Abstract
Background Cerebrospinal fluid (CSF) leak is widely recognized as a challenging and commonly occurring postoperative complication of transsphenoidal surgery (TSS).
The primary objective of this study is to benchmark the current prevalence of CSF leak after TSS in the adult population.
Methods The authors followed the PRISMA guidelines. The PubMed, Embase, and Cochrane Library databases were searched for articles reporting CSF leak after TSS in the adult population. Meta-analysis was performed using the Untransformed Proportion metric in OpenMetaAnalyst. For two between-group comparisons a generalized linear mixed model was applied.
Results We identified 2,408 articles through the database search, of which 70, published since 2015, were included in this systematic review. These studies yielded 24,979 patients who underwent a total of 25,034 transsphenoidal surgeries. The overall prevalence of postoperative CSF leak was 3.4% (95% confidence interval or CI 2.8–4.0%). The prevalence of CSF leak found in patients undergoing pituitary adenoma resection was 3.2% (95% CI 2.5–4.2%), whereas patients who underwent TSS for another indication had a CSF leak prevalence rate of 7.1% (95% CI 3.0–15.7%) (odds ratio [OR] 2.3, 95% CI 0.9–5.7). Patients with cavernous sinus invasion (OR 3.0, 95% CI 1.1–8.7) and intraoperative CSF leak (OR 5.9, 95% CI 3.8–9.0) have increased risk of postoperative CSF leak. Previous TSS and microscopic surgery are not significantly associated with postoperative CSF leak.
Conclusion The overall recent prevalence of CSF leak after TSS in adults is 3.4%. Intraoperative CSF leak and cavernous sinus invasion appear to be significant risk factors for postoperative CSF leak.
Keywords: complications, CSF leak, endonasal, liquorrhea, pituitary adenoma surgery, skull base
Introduction
Cerebrospinal fluid (CSF) leak is still widely recognized as a commonly occurring postoperative complication of transsphenoidal surgery (TSS). CSF leak is associated with various complications including meningitis, intracranial infection, and CSF hypotension syndrome. These complications often lead to additional health care costs and substantial morbidity as they may require prolonged hospitalization, reoperation, and external lumbar drainage (ELD). 1 2 Grotenhuis reports an additional cost of €10.243 per patient with postoperative CSF leak for transsphenoidal procedures. 2 The prevalence of postoperative CSF leak seems increased in patients with an elevated body mass index (BMI) and/or increased intracranial pressure. 3 However, the exact risk of this complication and variables of influence are not clearly defined and reported prevalence rates vary widely (0–40%). 4 CSF leak rates among patients undergoing TSS are regarded to be higher than for transcranial neurosurgical procedures due to additional risk factors, such as gravity and a lack of anatomical barriers provided by watertight dural closure and subcutaneous and cutaneous closure. 5 However, techniques of closure have been significantly improved by using a vital nasoseptal mucosal flap, the use of sealing materials, and improved neurosurgical techniques. 6 7 8 9 TSS has been an evolving field over the last decades, therefore complication rates should be investigated in recent literature and frequently updated as advancements in the surgical technique continue. The objectives of this study are to benchmark the prevalence of CSF leak after TSS in the adult population in the past 5 years, and to define variables affecting this risk.
Methods
The authors followed the PRISMA guidelines for this systematic review and meta-analysis. 10
Search Strategy and Study Selection
We performed a literature search in the PubMed, Embase, and Cochrane Library databases for articles reporting CSF leak after TSS until April 1, 2020. A combination of free, controlled, and Mesh/Emtree terms for TSS and CSF leak, such as “Transsphenoidal” OR “Endoscopic endonasal” AND “Cerebrospinal fluid leak” OR “Cerebrospinal fluid rhinorrhea,” was used to form a search string ( Appendices A–C [available in the online version], for the search strings per database).
Articles reporting original studies published since 2015 on the adult population reporting CSF leak rates after TSS written in English or Dutch were included. The timeframe 2015 to 2020 was chosen with the aim to provide an up-to-date analysis of CSF leak after TSS and to expand on the existing literature on this topic. 4 Extended procedures and use of dural sealants were no restriction for inclusion. Studies including CSF fistula repairs or biopsies were excluded. Furthermore, case reports ( n <30) were excluded, as these were not considered strong evidence due to the risk of publication bias and selected populations.
Two authors (R.S. and E.M.H.S.) independently screened titles and abstracts for eligibility, after which full-texts of all potentially eligible studies were assessed for inclusion. No disagreement regarding the inclusion of an article after full-text assessment was encountered. The reference lists of all included studies and relevant reviews were cross-checked for additional eligible articles.
Data Collection
We extracted the following data from the included studies: study characteristics (authors, publication year, inclusion period, design, country, center name, total number of patients, total number of surgeries); patient characteristics (mean age at surgery, number of females, mean BMI, mean follow-up duration, previous surgery at same site, type of sphenoid sinus, preoperative diabetes mellitus, use of immunosuppressive medication, use of blood thinners, preoperative hydrocephalus, preoperative pneumocephalus, history of skull base radiation, length of stay); surgery characteristics (indication [e.g., pituitary adenoma or craniopharyngioma resection], approach, extended or conventional [based on the article's definition], reconstruction technique, use of sealant, intraoperative placement of a CSF diversion shunt); tumor characteristics (type of tumor, maximal tumor diameter, invasive [Knosp grades 3 and 4] or not, suprasellar extension); outcome parameters (rate of intraoperative CSF leak and rate of postoperative CSF leak, as defined by the article). Studies with a noncomparative design were defined as case series. 11 The study quality of case series was assessed using the National Heart, Lung and Blood Institute of National Institutes of Health (NIH) quality assessment tool for case series studies, 12 whereas the Newcastle Ottawa Scale 13 was used for the quality assessment of cohort studies. Studies with fewer than six points were judged to be of poor quality, studies with six or seven points were deemed of fair quality and studies with more than seven points were classified as being of good quality. Each item was awarded one point if answered with “Yes” or a star.
Statistical Analysis
We performed a meta-analysis of prevalence using the Untransformed Proportion metric in OpenMetaAnalyst for Sierra, version 10.12. A binary random effects analysis using the DerSimonian-Laird method was applied if heterogeneity across studies was significant ( p <0.05). For nonsignificant heterogeneity across studies the binary fixed effects inverse variance model was used. For two between-group comparisons (microscopic versus endoscopic surgery and pituitary adenoma resection vs. other indication) a generalized linear mixed model was applied, using SAS version 9.4 (SAS Institute Inc), as these analyses involved comparisons of groups on study level. Heterogeneity across studies was ascertained through Higgins I. 2 14
The prevalence of CSF leak after TSS with 95% confidence interval (CI) was the primary outcome measure in this study. For between group comparisons of patients with and without certain risk factors for CSF leak the outcome measures were odds ratios (ORs) with 95% CI. We performed three sensitivity analyses: (1) excluding Pines et al, 15 as this publication accounts for almost half of the total population, (2) a comparison between studies published between 2015 to 2017 and 2018 to 2020 to evaluate a learning curve, (3) high quality studies only.
Results
Included Studies
We identified 2,408 articles through the initial database searches after removing duplicates. Seventy articles met the inclusion criteria for this systematic review. Eight articles were excluded from the meta-analysis due to an overlapping population with another included article (the study with the largest sample size was included). 16 17 18 19 20 21 22 23 One article was manually added by hand-searching the reference lists of all included articles. The study selection process and reasons for exclusion are shown in Fig. 1 .
Fig. 1.
