Abstract
Endoplasmic reticulum (ER) stress contributes to pancreatic beta-cell apoptosis in diabetes, but the factors involved are still not fully elucidated. Growth differentiation factor 15 (GDF15) is a stress response gene and has been reported to be increased and play an important role in various diseases. However, the role of GDF15 in beta cells in the context of ER stress and diabetes is still unclear. In this study, we have discovered that GDF15 promotes ER stress-induced beta-cell apoptosis and that downregulation of GDF15 has beneficial effects on beta-cell survival in diabetes. Specifically, we found that GDF15 is induced by ER stress in beta cells and human islets, and that the transcription factor C/EBPβ is involved in this process. Interestingly, ER stress-induced apoptosis was significantly reduced in INS-1 cells with Gdf15 knockdown and in isolated Gdf15 knockout mouse islets. In vivo, we found that Gdf15 deletion attenuates streptozotocin-induced diabetes by preserving beta cells and insulin levels. Moreover, deletion of Gdf15 significantly delayed diabetes development in spontaneous ER stress-prone Akita mice. Thus, our findings suggest that GDF15 contributes to ER stress-induced beta-cell apoptosis and that inhibition of GDF15 may represent a novel strategy to promote beta-cell survival and treat diabetes.
Keywords: diabetes, beta cells, ER stress, apoptosis, GDF15
In pancreatic beta cells, insulin is folded and matured in the endoplasmic reticulum (ER). Insulin mutations or need for excessive insulin production such as in response to hyperglycemia lead to accumulation of misfolded and unfolded insulin in the ER, resulting in ER stress (1-3). This triggers the unfolded protein response (UPR), which involves activation of 3 transmembrane proteins (IRE1, PERK, and ATF6), to alleviate ER stress (2, 3). However, prolonged ER stress converts this homeostatic response to terminal UPR, leading to beta-cell apoptosis and diabetes development (2, 3). Some processes involved in beta-cell ER stress/UPR have been extensively studied, but others are still not fully understood. To develop efficient strategies to halt beta-cell death and treat diabetes, a better understanding of the factors involved in beta-cell ER stress/UPR is needed.
Growth differentiation factor 15 (GDF15), a distant member of the TGFβ family (4), is also known as macrophage inhibitory cytokine-1 (4), nonsteroidal anti-inflammatory drug-activated gene (5), and prostate-derived factor (6). GDF15 is a stress response gene, and its increase has been associated with cancers (7, 8), cardiovascular disease (9,10), insulin resistance (11, 12), diabetes, and diabetic complications (13-18). Until now, the function of GDF15 has mainly been studied in the context of its role as a circulating factor in regulating food intake and body weight through binding and activation of its unique brain-restricted receptor, GDNF Family Receptor Alpha Like (19-22). In terms of intracellular GDF15, several studies have shown that various antitumor factors induce the expression of GDF15, which in turn promotes cancer cell apoptosis (23-29). In this context, GDF15 has been shown to translocate into mitochondria and induce reactive oxygen species production (30). In addition, GDF15 has also been shown to accumulate in the nucleus and modulate gene expression (31).
Surprisingly, a recent study reported that treatment with exogenous GDF15 could decrease cytokine-induced beta-cell death and that administration of GDF15 could reduce the incidence of diabetes in nonobese diabetic (NOD) mice (32). This is in contrast to the pro-apoptotic effects of GDF15 observed in tumors (25-29) and the elevated circulating GDF15 levels found in subjects with diabetes (16-18). These seemingly contrasting effects raise the question as to what role endogenous, and especially intracellular, GDF15 plays in beta-cell death and diabetes development. The present study was therefore aimed at elucidating the role of intracellular GDF15 and its downregulation in the context of diabetes. Using human and mouse islets, INS-1 cells, Akita cells, Gdf15 knockout mice, and 2 diabetes mouse models, we found that intracellular GDF15 contributes to ER stress-mediated beta-cell apoptosis and that downregulation of intracellular GDF15 has beneficial effects on beta-cell survival and delays diabetes development.
Materials and Methods
Tissue Culture
INS-1 cells were grown in RPMI 1640 (Thermo Fisher Scientific) with 11.1 mmol/L glucose, 10% fetal bovine serum, 1% penicillin/streptomycin, 1 mmol/L sodium pyruvate, 2 mmol/L L-glutamine, 10 mmol/L HEPES, and 0.05 mmol/L 2-mercaptoethanol, as previously described (33, 34). To induce ER stress, INS-1 cells were treated with a commonly used ER stress inducer, thapsigargin (TG), which inhibits the sarco/ER Ca2+-ATPase and induces cell death via ER Ca2+ depletion and the UPR (35). To study the effect of pro-inflammatory cytokines on Gdf15 expression, INS-1 cells were treated with cytokine cocktail (0.5 ng/mL TNFα, 0.5 ng/mL interferon γ, and 0.1 ng/mL IL-1β) or PBS for 16 hours. To knockdown gene expression, INS-1 cells were transfected with siRNA (Horizon Discovery) using DharmaFECT 1 transfection reagent (Horizon Discovery). The Akita and wild-type (WT) cells were grown in DMEM (Thermo Fisher Scientific) supplemented with 15% fetal bovine serum and 1% penicillin/streptomycin as previously described (36, 37). Human islets were obtained from the Integrated Islet Distribution Program (Table 1); islets from the same donor were treated with vehicle or drug. A minimum of 3 donor islet preparations were used per experiment. To induce ER stress, human islets were treated with 1 µM TG for 24 hours. Mouse pancreatic islets were isolated by collagenase digestion (33) and used for apoptosis assays.
Table 1.
Human islet preparations used in the study
| Islet prep | Origin | Islet isolation center | Unique identifier | Age (y) | Sex | BMI (kg/ m2) | HbA1c (%)/blood glucose (mg/dL) | Hx of DM | Cause of death | Cold ischemia (h) | Purity (%) | Viability (%) | Handpicked |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | IIDP | Southern California | ADGU064 | 30 | M | 35.9 | NA/176.4 | N | Stroke | 12.5 | 90 | 95 | Y |
| 2 | IIDP | Southern California | SAMN11791244 | 47 | M | 36.1 | NA/166.2 | N | Stroke | 6.5 | 95 | 95 | Y |
| 3 | IIDP | University of Miami | SAMN12274306 | 37 | M | 25.3 | NA/192.8 | N | Anoxia | 152 | 80 | 80 | Y |
| 4 | IIDP | University of Wisconsin | SAMN12670838 | 40 | M | 30.7 | NA/152.6 | N | Head trauma | 6.2 | 90 | 98 | Y |
| 5 | IIDP | Southern California | SAMN12713942 | 41 | M | 23.5 | NA/144.0 | N | Stroke | 10.7 | 85 | 95 | Y |
Abbreviations: BMI, body mass index; DM, diabetes mellitus; HbA1c, glycated hemoglobin; Hx, history; IIDP, Integrated Islet Distribution Program; M, male; N, no; NA, not available; Y, yes.
