Abstract
Study Objectives
Brain iron deficiency has been reported to be associated with the restless legs syndrome (RLS). However, 30%–50% of RLS patients do not respond to iron therapy, indicating that mechanisms other than brain iron deficiency may also participate in this disease. The striatum is known to be involved in the modulation of motor activity. We speculated that dysfunction of the striatum may induce RLS.
Methods
Two groups, wild-type (WT) and iron-deficient (ID) rats were used. Each group was divided into two subgroups, control and N-methyl-d-aspartate striatal-lesioned. After baseline recording, striatal-lesioned wild-type (WT-STL) and striatal-lesioned iron-deficient (ID-STL) rats were given pramipexole and thioperamide injections. Iron-deficient and ID-STL rats were then given a standard rodent diet for 4 weeks, and their sleep and motor activity were recorded.
Results
WT-STL rats showed periodic leg movements (PLM) in wake, an increase in PLM in slow wave sleep (SWS), a decrease in rapid-eye-movement sleep, and a decrease in the daily average duration of episodes in SWS. The sleep–wake pattern and motor activity did not differ between ID and ID-STL rats. Thioperamide or pramipexole injection decreased PLM in sleep and in wake in WT-STL rats and ID-STL rats. Unlike ID rats, whose motor hyperactivity can be reversed by iron replacement, PLM in wake and in sleep in ID-STL rats were not fully corrected by iron treatment.
Conclusions
Lesions of the striatum generate RLS-like activity in rats. Dysfunction of the striatum may be responsible for failure to respond to iron treatment in some human RLS patients.
Keywords: pramipexole, thioperamide, periodic leg movement, iron deficient, iron therapy, neurotoxic lesions
Statement of Significance.
Neurotoxic lesions of the striatum in wild-type rats produces restless legs syndrome (RLS)-like activity. The sleep–wake pattern and motor activity does not differ between iron-deficient (ID) and striatal-lesioned ID rats. Pramipexole or thioperamide are effective in the treatment of RLS-like activity in the striatal-lesioned wild-type and striatal-lesioned ID rat. In contrast to the ID rat, in which symptoms can be reversed by iron replacement (IR), motor hyperactivity in striatal-lesioned ID rats is not corrected by IR. 30%–50% of RLS patients do not have a positive response to iron therapy. We hypothesize that dysfunction of the striatum is responsible for failure to respond to iron treatment in some human RLS patients.
Introduction
The circuitry of the basal ganglia has been implicated in the pathogenesis of human restless legs syndrome (RLS). Single-photon emission computed tomography showed a reduced cerebral blood flow [1] and a decrease in postsynaptic dopamine D2 receptor density in the striatum [2, 3] of RLS patients. Postmortem examination found that RLS patients have an elevated level of tyrosine hydroxylase and phosphorylated tyrosine hydroxylase in the putamen and in the substantia nigra (SN), as well as a decrease in dopamine D2 receptor in the putamen [4]. We showed that GABAergic transmission in the SN pars reticulata (SNR) is important in the inhibition of motor activity in quiet wake and in sleep. Inhibition of SNR neuronal activity, resulting from an increase in GABA release into the SNR, produced RLS-like activity in rats [5].
Brain iron deficiency has been associated with RLS [6–8]. However, 30%–50% RLS patients either do not respond or partially respond to iron therapy. Clinical studies revealed that iron therapy, oral iron sulfate or intravenous infusion of ferric carboxymaltose or iron dextran, is effective in the treatment of RLS in 46%–68% patients [9–12]. Mechanisms underlying a lack of symptom improvement to iron therapy in many RLS patients are not clear. We have shown that the increased striatal levels of dopamine transporter and histamine H3 receptor level in the iron deficient (ID) rat, who were fed with low iron (4 ppm) rodent diet starting 21-day old, can be corrected by iron replacement (IR) [13, 14]. On the other hand, the decreased brain level of ferritin [15] and the striatal dopamine D2 receptor density [16], and the increased tyrosine hydroxylase and phosphorylated tyrosine hydroxylase in the midbrain ventral tegmentum [4], induced by a low iron diet in early life, cannot be corrected by iron supplementation in the rat. Thus, we speculated that abnormalities of the basal ganglia, perhaps resulting from iron deficiency, cannot be reversed with iron supplementation, resulting in a lack of response to iron therapy in these RLS patients.
Lee et al. [17]. reported that patients with unilateral striatal stroke often have RLS. Thus, we first wanted to examine whether lesions of the striatum produce RLS-like activity in the wild-type (WT) rat, and whether these RLS-like activities respond to drug treatment. Our previous studies showed that RLS-like activity in the ID rat can be reversed by IR [13]. Therefore, our second experiment aimed to determine whether RLS-like activity in the ID rat with striatal lesions can be fully corrected by IR. We have reported that pramipexole, a dopamine agonist, and thioperamide, an histamine H3 receptor antagonist, are effective in the treatment of RLS-like activities in the ID rats [13, 14]. Thus, we wanted to test the efficacy of both drugs in the treatment of RLS-like activities in the striatal-lesioned wild-type (WT-STL) and striatal-lesioned ID (ID-STL) rats.
Methods
All procedures were approved by the Institutional Animal Care and Use Committee, the VA Greater Los Angeles Healthcare System.
Development of wild-type and iron-deficient rats
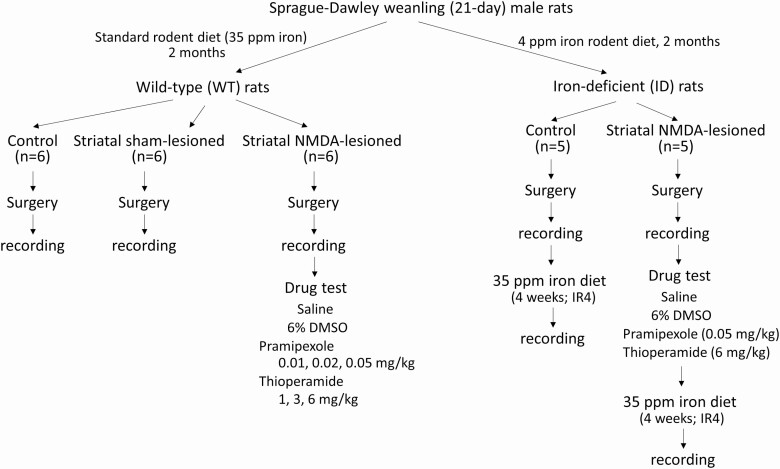
The experimental design is shown in Figure 1. Sprague-Dawley weanling male rats (21 days old) were purchased from Charles River Laboratory. The pups were divided into two groups, with one group (WT rat) fed with standard rodent diet, containing 35 ppm iron, and the other group (ID rat) fed with low iron rodent diet, containing 4 ppm iron (TD.80396, Harlan Teklad Lab), for 2 months. Rats were then surgically implanted with electrodes for sleep and motor activity recordings.
