Abstract
Background The superior petrosal vein (SPV) often obscures the surgical field or bleeds during microvascular decompression (MVD) for the treatment of trigeminal neuralgia. Although SPV sacrifice has been proposed, it is associated with multiple complications. We have performed more than 4,500 MVDs, including approximately 400 cases involving trigeminal neuralgia. We aimed to describe our operative technique and nuances to avoid SPV injury.
Methods We have provided a detailed description of our institutional protocol, including the anesthesia technique, neurophysiologic monitoring, patient positioning, surgical approach, and SPV management. The surgical outcomes and treatment-related complications were retrospectively analyzed.
Results No SPVs were sacrificed intentionally or accidentally during our MVD protocol for trigeminal neuralgia. In the 344 operations performed during 2006 to 2020, 269 (78.2%) patients did not require medication postoperatively, 58 (16.9%) tolerated the procedure with adequate medication, and 17 (4.9%) did not respond to MVD. Postoperatively, 35 (10.2%), 1 (0.3%), and 0 patients showed permanent trigeminal, facial, or vestibulocochlear nerve dysfunction, respectively. Wound infection occurred in five (1.5%) patients, while cerebrospinal fluid leaks occurred in three (0.9%) patients. Hemorrhagic complications appeared in four (1.2%) patients but these were unrelated to SPV injury. No surgery-related mortalities were reported.
Conclusion MVD for the treatment of trigeminal neuralgia can be achieved safely without sacrificing the SPV. A key step is positioning the patient's vertex at a 10-degree elevation from the floor, which can ease venous return and loosen the SPV, making it less fragile to manipulation and providing a wider surgical corridor.
Keywords: microvascular decompression, superior petrosal vein, trigeminal neuralgia, complications
Introduction
Trigeminal neuralgia (TN) is a chronic neuropathic condition involving one or more divisions of the ipsilateral trigeminal nerve with a characteristic pain triggered by innocuous stimuli and commonly described as stabbing, sharp, shooting, electric shock like, or ice prick like. The neural pathology generally involves the root entry zone of the trigeminal nerve as a result of its mechanical compression by a culprit vessel or tumor 1 2 3 which causes both morphological changes 1 2 and microstructural disruption. 4 5 A lifetime prevalence of 0.16 to 0.3% 6 7 and an incidence of 12.6 to 27.0 per 100,000 person-years 8 9 have been reported in population-based European studies, with a reported average onset age of 53 to 57 years. 1 10 The disease has been reported to affect mental health, 10 as well as the quality of life, by causing conditions such as increased anxiety, depression, and poor sleep. In addition to medical treatment, percutaneous procedures and stereotactic radiosurgery are widely performed in clinical practice.
Microvascular decompression (MVD) is the surgical treatment of choice for patients with TN that demonstrates vascular or tumoral compression along with morphological changes of the trigeminal nerve root on magnetic resonance imaging or during surgery. According to the guideline of the European Academy of Neurology, 11 an analysis including 5,149 patients reported that 62 to 89% of patients who underwent MVD were pain free at the 3 years' follow-up. With respect to complications, severe complications, such as mortality, cerebral edema, or stroke, were rare and occurred only in approximately 1% of the cases.
The superior petrosal vein (SPV, the vein of Dandy) is often encountered during a typical retrosigmoid approach for MVD, 12 and it obstructs the operating field of view 13 in addition to limiting the extent of cerebellar retraction. Therefore, some series in the literature have suggested sacrificing the SPV during this approach. 13 14 However, multiple studies have reported SPV-related complications including cerebellar swelling, 15 contralateral hearing loss, 16 peduncular hallucinosis, 17 18 and even surgical mortality. 19 Moreover, two recent studies presented conflicting conclusions of increased 20 and unchanged 21 rates of complications after SPV sacrifice during MVD.
To address this point, based on a 20-year experience with more than 4,500 MVD procedures for cranial nerve rhizopathies, we present our operative technique and nuances for SPV preservation and discuss our clinical outcomes.
