Abstract
We describe splenic infarction (SI), an infrequent condition, in an 82‐year‐old COVID‐19 patient with chronic atrial fibrillation (AF). COVID‐19 may cause thrombosis, and AF is a predisposing factor for splenic infarction. Suspicion of SI may be warranted in COVID‐19 patients with abdominal pain, especially if a predisposing factor exists.
Keywords: atrial fibrillation, COVID‐19, SARS‐CoV‐2, splenic infarction, thrombosis
We describe splenic infarction (SI), an infrequent condition, in an 82‐year‐old COVID‐19 patient with chronic atrial fibrillation (AF). COVID‐19 may cause thrombosis, and AF is a predisposing factor for splenic infarction. Suspicion of SI may be warranted in COVID‐19 patients with abdominal pain, especially if a predisposing factor exists.
![]()
1. INTRODUCTION
Coronavirus disease of 2019 (COVID‐19) appears to have a high propensity for arterial and venous thrombosis, theorized to originate from interactions between the immunological and inflammatory systems and the coagulation system. 1 , 2 The lungs are the most common site of these coagulopathic events in COVID‐19. However, these events have been observed in other organs, such as the abdominal visceral organs, with a lower frequency. 3 , 4 New clinical presentations related to severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection are identified every day. The lack of previous experience with COVID‐19 has resulted in uncertainty about the management of the complications. COVID‐19 cases with splenic infarction (SI) have been uncommon in the literature so far. SI is a rare disorder that can present as abdominal pain and occur as a result of splenic ischemia due to thrombosis or embolism. 3 , 5 , 6 Here, we describe a case of SI, which occurred in an 82‐year‐old woman patient with COVID‐19 and a history of atrial fibrillation (AF). Reporting these types of cases can help to become more familiar with the possible complications of this viral disease and its better management.
2. CASE PRESENTATION
An 82‐year‐old woman was admitted to the emergency department due to generalized abdominal pain. She had a history of hypertension, chronic atrial fibrillation, ischemic heart disease, and chronic obstructive pulmonary disease (COPD). Her daily medications included losartan, warfarin, Seroflo (fluticasone + salmeterol), Spiriva (tiotropium), and montelukast. Sixteen days ago, the patient had headache, myalgia, chills, fever, productive cough, nausea, and vomiting, with no dyspnea and was diagnosed with COVID‐19 by RT‐PCR test for SARS‐CoV‐2 infection using an oropharyngeal swab, and received two outpatient courses of doxycycline, levofloxacin, bromhexine, and theophylline. The antibiotics were used for pneumonia symptoms.
At the time of admission, the patient was well‐appearing, alert, and afebrile (35.8°C) with a respiratory rate of 20 breaths/min, heart rate of 65 beats/min, blood pressure of 165/90 mm Hg, and peripheral oxygen saturation of 85% in room air and 96% while using a mask with reservoir bag. Her lungs were clear on auscultation. Her abdomen was soft without scar, tenderness, and organomegaly.
Laboratory studies showed normal bilirubin, lactate dehydrogenase, creatine phosphokinase, and liver function tests. However, there were elevated levels of white blood cell (WBC) count (12,700/mm3, reference value: 4–10.5), erythrocyte sedimentation rate (26 mm/h, normal <20), qualitative C‐reactive protein (CRP) testing (2+, normal: negative), D‐Dimer (1940 ng/ml, normal < 500), and ferritin (670 ng/ml, reference value: 8–350). The activated partial thromboplastin time and prothrombin time INR (international normalized ratio) were 57 s (reference value: 25–45) and 1.4 (reference value: 0.95–1.2), respectively.
Spiral pulmonary computed tomography (CT) angiography with a low dose high‐resolution computed tomography (HRCT) using the COVID‐19 protocol showed peripheral and peribronchovascular distribution of ground‐glass opacities, affecting 40% of the parenchyma, suggestive of SARS‐CoV‐2 infection (Figure 1). Findings of lower extremity veins on color and spectral doppler ultrasonography were unremarkable. The pelvic and abdominal ultrasound images revealed a hypoechoic area (approximately 75 × 67 × 46 mm) in the inferior pole of the spleen. For further investigation, CT scan was recommended by the radiologist. Spiral abdominal CT scan and multislice abdominal and pelvic CT scan with contrast demonstrated a wedge‐shaped hypodense region in the inferior pole of the spleen (approximately 90 × 46 mm), suggestive of splenic infarction (Figure 2). CT scan also revealed the presence of renal scarring in the left kidney. There were no clinical signs of gastrointestinal tract infection or any other localized infections. Electrocardiogram exhibited atrial fibrillation. Transthoracic echocardiography showed a normal heart with a left ventricular ejection fraction (LVEF) of 50% and no sign of endocarditis.