Figure 1.
Diagram showing the experimental design. Two groups, wild-type (WT) and iron-deficient (ID), of male rats were used for the study. IR4: iron replacement for 4 weeks. See details in the Method section.
Surgery for sleep–wake and motor activity recording
Eighteen WT rats and 10 ID rats were used. Among WT rats, six were used for control, six for NMDA striatal-lesioned, and six for striatal sham-lesioned groups. Five ID rats were used for control and five ID rats were used as striatal-lesioned. Striatal NMDA-lesion or sham-lesion was performed in one side of the striatum. Surgery was performed to implant electrodes for electroencephalograph (EEG) and electromyogram (EMG) recordings. Under isoflurane (1.5%) anesthesia, a small hole over the skull was made in striatal NMDA-lesioned and striatal sham-lesioned rats. Then, a Hamilton 1 μL microsyringe (80100, Reno, Nevada) filled with 0.5 M NMDA (striatal-lesioned) or artificial cerebrospinal fluid (CSF, striatal sham-lesioned) was slowly lowered to the striatum (3.5 mm lateral from the midline, 8.7 mm anterior, and 4.6 mm dorsal to the interaural zero). Ten nanoliters of NMDA or CSF were injected over 1 min. Upon completion of the first injection, the injection needle was withdrawn 0.3 mm dorsal to the original site and the injection procedure repeated. This procedure was repeated five times so that a total of 50 nL NMDA or CSF was injected. Upon completion of the injection, the injection needle was retained at the final injection position for an additional 15 min. The hole in the skull was filled with Gelfoam (83176, Pfizer) and covered with dental acrylic. Rats were then implanted with electrodes for EEG and EMG recording, as described in our previous study [13]. Briefly, four jewelers’ screws were implanted over the cortex for cortical EEG recording. Flexible multi-stranded stainless steel wires (7935, A-M Systems, Inc., Carlsborg, WA) were inserted into the nuchal and hindlimb (anterior tibialis and gastrocnemius) musculature bilaterally for EMG recording. Wires from all electrodes were soldered to a 14-pin Amphenol strip connector and encased in an acrylic head plug.
Sleep and motor activity recordings and drug injection experiments
Sleep and motor activity recordings were performed at least 7 days after surgery, by which time rats had shown normal motor behaviors, eating, drinking, walking and running. Animals were then individually housed in a sound-attenuated chamber (BRS/LVE, Inc., Laurel, MD) with a light–dark cycle of 12:12, and were allowed to adapt to the Raturn rodent bowl (MD-1404, BioAnalytical System, West Lafayette, IN). The Raturn system counter-rotates the bowl to prevent tangling of the recording cables. Food and water were provided for ad libitum consumption. The electrophysiological signals were collected and amplified through a polygraph (Model 78E or Model 15LT, Grass, MA), and then digitized and recorded via a Micro 1401 (Cambridge Electronics Design, Cambridge, UK). Infrared cameras were used for video recordings. Video images were captured digitally and recorded (Q-See QSPDVR04; RapidOS, New Taipei City, Taiwan) with timestamps matched to polysomnographic recordings.
After 3 days of baseline polysomnographic recordings, the WT-STL rats were intraperitoneally (i.p.) injected with pramipexole (0.01, 0.02, and 0.05 mg/kg) and thioperamide (1, 3, and 6 mg/kg), one dose of every other day (Figure 1), while the ID-STL rats were given (i.p.) one dose each of pramipexole (0.05 mg/kg) and thioperamide (6 mg/kg; Figure 1). Pramipexole was dissolved in Ringer’s saline, while thioperamide was dissolved in 30% dimethyl sulfoxide (DMSO) and then diluted with Ringer’s saline, immediately before injection. Ringer’s saline and 6% DMSO in saline (DMSO-saline) were given to both WT-STL rats and ID-STL rats. Saline, DMSO-saline and test drugs were given to the animal at Zeitgeber Time (ZT) 2. Sleep and motor activity recordings were continued for 24 hours after injection. Recordings obtained during the first 8-hour after the injection of test substances were analyzed to determine the drug effect on sleep and motor activity.
IR experiment in ID and striatal-lesioned ID rats
The ID rats were fed a standard rodent diet containing 35 ppm iron after baseline sleep and motor activity recordings (Figure 1), whereas the ID-STL rats were fed a standard rodent diet after the test drug injection experiment (Figure 1). Sleep and motor activity were recorded in both ID and ID-STL rats for 3 days after they had been fed a standard rodent diet for 4 weeks (IR4).
At the end of the experiment, animals were deeply anesthetized with an overdose of sodium pentobarbital (100 mg/kg, i.p.). Animals were then intracardially perfused with 0.1 M phosphate buffer saline (PBS), pH 7.4, followed by 4% paraformaldehyde solution. Brains were removed and kept in 30% sucrose–PBS at 4°C. The brains were cut into 40 μL sections, and then stained with cresyl violet.
Data analysis
The CED 1401 Spike2 program (Cambridge Electronics Design) was used to analyze EEG power spectra and to detect phasic motor activity during quiet wake and sleep. A script (SUDSA2) from CED was used to perform the Fast Fourier Transform with a resolution of 512 on each 10-sec epoch of the EEG. The spectral powers were calculated for each 2-Hz frequency band from 1 to 50 Hz, and then analyzed in Microsoft Excel in conjunction with sleep stage. Only movement artifact free epochs were included in the spectral power analysis. In order to compensate for the difference of mean amplitude in EEG recording between animals, the average 24-hour total power in each animal was used as a normalizing factor for all frequency bins before comparing them between animals. All statistical analysis was performed in IBM SPSS.