Methods
This study was conducted after receiving approval from the institutional review board. The requirement for informed consent was waived because of the retrospective nature of the study and no patient-identifying data were collected for this study. One patient consented to the publication of her medical photos. We retrospectively reviewed the medical records of patients who underwent MVD for TN between 2006 and 2020. All patients were followed-up postoperatively at the outpatient clinic at regular intervals of 2 to 3 months. Examinations were performed by the same person (J.A.L.) during this period. The Barrow Neurological Institute (BNI) pain intensity score system was adopted for grading postoperative outcomes. The BNI score at the last visit was used for patients whose symptoms continued to change during the follow-up. Descriptive statistics are expressed as medians and ranges.
Anesthesia and Neurophysiological Monitoring
Anesthetic management and neurophysiological monitoring were performed in accordance with the standard institutional protocol which has been previously described in detail. 22 In brief, anesthesia was induced using intravenous sodium thiopental (5 mg/kg body weight) and maintained with continuous infusion of intravenous propofol (3–5.5 µg/kg body weight) and remifentanil (1–4 ng/mL). For consistent neurophysiological monitoring, a bispectral index level of 40 to 60 and the response to train of four stimulations of a 50% twitch height compared with that at baseline were maintained.
All surgeries were conducted under continuous monitoring of brainstem auditory evoked potential 23 from the time of anesthetic induction to dural closure. In the stimulus mode, we used alternating polarity clicks with a stimulus intensity of 120 dB, and white noise at 80 dB was delivered to the contralateral ear. The subdermal electrodes were inserted at the vertex and over both earlobes for recording. The bandpass of the amplifier was set to 150 to 3,000 Hz at all channels. We set a stimulation rate of 43.9 Hz for 400 averaged trials to obtain the result within 9.1 seconds. 24 The intraoperative neuromonitoring technician alerted the surgeon, with a warning criterion of 1-ms latency prolongation or a 50% decrement of wave V. The operator performed immediate corrective maneuvers in response to wave-V changes.
Patient Positioning
The authors believe that the patient's position is the most important factor influencing SPV preservation during MVD for TN. Our surgical technique was based on landmark studies in the past. 25 26 The patient was placed on the operating table with the head at the foot of the bed to maximize the operator's comfort. After induction of general anesthesia, the patient was placed in a park-bench position with appropriate padding at the pressure points, and the head was secured with a three-point head holder. The neck was flexed, and the shoulder was taped down to free the surgical field.
We preferred to raise the patient's upper body and elevate the vertex from the floor such that the angle between the superior–inferior axis of the head and the floor was approximately 10 degrees ( Fig. 1 ). Raising the patient's upper body and vertex facilitated venous return, reducing bleeding during the surgical approach. Additionally, less cerebellar bulging through the craniotomy window reduced the risk of cerebellar cortical injury, and as the cerebrospinal fluid could be released from the lateral medullary cistern rather than cisterna magna, craniotomy in the inferior direction could be minimized ( Fig. 2 ). As we did not require access inferiorly to reach the foramen magnum or cisterna magna, the risk of bleeding from the venous plexus near the foramen magnum could be avoided.
Fig. 1.

Patient positioning during microvascular decompression for trigeminal neuralgia. ( A ) The patient's upper body and vertex are elevated from the floor such that the angle between the vertex and the floor is approximately 10 degrees. ( B ) The head is not rotated to the contralateral side from the affected side, and the anterior–posterior axis of the head is maintained parallel to the floor.
Fig. 2.
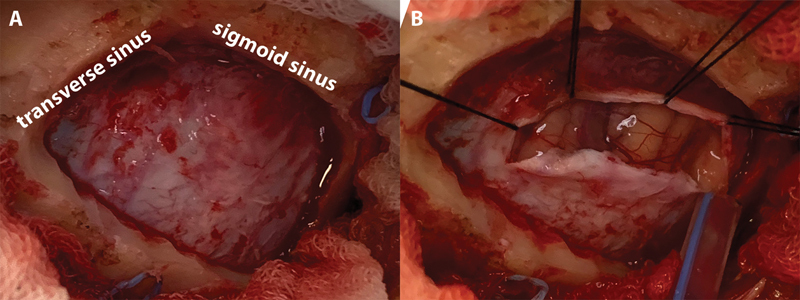
Craniotomy and cerebrospinal fluid drainage. ( A ) The junction of the transverse and sigmoid sinus is visualized. Craniotomy was not required to extend too inferiorly to approach the cisterna magna. ( B ) The cerebellum does not bulge through the craniotomy window even before cerebrospinal fluid release from the lateral medullary cistern.
