Abstract
Coronavirus disease 2019 (COVID-19) has so far killed many people; with the majority of deaths occurring in people over the age of 65 years. It has been noted that the severity and outcome of COVID-19 depends in part on the patient’s age. The combination of three factors could explain this ascertainment: the first is linked the lung aging, the second is the associated comorbidities in elderly subjects and the third is the particularities of COVID-19. Here we emphasize the modifications linked to pulmonary aging and their role in worsening the COVID-19 disease.
Keywords: COVID-19, lung aging, pulmonary functions, ACE2, co-morbidities
Résumé
Le coronavirus 2019 (Covid-19) a jusqu'à présent tué de nombreuses personnes. La majorité des décès est survenue chez des personnes de plus de 65 ans. Une des constatations les plus fréquentes est que la gravité et l’évolution du Covid-19 dépendent en grande partie de l’âge du patient. La combinaison de trois facteurs pourrait expliquer ce constat : le premier est lié au vieillissement pulmonaire, le second est lié aux comorbidités associées chez le sujet âgé et le troisième est lié aux particularités de Covid-19. Dans cette mise au point, nous soulignons les modifications liées au vieillissement pulmonaire physiologiques et leur rôle dans l'aggravation de la maladie covid19 chez le sujet âgé.
Mots Clés: Covid-19, vieillissement pulmonaire, fonction respiratoire, ACE2, Co-morbidités
Introduction
Among COVID-19 patients, elderly patients have a higher mortality rate and symptomatic infection rate. Approximately 80% and 90% of deaths have occurred in patients aged >70 years and ≥60 years in Korea and Italy, respectively (1 , 2 ).
Why is the high mortality of COVID-19 so strongly associated with age? The answer probably lies in the combination of three factors: the first factor is linked to the aging process especially of the lungs (pulmonary aging), the second is linked to associated co morbidities in elderly subjects and the third is linked to the particularities of COVID-19.
Pulmonary aging can be of intrinsic (genetic) or extrinsic (environmental) origin. The lung is the most vulnerable organ to extrinsic aging since it is in direct contact with environmental factors: tobacco, air pollution, occupational exposure. Indeed exposure to cigarette smoke and other environmental stressors over the life span accelerate biologic processes associated with normal aging. In addition, the elderly are often the subject of several chronic diseases (obesity, diabetes, and cardiovascular conditions) which tend to accelerate the general aging of the individual (3, 4, 5 ).
Methods
A literature search, which covered the period 1993 to September 2020, was conducted using the Pubmed and Google. The search strategy had used the following MeSH words: “Spirometry”, “COVID-19”, “ACE2”, “immunosenescence” AND “Aging”. The studies results’ were presented in the context of all other available evidence. Only studies written in English, were included.
Current state of knowledge
There is a progressive, age-associated decrease in lung function, the forced expiratory volume at the first second (FEV1) declines by ~30 mL per year in men and women, whereas the forced vital capacity (FVC) begins to decline later and at a slower rate (20 mL per year) resulting in a decrease in the FEV1/FVC ratio (6 ).