Sleep and periodic leg movements (PLM) in slow wave sleep (SWS; PLMS) and in quiet wake (PLMW) were visually scored offline with a tailored script in Spike2. PLMW and PLMS were analyzed by adapting the criteria described in our previous study [13]. In brief, phasic leg movements satisfying the following criteria were counted as PLM: (1) amplitude was twice of the tonic background activity, (2) duration ranged between 0.2 and 5 s, (3) interval between jerks was less than 90 s, and (4) at least four consecutive jerks fulfilled criteria described above. Phasic motor events in sleep, not meeting the criteria for PLMS were counted as isolated leg movements in sleep (ILMS). PLM epochs in SWS typically ended when the animal was awakened from sleep or the inter-leg movement interval was longer than 90 s. The index of PLMW (PLMWI) and PLMS (PLMSI) was calculated as the total number of periodic motor movements divided by total time in quiet wake or in SWS, respectively. Similarly, the index of ILMS (ILMSI) was calculated as the total number of ILM in SWS divided by total time in SWS.
To examine whether striatal lesions changed the sleep–wake pattern and motor activity, data were analyzed over a 24-hour period. In the WT rats, the differences among control, sham-lesioned, and striatal-lesioned rats in sleep–wake pattern and motor activity were tested by one-way ANOVA followed by Bonferroni corrected t-test, while a t-test was performed to determine the difference between ID control and ID-STL rats. To determine whether test drug injection affected sleep–wake pattern and motor activity, data were analyzed over the first 8 hours after each injection with repeated measure ANOVA on baseline, saline or DMSO, and 3 doses of each drug (WT-STL rats) or one dose of each drug (ID-STL rats) for within-subject effects, followed by Bonferroni corrected t-test. To determine whether sleep pattern and motor activity in the ID-STL rats can be reversed by iron replacement, a t-test was used to compare the difference between ID-STL-IR4 to WT rat. A t-test was also used to compare the drug efficacy and iron replacement on motor activity in the ID-STL rat (ID-STL-IR4 vs. pramipexole injection, and ID-STL-IR4 vs. thioperamide injection). Analyses were carried out using Microsoft Excel and IBM SPSS Statistics software.
Results
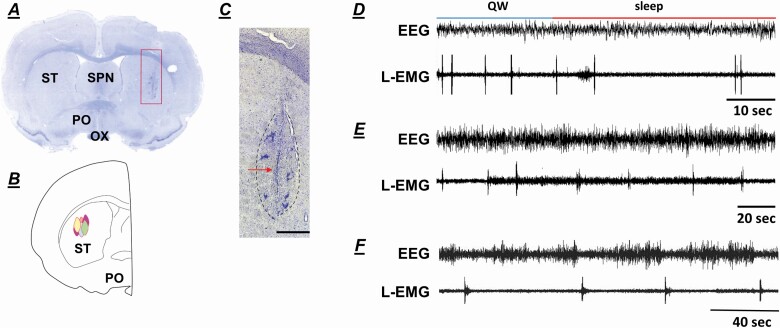
The NMDA-lesioned and sham-lesioned sites were in the central-lateral portion of the striatum (Figure 2A–C), the area that receives corticospinal projections to the limbs from the motor and supplemental motor cortex [18].
Figure 2.
(A) Photomicrographs showing the lesion site in the striatum. (B) Reconstructed histology showing the NMDA-lesioned sites in the striatum. (C) Higher magnification photomicrograph, taken of the square area shown in (A), showing the lesion area circled with black dots. The arrow shown in (C) indicates the injection needle tract. Brain tissue was stained with cresyl violet. (D) and (E) example of periodic leg movements (PLM) in quiet wake (QW; D) and in sleep (E) observed in the striatal-lesioned wild-type rats. (F) example of PLM recorded in wild-type rat. EEG: electroencephalogram, L-EMG: leg electromyogram, OX: optic tract, PO: preoptic area, SPN: septal nucleus, ST: striatum. Calibration (C) 90 μL.
Wild-type (WT) rat lesion experiments
Effect of neurotoxic lesions of the striatum on sleep and motor activity in WT rats.
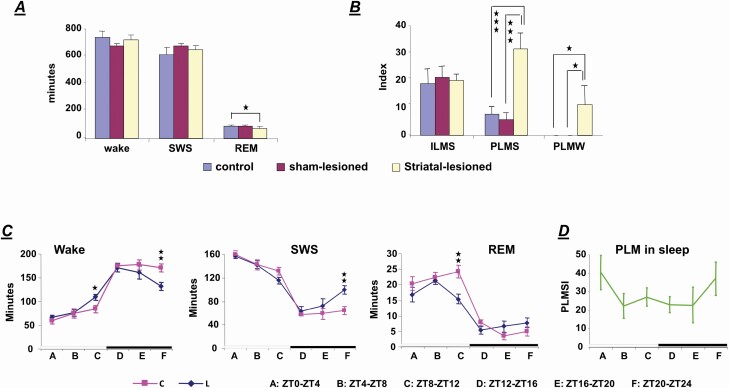
Neurotoxic striatal lesions in the WT rats produced a decrease in rapid-eye-movement sleep (REM; WT, WT-striatal-sham-lesioned, and WT-STL: F = 4.646, df = 2, p = .032, ANOVA; WT vs. WT-STL rat, p = .032, df = 10, t-test) with no change in wake and SWS time in a 24-hour recording (Figure 3A). However, statistical analysis showed that a decrease in the daily average duration of episodes in SWS (WT vs. WT-STL: p = .013, df = 10, t-test), but not in waking (WT vs. WT-STL: p = .682, df = 10, t-test) and in REM sleep (WT vs. WT-STL: p = .133, df = 10, t-test) is observed in the WT-STL rats, compared with WT rats. EEG power spectral analysis showed that delta power (1–4 Hz) during SWS in WT-STL rats is smaller, but does not significantly differ from WT rats (p = .053, df = 9, t-test). Similar to the ID rat [19], a decrease in wake time (WT vs. WT-STL: t-test, p = .008, df = 10, Figure 3C) and an increase in SWS time (WT vs. WT-STL: t-test, p = .006, df = 10, Figure 3C) were seen during the last 4 hours of the dark phase in WT-STL rats, compared with WT rats. An increase in wake (WT vs. WT-STL: t-test, p = .027, df = 10, Figure 3C) and REM sleep time (WT vs. WT-STL: t-test, p = .007, df = 10, Figure 3C) was also found during the last 4 hours of the light phase in the WT-STL rats. Motor behaviors, running, grooming, standing, and eating, were normal in the WT-STL rats. However, PLM in wake (Figures 2D and 3B), which was not observed in WT rats, was found in the WT-STL rats. A significant increase in PLMS (WT, WT-striatal-sham-lesioned, and WT-STL: ANOVA, F = 111.645, df = 2, p < .001; WT vs. WT-STL: t-test, p < .001, df = 10, Figures 2E and 3B) was also found in the WT-STL rats. The increased PLMS was maximal during the first 4 hours of light phase and the last 4 hours of dark phase (Figure 3D). PLMS was also observed in the WT rats (Figure 2F). However, the interval between jerks in WT rats was longer than that in the WT-STL rats, as we reported in previous studies [5, 19]. Isolated leg movements in SWS were not changed by the striatal lesion in the WT rats (WT, WT-striatal-sham-lesioned, and WT-STL: F =0.687, df = 2, p = .518, ANOVA, Figure 3B).