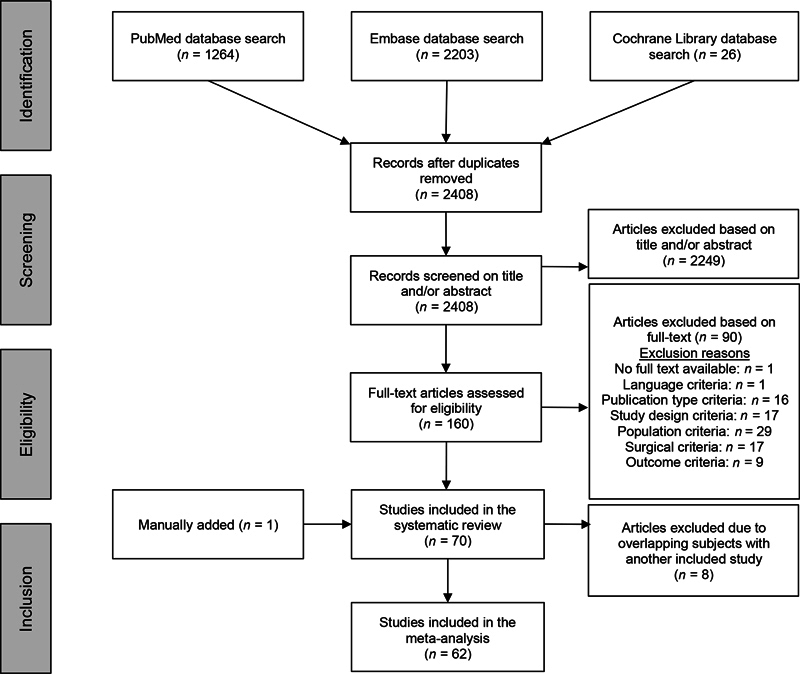
Flowchart demonstrating the study selection process.
The included studies yielded 24,979 patients who underwent a total of 25,034 transsphenoidal surgeries as some subjects had more than one surgery. This includes 262 extended procedures and 2,104 conventional procedures. In the remaining 58 articles insufficient information is provided to determine the number of extended and conventional surgeries. An overview of study characteristics is presented in Table 1 . Nineteen studies were judged to be of good quality, 36 studies of fair quality, and 15 studies of poor quality ( Supplementary Material 1 [available in the online version], for an overview of quality assessment).
Table 1. Overview of study characteristics of included studies.
| Authors | Year | Study design | Approach | Indication | Population ( N = 24,979) | Surgeries ( N = 25,034) | CSF leak (%) | Study quality |
|---|---|---|---|---|---|---|---|---|
| Gondim et al 39 | 2015 | RC | Endoscopic | Pituitary tumor | 374 | 374 | 3.7 | Good |
| Fathalla et al 40 | 2015 | RC | Endoscopic and microscopic | Pituitary tumor | 65 | 65 | 6.2 | Good |
| Wang et al 22 a | 2015 | CS | Endoscopic | Pituitary tumor | 1,166 | 1,166 | 0.6 | Fair |
| Nie et al 41 | 2015 | CS | Endoscopic | Pituitary tumor | 52 | 52 | 0.0 | Fair |
| Zhan et al 42 | 2015 | RC | Endoscopic | Pituitary tumor | 313 | 318 | 3.8 | Fair |
| Ishii et al 25 | 2015 | RC | Endoscopic | Pituitary tumor, craniopharyngioma, meningioma, chordoma, ependymoma | 48 | 48 | 6.3 | Poor |
| Park et al 24 | 2015 | CS | Endoscopic | Pituitary tumor, Rathke cleft cyst, craniopharyngioma, meningioma, chordoma/chondrosarcoma, other | 188 | 197 | 0.0 | Poor |
| Chabot et al 43 | 2015 | CS | Endoscopic | Pituitary tumor | 39 | 39 | 10.3 | Fair |
| Pinar et al 44 | 2015 | CS | Endoscopic | Pituitary tumor | 32 | 32 | 9.4 | Fair |
| Sanders-Taylor et al 45 | 2015 | RC | Endoscopic | Pituitary tumor | 264 | 264 | 1.9 | Poor |
| Pines et al 15 | 2015 | RC | Unknown | Pituitary tumor | 12,938 | 12,938 | 1.7 | Poor |
| Xie et al 46 | 2016 | RC | Endoscopic | Pituitary tumor | 43 | 43 | 14.0 | Good |
| Gao et al 47 | 2016 | RC | Endoscopic and microscopic | Pituitary tumor | 105 | 105 | 10.5 | Fair |
| Freyschlag et al 48 | 2016 | CS | Endoscopic and microscopic | Pituitary tumor | 50 | 50 | 0.0 | Fair |
| Park et al 49 | 2016 | RC | Endoscopic | Pituitary tumor, craniopharyngioma, meningioma, chordoma, Rathke cleft cyst, other | 106 | 106 | 9.4 | Fair |
| Jang et al 50 | 2016 | CS | Endoscopic | Pituitary tumor | 331 | 331 | 1.8 | Good |
| Zaidi et al 34 | 2016 | RC | Endoscopic and microscopic | Pituitary tumor | 135 | 135 | 1.5 | Fair |
| Gondim, Albuquerque et al 18 b | 2017 | CS | Endoscopic | Pituitary apoplexy | 39 | 39 | 0.0 | Poor |
| Fnais et al 51 | 2017 | RC | Endoscopic | Pituitary tumor, pituitary apoplexy, Rathke cleft cyst, craniopharyngioma, other | 145 | 138 | 11.6 | Fair |
| Ye et al 52 | 2017 | RC | Endoscopic | Pituitary tumor | 1,281 | 1,281 | 0.5 | Poor |
| Karki et al 53 | 2017 | RC | Microscopic | Pituitary tumor | 123 | 123 | 15.4 | Good |
| Wang et al 54 | 2017 | RC | Microscopic | Pituitary tumor | 51 | 51 | 0.0 | Good |
| Sun et al 55 | 2017 | CS | Endoscopic | Pituitary tumor | 42 | 42 | 9.5 | Poor |
| Ding et al 26 | 2017 | RC | Endoscopic | Craniopharyngioma | 33 | 33 | 18.2 | Good |
| Zhou et al 56 | 2017 | RC | Endoscopic | Pituitary tumor | 492 | 492 | 1.2 | Fair |
| Cebula et al 57 | 2017 | PC | Endoscopic | Pituitary tumor | 230 | 230 | 0.0 | Fair |
| Levi et al 37 | 2017 | RC | Endoscopic and microscopic | Pituitary tumor | 221 | 221 | 5.9 | Poor |
| Zoli et al 58 | 2017 | CS | Endoscopic | Pituitary tumor | 75 | 75 | 1.3 | Fair |
| Fujimoto et al 59 | 2017 | RC | Endoscopic | Pituitary tumor | 161 | 162 | 4.9 | Poor |
| Yano et al 23 c | 2017 | CS | Endoscopic | Pituitary tumor | 32 | 34 | 5.9 | Fair |
| Sasagawa et al 60 | 2017 | RC | Endoscopic and microscopic | Pituitary tumor | 78 | 78 | 1.3 | Fair |
| Fishpool et al 61 | 2017 | CS | Endoscopic | Pituitary tumor | 32 | 32 | 0.0 | Poor |
| Ajlan et al 62 | 2017 | RC | Endoscopic | Pituitary tumor | 176 | 176 | 4.5 | Fair |
| Przybylowski et al 63 | 2017 | RC | Endoscopic | Pituitary tumor | 96 | 96 | 4.2 | Good |
| Negm et al 19 d | 2017 | PC | Endoscopic | Pituitary tumor | 41 | 41 | 2.4 | Good |
| Shin et al 64 | 2017 | CS | Endoscopic | Pituitary tumor | 50 | 50 | 4.0 | Fair |