Animal Studies
All mouse studies were approved by the University of Alabama at Birmingham Animal Care and Use Committee. NOD mice and nonobese diabetes-resistant mice were purchased from the Jackson Laboratory. To follow the development of diabetes in mice, blood glucose was measured using a glucometer (Life Scan), and mice were considered diabetic if their blood glucose was >250 mg/dL on 2 consecutive days. Gdf15 knockout mice on a C57BL/6N background were generated as previously described (38). To induce diabetes, 13-week-old male Gdf15 knockout mice and WT C57BL/6N control mice (Charles River Laboratories) received multiple low-dose injections of streptozotocin (STZ: 40 mg/kg body weight per day for 5 days, prepared in 0.1 mmol/L sodium citrate at pH 4.5). To generate Gdf15-/-Ins2+/Akita (double mutant) mice, we crossed Gdf15 knockout mice with Ins2+/Akita (Akita) mice (The Jackson Laboratory).
Quantitative Real-time PCR
Total RNA was extracted using the miRNeasy Mini Kit (Qiagen) according to the manufacturer’s instructions. RNA was reverse transcribed to cDNA using the first-strand cDNA synthesis kit (Roche). Quantitative real-time PCR was performed on a LightCycler 480 System (Roche) using SYBR Green (Thermo Fisher Scientific). All primers used are listed in Table 2. All samples were corrected for the 18S ribosomal subunit run as an internal standard.
Table 2.
Primers used in the study
| Description | Sequences (5′-3′) |
|---|---|
| 1.18s qPCR 5′ primer | AGTCCCTGCCCTTTGTACACA |
| 2.18s qPCR 3′ primer | GATCCGAGGGCCTCACTAAAC |
| 3.Human GDF15 qPCR 5′ primer | TCCCGGGACCCTCAGAGT |
| 4.Human GDF15 qPCR 3′ primer | TCGTAGCGTTTCCGCAACT |
| 5.Mouse Gdf15 qPCR 5′ primer | ACCCCGGTGGTTCTTATGC |
| 6.Mouse Gdf15 qPCR 3′ primer | CAGGTCATCATAAGTCTGCAGTGA |
| 7.Rat Gdf15 qPCR 5′ primer | GGAGGACTCGAACTCAGAACCA |
| 8.Rat Gdf15 qPCR 3′ primer | TCGCACCTCTGGACTGAGTATC |
| 9.Human GDF15 promoter 5′ primer | CACACGCGTCAGGCATGTAATCCCACCACCAA |
| 10.Human GDF15 promoter 3′ primer-1 | CACAGATCTGCGGCAGCCACGAGAGCA |
| 11.Human GDF15 promoter 3′ primer-2 | CACAGATCTGGCTGTGCAGGTTGCGGCTC |
| 12.Human C/EBPβ qPCR 5′ primer | CAAGAAGACCGTGGACAAGCA |
| 13.Human C/EBPβ qPCR 3′ primer | CGCACGGCGATGTTGTT |
| 14.Mouse C/ebpβ qPCR 5′ primer | AGCGGCTGCAGAAGAAGGT |
| 15.Mouse C/ebpβ qPCR 3′ primer | GGCAGCTGCTTGAACAAGTTC |
| 16.Rat C/ebpβ qPCR 5′ primer | ACGAGCGGCTGCAGAAGA |
| 17.Rat C/ebpβ qPCR 3′ primer | GGCAGCTGCTTGAACAAGTTC |
| 18.Rat Gadd34 qPCR 5′ primer | GCTGGCCTGCCTACACTACAG |
| 19.Rat Gadd34 qPCR 3′ primer | CCACCTCCCCAACTTTCTTATCT |
Western Blotting
Protein extracts from WT and Akita cells were prepared using lysis buffer containing HEPES (50 mM), Nonidet P-40 (10%), sodium fluoride (100 mM), sodium pyrophosphate (10 mM), EDTA (4 mM), phenylmethanesulphonyl fluoride (1 mM), leupeptin (2 μM), activated sodium orthovanadate (2 mM), and okadaic acid (100 nM). Protein concentrations were measured by Pierce BCA protein assay (Thermo Fisher Scientific, Hudson, NH) and equal amounts of protein were loaded. Bands were visualized by ECL plus (GE Healthcare). The following antibodies were used: rabbit anti-GDF15 IgG (Bioss Antibodies, catalog no. bs-3818R-TR, RRID: AB_10857699) (39), mouse anti-beta-actin IgG (Cell Signaling Technology, catalog no. 3700S, RRID: AB_2242334) (40), horse anti-mouse IgG (Cell Signaling Technology, catalog no. 7076S, RRID: AB_330924) (41), and goat anti-rabbit IgG (Cell Signaling Technology, catalog no. 7074S, RRID: AB_2099233) (42).
Plasmid Construction, Transfection, and Luciferase Assay
The human GDF15 proximal promoter region was amplified by 2-round PCR using the primers listed in Table 2. The PCR product was subcloned into the Mlu І and Bgl II sites of the pGL3-Enhancer luciferase reporter plasmid (Promega). The reporter plasmid containing the human GDF15 promoter region was confirmed by sequencing. For luciferase assays, HEK293 cells were plated in 12-well plates and grown overnight to ~60% confluence. Cells were transfected with the reporter plasmid and the internal control plasmid pRL-TK (Promega) using DharmaFECT Duo transfection reagent (Horizon Discovery). Eight hours after transfection, the cells were changed to new medium containing 0.5 µM of TG or DMSO for another 16 hours. At 24 hours of transfection, firefly as well as Renilla luciferase activity were determined using the Dual Luciferase Assay Kit (Promega).
Apoptosis Assay
Apoptosis was analyzed by using the Cell Death Detection ELISAPLUS kit (Roche, catalog no. 11774425001, RRID: AB_2909412) (43) according to the manufacturer’s instruction and as previously described (44). In brief, INS-1 cells were seeded in 6-well plates and transfected with rat Gdf15 siRNA or scrambled control (Horizon Discovery) for 72 hours using DharmaFECT 1 transfection reagent (Horizon Discovery). For the last 16 hours of the transfection, the cells were treated with 0.1 µM of TG or DMSO. The cells were then lysed in 1 mL of lysis buffer and 20 µL of 5 time-diluted cell lysate was used for the apoptosis assay. The results were normalized to DNA content by using the Quant-iT PicoGreen dsDNA Reagent and Kit (Thermo Fisher Scientific). To detect apoptosis in mouse islets, 50 islets were treated with 1 µM of TG or DMSO for 24 hours. The islets were lysed in 100 µL of lysis buffer and 20 µL of cell lysate were used for the apoptosis assay.