FIGURE 1.
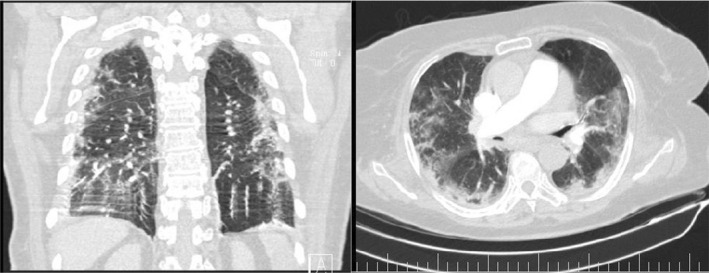
Ground‐glass opacities suggestive of SARS‐CoV‐2 infection
FIGURE 2.
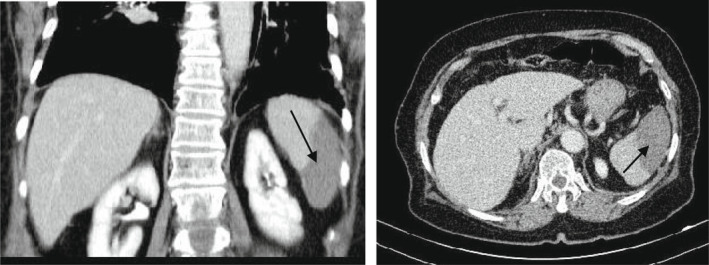
Abdominopelvic CT with IV and oral contrast showing wedge‐shaped hypodense region (black arrows) in the inferior pole of the spleen suggestive of splenic infarction
Warfarin was discontinued from the beginning of the hospitalization, and intravenous heparin was started. The patient was also treated with therapeutic doses of Seroflo, Spiriva, dexamethasone, insulin, bromhexine, metronidazole, pantoprazole, and metoprolol. Due to the patient's old age and female gender, the cardiologist recommended twice‐daily administration of 5 mg of apixaban and discontinuation of intravenous heparin. The patient tolerated the treatment. The abdominal pain gradually decreased, and the patient was discharged on the eighth day. She was also recommended to continue apixaban.
3. DISCUSSION
In the setting of acute SI, conventional ultrasonography has low sensitivity, whereas an abdominal CT scan with intravenous contrast is the imaging modality of choice. 5 , 6 The CT findings in our SARS‐CoV‐2 infected patient showed splenic infarction (SI). SI occurs as a result of splenic ischemia caused by thrombosis or embolism. 5 , 6 An abdominal pain, especially in the hypochondrium or the left flank, with a predisposing factor such as hypercoagulable state, structural heart disease with atrial fibrillation, blood‐borne malignancy, trauma, and pancreatic disorders could be the typical presentation of SI. 3 , 5 , 6 , 7
Hypercoagulability is one of the most typical features of the pathogenesis of SARS‐CoV‐2 infection. 6 According to the studies, the hypercoagulability in COVID‐19 is caused by interactions between the immune systems and the coagulation system. 1 Individuals infected with this virus often show arterial and venous thrombosis even after prophylactic or therapeutic anticoagulation. 2 , 3 , 6 Therefore, COVID‐19 may have contributed to the onset of SI in our patient by inducing immune system activation and inflammation (the elevated WBC count and CRP level) and consequently activating the coagulation system (increased D‐Dimer level). Although pulmonary embolism is the most common event associated with COVID‐19, thrombotic events have also been recorded in other organs and systems such as abdominal visceral organs, including intestine, kidney, and spleen. 3 , 4
In addition, our patient had AF, which is one of the common predisposing factors for infarction of visceral organs such as kidney and spleen. Indeed, renal scarring in our patient could indicate a previous infarction of this organ due to AF. Furthermore, the optimal INR level for avoiding thrombosis in a patient with AF is 2.0 to 3.0 8 ; however, our patient's INR level was subtherapeutic (INR: 1.4). Therefore, the patient was not appropriately anticoagulated for an AF context and was already prone to thromboembolism; the addition of COVID‐19 to this state may have increased the risk of splenic infarction. Although studies indicate that a considerable proportion of thromboses in SARS‐CoV‐2 infection occur in patients with no history of predisposing factors, 6 it cannot be ruled out that AF may have played a role in this case. The fact that 40% of patients with SI were found to have had more than one plausible predisposing factor 6 , 8 strengthens our assumption. Although the exact prevalence of SI, as a rare cause of abdominal pain, is unclear, 5 it seems that its occurrence is higher in patients with COVID‐19 than in patients admitted to hospitals for other reasons. 5 , 6
