Abstract
Objective Diagnostic criteria for otogenic skull base osteomyelitis (SBO) have been conflicting among researchers. We aimed to propose clinically useful diagnostic criteria and a staging system for otogenic SBO that is associated with infection control and mortality.
Design The present study is designed as a retrospective one.
Setting This study was conducted at the University Hospital.
Participants Thirteen patients with otogenic SBO who met the novel rigorous diagnostic criteria consisted of symptomatic and radiological signs on high-resolution computed tomography (HRCT) and magnetic resonance imaging (MRI). Simple refractory external otitis was not included. A staging system according to disease extent revealed by HRCT and MRI is proposed: lesions limited to the temporal bone (stage 1), extending to less than half (stage 2), exceeding the midline (stage 3), and extending to the whole of the clivus (stage 4). All patients received long-term antibiotic therapy. Patients were divided into infection-uncontrolled or -controlled groups based on symptoms, otoscopic findings, and C-reactive protein level at the last follow-up. The mean follow-up period was 27.7 months.
Main Outcome Measures Possible prognostic factors, such as immunocompromised status and symptoms, including cranial nerve palsy, pretreatment laboratory data, and treatments, were compared between the infection-uncontrolled and -controlled groups. Disease stages were correlated with infection control and mortality.
Results The infection-uncontrolled rate and mortality rate were 38.5 and 23.1%, respectively. There were no significant differences in possible prognostic factors between the infection-uncontrolled and -controlled groups. HRCT-based stages significantly correlated with infection control and mortality.
Conclusion We proposed here the clinically useful diagnostic criteria and staging systems that can predict infection control and prognosis of otogenic SBO.
Keywords: skull base osteomyelitis, malignant external otitis, diagnostic criteria, infection control, mortality, HRCT, MRI, staging
Introduction
In 1959, Meltzer and Kelemen first reported a case of fatal osteomyelitis caused by Pseudomonas aeruginosa which spread from the temporal bone to the mandible and zygoma. 1 Chandler subsequently reported a case series of severe necrotizing external otitis, involving multiple cranial nerves by the same single pathogen in elderly diabetic patients. 2 He coined the term “malignant external otitis (MEO)” for the condition where “malignant” refers to a high mortality rate. Later, skull base osteomyelitis (SBO) is considered as a condition in which MEO involves not only the temporal bone but also extends to the occipital and sphenoid bones. 3 Clinical characteristics of MEO and the otogenic SBO, including the infection control rate, recurrence rate, mortality rate, and prognostic factors, vary among reports, 4 5 6 7 8 9 10 11 12 probably because diagnostic criteria for the diseases have been conflicting among researchers, due to which various conditions from mild-to-severe status are mixed in previous reports. Moreover, although half a century has passed since Chandler reported that long-term antibiotic therapy is necessary for MEO, 4 the treatment is not uniformly administered. 13 Lack of diagnostic criteria and an appropriate staging system of the diseases extent may be a cause of nonuniform treatment strategy being performed. In the present study, we aimed to establish novel rigorous diagnostic criteria that require radiological signs on high-resolution computed tomography (HRCT) and magnetic resonance imaging (MRI), and a staging system by anatomical disease extent for otogenic SBO that can predict the infection control and mortality.
Materials and Methods
This study was conducted after approval by the Institutional Review Board of the Niigata University (approval number no.: 2019–0306).