ELISA
Serum GDF15 in mice and GDF15 protein levels in cells were measured by using 50 µL of diluted serum or cell lysate and the Mouse/Rat GDF15 Quantikine ELISA kit (R&D Systems, catalog no. MGD150, RRID: AB_2909411) (45) according to the manufacturer’s instructions. Mouse serum insulin levels were assessed by using the Ultra Sensitive Mouse Insulin ELISA Kit (Crystal Chem, catalog no. 90080, RRID: AB_2783626) (46) as previously described (47, 48).
Glucose Tolerance Test and Insulin Tolerance Test
Glucose tolerance tests and insulin tolerance tests were performed in Gdf15 knockout (KO) mice and control mice as previously described (33, 49). Briefly, age- and sex-matched mice were fasted for 4 hours and then given glucose (3 g/kg) or regular insulin (1 U/kg) by IP injection. Blood glucose was measured at 0, 15, 30, 60, 90, and 120 minutes.
Immunohistochemistry
Insulin staining was performed as previously described (33). The guinea pig anti-insulin antibody (Abcam, catalog no. ab7842, RRID: AB_306130) (50) and the Alexa Fluor 594 AffiniPure donkey anti-guinea pig IgG (Jackson ImmunoResearch, catalog no. 706-065-148, RRID: AB_2340451) (51) were used. After staining, the Vectashield Mounting Solution with 4′,6-diamidino-2-phenylindole (Vector Labs) was used for visualization of nuclei.
Statistical Analysis
The Student t test were used to calculate the significance of a difference between 2 groups. One-way ANOVA was used for temporal analyses of datasets containing 2 or more groups. Log-rank test was used to compare disease-free survival in 2 groups. A P value < 0.05 was used to indicate significant difference between groups.
RESULTS
Beta-cell GDF15 Is Induced by ER Stress
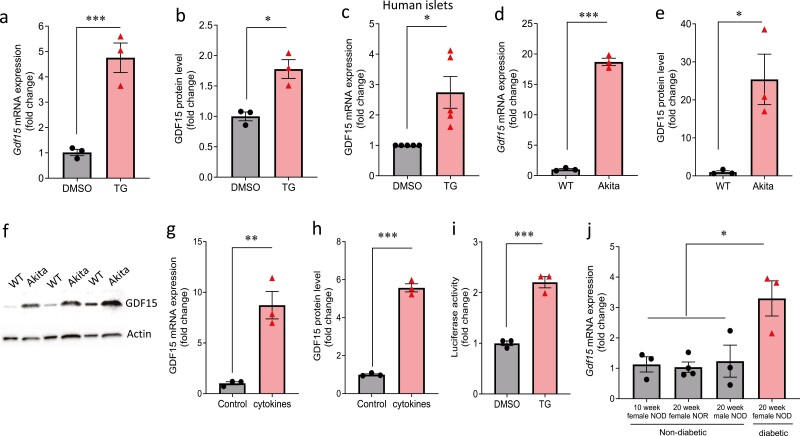
To test whether GDF15 expression responds to ER stress in beta cells, we treated INS-1 cells with TG. We found that both Gdf15 mRNA (Fig. 1A) and GDF15 protein (Fig. 1B) were significantly induced by TG. Of note, a similar induction of GDF15 expression was recapitulated in human islets (Fig. 1C). Next, we assessed GDF15 expression in Akita cells. These cells harbor a mutation in the Ins2 gene, which causes misfolding and accumulation of insulin in the ER, leading to spontaneous ER stress and beta-cell death (36). Gdf15 mRNA (Fig. 1D) and GDF15 protein (Fig. 1E and 1F) were also significantly increased in the Akita cells compared with WT cells. Pro-inflammatory cytokines have been shown to induce ER stress and contribute to beta-cell death in type 1 diabetes (52, 53). To test whether the expression of GDF15 is regulated by type 1 diabetes-associated cytokines, we treated rat INS-1 cells with a cytokine cocktail (TNFα, interferon γ, and IL-1β). We found that the levels of GDF15 mRNA and protein were also significantly induced by cytokine treatment (Fig. 1G and 1H). In addition, to investigate the mechanism of GDF15 regulation by ER stress, we cloned a fragment of the proximal promoter of human GDF15 (-1082 to -1) into a luciferase reporter plasmid (pGL3-Enhancer Vector). We found that TG treatment significantly induced luciferase activity driven by the human GDF15 promoter (Fig. 1I), suggesting that ER stress induces GDF15 at the transcriptional level. Furthermore, to test the effect in vivo, we measured the expression of Gdf15 in islets of NOD mice, whose beta cells have been shown to be exposed to ER stress during diabetes progression (1, 54). The results showed that Gdf15 mRNA levels are significantly increased in islets of diabetic NOD mice compared with nondiabetic controls (Fig. 1J). Together, these results suggest that the expression of beta-cell GDF15 is induced by ER stress.
Figure 1.
Effects of ER stress on GDF15 expression. (A) Gdf15 mRNA and (B) GDF15 protein expression by ELISA were assessed in INS-1 cells treated with 0.5 µM TG or DMSO for 16 hours. (C) GDF15 mRNA expression was assessed in nondiabetic isolated human islets treated with 1 µM TG or DMSO for 24 hours. (D) Gdf15 mRNA and (E) GDF15 protein expression by ELISA and (F) by Western blot were assessed in wild-type (WT) and Akita beta cells. (G) Gdf15 mRNA and (H) GDF15 protein expression by ELISA were measured in INS-1 cells treated with cytokine cocktail (0.5 ng/mL TNFα, 0.5 ng/mL IFNγ, and 0.1 ng/mL IL-1β) for 16 hours. (I) Luciferase activity driven by human GDF15 promoter was analyzed in HEK293 cells treated with 0.5 µM TG or DMSO for 16 hours. (J) Gdf15 mRNA expression levels were measured in islets of 10-week female nondiabetic NOD mice, 20-week female NOR mice, 20-week male non-diabetic NOD mice and 20-week female diabetic NOD mice. All data are shown as the mean ± standard error of the mean of at least 3 independent experiments or 3 mice; n = 5 human islet donors. *P < 0.05, **P < 0.01, and ***P < 0.001.
