Abstract
The bone marrow-derived multipotent mesenchymal cells (MSCs) have captured scientific interest due to their multi-purpose features and clinical applications. The operational dimension of MSCs is not limited to the bone marrow reservoir, which exerts bone-building and niche anabolic tasks; they also meet the needs of quenching inflammation and restoring inflamed tissues. Thus, the range of MSC activities extends to conditions such as neurodegenerative diseases, immune disorders and various forms of osteopenia. Steering these cells towards becoming an effective therapeutic tool has become mandatory. Many laboratories have employed distinct strategies to improve the plasticity and secretome of MSCs. We aimed to present how photobiomodulation therapy (PBM-t) can manipulate MSCs to render them an extraordinary anti-inflammatory and osteogenic instrument. Moreover, we discuss the outcomes of different PBM-t protocols on MSCs, concluding with some perplexities and complexities of PBM-t in vivo but encouraging and feasible in vitro solutions.
Keywords: Bone marrow mesenchymal cells, light therapy, low-level laser therapy, mesenchymal stem cell
Introduction
Cell-based engineering is continuously gaining ground in basic and clinical research due to the multiple actions that cells can exert against inflammation and tissue degeneration. In this regard, autologous, allogeneic or xenogeneic natural biomaterials and synthetic tissue-specific graft devices, usually combined with multipotent stem cells, have been employed for the treatment of inflammation-based diseases comprising various forms of osteopenia and osteoporosis.1 –3 There is a great need for efficient anti-inflammatory/anti-osteoporotic treatment development, considering that by 2050, the medical costs of osteoporosis shall reach 20 billion dollars annually and the number of hip fractures worldwide will increase to 4.5 million cases. 4
Currently, bone marrow-derived multipotent mesenchymal cells (MSCs) are considered a well-established and effective operational tool in regenerative medicine. Infused MSCs can reach the injured or inflamed areas, exerting immunomodulatory effects and enhancing tissue healing. The trump card of these multipotent cells is the nature of the biofactors released and the possibility to influence and manipulate their secretome quantity and quality. 5 Increasing evidence has implicated the importance of mitochondrial activities on this stem cell activation, their cell fate and the defence against senescence, suggesting its target role for future therapies. 6
Photobiomodulation therapy (PBM-t) has been indicated as very promising support for MSC handling, able to enhance the young MSC secretome or rejuvenate aged MSCs.7,8 Moreover, PBM-t can interact with cellular photoacceptors in the mitochondria via different light parameters and modulate cell metabolism. 9 In this way, PBM-t may modify differently the MSC bias, prompting these cells towards a complete homeostatic reorganisation. 10 The outcomes of MSC manipulation via PBM meet the needs of functional anti-inflammatory drugs and therapeutic platforms for inflammatory-based and aged diseases such as – but not only – osteoporosis and osteoarthritis.
This report aims to provide up-to-date knowledge regarding MSC efficacy and skillfulness after light irradiation. We wonder whether it is time to consider PBM as an optimal instrument for successful MSC-based therapy.
MSCs physiology and therapeutical features at a glance
The diversity and physiological and clinical features of MSCs have gradually caught the attention of modern research. In the last two decades, a debate has flared regarding the stemness, secretome and functional behaviour of these multi-skilled fibroblast-like cells. MSCs are a source of interest due to their ability to self-renew, giving rise to three distinct progenies – osteoblast, chondrocyte and adipocyte – and they can adhere to plastic when cultured. 11 Besides, MSCs express specific cell surface markers, comprising the cluster of differentiation (CD) 73, CD90 and CD105 while lacking CD11b, CD14, CD19, CD34, CD45, CD79a and major histocompatibility complex class II.1,12
Current workshops on MSC nomenclature overseen by the International Society for Cellular Therapy (ISCT) have disclosed that the term ‘mesenchymal stem cell’ demarcates a cohort of bone marrow-derived stem cells capable of multilineage-differentiation and self-renewal. Conversely, the moniker ‘mesenchymal stromal cells’ designates bulk unfractionated populations with plastic-adherent properties, employed mainly in immunomodulatory and tissue regeneration studies or clinical trials. These mesenchymal stromal cell cohorts comprise fibroblasts, myofibroblasts and a distinct pool of stem/progenitor cells, excluding haematopoietic and endothelial cells.13,14
A major feature of MSCs is their ability to act locally, regulating bone homeostasis and intramural inflammation, or to act systemically through chemotaxis to reach inflamed hotbeds and quench pro-inflammatory T cells and B cells and natural killer (NK) cell signals.15,16 The plasticity, the secretome content and the ‘hit and run’ mode of action have framed MSCs as an extraordinary immunomodulatory and tissue trophic tool.
The multidimensional nature of MSCs has laid the groundwork for their use in a vast range of clinical applications. Researchers have employed MSC-based therapeutic protocols for neurodegenerative diseases such as amyotrophic lateral sclerosis, Alzheimer’s disease and Parkinson’s disease17,18; immune disorders including rheumatoid arthritis and type I diabetes 19 ; and bone diseases.16,20 –22 Notably, there are more than 1000 phases I/II clinical trials and 115 phases III/IV clinical trials that have been completed or are ongoing, indicating that MSCs could be used clinically against a series of inflammation-based or metabolic disorders. 5
MSC-based therapy is contingent upon their ability to migrate and adhere to the target tissue and to exert their regulatory/anabolic effects. 23 The paracrine, immunomodulatory and regenerative properties of MSCs may be manipulated before administration. An important criterion to consider is that the functional commitment of MSCs depends on the donor age and clinical status as well as the in vitro protocols for MSC expansion.24,25 Indeed, the MSCs pool within the bone marrow is very low (0.0017%–0.0201% of nucleated cells or 0.42% of plastic-adherent cells according to other studies),26,27 although MSCs can be amplified 500-fold in culture. The limitations of the MSC expansion methods are the excessive number of passages required for a sufficient cell number (50 passages are necessary for the 500-fold increase). 28 A high number of in vitro passages introduce genetic instability such as DNA damage accumulation and transformation concerns. 29 Accordingly, long-term replated MSCs (passage 25 has been considered critical) show lower proliferative and differentiation potential along with disrupted homing and cytokine/chemokine release capacity.30,31 Besides, MSCs derived from aged mice exhibit increased DNA damage checkpoint and cellular senescence markers, rendering autologous transplantation a difficult clinical obstacle to overcome. 32 Aged MSCs are also characterised by a compromised osteogenic schedule due to their reduced differentiation capacity towards bone cells. 33 Thus, refining MSCs and initiating them towards a more efficient homeostatic bias appears to be mandatory. Invigorating and ‘pledging’ MSCs to obtain a desirable cell pool for healing/regenerative purposes has become an exciting procedural conundrum that investigators have worked to solve. Moreover, weakened pluripotent MSCs from aged or non-healthy individuals could be ‘reformatted’ and rejuvenated via various strategies, which amend the mechanistic features and operational signature of these cells. MSCs derived from healthy donors may be preconditioned and directed towards a more effective operational schema.
The operational agenda of MSCs
MSCs receive accurate micro-environmental stimuli and undertake a distinctive bias to carry out their homeostatic agenda. This remains possible because they express many receptors on their surface and secrete numerous bioactive molecules. Thus, the receptor-ligand affinities and secretome characteristics determine the existential and operational fate of MSCs, such as niche-forming/homing ability, sub-population maintenance, trilineage differentiation and egression.16,20 For example, adhesion mediators like β1 integrin, α1α6 integrin, CD44 and members of the vascular cell adhesion molecule (VCAM) family have been found on MSCs, managing their homing or migratory process.23,34 Likewise, CC chemokine receptor (CCR) and C-X-C chemokine receptors (CCXR) families are involved in MSC chemotaxis and migration. 35 MSCs can also secrete angiogenic, differentiating, chemoattractant, immunomodulatory and pro-survival/autophagy-related mediators, which can act via an autocrine and/or paracrine manner.16,20,36 Consistently, ligands including chemokines, cytokines and growth factors released by bone marrow progenitor/mature cells or endothelial cells orchestrate MSC homing, chemotaxis, migration and adhesion within medullary stroma or in target inflamed tissues.16,20,23 Specifically, pro-inflammatory interleukins (IL) such as IL-1β, IL-6 and IL-8 and growth factors such as vascular endothelial-derived growth factor-A (VEGF-A), fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), transforming growth factor-β1 (TGF-β1), but also monocyte chemoattractant protein-1 (MCP-1) and macrophage inflammatory protein-1a (MIP-1a), enhance MSC chemotaxis and direct these cells to the ‘burning’ areas.23,37,38 Inflammatory cytokines, predominantly IL-1β and tumour necrosis factor-α (TNF-α), promote MSCs to release proteolytic matrix metalloproteinases (MMPs). These enzymes can modify the extracellular matrix, permitting MSC egression and migration. 39 Hence, MMPs have been widely recognised as the operational fate inducers of MSCs. 40 Overall, MSCs can secrete bioactive factors, the amount of which could significantly alter the tissue homeostatic path in steady-state or pathological scenarios.
A substantial area of interest focuses on the following question: how can we influence MSCs to render them even more effective anabolic platforms? In response, current research involves considerable efforts to delve deeper into the unspecified facets of MSCs manipulation.