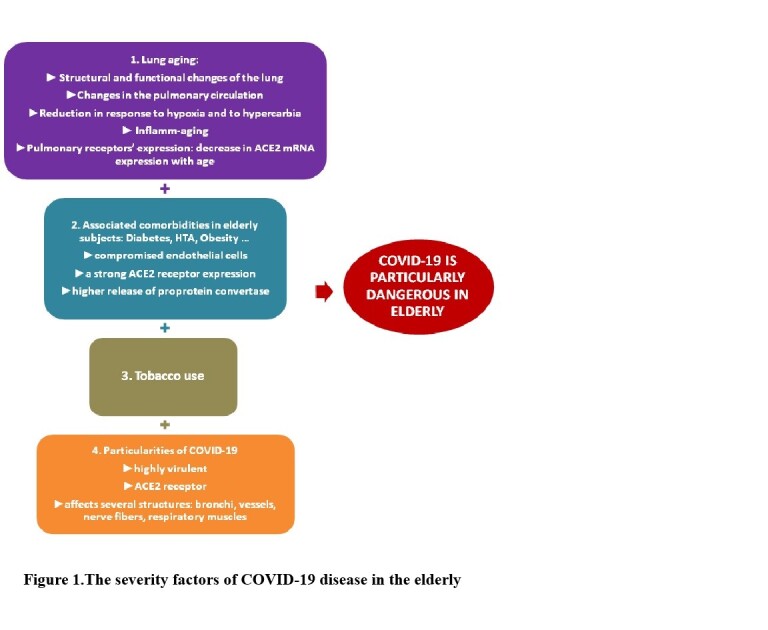
The losses in volumes and flow rates due to aging are due to structural and functional changes of the lung:the alteration of the elasticity of thoraco-pulmonary tissues,the kyphosis or curvature of the spine, the decrease in the strength of the respiratory muscles, the changes in the pulmonary circulation, the increase in ventilation-perfusion inequality, the reduction in response to hypoxia and to hypercarbia, the immunosenescence which can cause a low-grade systemic inflammation described as inflamm-aging and the regulation of pulmonary receptors expression (beta2 and Angiotensin converting enzyme-2 (ACE2) ( Figure 1 )(3, 6, 7 ). All these changes weaken the respiratory system and make it vulnerable to serious infections such as COVID-19 infection.
Figure1: The severity factors of COVID-19 disease in the elderly.
The decrease in the strength of the respiratory muscles with age is related to changes in skeletal muscle structure including the diaphragm (8 ). Physical inactivity and smoking exposure of many elderly subjects worsens skeletal muscle dysfunction by aggravating proteolysis and inhibiting protein synthesis, leading to loss of muscle mass (9 ).
In the study of Zhonghua Shi et al., they provide unique evidence for ACE2 expression in the human diaphragm and severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) viral infiltration in the diaphragm of a subset of COVID-19 patients. In COVID-19 patients, we report increased expression of genes involved in fibrosis and histological evidence for the development of fibrosis in the diaphragm (10 ).
Age-related changes in the pulmonary circulation result in an increase in pulmonary artery systolic pressure, increased ventilation-perfusion inequality and a progressive decrease in the diffusing capacity for carbon monoxide (DLCO) in elderly. The reduction in DLCO may be due to declines in the alveolar surface area and, possibly, in the density of lung capillaries (11 12 ). These changes in pulmonary circulation and alveolar ventilation could explain desaturation in the event of infection by COVID-19.
A reduction in response to hypoxia and to hypercarbia is noted in elderly subjects. The hypoxic ventilatory response is substantially reduced in smokers. So aging when associated to tobacco use leads to a loss of potentially protective mechanisms making elderly subjects more vulnerable to infections with COVID-19 (13 ).
The immune system also undergoes an aging process termed immunosenescence which can cause an inflamm-aging. Both innate immunity and adaptive immunity are affected by aging. Declining function of innate immune cells with aging also contributes to the dysregulation of the adaptive immune system via molecular cross-talk ( 14 15 ). Humoral immune function also changes significantly with aging. These changes include decreased antibody responses and diminished production of high-affinity antibodies related to defective surface immunoglobulin/B-cell receptor affinity, decreased signaling, and reduced B-cell proliferation. There is also a loss of naïve B-cells and an increase in memory cells with age,resulting in a reduced ability to respond to new antigens (16 17 18 ). The COVID-19 pandemic serves as a potent reminder that older people are at very high risk of adverse outcomes from infectious disease because of comorbidities associated with ageing and decreased immunological competence (immunosenescence). Some authors suggest tackling immunosenescence to improve COVID-19 outcomes and vaccine response in older adults (18 ).