The treatment of SI is generally conservative and is primarily based on the cause of infarction. In many cases, no treatment is required, and the abdominal pain will subside in 7–14 days. 5 Surgery is rarely required and is only considered in people with persistent symptoms. 6 Full anticoagulation is the recommended treatment for SI in cases with COVID‐19, as well as in situations of hypercoagulability and severe thrombosis. 6 , 9 Our patient was discharged following COVID‐19 treatment and apixaban administration on the eighth day. Apixaban is a direct oral anticoagulant (DOAC) that inhibits factor Xa directly. Studies have definitely established the DOACs as the first‐line agents for preventing stroke and systemic embolism in patients with non‐valvular AF. 10
4. CONCLUSION
The current case provides more evidence that COVID‐19 can be associated with splenic infarction. As a predisposing factor, the presence of AF in combination with COVID‐19 may have contributed to this condition, especially that the patient was not appropriately anticoagulated for an AF context. Although SI is a rare condition, suspicion of SI could be appropriate in the presence of abdominal pain in individuals with COVID‐19, particularly if there is also another risk factor for thrombosis, such as AF.
AUTHOR CONTRIBUTIONS
HS was involved in the diagnosis and treatment of the patient. SH involved in supervision and writing of the manuscript. RS revised the manuscript. RG and MK were involved in laboratory diagnosis of the patients and data collection. ALL authors read and approved the final manuscript.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
CONSENT
Written informed consent was obtained from the patient's next of kin for publication of this case report and any accompanying images.
ACKNOWLEDGMENTS
Not applicable.
Sarmadian R, Ghasemikhah R, Sarmadian H, Khosravi M, Hassani S. Post‐COVID‐19 splenic infarction in a patient with chronic atrial fibrillation: A case report. Clin Case Rep. 2022;10:e06011. doi: 10.1002/ccr3.6011
DATA AVAILABILITY STATEMENT
Not applicable.
REFERENCES
- 1. Spyropoulos AC. The management of venous thromboembolism in hospitalized patients with COVID‐19. Blood Adv. 2020;4(16):4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Santos Leite Pessoa M, Franco Costa Lima C, Farias Pimentel AC, Godeiro Costa JC, Bezerra Holanda JL. Multisystemic infarctions in COVID‐19: focus on the spleen. Eur J Case Rep Intern Med. 2020;7(7):001747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Castro GRA, Collaço IA, Dal Bosco CLB, Corrêa GG, Dal Bosco GB, Corrêa GL. Splenic infarction as a complication of COVID‐19 in a patient without respiratory symptoms: a case report and literature review. IDCases. 2021;24:e01062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Al‐Mashdali AF, Alwarqi AF, Elawad SM. Simultaneous renal infarction and splenic infarction as a possible initial manifestation of COVID‐19: a case report. Clin Case Rep. 2021;9(11):e04819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chapman J, Helm TA, Kahwaji CI. Splenic infarcts. [updated 2021 Jul 21]. StatPearls [Internet]. StatPearls Publishing; 2021. Jan. Available from. https://www.ncbi.nlm.nih.gov/books/NBK430902/ [PubMed] [Google Scholar]
- 6. Sztajnbok J, Brasil L, Romero LA, et al. Splenic infarction with aortic thrombosis in COVID‐19. Am J Med Sci. 2021;362:418‐423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Im JH, Chung M‐H, Lee H‐J, et al. Splenic infarction and infectious diseases in Korea. BMC Infect Dis. 2020;20(1):915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brett AS, Azizzadeh N, Miller EM, Collins RJ, Seegars MB, Marcus MA. Assessment of clinical conditions associated with splenic infarction in adult patients. JAMA Intern Med. 2020;180(8):1125‐1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schattner A, Adi M, Kitroser E, Klepfish A. Acute splenic infarction at an academic general hospital over 10 years: presentation, etiology, and outcome. Medicine (Baltimore). 2015;94(36):e1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hoffbrand AV, Steensma DP. Hoffbrand's Essential Haematology. John Wiley & Sons Ltd.; 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