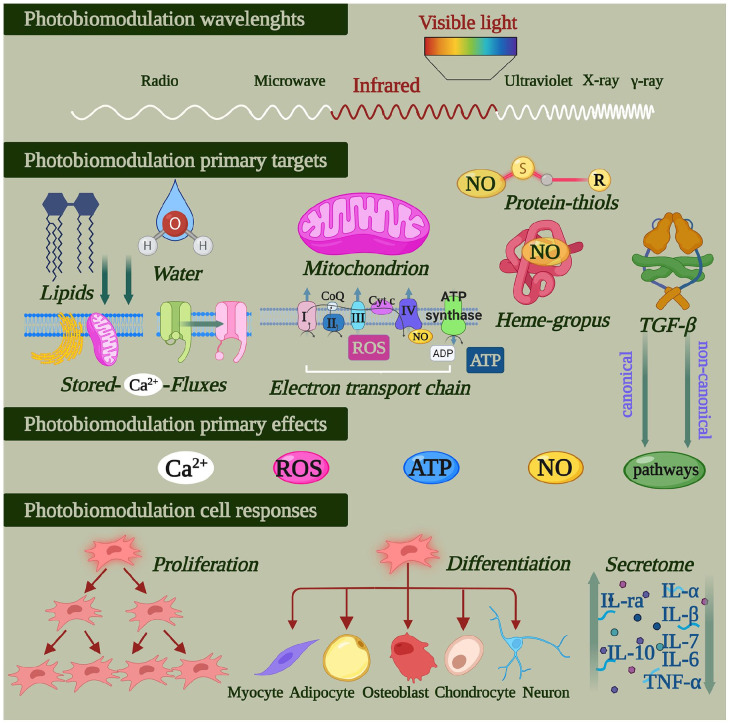
MSC handling for therapeutic purposes represents a promising area in tissue engineering and regenerative medicine. 41 The effectiveness of this procedure is being refined and improved continuously via diverse experimental protocols.42,43 Current research has highlighted methods to prevent MSC apoptosis; to improve their survival, adhesion and homing abilities; and alter the cytokines/chemokines, microRNA (miRNA) and other homeostatic mediators that they secrete.16,44,45 Until now, different approaches have been employed to precondition these multipotent cells: three-dimensional (3D) cell culture techniques, growth factors or transfection with specific DNA plasmid(s) and cytokine/chemokine pre-treatments showed promising results.16,36,44,46 Additionally, the miRNAs’ regulatory effects on post-transcriptional gene expression and the proper stem cell maintenance and function are known, 47 as also the instability that limits their employment. 48 Therefore, innovative approaches through therapeutic delivery vehicles and nanostructures were designed to preserve miRNAs and offer gene expression modulatory therapies for various biomedical applications.48 –50 Rahman et al. 51 suggest that the application of biomaterial nanopatterning and controlled-release morphogens could enhance MSC differentiation towards an osteogenic bias. Hence, ‘training’ MSCs towards a specific functional identity, improving their intramural achievements or strengthening the MSC transplantation protocols have become imperative. Recently, a novel approach was proposed to improve the effectiveness of MSCs through low-level laser [light] therapy parameters. The medical applications known as PBM-t, have increased dramatically in the last 10 years, and there have been promising clinical outcomes to standardise the administration of the therapy.16,52 –57 PBM can stimulate or overwhelm essential cellular homeostatic features such as viability, proliferation or differentiation depending on the irradiation parameters, namely the wavelength, power, the time and the number of applications, the fluence as well as the mode/device applied. 9 The physical photon stimulus on MSCs alters signalling cascades, which prompt the release of cytokines/chemokines, growth factors and other mediators (Figure 1). These molecules may orchestrate the functional MSC agenda and improve its tissue-anabolic and anti-inflammatory tasks. 58 The following sub-sections aim to elucidate the PBM mode of action at the cellular level, the recent findings of PBM on the MSCs idiosyncrasies and the new opportunities for PBM-treated MSC therapeutic applications.
Figure 1.
Photobiomodulation concerns the transformation of photophysical energy contained in visible and (near)-infrared light into chemical energy. It activates the primordial photoacceptive properties of some molecules naturally involved in the metabolism and physiology of animal/human cells. When activated, these primary light targets influence cell homeostasis. Photobiomodulation can moderate a cell’s responses and influence mesenchymal/stromal cell proliferation, differentiation and its secretome (figure created with BioRender.com).
Influencing the fate of multipotent mesenchymal cells through PBM-t
PBM: Evolutionary and molecular notes
PBM-t works because visible and (near-)infrared light can modulate cell metabolism and homeostasis without causing significant thermal increases. Indeed, photons at specific non-ionising wavelengths can transfer their energy to molecules in a cell.52,58 Although some protozoa as well as animals and humans do not use light as an energy source, many molecules involved in their physiology have retained their primordial photoacceptive properties. 40 Researchers have described that during evolution, minerals such as iron, copper and sulphur may have been energised by radiation from the sun during the Hadean aeon of Earth. The chemical evolution and increasing complexity of the molecules that followed would have led to the emergence of flavins, pigments, pteridines, nitroso-proteins, cytochromes, haem-containing proteins and porphyrins and proto-chlorophylls. 59 These molecules inherited the ability to interact with light and spontaneously aggregated through fatty acids in microspheres. So, many molecules became photoacceptors expressed on the membranes of the first proto-cells and prokaryotes. Subsequently, according to the cell evolution theory that eukaryotes evolved through symbiosis with prokaryotes, these photoacceptors became parts of components of the mitochondrial electron transport chain and cellular pathways. Therefore, metals and molecules able to be energised by photons have been transmitted through evolution from the origin of life to the primordial broth to prokaryotic and eukaryotic cells. This phenomenon supports the use of PBM-t in the management of human and animal cell metabolism and physiology. 54
To ensure that PBM-t acts appropriately, the principle of photochemical activation (Grotthuss–Draper law) should be respected. Basically, during irradiation, only light absorbed by a system can bring about a photochemical change. Hence, Rieske proteins, 2Fe-2S clusters, haem moieties, copper centres, flavin and cytochromes 60 in mitochondria are considered the lead actors in photon-energy transfer to eukaryotic non-plant cells. 61 More precisely, we have described that 808-nm 63 and 980-nm 64 diode laser light can affect the mitochondrial respiratory chain and adenosine triphosphate (ATP) production by modulating complex IV and, in part, complex III. Moreover, 1064-nm light influenced complex I in addition to complexes III and IV. 64 The extrinsic mitochondrial membrane complex II does not respond to photons at these wavelengths.62 –64 However, visible light at 400–500 nm may excite flavoproteins 65 and, therefore, affect cell pigments and the respiratory complexes I and II. Additionally, Pastore et al. 66 demonstrated that complex IV acts as a photoacceptor at 632.8 nm and also has absorption peaks at 450, 620–680 and 760–895 nm.
The cells of an injured site experience high nitric oxide (NO) levels that could competitively inhibit ATP production via the mitochondria oxygen-respiration chain. The light could modulate mitochondrial activity by preventing NO from binding to cytochrome c oxidase (complex IV). 58 In other words, PBM-t could separate NO from complex IV and restore physiological mitochondrial energy metabolism. 67
Besides the mitochondrial respiratory chain complexes, porphyrins, heterocyclic organic compounds complexed into haemoglobin and cytochrome P450 (CYP) enzymes are also photoacceptors. They show an elective affinity to light at 400–420 nm and 450 nm. 58 Haem-, thiol- and di-nitrosyl iron-containing proteins, which form complexes with NO (i.e., NO-haemoglobin and S-nitrosothiols), may be modulated by a wide range of visible and near-infrared wavelengths. 55
Maximum absorption spectra of opsin (Opn) 3 and Opn 4 at wavelengths from 460 to 500 nm and from 420 to 480 nm, respectively have also been described, and blue light is considered responsible for photo-vasorelaxation in rat pulmonary arteries via the Opn pathway. 68 Additionally, photoactivation of the latent TGF-ß1 isoform, but not TGF-ß2 or TGF-ß3, has been described after irradiation with an 810-nm laser diode system; it occurred via a specific methionine (position 253 on TGF-ß1). 69
Lastly, near-infrared light seems to excite lipids, which show a mild but significant absorption peak in the range of 900–1000 nm, 70 and water, which affects calcium ion (Ca2+) channels and stores.61,71
These light–cell interactions (Figure 1), which represent the primary targets of PBM-t, are followed by the endogenous generation of NO and reactive oxygen species (ROS), ATP production, modulation of redox and Ca2+ homeostasis and the release of secondary messengers. These changes pave the way for secondary effects that modulate the cell life cycle.52,58 Therefore, considering the current scientific and technical knowledge, visible and (near-)infrared PBM-t could be considered a supportive therapy for the treatments of tissue dysfunctions and damage in many medical52 –59 and veterinary areas. 72
Possible PBM targets and pathways in multipotent mesenchymal cells
To better understand the supportive role of PBM-t in ‘stem-cell therapy’, the key role of the light targets in the metabolism and physiology of MSCs needs to be elucidated. Mitochondria are the site of many metabolic reactions in a cell. Apart from bioenergetic management, mitochondria are a major source of endogenous ROS as a consequence of electron transport chain activities. MSCs show higher lactate production rates as a consequence of using glycolysis to generate energy. 73 However, during cell differentiation, there are drastic metabolic changes. In particular, Chen et al. 74 revealed that during osteogenic differentiation, the levels of mitochondrial DNA (mtDNA), respiratory enzymes, messenger RNA (mRNA) associated with mitochondrial biogenesis, intracellular ATP and antioxidant enzymes as well as the oxygen consumption rate increase, while the level of intracellular ROS declines. The initiation of the functional decline of bone marrow-derived MSCs in ageing is supported by compromised mitochondrial metabolism due to telomere attrition. 75 Additionally, lower levels of biogenesis and a higher basal rate of mitochondrial degradation have been found in MSCs from patients with atypical Parkison’s disease; these changes impaired their differentiation. 76 So, MSCs and their mitochondrial fate are correlated through ‘thick and thin, whatever happens’.
In terms of NO, researchers have demonstrated that NO-synthase inhibition reduced the osteogenic differentiation of MSCs from normal rats, indicating the important role of NO in inducing MSC to differentiate into osteoprogenitor cells. 77 Besides, NO effectively augmented the ability of MSCs to produce regenerative, immunomodulatory and trafficking molecules responsible for their paracrine effect of tissue repair. 78 Mujoo et al. 79 concluded that ‘the effects of the NO-cGMP signalling pathway most likely involve temporal, compartmental/spatial and localised concentration-dependent autocrine and paracrine responses able to modulate MSC behaviours’.
