Abstract
Introduction Endoscopic endonasal surgery (EES) has become the preferred approach for pituitary tumor resection. Nevertheless, research on quality of life related to pituitary adenoma surgery is scarce.
Objective The aim of the study is to evaluate short-term quality of life in patients after endoscopic endonasal resection of pituitary tumors and to find predictors for poor quality of life (QOL) outcome.
Materials and Methods A prospective cohort study was conducted, including all patients who underwent EES for pituitary tumors in a tertiary medical referral center. Recruited patients completed the Anterior Skull Base Disease-Specific QOL (ASBS-Q) questionnaire and the Sinonasal Outcome Test 22 (SNOT-22) questionnaire before surgery, 2 and 4 to 6 months after surgery. Demographic and clinical data was collected.
Results Our study included 49 patients. The overall ASBS-Q scores significantly improved 4 to 6 months after surgery (4.46 vs. 4.2, p < 0.05). We found a significant improvement in QOL related to emotional state 2 months post surgery (4.41 vs. 3.87, p < 0.05), which became borderline significant 4 to 6 months post surgery. There was a significant improvement in pain (4.5 vs. 4.08, p < 0.05) and vitality (4.43 vs. 4.16, p < 0.05) domains 4 to 6 months post surgery. SNOT-22 scores did not change significantly postoperatively. Factors such as secreting and non-secreting tumors, tumor size, intraoperative cerebrospinal fluid leak, gross tumor resection, endocrine remission, and the use of nasoseptal flap reconstruction did not have a significant effect on QOL.
Conclusion We found that patients after EES reported improved QOL 4 to 6 months post surgery. Specific improvement was noted in the QOL related to pain and vitality.
Keywords: quality of life, endoscopic endonasal sinus surgery, pituitary adenoma
Introduction
Quality of life (QOL) is a fundamental goal of medical care and it is assessed in an effort to improve treatment and restore patients' daily function. Benign pituitary tumors are associated with pituitary dysfunction, either hypersecretion or hypopituitarism, due to compression or destruction of normal pituitary cells. Endocrine changes are associated with systemic injury, higher anxiety-related traits, and psychological personality changes. 1 Benign tumors may also cause headache or visual disturbances due to pressure on surrounding structures. 1 These tumors may frequently necessitate surgical intervention. Moreover, resection of benign skull base tumors may, in itself, be an independent cause of significant side effects and complications and may therefore lead to impaired QOL. 2 3 4 5 6 7 8
The endoscopic approach to the anterior skull base caused a revolution in the surgical treatment of pituitary tumors, diminishing morbidity, discomfort, and complications while increasing survival. 7 The main flaw in this approach is, that despite the technological advantages of a minimally invasive technique, it is still frequently required to surgically manipulate or resect normal uninvolved intranasal structures to gain adequate access for a functional surgical corridor. As a result, iatrogenic sinonasal sequelae, such as crusting, rhinosinusitis, epistaxis, or hyposmia become part of the disease burden of such patients, leading to significant physical and psychological post-treatment effects. 9 10 11
QOL is a patient-reported measure that aims to describe a patient's perception of well-being. Patient-reported outcome measures allow a more objective assessment of the patient's health-related QOL by providing insight into the patients' experience with their overall care or health care service. 12 Moreover, surgeons were found to overrate the levels of patients' QOL, and their impressions did not correlate with the patients' subjective QOL ratings. 8 In the setting of pituitary gland tumors, only a limited number of studies have sought to examine the effect of endoscopic endonasal pituitary adenoma surgery on sinonasal- and tumor-related QOL. 13 14 15 Earlier reports vary in the instrument/questionnaires that were used to address the disease-specific QOL, and some even used general QOL assessments.
The main objective of our study was to determine the effects of endoscopic endonasal surgery for pituitary tumors on patient-reported QOLs using validated, tumor-specific instruments administered both pre- and postoperatively. Our secondary objective was to identify potential predictors for poor QOL among these patients.
Materials and Methods
Ethics and Patient Selection
This prospective study was approved by the Institutional Ethics Committee (TLV-0761–19). All adult patients who underwent endoscopic endonasal surgery for benign pituitary gland tumor resection in our institution between November 2014 and December 2017 were enrolled in the study. They all underwent magnetic resonance imaging (MRI) prior to surgery, and their studies were evaluated for the purpose of surgical intervention by a multidisciplinary team, including a neurosurgeon (Z.R.), an endocrinologist, and an otolaryngology expert in rhinology (A.A.). The same interdisciplinary team performed all operations. All patients suffering from prolactinomas underwent surgery after failure of dopamine agonist therapy or suffered from serious adverse effects of treatment.
QOL Assessment
The psychological, social, and physical well-being of the patients were assessed by the Anterior Skull Base Disease-Specific QOL Questionnaire (ASBS-Q), a disease-specific multidimensional questionnaire dedicated to patients undergoing surgery due to tumors involving the anterior skull base. The questionnaires were completed by the patients at three intervals: 1 week prior to surgery, and 2 and 4 to 6 months postoperatively. Patients also filled in a Hebrew validated Sinonasal Outcome Test 22 (SNOT-22) questionnaire. 16 Tumor size was measured in the preoperative MRI scan and classified as microadenoma (< 1 cm), macroadenoma (> 1 cm), or huge adenoma (> 2.5 cm). The histology of each of the lesions was recorded, and the patients were divided into groups of secreting versus non-secreting tumors.
