Abstract
Objective The aim of the study is to summarize and analyze the efficacy of the multilayered skull base reconstruction using in situ bone flap in endoscopic endonasal approach (EEA) for craniopharyngiomas.
Methods A retrospective review of 65 patients who underwent resection of their histopathology confirmed craniopharyngiomas performed at a single institution. Based on the team's understanding and mastery of skull base reconstruction techniques, patients were divided into two groups according to the methods of reconstruction in two periods. First (March 2015 through August 2016), osseous reconstruction was not adopted and served as the control group (34 cases). Second (September 2016 through July 2019), in situ bone flap repair of the skull base (complete osseous reconstruction) served as observation group (31 cases). The length of hospitalization and nasal exudation, bed rest time of hospital discharge, the incidence of cerebrospinal fluid leaks, lumbar drainage, and intracranial/pulmonary infections were collected and compared.
Results Compared with the control group, patients in the observation group had obviously less lumbar drainage and CSF leakage ( p < 0.05), but had no significant difference in cases of re-operation, meningitis, and pulmonary infection. At the meantime, cases of nasal exudation, bed rest, and hospitalization of the observation group were significantly reduced ( p < 0.05) in the observation group.
Conclusion The multilayered reconstruction technique (especially using in situ bone flap, combined with vascularized pedicled nasoseptal flap) is a safe and effective method in achieving watertight closure after EEEA, and can significantly reduce the incidence of cerebrospinal fluid leaks, and facilitate rehabilitation in skull base reconstruction of craniopharyngiomas.
Keywords: extended endoscopic endonasal approach, craniopharyngioma, skull base reconstruction, multilayer, CSF leak
Background
Craniopharyngioma is a common type of nonmalignant epithelial tumor that develops in the sellar area, and accounts for approximately 2 to 5% of all intracranial tumors. 1 Craniopharyngioma originates along the midline skull base, usually ventral to the optic chiasm. Historically, neurosurgeons have adopted a variety of transcranial microsurgery corridors for reaching and removing these tumors, including the anterolateral pterional and orbitozygomatic, anterior subfrontal and bifrontal approaches. Radical tumor resection with preservation of neurological and endocrinological functions is the primary target in the treatment of craniopharyngioma. All craniotomy approaches share the risk of brain retraction and, importantly, place the optic nerve between the surgeon and the tumor. The optic nerves should be manipulated for exposure of the tumor, and the superior pole of the mass is placed within the operative blind spot. 2 These factors increase the risk of postoperative neurological deficits. The extended endoscopic endonasal approach (EEEA) uses a natural corridor to fully expose the tumor without brain tissue retraction, which allows for minimal trauma and faster recovery. 3 Over the last decade, EEEA has rapidly evolved and currently offers the possibility of treating median skull base lesions with good outcomes. Importantly, the tumor is exposed along its long axis, the tumor is debulked, and the optic apparatus is decompressed early before the nerves are handled; therefore, extended operative working angles are created for maximizing tumor resection while minimizing surgical blind spots. However, postoperative cerebrospinal fluid leaks and reconstruction of the skull base remain significant drawbacks. When determining the appropriate technique for skull base reconstruction, the surgeon must take into consideration the size and location of the lesion, presence, and degree of CSF leak, as well as the availability of donor tissue. A variety of reconstructive techniques have been described in the skull base literature. Multilayer reconstruction of skull base has always been a classical repair method. Especially, the use of pedicled nasoseptal flap has improved the reliability of repair. In the present study, we modified our reconstruction strategy according to clinical experience and adopted in situ bone flap in clinical works innovatively. The bone flap was intactly excised, preserved, and restored at the time of skull base reconstruction. We collected clinical data from 65 patients with craniopharyngioma that underwent EEEA treatment, and different methods of skull base reconstruction were analyzed and compared. The current manuscript reports our preliminary experience using in situ bone flap in skull base reconstruction of craniopharyngiomas in a series.