Similarly, to mature cells, Ca2+ homeostasis affects the growth and osteogenic differentiation of MSCs. Consistently, there was increased proliferation and differentiation in cells exposed to 1.8 mM Ca2+, but this treatment did not stimulate cell growth, inhibited differentiation and enhanced cell mineralisation. 79 Other authors have observed that dynamic changes in Ca2+ concentrations in bone remodelling surfaces could modulate MSC activities to stimulate bone regeneration. 80 Moreover, it has been reported that Ca2+ concentration oscillations serve as a bidirectional signal during MSC differentiation. Non-invasive electrical stimulation facilitated osteodifferentiation via Ca2+-dependent pathways. 81
On the other hand, these cell signals play a dual role in cellular physiology and are strictly connected and can influence PBM-t, although the effect is not predictable. 82 For example, TGF-β induces MSC differentiation, 83 but it can induce senescence through mitochondrial ROS production. 84 Other reports have indicated that an ATP autocrine/paracrine signalling pathway is involved in the Ca2+ oscillations, followed by rearrangements of undifferentiated MSCs. 85 Overall, these molecules affect and modulate the redox environment in stem cells, suggesting that mitochondria also influence differentiation. 86 Moreover, mitochondria from MSCs could be transferred to diseased or damaged cells to replace dysfunctional mitochondria.87,88
PBM-t switches the commitment of MSC differentiation
MSCs have been employed in several experimental and clinical protocols for bone regeneration – for example, in periodontology and implantology – while MSC-based therapy is currently applied in oro-maxillofacial reconstruction.89,90 It has been well established that low-intensity light in the visible and near-infrared range can stimulate cellular activities and, specifically, enhance MSC viability, migration, homing and secretome release after transplantation.91,92 Moreover, the efficacy of a laser-based therapy depends on the accuracy of each PBM operating parameter.7,93,94 Investigation of these parameters has paved the way for the development of improved laser treatments of MSCs.
Table 1 shortly reports data relative to the photobiomodulation switch-effect on MSCs’ commitment towards differentiation.
Table 1.
Photobiomodulation switches the commitment of mesenchymal stem/stromal cells differentiation; selected articles.
| Authors | Model/control | Laser/probe | Parameters | Therapy | Effects |
|---|---|---|---|---|---|
| Merigo et al. 95 | In vitro: mBMSCs from 10-week-old C57BL/6 mice long bones. | 532-nm KTiOPO4 laser | 0.78 W, 13 s, 2.4 cm2, 4 J/cm2; CW | Irradiation: every other day | Stimulation of osteoblast differentiation pathway: upregulation of Bsp2 and Bmp2; increase of mm |
| Control: not receive laser irradiation | Standard hand-piece | Treatment duration: 1–2–3–4 weeks | |||
| Eroglu et al. 8 | In vitro: mBMSCs from young (3-month-old) and aged (24-month-old) C57BL/6 mice long bones | 808-nm diode laser | 0.0167–0.0250–0.0333 W/cm2, 180–160 s, 3.0–4.5–6.0 J/cm2; CW | Irradiation: daily | PBM at 3 J/cm2 (3 days) rejuvenates aged mMSC: downregulation of p21 and upregulation of Sirt1. Oxygen consumption and ATP production were improved. Cells kept differentiation agenda through osteogenic, adipogenic and chondrogenic lineages |
| Control: not receive laser irradiation | Standard probe | Treatment duration: from 1 to 3 days | |||
| Amaroli et al. 7 | In vitro: mBMSCs from 3-month-old female Balb-c mice long bones. | 808-nm diode laser | 1 W, 60 s, 1 cm2, 60 J, 60 J/cm2, 1 W/cm2; CW | Irradiation: daily | Stimulation of osteoblast differentiation pathway: upregulation of Runx2, Osx; increase of ALP and mm. Inhibition of adipocyte differentiation pathway: Downregulation of PPARɤ |
| Control: not receive laser irradiation | Flat-top hand-piece | Treatment duration: 5, 10 or 15 days | |||
| Amaroli et al. 94 | In vitro: mBMSCs from 3-month-old female Balb-c mice long bones | 808-nm diode laser | 1 W, 60 s, 1 cm2, 60 J, 60 J/cm2, 1 W/cm2; CW | Irradiation: daily | Actin cytoskeleton reorganisation towards osteogenesis: upregulation of cortactin, N-WASP and Arp2/3. Upregulation of Runx2 and osterix |
| Control: not receive laser irradiation | Flat-top hand-piece | Treatment duration: 5, 10 or 15 days | |||
| Sefati et al. 101 | In vivo: hypothyroid wistar rats (mMSC and tibia) | 890-nm GaAlAs lasers | 1.5 J/cm2, 1200 s; 80 Hz | Irradiation: daily | Stimulation of osteoblast differentiation. Upregulation of mesenchymal surface markers (CD44, CD90). Increase of broken tibia bone performances: bending stiffness, maximum force, stress high load, energy absorption, trabecular bone volume |
| Control: healthy and hypothyroid animals that do not receive laser irradiation | Standard hand-piece | Treatment duration: 1 day; 3 points on broken tibia bone site | |||
| Wang et al. 102 | In vitro: hBMSCs from fresh cancellous bone fragments of orthognathic patients (normal and inflammatory condition) | 1064-nm Nd:YAG laser | 0.25 W, 20 s, 2–4–8–16 J/cm2; CW | Irradiation: every other day | Stimulation of osteoblast differentiation’s pathway (max effect 4 J/cm2): upregulation of Runx2, osteocalcin; an increase of ALP and mm. inhibition of osteoblast differentiation’s pathway (16 J/cm2) |
| Control: not receive laser irradiation | Standard hand-piece | Treatment duration: a week | |||
| Peat et al. 103 | In vitro: eBMSCs from sternal bone marrow of horses aged 2–4 years old | 1064-nm Nd:YAG laser | Mean output power 13 W, 1.33 cm2, pulse energy 1.3 J, 9.77 J/cm2, mean power density 9.77 W/cm2; pulsed-wave frequency 10 Hz | Irradiation: daily | Upregulation of VEGF angiogenic factor, indispensable for bone remodelling and repair |
| Standard hand-piece | Treatment duration: 1 day |
ALP, alkaline phosphatase; Arp2/3, actin-related protein; Bmp2, bone morphogenetic protein 2; CW, continuous wave; eBMSC, equine bone marrow-derived mesenchymal stem cell; hBMSCs, human bone marrow mesenchymal stem cells; mBMSCs, murine bone marrow mesenchymal stem cells; mm, matrix mineralisation; N-WASP, Wiskott–Aldrich syndrome protein; Nd:YAG, neodymium-doped yttrium aluminium garnet; Osx, osterix; protein p21, p21; PPARγ, adipogenic transcription factor; Runx2, Runt-related transcription factor 2; Sirtuin, Sirt1; VEGF, vascular-endothelial growth factor.
Bone marrow-derived MSCs treated with 532-nm green visible light are shifted to an osteogenic differentiation commitment. Consistently, applying a potassium-titanyl-phosphate KTiOPO4 (KTP) laser at a fluence of 4 J/cm2 to cultured MSCs, three times a week for 4 weeks, promoted osteoblastogenesis through extracellular matrix mineralisation and enhanced transcription of bone sialoprotein 2, bone morphogenetic protein 2 and collagen type 1α1. 95 However, for in vivo applications, one much considers the fact that green light does not penetrate tissues well while near-infrared wavelengths penetrate tissue relatively easily. 96
Researchers applied 808-nm photon energy to MSCs via a standard probe (Gaussian profile) at a fluence of 3.0, 4.5 and 6.0 J/cm2. 3 days of irradiation at 3 J/cm2 was able to rejuvenate aged murine MSCs. There was downregulation of p21 and upregulation of Sirt1. Additionally, oxygen consumption and ATP production were improved and the differentiation agenda into osteogenic, adipogenic and chondrogenic lineages was kept. 8 A recent in vitro report argued that the same 808-nm irradiation with higher power (1 W) and fluence (60 J/cm2) for 60 s, repeated daily for four different time points, changed the MSC differentiation schedule of murine MSC. Indeed, providing the therapy with a flat-top hand-piece in continuous wave (CW) mode on an area of ~1 cm2 increased the production of osteogenic runt-box-related transcription factor (Runx2) and osterix and slightly decreased the adipogenic protein peroxisome proliferator-activated receptor gamma (PPARγ), driving these cells to an osteogenic bias. 7 The authors also observed more alkaline phosphatase–positive colonies and Ca2+ deposition in laser-treated MSC cultures, mainly after 10 and 15 days of treatment. 7
Given that microarchitectural modifications play a significant role in MSC lineage commitment, 97 a recent study emphasised MSC cytoskeletal morphometric variations after laser treatment. 83 Irradiation with 808-nm diode laser light at the abovementioned 1 W in CW on 1 cm2 for 60 s, delivered with a flat-top profiled hand-piece, altered the actin cytoskeleton and promoted osteogenic cell shape adaptions. Five to fifteen days of laser therapy promoted the molecular apparatus involved in de novo actin polymerisation. Key proteins involved in actin nucleation such as cortactin, neuronal Wiskott–Aldrich syndrome protein (N-WASP) and actin-related protein (Arp2/3) (specifically the p34/ArpC2 subunit) were increased after 10 days of treatment. The actin reorganisation observed in laser-irradiated MSCs was related directly to the concurrent transcription of the osteoinductive factors Runx2 and osterix. These data suggest a scenario whereby PBM-t induces actin cytoskeletal modifications, induces morphological changes and increases the transcription of bone markers, all of which favour MSC differentiation into an osteo-lineage commitment. 94
The above-mentioned data undoubtedly indicate that PBM-t 808-nm light to MSCs is capable of initiating different homeostatic agendas. In this regard, 808-nm light has been shown to interact with mitochondria complex IV cytochromes and partially with complex III, thereby affecting the energetic metabolism of extracted mitochondria. 62 Eroglu et al. 8 have also described that effect on MSCs. In a unicellular model, light delivered at a fluence of 3 and 60 J/cm2 affected mitochondrial metabolism differently. A fluence of 60 J/cm2 led to greater efficiency in Paramecium mitochondrial respiratory chain activity than a fluence of 3 J/cm2. 98
Therefore, the 808-nm modulatory effect on cell metabolism could explain why PBM delivered with higher power and fluence favours an osteoblast differentiation agenda rather than an adipogenic programme.
