prevalence of IDA and ID, as well as their associated factors. The global pooled prevalence of IDA among under-five children was 16.42% (95% CI: 10.82, 22.01). When the prevalence of anemia was greater than 40%, 20%, or 5%, according to WHO categories, it was classed as severe, moderate, or mild anemia. 51 As a result, the current study’s IDA level was classed as a moderate public health problem among under-5 children.
The findings of the current study was comparable with a systematic review and meta-analysis studies conducted in Iran 18.2% 9 and Brazil 20.9%. 52 However, the findings was lower than the study conducted in Brazil 40.2%. 53 The reason might be due to the present study that was conducted across the globe, which includes studies conducted in developed countries, where IDA was not a threat. Where as in Brazil there might be increased poverty in some parts of the country which resulted in malnutrition. 54
Sub-group analysis was done on the prevalence of IDA among under-5 children based on the continent of a study conducted. Accordingly, the prevalence of IDA was relatively high in Asia (23.37% (95% CI: 12.88, 33.87)) and Africa (16.40% (95% CI: 7.07, 25.73)) when compared with studies conducted in North America (4.18% (95% CI: 0.99, 7.38)), and Europe (7.39% (95% CI: 5.10, 9.67)). The difference in the pooled estimate of IDA in Asia and Africa with Europe and North America might be due to the difference in socio-economic status between the study subjects, ways of life, and geographical difference between the study subjects. Besides, people living in developing region were vulnerable for nutrient deficiency and infection. 55 Sub-group analysis based on study setting showed that 12.91% (95% CI: 4.95, 20.87) in the community-based and 19.49% (95% CI: 10.68, 28.30) in the hospital-based studies. There was no significant difference between the 2 settings. Even though the difference was not significant, the subgroup pooled estimate in the hospital setting is higher than that of the community-based setting. The reason might be individuals in the hospital might suffering from a certain type of disease and they might in need of immediate treatment.
The global pooled prevalence of ID among under-5 children was 17.95% (95% CI: 13.49, 22.41) according to this study. The findings of this systematic review and meta-analysis was comparable with a national estimate conducted in Kuwait 17%. 56 However, the finding is higher than a national estimate conducted in North America 2.9%. 57 Hemoglobinopathies, chronic renal disease, and gastrointestinal bleeding accounted for a greater share of anemia burden in high-income countries than ID. 57 The findings was lower than a systematic review and meta-analysis conducted in Iran 27.7%, 9 WHO’s reports of ID in Africa 62.3%, and in Southeast Asia 53.8%. 51 This could be due to the reason that the current study covers the whole world and the result may be affected with extremely low prevalence reported from developed countries. The variation might be due to the difference in socio-economic status, ways of life, density of parasitic infection, and the feeding practice. 58
Sub-group analysis on the prevalence of ID among under-5 children based on continent of a study conducted showed that 15.84% (95% CI: 5.52, 26.16) in North America, 20.33% (95% CI: 8.77, 31.90) in Asia, 18.50% (95% CI: 7.51, 29.50) in Africa, and 16.59% (95% CI: 14.18, 19.00) were in Europe. There is no significant difference between the study continents. Besides sub-group analysis based on study setting also showed that 14.44% (95% CI: 9.63, 19.24) in the community-based and 20.73% (95% CI: 12.93, 28.52) in the hospital-based. Even though the difference was no significant, the pooled estimate of ID in the hospital setting was higher than that of community based, this might be due to the reason that individuals visiting the hospital might be relatively in need of treatment and suffering from a certain types of diseases. 59
In this study, children under the age of 2 were 1.26 times more likely to acquire IDA (OR = 1.26; 95% CI: 1.14, 1.38). This could be attributed to a higher need for iron in this age group as a result of rapid growth and development combined with a lack of iron intake. 30 Furthermore, because their immune is not well developed at this age, kids are at a significant risk of infection. 30 Similarly, children who lived in large families were 1.38 times more likely to have IDA than children who lived in small families (OR = 1.38; 95%: 1.18, 1.57). This finding was supported with a systematic review and meta-analysis conducted on Brazilian children 53 and WHO reports for risk factors of under-5 IDA. 51 If food production and family size do not increase with the same rate, it will result with poverty and end up with food insecurity with in the household. Large families have a negative influence on household food security, especially when it comes to nutrient-dense foods. 60