Figure 3.
Effect of striatal lesions on sleep–wake pattern and motor activity in wild-type (WT) rats. (A) A decrease in rapid-eye-movement (REM) sleep with no change in wake and slow wave sleep (SWS) was found in the striatal-lesioned wild-type (WT-STL) rats in 24 hour recordings. (B) Striatal lesions in the WT rat produced an increase in periodic leg movements (PLM) in sleep (PLMS), as well as generating PLM in quiet wake (PLMW) in 24 hour recordings. In contrast, isolated leg movements in sleep (ILMS) were not changed in the WT-STL rats. Indexes of PLMS and PLMW were calculated as the total number of PLM in 24 hours in sleep and wake divided by the total time in sleep and wake in 24 hours. Index of ILMS was calculated as the total number of isolated motor events in 24 hours divided by the total time in sleep in 24 hours. (C) Distribution of wake, SWS and REM sleep time in 4-hour epoch over 24-hour recording in the WT control (C) and WT striatal-lesioned (L) rats. (D) Distribution of PLMS index (PLMSI) over 24-hour recording in the striatal-lesioned wild-type rats. Periodic leg movements in sleep were maximal at Zeitgeber time (ZT) 0–4 and ZT19–ZT23. The white and black bars shown on the bottom (C and D) represent the light and dark phases, respectively. t-Test, df = 10, *p < .05, **p < .01, ***p < .001.
Effect of pramipexole on sleep and motor activity in WT-STL rats.
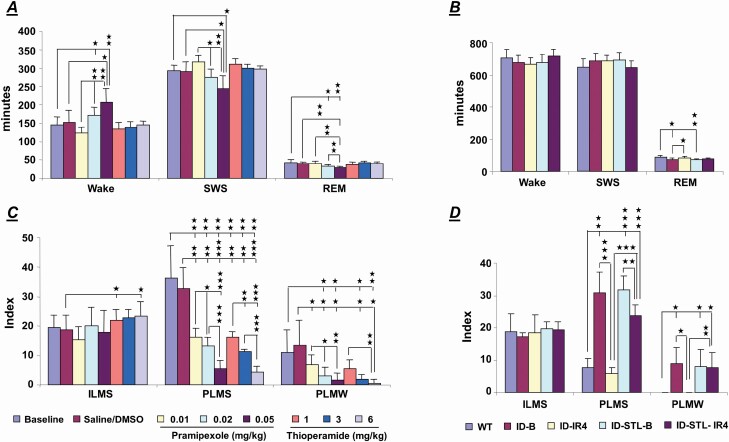
Three doses (0.01, 0.02, and 0.05 mg/kg) of pramipexole were used for the study. Systemic injection of pramipexole into the WT-STL rats produced a dose-dependent increase time in wake (baseline, saline and 0.01, 0.02, and 0.05 mg/kg; F = 11.556, p < .001, df = 4, ANOVA), and a dose-dependent decrease in SWS (baseline, saline and 0.01, 0.02, and 0.05 mg/kg; F = 9.172, p < .001, df = 4, ANOVA) and REM sleep (baseline, saline and 0.01, 0.02, and 0.05 mg/kg: F = 7.947, p = .001, df = 4, ANOVA) in the 8-hour of recording after pramipexole injection. Figure 4A showed a significant increase in wake time (baseline vs. each dose of pramipexole: t-test, df = 5; 0.02 mg/kg: p = .034; 0.05 mg/kg: p = .009), as well as a significant decrease in SWS (baseline vs. each dose of pramipexole: 0.05 mg/kg: p = .015, df = 5, t-test) and REM sleep (baseline vs. each dose of pramipexole: t-test, df = 5; 0.02 mg/kg: p = .035; 0.05 mg/kg: p = .003) during the 8-hour recording after pramipexole injection in the WT-STL rats. Pramipexole injection produced a dose-dependent decrease in PLMS (baseline, saline and 0.01, 0.02, and 0.05 mg/kg: F = 26.658, df = 4, p < .001, ANOVA). A significant decrease in PLMS (baseline vs. each dose of pramipexole: t-test, df = 5; 0.01 mg/kg: p = .009; 0.02 mg/kg: p = .006; 0.05 mg/kg: p = .003; Figure 4C) was found by pramipexole injection in the 8-hour of recording after injection. Pramipexole injections also dose-dependently suppressed PLMW (baseline, saline and 0.01, 0.02, and 0.05 mg/kg: F = 6.285, df = 4, p = .002, ANOVA) in the WT-STL rats. A significant decrease in PLMW (baseline vs. each dose of pramipexole: t-test, df = 5; 0.02 mg/kg: p = .021; 0.05 mg/kg: p = .021; Figure 4C) was found during the 8 hour post-pramipexole injection in the WT-STL rats. On the other hand, isolated leg movement in SWS was not changed by pramipexole injection (baseline, saline and 0.01, 0.02, and 0.05 mg/kg: F = 0.815, df = 4, p = .530, ANOVA, Figure 4C) in the WT-STL rats.
Figure 4.