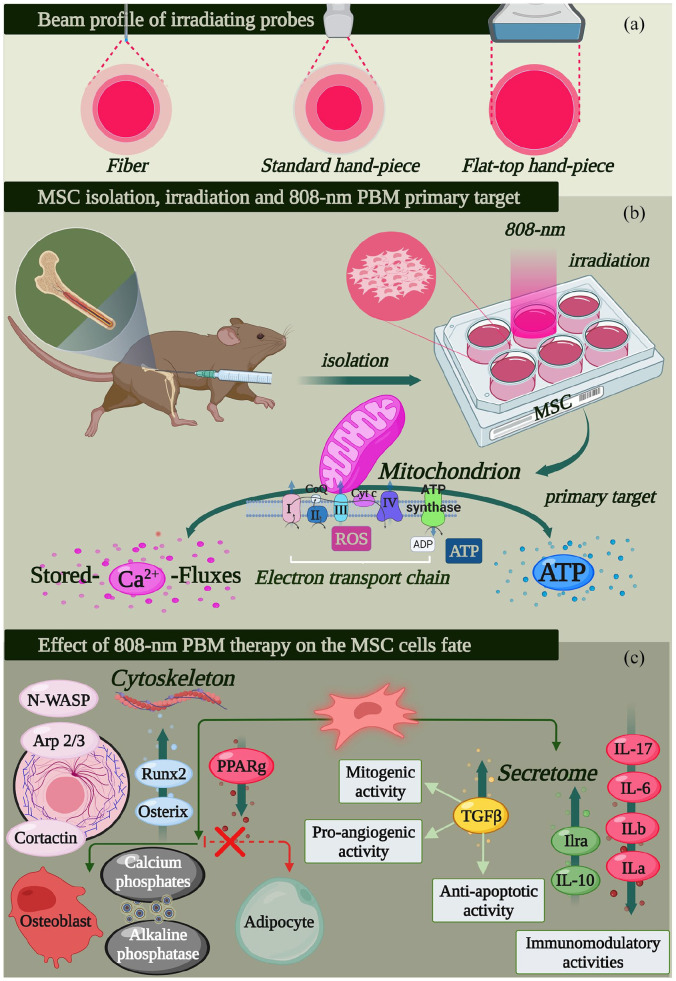
Of note, the hand-piece delivery system could influence the efficacy of PBM-t. According to previous studies, flat-top hand-piece delivery systems are more consistent. Compared with a standard (Gaussian profile) hand-piece, they provide a more homogeneous treatment of 808-nm and 980-nm laser light at the selected spot without causing collateral thermal damage (Figure 2(a)).99,100
Figure 2.
Design of promising PBM therapy7,94 to improve the anti-inflammatory and osteogenic capacity of bone marrow-derived multipotent mesenchymal cells (MSCs). The homogeneity of flat-top hand-piece irradiation (a) improves the PBM-t consistency, (b) in vitro irradiation of MSCs through 1 W and 60 J/cm2 irradiated in continuous-wave mode on an area of about 1 cm2 for 60 s with an 808-nm diode laser light (see Table 1), and the major cell target and (c) cellular pathway, secretome and cell agenda stimulated by the therapy (for further information see Tables 1 and 2), (figure created with BioRender.com).
To support in vitro data, Sefati et al. 101 used an interesting approach and reported that MSCs could act remotely and in combination with PBM-t to induce bone anabolic effects. Hypothyroid rats with bone defects were administrated MSC-conditioned medium and then irradiated with 890-nm light at a fluence of 1.5 J/cm2 for 20 s in pulsed mode (80 Hz), with an inter-treatment interval of 4 weeks. After this treatment, the researchers examined the long bone structure. Consistent with the authors’ hypothesis, the tibial analysis revealed improved bone healing as well as histological and biochemical parameters. 101 Overall, the laser-modulated MSC secretome, acting remotely, might be an effective remedial platform for bone regeneration and fracture repair. An ever-increasing number of curative methods use MSC-conditioned mediums for remote applications. Determining the proper photon energy combination could be a major asset against a wide spectrum of anabolic and/or inflammatory diseases.
The application of a higher wavelength of light – 1064-nm – to MSCs via a single-probe laser hand-piece at a fluence of up to 16 J/cm2 (0.25 W) for 20 s (CW) was able to ‘guide’ these multipotent cells towards an osteogenic fate. Indeed, PBM-t at 4 J/cm2 was sufficient to enhance the osteogenic potential of MSCs and to prompt transcription of alkaline phosphatase, Runx-2 and osteocalcin. 102 Of note, authors have shown that PBM-t can modulate mitochondrial metabolism and the relative oxidative stress production through a wide range of wavelengths other than 808-nm.63 –67 Indeed, irradiation of equine MSCs with 1064-nm light at a fluence of 9.77 J/cm2 in a pulsed-wave frequency of 10 Hz provoked a moderate increase in VEGF in the first 24 h of treatment. VEGF is a well-established angiogenic factor and is indispensable for bone remodelling and repair. 103 Hence, this treatment seems to be effective.
These results enrich the operational range of laser wavelengths available for MSC manipulation and point out the wide range of wavelengths, from visible to near-infrared light, that could be effective in bone rebuilding. Indeed, according to the effects on pre-osteoblasts and osteoblasts following the ATP increase, activation of the ERK1/2 and Wnt and β-catenin100,104 pathways by PBM and the influence on MSCs fate seems to strongly reflect the regulation of bone homeostasis by mechanical stimuli 105 and support preventive and regenerative medicine.
PBM regulates the MSC secretome
When preconditioned with microenvironmental variations comprising mechanical cues, ligand stimulation or transfection, cell engineering and light irradiation, MSCs could produce compounds to regulate the function of the local area or they could act systemically.7,25,45,106
Table 2 shortly reports data relative to the photobiomodulation switch-effect on MSCs’ secretome regulation.
Table 2.
Photobiomodulation regulates secretome of mesenchymal stem/stromal cells differentiation; selected articles.
| Authors | Model | Laser/probe | Parameters | Therapy | Effects |
|---|---|---|---|---|---|
| Amaroli et al. 7 | In vitro: mBMSCs from 3-month-old female Balb-c mice long bones. | 808-nm diode laser | 1 W, 60 s, 1 cm2, 60 J, 60 J/cm2, 1 W/cm2; CW | Irradiation: daily | Increase in the synthesis of TGF-β1. Down-regulation of pro-inflammatory cytokines: IL-6, and IL-17. Up-regulation of anti-inflammatory cytokines: IL-1rα, IL-10. |
| Control: not receive laser irradiation | Flat-top hand-piece | Treatment duration: 5, 10 and 15 days | |||
| Wang et al. 102 | In vitro: hBMSCs from fresh cancellous bone fragments of orthognathic patients (normal and inflammatory condition) | 1064-nm Nd:YAG laser | 0.25 W, 20 s, 2–4–8–16 J/cm2; CW | Irradiation: every other day | Suppression of TNF-α secretion |
| Control: not receive laser irradiation | Standard hand-piece | Treatment duration: a week | |||
| Peat et al. 103 | In vitro: eBMSCs from the sternal bone marrow of horses aged 2–4 years old | 1064-nm Nd:YAG laser | Mean output power 13 W, 1.33 cm2, pulse energy 1.3 J, 9.77 J/cm2, mean power density 9.77 W/cm2; pulsed-wave frequency 10 Hz | Irradiation: daily | Upregulation of VEGF and IL-10. No effect on TGF-β, IL-4, IL-17, IFN-ɤ and IFN-α. |
| Standard hand-piece | Treatment duration: 1 day |
CW, continuous wave; eBMSC, equine bone marrow-derived mesenchymal stem cell; hBMSCs, human bone marrow mesenchymal stem cells; IFN, interferon; IL, interleukin; mBMSCs, murine bone marrow mesenchymal stem cells; Nd:YAG, neodymium-doped yttrium aluminium garnet; TGF-β1, transforming growth factor β1; TNF-α, tumour necrosis factor alpha; VEGF, vascular-endothelial growth factor.
One of the most remarkable outcomes of the 808-nm PBM-t irradiation with a flat-top hand-piece in CW mode on an area of ~1 cm2 (1 W, 60 J/cm2 for 60 s) was the augmented release of the anti-inflammatory cytokines IL-1RA and IL-10 and concurrent reduction of the pro-inflammatory IL-1α, IL-1β, IL-6 and IL-17 in irradiated murine MSCs. The 808-nm PBM-t mechanism might involve TGF-β-mediated control of pro-inflammatory interleukins. 16 The anti-inflammatory action of PBM-t is also been supported by the results of human MSCs treated with a laser at a fluence of 4, 6 or 18 J/cm2, which exhibited reduced expression of TNF-α, a major pro-inflammatory factor. 102 Consistently, equine bone marrow–derived MSCs exposed to 1064-nm at a fluence of 9.77 J/cm2 showed increased VEGF and IL-10 release 24 h after irradiation. 103 IL-10 suppresses pro-inflammatory cytokines and is a prominent candidate for anti-inflammatory drug mixtures. 107 Conversely, there was no effect on TGF-β, IL-4, IL-17, interferon γ (IFN-ɤ) and IFN-α.
The tested PBM protocols modify the secretome of MSCs, with a shift towards an anti-inflammatory pattern. Indeed, most of the modern studies have shown that IL-10 released by irradiated MSCs probably acts as a watchdog for IL-17-induced release of IL-1β, TNF-α, IFN-γ and IL-1, thus quenching the phlogistic signals. Therefore, PBM not only guides MSCs to an osteogenic route but also modulates specific pro-inflammatory molecules, attenuating the local ‘burn’ of cells.
PBM preserves the viability and proliferative capacity of MSCs
It is well established that the proliferative ability and plasticity of stem cells decline with ageing. MSCs isolated from aged individuals show a weaker expansion potential, rendering the autologous transplantation of MSCs in older subjects a difficult problem.6,32 The impaired differentiation and growth agenda of aged MSCs can be attributed mostly to compromised mitochondrial function. Mitochondria orchestrate a series of fundamental cell homeostatic events, including cell growth, apoptosis, senescence and differentiation. 108 Thus, mitochondria are the crucial operatives and one of the primary therapeutic targets to counteract inflammation and rejuvenate MSCs. Laser light, acting on mitochondrial chromophores, may be an effective way to regulate mitochondrial behaviour and improve MSC metabolic activity.
Table 3 shortly reports data relative to the photobiomodulation preservation effect of MSCs’ viability and proliferative regulation.
Table 3.