The head was not rotated to the contralateral side from the affected side, and the anterior–posterior axis of the head was maintained parallel to the floor. A neutral position or rotation to the affected side of the head facilitates cerebellar retraction by gravity in patients undergoing MVD for TN. This provides wider access to Meckel's cave without the risk of injury from the introduction of brain retractors. The key steps in patient positioning are summarized in Table 1 .
Table 1. Key steps in patient positioning for SPV preservation during MVD for TN.
| A. Maintain the shoulder in the prone position rather than keeping it erect in the park-bench position to move the shoulder away from the surgical field. |
| B. Raise the patient's upper body and elevate the vertex from the floor, such that the angle between the superior–inferior axis of patient's head and the floor is 10 degrees. |
| C. Align the anterior–posterior axis of the patient's head parallel to the floor. |
Abbreviations: MVD, microvascular decompression; SPV, superior petrosal vein; TN, trigeminal neuralgia.
Skin Incision to Dural Opening
The iniomeatal line and an imaginary line lying on the digastric groove were used to define the transverse and sigmoid sinuses. The linear “lazy S-shaped” skin incision was placed just medial to the mastoid groove, approximately 1to 2 cm posterior and parallel to the hairline. This incision was adjusted more posteriorly (medially) in patients with a muscular neck, since a skin incision limits the surgical area in the linear retromastoid approach. 25 The subcutaneous and muscular layers were dissected with a monopolar cautery and fixed with the Adson cerebellar retractor.
The supralateral margin of the craniotomy should be positioned at the junction of the transverse sinus and sigmoid sinus. We preferred visualizing the two blue lines of both transverse and sigmoid sinuses to ensure that no bone overhang would limit the dural opening which prevented excessive cerebellar retraction and the consequent SPV traction. Craniotomy was not required to extend too inferiorly as we did not approach the cisterna magna. The size of the craniotomy was usually less than 3 cm in the superior–inferior direction and less than 2 cm in the anterior–posterior direction.
After dural incision, the cerebrospinal fluid was released from the lateral medullary cistern with gentle compression of the cerebellum with the brain retractor. In this step, cerebellar cortical injury occurred in some rare cases because of slackening of the cerebellum and widening of the space between the petrous bone and the cerebellum.
Management of Superior Petrosal Vein during the Intradural Procedure
While exposing the entire range of the trigeminal nerve, we used a brain retractor only in exceptional cases. The neurovascular structures were rarely coagulated or divided during the procedure and were instead dissected free from the arachnoid adhesions using microbayonet forceps and microscissors. The SPV became less tense owing to the elevated vertex, reducing its susceptibility to vascular injury by manipulation; the well-loosened SPV also allowed for establishment of working corridors.
In cases presenting with a high likelihood of SPV rupture during decompression of the culprit vessel, we covered and sealed the SPV along its trajectory with fibrin glue (Tisseel, Baxter Healthcare Corp., Deerfield, Illinois, United States) before decompression. Covering the opening of the superior petrosal sinus at the tentorial side when applying the glue was important because the junction of the vessels was more susceptible to stretching injuries. However, care must be taken to avoid immersing small vessels other than SPV in the glue because of the risk of unexpected vascular injury resulting from adhesion to the surrounding tissues or stretching.
Management of a Bleeding Superior Petrosal Vein
Bleeding of the SPV is treated using different approaches depending on the location. In our experience, most SPV-related bleeding events occurred at the junction of the SPV and the superior petrosal sinus. We could control the bleeding within minutes by raising the patient's vertex and padding a piece of Surgicel (knitted fabric of oxidized regenerated cellulose, Ethicon, Bridgewater, New Jersey, United States) on the avulsed site. Next, small pieces of Gelfoam (absorbable gelatin sponge, Pharmacia & Upjohn Company LLC, North Peapack, New Jersey, United States) were inserted into adjacent cisterns to act as barriers for blood accumulation and clot formation. These sponges were removed after bleeding control.