Diagnostic Criteria for Otogenic Skull Base Osteomyelitis
Table 1 shows our proposal for the diagnostic criteria for otogenic SBO. It consists of major (essential) and minor (adjunctive) signs. The major signs are as follows: nonacute, persistent, and severe pain (headache and/or otalgia); cortical bone destruction in the petrous portion of temporal bone and/or clival bone on HRCT; low signal intensity of bone marrow in the petrous portion of temporal bone and/or clival bone on T1-weighted images (T1WI) of MRI which is the most useful sequence to detect osteomyelitis 14 ; and the exclusion of other malignant and inflammatory diseases. Minor signs contain the signs that have been generally considered as findings of MEO and SBO and clinically useful findings to make differential diagnosis: immunocompromised status such as diabetes mellitus, old age (65+ years), P. aeruginosa as a pathogen, no fever higher than 38°C, refractory external otitis, abnormal erythrocyte sedimentation rate (ESR)/C-reactive protein (CRP), cranial nerve dysfunction, and bone involvement by imaging studies other than HRCT and MRI. All five major signs must be fulfilled for diagnosing otogenic SBO, while minor signs may help in making the diagnosis. Simple refractory external otitis showing neither cortical bone destruction on HRCT nor low signal intensity of bone marrow on T1WI MRI does not fulfil the diagnostic criteria for otogenic SBO.
Table 1. Diagnostic criteria for otogenic skull base osteomyelitis.
| Major signs (essential) |
| 1. Nonacute, persistent, and severe pain (headache or otalgia) |
| 2. Cortical bone destruction in the petrous portion of temporal bone and/or the clivus on HRCT |
| 3. Low signal intensity of bone marrow in the petrous portion of temporal bone and/or the clivus on T1WI MRI |
| 4. Exclusion of malignancy by tissue specimen • External auditory canal cancer • Nasopharyngeal cancer • Metastasis |
| 5. Exclusion of other inflammatory diseases by symptoms, otoscopic findings, blood exam, bacterial culture, CT, and MRI • Acute infection (acute mastoiditis, petrous apicitis, retropharyngeal abscess) • Cholesteatoma • Cholesterol granuloma • Tuberculosis • OMAAV |
| Minor signs (adjunctive) |
| 1. Immunocompromised status, including DM |
| 2. Old age (65+ years) |
| 3. Pseudomonas aeruginosa |
| 4. No fever higher than 38°C |
| 5. Refractory external otitis (exudate, edema, granulation) |
| 6. Abnormal ESR or CRP |
| 7. Cranial nerve dysfunction |
| 8. Bone involvement on RI scanning (Tc-99m or Ga-67) or PET (high RI/PET uptake in the petrous portion of temporal bone and/or the clivus) |
Abbreviations: CRP, C-reactive protein; CT, computed tomography; DM, diabetes mellitus; ESR, erythrocyte sedimentation rate; Ga, gallium; HRCT, high-resolution CT; OMAAV, otitis media with ANCA-associated vasculitis; PET, positron emission tomography; RI, radio isotope; T1WI, T1-weighted image; Tc, technetium.
Disease Stage of Otogenic Skull Base Osteomyelitis
Regarding the disease stages, they were classified into four stages by anatomical disease extent on HRCT and MRI ( Table 2 ): limited to the petrous portion of temporal bone (stage 1), extending to less than half of the clivus (stage 2), exceeding the midline (stage 3), and extending to the whole of the clivus (stage 4) ( Figs. 1 and 2 ). With regard to the HRCT, thin slice images (≤1 mm) were obtained with high-resolution bone filter scan protocol for all patients.
Table 2. HRCT- and MRI-based staging system of otogenic skull base osteomyelitis.
| Stage | Disease extent (cortical bone destruction on HRCT or low signal intensity of bone marrow on T1WI MRI) |
Diagnosis | |
|---|---|---|---|
| Petrous portion of temporal bone | Clivus | ||
| 1 | + | – | Mild SBO |
| 2 | + | Less than half | Moderate SBO |
| 3 | + | Exceeding the midline | Severe SBO |
| 4 | + | Whole | Very severe SBO |
Abbreviations: HRCT, high resolution computed tomography; MRI, magnetic resonance imaging; SBO, skull base osteomyelitis; T1WI, T1-weighted image.
Note: Central SBO lacks a temporal bone lesion, but this staging system can also be clinically applied to the central SBO by disease extent in the clivus.
Fig. 1.