Children who had low birth weight were 1.15 times more likely to develop ID than those having normal birth weight (OR = 1.15; 95% CI: 1.01, 1.30). This finding is supported by a study conducted in Estonia. 48 The majority of children born small for gestational age (SGA) catch up in weight and length within the first 2 years of life. As a result, the increased demand for iron during this period of rapid growth may result in ID. 61 Children born from anemic mother were 1.20 times more likely to develop ID than their counterparts (OR = 1.20; 95% CI: 1.05, 1.36). This finding was supported by a study conducted in Pakistan. 30 Hemoglobin, iron, and ferritin concentrations in the cord blood of anemic mothers were significantly lower, and there were linear connections between maternal hemoglobin and ferritin levels. 62 This might be due to anemia in mother’s results in low reserves of iron in the infants. 63 Similarly, children who did not drink iron-fortified milk were 1.28 times more likely to develop ID than their counterparts (OR = 1.28; 95% CI: 1.10, 1.46). This finding is supported by studies conducted in Singapore 26 and New Zealand. 49 Cow’s milk is lower in iron, produces little blood loss in the intestines, and makes iron absorption more difficult. As a result, it was well understood that cow’s milk was the primary dietary risk factor for ID in children under the age of 5. 64
Strengths and Limitations
An exhaustive search using different database and searching strategies were the strength of this study. The limitations of this study were, only including studies published in English language, and excluding others. Even though we performed subgroup and sensitivity analysis heterogeneity was still observed in all analysis. Due to the small number of papers published in some regions, the results for this region may be slightly biased.
Conclusion
This study found that the pooled prevalence of IDA and ID among under-5 children was found to be moderate, especially in developing countries. It is concluded that IDA and ID are a moderate public health problem in children under-5 years of age. Children less than 2 years old and those who are living in a large family size were at risk of developing IDA. Whereas children born from anemic mother, having low birth weight, and those don’t drink iron fortified milk were at higher risk of developing ID than their counter parts. To lower the risk of preterm and low birth weight, starting iron supplementation before or as soon as possible after pregnancy is advisable. Therefore, it needs a continuous screening program for ID and IDA for early detection and treatment. Supplementation and food fortification programs, as well as feeding a diverse diet and counseling parents on family planning, are all strongly advised to reduce under-5 ID and IDA.
Supplemental Material
Supplemental material, sj-docx-1-gph-10.1177_2333794X221110860 for Prevalence and Associated Factors of Iron Deficiency and Iron Deficiency Anemia Among Under-5 Children: A Systematic Review and Meta-Analysis by Solomon Gedfie, Solomon Getawa and Mulugeta Melku in Global Pediatric Health
Supplemental material, sj-docx-2-gph-10.1177_2333794X221110860 for Prevalence and Associated Factors of Iron Deficiency and Iron Deficiency Anemia Among Under-5 Children: A Systematic Review and Meta-Analysis by Solomon Gedfie, Solomon Getawa and Mulugeta Melku in Global Pediatric Health
Supplemental material, sj-docx-3-gph-10.1177_2333794X221110860 for Prevalence and Associated Factors of Iron Deficiency and Iron Deficiency Anemia Among Under-5 Children: A Systematic Review and Meta-Analysis by Solomon Gedfie, Solomon Getawa and Mulugeta Melku in Global Pediatric Health
Supplemental material, sj-docx-4-gph-10.1177_2333794X221110860 for Prevalence and Associated Factors of Iron Deficiency and Iron Deficiency Anemia Among Under-5 Children: A Systematic Review and Meta-Analysis by Solomon Gedfie, Solomon Getawa and Mulugeta Melku in Global Pediatric Health
Supplemental material, sj-docx-5-gph-10.1177_2333794X221110860 for Prevalence and Associated Factors of Iron Deficiency and Iron Deficiency Anemia Among Under-5 Children: A Systematic Review and Meta-Analysis by Solomon Gedfie, Solomon Getawa and Mulugeta Melku in Global Pediatric Health
Acknowledgments
We would like to acknowledge all the authors of the included studies in this review and meta-analysis.
Footnotes
Author Contributions: All authors involve in literature search, manuscript draft, review, statistical analysis, quality assessment, and final approval. The authors revised the manuscript critically and agreed to be responsible for all elements of the work.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Data Availability Statement: The datasets used in this investigation are given in the manuscript and its supplements.