A relatively small number of cases showed SPV bleeding from its main trunk. Elevating the patient's vertex was the key for hemostasis in these cases as well. In most cases, SPV bleeding was self-limiting as gentle compression of the bleeding focus with a neurosurgical pattie controlled the bleeding. Although SPV sacrifice is a justifiable surgical technique, we recommend preservation of the SPV whenever possible, even in cases with bleeding from the SPV.
Postprocedural Superior Petrosal Vein Protection
Fibrin glue was applied on the shredded Teflon felt (polytetrafluoroethylene, The Chemours Company, Wilmington, Delaware, United States) inserted between the trigeminal nerve and the culprit vessel after confirming that no vessel was in contact with the trigeminal nerve and its entry zone. We also covered the large-diameter bled vessels with fibrin glue to prevent delayed bleeding. The dura was then closed watertight, and the wound was sealed in layers. Details of our method are described in a previous report. 27
Results
Between 1997 and early 2020, 4,699 MVDs were performed for patients with hemifacial spasm ( n = 4,278) and TN ( n = 421) by the senior author. The study cohort consisted of 344 patients (115 men and 229 women; median age, 57 years; range, 16–87 years; 223 and 121 patients with right- and left-sided symptoms, respectively) who had previously undergone MVD for TN since 2006. The authors did not sacrifice the SPV intentionally or accidentally in any patient. Occasional bleeding from the SPV was successfully controlled using the aforementioned approaches.
The postoperative outcomes at the last follow-up examination showed BNI pain intensity scores of I and II, III and IV, and V for 269 (78.2%), 58 (16.9%), and 17 (4.9%) patients, respectively. The median follow-up period after surgery was 34 months (range, 1–161) with an interquartile range of 17 to 63 months. Postoperative motor and sensory dysfunction of the trigeminal nerve were observed in 3 (0.9%) and 165 (48.0%) patients, respectively, and the symptoms resulted in permanent motor and sensory impairment in 1 (0.3%) and 34 (9.9%) patients, respectively. Three (0.9%) patients complained of hearing impairment, which was determined to be partial hearing impairment. The pure tone average on the affected side was changed postsurgery from 25 to 40 dB, from 5 to 47 dB, and from 23 to 67 dB in each patient.
Among the four patients who reported facial weakness postoperatively, one acquired permanent facial asymmetry (House–Brackmann grade II). Wound infection was observed in five (1.5%) patients. Cerebrospinal fluid leakage was observed in three (0.9%) patients, who required additional surgery. No treatment-related mortality was identified in our cohort. However, 4 (1.2%) of the 344 patients showed postoperative hemorrhagic complications, including 1 patient with pin-site epidural hematoma at the frontal area, 2 patients with a small amount of subdural hematoma along the craniotomy site and tentorium of the affected side, and 1 with a faint hemorrhagic density near the surgical bed. None of these complications needed any additional surgical intervention or were related to SPV injury. The general demographic characteristics of the patients are described in Table 2 .
Table 2. Characteristics of the 344 surgeries for TN treated with MVD between 2006 and 2020.