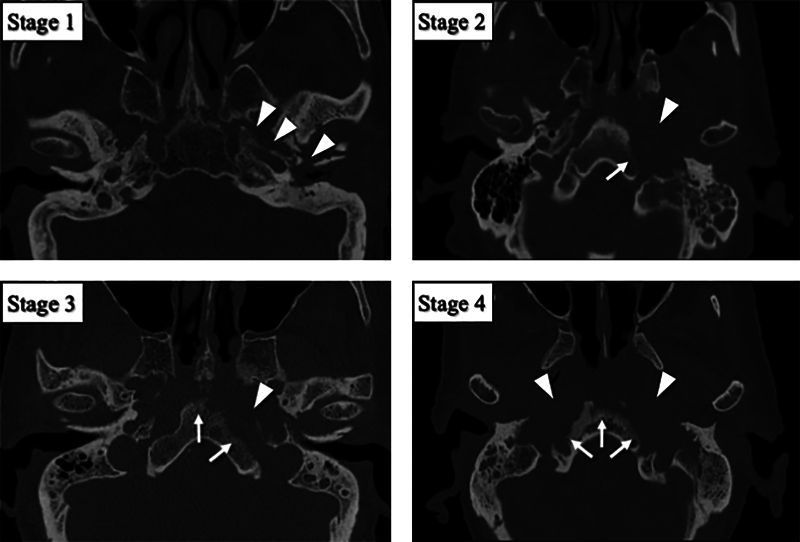
HRCT-based stages of otogenic skull base osteomyelitis. HRCT images were classified into four stages according to the cortical bone destruction. Arrow heads indicate areas of cortical bone destruction in the petrous portion of temporal bone. Arrows indicate areas of cortical bone destruction in the clivus. HRCT, high-resolution computed tomography.
Fig. 2.
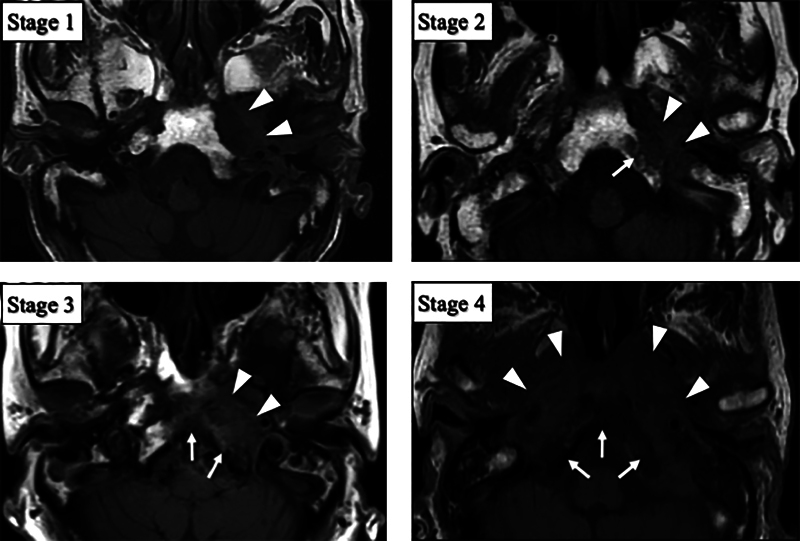
MRI-based stages of otogenic skull base osteomyelitis. MRI images were classified into four stages according to the low signal intensity area on T1WI. Arrow heads indicate areas of low signal intensity of bone marrow in the petrous portion of temporal bone and soft tissue in the infratemporal fossa. Arrows indicate areas of low signal intensity in the clivus. The images are from the same patients as in Fig. 1 . MRI, magnetic resonance imaging; T1WI, T1-weighted image.
Patients
Thirteen patients with otogenic SBO (11 males and 2 females) treated between 2008 and 2020, who met the newly proposed rigorous diagnostic criteria ( Table 1 ), were enrolled in this study. The mean follow-up period of the 13 patients was 27.7 months. Upon excluding three cases with early death (mean, 3.8 months), the mean follow-up period was 34.8 months.