| Patel et al 3 | 2018 | RC | Endoscopic | Pituitary tumor, Rathke cleft cyst, craniopharyngioma, other | 806 | 806 | 4.7 | Fair |
| Eseonu et al 65 | 2018 | CS | Endoscopic | Pituitary tumor | 275 | 275 | 3.6 | Good |
| Popov et al 66 | 2018 | RC | Endoscopic and microscopic | Pituitary tumor | 128 | 128 | 3.9 | Fair |
| Hansasuta et al 38 | 2018 | PC | Endoscopic | Pituitary tumor | 183 | 220 | 3.6 | Fair |
| Han et al 67 | 2018 | CS | Endoscopic | Pituitary tumor | 52 | 52 | 3.8 | Good |
| Guo et al 68 | 2018 | RC | Unknown | Pituitary tumor | 53 | 53 | 9.4 | Fair |
| Schuss et al 69 | 2018 | RC | Endoscopic and microscopic | Pituitary tumor | 255 | 255 | 6.7 | Poor |
| Hajdari et al 70 | 2018 | CS | Endoscopic | Pituitary tumor | 170 | 170 | 8.2 | Fair |
| Karamouzis et al 71 | 2018 | CS | Endoscopic | Pituitary tumor | 90 | 90 | 4.4 | Fair |
| Lofrese et al 72 | 2018 | RC | Endoscopic | Pituitary tumor | 95 | 95 | 5.3 | Fair |
| Cudal et al 73 | 2018 | CS | Unknown | Pituitary tumor, other | 47 | 47 | 6.4 | Poor |
| Robins et al 74 | 2018 | RC | Endoscopic | Pituitary tumor | 142 | 142 | 0.7 | Poor |
| Barger et al 35 | 2018 | CS | Endoscopic | Pituitary tumor | 43 | 43 | 2.3 | Good |
| Wilson et al 75 | 2018 | RC | Endoscopic | Pituitary tumor | 135 | 135 | 0.0 | Good |
| Rehman et al 76 | 2018 | CS | Endoscopic | Pituitary tumor | 63 | 63 | 15.9 | Fair |
| Xue et al 77 | 2019 | RC | Endoscopic | Pituitary apoplexy | 79 | 79 | 12.7 | Fair |
| Chen et al 16 e | 2019 | CS | Endoscopic | Pituitary tumor | 79 | 79 | 5.1 | Fair |
| Fallah et al 78 | 2019 | CS | Endoscopic | Pituitary tumor | 80 | 88 | 4.5 | Good |
| Spina et al 79 | 2019 | RC | Unknown | Pituitary tumor | 336 | 336 | 0.6 | Good |
| Shen et al 80 | 2019 | CS | Endoscopic | Pituitary tumor | 45 | 45 | 2.2 | Fair |
| Eichberg et al 17 f | 2019 | CS | Endoscopic | Pituitary tumor | 120 | 120 | 1.7 | Fair |
| Chen et al 81 | 2019 | RC | Endoscopic | Pituitary tumor | 131 | 131 | 8.4 | Good |
| Seltzer et al 21 g | 2019 | CS | Endoscopic and microscopic | Pituitary tumor | 52 | 52 | 1.9 | Fair |
| Azab et al 82 | 2019 | RC | Microscopic | Pituitary tumor | 205 | 205 | 2.9 | Good |
| Memel et al 83 | 2019 | RC | Unknown | Pituitary tumor | 115 | 115 | 2.6 | Fair |
| Rieley et al 84 | 2020 | RC | Endoscopic | Pituitary tumor, other | 427 | 427 | 13.1 | Poor |
| Liu et al 36 | 2020 | RC | Endoscopic | Pituitary tumor | 189 | 189 | 6.3 | Fair |
| Zhang et al 85 | 2020 | CS | Endoscopic | Pituitary tumor | 113 | 113 | 0.9 | Fair |
| Tardivo et al 86 | 2020 | RC | Endoscopic | Pituitary tumor | 81 | 81 | 4.9 | Good |
| Castaño-Leon et al 87 | 2020 | RC | Endoscopic and microscopic | Pituitary tumor, other | 187 | 187 | 5.3 | Fair |
| Pangal et al 20 g | 2020 | CS | Endoscopic | Pituitary apoplexy | 50 | 50 | 8.0 | Fair |
| Parikh et al 88 | 2020 | CS | Endoscopic | Pituitary tumor | 334 | 334 | 3.9 | Good |
| Tafreshi et al 89 | 2020 | CS | Endoscopic | Pituitary tumor, Rathke cleft cyst, arachnoid cyst, xanthogranuloma | 47 | 47 | 8.5 | Poor |
| Cappello et al 90 | 2020 | CS | Endoscopic | Pituitary tumor, craniopharyngioma, pituitary apoplexy, cyst, other | 125 | 125 | 3.2 | Fair |
Abbreviations: CS, case series; CSF, cerebrospinal fluid; N , number; PC, prospective cohort; RC, retrospective cohort.
Excluded from primary analysis due to overlapping population with Ye et al. 52
Excluded from primary analysis due to overlapping population with Gondim et al. 39
Excluded from primary analysis due to overlapping population with Fujimoto et al. 59
Excluded from primary analysis due to overlapping population with Wilson et al. 75
Excluded from primary analysis due to overlapping population with Zhang et al. 85
Excluded from primary analysis due to overlapping population with Chen, Sprau et al. 81
Excluded from primary analysis due to overlapping population with Memel et al. 83
There was insufficient data from the included studies to perform reliable analyses for several risk factors: suprasellar extension, dural invasion, BMI, preventative ELD, reconstruction technique, age at surgery, sex, diabetes mellitus, use of immunosuppressive medication, use of blood thinners, preoperative hydrocephalus, preoperative pneumocephalus, history of skull base radiation, and sealant use.
Outcome and Risk Factor Analysis
The overall prevalence of postoperative CSF leak was 3.4% (95% CI 2.8–4.0%) ( Fig. 2 ). Heterogeneity across studies was substantial ( I 2 81.7).
Fig. 2.
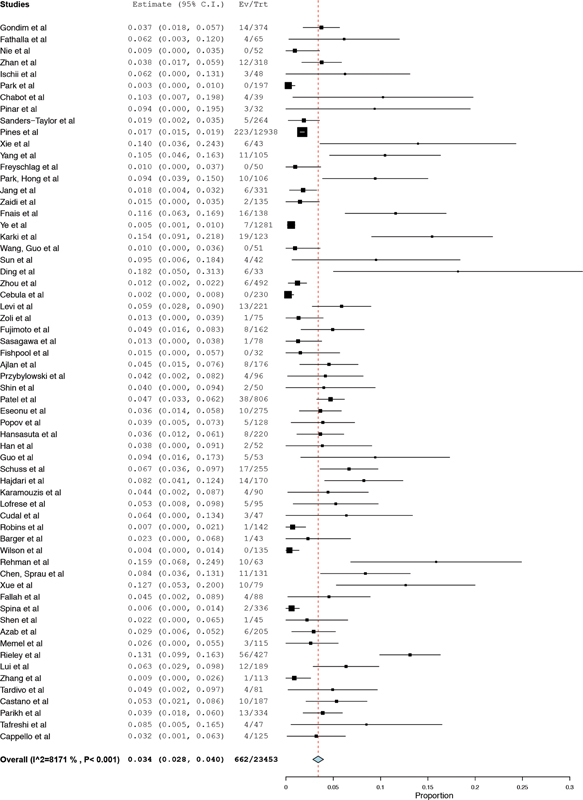
Forest plot prevalence of cerebrospinal fluid leak.