C/EBPβ Is Involved in the Induction of GDF15 Expression
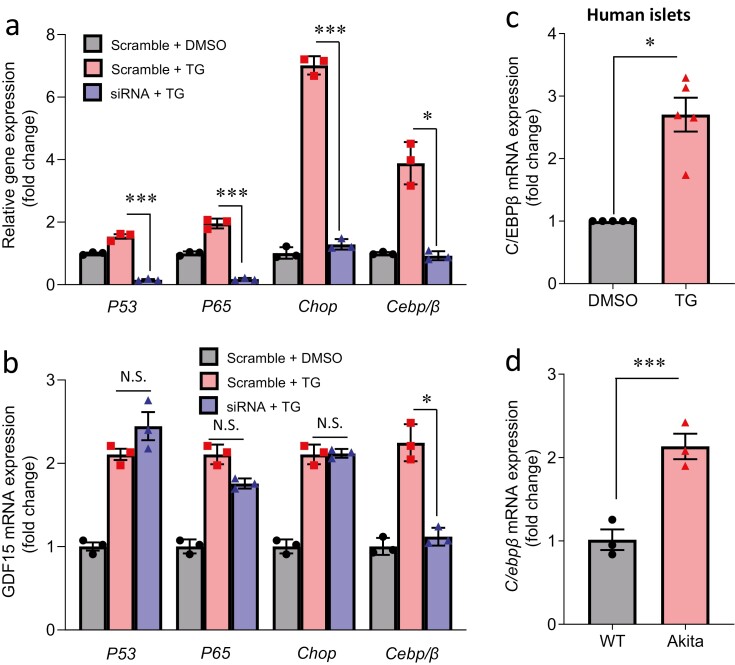
Previous studies have shown that GDF15 can be regulated by several transcription factors including nuclear factor-κB (55), CHOP (56), P53 (57), and C/EBPβ (26) in non-beta cells. We therefore tested these factors in beta cells and found that only C/EBPβ was able to regulate beta-cell GDF15 in the context of ER stress. Specifically, although siRNA transfection led to significant knockdown of P53, Nfκb (P65 subunit), Chop, and C/ebpβ, even in the presence of TG-induced ER stress (Fig. 2A), only knockdown of C/ebpβ could blunt TG-induced Gdf15 in INS-1 cells (Fig. 2B). In addition, C/EBPβ expression was increased in human islets treated with TG (Fig. 2C) and Akita cells (Fig. 2D), which was also associated with elevated GDF15 expression (Fig. 1C and 1D). These findings suggest that the transcription factor C/EBPβ is involved in the induction of GDF15 in beta cells.
Figure 2.
Role of C/EBPβ in the regulation of GDF15 expression. INS-1 cells were transfected with siP53, siP65, siChop, siCebp/β, or scrambled control, followed by treatment with TG (0.5 µM for 5 hours) or DMSO and the expression of these transcription factors (A) and of Gdf15 (B) was measured. (C) Nondiabetic isolated human islets treated with TG (1 µM for 24 hours) or DMSO, and (D) wild-type (WT) and Akita beta cells were analyzed for C/EBPβ mRNA expression. All data are shown as the mean ± standard error of the mean of 3 independent experiments; n = 5 islet donors. *P < 0.05 and ***P < 0.001.
GDF15 Promotes ER Stress-induced Beta-cell Apoptosis
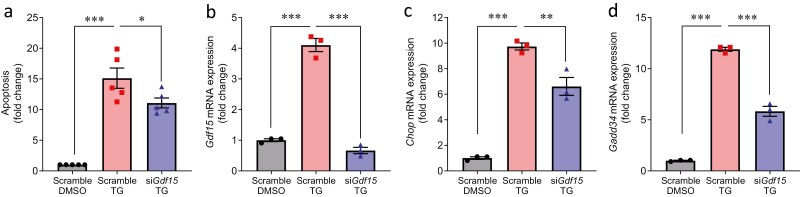
Next, we tested the possibility that GDF15 is not only induced by ER stress, but also plays a role in ER stress-mediated beta-cell apoptosis. Indeed, although TG treatment of INS-1 cells promoted apoptosis as expected, knockdown of Gdf15 significantly reduced ER stress-induced cell apoptosis (Fig. 3A). The activation of UPR is thought to be a cellular defense response to ER stress. However, prolonged activation of UPR also leads to cell apoptosis. To test whether GDF15 exerts its effects on beta-cell apoptosis via regulation of UPR, we analyzed the expression of CHOP, which is a common downstream factor of UPR (58, 59). Indeed, knockdown of Gdf15 effectively reduced TG-induced Gdf15 expression (Fig. 3B) and significantly decreased the TG-induced increase in Chop (Fig. 3C). Similarly, we discovered that knockdown of Gdf15 also significantly decreased TG-induced expression of Gadd34, which is a target of CHOP (60) (Fig. 3D). These results suggest that inhibition of GDF15 has beneficial effect on ER stress-induced beta-cell apoptosis, which is at least in part mediated by the regulation of CHOP signaling.
Figure 3.
Role of GDF15 in beta cell apoptosis and UPR. (A) INS-1 cells with knockdown of Gdf15 (siGdf15) or scrambled control followed by treatment with TG (0.1 µM for 16 hours) or DMSO were analyzed for apoptosis by Cell Death Detection ELISAPLUS. (B) Gdf15, (C) Chop, and (D) Gadd34 mRNA expression was measured in INS-1 cells with knockdown of Gdf15 followed by TG treatment (0.1 µM for 16 hours). All data are shown as the mean ± standard error of the mean of at least 3 independent experiments. *P < 0.05, **P < 0.01, and ***P < 0.001. N.S., not significant.
Gdf15 Deletion Has No Effect on Glucose Homeostasis Under Normal Conditions
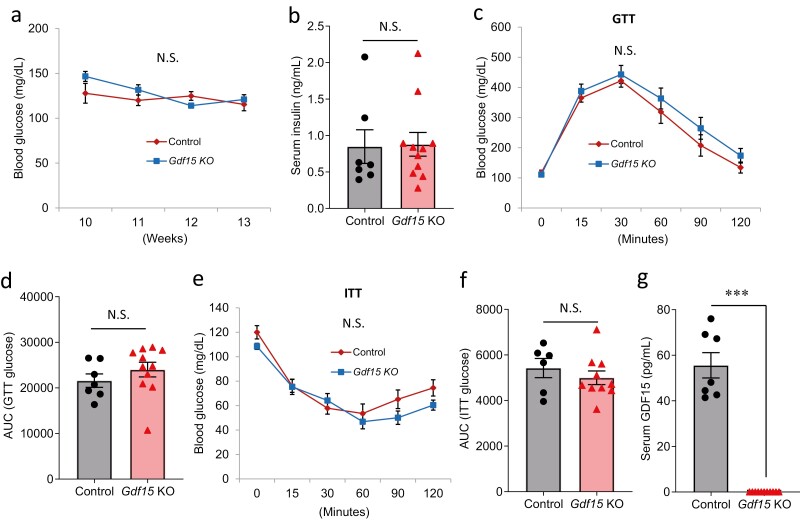
To study the in vivo role of GDF15 in glucose homeostasis, we obtained Gdf15KO (38). Gdf15KO mice are healthy and fertile as previously described (38). In addition, Gdf15KO mice showed no difference in glucose (Fig. 4A), insulin (Fig. 4B), glucose tolerance (Fig. 4C and 4D), and insulin sensitivity (Fig. 4E and 4F) compared with control mice, all while having no detectable GDF15 in their serum (Fig. 4G). These findings are consistent with previous reports (56, 61). The results further suggest that deletion of Gdf15 has no effect on glucose homeostasis under normal conditions.