| Demographics | |
|---|---|
| Number of patients | 344 |
| Age (y) Median (range) |
57 (16–87) |
| Sex | |
| Male | 115 (33.4%) |
| Female | 229 (66.6%) |
| Symptom laterality | |
| Right | 223 (64.8%) |
| Left | 121 (35.2%) |
| Surgical outcome in terms of BNI pain intensity score | |
| I–II (no medication required) | 269 (78.2%) |
| II–IV (controlled with medication) | 58 (16.9%) |
| V (severe pain, no pain relief) | 17 (4.9%) |
| Postoperative follow-up period (mo) | |
| Median | 34 |
| Range | 1–161 |
| Interquartile range | 17–63 |
| MVD-related complications | |
| Cranial nerve-V complications | |
| Sensory impairment | 165 (48.0%) |
| Temporary | 34 (9.9%) |
| Permanent | 131 (38.1%) |
| Motor impairment | 3 (0.9%) |
| Temporary | 2 (0.6%) |
| Permanent | 1 (0.3%) |
| Cranial nerve-VII complications | |
| Temporary | 3 (0.9%) |
| Permanent | 1 (0.3%) |
| Cranial nerve VIII complications | |
| Partial hearing impairment | 3 (0.9%) |
| Complete deafness | 0 |
| Wound infection | 5 (1.5%) |
| Cerebrospinal fluid leakage | 3 (0.9%) |
| Hemorrhagic complications | |
| Epidural | 1 (0.3%) |
| Subdural | 2 (0.6%) |
| Surgical bed | 1 (0.3%) |
| Mortality | 0 |
Abbreviations: BNI, Barrow Neurological Institute; MVD, microvascular decompression; TN, trigeminal neuralgia.
Discussion
In this study, we have described our surgical strategy for preserving the SPV during MVD for TN. Based on the standard surgical methods published in the past, 25 28 we established our own institutional standard protocol that aimed to preserve the SPV, as well as avoid complications. Our modification was primarily focused on allowing the patient's vertex elevation. This modification reduced the intracranial pressure, facilitated venous return, reduced cerebellar bulging throughout the craniotomy window, eased cerebrospinal fluid release from the lateral medullary cistern rather than the cisterna magna, and thus reduced the required range of craniotomy. Moreover, our modification did not limit the working corridor and instead loosened the vascular structures to reduce the need for a retractor. In the latter part of the protocol, we have described our strategy to address bleeding from the SPV. A retrospective review of our institutional data revealed acceptable clinical outcomes and rates of treatment-related complications.
The necessity of SPV sacrifice has been a topic of debate. Although expert reports have suggested that no serious sequelae result from routine sacrifice of the SPV, 13 14 25 29 30 the practice is associated with complications. 15 16 17 18 19 A future study investigating the predisposition of specific anatomic variants of petrosal venous anomalies to the risks of complications would allow risk stratification for individual patients. 20 However, our method could serve as an option to preserve the SPV regardless of individual variations in venous complexes 31 32 33 or culprit vessels. 34 35 36
The rates of treatment-related complications of MVD for TN in procedures performed by an experienced operator and surgical team are relatively low, with a few cases showing transient dysfunctions of the trigeminal, facial, and vestibulocochlear nerves. The rate of serious complications is 4%, while the mortality rate is less than 1%. 37 Various percutaneous procedures and stereotactic radiosurgery are also actively used as alternative treatment approaches in patients whose systemic comorbidities make the operative risk of MVD unacceptably high, even though MVD offers excellent long-term pain control rates as high as 72 to 85%. 38 39 40 41 Thus, development of surgical strategies to reduce the risk of surgery may facilitate the use of MVD as a safe option even in such high-risk patients.
Limitations
Nevertheless, our study had several limitations. First, this was a retrospective review based on a single-center experience. Second, the clinical outcomes reported in this study were a result of our institutional standard protocol, which may not be replicated in another clinical setting. Lastly, individual variations in surgical findings, including the anatomy of the SPV, which could be a key determinant in SPV preservation or sacrifice, were not observed or analyzed in this study. We were also unable to supply the exact number of cases with bleeding from the SPV, as this was not recorded. However, in our personal experience, bleeding from the SPV occurred in approximately 10% of cases; we were able to control the bleed with our protocol without SPV sacrifice.
Conclusion
This article presents the operative technique and nuances for SPV preservation and describes the corresponding clinical outcomes during MVD for the treatment of TN in 344 patients. We did not sacrifice the SPV intentionally or accidentally in any of the patients in our cohort, yielding acceptable clinical outcomes and treatment-related complications. A key aspect of our technique was patient positioning to elevate the patient's vertex from the floor, which facilitated the surgical process and proved advantageous for SPV preservation.
Funding Statement
Funding None.
Footnotes
Conflict of Interest None declared.