ORCID iDs: Solomon Gedfie  https://orcid.org/0000-0001-9964-1964
https://orcid.org/0000-0001-9964-1964
Solomon Getawa  https://orcid.org/0000-0003-2670-9547
https://orcid.org/0000-0003-2670-9547
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Pita GM, Jiménez S, Basabe B, et al. Anemia in children under five years old in eastern Cuba, 2005-2011. MEDICC Rev. 2014;16:16-23. [DOI] [PubMed] [Google Scholar]
- 2. Mahoney D. Iron deficiency in infants and young children: screening, prevention, clinical manifestations and diagnosis. UpToDate. 2015. [Google Scholar]
- 3. Beard J. Iron deficiency alters brain development and functioning. Nutr J. 2003;133(5 Suppl 1):1468S-1472S. [DOI] [PubMed] [Google Scholar]
- 4. WHO UNU. Iron Deficiency Anaemia: Assessment, Prevention, and Control. A Guide for Programme Managers. WHO; 2001. [Google Scholar]
- 5. Bharati S, Pal M, Chakrabarty S, Bharati P. Socioeconomic determinants of iron-deficiency anemia among children aged 6 to 59 months in India. Asia Pac J Public Health. 2015;27(2):NP1432-NP1443. [DOI] [PubMed] [Google Scholar]
- 6. Pasricha S-R, Drakesmith H, Black J, Hipgrave D, Biggs B-A. Control of iron deficiency anemia in low- and middle-income countries. Blood. 2013;121(14):2607-2617. [DOI] [PubMed] [Google Scholar]
- 7. Thankachan P, Walczyk T, Muthayya S, Kurpad AV, Hurrell RF. Iron absorption in young Indian women: the interaction of iron status with the influence of tea and ascorbic acid. Am J Clin Nutr. 2008;87(4):881-886. [DOI] [PubMed] [Google Scholar]
- 8. Stoltzfus R. Defining iron-deficiency anemia in public health terms: a time for reflection. Nutr J. 2001;131(2S-2):565S-567S. [DOI] [PubMed] [Google Scholar]
- 9. Nazari M, Mohammadnejad E, Dalvand S, Ghanei Gheshlagh R. Prevalence of iron deficiency anemia in Iranian children under 6 years of age: a systematic review and meta-analysis. J Blood Med. 2019;10:111-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barooti E, Rezazadehkermani M, Sadeghirad B, et al. Prevalence of iron deficiency anemia among Iranian pregnant women; a systematic review and meta-analysis. J Reprod Infertil. 2010;11(1):17-24. [PMC free article] [PubMed] [Google Scholar]
- 11. McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B. Worldwide prevalence of anaemia, WHO vitamin and mineral nutrition information system, 1993-2005. Public Health Nutr. 2009;12(4):444-454. [DOI] [PubMed] [Google Scholar]
- 12. Thane CW, Walmsley CM, Bates CJ, Prentice A, Cole TJ. Risk factors for poor iron status in British toddlers: further analysis of data from the National Diet and Nutrition Survey of children aged 1.5-4.5 years. Public Health Nutr. 2000;3(4):433-440. [DOI] [PubMed] [Google Scholar]
- 13. Lozoff B, Beard J, Connor J, Barbara F, Georgieff M, Schallert T. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr Rev. 2006;64(5 Pt 2):S34-S43; discussion S72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Domellöf M, Braegger C, Campoy C, et al. Iron requirements of infants and toddlers. J Pediatr Gastroenterol Nutr. 2014;58(1):119-129. [DOI] [PubMed] [Google Scholar]
- 15. Maraolo AE. Una bussola per le revisioni sistematiche: la versione italiana della nuova edizione del PRISMA statement. BMJ. 2021;372:n71. [Google Scholar]
- 16. Zhu YP, Liao QK. [Prevalence of iron deficiency in children aged 7 months to 7 years in China]. Zhonghua Er Ke Za Zhi. 2004;42(12):886-891. [PubMed] [Google Scholar]
- 17. Lind T, Lönnerdal B, Persson LA, Stenlund H, Tennefors C, Hernell O. Effects of weaning cereals with different phytate contents on hemoglobin, iron stores, and serum zinc: a randomized intervention in infants from 6 to 12 mo of age. Am J Clin Nutr. 2003;78(1):168-175. [DOI] [PubMed] [Google Scholar]
- 18. Suominen P, Virtanen A, Lehtonen-Veromaa M, et al. Regression-based reference limits for serum transferrin receptor in children 6 months to 16 years of age. Clin Chem. 2001;47(5):935-937. [PubMed] [Google Scholar]