Methods
Clinical Data
During the period of March 2015 to July 2019, 65 patients (30 men and 35 women; mean age 35.4 years, range 18–66 years; mean course of disease 2.7 years, range 20 days to 9 years) underwent surgery for the removal of craniopharyngioma by EEEA in our department (Department of Neurosurgery, Xijing Hospital, Fourth Military Medical University, Xi'an, China) and were enrolled in this retrospective analysis. Non craniopharyngioma midline suprasellar histopathologies and pediatric patients (<18 years of age) were excluded from the research. Adults undergoing EEA for recurrent tumors were excluded to avoid confounding bias in results. There were no significant differences in patient demographics, presenting symptoms, tumor subtype, or preoperative tumor volumes; no tumors had significant lateral or prechiasmatic extension. The electronic medical records were reviewed for all included patients. The series was divided into two epochs based on the team's understanding and mastery of skull base reconstruction techniques, so patients were divided into two groups according to the methods of reconstruction in different periods. In early epoch (March 2015 through August 2016), like other medical centers, no buttress was taken during the operation (no osseous reconstruction) which served as the control group (34 cases). With the development of endonasal endoscopy, we innovatively put forward the concept of in situ bone flap reconstruction and performed some clinical works. In post epoch (September 2016 through July 2019), in situ bone flap repair of the skull base (complete osseous reconstruction) served as the observation group (31 cases).
Imaging
Magnetic resonance imaging scanning was performed before surgery to provide excellent details about the tumor's size, location, and associated cysts, to analyze the relationship of the tumor and internal carotid artery, pituitary, optic chiasma, and the third ventricle. Computed tomography thin slice scanning was performed to reconstruct the shape of tumor, bones and blood vessels, and observe the outline of the internal carotid artery and its relationship with the tumor.
Surgical Technique and Skull Base Reconstruction
The EEEA (transtuberculum-transplanum) was used for the removal of these craniopharyngiomas. Patients were in a supine position with the head rotated to the right side by 15 to 20 degrees. To reduce mucosal hemorrhage, the nasal cavity is packed with adrenaline-soaked pledgets for 10 minutes. Care was taken to preserve the integrity and blood supply of the mucosal flap. The thigh is also prepared to harvest autologous fascia lata for reconstruction, in case the mucosal is defective. Both surgeons are positioned on the right side of the patient's head and use bi-nostril technique for the entire procedure. The middle turbinate was resected to enhance visibility, a needle electrode was used to make a pedicled nasoseptal flap with the base ranging from sphenoid ostium to the choanae. Then the mucosa with muco-perichondrium was lifted off and stored in the nasopharynx. The anterior and lateral walls of sphenoid sinus were carefully drilled to expose the sphenoid sinus cavity, and the posterior ethmoid sinus was removed using a Kerrison rongeur, thus sufficiently exposing the planum sphenoidale and tuberculum sellae. This exposure also provides enough space for rotating instruments flexibly. The bony septations sinus mucosa were taken out; this maneuver improves skull base landmarks visualization, increasing the nasoseptal flap adherence to the bone in the skull base reconstruction phase. The anatomical landmarks after exposure include the planum sphenoidale, tuberculum sellae, bilateral optic canal, and sellar floor (as shown in Fig. 1A ). The bone flap should be removed integrally with microdrill. In situ bone flap is a microbone, which consisted of planum sphenoidale, tuberculum sellae, partial optic canal, and sellar floor ( Fig. 1B ). The bones over the carotid arteries and optic canal were drilled with copious saline irrigation until an eggshell bone surface could be seen facilitating a complete bone resection. This is critical in reducing thermal damage to neurovascular tissues. The scope of the bone window can be extended outward using appropriate rongeur. We can also use micro-Doppler and neuronavigator to ensure security. The dura is incised along the central axis, and the margins of the incision should be smaller than that of the bone defect, especially for the vertical diameter. This ensures that the bone flap will not slip into the subdural space after surgery. The horizontal diameter could be adjusted according to the lesion's growth direction. After the dura incision, identify the location of optic chiasma and pituitary stalk first, open the arachnoid at the optic chiasm, and explore the supra and subchiasmatic spaces. Endoscopic craniopharyngioma removal follows the same steps as that of the standard transcranial microsurgery, which include identification of the tumor, internal tumor debulking, extracapsular dissection in the arachnoid-capsular plane, and protection of the neurovascular structures. 4 5 Certainly, intraoperative residual tumor is advisable if excessive dissection is likely to result in neurological morbidity.