Figure 4.
In vivo effects of GDF15 deletion on glucose homeostasis under normal conditions. (A) Blood glucose, (B) serum insulin, (C-D) glucose tolerance test (IP: 3 g/kg), (E-F) insulin tolerance test (IP: 1 U/kg), and (G) serum GDF15 levels were measured in Gdf15 KO mice and control mice. All data are shown as the mean ± standard error of the mean of at least 6 mice. ***P < 0.001. N.S., not significant.
Gdf15 Deletion Protects Mice From STZ-induced Diabetes and Islets From ER Stress-induced Apoptosis
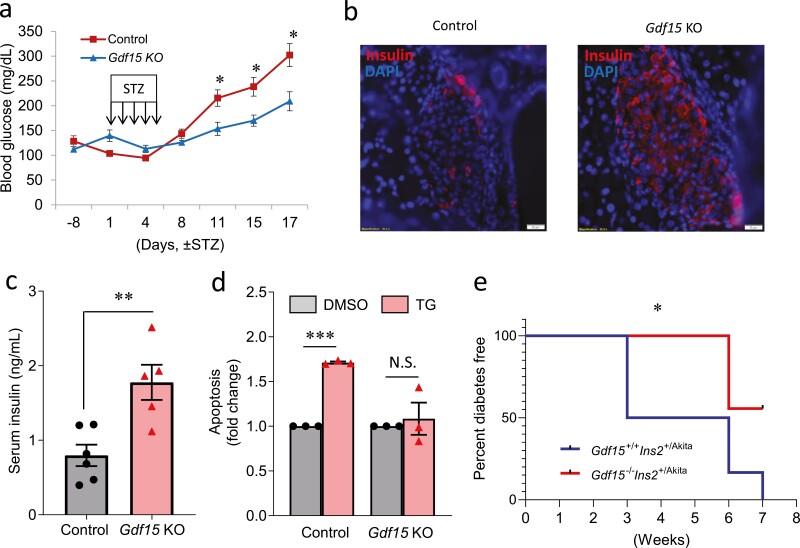
To test the in vivo role of Gdf15 in the context of diabetes, 13-week-old male Gdf15KO mice and WT controls were injected with multiple low-dose STZ. Although WT control mice developed overt diabetes (blood glucose >250 mg/dL), Gdf15KO mice maintained significantly lower blood glucose levels (Fig. 5A). Interestingly, although most beta cells were destroyed in the control mice, Gdf15KO mice still maintained healthy insulin-producing beta cells (Fig. 5B). In addition, Gdf15KO mice showed significantly higher serum insulin levels compared with controls (Fig. 5C). To test the effect of Gdf15 deletion on ER stress-induced apoptosis in islets, we treated islets isolated from Gdf15KO mice and WT control mice with TG. Although TG treatment significantly induced apoptosis in islets of control mice, this effect was completely abolished in islets of Gdf15KO mice (Fig. 5D). These results suggest that deletion of Gdf15 helps improve STZ-induced diabetes by maintaining functional beta cells and circulating insulin levels and helps prevent islets from ER stress-induced apoptosis.
Figure 5.
In vivo effects of Gdf15 deletion on STZ-induced diabetes and Akita diabetes. (A) Blood glucose, (B) islet immunohistochemistry, and (C) serum insulin in male Gdf15 knockout mice (n = 6) and control mice (n = 5) treated with multiple low-dose STZ injections (40 mg/kg/d) for 5 days. (D) Apoptosis analyzed by Cell Death Detection ELISAPLUS in primary mouse islets of Gdf15 KO mice and controls treated with TG (1 µM for 24 hours) or DMSO. (E) Kaplan-Meier survival curve of the diabetes incidence of female Akita mice (n = 6) and double mutant mice (n = 9) (diabetes is defined as blood glucose >250 mg/dL for 2 consecutive measurements). All data are shown as the mean ± standard error of the mean. Representative islet pictures are shown. *P < 0.05 and **P < 0.01.
Gdf15 Deletion Delays the Development of Diabetes in Akita Mice
To further test the in vivo role of Gdf15 in the context of an additional diabetes model, we crossed Gdf15KO mice with Ins2+/Akita (Akita) mice, whose Ins2 gene harbors a mutation leading to severe beta-cell ER stress and apoptosis, to create Gdf15-/-Ins2+/Akita (double mutant) mice. Consistent with the early onset and very severe diabetes found in male Akita mice reported previously (58), Gdf15 deletion was not able to change the disease course in males. However, interestingly, although half of the female Akita mice developed overt diabetes at 3 weeks, all of the same-aged female double mutant mice remained nondiabetic (Fig. 5F). In addition, although all the female Akita developed severe diabetes at 7 weeks, more than one-half of the same-aged double mutant mice still remained nondiabetic (Fig. 5F). These results suggest that deletion of Gdf15 significantly delays the development of diabetes in female Akita mice.
Discussion
In summary, our results demonstrate for the first time that GDF15 plays an important role in ER stress-induced beta-cell death, reveal a novel C/EBPβ-GDF15-CHOP signaling cascade, and indicate that deletion of Gdf15 has beneficial effect on beta-cell survival and diabetes.
ER stress/UPR has been shown to play an important role in beta-cell loss in diabetes. However, many of the factors involved in this process are still unknown. Here, we discovered that ER stress induced a dramatic increase in GDF15 expression in human islets and beta cells. Of note, this GDF15 elevation was not restricted to 1 condition, but was found in response to different stimuli (TG, cytokines) and in different diabetes models (Akita, NOD). We also elucidated the molecular mechanisms involved and found that the regulation of GDF15 in human isles and beta cells occurs at the transcriptional level and involves C/EBPβ. Furthermore, downregulation of Gdf15 decreased Chop and ER stress-induced beta-cell apoptosis and helped improve or delay diabetes in 2 different models of diabetes.
Interestingly, a previous study showed that ablation of C/ebpβ could also alleviate ER stress-induced beta-cell death and diabetes (62), which is in alignment with our current findings. In addition, our results suggest that GDF15-mediated ER stress-induced beta-cell apoptosis is at least in part mediated via regulation of the proapoptotic factor CHOP. Interestingly, deletion of Chop has also previously been shown to decrease ER stress-induced beta-cell death and delay the development of diabetes in Akita mice (58). In fact, we observed that Gdf15 deletion also has protective effects in Akita mice. Moreover, given the identified role of CHOP-mediated ER stress in this process, it is tempting to speculate that Gdf15 deletion would also have beneficial effects in the context of lipid overload, which previously has been shown to induce beta-cell apoptosis via the same pathway of CHOP-mediated ER stress (63-65). Taken together with our current findings, this suggests the existence of a previously unappreciated signaling cascade (C/EBPβ–GDF15-CHOP) that contributes to beta-cell death in the context of ER stress and diabetes.