References
- 1.Antonini G, Di Pasquale A, Cruccu G et al. Magnetic resonance imaging contribution for diagnosing symptomatic neurovascular contact in classical trigeminal neuralgia: a blinded case-control study and meta-analysis. Pain. 2014;155(08):1464–1471. doi: 10.1016/j.pain.2014.04.020. [DOI] [PubMed] [Google Scholar]
- 2.Maarbjerg S, Wolfram F, Gozalov A, Olesen J, Bendtsen L.Significance of neurovascular contact in classical trigeminal neuralgia Brain 2015138(Pt 2):311–319. [DOI] [PubMed] [Google Scholar]
- 3.Wei Y, Zhao W, Pu C et al. Clinical features and long-term surgical outcomes in 39 patients withtumor-related trigeminal neuralgia compared with 360 patients with idiopathic trigeminal neuralgia. Br J Neurosurg. 2017;31(01):101–106. doi: 10.1080/02688697.2016.1233321. [DOI] [PubMed] [Google Scholar]
- 4.Herweh C, Kress B, Rasche D et al. Loss of anisotropy in trigeminal neuralgia revealed by diffusion tensor imaging. Neurology. 2007;68(10):776–778. doi: 10.1212/01.wnl.0000256340.16766.1d. [DOI] [PubMed] [Google Scholar]
- 5.Lutz J, Linn J, Mehrkens J H et al. Trigeminal neuralgia due to neurovascular compression: high-spatial-resolution diffusion-tensor imaging reveals microstructural neural changes. Radiology. 2011;258(02):524–530. doi: 10.1148/radiol.10100477. [DOI] [PubMed] [Google Scholar]
- 6.Mueller D, Obermann M, Yoon M S et al. Prevalence of trigeminal neuralgia and persistent idiopathic facial pain: a population-based study. Cephalalgia. 2011;31(15):1542–1548. doi: 10.1177/0333102411424619. [DOI] [PubMed] [Google Scholar]
- 7.Sjaastad O, Bakketeig L S. The rare, unilateral headaches. Vågå study of headache epidemiology. J Headache Pain. 2007;8(01):19–27. doi: 10.1007/s10194-006-0292-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall G C, Carroll D, Parry D, McQuay H J.Epidemiology and treatment of neuropathic pain: the UK primary care perspective Pain 2006122(1,2):156–162. [DOI] [PubMed] [Google Scholar]
- 9.Koopman J S, Dieleman J P, Huygen F J, de Mos M, Martin C G, Sturkenboom M C.Incidence of facial pain in the general population Pain 2009147(1–3):122–127. [DOI] [PubMed] [Google Scholar]
- 10.Zakrzewska J M, Wu J, Mon-Williams M, Phillips N, Pavitt S H. Evaluating the impact of trigeminal neuralgia. Pain. 2017;158(06):1166–1174. doi: 10.1097/j.pain.0000000000000853. [DOI] [PubMed] [Google Scholar]
- 11.Bendtsen L, Zakrzewska J M, Abbott J et al. European Academy of Neurology guideline on trigeminal neuralgia. Eur J Neurol. 2019;26(06):831–849. doi: 10.1111/ene.13950. [DOI] [PubMed] [Google Scholar]
- 12.Broggi G, Broggi M, Ferroli P, Franzini A. Surgical technique for trigeminal microvascular decompression. Acta Neurochir (Wien) 2012;154(06):1089–1095. doi: 10.1007/s00701-012-1324-2. [DOI] [PubMed] [Google Scholar]
- 13.Pathmanaban O N, O'Brien F, Al-Tamimi Y Z, Hammerbeck-Ward C L, Rutherford S A, King A T. Safety of superior petrosal vein sacrifice during microvascular decompression of the trigeminal nerve. World Neurosurg. 2017;103:84–87. doi: 10.1016/j.wneu.2017.03.117. [DOI] [PubMed] [Google Scholar]
- 14.Choudhari K A. Superior petrosal vein in trigeminal neuralgia. Br J Neurosurg. 2007;21(03):288–292. doi: 10.1080/02688690701397773. [DOI] [PubMed] [Google Scholar]