Fig. 1.
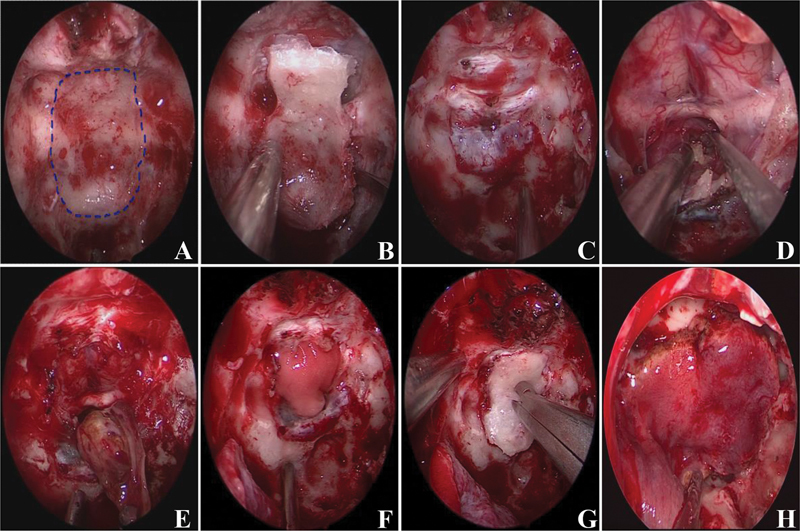
Multilayered skull base reconstruction using an in situ bone flap and pedicled nasoseptal flap (the observation group). ( A ) Blue line suggests the border of the bone window; ( B ) In situ bone flap was made; ( C ) Fully exposed bone window; ( D,E ) Piecemeal tumor was excised piece by piece; ( F ) Artificial biomembrane placed in subdural space (the first layer); ( G ) In situ bone flap placement (the second layer, complete osseous reconstruction); ( H ) Pedicled nasoseptal flap repair the defect completely (the third layer).
Reconstruction of the skull base is an important procedure after operation. We perform multilayered reconstruction to achieve a watertight closure. We place an absorbable artificial biomembrane in all cases as the first step ( Fig. 1F ), and put biomembrane inlay the subdural to complete the first membranous reconstruction was vital step to prevent a postoperative CSF leak, pneumocephalus, and other potential complications. In observation group, in situ bone flap was used on biomembrane to achieve osseous reconstruction maximumlly ( Fig. 1G ), then pedicled nasoseptal flap was covered on the bone flap for further reinforcement ( Fig. 1H ) and extended beyond its edges as far as possible with maximal contact on the bone adjacent to the defect. In contrast, bone reconstruction was not used in the control group and pedicled nasoseptal flap was turned on biomembrane to cover the entire defect. Surgicel and gelatin sponges were packed at the flap edges to prevent its displacement or migration, to further support the flap and accelerate healing. Silver ion gauze was used to fill the nasal cavity. The length of nasal exudation and hospitalization, the incidence of cerebrospinal fluid leaks, intracranial infection, and the number of lumbar drain were compared analyzing the advantages of skull base reconstruction using an in situ bone flap. Lumbar drainage was only performed if nasal exudation increased after surgery, not used as a preventive maneuver. To be prudent, patients were strictly placed in semi-sitting position after the operation to reduce intracranial pressure and pulsation over the skull base defect. Mannitol was given in hospitalization. Broad-spectrum antibiotic therapy was administered for 5 days. Silver ion gauze was removed 2 weeks after surgery ( https://www.youtube.com/watch?v=rZKq_EpDRMA ).
Statistical Analysis
SPSS software was used for the statistical analysis of the data. Data are presented as the mean ± standard deviation. Statistical significance between variables was determined utilizing Student's t -test and Fisher's exact test, p < 0.05 was considered statistically significant.
Results
No significant demographic differences in mean patient age, sex, or presenting symptoms were identified. Neurological deficits as a result of direct neural tissue trauma or vascular compromise, pituitary hormonal dysfunction, and visual changes were not described in detail as we mainly discussed the reconstruction of the skull base in the present study. The cases of gross total resection and recurrence of the craniopharyngiomas were not elaborated in detail. The mean follow-up period was 15.4 months, range 3 to 32 months. The postoperative complications of the patients were presented in Table 1 .