The prevalence of CSF leak found in patients undergoing pituitary adenoma resection was 3.2% (95% CI 2.5–4.2%), whereas patients who underwent TSS for another indication (i.e., craniopharyngioma, meningioma, Rathke cleft cyst) had a CSF leak prevalence rate of 7.1% (95% CI 3.0–15.7%) (OR 2.3, 95% CI 0.9–5.7). Data on further specified diagnosis subgroups is too limited to analyze its influence on CSF leak. In this dataset, there is one study reporting on CSF leak on Rathke cleft cyst separately in which none of the 19 cases had CSF leak. 24 Three small populations of craniopharyngioma's are included in which a total of six out of 49 patients had CSF leak (12.2%). 24 25 26 Two studies specify CSF leak in meningioma cases, of which one out of 15 patients had CSF leak (6.7%). 24 25 For 2,318 cases the CSF leak rate was not specified as per diagnosis subgroup.
Postoperative CSF leak was observed in 5.5% (95% CI 3.3–9.0%) of microscopically approached cases, as opposed to 4.0% (95% CI 3.0–5.2%) in endoscopic cases (OR 1.4, 95% CI 0.9–2.3).
CSF leak was present in 2.0% (95% CI 0.0–4.9%) of patients with previous TSS as compared with 0.4% (95% CI 0–1.0%) in patients without history of TSS (OR 0.9, 95% CI 0.2–4.5).
The prevalence of CSF leak in patients without intraoperative CSF leak was 0.7%, whereas 4.1% (OR 5.9, 95% CI 3.8–9.0) of patients with intraoperative CSF leak had a postoperative CSF leak. The prevalence of CSF leak in patients without cavernous sinus invasion was 0.5% (95% CI 0.0–1.1%), as opposed to 2.2% (95% CI 0.4–4.1) in patients with cavernous sinus invasion (OR 3.0, 95% CI 1.1–8.7) ( Table 2 ).
Table 2. Risk factors for postoperative CSF leak.
| Outcome | OR | Lower bound | Upper bound | p -Value |
|---|---|---|---|---|
| Pituitary adenoma resection vs. other | 2.3 | 0.9 | 5.7 | 0.07 |
| Microscopic vs. endoscopic | 1.4 | 0.9 | 2.3 | 0.18 |
| History of TSS vs. no history of TSS | 0.9 | 0.2 | 4.5 | 0.87 |
| Intraoperative CSF leak vs. no intraoperative CSF leak | 5.9 | 3.8 | 9.0 | 0.00 a |
| Cavernous sinus invasion vs. no cavernous sinus invasion | 3.0 | 1.1 | 8.7 | 0.04 a |
Abbreviations: CSF, cerebrospinal fluid; OR, odds ratio; TSS, transsphenoidal surgery.
Significant.
Sensitivity Analyses
When the study from Pines et al 15 is excluded from the overall analysis, the results are comparable (3.7%, 95% CI 3.1–4.4%) to the primary outcome analysis (3.4%, 95% CI 2.8–4.0%).
The sensitivity analysis only including high quality studies also shows comparable results to the primary outcome analysis with a CSF leak rate of 3.6% (95% CI 2.3–4.8%).
Analysis of studies between 2015 and 2017 shows a CSF leak rate of 2.5% (95%CI 1.9–3.1%). The CSF leak rate is 4.6% (95% CI 3.4–5.8%) in studies published between 2018 and 2020. This does not provide evidence for a learning curve in studies published between 2015 and 2020.
Discussion
This meta-analysis shows that postoperative CSF leak occurs in 3.4% of adults undergoing TSS. Patients with cavernous sinus invasion are significantly more likely to develop postoperative CSF leak compared with those without cavernous sinus invasion (OR 3.0). Another risk factor for postoperative CSF leak is the presence of an intraoperative CSF leak (OR 5.9).
Historically, TSS is thought to pose high risk of CSF leak. The leak rate found in this study is considerably lower compared with a previous meta-analysis including studies published until 2015. 4 This previous meta-analysis reports a CSF leak rate between 7.5 and 10.5% for endoscopic endonasal tumor resections (including invasive sinonasal tumors) and 5% for pituitary surgery. 4 A similar trend was observed in another recent meta-analysis CSF leak following extended endoscopic endonasal approach for anterior skull base meningioma. 27 In this study CSF leak decreased from 22 to 4% between 2004 and 2020. 27
The reduced CSF leak rate found in the current study most probably results from a combination of three factors. First, improved surgical techniques; approach, sealants, endoscopic visualization and more widely used vascularized nasoseptal mucosal flaps. Second, improved awareness for CSF leak due to initial experiences after more broad indications for (endoscopic) TSS. Third, improved indication for TSS. Endoscopic surgery is no longer chosen for part of the larger tuberculum sellae meningioma and craniopharyngioma (with lateral or suprachiasmatic extensions) cases in most centers. 28 29
No evidence for a learning curve is found within the timeframe of the current study (2015–2020). Analysis of subgroups based on publication year to define a learning curve is limited by the variation in inclusion periods of studies published in the same year, the difference in the number of publications from a certain time period reporting CSF leak and that no differentiation can be made on type of pathology based on year of publication which may influence results. Furthermore, publication bias cannot be excluded as a contributing factor to the difference in CSF leak rate observed between the current and previous meta-analyses. Yet, we do not believe this to be the main factor of influence, considering that publication bias may have also affected studies in the past. Furthermore, there is wide variance in leak rate reported in included studies and studies of small sample size, most vulnerable for publication bias, were excluded.
Moreover, the overall prevalence of postoperative CSF leak after TSS is considerably lower than that reported in meta-analyses for craniotomy (8%) and spinal surgery (14%). 30 31 However, this does not apply to all indications for TSS. CSF leak after TSS for other indications than pituitary adenoma resection is comparable to that found for cranial surgery, including infratentorial surgery, known to be more vulnerable to CSF leak. 30 The relatively low overall leak rate in this meta-analysis may be a result of the relatively high number of pituitary adenoma's included, which may represent a patient population with few additional risk factors, ameliorating the risk of postoperative CSF leak.
Furthermore, a broad range of leak prevalences (0.0–18.2%) was reported by the included studies, resulting in substantial heterogeneity in the meta-analysis. The variation between studies could be explained by the fact that we have included TSS for various indications, which may differ in the presence of patient and surgery-related risk factors. This is reflected by the results of our subgroup analyses in which we find a relatively low CSF leak rate of 3.2% for pituitary lesions and a substantially higher prevalence of 7.1% for other indications.
However, CSF leak prevalences vary considerably within different subgroups, for example, including standard extradural pituitary surgery only. This can theoretically be explained by different surgical techniques and closure techniques.
Despite the significant improvement in surgical techniques, cavernous sinus invasion is still a considerable factor in CSF leak due to its need for extensive surgery. 32 This may indicate that tumors infiltrating the cavernous sinus are likely to cross the diaphragm thereby increasing the risk of postoperative CSF leak. As definitions of cavernous sinus invasion may vary, we classified Knosp grade 3 and 4 as invasive for this meta-analysis. This finding also further explains the difference in CSF leak between various surgical indications. As craniopharyngiomas and meningiomas are intradural intra-arachnoid lesions, there will certainly be intraoperative leak and thus higher risk of postoperative CSF leak, compared with extra-arachnoid pathology such as pituitary adenomas.
It was postulated by other authors that reoperation in patients with previous TSS tends to result in incomplete repair of intraoperative CSF leak, which may result in higher rates of postoperative CSF leak. 33 Although CSF leak was present in 2.0% of patients with previous TSS as opposed to 0.4% in patients who underwent primary TSS, our meta-analysis does not find a significant association between previous TSS and postoperative CSF leak (OR 0.9, 95% CI 0.2–4.5). However, this effect may be influenced by the limited number of studies reporting TSS as a potential risk factor.
To our knowledge this systematic review includes the largest patient population thus far, including over 25,000 cases. Furthermore, it only includes publications from the past 5 years, thereby providing an up-to-date overview of the current situation with state-of-the-art techniques.