- 15.Masuoka J, Matsushima T, Hikita T, Inoue E. Cerebellar swelling after sacrifice of the superior petrosal vein during microvascular decompression for trigeminal neuralgia. J Clin Neurosci. 2009;16(10):1342–1344. doi: 10.1016/j.jocn.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 16.Strauss C, Naraghi R, Bischoff B, Huk W J, Romstöck J. Contralateral hearing loss as an effect of venous congestion at the ipsilateral inferior colliculus after microvascular decompression: report of a case. J Neurol Neurosurg Psychiatry. 2000;69(05):679–682. doi: 10.1136/jnnp.69.5.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koerbel A, Wolf S A, Kiss A.Peduncular hallucinosis after sacrifice of veins of the petrosal venous complex for trigeminal neuralgia Acta Neurochir (Wien) 200714908831–832., discussion 832–833 [DOI] [PubMed] [Google Scholar]
- 18.Tsukamoto H, Matsushima T, Fujiwara S, Fukui M. Peduncular hallucinosis following microvascular decompression for trigeminal neuralgia: case report. Surg Neurol. 1993;40(01):31–34. doi: 10.1016/0090-3019(93)90166-x. [DOI] [PubMed] [Google Scholar]
- 19.Anichini G, Iqbal M, Rafiq N M, Ironside J W, Kamel M. Sacrificing the superior petrosal vein during microvascular decompression. Is it safe? Learning the hard way. Case report and review of literature. Surg Neurol Int. 2016;7 14:S415–S420. doi: 10.4103/2152-7806.183520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liebelt B D, Barber S M, Desai V R et al. Superior petrosal vein sacrifice during microvascular decompression: perioperative complication rates and comparison with venous preservation. World Neurosurg. 2017;104:788–794. doi: 10.1016/j.wneu.2017.05.098. [DOI] [PubMed] [Google Scholar]
- 21.Xia Y, Kim T Y, Mashouf L A et al. Absence of ischemic injury after sacrificing the superior petrosal vein during microvascular decompression. Oper Neurosurg (Hagerstown) 2020;18(03):316–320. doi: 10.1093/ons/opz163. [DOI] [PubMed] [Google Scholar]
- 22.Lee S, Park S K, Lee J A et al. A new method for monitoring abnormal muscle response in hemifacial spasm: a prospective study. Clin Neurophysiol. 2018;129(07):1490–1495. doi: 10.1016/j.clinph.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 23.Park S K, Joo B E, Lee S et al. The critical warning sign of real-time brainstem auditory evoked potentials during microvascular decompression for hemifacial spasm. Clin Neurophysiol. 2018;129(05):1097–1102. doi: 10.1016/j.clinph.2017.12.032. [DOI] [PubMed] [Google Scholar]
- 24.Joo B E, Park S K, Cho K R, Kong D S, Seo D W, Park K. Real-time intraoperative monitoring of brainstem auditory evoked potentials during microvascular decompression for hemifacial spasm. J Neurosurg. 2016;125(05):1061–1067. doi: 10.3171/2015.10.JNS151224. [DOI] [PubMed] [Google Scholar]
- 25.McLaughlin M R, Jannetta P J, Clyde B L, Subach B R, Comey C H, Resnick D K. Microvascular decompression of cranial nerves: lessons learned after 4400 operations. J Neurosurg. 1999;90(01):1–8. doi: 10.3171/jns.1999.90.1.0001. [DOI] [PubMed] [Google Scholar]
- 26.Rhoton A L., JrThe cerebellopontine angle and posterior fossa cranial nerves by the retrosigmoid approach Neurosurgery 200047(3, suppl):S93–S129. [DOI] [PubMed] [Google Scholar]
- 27.Lee S, Park S K, Joo B E, Lee J A, Kong D S, Park K. A surgical strategy to prevent delayed epidural hematoma after posterior fossa surgery using lateral suboccipital retrosigmoid approach. J Clin Neurosci. 2018;52:156–158. doi: 10.1016/j.jocn.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 28.Jannetta P J, McLaughlin M R, Casey K F. Technique of microvascular decompression. Technical note. Neurosurg Focus. 2005;18(05):E5. [PubMed] [Google Scholar]