Surgical Technique
Extirpation of pituitary gland tumors was performed via the expanded endoscopic approach in all patients. Both middle turbinates were removed to facilitate a bi-manual approach. A partial anterior and posterior ethmoidectomy was performed on the right nasal cavity to create a wide corridor for the endoscope and the suction instruments. Resection of the posterior third of the superior turbinate was performed when a suprasellar approach was planned. A nasoseptal flap (NSF) was harvested at the beginning of the operation in cases where a high flow cerebrospinal fluid (CSF) leak was anticipated, then a wide sphenoidotomy was preformed after a partial posterior septectomy and resection of the vomer lowering the anterior wall of the sphenoid. When dissecting the posterior septal mucosa from the vomer, we usually preserved the posterior septal artery by reflecting down the mucosa of the posterior septum and the choana, in a manner that it resembles a minute “rescue flap.” This allows us to elevate an NSF at the end of the tumor resection when unanticipated high flow CSF leak occurred. The reconstruction method was tailored to the anticipated defect size and CSF leakage. 17 When we anticipated a high flow CSF leak (i.e., in cases when we planned also suprasellar approach, very high suprasellar tumors or proximity to third ventricle), we preferred to use an NSF prepared ahead with autologous fat. In low flow CSF leaks we used autologous fat. NSF was used only if raised/prepared one in the beginning of the surgery. Follow-up was conducted in the outpatient clinic at 2 months and between 4 and 6 months after the surgery.
Questionnaire
All patients completed the ASBS-Q at the abovementioned postoperative intervals. The questionnaire is a patient-based measurement designed for self-administration. It consists of six domains: the role of performance (eight items), physical function (seven items), vitality (six items), pain (three items), specific symptoms (seven items), and emotional state (five items), which yield a total of 36 questions with a nominal scale of five steps for each question 9 . A higher score means a higher satisfaction with regard to one's QOL. All questions have an identical level of importance. The domain of specific symptoms includes seven questions on several aspects that are most relevant to this patient population, such as altered taste, smell, and appearance, as well as epiphora, nasal secretions, and visual disturbances. The study population was divided into subgroups according to tumor characteristics, tumor size, intraoperative CSF leak, and reconstruction technique to identify patients who were likely to sustain poor QOL after surgery.
The He-SNOT-22 questionnaire 16 was completed by all patients at the abovementioned postoperative intervals. The questionnaire is a patient-based measurement designed for self-administration. The questionnaire's overall score ranges between 0 and 88, and the higher the score, the higher the impairment in QOL.
Statistical Analysis
Categorical variables were described as frequency and percentage. Continuous variables were evaluated for normal distribution by a histogram and Q–Q plot and reported as mean and standard deviation. The preoperative and postoperative overall score as well as selected questions were compared by the Wilcoxon signed ranks test. Comparisons between patients with secreting and non-secreting tumors, between patients with and without endocrine remission and between patients with and without gross tumor resection (GTR) were performed with the Mann-Whitney test for the continuous variables, and the chi-square test or Fisher's exact test for the categorical variables. All statistical tests were two-sided and a p -value < 0.05 was accepted as statistically significant. SPSS software (IBM SPSS Statistics for Windows, version 25, IBM corp., Armonk, New York, United Sates, 2017) was employed for all statistical analyses.
Results
A total of 49 patients met the inclusion criteria and agreed to participate in the study. The questionnaire response rate was 85% at 2 months following surgery and 53% at 4 to 6 months following surgery. The mean age of the cohort was 48.37 ± 16.96 years, and 23 (46.9%) were males. The patients' demographic data are shown in Table 1 . Twenty (40.8%) patients complained of preoperative visual impairments, and 21 (42.9%) complained of headaches. Fourteen (28.6%) patients had microadenomas (< 1 cm), 16 (32.7%) had macroadenomas, and 18 (36.7%) patients had huge adenomas (> 2.5 cm) according to their preoperative MRIs. Twelve (24.5%) surgeries were revision surgeries. Seventeen (34.7%) patients had intraoperative CSF leak for which they were treated intraoperatively. An NSF was used for reconstruction in 20 (40.8%) patients. Only three (6.1%) patients experienced major postoperative complications (meningitis [4.1%], major bleeding [2%]). Eight (16.3%) patients had transient diabetes insipidus (DI) during their postoperative course. On final pathology, eight (16.3%) patients had growth hormone-secreting tumors, 14 (28.6%) patients had adrenocorticotropic hormone-secreting tumors, 19 (38.8%) patients had nonfunctional tumors, and the remaining eight (16.3%) had prolactinomas or other secreting tumors ( Table 1 ).
Table 1. Demographic characteristics intraoperative and postoperative characteristics of the study population.
| Parameter | N |
|---|---|
| Age | 48.37 ± 16.96 ( N = 49) |
| Female sex ( N = 49) | 23 (46.9%) |
| Comorbidities ( N = 49) | 28 (57.1%) |
| Preoperative visual impairments ( N = 49) | 20 (40.8%) |
| Preoperative complaints of headache ( N = 49) | 21 (42.9%) |
| Preoperative endocrine impairment ( N = 49) | |
| Without medical treatment | 25 (51%) |
| With medical treatment | 11 (22.6%) |
| Revision surgery ( N = 47) | 12 (24.5%) |
| Intraoperative CSF leak ( N = 49) | 17 (34.7%) |
| NSF reconstruction ( N = 49) | 20 (40.8%) |
| Major postoperative complications ( N = 49) | 3 (6.1%) |
| Postoperative visual impairment ( N = 49) | 8 (16.3%) |
| Postoperative complaints of headache ( N = 49) | 13 (26.5%) |
| Postoperative endocrine impairment ( N = 49) | |
| Without medical treatment | 8 (16.3%) |
| With medical treatment | 26 (53.1%) |
| Postoperative DI ( N = 49) | 6 (12.2%) |
| Transient DI ( N = 49) | 8 (16.3%) |
| Final pathology ( N = 49) | |
| GH secreting (acromegaly) | 8 (16.3%) |
| Cortisol secreting (Cushing) | 14 (28.6%) |
| Nonfunctional or Rathke pouch | 19 (38.8%) |
| Prolactinoma or other secreting tumors | 8 (16.3%) |
| Tumor size ( N = 48) | |
| Microadenoma | 14 (28.6%) |
| Macroadenoma | 16 (32.7%) |
| Huge adenoma (>2.5 cm) | 18 (36.7%) |
| Endocrine biochemical remission in secreting tumors ( N = 22) | 18 (81.8%) |
| GTR in non-secreting tumors ( N = 27) | 19 (70.4%) |
Abbreviations: CSF, cerebrospinal fluids; DI, diabetes insipidus; GH, growth hormone; GTR, gross tumor resection; NSF, nasoseptal flap.