Although we cannot rule out the possibility that deletion of Gdf15 has additional beneficial effect on whole body glucose homeostasis in peripheral tissues such as liver and adipose tissue, it is important to note that the glucose tolerance tests and insulin tolerance tests remained unaltered in Gdf15 knockout mice and the phenotype only became apparent in the context of beta-cell stress such as STZ injections and Akita mutation. In addition, isolated islets of Gdf15KO were also protected ex vivo from ER stress-induced apoptosis, supporting the notion of an islet effect. This suggests that inhibition of beta-cell GDF15 may represent a novel strategy to help protect against beta-cell death and diabetes.
So far, the role of GDF15 in diabetes has remained controversial. Although a recent report suggested that administration of exogenous GDF15 could delay the development of diabetes in NOD mice (32) and decrease cytokine-induced beta-cell death, previous work has found that diabetes is actually associated with increased GDF15 levels (13-18) and that elevation of GDF15 promotes cell apoptosis (23-29). Our studies now demonstrate that downregulation of endogenous, intracellular GDF15 has beneficial effects in the context of diabetes and protects beta cells from ER stress-induced apoptosis. Of note, these beneficial effects were observed even in the face of unmeasurably low circulating GDF15 levels in the Gdf15 knockout mice, suggesting that low serum levels are not necessarily detrimental. This is consistent with the notion that the expression of the only known GDF15 receptor, GDNF Family Receptor Alpha Like, has been reported to be restricted to the brain (19-22) and therefore would not be anticipated to contribute to any peripheral systemic functions caused by circulating GDF15.
Taken together, we found that intracellular GDF15 contributes to ER stress-induced beta-cell death and that deletion of GDF15 can prevent or delay diabetes development in different mouse models. Hence, inhibition of intracellular GDF15 may represent a novel strategy to promote beta-cell survival and treat diabetes. In fact, we recently demonstrated that the principle of targeting beta-cell expression of a detrimental factor can be very promising, both by pursuing novel drug discovery using small molecule screening (47) and by repurposing existing drugs that then can be used more readily in clinical trials (66).
Obviously, there are also some limitations to our study. Although we were able to demonstrate that like GDF15, the transcription factor C/EBPβ is increased in Akita beta cells and in response to TG in human islets and INS-1 and that C/EBPβ knockdown completely blunted the GDF15 increase, we could not show direct C/EBPβ binding to the GDF15 promoter by chromatin immunoprecipitation because of the unavailability of a functioning C/EBPβ chromatin immunoprecipitation antibody. In addition, although our results clearly demonstrate that GDF15 deletion affects beta-cell survival and glucose homeostasis in the context of ER stress, we cannot rule out any contributions from GDF15 deficiency in other metabolically active tissues because we used a whole-body Gdf15 knockout mouse. Future studies using a beta-cell-specific Gdf15 knockout will therefore address the tissue specific effects of GDF15.
Glossary
Abbreviations
- ER
endoplasmic reticulum
- GDF15
growth differentiation factor 15
- NOD
nonobese diabetic
- STZ
streptozotocin
- TG
thapsigargin
- UPR
unfolded protein response
- WT
wild-type
Funding
This work was supported by R01DK078752 and Human Islet Research Network U01DK120379 granted to A.S. and UAB Diabetes Research Center Pilot & Feasibility Award (NIH P30 DK079626) granted to G.X. Human pancreatic islets and/or other resources were provided by the NIDDK-funded Integrated Islet Distribution Program (IIDP) at City of Hope, NIH grant #2UC4DK098085.
Author Contributions
G.X. conceived the project, designed, performed, and analyzed the experiments, wrote the manuscript, and is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. J.C. isolated the mouse islets and helped with the mouse experiments. S.J. and T.B.G. helped with the mouse experiments. S.R. and A.K. provided the Akita and wild-type cells and edited the manuscript. E.L.G.-L. and S.J.L. provided the Gdf15KO mice. A.S. supported the work and revised the manuscript.
Conflict of Interest
No potential conflicts of interest relevant to this article were reported.
Data Availability
The data sets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
- 1. Tersey SA, Nishiki Y, Templin AT, et al. . Islet beta-cell endoplasmic reticulum stress precedes the onset of type 1 diabetes in the nonobese diabetic mouse model. Diabetes. 2012;61:818-827. doi: 10.2337/db11-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Papa FR. Endoplasmic reticulum stress, pancreatic β-cell degeneration, and diabetes. Cold Spring Harb Perspect Med. 2012;2:a007666. doi: 10.1101/cshperspect.a007666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fonseca SG, Gromada J, Urano F. Endoplasmic reticulum stress and pancreatic β-cell death. Trends Endocrinol Metab. 2011;22:266-274. doi: 10.1016/j.tem.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bootcov MR, Bauskin AR, Valenzuela SM, et al. . MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc Natl Acad Sci USA. 1997;94:11514-11519. doi: 10.1073/pnas.94.21.11514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baek SJ, Wilson LC, Eling TE. Resveratrol enhances the expression of non-steroidal anti-inflammatory drug-activated gene (NAG-1) by increasing the expression of p53. Carcinogenesis. 2002;23:425-434. doi: 10.1093/carcin/23.3.425. [DOI] [PubMed] [Google Scholar]
- 6. Paralkar VM, Vail AL, Grasser WA, et al. . Cloning and characterization of a novel member of the transforming growth factor-beta/bone morphogenetic protein family. J Biol Chem. 1998;273:13760-13767. doi: 10.1074/jbc.273.22.13760. [DOI] [PubMed] [Google Scholar]
- 7. Welsh JB, Sapinoso LM, Kern SG, et al. . Large-scale delineation of secreted protein biomarkers overexpressed in cancer tissue and serum. Proc Natl Acad Sci USA. 2003;100:3410-3415. doi: 10.1073/pnas.0530278100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Welsh JB, Sapinoso LM, Su AI, et al. . Analysis of gene expression identifies candidate markers and pharmacological targets in prostate cancer. Cancer Res. 2001;61:5974-5978. [PubMed] [Google Scholar]
- 9. Xu J, Kimball TR, Lorenz JN, et al. . GDF15/MIC-1 functions as a protective and antihypertrophic factor released from the myocardium in association with SMAD protein activation. Circ Res. 2006;98:342-350. doi: 10.1161/01.RES.0000202804.84885.d0. [DOI] [PubMed] [Google Scholar]