- 29.Zhong J, Li S T, Zhu J et al. A clinical analysis on microvascular decompression surgery in a series of 3000 cases. Clin Neurol Neurosurg. 2012;114(07):846–851. doi: 10.1016/j.clineuro.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 30.Zhong J, Zhu J, Sun H et al. Microvascular decompression surgery: surgical principles and technical nuances based on 4000 cases. Neurol Res. 2014;36(10):882–893. doi: 10.1179/1743132814Y.0000000344. [DOI] [PubMed] [Google Scholar]
- 31.Basamh M, Sinning N, Kehler U. Individual variations of the superior petrosal vein complex and their microsurgical relevance in 50 cases of trigeminal microvascular decompression. Acta Neurochir (Wien) 2020;162(01):197–209. doi: 10.1007/s00701-019-04109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dumot C, Sindou M. Veins of the cerebellopontine angle and specific complications of sacrifice, with special emphasis on microvascular decompression surgery. A review. World Neurosurg. 2018;117:422–432. doi: 10.1016/j.wneu.2018.06.160. [DOI] [PubMed] [Google Scholar]
- 33.Feng B, Zheng X, Wang X, Wang X, Ying T, Li S. Management of different kinds of veins during microvascular decompression for trigeminal neuralgia: technique notes. Neurol Res. 2015;37(12):1090–1095. doi: 10.1080/01616412.2015.1115588. [DOI] [PubMed] [Google Scholar]
- 34.Gajski D, Dennis A R, Arnautović K I. Microsurgical decompression of trigeminal neuralgia caused by simultaneous double arterial (SCA and AICA) and petrosal vein complex compression. J Neurol Surg B Skull Base. 2018;79 05:S428–S430. doi: 10.1055/s-0038-1669968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hong W, Zheng X, Wu Z et al. Clinical features and surgical treatment of trigeminal neuralgia caused solely by venous compression. Acta Neurochir (Wien) 2011;153(05):1037–1042. doi: 10.1007/s00701-011-0957-x. [DOI] [PubMed] [Google Scholar]
- 36.Inoue T, Hirai H, Shima A, Suzuki F, Fukushima T, Matsuda M. Diagnosis and management for trigeminal neuralgia caused solely by venous compression. Acta Neurochir (Wien) 2017;159(04):681–688. doi: 10.1007/s00701-017-3085-4. [DOI] [PubMed] [Google Scholar]
- 37.Degn J, Brennum J. Surgical treatment of trigeminal neuralgia. Results from the use of glycerol injection, microvascular decompression, and rhizotomia. Acta Neurochir (Wien) 2010;152(12):2125–2132. doi: 10.1007/s00701-010-0840-1. [DOI] [PubMed] [Google Scholar]
- 38.Ko A L, Ozpinar A, Lee A, Raslan A M, McCartney S, Burchiel K J. Long-term efficacy and safety of internal neurolysis for trigeminal neuralgia without neurovascular compression. J Neurosurg. 2015;122(05):1048–1057. doi: 10.3171/2014.12.JNS14469. [DOI] [PubMed] [Google Scholar]
- 39.Pamir M N, Peker S. Microvascular decompression for trigeminal neuralgia: a long-term follow-up study. Minim Invasive Neurosurg. 2006;49(06):342–346. doi: 10.1055/s-2006-960487. [DOI] [PubMed] [Google Scholar]
- 40.Pollock B E, Stien K J.Posterior fossa exploration for trigeminal neuralgia patients older than 70 years of age Neurosurgery 201169061255–1259., discussion 1259–1260 [DOI] [PubMed] [Google Scholar]
- 41.Tyler-Kabara E C, Kassam A B, Horowitz M H et al. Predictors of outcome in surgically managed patients with typical and atypical trigeminal neuralgia: comparison of results following microvascular decompression. J Neurosurg. 2002;96(03):527–531. doi: 10.3171/jns.2002.96.3.0527. [DOI] [PubMed] [Google Scholar]