- 19. Joanna Briggs Institute. JBI critical appraisal checklist for analytical cross sectional studies. 2016. [Google Scholar]
- 20. Joanna Briggs Institute. JBI critical appraisal checklist for case control studies. Joanna Briggs Institute Reviewers’ Manual. 2016. [Google Scholar]
- 21. Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, et al. Chapter 7: Systematic reviews of etiology and risk. In: Aromataris E, Munn Z, eds. Joanna Briggs Institute Reviewer’s Manual. The Joanna Briggs Institute; 2017;5:217-269. [Google Scholar]
- 22. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539-1558. [DOI] [PubMed] [Google Scholar]
- 23. Shi X, Nie C, Shi S, Wang T, Yang H, Zhou Y, et al. Effect comparison between Egger’s test and Begg’s test in publication bias diagnosis in meta-analyses: evidence from a pilot survey. Int J Res Stud Biosci. 2017;5(5):14-20. [Google Scholar]
- 24. Faysal W, Zaidi ARZ, Al-Abdi S, Alhumaid S, AlShehery MZ, Al Mutair A. Hospital-based prevalence of iron deficiency anemia among pre-school children in Dubai. Cureus. 2020;12(10):e10894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Halib H, Muda WM, Dam PC, Mohamed HJ. Prevalence of iron deficiency and its associated risk factors among primary school children in Kelantan. J Fundam Appl Sci. 2018;9(2S):397-412. [Google Scholar]
- 26. Siti-Noor AS, Wan-Maziah WM, Narazah MY, Quah BS. Prevalence and risk factors for iron deficiency in Kelantanese pre-school children. Singapore Med J. 2006;47(11):935-939. [PubMed] [Google Scholar]
- 27. Sirdah MM, Yaghi A, Yaghi AR. Iron deficiency anemia among kindergarten children living in the marginalized areas of Gaza Strip Palestine. Rev Bras Hematol Hemoter. 2014;36(2):132-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wirth JP, Rajabov T, Petry N, et al. Micronutrient deficiencies, over- and undernutrition, and their contribution to anemia in Azerbaijani preschool children and non-pregnant women of reproductive age. Nutrients. 2018;10(10):1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Keikhaei B, Zandian K, Ghasemi A, Tabibi R. Iron-deficiency anemia among children in southwest Iran. Food Nutr Bull. 2007;28(4):406-411. [DOI] [PubMed] [Google Scholar]
- 30. Habib MA, Black K, Soofi SB, et al. Prevalence and predictors of iron deficiency anemia in children under five years of age in Pakistan, a secondary analysis of national nutrition survey data 2011-2012. PLoS One. 2016;11(5):e0155051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen C-M, Mu SC, Shih C-K, et al. Iron status of infants in the first year of life in northern Taiwan. Nutrients. 2020;12(1):139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Quaderi HR, Hoque MM, Ahmed NU, Begum D, Debnath B. Prevalence of anemia in children aged six months to thirty six months - a hospital based study. Banglad J Child Health. 2017;40(2):98-102. [Google Scholar]
- 33. Kadivar MR, Yarmohammadi H, Mirahmadizadeh AR, Vakili M, Karimi M. Prevalence of iron deficiency anemia in 6 months to 5 years old children in fars, Southern Iran. Med Sci Monit. 2003;9(2):CR100-CR104. [PubMed] [Google Scholar]
- 34. Khan JR, Awan N, Misu F. Determinants of anemia among 6-59 months aged children in Bangladesh: evidence from nationally representative data. BMC Pediatr. 2016;16(1):3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Joo EY, Kim KY, Kim DH, Lee J-E, Kim SK. Iron deficiency anemia in infants and toddlers. Blood Res. 2016;51(4):268-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kikafunda JK, Lukwago FB, Turyashemererwa F. Anaemia and associated factors among under-fives and their mothers in Bushenyi district, Western Uganda. Public Health Nutr. 2009;12(12):2302-2308. [DOI] [PubMed] [Google Scholar]