- 10. Kempf T, Eden M, Strelau J, et al. . The transforming growth factor-beta superfamily member growth-differentiation factor-15 protects the heart from ischemia/reperfusion injury. Circ Res. 2006;98:351-360. doi: 10.1161/01.RES.0000202805.73038.48. [DOI] [PubMed] [Google Scholar]
- 11. Lee SE, Kang SG, Choi MJ, et al. . Growth differentiation factor 15 mediates systemic glucose regulatory action of t-helper type 2 cytokines. Diabetes. 2017;66:2774-2788. doi: 10.2337/db17-0333. [DOI] [PubMed] [Google Scholar]
- 12. Vila G, Riedl M, Anderwald C, et al. . The relationship between insulin resistance and the cardiovascular biomarker growth differentiation factor-15 in obese patients. Clin Chem. 2011;57:309-316. doi: 10.1373/clinchem.2010.153726. [DOI] [PubMed] [Google Scholar]
- 13. Dominguez-Rodriguez A, Abreu-Gonzalez P, Avanzas P. Usefulness of growth differentiation factor-15 levels to predict diabetic cardiomyopathy in asymptomatic patients with type 2 diabetes mellitus. Am J Cardiol. 2014;114:890-894. doi: 10.1016/j.amjcard.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 14. Daniels LB, Clopton P, Laughlin GA, Maisel AS, Barrett-Connor E. Growth-differentiation factor-15 is a robust, independent predictor of 11-year mortality risk in community-dwelling older adults: the Rancho Bernardo Study. Circulation. 2011;123:2101-2110. doi: 10.1161/CIRCULATIONAHA.110.979740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chung JO, Park SY, Cho DH, Chung DJ, Chung MY. Relationship between plasma growth differentiation factor-15 levels and diabetic retinopathy in individuals with type 2 diabetes. Sci Rep. 2020;10:20568. doi: 10.1038/s41598-020-77584-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lajer M, Jorsal A, Tarnow L, Parving HH, Rossing P. Plasma growth differentiation factor-15 independently predicts all-cause and cardiovascular mortality as well as deterioration of kidney function in type 1 diabetic patients with nephropathy. Diabetes Care. 2010;33:1567-1572. doi: 10.2337/dc09-2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kempf T, Guba-Quint A, Torgerson J, et al. . Growth differentiation factor 15 predicts future insulin resistance and impaired glucose control in obese nondiabetic individuals: results from the XENDOS trial. Eur J Endocrinol. 2012;167:671-678. doi: 10.1530/EJE-12-0466. [DOI] [PubMed] [Google Scholar]
- 18. Dostálová I, Roubicek T, Bartlova M, et al. . Increased serum concentrations of macrophage inhibitory cytokine-1 in patients with obesity and type 2 diabetes mellitus: the influence of very low calorie diet. Eur J Endocrinol. 2009;161:397-404. doi: 10.1530/EJE-09-0417. [DOI] [PubMed] [Google Scholar]
- 19. Emmerson PJ, Wang F, Du Y, et al. . The metabolic effects of GDF15 are mediated by the orphan receptor GFRAL. Nat Med. 2017;23:1215-1219. doi: 10.1038/nm.4393. [DOI] [PubMed] [Google Scholar]
- 20. Mullican SE, Lin-Schmidt X, Chin CN, et al. . GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates. Nat Med. 2017;23:1150-1157. doi: 10.1038/nm.4392. [DOI] [PubMed] [Google Scholar]
- 21. Yang L, Chang CC, Sun Z, et al. . GFRAL is the receptor for GDF15 and is required for the anti-obesity effects of the ligand. Nat Med. 2017;23:1158-1166. doi: 10.1038/nm.4394. [DOI] [PubMed] [Google Scholar]
- 22. Hsu JY, Crawley S, Allan BB, et al. . Non-homeostatic body weight regulation through a brainstem-restricted receptor for GDF15. Nature. 2017;550:255-259. doi: 10.1038/nature24042. [DOI] [PubMed] [Google Scholar]
- 23. Corre J, Hebraud B, Bourin P. Concise review: growth differentiation factor 15 in pathology: a clinical role? Stem Cells Transl Med. 2013;2:946-952. doi: 10.5966/sctm.2013-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang X, Baek SJ, Eling TE. The diverse roles of nonsteroidal anti-inflammatory drug activated gene (NAG-1/GDF15) in cancer. Biochem Pharmacol. 2013;85:597-606. doi: 10.1016/j.bcp.2012.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Han M, Dai D, Yousafzai NA, et al. . CXXC4 activates apoptosis through up-regulating GDF15 in gastric cancer. Oncotarget. 2017;8:103557-103567. doi: 10.18632/oncotarget.21581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee SH, Krisanapun C, Baek SJ. NSAID-activated gene-1 as a molecular target for capsaicin-induced apoptosis through a novel molecular mechanism involving GSK3beta, C/EBPbeta and ATF3. Carcinogenesis. 2010;31:719-728. doi: 10.1093/carcin/bgq016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thapa D, Babu D, Park MA, et al. . Induction of p53-independent apoptosis by a novel synthetic hexahydrocannabinol analog is mediated via Sp1-dependent NSAID-activated gene-1 in colon cancer cells. Biochem Pharmacol. 2010;80:62-71. doi: 10.1016/j.bcp.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 28. Kim KS, Yoon JH, Kim JK, et al. . Cyclooxygenase inhibitors induce apoptosis in oral cavity cancer cells by increased expression of nonsteroidal anti-inflammatory drug-activated gene. Biochem Biophys Res Commun. 2004;325:1298-1303. doi: 10.1016/j.bbrc.2004.10.176. [DOI] [PubMed] [Google Scholar]
- 29. Zhang W, Hu C, Wang X, et al. . Role of GDF15 in methylseleninic acid-mediated inhibition of cell proliferation and induction of apoptosis in prostate cancer cells. PLoS One. 2019;14:e0222812. doi: 10.1371/journal.pone.0222812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang X, Kang Y, Huo T, et al. . GL-V9 induced upregulation and mitochondrial localization of NAG-1 associates with ROS generation and cell death in hepatocellular carcinoma cells. Free Radic Biol Med. 2017;112:49-59. doi: 10.1016/j.freeradbiomed.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 31. Min KW, Liggett JL, Silva G, et al. . NAG-1/GDF15 accumulates in the nucleus and modulates transcriptional regulation of the Smad pathway. Oncogene 2016;35:377-388. doi: 10.1038/onc.2015.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nakayasu ES, Syed F, Tersey SA, et al. . Comprehensive proteomics analysis of stressed human islets identifies GDF15 as a target for type 1 diabetes intervention. Cell Metab. 2020;31:363-374.e6. doi: 10.1016/j.cmet.2019.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xu G, Chen J, Jing G, Shalev A. Preventing beta-cell loss and diabetes with calcium channel blockers. Diabetes. 2012;61:848-856. doi: 10.2337/db11-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Xu G, Chen J, Jing G, Shalev A. Thioredoxin-interacting protein regulates insulin transcription through microRNA-204. Nat Med. 2013;19:1141-1146. doi: 10.1038/nm.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sehgal P, Szalai P, Olesen C, et al. . Inhibition of the sarco/endoplasmic reticulum (ER) Ca(2+)-ATPase by thapsigargin analogs induces cell death via ER Ca(2+) depletion and the unfolded protein response. J Biol Chem. 2017;292:19656-19673. doi: 10.1074/jbc.M117.796920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nozaki J, Kubota H, Yoshida H, et al. . The endoplasmic reticulum stress response is stimulated through the continuous activation of transcription factors ATF6 and XBP1 in Ins2+/Akita pancreatic beta cells. Genes Cells 2004;9:261-270. [DOI] [PubMed] [Google Scholar]