Treatment
As standard therapy, long-term intravenous administration of antibiotics (6–8 weeks) combined with hyperbaric oxygen therapy (HBOT) was administered under hospitalization. The choice of antibiotics depended on the pathogenic bacteria. When P. aeruginosa was thought to be the pathogen, a combination of a third-generation cephalosporin and an aminoglycoside or quinolone was used, according to the treatment strategy for osteomyelitis. 15 If fungi were detected, antifungal drugs were also administered. Following the intravenous administration of antibiotics, oral antibiotics and/or antifungal drugs were administered for at least 6 months after CRP became negative. Patients with a good general condition underwent tympanomastoid surgery for removing the inflammatory lesion as much as possible.
Outcome Assessment
Patients were divided into an infection-uncontrolled group and an infection-controlled group. Patients who had completed at least 6 months from the beginning of treatment without signs of infection (severe pain, otoscopic abnormal signs, and CRP-positive condition) at the last follow-up were considered as infection-controlled. Patients suspected of having persistent infection in any of the tests or those with SBO-related deaths were considered as infection-uncontrolled. Additionally, patients were categorized into an SBO-related death group and the others including cured, infection-bearing alive, or death by other causes patients at the last follow-up.
Correlation of Possible Prognostic Factors and Stages with Treatment Outcomes
The patients' background, symptoms, pretreatment laboratory data, treatment, and the disease stage based on HRCT and MRI were compared between the infection-uncontrolled and -controlled groups. The disease stage was also compared between the SBO-related death group and the others.
Statistics
Differences in each parameter were tested by the unpaired Student's t -test (age, hemoglobin A1c [HbA1c], ESR, and CRP), Welch's t -test after F-test (period from onset to the start of treatment), Fisher's exact test (sex, immunocompromised status, severe pain, fever, refractory external otitis, cranial nerve palsy, bacteria, intravenous antibiotic administration, surgery, HBOT, and infection control team [ICT] intervention), and the Mann–Whitney U -test (disease stage) using SPSS Statistics version 26.0 (IBM, Armonk, New York, United States). A p –value of <0.05 was considered to be statistically significant.
Results
Treatment Outcome
Thirteen patients were identified with our rigorous diagnostic criteria for otogenic SBO. The infection-uncontrolled group included five patients and the controlled group included eight. In the uncontrolled group, one patient survived, while one and three patients died of other cause and SBO, respectively. The SBO-related death group included 3 and the other group included 10 patients. Therefore, the infection-uncontrolled rate and SBO-related mortality rate were 38.5 and 23.1%, respectively.
Demographic and Clinical Characteristics of Infection-Uncontrolled and -Controlled Groups
Table 3 shows the patients' background (age, sex, and immunocompromised status), symptoms (severe pain, fever, refractory external otitis, cranial nerve palsy, and period from onset to the start of treatment), pretreatment laboratory data (HbA1c, ESR, CRP, and bacterial culture), and treatment (intravenous antibiotic administration, surgery, HBOT, and ICT intervention) for the infection-uncontrolled and -controlled groups. There were no significant differences in any of these factors between the groups.
Table 3. Demographic and clinical characteristics of the infection-uncontrolled and controlled groups.
