Abstract
Background A preoperative three-dimensional (3D) surgical field understanding remains a key factor to achieve safer endonasal transsphenoidal endoscopic approaches (ETSE). The aim of this article is to describe how we can get a reliable 3D sphenoidal anatomical reconstruction for surgical planning by using a user-friendly, accurate, and free image software.
Methods Free computer software (OSIRIX Medical Imaging Software) was used to create in a personal computer a three-dimensional (3D) reconstruction of the sphenoid sinus (SS) based on head computed tomography angiographies (CTAs) from a series of 67 patients who were operated for sellar tumors during a 4-year period (March 2016 to March 2020). The aim of the 3D reconstruction with OSIRIX was to reveal preoperatively the most important intrasphenoidal structures seen from the endonasal point of view.
Results The intraoperative visible sphenoidal structures were previously recognized in the virtual 3D reconstructed image with 100% of specificity (SP) and positive predictive value. The OSIRIX view by using region of interest points allowed us to see preoperatively the internal carotid artery parasellar course even in those cases in which it was hidden by bone or tumor. Moreover, the 3D reconstruction was able to provide a clear differentiation between the tumor and the pituitary gland when both structures were in contact with the sellar floor.
Conclusion Our experience with the OSIRIX software from CTA as preoperative planning for endonasal pituitary surgery was valuable, because it gave us access in simple way to a free and reliable 3D image of the SS.
Keywords: sphenoid sinus, pituitary surgery, endoscopic transsphenoidal approach, 3D reconstruction
Introduction
The transsphenoidal approach seemed to be the surgical corridor of choice for sellar pathologies and was first described by Cushing as early as 1907, 1 but he soon abandoned the technique because of poor light penetration, subtotal removals and high complication rates, and he returned to the transcranial route. 2 It was in 1962 with Hardy and the appearing of surgical microscopes that the transsphenoidal approach was reestablished as the gold standard for over 40 years with good outcomes. 3 But it is only from the early 2000, that the purely endonasal transsphenoidal endoscopic (ETSE) approach developed by Ho, Carrau, and Cappabianca became worldwide accepted and preferred. 4 5
The sphenoid sinus (SS) is located in the center of the skull base and the degree of its pneumatization can reveal recognizable bony prominences or recesses that correspond to important neurovascular structures, in particular the pituitary gland, the internal carotid artery (ICA) and the optic nerves. 6 The advantages of ETSE approach are the illumination to the SS structures in close range, the maneuverability in different angles to see tumor remnants in the parasellar and suprasellar compartments, and less trauma to nasal structures. However, ETSE technique requires a long learning curve to recognize surgical anatomical structures and to handle an endoscope that provides a two-dimensional (2D) view with peripheral distortion at high magnification (barrel effect). 7 Therefore, surgical planning and preoperative surgical field understanding and prediction have become very important to get used to the endoscope images in the field, and to better interpret the navigation systems. To overcome those potential risks, many expensive cadaveric courses are given and sophisticated virtual sphenoidal endoscopy software are developed. 7 The problem we faced at our institution before starting using our method was to get a three-dimensional (3D) reconstruction from a computed tomography (CT), because we were dependent on a neuroradiologist who needed the time to do it and had to guess what angle and view would be best suitable to us for the surgery.
That is the reason why we thought about a way to get a 3D reconstruction of the sphenoidal anatomy that should be easy, accurate, independent from a neuroradiologist, reliable, and free. We found all of those characteristics with the popular OSIRIX radiological viewer software. Therefore, the aim of this article is to describe how by using the OSIRIX software we can get a reliable 3D sphenoidal anatomy reconstruction for surgical planning and being at the same time simple to use, accurate, and free.
Patients and Methods
Patients and Surgical Technique
For this study, we assessed a series of 67 patients with pituitary pathology and selected for an ETSE approach at the Neurosurgical Department of the General University Hospital of Alicante between March 2016 through March 2020. Five cases were excluded because the SS was completely occupied by material such as bone, tumor, or fibrous tissue from previous surgeries. Intraoperatively the three major reasons making the recognition of the anatomy in the SS difficult were a low or a bad SS pneumatization, the presence of multiple Onodi (sphenoethmoidal) cells, and an extensive SS tumoral invasion involving one or both ICAs, because in these cases the SS anatomical structures cannot be identified by their bony prominences.
All cases of the study were operated by the same endoscopic skull base team composed by a neurosurgeon and an ENT using the binostril ETSE technique.
Imaging and 3D Reconstruction
Video 1 The video shows the reconstruction technique with the software OSIRIX. The three-dimensional volume of the skull is cut with the tool “crop” at the level of the sphenoid sinus.