One limitation of this study is that the outcome CSF leak is defined differently across studies, this may further explain the variation in reported leak rates across studies. For example, Zaidi et al 34 define CSF leak as “CSF leak requiring intervention,” for other studies CSF leak was taken into consideration only if confirmed by β2-transferrine testing. 35 36 Furthermore, the majority of included studies do not clearly describe their definition of CSF leak which may have caused differences in postoperative CSF leak percentages. Although, self-limiting CSF rhinorrhea is very rare, not all patients require intervention by reoperation, which may result in lower reporting of CSF leak in studies incorporating the need for surgical repair in their definition. 37 38
Second, the results of the current meta-analysis are mostly based on retrospective cohort studies and case series, of which a substantial number is of limited sample size. The outcome of this meta-analysis may be subject to publication bias, contributing to the striking difference in postoperative CSF leak rate found for TSS compared with cranial and spinal surgery, as well as previous meta-analyses on TSS.
Third, some of the analyses are based on a limited number of cases. The analysis comparing endoscopic versus microscopic surgery could be performed for a limited number of studies, showing a higher leak rate for microscopic surgery, yet no significant difference. This result should therefore be interpreted with some caution. We find a substantially higher prevalence of CSF leak for TSS for indications other than pituitary adenoma resection. Again, this result is not statistically significant. Yet, the effect may be underestimated by the relatively low number and small sample size of studies reporting on other indications than pituitary adenoma resection.
Fourthly, no meta-analyses could be performed for several potentially important factors due to insufficient data, for example: suprasellar extension, dural invasion, BMI, microadenoma versus macroadenoma, use of preventative ELD, or reconstruction technique. We did not exclude studies based on their skull base reconstruction technique, which means that all types of reconstruction were included. Many recent studies have focused on different sellar reconstruction techniques. In the current review no analyses were possible to compare specific techniques as there was insufficient data from the included studies. Nevertheless, this factor could be a cause of the broad range of leak prevalences. Similarly, factors such as BMI, especially in combination with increased intracranial pressure, and extension of the tumor may have an influence on CSF leak. The effects of these potential influences could not be studied in the current review which limits the generalizability of the overall results.
Lastly, studies with fewer than 30 subjects were excluded from this meta-analysis. Therefore, studies on patients with rare pathology (such as tuberculum sellae meningioma) specifically, may be underrepresented in the current meta-analysis. This may have led to an underestimation of the overall CSF leak incidence after TSS.
The results of this meta-analysis underline that CSF leak after TSS for intradural and invasive lesions, such as craniopharyngiomas or tuberculum sellae meningiomas is a clinically relevant problem. To further improve the advancement of TSS for these indications effective solutions to prevent postoperative CSF leak are warranted. Future research should focus on effective closure techniques including augmented dural repair to prevent intraoperative CSF leak for this type of surgery especially. The outcomes of this meta-analysis could serve as a benchmark for future prospective studies on novel techniques to prevent CSF leak after TSS.
Conclusion
The overall prevalence of CSF leak after TSS in the adult population is 3.4%. Variables of influence are the presence of intraoperative CSF leak and cavernous sinus invasion.
Acknowledgments
We thank N.P.A. Zuithoff, PhD, for his valuable guidance and contribution to the statistical analyses.
Funding Statement
Funding E.M.H.S. receives a research grant through Polyganics B.V.
Conflict of Interest T.P.C.v.D. is a consultant for Polyganics B.V. The other authors declare no conflict of interests.
Authors' Contributions
E.M.H.S., R.S., E.H.J.V. and T.P.C.v.D. contributed to the study conception and design. E.M.S.H. and R.S. performed the literature search and data collection. The first draft of the manuscript was written by E.M.H.S. and R.S. Supervision was done by T.P.C.v.D., E.H.J.V., and E.W.H. All authors critically revised the final manuscript.
The authors contributed equally to this manuscript.
Supplementary Material
References
- 1.van Aken M O, Feelders R A, de Marie S et al. Cerebrospinal fluid leakage during transsphenoidal surgery: postoperative external lumbar drainage reduces the risk for meningitis. Pituitary. 2004;7(02):89–93. doi: 10.1007/s11102-005-5351-3. [DOI] [PubMed] [Google Scholar]
- 2.Grotenhuis J A.Costs of postoperative cerebrospinal fluid leakage: 1-year, retrospective analysis of 412 consecutive nontrauma cases Surg Neurol 20056406490–493., discussion 493–494 [DOI] [PubMed] [Google Scholar]
- 3.Patel P N, Stafford A M, Patrinely J R et al. Risk factors for intraoperative and postoperative cerebrospinal fluid leaks in endoscopic transsphenoidal sellar surgery. Otolaryngol Head Neck Surg. 2018;158(05):952–960. doi: 10.1177/0194599818756272. [DOI] [PubMed] [Google Scholar]
- 4.Borg A, Kirkman M A, Choi D. Endoscopic endonasal anterior skull base surgery: a systematic review of complications during the past 65 years. World Neurosurg. 2016;95:383–391. doi: 10.1016/j.wneu.2015.12.105. [DOI] [PubMed] [Google Scholar]
- 5.Jeon C, Hong S D, Seol H J et al. Reconstructive outcome of intraoperative cerebrospinal fluid leak after endoscopic endonasal surgery for tumors involving skull base. J Clin Neurosci. 2017;45:227–231. doi: 10.1016/j.jocn.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 6.Hadad G, Bassagasteguy L, Carrau R L et al. A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. Laryngoscope. 2006;116(10):1882–1886. doi: 10.1097/01.mlg.0000234933.37779.e4. [DOI] [PubMed] [Google Scholar]
- 7.Hong C K, Kim Y B, Hong J B, Lee K S. Sealing of cerebrospinal fluid leakage during conventional transsphenoidal surgery using a fibrin-coated collagen fleece. J Clin Neurosci. 2015;22(04):696–699. doi: 10.1016/j.jocn.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 8.Sigler A C, D'Anza B, Lobo B C, Woodard T D, Recinos P F, Sindwani R. Endoscopic skull base reconstruction: an evolution of materials and methods. Otolaryngol Clin North Am. 2017;50(03):643–653. doi: 10.1016/j.otc.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 9.Zanation A M, Thorp B D, Parmar P, Harvey R J. Reconstructive options for endoscopic skull base surgery. Otolaryngol Clin North Am. 2011;44(05):1201–1222. doi: 10.1016/j.otc.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 10.PRISMA Group . Moher D, Liberati A, Tetzlaff J, Altman D G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(07):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mathes T, Pieper D. Clarifying the distinction between case series and cohort studies in systematic reviews of comparative studies: potential impact on body of evidence and workload. BMC Med Res Methodol. 2017;17(01):107. doi: 10.1186/s12874-017-0391-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Institutes of Health Study Quality Assessment Tools: quality Assessment Tool for Case Series Studies 2014https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. Accessed May 3, 2020