Effect of pramipexole and thioperamide on sleep–wake pattern (A) and motor activity (C) in the striatal-lesioned wild-type (WT-STL) rats. Data were taken 8 hours after test drug injection. High doses of pramipexole (0.02 mg/kg and 0.05 mg/kg) injection into the WT-STL rats produced an increase in wake and a decrease in slow wave sleep (SWS) and rapid eye movement (REM) sleep (A). Thioperamide injected into the WT-STL rats had no effect on the sleep–wake pattern (A). Motor hyperactivity, PLM in sleep (PLMS) and in quiet wake (PLMW), was suppressed by pramipexole or thioperamide injection into the WT-STL rats (C). Effect of striatal lesions and iron replacement (IR) in the iron-deficient (ID) rat on sleep–wake pattern and motor activity in 24 hour recording. (B) The total time in wake and SWS in 24 hour recording did not differ between wild-type (WT), ID, and striatal-lesioned ID (ID-STL), as well as ID (ID-IR4) and ID-STL (ID-STL-IR4) rats fed standard rodent diet for 4 weeks. The total amount of REM sleep time in ID and ID-STL rats was lower than that in WT rats; however, the lower amount of REM sleep time in ID and ID-STL rats was corrected by iron replacement (IR). (D) Iron replacement decreased PLMS in both ID and ID-STL rats. Iron replacement decreased PLMW in the ID rats, but not in the ID-STL rats. Furthermore, iron replacement fully corrected PLMS and PLMW in the ID rats, but not the ID-STL rats. Indexes of PLMS and PLMW were calculated as the total number of PLM in 8 (B) and 24 (D) hours in sleep and wake divided by the total time in sleep and wake in 8 (B) and 24 (D) hours, respectively. Index of ILMS was calculated as the total number of isolated motor events in 8 (B) and 24 (D) hours divided by the total time in sleep in 8 (B) and 24 (D) hours, respectively. ID-B: baseline of iron-deficient rat, ID-STL-B: baseline of striatal-lesioned iron-deficient rat, W: wake. t-Test, * p < .05, ** p < .01, *** p < .001. Number of animals: wild-type: 6, iron-deficient and striatal-lesioned iron-deficient: 5 each.
Effect of thioperamide on sleep and motor activity in WT-STL rats.
None of the doses (1, 3, and 6 mg/kg) of thioperamide changed the total time in wake or in sleep over the 8 hours recording after injection in the WT-STL rats (Figure 4A). Similar to pramipexole, thioperamide injections produced a dose-dependent decrease in PLMS (baseline, 6% DMSO and 1, 3, and 6 mg/kg: F = 34.107, df = 4, p < .001, ANOVA) in the WT-STL rats. Thioperamide at all doses significantly suppressed PLMS (baseline vs. each dose of thioperamide: t-test, df = 5; 1 mg/kg: p = 0.008; 3 mg/kg: p = .003; 6 mg/kg: p = .001; Figure 4C) in the WT-STL rats. A dose-dependent decrease in PLMW (baseline, 6% DMSO and 1, 3, and 6 mg/kg: F = 10.068, df = 4, p < .001, ANOVA) was also observed by thioperamide injection in the WT-STL rats. Moderate and high doses of thioperamide significantly decreased PLMW (baseline vs. each dose of thioperamide: t-test, df = 5; 3 mg/kg: p = .01; 6 mg/kg: p = .008; Figure 4C) in the WT-STL rats. Thioperamide at moderate dose significantly increased ILMS (baseline vs. 3 mg/kg thioperamide: p = .043, df = 5, t-test, Figure 4C) in the WT-STL rats.
Iron-deficient rat lesion experiments
Sleep–wake pattern, motor activity, and effect of test drugs on sleep and motor activity in ID-STL rats.
The sleep–wake pattern and motor activity over 24-hour did not differ between ID and ID-STL rats (Figure 4B and D).
Systemic injection of either pramipexole (0.05 mg/kg) or thioperamide (6 mg/kg) into the ID-STL rats did not alter time in wake, SWS, and REM sleep during the 8-hour after drug injection. However, systemic injection of pramipexole (baseline vs. pramipexole: t-test, df = 4, p = .002, Table 1) or thioperamide (baseline vs. thioperamide: t-test, df = 4, p = .001, Table 1) decreased PLM in sleep in the ID-STL rats. PLM in wake was also decreased by thioperamide (baseline vs. thioperamide: t-test, df = 4, p = .008, Table 1), but not by pramipexole (baseline vs. pramipexole: t-test, df = 4, p = .403, Table 1) injection in the ID-STL rats.
Table 1.
Comparison of the effect of iron replacement (IR) and drug on motor activity in the striatal-lesioned iron deficient rats1.
| WT | ID-STL | ||||
|---|---|---|---|---|---|
| B | IR4 | TP | PR | ||
| ILMSI | 20.5 ± 5.5 | 19.7 ± 2.56 | 18.1 ± 2.9 | 18.6 ± 4.4 | 21.9 ± 0.9 |
| PLMSI | 6.1 ± 2.8 | 35.5 ± 6.79a | 24.3 ± 4.5b,d | 4 ± 1.6e | 12.4 ± 0.7c,f,h |
| PLMWI | 0 | 11.6 ± 5.05a | 12.7 ± 10.7b | 0.8 ± 1.1e,g | 7.5 ± 7.3 |
1Data (mean ± SD) taken from 8 hour recording after drug injection. B: baseline, ID-STL: striatal-lesioned iron-deficient rat, ILMSI: index of isolated leg movements in sleep, IR4: iron deficient rats fed with standard rodent diet for 4 weeks, PLMSI and PLMWI: index of periodic leg movements (PLM) in sleep (PLMS) and in quiet wake (PLMW), respectively, PR: pramipexole, TP: thioperamide. WT: wild-type rat.
aSignificant difference between WT and ID-STL-B rats.
bSignificant difference between WT and ID-STL-IR4 rats.
cSignificant difference between WT and ID-STL-PR (pramipexole injection) rats.
dSignificant difference between ID-STL-B and ID-STL-IR4 rats.
eSignificant difference between ID-STL-B and ID-STL-TP (thioperamide injection) rats.
fSignificant difference between ID-STL-B and ID-STL-PR rats.
gSignificant difference between ID-STL-IR4 and ID-STL-TP rats.
hSignificant difference between ID-STL-IR4 and ID-STL-PR rats.
Effect of iron replacement (IR) on sleep–wake pattern and motor activity in the ID and ID-STL rats.