| Infection-uncontrolled ( n = 5) | Controlled ( n = 8) | p -Value | |||
|---|---|---|---|---|---|
| Patients' background | |||||
| Age (y) | 80.4 (72–90) | 70.4 (60–87) | 0.06 | ||
| Sex | Male | 5 | 6 | 0.36 | |
| Female | 0 | 2 | |||
| Immunocompromised status (diabetes mellitus, steroid use, etc.) | Yes | 5 | 5 | 0.20 | |
| No | 0 | 3 | |||
| Symptoms | |||||
| Period from onset to the start of treatment (days) | 192.0 (25–490) | 81.8 (18–157) | 0.29 | ||
| Severe pain (opioid use) | Yes | 4 | 5 | 0.49 | |
| No | 1 | 3 | |||
| Fever (>38°C) | Yes | 1 | 1 | 0.64 | |
| No | 4 | 7 | |||
| Refractory external otitis | Yes | 4 | 8 | 0.38 | |
| No | 1 | 0 | |||
| Cranial nerve palsy | Yes | 3 | 2 | 0.25 | |
| (Solitary VII palsy) | (1) | (1) | |||
| (Other nerve palsy [IX, X, XII]) | (2) | (1) | |||
| No | 2 | 6 | |||
| Pretreatment laboratory data | |||||
| HbA1c (%) | 6.9 (6.1–8.0) | 7.3 (5.2–12.2) | 0.74 | ||
| ESR (mm/h) | 105.0 (67–143) | 85.0 (58–111) | 0.39 | ||
| CRP (mg/dL) | 6.2 (0.35–12.6) | 7.0 (1.16–30.35) | 0.87 | ||
| Bacteria | Pseudomonas aeruginosa | Yes | 4 | 3 | 0.18 |
| No | 1 | 5 | |||
| Fungi | Yes | 2 | 2 | 0.51 | |
| No | 3 | 6 | |||
| Treatment | |||||
| Intravenous antibiotic administration | Yes | 5 | 7 | 0.62 | |
| No | 0 | 1 | |||
| Surgery | Yes | 0 | 3 | 0.20 | |
| No | 5 | 5 | |||
| Hyperbaric oxygen therapy | Yes | 2 | 5 | 0.41 | |
| No | 3 | 3 | |||
| Infection control team intervention | Yes | 3 | 6 | 0.51 | |
| No | 2 | 2 | |||
Abbreviations: CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; Hb, hemoglobin.
HRCT-Based Stages of Infection-Uncontrolled and -Controlled Groups
HRCT-based stages showed that there were one and four patients with stages 1 and 4, respectively, in the uncontrolled group, and five, one, one, and one patients with stages 1, 2, 3, and 4, respectively, in the infection-controlled group, demonstrating significant correlation between the stage and infection control ( p = 0.047; Table 4 ).
Table 4. HRCT-based stages of the infection-uncontrolled and -controlled groups.
| Infection uncontrolled ( n = 5) | Controlled ( n = 8) | p -Value | |||
|---|---|---|---|---|---|
| HRCT ( n = 13) | Stage | 1 | 1 | 5 | 0.047 * |
| 2 | 0 | 1 | |||
| 3 | 0 | 1 | |||
| 4 | 4 | 1 | |||
Abbreviation: HRCT, high resolution computed tomography.
Statistically significant.
HRCT-Based Stages of SBO-Related Death and the Others Groups
HRCT-based stages showed that there were three patients with stage 4 in the SBO-related death group, and six, one, one, and two patients with stages 1, 2, 3, and 4, respectively, in the others group, demonstrating significant correlation between the stage and mortality ( p = 0.027; Table 5 ).
Table 5. HRCT-based stages of SBO-related death and the others (including cured, infection-bearing alive, or death by other causes patients) groups.
| SBO-related death ( n = 3) | The others ( n = 10) | p- Value | |||
|---|---|---|---|---|---|
| HRCT ( n = 13) | Stage | 1 | 0 | 6 | 0.027 * |
| 2 | 0 | 1 | |||
| 3 | 0 | 1 | |||
| 4 | 3 | 2 | |||
Abbreviations: HRCT, high resolution computed tomography; SBO, skull base osteomyelitis.
Statistically significant.
MRI-Based Stages of Infection-Uncontrolled and -Controlled Groups
MRI-based stages showed that there were one and four patients with stages 1 and 4, respectively, in the uncontrolled group, and three, one, two, and two patients with stages 1, 2, 3, and 4, respectively, in the infection-controlled group, demonstrating no significant correlation between the stage and infection control ( p = 0.137; Table 6 ).