In addition to the pituitary magnetic resonance imaging (MRI) with contrast, a multi-slice of 1 mm head computed tomography angiography (CTA) was obtained from each patient. Then, CTA was downloaded in a Digital Imaging and Communications in Medicine (DICOM) format to a regular portable MacBook Pro (2,3 GHz intel Core i7 with a dedicated video card NVIDIA GeForce GT650M, 1024 MB [Apple, Austin, Texas, USA]). A 3D reconstruction was obtained from the CTA using the software OSIRIX v.3.8 64-bit (Pixmeo Sarl [Geneva, Switzerland]) which was easily downloaded from the Internet
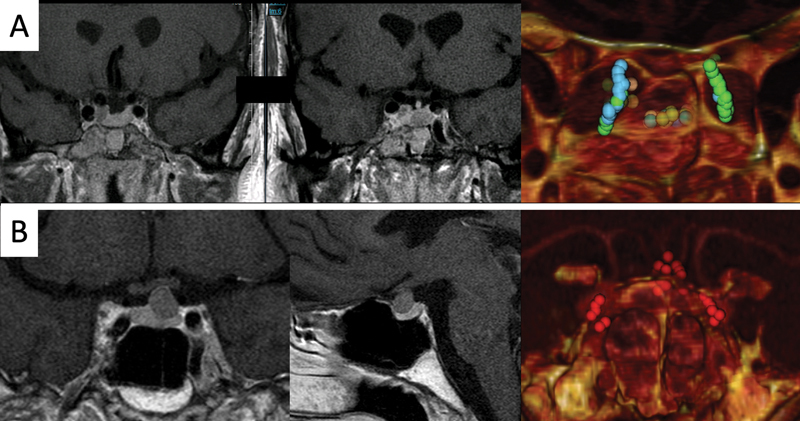
The reconstruction technique was quite easy to do ( Video 1 ). The time to get the image reconstruction varied between 3 and 5 minutes. The image data (CTA) consisted of DICOM files that were imported into the software by CD or external USB drives. Then, “3D volume rendering” tool was selected to obtain 3D volume of the skull from the axial slices. After that, we orientated the head with the eyes facing anteriorly and a slight inferior rotation. We used the tool “crop” to make a coronal cut perpendicular to the skull horizontal plane at a level immediately posterior to the anterior wall of the SS. With this plane, the inner aspect of the SS was exposed to our vision. Then we could do the changes we needed to get a better view of the intrasphenoidal anatomy, such as modifying the threshold, the rotation, and the zoom. Another interesting aspect was to mark with colored points some important region of interest (ROI) in the 2D axial slices that were automatically represented in our 3D reconstruction ( Fig. 1 ). This procedure was especially useful to show hidden structures, particularly marking the course of the ICAs in cases where they were covered by a septum or by the tumor itself and also to see the position of microadenomas that were not in direct contact with the sellar floor. In the latter cases, the hidden structures can be seen by transparency changing the threshold of the CTA.
Fig. 1.
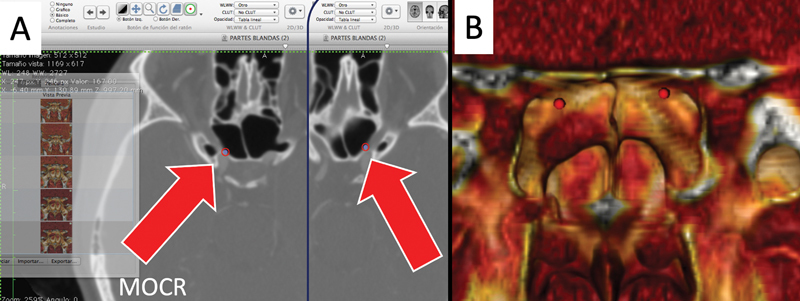
( A ) From the two-dimensional axial computed tomography angiography, we identify and mark with red region of interest points both medial optic-carotid recesses (MOCR). ( B ) The same references appear in the three-dimensional reconstruction from OSIRIX.
Intraoperative surgical videos were recorded with a 0 degree endoscope (Hopkins II-Karl Storz, Tuttligen, Germany).
The Study Method
The first step of the study consisted of making a 3D reconstruction of the sphenoidal region anatomy as explained above and was used as a presurgical planning ( Video 1 ).
-
The second step consisted of identifying the following intrasphenoidal anatomical references both with the 3D model (preoperatively) and with the endoscope in live surgery ( Fig. 2 ):
Sellar prominence
Right and left optic nerves
Right and left lateral optic-carotid recesses
Right and left medial optic-carotid recesses
Right and left ICA prominences
Clival recess
Tuberculum sellae
The intraoperative identification is performed just after performing the sphenoidotomy and before drilling the intrasphenoidal septa. We routinely used the navigation system (StealthStation S8 system Medtronic) to check the position of these points. The capacity of the 3D reconstruction to identify preoperatively each landmark is analyzed statistically in terms of sensitivity (SE), specificity (SP), positive predictive value (PPV), and negative predictive value (NPV) using SPSS version 22.0 for Macintosh.