Table 1. Patient information using different methods of skull base reconstruction.
| Title | Observation group ( n = 31) |
Control group ( n = 34) |
p -Value |
|---|---|---|---|
| Lumbar drainage | 1 | 4 | 0.358 |
| CSF leak | 0 | 6 | 0.025 a |
| Re-operation | 0 | 2 | 0.493 |
| Meningitis | 1 | 4 | 0.358 |
| Pulmonary infection | 1 | 3 | 0.612 |
| Nasal exudation | 3.1 ± 0.9 d | 6.1 ± 2.1 d | <0.0001 a |
| Bed rest | 4.5 ± 1.0 d | 7.3 ± 2.5 d | <0.0001 a |
| Hospitalization | 7.2 ± 0.7 d | 9.5 ± 1.4 d | <0.0001 a |
Abbreviation: CSF, cerebrospinal fluid.
p -Value < 0.05 for CSF leak and p < 0.0001 for nasal exudation, bed rest and hospitalization.
Note: Student's t -test or Fisher's exact test is performed if appropriate.
There was significant difference between the two groups in cerebrospinal fluid leaks ( p = 0.025). Six patients (17.6%) in the control group had obvious nasal exudation within 48 hours after surgery and no decreasing trend, which required lumbar drainage. The nasal exudation was significantly reduced in four patients after lumbar drainage, and they were cured and discharged after 1 week; two patients underwent secondary endoscopic surgery due to increased intracranial pneumatosis, although the difference of re-operation was not statistically significant ( p = 0.493). There was no significant difference between the two groups in meningitis and pulmonary infection ( p = 0.358; p = 0.612). In in situ bone flap group, there was one case having cough caused by pneumonia, that led to obvious nasal exudation 4 days postoperatively. Lumbar drainage was required for the sake of reliability and it cured after 5 days. Compared with the control group, the time of nasal exudation in patients who underwent in situ bone flap repair (observation group) was significantly shorter than that in the control group (3.1 ± 0.9 vs. 6.1 ± 2.1; p < 0.0001). All meningitis patients were given extra antibiotic therapy as per local guidelines, and they were discharged safely with no serious complications. The duration of hospitalization in the observation group was also shorter than control group ( p < 0.0001). The duration of bed-rest discharge was, on average, 4.5 days in the observation group, but longer (7.3 days) in the control group. No deaths occurred in the series. Representative images of the skull base after surgery are shown in Fig. 2 . The lesion was completely excised, the nasoseptal mucosal flap healed well, and no herniation of brain tissue was observed. The skull base heals well using in situ bone flap reconstruction as shown using 3D reconstruction. The bone flap remains in its original position of the skull base and is not absorbed.
Fig. 2.
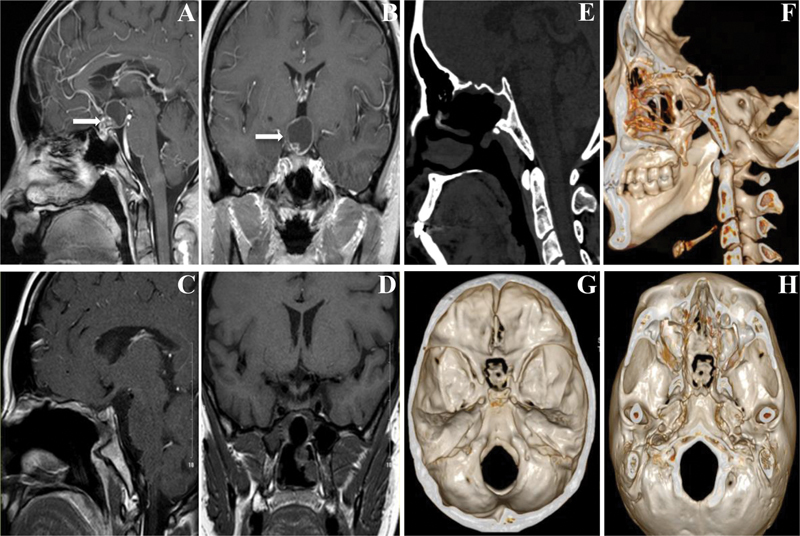
Representative images before and after surgery. ( A,B ) MRI results before surgery ( white arrows suggest the tumor ); ( C,D ) MRI results after surgery show complete resection of the tumor and good healing of the nasoseptal flap 1 month postoperatively; ( E–H ) 3D reconstruction of the skull base 1 year after surgery shows that bone window healed well using in situ bone flap and significant bone absorption was not seen. 3D, three-dimensional: MRI, magnetic resonance imaging.