Sinonasal-Related QOL Outcomes
No statistical difference was found in SNOT-22 scores before or after surgery at all tested time points. The mean SNOT-22 score was 18.85 ± 15.34 preoperatively, decreasing to 16.14 ± 16.04 at 2 months postoperatively, and increasing to 19.94 ± 17.68 at 4 to 6 months postoperatively. The mean difference was −1.00 ± 13.85 at 2 months post-surgery, and 2.45 ± 14.54 for 4 to 6 months post-surgery, both less than the minimal clinically important differences (MCID) value of SNOT-22 questionnaire 18 ( Table 2 , Fig. 1 ).
Table 2. Overall quality of life of the study cohort ( N = 49) preoperatively, and 2 and 4–6 mo after surgery .
| Preoperative ( N = 49) | 2-mo postoperative ( N = 42) | p -Value | 4–6-mo postoperative ( N = 26) | p -Value | |
|---|---|---|---|---|---|
| SNOT-22 | 18.85 ± 15.34 | 16.14 ± 16.04 | 0.29 | 19.94 ± 17.68 | 0.39 |
| Overall ASBS-Q | 4.20 ± 0.65 | 4.39 ± 0.62 | 0.15 | 4.46 ± 0.48 | 0.038 |
| Emotional state | 3.87 ± 1.03 | 4.41 ± 0.83 | 0.007 | 4.22 ± 0.94 | 0.051 |
| Specific symptoms | 4.35 ± 0.56 | 4.43 ± 0.67 | 0.35 | 4.35 ± 0.64 | 0.81 |
| Pain | 4.08 ± 1.04 | 4.27 ± 0.82 | 0.19 | 4.50 ± 0.76 | 0.016 |
| Vitality | 4.16 ± 0.92 | 4.40 ± 0.81 | 0.24 | 4.43 ± 0.74 | 0.045 |
| Physical function | 4.47 ± 0.67 | 4.54 ± 0.63 | 0.66 | 4.71 ± 0.37 | 0.61 |
| Role of performance | 4.20 ± 0.78 | 4.38 ± 0.68 | 0.19 | 4.45 ± 0.47 | 0.26 |
Abbreviations: ASBS-Q, Anterior Skull Base Disease-Specific QOL; SNOT-22, Sinonasal Outcome Test 22.
Note: Bold indicates significant difference in comparison to the preoperative score ( p > 0.05), italic indicates borderline significance.
Fig. 1.
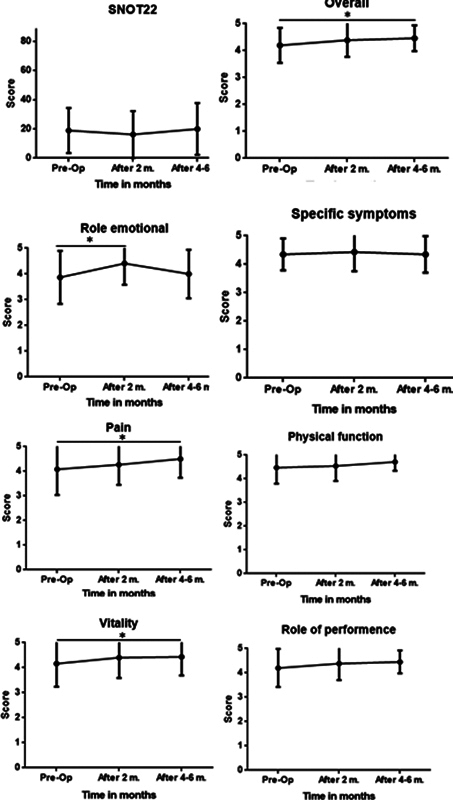
Estimates of sinonasal- and tumor-related quality of life scores before and after endoscopic pituitary adenoma tumor resection.
Anterior Skull Base Disease-Specific Questionnaire QOL Outcomes
The impact of surgery on the various aspects of QOL is summarized in Table 2 . Patients reported a significant improvement in the overall QOL score at 4 to 6 months postoperatively (from 4.20 ± 0.65 to 4.46 ± 0.48, p < 0.05), as opposed to the SNOT-22 scores mentioned above ( Table 2 , Fig. 1 ). Two domains of the questionnaire specifically contributed to the improved overall QOL score at 4 to 6 months postoperatively: the “pain-related” domain (subscale average score of 4.50 ± 0.76 vs. 4.08 ± 1.04, p < 0.05) and the “vitality-related” domain (average score of 4.43 ± 0.74 vs. 4.16 ± 0.92, p < 0.05). The scores of the specific symptom domain did not change post-surgery (4.35 ± 0.56 vs. 4.35 ± 0.64, p = NS). In other domains, there was a trend of QOL scores improvement although not significant, but it may result in a positive effect on the overall QOL scores. Interestingly, comparing the QOL scores at 2 months postoperatively to the preoperative QOL scores, a significant improvement was observed solely in the “emotional state impact” domain scores (average score of 4.41 ± 0.83 vs. 3.87 ± 1.03, p < 0.05).