The third step consisted of marking with red ROI points the complete course of the parasellar segment of each ICA, especially in cases where this structure was hidden by bone or tumor ( Fig. 3 ).
The fourth step consisted of checking the ability of our 3D reconstructions to show the differentiation between the tumor and normal pituitary gland in those cases where both the pituitary gland and the tumor are in contact with the sellar floor ( Fig. 4 ).
Fig. 2.
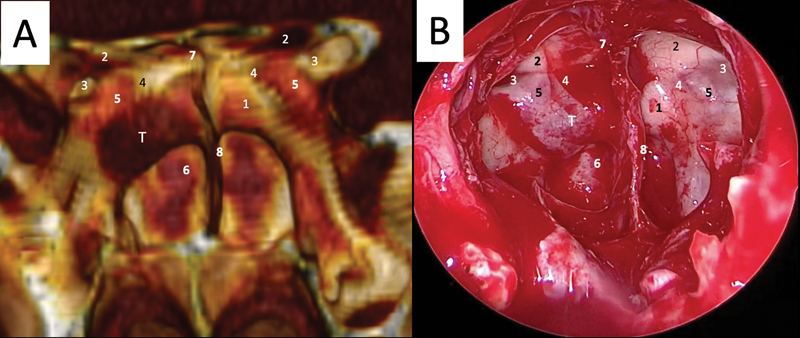
( A ) The three-dimensional computed tomography angiography reconstruction of the sphenoid sinus in a case of a sellar microadenoma. ( B ) Intraoperative picture of the sphenoid sinus opened in the same patient from an endonasal transsphenoidal endoscopic approach. (1) Sellar prominence; (2) optic nerves; (3) lateral optic-carotid recesses; (4) medial optic-carotid recess; (5) internal carotid artery prominence; (6) clival recess; (7) tuberculum sellae; (8) septa morphology; T: tumor (microadenoma located ventrally and lateralized at the right side of the sellar floor).
Fig. 3.
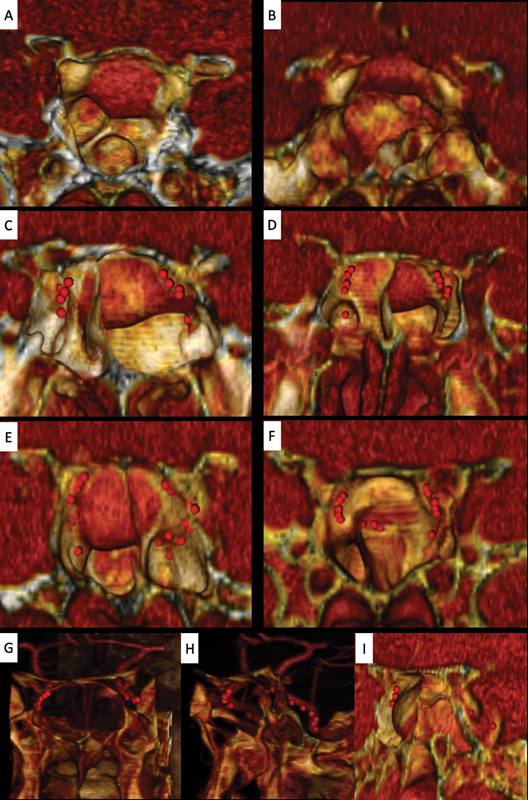
Examples of internal carotid artery (ICA) reconstruction based on OSIRIX. ( A ) and ( B ) With a good sphenoidal sinus pneumatization, the ICA prominences are naturally well demarcated without using region of interest (ROI) points. ( C–F ) Examples of cases where the ICAs are not clearly seen with the simple three-dimensional (3D) reconstructions because they are covered by any septum or are severely displaced by the tumor. The use of red ROI points previously marked in the two-dimensional axial CTA slices and automatically represented in our three-dimensional model allowing us to predict the position of the ICA preoperatively. Some anatomical variations can be identified beforehand, like a medialization of the ICA (i.e., right ICA of case F). ( G–I ) represents a macroadenoma with Knops 4 invasion of the left cavernous sinus. The ICA is marked by red ROI points and by using a combination of coronal and sagittal cuts and slight changes in the threshold or transparency of our 3D reconstruction, we are able to identify its complete course.
Fig. 4.