- 13.The Ottawa Hospital The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-AnalysesAccessed March 23, 2020 at:http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp2019
- 14.Higgins J PT, Thompson S G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 15.Pines M J, Raikundalia M D, Svider P F, Baredes S, Liu J K, Eloy J A. Transsphenoidal surgery and diabetes mellitus: an analysis of inpatient data and complications. Laryngoscope. 2015;125(10):2273–2279. doi: 10.1002/lary.25162. [DOI] [PubMed] [Google Scholar]
- 16.Chen S, Xu S, Lin F et al. Endoscopic surgical treatment of Cushing's disease: a single-center experience of cauterization of peritumoral tissues. Exp Ther Med. 2019;18(06):4420–4426. doi: 10.3892/etm.2019.8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eichberg D G, Richardson A M, Brusko G D et al. The use of dehydrated amniotic membrane allograft for augmentation of dural repair in transsphenoidal endoscopic endonasal resection of pituitary adenomas. Acta Neurochir (Wien) 2019;161(10):2117–2122. doi: 10.1007/s00701-019-04008-x. [DOI] [PubMed] [Google Scholar]
- 18.Gondim J A, de Albuquerque L AF, Almeida J P et al. Endoscopic endonasal surgery for treatment of pituitary apoplexy: 16 years of experience in a specialized pituitary center. World Neurosurg. 2017;108:137–142. doi: 10.1016/j.wneu.2017.08.131. [DOI] [PubMed] [Google Scholar]
- 19.Negm H M, Al-Mahfoudh R, Pai M et al. Reoperative endoscopic endonasal surgery for residual or recurrent pituitary adenomas. J Neurosurg. 2017;127(02):397–408. doi: 10.3171/2016.8.JNS152709. [DOI] [PubMed] [Google Scholar]
- 20.Pangal D J, Chesney K, Memel Z et al. Pituitary apoplexy case series: outcomes after endoscopic endonasal transsphenoidal surgery at a single tertiary center. World Neurosurg. 2020;137:e366–e372. doi: 10.1016/j.wneu.2020.01.204. [DOI] [PubMed] [Google Scholar]
- 21.Seltzer J, Wedemeyer M A, Bonney P A, Carmichael J D, Weiss M, Zada G. Outcomes following transsphenoidal surgical management of incidental pituitary adenomas: a series of 52 patients over a 17-year period. J Neurosurg. 2018;130(05):1–9. doi: 10.3171/2017.11.JNS171485. [DOI] [PubMed] [Google Scholar]
- 22.Wang F, Zhou T, Wei S et al. Endoscopic endonasal transsphenoidal surgery of 1,166 pituitary adenomas. Surg Endosc. 2015;29(06):1270–1280. doi: 10.1007/s00464-014-3815-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yano S, Hide T, Shinojima N. Efficacy and complications of endoscopic skull base surgery for giant pituitary adenomas. World Neurosurg. 2017;99:533–542. doi: 10.1016/j.wneu.2016.12.068. [DOI] [PubMed] [Google Scholar]
- 24.Park J H, Choi J H, Kim Y I, Kim S W, Hong Y K. Modified graded repair of cerebrospinal fluid leaks in endoscopic endonasal transsphenoidal surgery. J Korean Neurosurg Soc. 2015;58(01):36–42. doi: 10.3340/jkns.2015.58.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishii Y, Tahara S, Hattori Y, Teramoto A, Morita A, Matsuno A.Fascia patchwork closure for endoscopic endonasal skull base surgery Neurosurg Rev 20153803551–556., discussion 556–557 [DOI] [PubMed] [Google Scholar]
- 26.Ding H, Gu Y, Zhang X et al. Learning curve for the endoscopic endonasal approach for suprasellar craniopharyngiomas. J Clin Neurosci. 2017;42:209–216. doi: 10.1016/j.jocn.2017.04.021. [DOI] [PubMed] [Google Scholar]
- 27.Zamanipoor Najafabadi A H, Khan D Z, Muskens I S et al. Trends in cerebrospinal fluid leak rates following the extended endoscopic endonasal approach for anterior skull base meningioma: a meta-analysis over the last 20 years. Acta Neurochir (Wien) 2021;163(03):711–719. doi: 10.1007/s00701-020-04641-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giammattei L, Starnoni D, Cossu G et al. Surgical management of tuberculum sellae meningiomas: myths, facts, and controversies. Acta Neurochir (Wien) 2020;162(03):631–640. doi: 10.1007/s00701-019-04114-w. [DOI] [PubMed] [Google Scholar]
- 29.Fatemi N, Dusick J R, de Paiva Neto M A, Malkasian D, Kelly D F.Endonasal versus supraorbital keyhole removal of craniopharyngiomas and tuberculum sellae meningiomasNeurosurgery 2009;64(05, Suppl 2):269–284, discussion 284–286 [DOI] [PubMed]
- 30.Kinaci A, Algra A, Heuts S, O'Donnell D, van der Zwan A, van Doormaal T. Effectiveness of dural sealants in prevention of cerebrospinal fluid leakage after craniotomy: a systematic review. World Neurosurg. 2018;118:368–3760. doi: 10.1016/j.wneu.2018.06.196. [DOI] [PubMed] [Google Scholar]
- 31.Kinaci A, Moayeri N, van der Zwan A, van Doormaal T PC. Effectiveness of sealants in prevention of cerebrospinal fluid leakage after spine surgery: a systematic review. World Neurosurg. 2019;127:567–5750. doi: 10.1016/j.wneu.2019.02.236. [DOI] [PubMed] [Google Scholar]
- 32.Kitano M, Taneda M, Shimono T, Nakao Y. Extended transsphenoidal approach for surgical management of pituitary adenomas invading the cavernous sinus. J Neurosurg. 2008;108(01):26–36. doi: 10.3171/JNS/2008/108/01/0026. [DOI] [PubMed] [Google Scholar]
- 33.Nishioka H, Haraoka J, Ikeda Y.Risk factors of cerebrospinal fluid rhinorrhea following transsphenoidal surgery Acta Neurochir (Wien) 2005147111163–1166., discussion 1166 [DOI] [PubMed] [Google Scholar]
- 34.Zaidi H A, Awad A W, Bohl M A et al. Comparison of outcomes between a less experienced surgeon using a fully endoscopic technique and a very experienced surgeon using a microscopic transsphenoidal technique for pituitary adenoma. J Neurosurg. 2016;124(03):596–604. doi: 10.3171/2015.4.JNS15102. [DOI] [PubMed] [Google Scholar]
- 35.Barger J, Siow M, Kader M et al. The posterior nasoseptal flap: a novel technique for closure after endoscopic transsphenoidal resection of pituitary adenomas. Surg Neurol Int. 2018;9:32. doi: 10.4103/sni.sni_192_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu B, Wang Y, Zheng T et al. Effect of intraoperative lumbar drainage on gross total resection and cerebrospinal fluid leak rates in endoscopic transsphenoidal surgery of pituitary macroadenomas. World Neurosurg. 2020;135:e629–e639. doi: 10.1016/j.wneu.2019.12.100. [DOI] [PubMed] [Google Scholar]
- 37.Levi V, Bertani G A, Guastella C et al. Microscopic versus endoscopic transsphenoidal surgery for pituitary adenoma: analysis of surgical safety in 221 consecutive patients. Clin Otolaryngol. 2017;42(02):466–469. doi: 10.1111/coa.12631. [DOI] [PubMed] [Google Scholar]