Discussion
The extended endoscopic endonasal approaches (EEEAs) offer an expanded visual operative field with the potential to resect lesions of the skull base. With the development of technology, both the degree of resection and complication rates have proven comparable to those for transcranial approaches, even visual outcomes may be better via endoscopic endonasal surgery. Along with the development of a variety of endonasal approaches, numerous techniques and materials have been used in effort to achieve a resilient and reliable method of skull base reconstruction. After the pedicled nasoseptal flap development, we observed a striking decrease of CSF leak rates in the expanded endonasal approach series ranging from 14.6 to 4% 6 , 58 to 23.4% 7 for craniopharyngiomas. As the success rates vary widely based on pathology, size of the cranial base defect, experience, and technique, in our study all cases were performed by the same surgeon (Dakuan Gao). The EEEA has become more popular among clinicians. 8 9 Skull base reconstruction, which plays an essential role in preventing postoperative cerebrospinal fluid leaks, brain herniation, tension pneumocephalus, and meningitis, severely restricted the application and development of the extended endonasal approaches. The multilayer reconstruction technique 10 (fat, fascia lata inlay, and onlay, nasoseptal flap covering the whole defect, plus Dura Seal or other fibrin glue) is predominant method in many countries around the world. In this study, we used the biomembrane inlay inside of the dura, served as the first layer to achieve watertight closure. Craniopharyngioma transtuberculum-transplanum approach is a classic representative of endoscopic endonasal surgery 11 . The EEA of CP is relatively stable, the resection range of bone and dura mater could be approximately determined 12 , postoperative cerebrospinal fluid leaks were similar, and data deviation of skull base reconstruction between groups is relatively small. However, when it comes to meningioma in tuberculum sellae, the range of bony defect and dura resection should be adjusted according to the size of the tumor. There is significant variation in the rate of postoperative cerebrospinal fluid leaks. Therefore, these patients were not enrolled. In the present study, artificial biomembrane as the first layer was placed into the subdural space and it adhered to the dura mater due to the pressure from cerebrospinal fluid and brain tissue. This maneuver, similar to dura repair in craniotomy, serves as the first layer to achieve watertight closure. The effective membranous reconstruction of the osteodural defect is critical to restore the integrity of the skull base. 12 Fascia lata has advantages in the flat or regular area of the skull base reconstruction. For the irregular CP surgical area, artificial biomembrane has advantages in both compliance and adhesiveness, and does not require an incision in the lateral thigh.
The structure of the skull base is irregular with an angle of approximately 115 ± 6.5 degrees at the planum sphenoidal joint of the anterior skull base and sellar floor ( Fig. 3A ). Besides, the surface of sellar floor is roughly spherical, making it difficult to attach external repair materials to it. Through cadaver anatomy and clinical experience accumulation, we creatively used grinding drills to make in situ bone flap (tuberculum—planum-seller micro-bone flap in cadaver anatomy, Fig. 3B,C ). As an autologous tissue, in situ bone flap fits well with the skull base and maximally restore anatomical structure. It is easy to harvest, and biocompatible with a minimal risk of rejection. In addition, the application of in situ bone flap repair could efficiently convert high flow CSF leaks into low flow ones, which is essential for watertight closures, and in general accelerates the recovery of patients. The extent of vertical resection of the dura mater was smaller than the bone defect, and the diameter of the bone flap was longer than that of dura mater in all directions, thus providing a similar “cap” effect to ensure that the bone flap does not slide into the intracranial space 13 . The resection of the dura matter should not reach the margins of the defect, or it will increase the difficulty of repair. A retrospective review data suggest that buttresses are beneficial for the repair of most of the grades of CSF leaks 14 , and also suggested that it is useful to harvest septal bone or vomer if available during the approach of nasal phase. Such bone should be removed as large piece as possible, so that it can be tailored for skull base repair. In any case, the tailored bone cannot be attached to the skull base in situ, also maybe slide into cranial cavity and cause damage to neurovascular structure. The pedicled nasoseptal flap covered the bone flap as the third layer and achieved skull base reconstruction completely.