- 37. Lei X, Zhang S, Barbour SE, et al. . Spontaneous development of endoplasmic reticulum stress that can lead to diabetes mellitus is associated with higher calcium-independent phospholipase A2 expression: a role for regulation by SREBP-1. J Biol Chem. 2010;285:6693-6705. doi: 10.1074/jbc.M109.084293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hsiao EC, Koniaris LG, Zimmers-Koniaris T, et al. . Characterization of growth-differentiation factor 15, a transforming growth factor beta superfamily member induced following liver injury. Mol Cell Biol. 2000;20:3742-3751. doi: 10.1128/MCB.20.10.3742-3751.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. RRID: AB_10857699. https://scicrunch.org/resolver/AB_10857699. [Google Scholar]
- 40. RRID: AB_2242334. https://scicrunch.org/resolver/AB_2242334. [Google Scholar]
- 41. RRID: AB_330924. https://scicrunch.org/resolver/AB_330924. [Google Scholar]
- 42. RRID: AB_2099233. https://scicrunch.org/resolver/AB_2099233. [Google Scholar]
- 43. RRID: AB_2909412. https://scicrunch.org/resolver/AB_2909412. [Google Scholar]
- 44. Xu G, Thielen LA, Chen J, et al. . Serum miR-204 is an early biomarker of type 1 diabetes-associated pancreatic beta-cell loss. Am J Physiol Endocrinol Metab. 2019;317:E723-E730. doi: 10.1152/ajpendo.00122.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. RRID: AB_2909411. https://scicrunch.org/resolver/AB_2909411. [Google Scholar]
- 46. RRID: AB_2783626. https://scicrunch.org/resolver/AB_2783626. [Google Scholar]
- 47. Thielen LA, Chen J, Jing G, et al. . Identification of an anti-diabetic, orally available small molecule that regulates TXNIP expression and glucagon action. Cell Metab. 2020;32:353-365.e8. doi: 10.1016/j.cmet.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jo S, Chen J, Xu G, et al. . miR-204 controls glucagon-like peptide 1 receptor expression and agonist function. Diabetes. 2018;67:256-264. doi: 10.2337/db17-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chen J, Hui ST, Couto FM, et al. . Thioredoxin-interacting protein deficiency induces Akt/Bcl-xL signaling and pancreatic beta-cell mass and protects against diabetes. FASEB J. 2008;22:3581-3594. doi: 10.1096/fj.08-111690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. RRID: AB_306130. https://scicrunch.org/resolver/AB_306130. [Google Scholar]
- 51. RRID: AB_2340451. https://scicrunch.org/resolver/AB_2340451. [Google Scholar]
- 52. Brozzi F, Nardelli TR, Lopes M, et al. . Cytokines induce endoplasmic reticulum stress in human, rat and mouse beta cells via different mechanisms. Diabetologia. 2015;58:2307-2316. doi: 10.1007/s00125-015-3669-6. [DOI] [PubMed] [Google Scholar]
- 53. Cardozo AK, Ortis F, Storling J, et al. . Cytokines downregulate the sarcoendoplasmic reticulum pump Ca2+ ATPase 2b and deplete endoplasmic reticulum Ca2+, leading to induction of endoplasmic reticulum stress in pancreatic beta-cells. Diabetes. 2005;54:452-461. doi: 10.2337/diabetes.54.2.452. [DOI] [PubMed] [Google Scholar]
- 54. Morita S, Villalta SA, Feldman HC, et al. . Targeting ABL-IRE1α signaling spares ER-stressed pancreatic β cells to reverse autoimmune diabetes. Cell Metab. 2017;25:883-897.e8. doi: 10.1016/j.cmet.2017.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ratnam NM, Peterson JM, Talbert EE, et al. . NF-kappaB regulates GDF-15 to suppress macrophage surveillance during early tumor development. J Clin Invest. 2017;127:3796-3809. doi: 10.1172/JCI91561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chung HK, Ryu D, Kim KS, et al. . Growth differentiation factor 15 is a myomitokine governing systemic energy homeostasis. J Cell Biol. 2017;216:149-165. doi: 10.1083/jcb.201607110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Osada M, Park HL, Park MJ, et al. . A p53-type response element in the GDF15 promoter confers high specificity for p53 activation. Biochem Biophys Res Commun. 2007;354:913-918. doi: 10.1016/j.bbrc.2007.01.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Oyadomari S, Koizumi A, Takeda K, et al. . Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J Clin Invest. 2002;109:525-532. doi: 10.1172/JCI14550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381-389. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- 60. Marciniak SJ, Yun CY, Oyadomari S, et al. . CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18:3066-3077. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tran T, Yang J, Gardner J, Xiong Y. GDF15 deficiency promotes high fat diet-induced obesity in mice. PLoS One. 2018;13:e0201584. doi: 10.1371/journal.pone.0201584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Matsuda T, Kido Y, Asahara S-I, et al. . Ablation of C/EBPbeta alleviates ER stress and pancreatic beta cell failure through the GRP78 chaperone in mice. J Clin Invest. 2010;120:115-126. doi: 10.1172/JCI39721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Chen J, Fontes G, Saxena G, Poitout V, Shalev A. Lack of TXNIP protects against mitochondria-mediated apoptosis but not against fatty acid-induced ER stress-mediated beta-cell death. Diabetes. 2010;59:440-447. doi: 10.2337/db09-0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Laybutt DR, Preston AM, Akerfeldt MC, et al. . Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes. Diabetologia. 2007;50:752-763. doi: 10.1007/s00125-006-0590-z. [DOI] [PubMed] [Google Scholar]
- 65. Kharroubi I, Ladriere L, Cardozo AK, et al. . Free fatty acids and cytokines induce pancreatic beta-cell apoptosis by different mechanisms: role of nuclear factor-kappaB and endoplasmic reticulum stress. Endocrinology. 2004;145:5087-5096. doi: 10.1210/en.2004-0478. [DOI] [PubMed] [Google Scholar]
- 66. Ovalle F, Grimes T, Xu G, et al. . Verapamil and beta cell function in adults with recent-onset type 1 diabetes. Nat Med. 2018;24:1108-1112. doi: 10.1038/s41591-018-0089-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.