- 37. Donahue Angel M, Berti P, Siekmans K, Tugirimana PL, Boy E. Prevalence of iron deficiency and iron deficiency anemia in the northern and southern provinces of Rwanda. Food Nutr Bull. 2017;38(4):554-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Harvey-Leeson S, Karakochuk CD, Hawes M, et al. Anemia and micronutrient status of women of childbearing age and children 6-59 months in the Democratic Republic of the Congo. Nutrients. 2016;8(2):98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bahizire E, Bahwere P, Donnen P, et al. High prevalence of anemia but low level of iron deficiency in preschool children during a low transmission period of malaria in rural Kivu, Democratic Republic of the Congo. Am J Trop Med Hyg. 2017;97(2):489-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wirth JP, Rohner F, Woodruff BA, et al. Anemia, micronutrient deficiencies, and malaria in children and women in Sierra Leone prior to the Ebola outbreak - findings of a cross-sectional study. PLoS One. 2016;11(5):e0155031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kessy J, Philemon R, Lukambagire A, et al. Iron depletion, Iron deficiency, and Iron deficiency anaemia among children under 5 years old in Kilimanjaro, northern Tanzania: a hospital-based cross-sectional study. EA Health Research Journal. 2019;3(1):42-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Akodu OS, Disu EA, Njokanma OF, Kehinde OA. Iron deficiency anaemia among apparently healthy pre-school children in Lagos, Nigeria. Afr Health Sci. 2016;16(1):61-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Innis SM, Nelson CM, Wadsworth LD, MacLaren IA, Lwanga D. Incidence of iron-deficiency anaemia and depleted iron stores among nine-month-old infants in Vancouver, Canada. Can J Public Health. 1997;88(2):80-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gupta PM, Perrine CG, Mei Z, Scanlon KS. Iron, anemia, and iron deficiency anemia among young children in the United States. Nutrients. 2016;8(6):330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Weiler HA, Jean-Philippe S, Cohen TR, Vanstone CA, Agellon S. Depleted iron stores and iron deficiency anemia associated with reduced ferritin and hepcidin and elevated soluble transferrin receptors in a multiethnic group of preschool-age children. Appl Physiol Nutr Metab. 2015;40(9):887-894. [DOI] [PubMed] [Google Scholar]
- 46. Ayoya MA, Ngnie-Teta I, Séraphin MN, et al. Prevalence and risk factors of anemia among children 6-59 months old in Haiti. Anemia. 2013;2013:502968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Uijterschout L, Vloemans J, Vos R, et al. Prevalence and risk factors of iron deficiency in healthy young children in the southwestern Netherlands. J Pediatr Gastroenterol Nutr. 2014;58(2):193-198. [DOI] [PubMed] [Google Scholar]
- 48. Vendt N, Grünberg H, Leedo S, Tillmann V, Talvik T. Prevalence and causes of iron deficiency anemias in infants aged 9 to 12 months in Estonia. Medicina. 2007;43(12):947-952. [PubMed] [Google Scholar]
- 49. Soh P, Ferguson EL, McKenzie JE, Homs MY, Gibson RS. Iron deficiency and risk factors for lower iron stores in 6-24-month-old New Zealanders. Eur J Clin Nutr. 2004;58(1):71-79. [DOI] [PubMed] [Google Scholar]
- 50. Tympa-Psirropoulou E, Vagenas C, Psirropoulos D, Dafni O, Matala A, Skopouli F. Nutritional risk factors for iron-deficiency anaemia in children 12-24 months old in the area of Thessalia in Greece. Nutr Food Sci Int J. 2005;56(1):1-12. [DOI] [PubMed] [Google Scholar]
- 51. Hong L-F, Li XL, Luo S-H, et al. Relation of leukocytes and its subsets counts with the severity of stable coronary artery disease in patients with diabetic mellitus. PLoS One. 2014;9(3):e90663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Collier A, Jackson M, Bell D, et al. Neutrophil activation detected by increased neutrophil elastase activity in type 1 (insulin-dependent) diabetes mellitus. Diabetes Res. 1989;10(3):135-138. [PubMed] [Google Scholar]