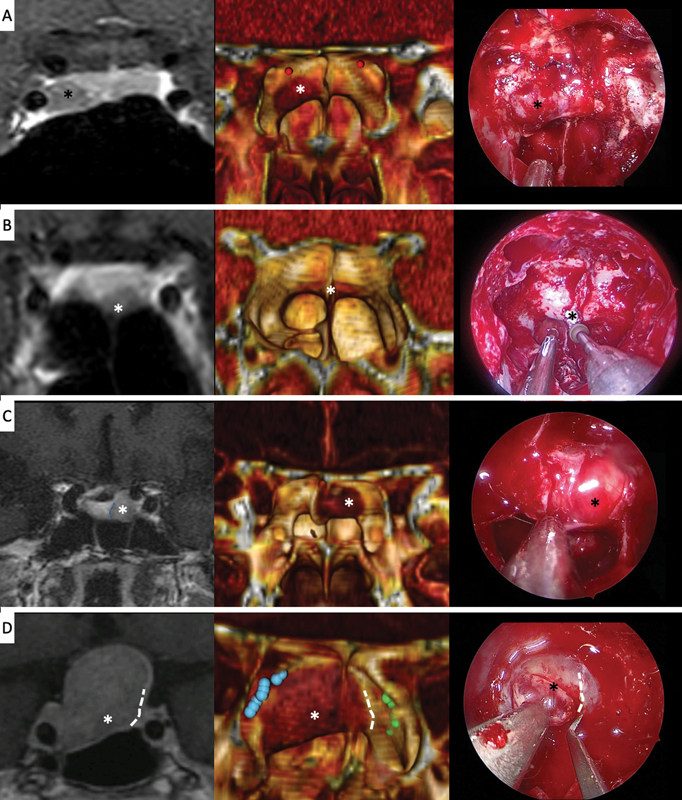
Examples of cases where the three-dimensional (3D) reconstruction allows us to predict the differentiation of tumor and pituitary gland at the sellar floor level. On the left the pituitary coronal magnetic resonance imaging section, in the middle the 3D model and on the right an intraoperative picture of the sphenoid sinus are showed. The 3D OSIRIX reconstruction allows us to locate and perform the sellar opening in a more accurate and a safer way. Most cases are microadenomas (cases A–C), but some macroadenomas (case D) show the same behavior (D). (*): Tumor, discontinuous white line: tumor-gland limit.
Results
Demographic data for the patients and the characteristics of the lesions are given in detail in Table 1 . Based on the 3D model images, the eleven previously described sphenoidal landmarks were evaluated and compared with the actual intraoperative endoscopic views ( Table 2 ). Preoperative and intraoperative findings matched with high accuracy in all 67 patients. The validation of the 3D model to identify preoperatively anatomical references is shown in Table 3 . The model showed perfect SP and NPV to identify all the landmarks. In terms of SE and PPV, the obtained values were also 100% for sellar prominence, clival recess, and tuberculum sellae. However, the 3D model was not able to identify the rest of the references in a few cases. We analyzed these cases and found two main factors: low quality reconstruction in two cases and complex pattern of septation hiding the anatomical structures in four cases. The second factor is obviously overcome with the use of the high-definition endoscope in real surgery. The better image obtained with the endoscope and its capacity of entering inside small and narrow cavities of the SS explain the small differences.
Table 1. Patients and tumor characteristics.
| Number of patients | 67 |
| Male:female ratio | 37:30 |
| Age (yr): median (range) | 55.5 (14–83) |
| Pathological features, n (%) | |
| Pituitary adenomas | 62 (92.5%) |
| Nonfunctioning adenomas | 36 (53.7%) |
| GH-cell adenomas | 13 (19.4%) |
| ACTH-cell adenomas | 7 (10.4%) |
| PRL-cell adenomas | 6 (8.9%) |
| Meningiomas | 2 (2.9%) |
| Craniopharyngiomas | 1 (1.4%) |
| Arachnoid cysts | 1 (1.4%) |
| Rathke cleft cysts | 1 (1.4%) |
| Giant: macro:micro (adenomas) ratio | 2:48:12 |
Abbreviations: ACTH, adrenocorticotropic hormone; GH, growth hormone; PRL, prolactin.