Photobiomodulation preserves the viability and proliferative capacity of mesenchymal stem/stromal cells; selected articles.
| Authors | Model | Laser/Probe | Parameters | Therapy | Effects |
|---|---|---|---|---|---|
| Fallahnezhad et al. 109 | In vitro: mBMSCs or (OVX) mBMSCs from 14-week-old female Wistar rats’ long bones | 632.8 He–Ne laser | 0.003/0.05 W; 378, 756 or 1512 s/23, 45 or 84 s; 0.6, 1.2 and 2.4 J∕cm2, 1.91 cm2, distance of 15 and 10 cm | Thrice every other day | 0.6 J/cm2 showed a conspicuous increase in viability parameters |
| Control: healthy and osteoporotic animals that do not receive laser irradiation | 808-nm diode laser | ||||
| Standard probe | |||||
| Eroglu et al. 8 | In vitro: mBMSCs from young (3-month-old) and aged (24-month-old) C57BL/6 mice long bones | 808-nm diode laser | 0.0167–0.0250–0.0333 W/cm2, 180–160 s, 3.0–4.5–6.0 J/cm2; CW | Irradiation: daily | PBM at 3 J/cm2 (3 days) rejuvenates aged mMSC: downregulation of p21 and upregulation of Sirt1 |
| Control: not receive laser irradiation | Standard probe | Treatment duration: from 1 to 3 days | Oxygen consumption and ATP production were improved | ||
| Cells kept differentiation agenda through osteogenic, adipogenic and chondrogenic lineages | |||||
| Wang et al. 102 | In vitro: hBMSCs from fresh cancellous bone fragments of orthognathic patients (normal and inflammatory condition) | 1064-nm Nd:YAG laser | 0.25 W, 20 s, 2–4–8–16 J/cm2; CW | Irradiation: every other day | Stimulation of cell proliferation pathway (4 J/cm2 and 8 J/cm2): |
| Control: not receive laser irradiation | Standard hand-piece | Treatment duration: a week | Inhibition of osteoblast proliferation pathway (16 J/cm2) | ||
| Leonida et al. 110 | In vitro: hBMSCs from the posterior iliac crest of patients. The cells are inserted into collagen scaffolds | 1064-nm Nd:YAG laser | Test 1: 1.5 W, 100 mJ, 15 Hz; 0.84 J/cm2 each cycle, with a total amount of irradiation of 2.52 J/cm2 | Irradiation: tests 1 and 2 received three irradiation cycles of 30 s each, separated by 30-sintervals | After 7 days, proliferation was significantly increased in scaffolds treated with laser |
| Control: not receive laser irradiation | Standard hand-piece | Test 2: 2.25 W, 150 mJ, 15 Hz; 1.27 J/cm2 each cycle with a total amount of 3.81 J/cm2 | |||
| The distance between the laser head and scaffolds was 5 mm and laser divergence were 0.0042 rad; 5 mm on defocalization |
ATP, adenosine triphosphate; CW, continuous wave; hBMSCs, human bone marrow mesenchymal stem cells; mBMSCs, murine bone marrow mesenchymal stem cells; Nd:YAG, neodymium-doped yttrium aluminium garnet; protein p21, p21; Sirtuin, Sirt1.
Pastore et al. 66 described the specific 638.2-nm helium-neon laser sensitivity of the purified cytochrome c oxidase and Amaroli et al. 62 demonstrated 808-nm photobiostimulated the mitochondria respiratory chain without uncoupling them; increment in the ATP production was induced. Interestingly, MSCs extracted from ovariectomised rats and exposed three times to 632.8-nm He-Ne laser energy at a fluence of 0.6 J/cm2 showed a conspicuous increase in viability parameters compared with the healthy ‘control’ MSC group. 109 Moreover, He-Ne and 808-nm light stimulation (1.2 J/cm2) of MSCs enhanced their viability and proliferative capacity. Given the in vitro He-Ne laser regenerative outcomes on aged and ‘osteoporotic’ MSCs, this protocol may be considered in allogenic transplantation protocols against various forms of osteopenia in humans or veterinary medicine. Additionally, as described above, young and aged MSCs irradiated with near-infrared light at 808-nm and a fluence of 3 J/cm2 for 3 h exhibited improvement in mitochondrial activities. 8 Moreover, two or three consecutive laser treatments considerably enhanced the proliferative capacity of both young and aged MSCs on day 7. These findings suggest that pertinent laser therapy re-establishes mitochondrial respiration and facilitates the expansion of aged MSCs. 8
In a recent study, 1064-nm PBM-t at a fluence of 2–4 J/cm2 promoted MSC proliferation, while a slightly higher fluence (16 J/cm2) suppressed MSC expansion. 102 Tissue engineering approaches involving the growth of laser-irradiated MSCs in two-dimensional (2D)/3D scaffolds have also been contemplated for bone regeneration. MSCs cultured on collagen type I sponges and irradiated once with 1064-nm at a fluence of 2.52 or 3.81 J/cm2 displayed an enhanced proliferative capacity, and the cells had covered the biomaterial surface after 7 days of treatment. 110 The accelerated MSC proliferation on bio-scaffolds after PBM meets the needs of periodontal regenerative therapy protocols and bone healing strategies.
Therefore, specific PBM-t parameters efficiently enhanced multipotent stem cell viability and in vitro expansion. Of note, similar effects have been observed in osteo-committed lineages under in vitro and in vivo strictly irradiated conditions.100,104,111
Restrictions and perplexities of PBM-t: Some broader considerations
For a long time, PBM had been considered free of side effects because it does not involve drugs. However, as appropriately reviewed by Karu, 112 after 50 years of research, it might be time to consider PBM a drug equivalent. PBM is not a chemical and therefore patients do not experience the classical collateral effects that occur during drug administration. However, as mentioned before, PBM can modulate metabolic and oxidative pathways as well as the cell signals responsible for the survival/death fate of cells. Moreover, currently unknown photoacceptors might be activated by light and affect the outcome of PBM-t, leading to unexpected results, and cancer and prokaryotic cells may be undesirable targets.52,53 The PBM effects on patients affected by cell metabolic dysfunctions (e.g. Fanconi anaemia) have not been investigated extensively.
From an operative/clinical point of view, the main problems related to PBM-t are its repeatability in the patient as well as the homogeneity of the irradiation of the therapy to the whole tissue area. 94 Two major variables influence PBM-t success. The first is the variation in the number of mitochondria in a cell and the optical properties of a tissue. 113 Photons could scatter before being absorbed by mitochondrial components, and skin colour and thickness may drastically affect the absorbance, transmittance and reflectance of light.114,115 Second, the probes (fibre and standard hand-piece) have discrete and non-homogeneous light-beam distribution characteristics (Figure 2(a)). This factor affects the cell (mitochondrial) activity with respect to its position within the treated areas. 94 Additionally, researchers have pointed out that uniform and constant power density, larger area (spot) and sufficiently high irradiances have a greater chance of penetrating and reaching the target. 116 The issue of homogeneity, spot area and higher irradiance with limited temperature increases seem to have been overcome through the realisation and patenting of the new flat-top profiled hand-piece. 94 However, a better understanding of the behaviour of photons in tissues and identification of predictive mathematical models are some important challenges of precision medicine. 112 Indeed, many of the low-level power/fluence therapies and the wavelengths used in vitro cannot reach the target in vivo or reach it at very different doses.
PBM sometimes appears to work according to hormesis but in other cases, window effects have been described. 63 For example, mitochondria subjected to irradiation at 980 nm and 0.1 W power experienced higher oxidative stress and a drastic inhibition of ATP production due to uncoupling. Conversely, irradiation at 980 nm and 0.8 W power did not induce mitochondrial uncoupling and induced an increase in ATP production. No effect was observed when using 0.5 W power. 63 Overall, there could be notable variability in the efficacy of PBM-t from patient to patient. Indeed, in a recent commentary Lanzafame 116 argued that ‘concerning PBM, it is necessary to take a number of variables into consideration and to understand that the wavelength, the intended target and the method of light delivery are of great importance. The gender of the patient, although often neglected, may well also affect results and may partially explain variability in published study results and our clinical outcomes’. The restrictions and perplexities of laser therapy on MSCs are mostly experienced in vivo because under in vitro conditions, researchers can control relevant variables.
PBM-t safety
Now that photobiomodulation emerged in clinical practice has to increase the attention on safety. Photobiomodulation is not dangerous per se but undesirable and sometimes dangerous collateral effects can occur. Indeed, clinically relevant understanding of both the biological interactions and results of applying laser light to a variety of tissues, and the appropriate means of delivering and controlling the energy to obtain desired outcomes, are not clearly understood. Indeed, the properties of both lasers and wavelengths, the specific absorbing chromophores of each wavelength, the dosimetry (power, power density, parameters, fluence, energy density, pulse, continuous wave, spot size, etc.), the delivery systems and instrumentation and the desired clinical application (medical and surgical), must be correctly managed. 117
In other words, the laser devices prevalently used in PBM work as Class 3b or Class 4 healthcare laser systems that, at the parameters employed, can be able to damage structures in the eye if it is unprotected. 118 Therefore, the risks for patients, physicians, assistants and nurse practitioners, have to be prevented through specific goggles. Additionally, laser effects on the skin have to be taken into account. Different wavelengths of light may penetrate the skin and generally the tissues in different ways and the endogenous or exogenous pigments, blood circulation, the power density of the incident beam, time of exposure and the area and heat conduction in the affected zone are variables that can influence it. These interactions can lead to immediate biological damage through the burning of the tissue as well as undesired effects on cancer cells and pathogens microbes are debated. 117
More simply, the skin phototype has to be considered before PBM-t irradiation. Additionally, in the section ‘PBM: evolutionary and molecular notes’ we described the PBM-t targets in the cellular universal pathway involved in cell proliferation/viability/apoptosis. Therefore, PBM-t can interact with normal cells but also with cancer and pathogens organisms. The Warburg effect has, for many years, suggested a no-supportive effect of PBM on tumour growth. However, in the last years, the role of mitochondria energetic metabolism in cancer homeostasis gained attention. 53 Particularly, ATP increase through PBM-t therapy irradiation can lead to pro-apoptotic cytotoxic stimuli, inducing energy-dependent cell death programmes but also on PhosphatidylInositol 3-Kinase (PI3K)/Protein kinase B (AKT)/mechanistic target of rapamycin (mTOR), TGF-β, MAPK, that can support the cancer growth and differentiation. Recently, the effect of an 808-nm PBM-t irradiated with a flat-top hand-piece at 1 W, 60 J/cm2 for the 60 s on a spot area of 1 cm2 on head and neck squamous carcinoma was described; PBM was able to kill carcinoma through inhibition of catalase activities that made the cell unable to counteract oxidative stress. 53 Of note, Bensadoun et al. 119 showed as growing literature indicates that PBM-t could be considered relatively safe and effective, despite further elucidation has to be provided regarding PBM use in oncology.