Subgroup Analysis
To detect possible predictors of a poor QOL, we compared different subgroup populations: secreting versus non-secreting tumors, tumor size, and patients with NSF reconstruction ( Table 3 ) and found no correlation between any of those factors and poor QOL outcome. We then compared the subgroup of patients with secreting tumors who achieved endocrine remission to those who did not and, again, there were no significant differences in these subgroups ( Table 4 ). We did not observe any significant difference between patients with non-secreting tumors who achieved GTR to those who did not ( Table 4 ). Moreover, there was no significant difference in QOL when we combined the groups of non-secreting tumors with GTR to those with secreting tumors with endocrine biochemical remission to a “surgical success” group and compared it to the patients who did not achieve surgical “success,” ( Table 4 ). When we analyzed the QOL scores according to CSF leak intensity (no CSF leak, intraoperative low flow CSF leak, and intraoperative high flow CSF leak), we did not find any effect on QOL scores over time ( Table 5 ).
Table 3. Analysis of overall quality of life and domains among different subgroups of secreting vs. non-secreting tumors; microadenomas and macroadenomas vs. huge adenomas; with and without nasoseptal flap reconstruction.
| SNOT-22 | Overall ASBS-Q score | Emotional state | Specific symptoms | Pain | Vitality | Physical function | Role of performance | ||
|---|---|---|---|---|---|---|---|---|---|
| Non-secreting tumors ( N = 27) | Preop ( N = 27) | 17.12 ± 15.92 | 4.33 ± 0.62 | 4.07 ± 1.02 | 4.41 ± 0.55 | 4.21 ± 0.90 | 4.37 ± 0.69 | 4.51 ± 0.65 | 4.23 ± 0.80 |
| After 2 mo ( N = 25) | 17.72 ± 16.63 | 4.45 ± 0.60 | 4.37 ± 0.77 | 4.35 ± 0.73 | 4.20 ± 0.95 | 4.52 ± 0.64 | 4.59 ± 0.61 | 4.46 ± 0.61 | |
| After 4–6 mo ( N = 13) | 14.63 ± 8.89 | 4.55 ± 0.42 | 4.40 ± 0.72 | 4.43 ± 0.55 | 4.62 ± 0.68 | 4.73 ± 0.30 | 4.63 ± 0.45 | 4.46 ± 0.55 | |
| Secreting tumors ( N = 22) | Preop ( N = 22) | 20.90 ± 14.74 | 4.12 ± 0.68 | 3.63 ± 1.03 | 4.29 ± 0.59 | 3.94 ± 1.21 | 3.91 ± 1.12 | 4.44 ± 0.71 | 4.17 ± 0.78 |
| After 2 mo ( N = 19) | 13.69 ± 15.27 | 4.33 ± 0.68 | 4.23 ± 0.95 | 4.33 ± 0.59 | 4.39 ± 0.62 | 4.23 ± 1.02 | 4.47 ± 0.70 | 4.28 ± 0.80 | |
| After 4–6 mo ( N = 13) | 24.50 ± 22.04 | 4.40 ± 0.55 | 4.03 ± 1.13 | 4.28 ± 0.74 | 4.38 ± 0.85 | 4.14 ± 0.94 | 4.80 ± 0.28 | 4.44 ± 0.41 | |
| Microadenomas and macroadenomas ( N = 30) | Preop ( n = 30) | 19.29 ± 15.50 | 4.20 ± 0.68 | 3.83 ± 1.04 | 4.38 ± 0.59 | 3.98 ± 1.11 | 4.08 ± 1.05 | 4.43 ± 0.67 | 4.20 ± 0.76 |
| After 2 mo ( n = 26) | 14.00 ± 14.50 | 4.46 ± 0.59 | 4.43 ± 0.80 | 4.43 ± 0.56 | 4.41 ± 0.60 | 4.48 ± 0.86 | 4.55 ± 0.68 | 4.41 ± 0.70 | |
| After 4–6 mo ( n = 15) | 20.13 ± 21.01 | 4.44 ± 0.57 | 4.13 ± 1.12 | 4.43 ± 0.73 | 4.36 ± 0.88 | 4.20 ± 0.93 | 4.79 ± 0.28 | 4.48 ± 0.45 | |
| Huge adenomas ( N = 18) | Preop ( n = 18) | 18.12 ± 15.99 | 4.27 ± 0.64 | 3.89 ± 1.08 | 4.31 ± 0.55 | 4.22 ± 0.94 | 4.30 ± 0.70 | 4.53 ± 0.69 | 4.22 ± 0.85 |
| After 2 mo ( n = 16) | 19.18 ± 18.01 | 4.34 ± 0.69 | 4.13 ± 0.88 | 4.21 ± 0.82 | 4.06 ± 1.09 | 4.28 ± 0.74 | 4.54 ± 0.59 | 4.54 ± 0.59 | |
| After 4–6 mo ( n = 7) | 16.75 ± 8.77 | 4.49 ± 0.40 | 4.35 ± 0.68 | 4.31 ± 0.56 | 4.64 ± 0.60 | 4.68 ± 0.30 | 4.58 ± 0.46 | 4.41 ± 0.54 | |