The pulmonary receptors’ expression changes with age. ACE2 was predominantly expressed in alveolar epithelium (alveolar cells type 2), bronchiolar epithelium, endothelium and smooth muscle cells of pulmonary vessels (19 ). ACE2 expression is dramatically reduced with aging in both genders (20 ). Sina Booeshaghi and LiorPachter found that the decrease in ACE2 mRNA expression with age in lungs is likely due to two underlying phenomena: a reduction of ACE2 mRNA in ciliated cells, and a shift in ciliated cell abundance with age (21 ). SARS CoV-2 can infect human target sites through the cellular angiotensin-converting enzyme II (ACE2) receptor in the respiratory tract. ACE2 was recently identified as a functional receptor for COVID-19 and is therefore a prime target for pathogenesis and pharmacological intervention (19 ). Certain comorbidities (diabetes, chronic obstructive pulmonary disease (COPD), cardiovascular diseases (CVD), hypertension, malignancies, HIV and obesity) are associated with a strong ACE2 receptor expression and higher release of proprotein convertase that enhances the viral entry into the host cells. The comorbidities lead to the COVID-19 patient into a vicious infectious circle of life and are substantially associated with significant morbidity and mortality (22 ).
In healthy individuals, endothelial cells help to regulate blood pressure, prevent inflammation, and inhibit clotting, in part through the continual production of nitric oxide (NO); they also serve as gatekeepers for molecules passing in and out of the bloodstream. Some elderly patients who have obesity, diabetes, and cardiovascular conditions have compromised endothelial cells. By attacking those cells, COVID-19 infection causes leaking fluids out of the vessels and blood clotting. Those changes spark inflammation throughout the body and fuel the acute respiratory distress syndrome (ARDS) responsible for most patient deaths ( Figure 1 )(23 ).
Conclusion
These changes related to age may expose elderly subjects to an increased risk of serious infections by COVID-19, decompensation of chronic diseases, addiction and death.
- The author declares that there are no conflicts of interest.
References
- Guan W J, Ni Z Y, Hu Y. for the China Medical Treatment Expert Group for COVID-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1728. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COVID-19 Surveillance Group. Characteristics of COVID-19 patients dying in Italy: report based on available data on March 20th, 2020. Rome, Italy: InstitutoSuperiore Di Sanita; 2020 . https://www.epicentro.iss.it/coronavirus/bollettino/ReportCOVID-2019_20_marzo_eng.pdfpdf
- Guénard H, Rouatbi S. Physiological aspects of the decline of pulmonary function with age. Revue des maladies respiratoires. 2004;21(5 Pt 3) [PubMed] [Google Scholar]
- Partridge López-Otín C Blasco MA. L. Serrano M. Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepeule Johanna, Litonjua Augusto A, Gasparrini Antonio, Koutrakis Petros, Sparrow David, Vokonas Pantel S, Schwartz Joel. Environmental research. Vol. 165. Elsevier; 2018. Lung function association with outdoor temperature and relative humidity and its interaction with air pollution in the elderly; pp. 110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culham Elsie G, Jimenez Hilda A I, King Cheryl E. Spine. 11. Vol. 19. Ovid Technologies (Wolters Kluwer Health); 1994. Thoracic Kyphosis, Rib Mobility, and Lung Volumes in Normal Women and Women With Osteoporosis; pp. 1250–1255. [DOI] [PubMed] [Google Scholar]
- Cho Won-Kyung K, Lee Chun Geun, Kim Lark Kyun. Yonsei Medical Journal. 5. Vol. 60. Yonsei University College of Medicine; 2019. COPD as a Disease of Immunosenescence; pp. 407–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouatbi S, Moussa Ben, Guezguez S, F, Saad Ben, H. Muscle dysfunction in case of active tobacco consumption. Science & Sports. 2017;32(4):119–145. [Google Scholar]