Table 6. MRI-based stages of the infection-uncontrolled and -controlled groups.
| Infection uncontrolled ( n = 5) | Controlled ( n = 8) | p -Value | |||
|---|---|---|---|---|---|
| MRI ( n = 13) | Stage | 1 | 1 | 3 | 0.137 |
| 2 | 0 | 1 | |||
| 3 | 0 | 2 | |||
| 4 | 4 | 2 | |||
Abbreviation: MRI, magnetic resonance imaging.
MRI-Based Stages of SBO-Related Death and the Others Groups
MRI-based stages showed that there were three patients with stage 4 in the SBO-related death group, and four, one, two, and three patients with stages 1, 2, 3, and 4, respectively, in the others group, demonstrating no significant correlation between the stage and mortality ( p = 0.058; Table 7 ).
Table 7. MRI-based stages of SBO-related death and the others (including cured, infection-bearing alive, or death by other causes patients) groups.
| SBO-related death ( n = 3) | The others ( n = 10) | p -Value | |||
|---|---|---|---|---|---|
| MRI ( n = 13) | Stage | 1 | 0 | 4 | 0.058 |
| 2 | 0 | 1 | |||
| 3 | 0 | 2 | |||
| 4 | 3 | 3 | |||
Abbreviations: MRI, magnetic resonance imaging; SBO, skull base osteomyelitis.
Discussion
Treatment Outcome
The infection uncontrolled rate and mortality rate of otogenic SBO using the novel rigorous diagnostic criteria requiring bone involvements on HRCT and MRI were 38.5 and 23.1%, respectively. Because previous reports mixedly included various clinical conditions ranging from mild-to-severe SBO/MEO, it is difficult to directly compare the results of the current study with those of previous reports. 5 16 17 18 19 20 Among these reports, MEO mortality was reported as 4.4 to 19% 5 6 7 8 9 10 and SBO mortality was reported as 0 to 15%. 16 17 18 21 Although the uniform intensive therapies were applied to all patients in our study, the mortality rate (23.1%) was a little high, indicating that mortality rate of otogenic SBO is still high under rigorous diagnostic criteria requiring bone involvements on HRCT and MRI. The infection-uncontrolled rate (38.5%) was almost the same as that reported previously. 10
Possible Prognostic Factors for Otogenic Skull Base Osteomyelitis
Facial palsy, 5 7 8 16 cranial nerve involvement, 10 11 and immunocompromised conditions, including diabetes, 5 8 10 bilateral symptoms, 7 16 elderly age, 8 12 extensive granulation in the external auditory canal, 11 detection of fungi, 11 and surgical treatment, 12 have been proposed as prognostic factors for SBO/MEO. However, treatment outcomes were not actually correlated with each factor in most of these reports. In the present study, we statistically tested the correlation between possible predictive factors and infection control in patients with otogenic SBO under novel solid diagnostic criteria. As a result, all of the previously reported factors (age, immunocompromised condition, cranial nerve involvement, refractory external otitis, detection of fungi, and surgery) revealed no association with infection control ( Table 3 ). Moreover, other possible prognostic factors, such as sex, period from onset to the start of treatment, severe pain, fever, pretreatment laboratory data, and treatments, revealed no association with infection control ( Table 3 ). These no correlations might be at least partly due to a low number of patients; however, HRCT-based stages of otogenic SBO showed significant correlation with infection control and mortality. Meanwhile, MRI-based stages showed no correlation.
The Staging System for Otogenic Skull Base Osteomyelitis
HRCT-based stages significantly correlated with both infection control and mortality ( Tables 4 and 5 ), suggesting that the HRCT-based staging system is clinically useful for predicting infection control and mortality of otogenic SBO patients. To date, although uniform intensive therapies have been recommended for SBO/MEO, 4 treatment intensities can be decided according to the disease stage in the future. In contrast, MRI-based stages showed no correlation with infection control ( Table 6 ) and mortality ( Table 7 ), possibly because the MRI-based stages tended to be higher than the CT stages. It is suggested that the MRI-based staging system may be useful for the early detection of SBO but would have a risk of overstaging in predicting infection control and prognosis.