Lastly, in a recent review, Amaroli et al. 52 pointed out the effectiveness of PBM-t on prokaryotes; PBM can affect bacteria metabolism, homeostasis, defence against stress and life-and-death mechanisms. Therefore, the possible light-bacteria interaction and microbiota management in health and illness patients need attention before PBM-t delivery.
Conclusions and perspectives
There has been a significant advance in understanding the effects of photons on MSC properties. Indeed, the literature suggests that the effects on both MSCs and cells with competence for and osteogenic behaviour seem to be carried out similarly by physical stimuli (e.g. mechanical and photonics). As a well-established therapeutic tool, laser pre-conditioning could substantially improve the anti-inflammatory and osteogenic capacity of MSCs. On the other hand, PBM-t protocols not only improve MSC viability and proliferation but, in accordance with precise parameters, also prompt these multipotent stem cells towards a predefined lineage commitment and secretome. Unfortunately, because of limited data in vitro approaches cannot be translated easily in vivo in humans and animals. Additional investigation to optimise the functional schedule of PBM-t is needed. From an instrumental point of view, researchers have to invest in the development of novel devices for consistent therapeutic approaches, which take into account the physical property of light, the reliability of laser parameter irradiation and the possibility to perform individual PBM-t, with the aim to improve medical decisions, treatments and standardisation of practices. From a biological/clinical point of view, we are interested in moving away from collecting pre-clinical evidence to establishing reliable therapies. As with drugs, this transition will require determining the effectiveness, safeness, posology and clinical utility of PBM-t protocols.
Because of the current limits of PBM-t, we cannot provide a definitive conclusion regarding the parameters that provide the most effective therapy. Moreover, the best parameters – including the wavelength of light, fluence and power – will likely differ depending on the specific application. Certainly, there are effective fluences for MSC manipulation, which seem to include the range from 0.6 to 3 J/cm2 when 808-nm infrared light is provided with a standard (Gaussian) hand-piece. On the other hand, a fluence of 0.84–9.77 J/cm2 provides similar delivery of 1064-nm light. Another factor is the equipment used to deliver irradiation. A novel flat-top hand-piece leads to greater irradiance with uniform and constant power density on a ‘larger’ area than standard (Figure 2(a)). Therefore, 808-nm light delivered at 1 W and 60 J/cm2 appears to be suitable for experimental in vivo translation, based on the results on MSC differentiation and secretome release (Figure 2(b) and (c)) as well as the lack of adverse effects during 1-year follow-up in patients affected by Bell’s palsy and major aphthae who had been irradiated and had recovered57,120; the therapy was able to kill carcinoma cells. 53
To summarise, PBM-t can significantly modulate MSC physiology, rendering these multipotent cells efficient bone-building elements and concurrently serves as a suitable anti-inflammatory platform. However, to switch from in vitro to in vivo, studies in-depth are necessary to reach unequivocal conclusions.
Footnotes
Author contributions: All the authors conceived this review. A.A and D.A. coordinated the literature search and review design. A.A., D.A., C.P., A.M. and M.G.S. performed the literature search. D.A and A.A. wrote the paper. Personal support with funding: S.B. and A.Z. All the authors revised the manuscript and participated in the discussion. All authors agreed to the published version of the manuscript.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Dimitrios Agas  https://orcid.org/0000-0002-7809-3601
https://orcid.org/0000-0002-7809-3601
References
- 1. Wang YL, Hong A, Yen TH, et al. Isolation of mesenchymal stem cells from human alveolar periosteum and effects of vitamin D on osteogenic activity of periosteum-derived cells. J Vis Exp 2018; 135: 57166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abdelgawad LM, Abdelaziz AM, Sabry D, et al. Influence of photobiomodulation and vitamin D on osteoblastic differentiation of human periodontal ligament stem cells and bone-like tissue formation through enzymatic activity and gene expression. Biomol Concepts 2020; 11: 172–181. [DOI] [PubMed] [Google Scholar]
- 3. Chahal J, Gómez-Aristizábal A, Shestopaloff K, et al. Bone marrow mesenchymal stromal cell treatment in patients with osteoarthritis results in overall improvement in pain and symptoms and reduces synovial inflammation. Stem Cells Transl Med 2019; 8: 746–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Becker DJ, Kilgore ML, Morrisey MA. The societal burden of osteoporosis. Curr Rheumatol Rep 2010; 12: 186–191. [DOI] [PubMed] [Google Scholar]
- 5. Chu DT, Phuong TNT, Tien NLB, et al. An update on the progress of isolation, culture, storage, and clinical application of human bone marrow mesenchymal stem/stromal cells. Int J Mol Sci 2020; 21: 708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang H, Menzies KJ, Auwerx J. The role of mitochondria in stem cell fate and aging. Development 2018; 145: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Amaroli A, Agas D, Laus F, et al. The effects of photobiomodulation of 808 nm diode laser therapy at higher fluence on the in vitro osteogenic differentiation of bone marrow stromal cells. Front Physiol 2018; 9: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eroglu B, Genova E, Zhang Q, et al. Photobiomodulation has rejuvenating effects on aged bone marrow mesenchymal stem cells. Sci Rep 2021; 11: 13067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hamblin MR. Mechanisms and mitochondrial redox signaling in photobiomodulation. Photochem Photobiol 2018; 94: 199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sleep SL, Skelly D, Love RM, et al. Bioenergetics of photobiomodulated osteoblast mitochondrial cells derived from human pulp stem cells: systematic review. Lasers Med Sci 2022; 37: 1843–1853. [DOI] [PubMed] [Google Scholar]
- 11. Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006; 8: 315–317. [DOI] [PubMed] [Google Scholar]
- 12. Krampera M, Galipeau J, Shi Y, et al. Immunological characterization of multipotent mesenchymal stromal cells–The International Society for Cellular Therapy (ISCT) working proposal. Cytotherapy 2013; 15: 1054–1061. [DOI] [PubMed] [Google Scholar]
- 13. Nolta JA, Galipeau J, Phinney DG. Improving mesenchymal stem/stromal cell potency and survival: Proceedings from the International Society of Cell Therapy (ISCT) MSC preconference held in May 2018, Palais des Congrès de Montréal, organized by the ISCT MSC Scientific Committee. Cytotherapy 2020; 22: 123–126. [DOI] [PubMed] [Google Scholar]
- 14. Viswanathan S, Shi Y, Galipeau J, et al. Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT®) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy 2019; 21: 1019–1024. [DOI] [PubMed] [Google Scholar]
- 15. De Miguel M, Fuentes-Julian S, Blazquez-Martinez A, et al. Immunosuppressive properties of mesenchymal stem cells: advances and applications. Curr Mol Med 2012; 12: 574–591. [DOI] [PubMed] [Google Scholar]
- 16. Agas D, Sabbieti MG. Archetypal autophagic players through new lenses for bone marrow stem/mature cells regulation. J Cell Physiol 2021; 236(9): 6101–6114. [DOI] [PubMed] [Google Scholar]
- 17. Yamout B, Hourani R, Salti H, et al. Bone marrow mesenchymal stem cell transplantation in patients with multiple sclerosis: a pilot study. J Neuroimmunol 2010; 227: 185–189. [DOI] [PubMed] [Google Scholar]
- 18. Wei Y, Xie Z, Bi J, et al. Anti-inflammatory effects of bone marrow mesenchymal stem cells on mice with Alzheimer’s disease. Exp Ther Med 2018; 16: 5015–5020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Markov A, Thangavelu L, Aravindhan S, et al. Mesenchymal stem/stromal cells as a valuable source for the treatment of immune-mediated disorders. Stem Cell Res Ther 2021; 12: 192. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20. Agas D, Marchetti L, Douni E, et al. The unbearable lightness of bone marrow homeostasis. Cytokine Growth Factor Rev 2015; 26(3): 347–359. [DOI] [PubMed] [Google Scholar]
- 21. Jayaram P, Ikpeama U, Rothenberg JB, et al. Bone marrow-derived and adipose-derived mesenchymal stem cell therapy in primary knee osteoarthritis: a narrative review. PM R 2019; 11: 177–191. [DOI] [PubMed] [Google Scholar]
- 22. Lin H, Sohn J, Shen H, et al. Bone marrow mesenchymal stem cells: aging and tissue engineering applications to enhance bone healing. Biomaterials 2019; 203: 96–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Szydlak R. Biological, chemical and mechanical factors regulating migration and homing of mesenchymal stem cells. World J Stem Cells 2021; 13(6): 619–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Haque N, Abu Kasim NH. Pooled human serum increases regenerative potential of in vitro expanded stem cells from human extracted deciduous teeth. Adv Exp Med Biol 2018; 1083: 29–44. [DOI] [PubMed] [Google Scholar]
- 25. Choudhery MS. Strategies to improve regenerative potential of mesenchymal stem cells. World J Stem Cells 2021; 13(12): 1845–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alvarez-Viejo M, Menendez-Menendez Y, Blanco-Gelaz MA, et al. Quantifying mesenchymal stem cells in the mononuclear cell fraction of bone marrow samples obtained for cell therapy. Transplant Proc 2013; 45(1): 434–439. [DOI] [PubMed] [Google Scholar]
- 27. Jang Y, Koh YG, Choi YJ, et al. Characterization of adipose tissue-derived stromal vascular fraction for clinical application to cartilage regeneration. Vitro Cell. Dev.Biol. Anim 2015; 51: 142–150. [DOI] [PubMed] [Google Scholar]