Table 2. Number of intrasphenoidal landmarks identified by 3D model and real surgery.
| Intrasphenoidal reference | Frequency in 3D model (%) | Frequency in real surgery (%) |
|---|---|---|
| Sellar prominence | 50/67 (74.6%) | 50/67 (74.6%) |
| Right optic nerve | 57/67 (85%) | 59/67 (88%) |
| Left optic nerve | 55/67 (82%) | 58/67 (86.5%) |
| Right lateral optic-carotid recess | 34/67 (50.7%) | 36/67 (53.7%) |
| Left lateral optic-carotid recess | 30/67 (44.7%) | 31/67 (46.2%) |
| Right medial optic-carotid recess | 55/67 (82%) | 58/67 (86.5%) |
| Left medial optic-carotid recess | 54/67 (80.6%) | 57/67 (85%) |
| Right ICA prominence | 60/67 (89.5%) | 61/67 (91%) |
| Left ICA prominence | 55/67 (82%) | 57/67 (85%) |
| Clival recess | 50/67 (74.6%) | 50/67 (74.6%) |
| Tuberculum sellae | 67/67 (100%) | 67/67 (100%) |
Abbreviations: 3D, three-dimensional; ICA, internal carotid artery.
Table 3. Validation of 3D model to identify references seen in real surgery.
| Intrasphenoidal references | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| Sellar prominence | 100% | 100% | 100% | 100% |
| Right optic nerve | 96.6% | 100% | 100% | 80% |
| Left optic nerve | 94.8% | 100% | 100% | 75% |
| Right lateral optic-carotid recess | 94.4% | 100% | 100% | 93.9% |
| Left lateral optic-carotid recess | 96.8% | 100% | 100% | 97.3% |
| Right medial optic-carotid recess | 94.8% | 100% | 100% | 75% |
| Left medial optic-carotid recess | 94.7% | 100% | 100% | 76.9% |
| Right ICA prominence | 98.4% | 100% | 100% | 85.7% |
| Left ICA prominence | 96.5% | 100% | 100% | 83.3% |
| Clival recess | 100% | 100% | 100% | 100% |
| Tuberculum sellae | 100% | 100% | 100% | 100% |
Abbreviations: 3D, three-dimensional; ICA, internal carotid artery; NPV, negative predictive value; PPV, positive predictive value.
The results reinforce the mental concept that the surgeon had and then facilitating the pure tumor resections, sparing safely the surrounding healthy neurovascular structures.
The anatomical characteristics of the SS were described in Table 4 : the pneumatization in the sagittal plane, the sphenoid septa in contact with ICAs, the visibility of parasellar ICAs from our anterior endonasal view, the patients in which the tumor is visible in our reconstruction, and the cases where we are able to see both the pituitary gland and the tumor lying on the sellar floor.
Table 4. Anatomical–radiological findings.
| Anatomical finding | Number (%) |
|---|---|
| Pneumatization (sagittal plane) | |
| Sellar type | 50 (74.6%) |
| Presellar type | 17 (25.4%) |
| Conchal type | 0 |
| Patients with 1 or more septa in contact with ICA | 37 (55%) |
| Right ICA invisible | 7 (10.4%) |
| Left ICA invisible | 12 (17.9%) |
| Tumor visible | 56 (83.5%) |
| Pituitary gland–tumor differentiation | 18 (26.8%) |
Abbreviation: ICA, internal carotid artery.
The OSIRIX view allowed us to localize the ICA course in all cases. Particularly when this structure was hidden by either a septum or the tumor (7/67 right ICA and 12/67 left ICA) our method consisting in marking ROI points, could easily identify its morphology ( Fig. 3 ).
Moreover, the reconstruction was able to show the differentiation between the tumor and the pituitary gland in the cases (18/67) where both structures were in contact with the sellar floor ( Fig. 4 ).
Finally, as previously described, we have found this tool very useful to mark the position of microadenomas that are not in direct contact with the sellar floor. In these cases, and similar to the ICAs, the hidden tumors can be seen by transparency just modifying the threshold of the CT ( Fig. 5 ).
Fig. 5.
Examples of cases where the tumor is not in direct contact with the sellar floor. The use of region of interest points marking the tumor and changes in threshold/transparency allow us to predict the location of the tumor. ( A ) Case of a patient with acromegaly showing two separated tumoral nodules: one in the center of the pituitary gland and the other in contact with the right cavernous sinus behind the parasellar segment of the right internal carotid artery. ( B ) Case of a patient with Cushing disease showing a microadenoma close to the pituitary stalk. LHP, ---; RFA,---
Discussion
Introduction of an endoscope in transsphenoidal surgery provides surgeons with a wide panoramic view, and use of various angled endoscopes presents multidirectional views, facilitating a much better understanding of anatomical relationships of the sella and surrounding vital structures such as the ICAs and optic nerves. However, the disadvantage of this approach is that, with wide opening of the sellar floor, there is a risk that the surgeon may come extremely close to the mentioned vital structures. Estimation of the safety margin in the opening of the sellar floor both before and during surgery is therefore critical. 8 Experience, special training, and preoperative planning are crucial for the safe use of this technique. 9
Anatomical abnormalities in the SS are common in pituitary surgery. Lack of knowledge of those variations leads to increased surgical complications and poor outcomes. 10 11 CTA reconstruction of the anatomical structures of pituitary tumors can avoid potential damage on nerves and blood vessels in patients with anatomical variations. 12 When surgeons preoperatively simulate surgical procedures, they usually have to perform an imaginary surgical plan by mentally reconstructing 3D images based on preoperative 2D CT or MRI. Such imaginary reconstructions do not always represent the actual anatomy, particularly when the surgeon is not completely familiar with the surgical field. 8 As CT and MRI data are networked in hospital information systems and are able to be viewed on computer monitors, the surgeon can nowadays work with user-friendly softwares to build 3D models of the surgical field during preoperative evaluation of the images.