- 38.Hansasuta A, Pokanan S, Punyawai P, Mahattanakul W. Evolution of technique in endoscopic transsphenoidal surgery for pituitary adenoma: a single institution experience from 220 procedures. Cureus. 2018;10(01):e2010. doi: 10.7759/cureus.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gondim J A, Almeida J P, de Albuquerque L AF, Gomes E, Schops M, Mota J I. Endoscopic endonasal transsphenoidal surgery in elderly patients with pituitary adenomas. J Neurosurg. 2015;123(01):31–38. doi: 10.3171/2014.10.JNS14372. [DOI] [PubMed] [Google Scholar]
- 40.Fathalla H, Cusimano M D, Di Ieva Aet al. Endoscopic versus microscopic approach for surgical treatment of acromegaly Neurosurg Rev 20153803541–548., discussion 548–549 [DOI] [PubMed] [Google Scholar]
- 41.Nie S, Li K, Huang Y, Zhao J, Gao X, Sun J. Endoscopic endonasal transsphenoidal surgery for treating pituitary adenoma via a sub-septum mucosa approach. Int J Clin Exp Med. 2015;8(04):5137–5143. [PMC free article] [PubMed] [Google Scholar]
- 42.Zhan R, Ma Z, Wang D, Li X. Pure endoscopic endonasal transsphenoidal approach for nonfunctioning pituitary adenomas in the elderly: Surgical outcomes and complications in 158 patients. World Neurosurg. 2015;84(06):1572–1578. doi: 10.1016/j.wneu.2015.08.035. [DOI] [PubMed] [Google Scholar]
- 43.Chabot J D, Chakraborty S, Imbarrato G, Dehdashti A R. Evaluation of outcomes after endoscopic endonasal surgery for large and giant pituitary macroadenoma: a retrospective review of 39 consecutive patients. World Neurosurg. 2015;84(04):978–988. doi: 10.1016/j.wneu.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 44.Pinar E, Yuceer N, Imre A, Guvenc G, Gundogan O. Endoscopic endonasal transsphenoidal surgery for pituitary adenomas. J Craniofac Surg. 2015;26(01):201–205. doi: 10.1097/SCS.0000000000001240. [DOI] [PubMed] [Google Scholar]
- 45.Sanders-Taylor C, Anaizi A, Kosty J, Zimmer L A, Theodosopoulos P V. Sellar reconstruction and rates of delayed cerebrospinal fluid leak after endoscopic pituitary surgery. J Neurol Surg B Skull Base. 2015;76(04):281–285. doi: 10.1055/s-0034-1544118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xie T, Liu T, Zhang X et al. Time to revive the value of the pseudocapsule in endoscopic endonasal transsphenoidal surgery for growth hormone adenomas. World Neurosurg. 2016;89:65–71. doi: 10.1016/j.wneu.2016.01.036. [DOI] [PubMed] [Google Scholar]
- 47.Gao Y, Zheng H, Xu S et al. Endoscopic versus microscopic approach in pituitary surgery. J Craniofac Surg. 2016;27(02):e157–e159. doi: 10.1097/SCS.0000000000002401. [DOI] [PubMed] [Google Scholar]
- 48.Freyschlag C F, Goerke S A, Obernauer J, Kerschbaumer J, Thomé C, Seiz M. A sandwich technique for prevention of cerebrospinal fluid rhinorrhea and reconstruction of the sellar floor after microsurgical transsphenoidal pituitary surgery. J Neurol Surg A Cent Eur Neurosurg. 2016;77(03):229–232. doi: 10.1055/s-0035-1547357. [DOI] [PubMed] [Google Scholar]
- 49.Park W, Hong S D, Nam D H et al. Nasoseptal flap elevation in patients with history of septal surgery: does it increase flap failure or cerebrospinal fluid leakage? World Neurosurg. 2016;93:164–167. doi: 10.1016/j.wneu.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 50.Jang J H, Kim K H, Lee Y M, Kim J S, Kim Y Z. Surgical results of pure endoscopic endonasal transsphenoidal surgery for 331 pituitary adenomas: a 15-year experience from a single institution. World Neurosurg. 2016;96:545–555. doi: 10.1016/j.wneu.2016.09.051. [DOI] [PubMed] [Google Scholar]
- 51.Fnais N, Maio S D, Edionwe S et al. Hemi-transseptal approach for pituitary surgery: a follow-up study. J Neurol Surg B Skull Base. 2017;78(02):145–151. doi: 10.1055/s-0036-1593816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ye Y, Wang F, Zhou T, Luo Y. Low complication rate of sellar reconstruction by artificial dura mater during endoscopic endonasal transsphenoidal surgery. Medicine (Baltimore) 2017;96(52):e9422. doi: 10.1097/MD.0000000000009422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karki M, Sun J, Yadav C P, Zhao B. Large and giant pituitary adenoma resection by microscopic trans-sphenoidal surgery: surgical outcomes and complications in 123 consecutive patients. J Clin Neurosci. 2017;44:310–314. doi: 10.1016/j.jocn.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 54.Wang Q, Guo X, Gao L et al. Surgical outcome of growth hormone-secreting pituitary adenoma with empty sella using a new classification. World Neurosurg. 2017;105:651–658. doi: 10.1016/j.wneu.2017.06.071. [DOI] [PubMed] [Google Scholar]
- 55.Sun G, Cao Y, Jiang N et al. Binostril endoscopic transsphenoidal neurosurgery for pituitary adenomas: experience with 42 patients. Oncotarget. 2017;8(40):69020–69024. doi: 10.18632/oncotarget.16976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou Q, Yang Z, Wang X et al. Risk factors and management of intraoperative cerebrospinal fluid leaks in endoscopic treatment of pituitary adenoma: analysis of 492 patients. World Neurosurg. 2017;101:390–395. doi: 10.1016/j.wneu.2017.01.119. [DOI] [PubMed] [Google Scholar]
- 57.Cebula H, Baussart B, Villa C et al. Efficacy of endoscopic endonasal transsphenoidal surgery for Cushing's disease in 230 patients with positive and negative MRI. Acta Neurochir (Wien) 2017;159(07):1227–1236. doi: 10.1007/s00701-017-3140-1. [DOI] [PubMed] [Google Scholar]
- 58.Zoli M, Milanese L, Faustini-Fustini M et al. Endoscopic endonasal surgery for pituitary apoplexy: evidence on a 75-case series from a tertiary care center. World Neurosurg. 2017;106:331–338. doi: 10.1016/j.wneu.2017.06.117. [DOI] [PubMed] [Google Scholar]
- 59.Fujimoto K, Yano S, Shinojima N, Hide T, Kuratsu J-I. Endoscopic endonasal transsphenoidal surgery for patients aged over 80 years with pituitary adenomas: surgical and follow-up results. Surg Neurol Int. 2017;8:213. doi: 10.4103/sni.sni_189_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sasagawa Y, Hayashi Y, Tachibana O et al. Clinical characteristics of acromegalic patients with empty sella and their outcomes following transsphenoidal surgery. Pituitary. 2017;20(04):403–408. doi: 10.1007/s11102-017-0798-6. [DOI] [PubMed] [Google Scholar]
- 61.Fishpool S JC, Amato-Watkins A, Hayhurst C. Free middle turbinate mucosal graft reconstruction after primary endoscopic endonasal pituitary surgery. Eur Arch Otorhinolaryngol. 2017;274(02):837–844. doi: 10.1007/s00405-016-4287-8. [DOI] [PubMed] [Google Scholar]
- 62.Ajlan A, Achrol A S, Albakr A et al. Cavernous sinus involvement by pituitary adenomas: clinical implications and outcomes of endoscopic endonasal resection. J Neurol Surg B Skull Base. 2017;78(03):273–282. doi: 10.1055/s-0036-1598022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Przybylowski C J, Dallapiazza R F, Williams B J et al. Primary versus revision transsphenoidal resection for nonfunctioning pituitary macroadenomas: matched cohort study. J Neurosurg. 2017;126(03):889–896. doi: 10.3171/2016.3.JNS152735. [DOI] [PubMed] [Google Scholar]
- 64.Shin S S, Gardner P A, Ng J et al. Endoscopic endonasal approach for adrenocorticotropic hormone-secreting pituitary adenomas: outcomes and analysis of remission rates and tumor biochemical activity with respect to tumor invasiveness. World Neurosurg. 2017;102:651–6580. doi: 10.1016/j.wneu.2015.07.065. [DOI] [PubMed] [Google Scholar]