Fig. 3.
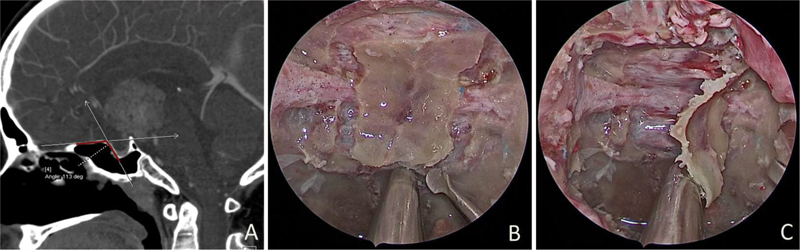
Anatomical study of in situ bone flap in cadaver. ( A ) There is an angle approximately 113 degrees between the planum sphenoidal and sellar floor. ( B ) In situ bone flap consisted of planum sphenoidale, tuberculum sellae, partial optic canal, and sellar floor. ( C ) The bone flap was completely excised and retained to skull base reconstruction.
The skull base reconstruction of control group only utilized biomembrane and pedicled nasoseptal flap, because many studies have shown that osseous reconstruction is not required. This is what we did in the early clinical works. No herniation of brain tissue was found in all cases. Whether osseous reconstruction is necessary needs further confirmation. The in-situ bone flap adopted could relieve the dislodgement of the repair construct at the skull base, including bucking on the endotracheal tube, nausea, and vomiting. In the present study, in situ bone flap repair protocol has advantages over the control group in terms of the incidence of postoperative lumbar drainage, nasal exudation, meningitis, and period of hospitalization, and can also significantly reduce the medical costs. After all, the presence of postoperative repair failure was strongly associated with meningitis. Two recent meta-analyses and a systematic review article failed to find benefit of perioperative lumbar drainage in patients undergoing EEA, and emphasized potential risk of major complications, including meningitis and ventriculitis. 15 In terms of the treatment of cerebrospinal fluid leakage, we dare not to arbitrarily ignore and still use lumbar drainage to reduce intracranial pressure and promote mucosal flap healing.
There is great heterogeneity in the operative care of craniopharyngiomas across different institutions, 16 with limited evidence regarding the comparative complications. Clinical selection of repair material and method is currently based on experience. 17 18 19 For high flow leaks, the curative effect of pedicled mucosal flap repair is clear. 20 We have summarized four levels of skull base repair protocol: for the first layer, place an artificial biomembrane lining the inner surface of the dura and completely cover the defect area; for the second layer, bony repair material should be placed between the dura mater and skull base to achieve osseous reconstruction; for the third layer, completely cover the defect of the skull base using a pedicled nasoseptal flap; and final for the fourth layer, use support materials such as gelatin sponge or silver gauze. There are also reports of using autologous fibrin glue (Dura Seal) 21 in America or other countries, which was used on the exterior of the nasoseptal flap to further strengthen the reconstruction. These products, however, are currently unavailable in China and the effect of Chinese-made products needs to be further verified. We strongly do not recommend using chemical glue as it may cause serious infection or graft rejection.
Limitations of This Study
There are a few limitations in this study. It mainly compared and analyzed the different methods of skull base reconstruction in a retrospective study. The sample size was relatively small and not clearly randomized. The amount of nasal exudation always depends on the patient's chief complaint, it lacks objective judgment, and results in the quantity of lumbar drainage may not be reliable. The incident cases of complication were so low as to render any meaningful conclusion impossible, and this requires further discussion. The present cases employed multilayered repair with dura substitute, pedicled nasoseptal flap, surgical and merocele sponges; it is hard to distinguish which element contributed most to successful repair. On the positive side, the retrospective clinical data we collected in the current study showed that utilization of in situ bone flap in skull base reconstruction had obvious superiority in each term. It was clearly noted that patients who underwent the EEEA procedure using in situ bone flap and pedicled nasoseptal flap, had lower incidence of cerebrospinal fluid leaks, and amount of lumbar drainage. Additionally, they also had obviously shorter periods of hospitalization. There is, of course, a learning curve of utilizing in situ bone flap, experienced surgeons can reduce the risks involved in creating the in situ bone flap.