- 28. Nasef A, Fouillard L, El-Taguri A, et al. Human bone marrow-derived mesenchymal stem cells. Libyan J Med 2007; 2: 190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Neri S. Genetic stability of mesenchymal stromal cells for regenerative medicine applications: a fundamental biosafety aspect. Int J Mol Sci 2019; 20: 2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sensebé L, Krampera M, Schrezenmeier H, et al. Mesenchymal stem cells for clinical application. Vox Sang 2010; 98: 93–107. [DOI] [PubMed] [Google Scholar]
- 31. Bernardo ME, Zaffaroni N, Novara F, et al. Human bone marrow–derived mesenchymal stem cells do not undergo transformation after long-term in vitro culture and do not exhibit telomere maintenance mechanisms. Cancer Res 2007; 67: 9142–9149. [DOI] [PubMed] [Google Scholar]
- 32. Shen J, Tsai YT, Dimarco NM, et al. Transplantation of mesenchymal stem cells from young donors delays aging in mice. Sci Rep 2011; 1: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Andrzejewska A, Lukomska B, Janowski M. Concise review: mesenchymal stem cells: from roots to boost. Stem Cells 2019; 37: 855–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chamberlain G, Fox J, Ashton B, et al. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 2007; 25: 2739–2749. [DOI] [PubMed] [Google Scholar]
- 35. Ringe J, Strassburg S, Neumann K, et al. Towards in situ tissue repair: human mesenchymal stem cells express chemokine receptors CXCR1, CXCR2 and CCR2, and migrate upon stimulation with CXCL8 but not CCL2. J Cell Biochem 2007; 101: 135–146. [DOI] [PubMed] [Google Scholar]
- 36. Agas D, Sabbieti MG. Autophagic mediators in bone marrow niche homeostasis. Adv Exp Med Biol 2022; 1376: 61–75. [DOI] [PubMed] [Google Scholar]
- 37. Eseonu OI, De Bari C. Homing of mesenchymal stem cells: mechanistic or stochastic? Implications for targeted delivery in arthritis. Rheumatology 2015; 54: 210–218. [DOI] [PubMed] [Google Scholar]
- 38. Leibacher J, Henschler R. Biodistribution, migration and homing of systemically applied mesenchymal stem/stromal cells. Stem Cell Res Ther 2016; 7: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sohni A, Verfaillie CM. Mesenchymal stem cells migration homing and tracking. Stem Cells Int 2013; 2013: 130763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Almalki SG, Agrawal DK. Effects of matrix metalloproteinases on the fate of mesenchymal stem cells. Stem Cell Res Ther 2016; 7: 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mazini L, Rochette L, Amine M, et al. Regenerative capacity of adipose derived stem cells (ADSCs), comparison with mesenchymal stem cells (MSCs). Int J Mol Sci 2019; 20: 2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Van Rensburg MJ, Crous A, Abrahamse H. Potential of photobiomodulation to induce differentiation of adipose- derived mesenchymal stem cells into neural cells. Curr Stem Cell Res Ther 2021; 16(3): 307–322. [DOI] [PubMed] [Google Scholar]
- 43. Crous A, Jansen van Rensburg M, Abrahamse H. Single and consecutive application of near-infrared and green irradiation modulates adipose derived stem cell proliferation and affect differentiation factors. Biochimie 2022; 196: 225–233. [DOI] [PubMed] [Google Scholar]
- 44. Lee S, Choi E, Cha MJ, et al. Cell adhesion and long-term survival of transplanted mesenchymal stem cells: a prerequisite for cell therapy. Oxid Med Cell Longev 2015; 2015: 632902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Levy O, Kuai R, Siren EMJ, et al. Shattering barriers toward clinically meaningful MSC therapies. Sci Adv 2020; 6(30): eaba6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lacava G, Laus F, Amaroli A, et al. P62 deficiency shifts mesenchymal/stromal stem cell commitment toward adipogenesis and disrupts bone marrow homeostasis in aged mice. J Cell Physiol 2019; 234: 16338–16347. [DOI] [PubMed] [Google Scholar]
- 47. Hatfield S, Ruohola-Baker H. MicroRNA and stem cell function. Cell Tissue Res 2008; 331: 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Li S, Liu Y, Tian T, et al. Bioswitchable delivery of microRNA by framework nucleic acids: application to bone regeneration. Small 2021; 17: e2104359. [DOI] [PubMed] [Google Scholar]
- 49. Zhang T, Tian T, Lin Y. Functionalizing framework nucleic-acid-based nanostructures for biomedical application. Adv Mater Weinheim 2021; e2107820. [DOI] [PubMed] [Google Scholar]
- 50. Zhang B, Tian T, Xiao D, et al. Facilitating in situ tumor imaging with a tetrahedral DNA framework-enhanced hybridization chain reaction probe. Adv Funct Mater 2022; 32: 2109728. [Google Scholar]
- 51. Rahman SU, Ponnusamy S, Nagrath M, et al. Precision-engineered niche for directed differentiation of MSCs to lineage-restricted mineralized tissues. J Tissue Eng 2022; 13: 20417314211073934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Amaroli A, Ravera S, Zekiy A, et al. A narrative review on oral and periodontal bacteria Microbiota photobiomodulation, through visible and near-infrared light: from the origins to modern therapies. Int J Mol Sci 2022; 23(3): 1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ravera S, Bertola N, Pasquale C, et al. 808-nm photobiomodulation affects the viability of a head and neck squamous carcinoma cellular model, acting on energy metabolism and oOxidative stress production. Biomedicines 2021; 9(11): 1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ravera S, Colombo E, Pasquale C, et al. Mitochondrial bioenergetic, pPhotobiomodulation and Trigeminal ranches nerve damage, what’s the connection? a review. Int J Mol Sci 2021; 22(9): 4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Colombo E, Signore A, Aicardi S, et al. Experimental and clinical applications of red and near-infrared photobiomodulation on endothelial dysfunction: a review. Biomedicines 2021; 9(3): 274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Amaroli A, Colombo E, Zekiy A, et al. Interaction between laser light and osteoblasts: photobiomodulation as a trend in the management of socket bone preservation-a review. Biology 2020; 9(11): 409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pasquale C, Colombo E, Benedicenti S, et al. 808-Nm near-infrared laser photobiomodulation versus switched-off laser placebo in major aphthae management: a randomized double-blind controlled trial. Appl Sci 2021; 11: 4717. [Google Scholar]
- 58. de Freitas LF, Hamblin MR. Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J Sel Top Quantum Electron 2016; 22: 7000417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Marijuán PC, Navarro J. From molecular recognition to the “vehicles” of evolutionary complexity: an informational approach. Int J Mol Sci 2021; 22: 11965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Guo R, Gu J, Zong S, et al. Structure and mechanism of mitochondrial electron transport chain. Biomed J 2018; 41: 9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ferrando S, Agas D, Mirata S, et al. The 808 nm and 980 nm infrared laser irradiation affects spore germination and stored calcium homeostasis: a comparative study using delivery hand-pieces with standard (Gaussian) or flat-top profile. J Photochem Photobiol B 2019; 199: 111627. [DOI] [PubMed] [Google Scholar]
- 62. Amaroli A, Ravera S, Parker S, et al. An 808-nm diode laser with a flat-top handpiece positively photobiomodulates mitochondria activities. Photomed Laser Surg 2016; 34: 564–571. [DOI] [PubMed] [Google Scholar]
- 63. Amaroli A, Pasquale C, Zekiy A, et al. Photobiomodulation and oxidative stress: 980 nm diode laser light regulates mitochondrial activity and reactive oxygen species production. Oxid Med Cell Longev 2021; 2021: 6626286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ravera S, Ferrando S, Agas D, et al. 1064 nm Nd:YAG laser light affects transmembrane mitochondria respiratory chain complexes. J Biophotonics 2019; 12: e201900101. [DOI] [PubMed] [Google Scholar]
- 65. Swartz TE, Corchnoy SB, Christie JM, et al. The photocycle of a flavin-binding domain of the blue light photoreceptor phototropin. J Biol Chem 2001; 276: 36493–36500. [DOI] [PubMed] [Google Scholar]
- 66. Pastore D, Greco M, Passarella S. Specific helium-neon laser sensitivity of the purified cytochrome c oxidase. Int J Radiat Biol 2000; 76: 863–870. [DOI] [PubMed] [Google Scholar]
- 67. Passarella S, Karu T. Absorption of monochromatic and narrow band radiation in the visible and near IR by both mitochondrial and non-mitochondrial photoacceptors results in photobiomodulation. J Photochem Photobiol B 2014; 140: 344–358. [DOI] [PubMed] [Google Scholar]
- 68. Barreto Ortiz S, Hori D, Nomura Y, et al. Opsin 3 and 4 mediate light-induced pulmonary vasorelaxation that is potentiated by G protein-coupled receptor kinase 2 inhibition. Am J Physiol Lung Cell Mol Physiol 2018; 314: L93–L106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Khan I, Rahman SU, Tang E, et al. Accelerated burn wound healing with photobiomodulation therapy involves activation of endogenous latent TGF-β1. Sci Rep 2021; 11: 13371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Jansen K, Wu M, van der Steen AF, et al. Photoacoustic imaging of human coronary atherosclerosis in two spectral bands. Photoacoustics 2014; 2: 12–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wang Y, Huang YY, Wang Y, et al. Photobiomodulation of human adipose-derived stem cells using 810 nm and 980 nm lasers operates via different mechanisms of action. Biochim Biophys Acta Gen Subjects 2017; 1861: 441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hochman L. Photobiomodulation therapy in veterinary medicine: a review. Top Companion Anim Med 2018; 33(3): 83–88. [DOI] [PubMed] [Google Scholar]
- 73. Kondoh H, Lleonart ME, Nakashima Y, et al. A high glycolytic flux supports the proliferative potential of murine embryonic stem cells. Antioxid Redox Signal 2007; 9(3): 293–239. [DOI] [PubMed] [Google Scholar]
- 74. Chen CT, Shih YR, Kuo TK, et al. Coordinated changes of mitochondrial biogenesis and antioxidant enzymes during osteogenic differentiation of human mesenchymal stem cells. Stem Cells 2008; 26(4): 960–968. [DOI] [PubMed] [Google Scholar]
- 75. Sui B, Hu C, Jin Y. Mitochondrial metabolic failure in telomere attrition-provoked aging of bone marrow mesenchymal stem cells. Biogerontology 2016; 17(2): 267–279. [DOI] [PubMed] [Google Scholar]