The idea to have 3D reconstructed images before and during surgery has been defended by many authors before. They concluded that a 3D image of the sphenoidal anatomy allowed them performing bigger sellar openings and achieving a higher number of complete tumor removals in a safer way. 8 12 13
We propose the free available software named OSIRIX for the reconstruction of the images and realization of the virtual endoscopy as a reliable alternative to the dedicated software and systems made for that purpose. OSIRIX is an opensource DICOM viewer with the powerful capability of 3D volumetric rendering. OSIRIX has gained significant attention in recent years as a tool of research in neurosurgery. 14 15 16 17 18 19 20 The images obtained using this method are high quality and provide a good resemblance with the intraoperative images. The reliability of the method is sustained by the high correlation obtained after comparing the virtual and intraoperative images.
The main advantage brought by this method is the possibility of getting familiar with the specific endoscopic anatomy, and also offering prospective information about the anatomic variations in the individual patient.
One of the most important landmarks that need to be assessed at the level of SS are the sphenoid septa. Knowledge of their appropriate orientation within the sinus and their insertion is crucial because it is not uncommon for the sphenoid septa to have a lateral attachment in close relation with the ICA wall. 21 Fernandez-Miranda et al 22 reported from a total of 54 SS studied, 47 (87%) had at least one septum related to the ICA, and only 13% presented a typical isolated midline septation. Our results are significantly different, showing less cases (55%) with this septum-ICA relation.
Other important landmarks are the carotid prominences and the medial optic-carotid recesses, because these structures are the borders that confine sellar opening in the lateral direction. 23 It is important to know their position to avoid injuries of the ICA and the optic nerves. In the literature, the cited incidence of ICA injury during endoscopic endonasal skull base surgery ranges from 0.3 to 2%. 24 Talala et al 25 were the first to describe the use of virtual endoscopy for visualization of the ICA inside the SS. This technique is important for appreciation of their correct position at this level, especially in cases with low inter-carotid distance.
The differentiation between the tumor and the pituitary gland can be easily seen in MRI. However, the use of the OSIRIX reconstruction for this objective allows us to see the relation of the tumor surface with the bone landmarks in a 3D reconstruction. It is difficult to measure how this method has changed the management of our patients. In our experience, the 3D model allows the surgeon to perform the sellar opening more accurately. Figs. 1 , 2 , and Video 1 have been based on an illustrative case. This case showed a microprolactinoma of a 40 years old female. In this case, the 3D reconstruction allowed us to perform a small and accurate sellar opening with a clear orientation of the tumor in relation with the ICA and the healthy pituitary gland. These advantages translate into safer and faster surgery.
Rotariu et al 9 also used OSIRIX to create a virtual endoscopy method that showed a good correlation for the structures located at the level of the nasal cavity and SS. This study, similar to our method, was highly influenced by the degree of pneumatization of the containing structures, obtaining a poor correlation in cases where there was an invasion of the SS or an absence of an adequate pneumatization. But one major difference between our SS 3D reconstruction and Rotariu's et al study was that they reconstructed the SS anatomy based on a simple CT and not on a CTA, which allowed us to identify bony septa and vascular structures (ICA) at the same time. Moreover, our method offers the benefit of getting a better visualization of the structures located beyond the sellar and parasellar floor by using ROIs and modifying the threshold. However, we focused our reconstruction only on the intrasphenoidal anatomy instead of the whole nasal cavity like in the aforementioned article.
Obviously, all these methods are complementary to the navigation systems. Reconstructed 3D models may be greatly superior to the navigation system in drawing the contours of the SS and presenting the anatomical relationships of the sella and surrounding structures. 8 We do emphasize once more that this software is free and user-friendly. The only thing the surgeon needs is a computer to run the software and 3 to 5 minutes to reconstruct the sphenoidal anatomy.