- Jackman Robert W, Kandarian Susan C. American Journal of Physiology-Cell Physiology. 4. Vol. 287. American Physiological Society; 2004. The molecular basis of skeletal muscle atrophy; pp. C834–C843. [DOI] [PubMed] [Google Scholar]
- Shi Zhonghua, De Vries Heder J, Vlaar Alexander P J, Van Der Hoeven Johannes, Boon Reinier A, Heunks Leo M A, Ottenheijm Coen A C. JAMA Internal Medicine. 1. Vol. 181. American Medical Association (AMA); 2021. Diaphragm Pathology in Critically Ill Patients With COVID-19 and Postmortem Findings From 3 Medical Centers; pp. 122–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin David L, Buxton Richard B, Spiess James P, Arai Tatsuya, Balouch Jamal, Hopkins Susan R. Journal of Applied Physiology. 5. Vol. 102. American Physiological Society; 2007. Effects of age on pulmonary perfusion heterogeneity measured by magnetic resonance imaging; pp. 2064–2070. [DOI] [PubMed] [Google Scholar]
- Rouatbi S, Ouahchi YF, Harrabi I, Tabka Z, Guénard H. Physiological factors influencing pulmonary capillary volume and membrane diffusion. Revue des maladies respiratoires. 2006;23(3 Pt 1):211–218. doi: 10.1016/s0761-8425(06)71570-4. [DOI] [PubMed] [Google Scholar]
- Lalley P M. Respiratory Physiology & Neurobiology. 3. Vol. 187. Elsevier BV; 2013. The aging respiratory system—Pulmonary structure, function and neural control; pp. 199–210. [DOI] [PubMed] [Google Scholar]
- Baylis Daniel, Bartlett David B, Patel Harnish P, Roberts Helen C. Longevity & Healthspan. 1. Vol. 2. Springer Science and Business Media LLC; 2013. Understanding how we age: insights into inflammaging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelo-Branco Camil, Soveral Iris. Gynecological Endocrinology. 1. Vol. 30. Informa UK Limited; 2014. The immune system and aging: a review; pp. 16–22. [DOI] [PubMed] [Google Scholar]
- Cox Lynne S, Bellantuono Ilaria, Lord Janet M, Sapey Elizabeth, Mannick Joan B, Partridge Linda, Gordon Adam L, Steves Claire J, Witham Miles D. The Lancet Healthy Longevity. 2. Vol. 1. Elsevier BV; 2020. Tackling immunosenescence to improve COVID-19 outcomes and vaccine response in older adults; pp. e55–e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solana Rafael, Tarazona Raquel, Gayoso Inmaculada, Lesur Olivier, Dupuis Gilles, Fulop Tamas. Seminars in Immunology. 5. Vol. 24. Elsevier BV; 2012. Innate immunosenescence: Effect of aging on cells and receptors of the innate immune system in humans; pp. 331–341. [DOI] [PubMed] [Google Scholar]
- Whisler R L, Grants I S. Mechanisms of Ageing and Development. 1-2. Vol. 71. Elsevier BV; 1993. Age-related alterations in the activation and expression of phosphotyrosine kinases and protein kinase C (PKC) among human B cells☆; pp. 31–46. [DOI] [PubMed] [Google Scholar]
- Gheblawi Mahmoud, Wang Kaiming, Viveiros Anissa, Nguyen Quynh, Zhong Jiu-Chang C, Turner Anthony J, Raizada Mohan K, Grant Maria B, Oudit Gavin Y. Circulation Research. 10. Vol. 126. Ovid Technologies (Wolters Kluwer Health); 2020. Angiotensin-Converting Enzyme 2: SARS-CoV-2 Receptor and Regulator of the Renin-Angiotensin System; pp. 1456–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xudong Xie, Junzhu Chen, Xingxiang Wang, Furong Zhang, Yanrong Liu. Life Sciences. 19. Vol. 78. Elsevier BV; 2006. Age- and gender-related difference of ACE2 expression in rat lung; pp. 2166–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booeshaghi A Sina, Pachter Lior. biorxiv. Cold Spring Harbor Laboratory; 2020. Decrease in ACE2 mRNA expression in aged mouse lung. [Google Scholar]
- Ejaz Hasan, Alsrhani Abdullah, Zafar Aizza, Javed Humera, Junaid Kashaf, Abdalla Abualgasim E, Abosalif Khalid OA, Ahmed Zeeshan, Younas Sonia. Journal of infection and public health. 12. Vol. 13. Elsevier; 2020. COVID-19 and comorbidities: Deleterious impact on infected patients; pp. 1833–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco Del, Vianello S, Ragusa A M, Caselli R, Bastaa C, G. COVID-19 and cardiovascular consequences: Is the endothelial dysfunction the hardest challenge? Thromb Res. 2020;196:143–51. doi: 10.1016/j.thromres.2020.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]