Consistent with our previous study [13], the total time in wake and SWS were not significantly changed after the ID rats were fed standard rodent diet for 4 weeks (ID-IR4, Figure 4B), whereas, REM sleep time was increased (ID baseline vs. ID-IR4: t-test, df = 4, p = .01; Figure 4B) in ID-IR4 rats. Motor hyperactivity, PLM in sleep (ID baseline vs. ID-IR4: t-test, df = 4, p < .001; Figure 4D) and PLM in wake (ID baseline vs. ID-IR4: t-test, df = 4, p = .011; Figure 4D), were also significantly decreased in ID-IR4 rats in 24 hour recordings.
Total time in wake, SWS, and REM sleep was not changed after the ID-STL rats were fed with standard rodent diet for 4 weeks (ID-STL-IR4; Figure 4B). Periodic leg movements in sleep (ID-STL baseline vs. ID-STL-IR4: t-test, df = 4, p = .003, Figure 4D), but not PLMW (ID-STL baseline vs. ID-STL-IR4: t-test, df = 4, p = .892, Figure 4D) were improved in ID-STL-IR4 rats.
Comparison of the efficacy of iron replacement on motor activity in the ID rats and in the ID-STL rats in 24 hour recording.
Though PLM in sleep improved with iron replacement in both ID and ID-STL rats, the response of motor activity to iron replacement differed between the ID and ID-STL rats. PLM in sleep in the ID-STL-IR4 rats was higher than that in ID-IR4 rats (ID-IR4 vs. ID-STL-IR4: t-test, df = 8, p < .001; Figure 4D). Furthermore, PLM in sleep in ID rats was completely corrected by iron replacement (WT rats vs. ID-IR4 rats, t-test, df = 9, p = .161; Figure 4D). In contrast, PLM in sleep in the ID-STL rats was not corrected after the ID-STL rats were fed the standard rodent diet for 4 weeks (WT rats vs. ID-STL-IR4 rats, t-test, df = 9, p < .001; Figure 4D). Similarly, PLM in quiet wake was also significantly higher in ID-STL-IR4 rats than that in ID-IR4 rats (ID-IR4 vs. ID-STL-IR4: t-test, df = 8, p = .003; Figure 4D). PLM in quiet wake in the ID rats (Figure 4D), but not in the ID-STL rats was reversed by iron replacement (WT rats vs. ID-STL-IR4 rats, t-test, df = 9, p = .019; Figure 4D).
Comparison of the efficacy of iron replacement and drug treatment in the ID-STL rats.
PLMS and PLMW were analyzed in WT rats, ID-STL-IR4, and pramipexole/thioperamide injection in the ID-STL rats during 8 hour recording after drug injection, to compare the efficacy of iron replacement and drug treatment on motor activity. Both PLMS (WT vs. ID-STL-IR4 rats; t-test, df = 6.618, p < .001; Table 1) and PLMW (WT vs. ID-STL-IR4 rats; t-test, df = 4, p = .015; Table 1) were higher in the ID-STL-IR4 rats than in the WT rats, indicating PLMS and PLMW were not corrected by iron replacement in the ID-STL rats. On the other hand, PLMS (WT vs. ID-STL-thioperamide; t-test, df = 9, p = .146; Table 1) and PLMW (WT vs. ID-STL-thioperamide; t-test, df = 4, p = .179; Table 1) did not differ between WT and thioperamide treated ID-STL rats. Pramipexole injection into the ID-STL rats completely corrected PLMW (WT rats vs. ID-STL-pramipexole rats; t-test, df = 4, p = .085, Table 1), but not PLMS (WT rats vs. ID-STL-pramipexole rats; t-test, df = 5.857, p = .001, Table 1).
Discussion
We came to five conclusions. First, lesion of the striatum in the wild-type rat generated RLS-like activity. Second, striatal lesion induced RLS-like activity in the WT rat can be improved by systemic administration of pramipexole or thioperamide. Third, iron therapy, which corrects RLS-like activity in the ID rat, failed to reverse motor symptoms of the striatal-lesioned ID rat. Fourth, thioperamide was effective for the treatment of motor symptoms in ID rats with striatal lesion. Finally, pramipexole had a partial effect on motor activity in the striatal-lesioned ID rat.
The total wake and SWS time in 24 hours in the WT-STL rats did not differ from that in the WT rats, but there was a decrease in REM sleep in the WT-STL rats. A decrease in REM sleep was reported in RLS patients [1]. The sleep–wake pattern in the WT-STL rats was similar to that seen in the ID rat [13]. EEG power analysis showed that delta power in SWS in the WT-STL rats does not significantly change, compared with the WT rats. An increase in delta power [20] or no change in delta power [21] was reported in RLS patients. Although the total SWS time in WT-STL rats did not differ from the WT rats, an increase in SWS time during the last 4 hours of dark phase was found in the WT-STL rats. This suggested that the WT-STL rats may experience an increase in daytime sleepiness. Furthermore, the daily average duration of SWS was shorter in the WT-STL rats than that in WT rats indicating that WT-STL rats show sleep fragmentation. We showed in the present study that PLMS index is high in the first 4 hours of light phase in the WT-STL rats. PLM in sleep was maximal during the first half of the night sleep in RLS patients [22]. Thus, the sleep pattern and motor hyperactivity in quiet wake and in sleep seen in WT-STL rats resemble symptoms in RLS patients. Our present study showed that a unilateral lesion of the striatum generated RLS-like activity. This result is consistent with the clinical finding [1, 17] that dysfunction of just one side of the striatum is capable of generating RLS.