The otogenic SBO usually involves the petrous portion of temporal bone as the primary site. Since the clivus, which is composed of the occipital and sphenoid bones, is just adjacent to the petrous portion of the temporal bone; clivus is the most likely site outside the temporal bone to be involved by otogenic SBO. Therefore, when defining stages of otogenic SBO by anatomical disease extent, we chose the petrous portion of temporal bone and clivus as key anatomical structures. As a result, HRCT-based stages showed significant correlation with infection control and mortality ( Tables 4 and 5 ).
In rare cases known as central SBO, the clivus is involved without temporal bone destruction. Nonetheless, it usually has a poor clinical course. 22 Although the central SBO lacks a temporal bone lesion, we allocated the central SBO into the stage 2 or higher in our staging system ( Table 2 ). Therefore, this staging system can also be applied to the central SBO, so that stages would match the known poor clinical outcome of the central SBO.
Limitations
Under rigorous diagnostic criteria requiring bone involvements on HRCT and MRI, the number of patients in the present study was relatively small. Therefore, it was unable to perform multivariant analysis to identify significant prognostic predictors or to compare the mortality between disease stages by Kaplan–Meier analysis. Multicenter prospective studies with a large number of patients to evaluate the diagnostic criteria and HRCT and MRI-based staging system of otogenic SBO are essential.
Conclusion
We developed novel rigorous diagnostic criteria that require bone involvements on HRCT and MRI and a staging system for otogenic SBO. Among patients with otogenic SBO who met the diagnostic criteria, the infection uncontrolled rate and mortality rate were 38.5 and 23.1%, respectively. While the patients' background, symptoms, pretreatment laboratory data, and treatment did not show any correlation with infection control, the HRCT-based staging system correlated with infection control and mortality.
Acknowledgments
The authors thank for Dr. Shodayu Takashima for his valuable comments and critical reading of the manuscript.
Funding Statement
Funding None.
Conflict of Interest None declared.
Note
This work was approved by the Institutional Review Board of the Niigata University Medical and Dental Hospital (approval no.: 2019–0306).
References
- 1.Meltzer P, Kelemen G. Pyocyaneous osteomyelitis of the temporal bone, mandible and zygoma. Laryngoscope. 1959;69:1300–1316. [Google Scholar]
- 2.Chandler J R.Malignant external otitis Laryngoscope 196878081257–1294.4970362 [Google Scholar]
- 3.Chandler J R, Grobman L, Quencer R, Serafini A. Osteomyelitis of the base of the skull. Laryngoscope. 1986;96(03):245–251. doi: 10.1288/00005537-198603000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Chandler J R. Pathogenesis and treatment of facial paralysis due to malignant external otitis. Ann Otol Rhinol Laryngol. 1972;81(05):648–658. doi: 10.1177/000348947208100505. [DOI] [PubMed] [Google Scholar]
- 5.Franco-Vidal V, Blanchet H, Bebear C, Dutronc H, Darrouzet V. Necrotizing external otitis: a report of 46 cases. Otol Neurotol. 2007;28(06):771–773. doi: 10.1097/MAO.0b013e31805153bd. [DOI] [PubMed] [Google Scholar]
- 6.Chen C-N, Chen Y-S, Yeh T H, Hsu C J, Tseng F Y. Outcomes of malignant external otitis: survival vs mortality. Acta Otolaryngol. 2010;130(01):89–94. doi: 10.3109/00016480902971247. [DOI] [PubMed] [Google Scholar]