| No NSF reconstruction ( N = 29) | Preop ( n = 29) | 17.36 ± 13.68 | 4.31 ± 0.55 | 3.94 ± 0.98 | 4.38 ± 0.52 | 4.13 ± 0.98 | 4.30 ± 0.65 | 4.53 ± 0.63 | 4.31 ± 0.59 |
| After 2 mo ( n = 27) | 12.92 ± 14.26 | 4.43 ± 0.53 | 4.38 ± 0.74 | 4.38 ± 0.52 | 4.20 ± 0.93 | 4.53 ± 0.51 | 4.56 ± 0.60 | 4.41 ± 0.61 | |
| After 4–6 mo ( n = 10) | 20.73 ± 18.40 | 4.45 ± 0.55 | 3.96 ± 1.29 | 4.42 ± 0.51 | 4.30 ± 0.96 | 4.33 ± 0.97 | 4.82 ± 0.19 | 4.49 ± 0.46 | |
| With NSF reconstruction ( N = 20) | Preop ( n = 20) | 21.17 ± 17.80 | 4.13 ± 0.78 | 3.78 ± 1.14 | 4.32 ± 0.64 | 4.03 ± 1.16 | 3.96 ± 1.21 | 4.40 ± 0.73 | 4.05 ± 1.00 |
| After 2 mo ( n = 15) | 21.73 ± 17.88 | 4.33 ± 0.78 | 4.20 ± 1.00 | 4.28 ± 0.90 | 4.42 ± 0.61 | 4.18 ± 1.17 | 4.53 ± 0.72 | 4.34 ± 0.83 | |
| After 4–6 mo ( n = 13) | 19.37 ± 17.77 | 4.48 ± 0.46 | 4.38 ± 0.68 | 4.32 ± 0.73 | 4.63 ± 0.61 | 4.50 ± 0.59 | 4.64 ± 0.45 | 4.42 ± 0.50 |
Abbreviations: SNOT-22, Sinonasal Outcome Test 22; NSF, nasoseptal flap.
Table 4. Subgroup analysis of overall quality of life and domains among patients with secreting tumors who achieved endocrine remission vs. those who did not ( N = 22); non-secreting tumors who achieved gross tumor resection vs. those who did not ( N = 27) and combined group of patients with non-secreting tumors who achieved gross tumor resection and patients with secreting tumors who achieved endocrine remission vs. those who did not ( N = 49) .
| SNOT-22 | Overall ASBS-Q score | Emotional state | Specific symptoms | Pain | Vitality | Physical function | Role of performance | ||
|---|---|---|---|---|---|---|---|---|---|
| No endocrine remission ( n = 4) | Preop ( n = 4) | 21.25 ± 12.92 | 4.48 ± 0.32 | 4.35 ± 0.44 | 4.58 ± 0.29 | 4.25 ± 0.5 | 4.58 ± 0.35 | 4.54 ± 0.54 | 4.36 ± 0.35 |
| After 2 mo ( n = 3) | 24.67 ± 19.35 | 4.09 ± 0.55 | 4.13 ± 0.81 | 4.0 ± 0.50 | 3.78 ± 0.38 | 4.38 ± 0.40 | 4.21 ± 0.76 | 3.84 ± 0.61 | |
| After 4–6 mo ( n = 2) | 24.5 ± 34.65 | 4.7 ± 0.14 | 5 ± 0 | 4.42 ± 0.82 | 4.67 ± 0.47 | 4.9 ± 0.14 | 4.93 ± 0.1 | 4.56 ± 0.09 | |
| With endocrine remission ( n = 18) | Preop ( n = 18) | 20.82 ± 15.50 | 4.04 ± 0.72 | 3.47 ± 1.06 | 4.22 ± 0.63 | 3.87 ± 1.31 | 3.76 ± 1.18 | 4.42 ± 0.75 | 4.13 ± 0.85 |
| After 2 mo ( n = 14) | 11.15 ± 13.86 | 4.38 ± 0.71 | 4.25 ± 1.0 | 4.4 ± 0.60 | 4.52 ± 0.58 | 4.2 ± 1.11 | 4.53 ± 0.70 | 4.37 ± 0.82 | |
| After 4–6 mo ( n = 12) | 24.5 ± 21.57 | 4.34 ± 0.58 | 3.95 ± 1.15 | 4.26 ± 0.77 | 4.33 ± 0.91 | 4 ± 0.95 | 4.77 ± 0.3 | 4.41 ± 0.45 | |
| No GTR ( n = 8) | Preop ( n = 8) | 15.43 ± 15.41 | 4.3 ± 0.72 | 4.01 ± 1.26 | 4.35 ± 0.46 | 4.04 ± 1.09 | 4.6 ± 0.54 | 4.39 ± 0.80 | 4.14 ± 1.11 |
| After 2 mo ( n = 7) | 22 ± 18.92 | 4.29 ± 0.744 | 4.0 ± 1.04 | 4.38 ± 0.79 | 3.80 ± 1.38 | 4.51 ± 0.68 | 4.42 ± 0.61 | 4.3 ± 0.72 | |
| After 4–6 mo ( n = 3) | 18.83 ± 10.42 | 4.41 ± 0.43 | 4.38 ± 0.51 | 4.41 ± 0.25 | 4.33 ± 1.15 | 4.75 ± 0.13 | 4.33 ± 0.81 | 4.18 ± 0.80 | |
| With GTR ( n = 19) | Preop ( n = 19) | 17.78 ± 16.5 | 4.33 ± 0.59 | 4.09 ± 0.94 | 4.43 ± 0.59 | 4.28 ± 0.28 | 4.27 ± 0.73 | 4.55 ± 0.58 | 4.27 ± 0.66 |
| After 2 mo ( n = 18) | 16.05 ± 15.92 | 4.5 ± 0.54 | 4.51 ± 0.60 | 4.34 ± 0.73 | 4.35 ± 0.71 | 4.52 ± 0.55 | 4.66 ± 0.60 | 4.51 ± 0.56 | |