Conclusion
The EEEA is an amenable and minimally invasive option to the midline suprasellar. The clinical efficacy of in situ bone flap reconstruction technique is an effective method in achieving a watertight closure after EEEA. Despite this study reporting a preliminary experience in small series of patients, it seems that this method can reduce CSF leakage and incision rates in abdomen or thigh, thus decreasing meningitis, hospitalization, and additional trip to the operating room. After all, lack of buttress is an obvious factor in postoperative CSF leaks. 14 Our preliminary results recommend that in situ bone flap combined with pedicled nasoseptal flap may be considered an approach of choice for skull base reconstruction. Further prospective, randomized, multi-institutional collaboration, and larger volume series are required to develop and refine the reconstruction strategies to fully evaluate the appropriate approach for craniopharyngiomas. However, we feel confident that the facility and practicality of this technique will allow for reproducibility of results when performed under similar situations.
Funding Statement
Funding This research was supported by Natural Science Foundation of China (No. 81627806).
Conflict of Interest None declared.
Authors' Contributions
D.G. contributed toward conception, design, and surgical operation. Y.Z., Y.H., and T.J. contributed toward data collection and analysis. Y.Z. and Y.H. drafted the article. J.X., D.G., and W.L. revised the article. D.G. approved the final version on behalf of all the authors. J.M.S. and D.F. contributed to language polishing. W.L. provided technical support and other help.
These authors contributed equally to this work.
References
- 1.Zhang S, Fang Y, Cai B W, Xu J G, You C. Intracystic bleomycin for cystic craniopharyngiomas in children. Cochrane Database Syst Rev. 2016;7:CD008890. doi: 10.1002/14651858.CD008890.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Komotar R J, Starke R M, Raper D M, Anand V K, Schwartz T H. Endoscopic endonasal compared with microscopic transsphenoidal and open transcranial resection of craniopharyngiomas. World Neurosurg. 2012;77(02):329–341. doi: 10.1016/j.wneu.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 3.Castelnuovo P, Dallan I, Battaglia P, Bignami M. Endoscopic endonasal skull base surgery: past, present and future. Eur Arch Otorhinolaryngol. 2010;267(05):649–663. doi: 10.1007/s00405-009-1196-0. [DOI] [PubMed] [Google Scholar]
- 4.Elliott R E, Jane J A, Jr, Wisoff J H.Surgical management of craniopharyngiomas in children: meta-analysis and comparison of transcranial and transsphenoidal approaches Neurosurgery 20116903630–643., discussion 643 [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Navarro V, Anand V K, Schwartz T H. Gasket seal closure for extended endonasal endoscopic skull base surgery: efficacy in a large case series. World Neurosurg. 2013;80(05):563–568. doi: 10.1016/j.wneu.2011.08.034. [DOI] [PubMed] [Google Scholar]
- 6.Cavallo L M, Frank G, Cappabianca P et al. The endoscopic endonasal approach for the management of craniopharyngiomas: a series of 103 patients. J Neurosurg. 2014;121(01):100–113. doi: 10.3171/2014.3.JNS131521. [DOI] [PubMed] [Google Scholar]
- 7.Koutourousiou M, Gardner P A, Fernandez-Miranda J C, Tyler-Kabara E C, Wang E W, Snyderman C H. Endoscopic endonasal surgery for craniopharyngiomas: surgical outcome in 64 patients. J Neurosurg. 2013;119(05):1194–1207. doi: 10.3171/2013.6.JNS122259. [DOI] [PubMed] [Google Scholar]