- 76. Angelova PR, Barilani M, Lovejoy C, et al. Mitochondrial dysfunction in Parkinsonian mesenchymal stem cells impairs differentiation. Redox Biol 2018; 14: 474–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ocarino NM, Boeloni JN, Goes AM, et al. Osteogenic differentiation of mesenchymal stem cells from osteopenic rats subjected to physical activity with and without nitric oxide synthase inhibition. Nitric Oxide 2008; 19: 320–325. [DOI] [PubMed] [Google Scholar]
- 78. Chen H, Min XH, Wang QY, et al. Pre-activation of mesenchymal stem cells with TNF-α, IL-1β and nitric oxide enhances its paracrine effects on radiation-induced intestinal injury. Sci Rep 2015; 5: 8718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mujoo K, Krumenacker JS, Murad F. Nitric oxide-cyclic GMP signaling in stem cell differentiation. Free Radic Biol Med 2011; 51(12): 2150–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lee MN, Hwang HS, Oh SH, et al. Elevated extracellular calcium ions promote proliferation and migration of mesenchymal stem cells via increasing osteopontin expression. Exp Mol Med 2018; 50(11): 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Sun S, Liu Y, Lipsky S, et al. Physical manipulation of calcium oscillations facilitates osteodifferentiation of human mesenchymal stem cells. FASEB J 2007; 21(7): 1472–1480. [DOI] [PubMed] [Google Scholar]
- 82. Amaroli A, Ferrando S, Benedicenti S. Photobiomodulation affects key cellular pathways of all life-forms: considerations on old and new laser light targets and the calcium issue. Photochem Photobiol 2019; 95: 455–459. [DOI] [PubMed] [Google Scholar]
- 83. Burova E, Borodkina A, Shatrova A, et al. Sublethal oxidative stress induces the premature senescence of human mesenchymal stem cells derived from endometrium. Oxid Med Cell Longev 2013; 2013: 474931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Wu J, Niu J, Li X, et al. TGF-β1 induces senescence of bone marrow mesenchymal stem cells via increase of mitochondrial ROS production. BMC Dev Biol 2014; 14: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kawano S, Otsu K, Kuruma A, et al. ATP autocrine/paracrine signaling induces calcium oscillations and NFAT activation in human mesenchymal stem cells. Cell Calcium 2006; 39(4): 313–324. [DOI] [PubMed] [Google Scholar]
- 86. Li Q, Gao Z, Chen Y, et al. The role of mitochondria in osteogenic, adipogenic and chondrogenic differentiation of mesenchymal stem cells. Protein Cell 2017; 8(6): 439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Paliwal S, Chaudhuri R, Agrawal A, et al. Regenerative abilities of mesenchymal stem cells through mitochondrial transfer. J Biomed Sci 2018; 25: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Li C, Cheung MKH, Han S, et al. Mesenchymal stem cells and their mitochondrial transfer: a double-edged sword. Biosci Rep 2019; 39: BSR20182417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Egusa H, Sonoyama W, Nishimura M, et al. Stem cells in dentistry–part II: clinical applications. J Prosthodont Res 2012; 56: 229–248. [DOI] [PubMed] [Google Scholar]
- 90. Zigdon-Giladi H, Bick T, Lewinson D, et al. Mesenchymal stem cells and endothelial progenitor cells stimulate bone regeneration and mineral density. J Periodontol 2014; 85: 984–990. [DOI] [PubMed] [Google Scholar]
- 91. Lipovsky A, Oron U, Gedanken A, et al. Low-level visible light (LLVL) irradiation promotes proliferation of mesenchymal stem cells. Lasers Med Sci 2013; 28: 1113–1117. [DOI] [PubMed] [Google Scholar]
- 92. Kushibiki T, Hirasawa T, Okawa S, et al. Low reactive level laser therapy for mesenchymal stromal cells therapies. Stem Cells Int 2015; 2015: 974864–974912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Jenkins PA, Carroll JD. How to report low-level laser therapy (LLLT)/photomedicine dose and beam parameters in clinical and laboratory studies. Photomed Laser Surg 2011; 29: 785–787. [DOI] [PubMed] [Google Scholar]
- 94. Amaroli A, Sabbieti MG, Marchetti L, et al. The effects of 808-nm near-infrared laser light irradiation on actin cytoskeleton reorganization in bone marrow mesenchymal stem cells. Cell Tissue Res 2021; 383: 1003–1016. [DOI] [PubMed] [Google Scholar]
- 95. Merigo E, Bouvet-Gerbettaz S, Boukhechba F, et al. Green laser light irradiation enhances differentiation and matrix mineralization of osteogenic cells. J Photochem Photobiol B 2016; 155: 130–136. [DOI] [PubMed] [Google Scholar]
- 96. Hamblin MR, Ferraresi C, Huang Y-Y, et al. Low-level light therapy: photobiomodulation. Washington: Society of Photo-Optical Instrumentation Engineers (SPIE), 2018. [Google Scholar]
- 97. Treiser MD, Yang EH, Gordonov S, et al. Cytoskeleton-based forecasting of stem cell lineage fates. Proc Natl Acad Sci U S A 2010; 107: 610–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Amaroli A, Ravera S, Parker S, et al. 808-nm laser therapy with a flat-top handpiece photobiomodulates mitochondria activities of Paramecium primaurelia (Protozoa). Lasers Med Sci 2016; 31: 741–747. [DOI] [PubMed] [Google Scholar]
- 99. Amaroli A, Arany P, Pasquale C, et al. Improving consistency of photobiomodulation therapy: A novel flat-top beam hand-piece versus standard gaussian probes on mitochondrial activity. Int J Mol Sci 2021; 22: 7788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Hanna R, Agas D, Benedicenti S, et al. A comparative study between the effectiveness of 980 nm photobiomodulation delivered by hand-piece with Gaussian vs. flat-top profiles on osteoblasts maturation. Front Endocrinol 2019; 10: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Sefati N, Abbaszadeh HA, Fadaei Fathabady F, et al. The combined effects of mesenchymal stem cell conditioned media and low-level laser on stereological and biomechanical parameter in hypothyroidism rat model. J Laser Med Sci 2018; 9: 243–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Wang L, Wu F, Liu C, et al. Low-level laser irradiation modulates the proliferation and the osteogenic differentiation of bone marrow mesenchymal stem cells under healthy and inflammatory condition. Lasers Med Sci 2019; 34: 169–178. [DOI] [PubMed] [Google Scholar]
- 103. Peat FJ, Colbath AC, Bentsen LM, et al. In vitro effects of high-intensity laser photobiomodulation on equine bone marrow-derived mesenchymal stem cell viability and cytokine expression. Photomed Laser Surg 2018; 36: 83–91. [DOI] [PubMed] [Google Scholar]
- 104. Agas D, Hanna R, Benedicenti S, et al. Photobiomodulation by near-infrared 980-nm wavelengths regulates pre-osteoblast proliferation and viability through the PI3K/Akt/Bcl-2 pathway. Int J Mol Sci 2021; 22: 7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Robling AG, Turner CH. Mechanical signaling for bone modeling and remodeling. Crit Rev Eukaryot Gene Expr 2009; 19: 319–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Agas D, Amaroli A, Lacava G, et al. Loss of p62 impairs bone turnover and inhibits PTH-induced osteogenesis. J Cell Physiol 2020; 235(10): 7516–7529. [DOI] [PubMed] [Google Scholar]
- 107. Asadullah K, Sterry W, Volk HD. Interleukin-10 therapy-review of a new approach. Pharmacol Rev 2003; 55: 241–269. [DOI] [PubMed] [Google Scholar]
- 108. Khacho M, Clark A, Svoboda DS, et al. Mitochondrial dynamics impacts stem cell identity and fate decisions by regulating a nuclear transcriptional program. Cell Stem Cell 2016; 19: 232–247. [DOI] [PubMed] [Google Scholar]
- 109. Fallahnezhad S, Piryaei A, Tabeie F, et al. Low-level laser therapy with helium-neon laser improved viability of osteoporotic bone marrow-derived mesenchymal stem cells from ovariectomy-induced osteoporotic rats. J Biomed Opt 2016; 21: 98002. [DOI] [PubMed] [Google Scholar]
- 110. Leonida A, Paiusco A, Rossi G, et al. Effects of low-level laser irradiation on proliferation and osteoblastic differentiation of human mesenchymal stem cells seeded on a three-dimensional biomatrix: in vitro pilot study. Lasers Med Sci 2013; 28: 125–132. [DOI] [PubMed] [Google Scholar]
- 111. Abdel Hamid MA, Zaied AA, Zayet MK, et al. Efficacy of Flat-Top hand-piece using 980 nm diode laser photobiomodulation on socket healing after extraction: split-mouth experimental model in dogs. Photochem Photobiol 2021; 97: 627–633. [DOI] [PubMed] [Google Scholar]
- 112. Karu T. Is it time to consider photobiomodulation as a drug equivalent? Photomed Laser Surg 2013; 31(5): 189–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Amaroli A, Benedicenti S, Bianco B, et al. Electromagnetic dosimetry for isolated mitochondria exposed to near-infrared continuous-wave illumination in photobiomodulation experiments. Bioelectromagnetics 2021; 42(5): 384–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Salehpour F, Cassano P, Rouhi N, et al. Penetration profiles of visible and near-infrared lasers and light-emitting diode light through the head tissues in animal and human species: a review of literature. Photobiomodul Photomed Laser Surg 2019; 37: 581–595. [DOI] [PubMed] [Google Scholar]
- 115. Souza-Barros L, Dhaidan G, Maunula M, et al. Skin color and tissue thickness effects on transmittance, reflectance, and skin temperature when using 635 and 808 nm lasers in low intensity therapeutics. Lasers Surg Med 2018; 50: 291–301. [DOI] [PubMed] [Google Scholar]
- 116. Lanzafame R. Light dosing and tissue penetration: it is complicated. Photobiomodul Photomed Laser Surg 2020; 38: 393–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Smalley PJ. Laser safety: risks, hazards, and control measures. Laser Ther 2011; 20: 95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Barat K. Ken Barat Laser Safety in specialized applications. Melville, New York: AIP Publishing; (online), 2021. [Google Scholar]
- 119. Bensadoun RJ, Epstein JB, Nair RG, et al.; World Association for Laser Therapy (WALT). Safety and efficacy of photobiomodulation therapy in oncology: a systematic review. Cancer Med 2020; 9: 8279–8300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Pasquale C, Utyuzh A, Mikhailova MV, et al. Recovery from idiopathic facial paralysis (Bell’s palsy) using photobiomodulation in patients non-responsive to standard treatment: a case series study. Photonics 2021; 8: 341. [Google Scholar]