- 53. Silveira VNC, Carvalho CA, Viola PCAF, et al. Prevalence of iron-deficiency anaemia in Brazilian children under 5 years of age: a systematic review and meta-analysis. Br J Nutr. 2021;126:1257-1269. [DOI] [PubMed] [Google Scholar]
- 54. Muniz PT, Castro TG, Araújo TS, et al. Child health and nutrition in the western Brazilian Amazon: population-based surveys in two counties in Acre State. Cad Saúde Pública. 2007;23:1283-1293. [DOI] [PubMed] [Google Scholar]
- 55. Osungbade KO, Oladunjoye AO. Anaemia in developing countries: burden and prospects of prevention and control. Anemia. 2012;3:116-129. [Google Scholar]
- 56. Al Zenki S, Alomirah H, Al Hooti S, et al. Prevalence and determinants of anemia and iron deficiency in Kuwait. Int J Environ Res Public Health. 2015;12(8):9036-9045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kassebaum NJ, Jasrasaria R, Naghavi M, et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood. 2014;123(5):615-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yu XY, Chen H-M, Liang J-L, et al. Hyperglycemic myocardial damage is mediated by proinflammatory cytokine: macrophage migration inhibitory factor. PLoS One. 2011;6(1):e16239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Randi ML, Bertozzi I, Santarossa C, et al. Prevalence and causes of anemia in hospitalized patients: impact on diseases outcome. J Clin Med. 2020;9(4):950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Olayemi AO. Effects of family size on household food security in Osun State, Nigeria. Asian J Agric Rural Dev. 2012;2(2):136-141. [Google Scholar]
- 61. Thorsdottir I, Gunnarsson BS, Atladottir H, Michaelsen KF, Palsson G. Iron status at 12 months of age — effects of body size, growth and diet in a population with high birth weight. Eur J Clin Nutr. 2003;57(4):505-513. [DOI] [PubMed] [Google Scholar]
- 62. Sweet DG, Savage G, Tubman TR, Lappin TR, Halliday HL. Study of maternal influences on fetal iron status at term using cord blood transferrin receptors. Arch Dis Child Fetal Neonatal Ed. 2001;84(1):F40-F43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ahmed A, Ahmad A, Khalid N, et al. A question mark on iron deficiency in 185 million people of Pakistan: its outcomes and prevention. Crit Rev Food Sci Nutr. 2014;54(12):1617-1635. [DOI] [PubMed] [Google Scholar]
- 64. Tefferi A, Hanson CA, Inwards DJ. How to interpret and pursue an abnormal complete blood cell count in adults. Mayo Clin Proc. 2005;80(7):923-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-gph-10.1177_2333794X221110860 for Prevalence and Associated Factors of Iron Deficiency and Iron Deficiency Anemia Among Under-5 Children: A Systematic Review and Meta-Analysis by Solomon Gedfie, Solomon Getawa and Mulugeta Melku in Global Pediatric Health
Supplemental material, sj-docx-2-gph-10.1177_2333794X221110860 for Prevalence and Associated Factors of Iron Deficiency and Iron Deficiency Anemia Among Under-5 Children: A Systematic Review and Meta-Analysis by Solomon Gedfie, Solomon Getawa and Mulugeta Melku in Global Pediatric Health
Supplemental material, sj-docx-3-gph-10.1177_2333794X221110860 for Prevalence and Associated Factors of Iron Deficiency and Iron Deficiency Anemia Among Under-5 Children: A Systematic Review and Meta-Analysis by Solomon Gedfie, Solomon Getawa and Mulugeta Melku in Global Pediatric Health
Supplemental material, sj-docx-4-gph-10.1177_2333794X221110860 for Prevalence and Associated Factors of Iron Deficiency and Iron Deficiency Anemia Among Under-5 Children: A Systematic Review and Meta-Analysis by Solomon Gedfie, Solomon Getawa and Mulugeta Melku in Global Pediatric Health
Supplemental material, sj-docx-5-gph-10.1177_2333794X221110860 for Prevalence and Associated Factors of Iron Deficiency and Iron Deficiency Anemia Among Under-5 Children: A Systematic Review and Meta-Analysis by Solomon Gedfie, Solomon Getawa and Mulugeta Melku in Global Pediatric Health