RLS-like activity induced by lesions of the striatum, as seen in the present study, may result from an alteration of neurotransmission. Using cell type-specific deletion of the striatal signaling protein dopamine- and cAMP-regulated phosphoprotein Mr 32 kDa (DARPP-32), Bateup et al. [23] demonstrated that mice with a loss of DARPP-32 in striatopallidal neurons, which contain the dopamine D2 receptor, show a robust increase in locomotor activity. Striatopallidal neurodegeneration causes pallidal hyperactivity, and consequently increases GABA release onto the SNR, decreases SNR neuronal activity, and causes insomnia and motor hyperactivity [5]. Sperk et al. [24] reported that unilateral lesion of the striatum, induced by injection of kainic acid, increases histamine release. Our previous study [14] discussed the hypothetical role of the striatal histamine H3 receptor in the modulation of motor activity. The histamine H3 receptor in the striatum has been shown to be present both pre- and postsynaptically. The presynaptic histamine H3 receptor not only suppresses neurotransmitter release [25] but also forms heteromers with the adenosine A2a receptor (A2aR) [26]. Activation of the postsynaptic histamine H3 receptor decreases the affinity of postsynaptic dopamine D2 receptor for its agonists in the striatum [27]. We hypothesize that the increased histamine release induced by striatal lesions overstimulates striatal presynaptic and postsynaptic histamine H3 receptor activity, which in turn increases A2aR activity and decreases dopamine D2 receptor activity. Thus, application of the histamine H3 receptor antagonist, thioperamide, restores the function of A2aR and dopamine D2 receptor and reverses motor hyperactivity in the WT-STL rat.
Pramipexole was also shown to be effective in the treatment of RLS-like activity in the WT-STL rat. The effect of pramipexole on RLS-like activity in the WT-STL rat may be via its action on the striatum and spinal cord. First, the decreased dopamine D2 receptor in the striatum reported in RLS patients is presynaptic [3]. On the other hand, neurotoxic lesion damages to the neurons but not the nerve fibers innervating the striatum. Second, pramipexole may exert its effect on the spinal cord. The density of dopamine D2 and D3 receptors is very high in the dorsal horn [28], which contains second order neurons of the somato-sensory system.
The major finding in this study was that motor hyperactivity in quiet wake and in sleep seen in the ID-STL rat is not corrected by iron replacement. Clinical studies revealed that 30%–50% RLS patients do not respond to iron therapy [9–12]. Trotti and Becker [29] searched clinical studies on iron treatment in adult RLS patients, and found that iron treatment does not improve PLM in sleep. Simakajornboon et al. [30] showed that children who had PLMSI at 27.6 per hour on average, in 6 out of 25 patients, had no response to iron therapy. The mechanisms underlying the lack of response to iron therapy in RLS patients and ID-STL rats are not clear. We have shown that high striatal levels of dopamine transporter and histamine H3 receptor in ID rats, who were fed a low iron diet at weaning can be corrected by iron replacement [13, 14]. Ward et al. [31] also reported that iron replacement reverses abnormal striatal metabolome observed in the ID rats, who were fed a low iron diet at weaning. However, some abnormalities of neural structures induced by iron deficiency during gestation and/or before weaning, may not be correctable by iron supplementation. Connor et al. [4] reported that substantia nigra levels of tyrosine hydroxylase and phosphorylated tyrosine hydroxylase remain high in ID rats, who are iron deficient during gestation, after the animals fed with iron sufficient diet. Ben-Shachar et al. [16] showed that the effect of iron supplementation on the neural abnormality induced by iron deficiency depends on the age, at which iron deprivation occurred. They found that striatal dopamine D2 receptor density can be restored by iron replacement in ID rats fed with low iron diet at weanling [16]. In contrast, striatal dopamine D2 receptor density did not normalize with iron replacement when the animals were exposed to a low iron diet starting at 10 days of age [16]. A decrease in striatal dopamine D2 receptor has been reported in RLS patients [2–4]. Thus, it is likely that RLS patients who do not respond or partially respond to iron therapy may have an iron deficiency early in life, such as during gestation. The motor disorder that was not reversed by iron therapy in our ID-STL rats may result from striatum lesions that increase histamine release in the striatum.
In conclusion, wild-type rats with striatal lesions show a decrease in REM sleep, an increase in PLM in sleep, generate PLM in quiet wake, and have a positive response to pramipexole or thioperamide treatment. Thus, striatal-lesioned wild-type rats can serve as an animal model of RLS. Though RLS-like activities in the striatal-lesioned ID rats cannot be fully corrected by iron replacement, they can be reversed by pramipexole or thioperamide injection.
Contributor Information
Yuan-Yang Lai, Department of Psychiatry & Biobehavioral Sciences, David Geffen School of Medicine, University of California, Los Angeles, CA, USA; VA Greater Los Angeles HealthCare System, Sepulveda, 16111 Plummer Street, North Hills, CA, USA; Greater Los Angeles Veterans Research and Education Foundation, 11301 Wilshire Blvd, Los Angeles, CA, USA.
Kung-Chiao Hsieh, VA Greater Los Angeles HealthCare System, Sepulveda, 16111 Plummer Street, North Hills, CA, USA.
Keng-Tee Chew, VA Greater Los Angeles HealthCare System, Sepulveda, 16111 Plummer Street, North Hills, CA, USA.
Darian Nguyen, Greater Los Angeles Veterans Research and Education Foundation, 11301 Wilshire Blvd, Los Angeles, CA, USA.
Jerome M Siegel, Department of Psychiatry & Biobehavioral Sciences, David Geffen School of Medicine, University of California, Los Angeles, CA, USA; VA Greater Los Angeles HealthCare System, Sepulveda, 16111 Plummer Street, North Hills, CA, USA; Brain Research Institute, University of California, Los Angeles, CA, USA.
Funding
This work is supported by the National Institute of Health, National Institute of Neurological Disorders and Stroke, NS082242 (YYL) and National Institute on Drug Abuse, DA034748 (JMS), and the Medical Research Service of the Department of Veterans Affairs (JMS).
Disclosure Statement
None declared.