- 7.Soudry E, Hamzany Y, Preis M, Joshua B, Hadar T, Nageris B I. Malignant external otitis: analysis of severe cases. Otolaryngol Head Neck Surg. 2011;144(05):758–762. doi: 10.1177/0194599810396132. [DOI] [PubMed] [Google Scholar]
- 8.Stern Shavit S, Soudry E, Hamzany Y, Nageris B. Malignant external otitis: Factors predicting patient outcomes. Am J Otolaryngol. 2016;37(05):425–430. doi: 10.1016/j.amjoto.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Glikson E, Sagiv D, Wolf M, Shapira Y. Necrotizing otitis externa: diagnosis, treatment, and outcome in a case series. Diagn Microbiol Infect Dis. 2017;87(01):74–78. doi: 10.1016/j.diagmicrobio.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 10.Lee S K, Lee S A, Seon S W et al. Analysis of prognostic factors in malignant external otitis. Clin Exp Otorhinolaryngol. 2017;10(03):228–235. doi: 10.21053/ceo.2016.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eveleigh M O, Hall C E, Baldwin D L. Prognostic scoring in necrotising otitis externa. J Laryngol Otol. 2009;123(10):1097–1102. doi: 10.1017/S0022215109990491. [DOI] [PubMed] [Google Scholar]
- 12.Peled C, El-Seid S, Bahat-Dinur A, Tzvi-Ran L R, Kraus M, Kaplan D. Necrotizing otitis externa-analysis of 83 cases: clinical findings and course of disease. Otol Neurotol. 2019;40(01):56–62. doi: 10.1097/MAO.0000000000001986. [DOI] [PubMed] [Google Scholar]
- 13.Strauss M, Aber R C, Conner G H, Baum S. Malignant external otitis: long-term (months) antimicrobial therapy. Laryngoscope. 1982;92(04):397–406. doi: 10.1288/00005537-198204000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Lesser F D, Derbyshire S G, Lewis-Jones H. Can computed tomography and magnetic resonance imaging differentiate between malignant pathology and osteomyelitis in the central skull base? J Laryngol Otol. 2015;129(09):852–859. doi: 10.1017/S0022215115001991. [DOI] [PubMed] [Google Scholar]
- 15.Lew D P, Waldvogel F A.Osteomyelitis Lancet 2004364(9431):369–379. [DOI] [PubMed] [Google Scholar]
- 16.Spielmann P M, Yu R, Neeff M. Skull base osteomyelitis: current microbiology and management. J Laryngol Otol. 2013;127 01:S8–S12. doi: 10.1017/S0022215112002356. [DOI] [PubMed] [Google Scholar]
- 17.Ridder G J, Breunig C, Kaminsky J, Pfeiffer J. Central skull base osteomyelitis: new insights and implications for diagnosis and treatment. Eur Arch Otorhinolaryngol. 2015;272(05):1269–1276. doi: 10.1007/s00405-014-3390-y. [DOI] [PubMed] [Google Scholar]
- 18.Sokołowski J, Lachowska M, Karchier E, Bartoszewicz R, Niemczyk K. Skull base osteomyelitis: factors implicating clinical outcome. Acta Neurol Belg. 2019;119(03):431–437. doi: 10.1007/s13760-019-01110-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobsen L M, Antonelli P J. Errors in the diagnosis and management of necrotizing otitis externa. Otolaryngol Head Neck Surg. 2010;143(04):506–509. doi: 10.1016/j.otohns.2010.06.924. [DOI] [PubMed] [Google Scholar]
- 20.Lee S, Hooper R, Fuller A, Turlakow A, Cousins V, Nouraei R. Otogenic cranial base osteomyelitis: a proposed prognosis-based system for disease classification. Otol Neurotol. 2008;29(05):666–672. doi: 10.1097/MAO.0b013e318179972f. [DOI] [PubMed] [Google Scholar]
- 21.Johnson A K, Batra P S. Central skull base osteomyelitis: an emerging clinical entity. Laryngoscope. 2014;124(05):1083–1087. doi: 10.1002/lary.24440. [DOI] [PubMed] [Google Scholar]
- 22.Clark M PA, Pretorius P M, Byren I, Milford C A. Central or atypical skull base osteomyelitis: diagnosis and treatment. Skull Base. 2009;19(04):247–254. doi: 10.1055/s-0028-1115325. [DOI] [PMC free article] [PubMed] [Google Scholar]