Our method of reconstruction has several limitations. For example, when the SS is poorly pneumatized, it shows a complex pattern of septation or is occupied by a downward-extended tumor, bony structures including the carotid prominences cannot be identified. In such cases, it is very important to accurately identify the ICAs in the early stage of surgery to remove most of the tumor without injuring the ICAs. We have different intraoperative ways to find the ICA such as using the navigation systems, the Doppler or the fluorescence detection by indocyanine green. Preoperatively, our method minimizes this problem by marking the ICA with colored ROIs that can be seen just by changing the threshold of the model.
Moreover, major obstacles exist to acquire CT images and create these 3D models, such as an excessive radiation exposure and the need for iodine contrast agent administration to depict bilateral ICAs. Recently, zero-echo-time (ZTE)-based segmentation methods on MRI have been suggested as a new bone-identification technique. 26 27 However, the CT still remains as the current gold standard imaging technique to reveal bone structures, because the preoperative 3D ZTE-based MRI model does not so accurately correlate with the intraoperative images as CT-based 3D imaging.
Conclusion
Our experience with the OSIRIX software from CTA as preoperative planning for endonasal pituitary surgery was valuable, because it gave us access in simple way to a free and reliable 3D image of the SS. To be able to recognize the structures in 3D beforehand, improve the decision making, the surgical strategy, and the orientation in the surgical field.
Funding Statement
Funding None.
Conflict of Interest All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers' bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or nonfinancial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Note
The paper has not been published previously.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
For this type of study, formal consent is not required.
References
- 1.O'Malley B W, Jr, Grady M S, Gabel B C et al. Comparison of endoscopic and microscopic removal of pituitary adenomas: single-surgeon experience and the learning curve. Neurosurg Focus. 2008;25(06):E10. doi: 10.3171/FOC.2008.25.12.E10. [DOI] [PubMed] [Google Scholar]
- 2.Gendeh B S, Doi M, Selladurai B M, Khalid B A, Jegan T, Misiran K. The role of endoscopic endonasal approach to pituitary tumours: HUKM experience. Med J Malaysia. 2006;61(03):343–348. [PubMed] [Google Scholar]
- 3.Ciric I, Ragin A, Baumgartner C, Pierce D.Complications of transsphenoidal surgery: results of a national survey, review of the literature, and personal experience Neurosurgery 19974002225–236., discussion 236–237 Review [DOI] [PubMed] [Google Scholar]
- 4.Jho H D, Carrau R L. Endoscopy assisted transsphenoidal surgery for pituitary adenoma. Technical note. Acta Neurochir (Wien) 1996;138(12):1416–1425. doi: 10.1007/BF01411120. [DOI] [PubMed] [Google Scholar]
- 5.Cappabianca P, Alfieri A, de Divitiis E. Endoscopic endonasal transsphenoidal approach to the sella: towards functional endoscopic pituitary surgery (FEPS) Minim Invasive Neurosurg. 1998;41(02):66–73. doi: 10.1055/s-2008-1052019. [DOI] [PubMed] [Google Scholar]
- 6.Wang J, Bidari S, Inoue K, Yang H, Rhoton A., Jr Extensions of the sphenoid sinus: a new classification. Neurosurgery. 2010;66(04):797–816. doi: 10.1227/01.NEU.0000367619.24800.B1. [DOI] [PubMed] [Google Scholar]
- 7.Wolfsberger S, Neubauer A, Bühler Ket al. Advanced virtual endoscopy for endoscopic transsphenoidal pituitary surgery Neurosurgery 200659051001–1009., discussion 1009–1010 [DOI] [PubMed] [Google Scholar]
- 8.Inoue A, Ohnishi T, Kohno S et al. Utility of three-dimensional computed tomography for anatomical assistance in endoscopic endonasal transsphenoidal surgery. Neurosurg Rev. 2015;38(03):559–565. doi: 10.1007/s10143-015-0625-3. [DOI] [PubMed] [Google Scholar]
- 9.Rotariu D I, Ziyad F, Budu A, Poeata I. The role of OSIRIX based virtual endoscopy in planning endoscopic transsphenoidal surgery for pituitary adenoma. Turk Neurosurg. 2017;27(03):339–345. doi: 10.5137/1019-5149.JTN.16311-15.2. [DOI] [PubMed] [Google Scholar]