References
- 1. Joo EY, et al. . Reduced cerebral blood perfusion of putamen and insular cortex in patients with idiopathic restless legs syndrome. J Korea Sleep Res Soc. 2012;9(1):10–14. doi: 10.13078/jksrs.12003. [DOI] [Google Scholar]
- 2. Earley CJ, et al. . Increased synaptic dopamine in the putamen in restless legs syndrome. Sleep 2013;36(1):51–57. doi: 10.5665/sleep.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Michaud M, et al. . SPECT imaging of striatal pre- and postsynaptic dopaminergic status in restless legs syndrome with periodic leg movements in sleep. J Neurol. 2002;249(2):164–170. doi: 10.1007/pl00007859. [DOI] [PubMed] [Google Scholar]
- 4. Connor JR, et al. . Altered dopaminergic profile in the putamen and substantia nigra in restless leg syndrome. Brain 2009;132(9):2403–2412. doi: 10.1093/brain/awp125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lai YY, et al. . Substantia nigra pars reticulata-mediated sleep and motor activity regulation. Sleep 2021;44(1):1–8. doi: 10.1093/sleep/zsaa151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li X, et al. . Brain iron deficiency in idiopathic restless legs syndrome measured by quantitative magnetic susceptibility at 7 tesla. Sleep Med. 2016;22:75–82. doi: 10.1016/j.sleep.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mizuno S, et al. . CSF iron, ferritin and transferrin levels in restless legs syndrome. J Sleep Res. 2005;14(1):43–47. doi: 10.1111/j.1365-2869.2004.00403.x. [DOI] [PubMed] [Google Scholar]
- 8. Rizzo G, et al. . Low brain iron content in idiopathic restless legs syndrome patients detected by phase imaging. Move Disord. 2013;28(13):1886–1890. doi: 10.1002/mds.25576. [DOI] [PubMed] [Google Scholar]
- 9. Allen RP, et al. . Clinical efficacy and safety of IV ferric carboxymaltose (FCM) treatment of RLS: a multi-centred, placebo-controlled preliminary clinical trial. Sleep Med. 2011;12(9):906–913. doi: 10.1016/j.sleep.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 10. Cho YW, et al. . Lower molecular weight intravenous iron dextran for restless legs syndrome. Sleep Med. 2013;14(3):274–277. doi: 10.1016/j.sleep.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 11. Lee CS, et al. . Comparison of the efficacies of oral iron and pramipexole for the treatment of restless legs syndrome patients with low serum ferritin. Eur J Neurol. 2014;21(2):260–266. doi: 10.1111/ene.12286. [DOI] [PubMed] [Google Scholar]
- 12. Park HR, et al. . Allen RP. Patient characteristics predicting responses to intravenous ferric carboxymaltose treatment of restless legs syndrome. Sleep Med. 2020;75(11):81–87. doi: 10.1016/j.sleep.2020.02.027. [DOI] [PubMed] [Google Scholar]
- 13. Lai YY, et al. . Motor hyperactivity of the iron deficient rat—an animal model of restless legs syndrome. Move Disord. 2017;32(12):1687–1693. doi: 10.1002/mds.27133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lai YY, et al. . Striatal histamine mechanism in the pathogenesis of restless legs syndrome. Sleep 2020;43(2):1–9. doi: 10.1093/sleep/zsz223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dallman PR, et al. . Brain iron: persistent deficiency following short-term iron deprivation in the young rat. Br J Haemotol. 1975;31:209–215. doi: 10.1111/j.1365-2141.1975.tb00851.x. [DOI] [PubMed] [Google Scholar]
- 16. Ben-Shachar D, et al. . Long-term consequence of early iron-deficiency on dopaminergic neurotransmission in rats. Int J Devl Neurosci. 1986;4(1):81–88. doi: 10.1016/0736-5748(86)90019-5. [DOI] [PubMed] [Google Scholar]
- 17. Lee SJ, et al. . Poststroke restless legs syndrome and lesion location: anatomical considerations. Move Disord. 2009;24(1):77–84. doi: 10.1002/mds.22303. [DOI] [PubMed] [Google Scholar]
- 18. Nambu A. Somatotopic organization of the primate basal ganglia. Front Neuroanat. 2011;5:1–9. doi: 10.3389/fnana.2011.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lai YY, et al. . Reply: The iron-deficient rat as a model of restless legs syndrome: was anything lost in translation? Move Disord. 2018;33(1):182–183. doi: 10.1002/mds.27263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Choi JW, et al. . Abnormal sleep delta rhythm and interregional phase synchrony in patients with restless legs syndrome and their reversal by dopamine agonist treatment. J Clin Neurol. 2017;13(4):340–350. doi: 10.3988/jcn.2017.13.4.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hornyak M, et al. . Spectral analysis of sleep EEG in patients with restless legs syndrome. Clin Neurophysiol. 2005;116:1265–1272. doi: 10.1016/j.clinph.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 22. Hening W. The clinical neurophysiology of the restless legs syndrome and periodic leg movements. Part I: diagnosis, assessment, and characterization. Clin Neurophysiol. 2004;115(9):1965–1974. doi: 10.1016/j.clinph.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 23. Bateup HS, et al. . Distinct subclasses of medium spiny neurons differentially regulate striatal motor behaviors. PNAS 2010;107(33):14845–14850. doi: 10.1073/pnas.1009874107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sperk G, et al. . Changes in histamine in the rat striatum following local injection of kainic acid. Neuroscience. 1981;12(6):2669–2675. doi: 10.1016/0306-4522(81)90111-1. [DOI] [PubMed] [Google Scholar]
- 25. Molina-Hernandez A, et al. . Histamine H3 receptor activation inhibits glutamate release from rat striatal synaptosomes. Neuropharmacology. 2001;41(8):928–934. doi: 10.1016/s0028-3908(01)00144-7. [DOI] [PubMed] [Google Scholar]
- 26. Marquez-Gomez R, et al. . Functional histamine H3 and adenosine A2A receptor heteromers in recombinant cells and rat striatum. Pharmacol Res. 2018;129:515–525. doi: 10.1016/j.phrs.2017.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ferrada C, et al. . Interactions between histamine H3 and dopamine D2 receptors and the implications for striatal function. Neuropharmacology. 2008;55(2):190–197. doi: 10.1016/j.neuropharm.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kaur J, et al. . Spinal cord dopamine D2/D3 receptors: in vivo and ex vivo imaging in the rat using 18F/11C-fallypride. Nucl Med Biol. 2014;41(10):841–847. doi: 10.1016/j.nucmedbio.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Trotti LM, Becker LA. Iron for the treatment of restless legs syndrome. Cochrane Database Syst Rev. 2019;1. Art. No.: CD007834,1–52. doi: 10.1002/14651858.CD007834.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Simakajornboon N, et al. . Periodic limb movements in sleep and iron status in children. Sleep 2003;26(6):735–738. doi: 10.1093/sleep/26.6.735 [DOI] [PubMed] [Google Scholar]
- 31. Ward KL, et al. . Gestational and lactational iron deficiency alters the developing striatal metabolome and associated behaviors in young rats. J Nutrition 2007;137(4):1043–1049. doi: 10.1093/jn/137.4.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]