| After 4–6 mo ( n = 10) | 13.22 ± 8.52 | 4.58 ± 0.42 | 4.41 ± 0.79 | 4.43 ± 0.62 | 4.7 ± 0.53 | 4.71 ± 0.34 | 4.71 ± 0.28 | 4.54 ± 0.47 | |
| No success ( n = 12) | Preop ( n = 12) | 17.55 ± 14.19 | 4.36 ± 0.61 | 4.13 ± 1.05 | 4.43 ± 0.42 | 4.11 ± 0.91 | 4.59 ± 0.47 | 4.44 ± 0.71 | 4.22 ± 0.91 |
| After 2 mo ( n = 10) | 22.80 ± 17.99 | 4.23 ± 0.67 | 4.04 ± 0.94 | 4.27 ± 0.72 | 3.80 ± 1.15 | 4.48 ± 0.50 | 4.37 ± 0.63 | 4.16 ± 0.70 | |
| After 4–6 mo ( n =) | 21.10 ± 19.08 | 4.53 ± 0.35 | 4.54 ± 0.52 | 4.42 ± 0.45 | 4.47 ± 0.87 | 4.81 ± 0.14 | 4.57 ± 0.66 | 4.34 ± 0.61 | |
| Success ( n = 37) | Preop ( n = 37) | 19.26 ± 15.86 | 4.19 ± 0.67 | 3.79 ± 1.04 | 4.33 ± 0.61 | 4.08 ± 1.10 | 4.02 ± 0.99 | 4.49 ± 0.67 | 4.20 ± 0.75 |
| After 2 mo ( n = 32) | 14 ± 15.06 | 4.45 ± 0.61 | 4.40 ± 0.80 | 4.37 ± 0.67 | 4.43 ± 0.65 | 4.38 ± 0.90 | 4.61 ± 0.64 | 4.45 ± 0.68 | |
| After 4–6 mo ( n =) | 19.67 ± 17.82 | 4.45 ± 0.52 | 4.17 ± 1.00 | 4.34 ± 0.69 | 4.51 ± 0.76 | 4.34 ± 0.80 | 4.75 ± 0.29 | 4.48 ± 0.45 |
Abbreviations: CSF, cerebrospinal fluids; GTR, gross tumor resection; NSF, nasoseptal flap; SNOT-22, Sinonasal Outcome Test 22.
Table 5. Analysis of overall quality of life and domains among different subgroups of: no CSF leak, low flow CSF leak, high flow CSF leak.
| SNOT-22 | Overall ASBS-Q score | ||
|---|---|---|---|
| No CSF leak ( N = 32) | Preop ( N = 32) | 19.19 ± 15.87 | 4.22 ± 0.66 |
| After 2 mo ( N = 28) | 14.71 ± 15.75 | 4.46 ± 0.58 | |
| After 4–6 mo ( N = 16) | 24.06 ± 20.07 | 4.34 ± 0.54 | |
| Low flow CSF leak ( N = 12) | Preop ( N = 12) | 24.60 ± 13.46 | 4.07 ± 0.69 |
| After 2 mo ( N = 10) | 22.77 ± 17.58 | 4.07 ± 0.71 | |
| After 4–6 mo ( N = 7) | 14.50 ± 9.83 | 4.59 ± 0.38 | |
| High flow CSF leak ( N = 5) | Preop ( N = 5) | 5.2 ± 5.97 | 4.68 ± 0.24 |
| After 2 mo ( N = 4) | 11.25 ± 13.89 | 4.92 ± 0.10 | |
| After 4–6 mo ( N = 4) | 10.67 ± 15.04 | 4.78 ± 0.27 |
Abbreviations: ASBS-Q, Anterior Skull Base Disease-Specific QOL; CSF, cerebrospinal fluids; preop, preoperative; SNOT-22, Sinonasal Outcome Test 22.
Discussion
The effectiveness of endonasal surgical techniques for the extirpation of pituitary tumors has been established over the past two decades. The endonasal approach, however, has unique associated morbidities related to the formation of the endonasal corridor and the various reconstruction techniques, such as the use of NSF, which causes injury to healthy nasal tissues and may therefore affect sinonasal-related QOL. Interestingly, literature on the impact of pituitary surgery on a patient's QOL is sparse, and the objective of this study was therefore to assess and reveal the impact of endonasal endoscopic approach to pituitary lesions on the patients' QOL.
Since the currently available instruments (e.g., the SF-36) for estimating QOL are not designed for endonasal pituitary surgeries, we used two questionnaires: the SNOT-22 validated Hebrew version, which is routinely used to assess sinonasal-related QOL in patients undergoing endoscopic sinus surgery, and the ASBS-Q, a disease-specific instrument validated in patients who had undergone endonasal skull base tumor resection. These two questionnaires are complementary in evaluating all QOL-related aspects in patients undergoing endonasal pituitary surgery.