- 65.Eseonu C I, ReFaey K, Pamias-Portalatin E et al. Three-hand endoscopic endonasal transsphenoidal surgery: experience with an anatomy-preserving mononostril approach technique. Oper Neurosurg (Hagerstown) 2018;14(02):158–165. doi: 10.1093/ons/opx110. [DOI] [PubMed] [Google Scholar]
- 66.Popov D, Hadzhiyanev A, Bussarsky A, Fernidandov D. Short-term outcome of endoscopic versus microscopic pituitary adenoma surgery in a single center. Biomed Res (Aligarh) 2018;29(14):2971–2974. [Google Scholar]
- 67.Han Y-L, Chen D-M, Zhang C, Pan M, Yang X P, Wu Y G. Retrospective analysis of 52 patients with prolactinomas following endoscopic endonasal transsphenoidal surgery. Medicine (Baltimore) 2018;97(45):e13198. doi: 10.1097/MD.0000000000013198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guo Z, Liu C, Hou H et al. Preoperative computed tomography (CT) evaluation of anatomical abnormalities in endonasal transsphenoidal approach in pituitary adenoma. Med Sci Monit. 2018;24:1268–1275. doi: 10.12659/MSM.904402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schuss P, Hadjiathanasiou A, Klingmüller D, Güresir Á, Vatter H, Güresir E. Transsphenoidal pituitary surgery: comparison of two sellar reconstruction techniques and their effect on postoperative cerebrospinal fluid leakage. Neurosurg Rev. 2018;41(04):1053–1058. doi: 10.1007/s10143-018-0949-x. [DOI] [PubMed] [Google Scholar]
- 70.Hajdari S, Kellner G, Meyer A, Rosahl S, Gerlach R. Endoscopic endonasal surgery for removal of pituitary adenomas: a surgical case series of treatment results using different 2- and 3-dimensional visualization systems. World Neurosurg. 2018;119:e80–e86. doi: 10.1016/j.wneu.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 71.Karamouzis I, Caputo M, Mele C et al. Transsphenoidal surgery for pituitary adenomas: early results from a single center. Hormones (Athens) 2018;17(04):551–556. doi: 10.1007/s42000-018-0082-9. [DOI] [PubMed] [Google Scholar]
- 72.Lofrese G, Vigo V, Rigante M et al. Learning curve of endoscopic pituitary surgery: experience of a neurosurgery/ENT collaboration. J Clin Neurosci. 2018;47:299–303. doi: 10.1016/j.jocn.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 73.Cudal B IB, Montano C N, Pontillas A E, Baraoidan R ZP. Post-operative complications of trans-sphenoidal surgery in a local tertiary hospital during hospital stay. Phillippine J Intern Med. 2018;56(01):15–18. [Google Scholar]
- 74.Robins J MW, Alavi S A, Tyagi A K, Nix P A, Wilson T M, Phillips N I. The learning curve for endoscopic trans-sphenoidal resection of pituitary macroadenomas. A single institution experience, Leeds, UK. Acta Neurochir (Wien) 2018;160(01):39–47. doi: 10.1007/s00701-017-3355-1. [DOI] [PubMed] [Google Scholar]
- 75.Wilson P J, Omay S B, Kacker A, Anand V K, Schwartz T H. Endonasal endoscopic pituitary surgery in the elderly. J Neurosurg. 2018;128(02):429–436. doi: 10.3171/2016.11.JNS162286. [DOI] [PubMed] [Google Scholar]
- 76.Rehman L, Rehman U L, Jabeen R, Rizvi R. Endoscopic trans-sphenoidal surgery: efficacy and response in pituitary adenoma. Pak J Med Sci. 2018;34(02):412–417. doi: 10.12669/pjms.342.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xue H, Yang Z, Liu J, Wang X, Bi Z, Liu P. Continuous dural suturing for closure of grade 3 leaks after tumor removal via an endoscopic endonasal approach. Neurosurg Rev. 2021;44(01):373–380. doi: 10.1007/s10143-019-01199-w. [DOI] [PubMed] [Google Scholar]
- 78.Fallah N, Taghvaei M, Sadaghiani S, Sadrhosseini S M, Esfahanian F, Zeinalizadeh M. Surgical outcome of endoscopic endonasal surgery of large and giant pituitary adenomas: an institutional experience from the Middle East. World Neurosurg. 2019;132:e802–e811. doi: 10.1016/j.wneu.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 79.Spina A, Losa M, Mortini P. Pituitary adenomas in elderly patients: clinical and surgical outcome analysis in a large series. Endocrine. 2019;65(03):637–645. doi: 10.1007/s12020-019-01959-0. [DOI] [PubMed] [Google Scholar]
- 80.Shen S C, Shen C C, Pu T W, Cheng W Y. Long-term effects of intracapsular debulking and adjuvant somatostatin analogs for growth hormone-secreting pituitary macroadenoma: 10 years of experience in a single institute. World Neurosurg. 2019;126:e41–e47. doi: 10.1016/j.wneu.2019.01.125. [DOI] [PubMed] [Google Scholar]
- 81.Chen S H, Sprau A, Chieng L et al. Transsphenoidal approach for pituitary adenomas in elderly patients. World Neurosurg. 2019;121:e670–e674. doi: 10.1016/j.wneu.2018.09.187. [DOI] [PubMed] [Google Scholar]
- 82.Azab M A, O'Hagan M, Abou-Al-Shaar H, Karsy M, Guan J, Couldwell W T. Safety and outcome of transsphenoidal pituitary adenoma resection in elderly patients. World Neurosurg. 2019;122:e1252–e1258. doi: 10.1016/j.wneu.2018.11.024. [DOI] [PubMed] [Google Scholar]
- 83.Memel Z, Chesney K, Pangal D J, Bonney P A, Carmichael J D, Zada G. Outcomes following transsphenoidal pituitary surgery in the elderly: a retrospective single-center review. Oper Neurosurg (Hagerstown) 2019;16(03):302–309. doi: 10.1093/ons/opy109. [DOI] [PubMed] [Google Scholar]
- 84.Rieley W, Askari A, Akagami R, Gooderham P A, Swart P A, Flexman A M. Immediate use of continuous positive airway pressure in patients with obstructive sleep apnea following transsphenoidal pituitary surgery: a case series. J Neurosurg Anesthesiol. 2020;32(01):36–40. doi: 10.1097/ANA.0000000000000573. [DOI] [PubMed] [Google Scholar]
- 85.Zhang C, Yang N, Mu L et al. The application of nasoseptal “rescue” flap technique in endoscopic transsphenoidal pituitary adenoma resection. Neurosurg Rev. 2020;43(01):259–263. doi: 10.1007/s10143-018-1048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tardivo V, Penner F, Garbossa D et al. Surgical management of pituitary adenomas: does age matter? Pituitary. 2020;23(02):92–102. doi: 10.1007/s11102-019-01014-1. [DOI] [PubMed] [Google Scholar]
- 87.Castaño-Leon A M, Paredes I, Munarriz P M et al. Endoscopic transnasal trans-sphenoidal approach for pituitary adenomas: a comparison to the microscopic approach cohort by propensity score analysis. Neurosurgery. 2020;86(03):348–356. doi: 10.1093/neuros/nyz201. [DOI] [PubMed] [Google Scholar]
- 88.Parikh A, Adapa A, Sullivan S E, McKean E L. Predictive factors, 30-day clinical outcomes, and costs associated with cerebrospinal fluid leak in pituitary adenoma resection. J Neurol Surg B Skull Base. 2020;81(01):43–55. doi: 10.1055/s-0039-1679896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tafreshi A R, Du R, Rutkowski M J et al. Differential clinical presentation, intraoperative management strategies, and surgical outcomes after endoscopic endonasal treatment of cystic sellar masses. World Neurosurg. 2020;133:e241–e251. doi: 10.1016/j.wneu.2019.08.234. [DOI] [PubMed] [Google Scholar]
- 90.Cappello Z J, Tang D M, Roxbury C R et al. Utility of the nasoseptal “rescue” flap approach: analysis of 125 consecutive patients and implications for routine transsphenoidal surgery. Am J Rhinol Allergy. 2020;34(02):269–275. doi: 10.1177/1945892419892164. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