- 10.Oertel J, Gaab M R, Linsler S. The endoscopic endonasal transsphenoidal approach to sellar lesions allows a high radicality: the benefit of angled optics. Clin Neurol Neurosurg. 2016;146:29–34. doi: 10.1016/j.clineuro.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 11.Kassam A B, Prevedello D M, Carrau R L et al. Endoscopic endonasal skull base surgery: analysis of complications in the authors' initial 800 patients. J Neurosurg. 2011;114(06):1544–1568. doi: 10.3171/2010.10.JNS09406. [DOI] [PubMed] [Google Scholar]
- 12.Guo Z, Liu C, Hou H et al. Preoperative computed tomography (CT) evaluation of anatomical abnormalities in endonasal transsphenoidal approach in pituitary adenoma. Med Sci Monit. 2018;24:1268–1275. doi: 10.12659/MSM.904402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu J, Liu S, Heng X et al. The values of thin sections and three-dimensional reconstruction in the sellar region. World Neurosurg. 2012;78(05):510–515. doi: 10.1016/j.wneu.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Spiriev T, Nakov V, Laleva L, Tzekov C. OsiriX software as a preoperative planning tool in cranial neurosurgery: a step-by-step guide for neurosurgical residents. Surg Neurol Int. 2017;8:241. doi: 10.4103/sni.sni_419_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bruneau M, Kamouni R, Schoovaerts F, Pouleau H B, De Witte O. Simultaneous image-guided skull bone tumor resection and reconstruction with a preconstructed prosthesis based on an OsiriX virtual resection. Oper Neurosurg (Hagerstown) 2015;11(04):484–490. doi: 10.1227/NEU.0000000000000971. [DOI] [PubMed] [Google Scholar]
- 16.Harput M V, Gonzalez-Lopez P, Türe U.Three-dimensional reconstruction of the topographical cerebral surface anatomy for presurgical planning with free OsiriX Software Neurosurgery 20141003426–435., discussion 435 [DOI] [PubMed] [Google Scholar]
- 17.Jaimovich S G, Guevara M, Pampin S, Jaimovich R, Gardella J L. [Neurosurgical planning using OsiriX software] Surg Neurol Int. 2014;5 05:S267–S271. doi: 10.4103/2152-7806.137970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jalbert F, Paoli J R. [Osirix: free and open-source software for medical imagery] Rev Stomatol Chir Maxillofac. 2008;109(01):53–55. doi: 10.1016/j.stomax.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 19.Mandel M, Amorim R, Paiva W, Prudente M, Teixeira M J, Andrade A F. 3D preoperative planning in the ER with OsiriX®: when there is no time for neuronavigation. Sensors (Basel) 2013;13(05):6477–6491. doi: 10.3390/s130506477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosset A, Spadola L, Ratib O, Osiri X. OsiriX: an open-source software for navigating in multidimensional DICOM images. J Digit Imaging. 2004;17(03):205–216. doi: 10.1007/s10278-004-1014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamid O, El Fiky L, Hassan O, Kotb A, El Fiky S. Anatomic variations of the sphenoid sinus and their impact on transsphenoid pituitary surgery. Skull Base. 2008;18(01):9–15. doi: 10.1055/s-2007-992764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernandez-Miranda J C, Prevedello D M, Madhok R et al. Sphenoid septations and their relationship with internal carotid arteries: anatomical and radiological study. Laryngoscope. 2009;119(10):1893–1896. doi: 10.1002/lary.20623. [DOI] [PubMed] [Google Scholar]
- 23.Jane J A, Jr, Han J, Prevedello D M, Jagannathan J, Dumont A S, Laws E R., Jr Perspectives on endoscopic transsphenoidal surgery. Neurosurg Focus. 2005;19(06):E2. doi: 10.3171/foc.2005.19.6.3. [DOI] [PubMed] [Google Scholar]
- 24.Gardner P A, Tormenti M J, Pant H, Fernandez-Miranda J C, Snyderman C H, Horowitz M B.Carotid artery injury during endoscopic endonasal skull base surgery: incidence and outcomes Neurosurgery 201373(2, Suppl Operative) discussion ons269–ons270ons261–ons269. [DOI] [PubMed] [Google Scholar]
- 25.Talala T, Pirilä T, Karhula V, Ilkko E, Suramo I. Preoperative virtual endoscopy and three-dimensional imaging of the surface landmarks of the internal carotid arteries in trans-sphenoidal pituitary surgery. Acta Otolaryngol. 2000;120(06):783–787. doi: 10.1080/000164800750000351. [DOI] [PubMed] [Google Scholar]
- 26.Inoue A, Kohno S, Nishida N et al. Clinical utility of new three-dimensional model using a zero-echo-time sequence in endoscopic endonasal transsphenoidal surgery. Clin Neurol Neurosurg. 2020;190:105743. doi: 10.1016/j.clineuro.2020.105743. [DOI] [PubMed] [Google Scholar]
- 27.Delso G, Wiesinger F, Sacolick L I et al. Clinical evaluation of zero-echo-time MR imaging for the segmentation of the skull. J Nucl Med. 2015;56(03):417–422. doi: 10.2967/jnumed.114.149997. [DOI] [PubMed] [Google Scholar]