Our study included 49 patients who underwent endoscopic transsphenoidal surgery for pituitary lesions between November 2014 and December 2017. The principal result of this prospective study is that the endoscopic approach preserves the patient's QOL. Furthermore, most of the patients reported improvement in their QOL at 4 to 6 months after surgery, as demonstrated in the significantly improved overall ASBS-Q scores assessed at that time point. Although below MCID value for ASBS-Q (> 0.4), 5 it indicates a positive trend of preservation of good QOL among patients undergoing EEA for benign pituitary lesions. Two of the questionnaire domains, “pain” and “vitality” showed significant improvement, which contributed to the overall improved QOL scores. On the other hand, the SNOT-22 scores did not alter significantly during the full extent of the follow-up period: we interpret this as a meaning that the endonasal procedure did not result in QOL improvement or deterioration related to chronic sinonasal conditions. That finding may be due to the fact that most of our study patients had not sustained chronic sinus disease to begin with.
Interestingly, QOL improvement was also attributed to a significant score improvement of the ASBS-Q “pain” domain 6 months post-surgery. This may reflect the reported improvement of headaches after surgery ( Table 1 ). The prevalence of headaches in pituitary gland tumors is variable, and may reach up to 70% of the patients, 19 20 21 and is particularly frequent in prolactinomas, 19 which were 16% of our cohort. The presence of headache in pituitary tumor is related to a combination of many factors, such as tumor extension, relationship with the sellar structures, etc. 20
Middle turbinectomy among other intranasal surgical interventions, may cause olfaction loss, 22 that may worsen QOL. However, the data regarding anosmia and parosmia is contradicting. 23 24 25 The ASBS-specific symptoms domain includes items regarding the sense of smell, which did not deteriorate significantly among our cohort. The latter is interesting in the context of the conflicting data regarding the effect of middle turbinectomy on QOL. Soler et al addressed this topic in a multicenter study on patients with chronic sinusitis and polyposis; their investigation found no difference in QOL outcomes in patients with bilateral middle turbinectomy preservation versus resection. 26 Another research by Delarestaghi et al, suggested that the addition of partial middle-turbinectomy in endoscopic sinus surgery improves QOL of patients. 27 de Carvalho et al evaluated olfactory loss in patients who underwent expanded endoscopic approach with partial middle turbinectomy and NSF reconstruction and they found that the deterioration in the sense of smell was transient up to 3 months. 28
Several studies utilized the SNOT-22 to evaluate QOL in patients operated for pituitary lesions. Pant et al examined the trend over time of postoperative SNOT-22 scores in patients after endonasal anterior skull base tumor extirpation ( n = 51), including pituitary gland lesions and reported a significant improvement in the SNOT-22 scores. The best postoperative SNOT-22 score was achieved by 6 to 12 months postoperatively. 13 Similar to our study, patients with pituitary gland lesions reported better SNOT-22 scores in comparison with other anterior skull base patients in the study population. However, Pant et al did not obtain the preoperative SNOT-22 scores, and therefore could not assess the direct effect of surgery on the patients' endonasal related-QOL. McCoul et al assessed the QOL of 81 adult patients who underwent endoscopic endonasal resection of pituitary adenomas and reported results similar to ours, with postoperative SNOT-22 scores remaining insignificantly changed in comparison with preoperative levels. 14 Alobid et al used the rhinosinusitis outcome measure (RSOM) to measure QOL in 69 patients after pituitary tumor resection, and their results revealed an impairment in QOL scores related to headache and smell at 3 months following surgery. Among our cohort, the sense of smell slightly decreased (5 ± 0.81 preoperatively vs. 4 ± 1.03 4 to 6 months postop, p = NS). 15 Similar to our results, they found that the overall QOL was preserved after the surgery. Interestingly, their patients who underwent an expanded endoscopic approach to the anterior skull base (which required flap reconstruction) reported more nasal-related complaints (smell loss and posterior nasal discharge). In this context, it is important to stress that the SNOT-22 questionnaire was developed for evaluating chronic sinusitis patients. Our clinical impression is that it may not adequately address pituitary tumor resection parameters (as does the RSOM questionnaire used for the abovementioned study). To overcome this shortcoming, we used the ASBS-Q questionnaire, which is a validated specific QOL questionnaire designed for patients with skull base tumors, in conjunction with SNOT-22. The analysis of ASBS-Q scores did not detect any significant predictors for poor QOL in our study. A previous multicenter study by Little et al, 29 used the Anterior Skull Base Nasal Inventory-12 to examine the effect of endoscopic endonasal surgery for pituitary lesions on patient-reported sinonasal QOL. They demonstrated a decrease in general and nasal QOL 2 weeks after surgery, which improved to presurgery level at 3- and 6-month post surgery. These findings correspond with our results that reported sinonasal QOL is back to preoperative surgery at 2 months. This may reflect that the sinonasal healing process is rapid and generally very good. We compared subgroups of patients who differed in factors related to the tumor itself and to the extent of surgery, such as tumor size, revision surgery, NSF reconstruction, intraoperative CSF leak, tumor characteristics (secreting or non-secreting tumor type), extent of resection, and endocrine remission. However, none of the above variable s affected the patients' QOL ( Table 4 ).
According to our findings, QOL improvement was related mostly to the scores of two subdomains of “vitality” and “pain.” McCoul et al applied the ASBS-Q in pituitary surgery and also observed an improved overall postoperative disease-specific QOL. 17 They found a similar significant improvement after 3 months in both the “vitality” and “pain” domains. Unlike our results, they also detected significant improvement in the “physical function,” “emotional impact,” and “performance” domains. Their results demonstrated that partial resection correlated with worse QOL, both overall and among patients with hypersecreting tumors. 14 Extrasellar tumor extension, intraoperative CSF leakage, and reconstruction technique during surgery did not impact postoperative QOL in either their study or ours. Improvement in QOL was independent of the occurrence of endocrine remission.