- 8.Esposito F, Dusick J R, Fatemi N, Kelly D F.Graded repair of cranial base defects and cerebrospinal fluid leaks in transsphenoidal surgery Oper Neurosurg (Hagerstown) 200760(04, Suppl 2): discussion 303–304295–303. [DOI] [PubMed] [Google Scholar]
- 9.Hara T, Akutsu H, Yamamoto T et al. Cranial base repair using suturing technique combined with a mucosal flap for cerebrospinal fluid leakage during endoscopic endonasal surgery. World Neurosurg. 2015;84(06):1887–1893. doi: 10.1016/j.wneu.2015.08.025. [DOI] [PubMed] [Google Scholar]
- 10.Silveira-Bertazzo G, Manjila S, Carrau R et al. Expanded endoscopic endonasal approach for extending suprasellar and third ventricular lesions. Acta Neurochir (Wien) 2020;162(10):2403–2408. doi: 10.1007/s00701-020-04368-9. [DOI] [PubMed] [Google Scholar]
- 11.Wang A J, Zaidi H A, Laws E D., Jr History of endonasal skull base surgery. J Neurosurg Sci. 2016;60(04):441–453. [PubMed] [Google Scholar]
- 12.Cavallo L M, Solari D, Somma T, Cappabianca P. The 3F (fat, flap, and flash) technique for skull base reconstruction after endoscopic endonasal suprasellar approach. World Neurosurg. 2019;126:439–446. doi: 10.1016/j.wneu.2019.03.125. [DOI] [PubMed] [Google Scholar]
- 13.Steno J, Malácek M, Bízik I.Tumor-third ventricular relationships in supradiaphragmatic craniopharyngiomas: correlation of morphological, magnetic resonance imaging, and operative findings Neurosurgery 200454051051–1058., discussion 1058–1060 [DOI] [PubMed] [Google Scholar]
- 14.Conger A, Zhao F, Wang X et al. Evolution of the graded repair of CSF leaks and skull base defects in endonasal endoscopic tumor surgery: trends in repair failure and meningitis rates in 509 patients. J Neurosurg. 2018;130(03):861–875. doi: 10.3171/2017.11.JNS172141. [DOI] [PubMed] [Google Scholar]
- 15.Ivan M E, Iorgulescu J B, El-Sayed I et al. Risk factors for postoperative cerebrospinal fluid leak and meningitis after expanded endoscopic endonasal surgery. J Clin Neurosci. 2015;22(01):48–54. doi: 10.1016/j.jocn.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Wang E W, Gardner P A, Zanation A M.International consensus statement on endoscopic skull-base surgery: executive summary Int Forum Allergy Rhinol 20199(S3)S127–S144. [DOI] [PubMed] [Google Scholar]
- 17.Hertel V, Schick B. Diagnosis and treatment of frontobasal cerebrospinal fluid fistulas. Laryngorhinootologie. 2012;91(09):585–597. doi: 10.1055/s-0032-1316382. [DOI] [PubMed] [Google Scholar]
- 18.Kim B Y, Shin J H, Kim S Wet al. Risk factors predicting nasoseptal flap failure in the endoscopic endonasal transsphenoidal approachJ Craniofac Surg2016 [DOI] [PubMed]
- 19.Korshunov A, Jakobiec F A, Eberhart C G et al. Comparative integrated molecular analysis of intraocular medulloepitheliomas and central nervous system embryonal tumors with multilayered rosettes confirms that they are distinct nosologic entities. Neuropathology. 2015;35(06):538–544. doi: 10.1111/neup.12227. [DOI] [PubMed] [Google Scholar]
- 20.El-Banhawy O A, Halaka A N, Altuwaijri M A, Ayad H, El-Sharnoby M M. Long-term outcome of endonasal endoscopic skull base reconstruction with nasal turbinate graft. Skull Base. 2008;18(05):297–308. doi: 10.1055/s-0028-1086055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie T, Zhang X B, Yun H, Hu F, Yu Y, Gu Y. 3D-FIESTA MR images are useful in the evaluation of the endoscopic expanded endonasal approach for midline skull-base lesions. Acta Neurochir (Wien) 2011;153(01):12–18. doi: 10.1007/s00701-010-0852-x. [DOI] [PubMed] [Google Scholar]