Pant et al used the ASBS-Q questionnaire and reported that endoscopic surgery for anterior skull base lesions, including pituitary adenomas, did not lower patients' QOL postoperatively. Similar to our results, the overall postoperative QOL was very good. 13 Their results showed that 75% of their patients had a mean score of 4.0/5.0 or higher for the overall QOL and by 1 to 3 months postoperatively, and comparable scores for all domains except for those associated with “emotion” and “specific symptoms.” 13 Interestingly, our study showed the opposite for the “emotional impact” domain: our patients demonstrated significant improvement in short-term (2 months) after the surgery compared with their initial preoperative scores. This difference may be attributed to the fact that Pant et al did not assess the initial preoperative scores but rather only the postoperative ones.
Our patients reported a significant improvement in the “emotional impact” domain at 2 months postoperatively. This improvement may be attributed to the relief of having undergone the operation. This improvement was also noted in a prospective study conducted by our team which studied QOL using the ASBS-Q in patients undergoing extirpation of skull base tumors. 6 In that study, patients with benign lesions reported a significant improvement in the “emotional impact” domain both at 6 months and 12 months postoperatively.
Strengths and Weaknesses
Our study has several limitations that bear mention. First, it was performed in a single tertiary referral center, raising the potential for selection bias. Second, smaller sample sizes were obtained for later time points during follow-up/data on certain potential confounding factors, such as socioeconomic status and educational level, were not collected and therefore not controlled for in this analysis. Sinonasal morbidity, as reflected by the SNOT-22 and the “specific symptoms” domain, may have been adversely affected by the inclusion of partial middle turbinectomy in all cases. Its strengths lie in the prospective nature and the compatible and meticulous tumor-related assessment of QOL via compatible questionnaire tailored for skull base tumors.
Conclusion
We found that the endoscopic endonasal approach for pituitary lesions in not associated with a negative impact on the patients' QOL. Rather, our patients' overall QOL at 4 to 6 months postoperatively improved, with specific significant improvement in the domains of “pain” and “vitality” at 3 to 6 months postoperatively.
Footnotes
Conflict of Interest None declared.
References
- 1.Crespo I, Santos A, Resmini E, Valassi E, Martínez-Momblán M A, Webb S M. Improving quality of life in patients with pituitary tumours. Eur Endocrinol. 2013;9(01):32–36. doi: 10.17925/EE.2013.09.01.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohsenipour I, Deusch E, Gabl M, Hofer M, Twerdy K. Quality of life in patients after meningioma resection. Acta Neurochir (Wien) 2001;143(06):547–553. doi: 10.1007/s007010170059. [DOI] [PubMed] [Google Scholar]
- 3.Kelleher M O, Fernandes M F, Sim D W, O'Sullivan M G. Health-related quality of life in patients with skull base tumours. Br J Neurosurg. 2002;16(01):16–20. doi: 10.1080/02688690120114183. [DOI] [PubMed] [Google Scholar]
- 4.Abergel A, Fliss D M, Margalit N, Gil Z. A prospective evaluation of short-term health-related quality of life in patients undergoing anterior skull base surgery. Skull Base. 2010;20(01):27–33. doi: 10.1055/s-0029-1242982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amit M, Abergel A, Fliss D M, Gil Z. The clinical importance of quality-of-life scores in patients with skull base tumors: a meta-analysis and review of the literature. Curr Oncol Rep. 2012;14(02):175–181. doi: 10.1007/s11912-012-0222-3. [DOI] [PubMed] [Google Scholar]
- 6.Gil Z, Abergel A, Spektor S et al. Quality of life following surgery for anterior skull base tumors. Arch Otolaryngol Head Neck Surg. 2003;129(12):1303–1309. doi: 10.1001/archotol.129.12.1303. [DOI] [PubMed] [Google Scholar]
- 7.Cavel O, Abergel A, Margalit N, Fliss D M, Gil Z. Quality of life following endoscopic resection of skull base tumors. J Neurol Surg B Skull Base. 2012;73(02):112–116. doi: 10.1055/s-0032-1301392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gil Z, Abergel A, Spektor S, Khafif A, Fliss D M. Patient, caregiver, and surgeon perceptions of quality of life following anterior skull base surgery. Arch Otolaryngol Head Neck Surg. 2004;130(11):1276–1281. doi: 10.1001/archotol.130.11.1276. [DOI] [PubMed] [Google Scholar]
- 9.Heald A H, Ghosh S, Bray S et al. Long-term negative impact on quality of life in patients with successfully treated Cushing's disease. Clin Endocrinol (Oxf) 2004;61(04):458–465. doi: 10.1111/j.1365-2265.2004.02118.x. [DOI] [PubMed] [Google Scholar]
- 10.van Beek A P, van den Bergh A CM, van den Berg L M et al. Radiotherapy is not associated with reduced quality of life and cognitive function in patients treated for nonfunctioning pituitary adenoma. Int J Radiat Oncol Biol Phys. 2007;68(04):986–991. doi: 10.1016/j.ijrobp.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 11.Kuan E C, Yoo F, Chyu J, Oh A, Bergsneider M, Wang M B. Quality of Life before and after endoscopic pituitary surgery as measured by the Short-Form-36. J Neurol Surg B Skull Base. 2018;79(03):314–318. doi: 10.1055/s-0037-1608648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weldring T, Smith S MS. Patient-Reported Outcomes (PROs) and Patient-Reported Outcome Measures (PROMs) Health Serv Insights. 2013;6:61–68. doi: 10.4137/HSI.S11093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pant H, Bhatki A M, Snyderman C H et al. Quality of life following endonasal skull base surgery. Skull Base. 2010;20(01):35–40. doi: 10.1055/s-0029-1242983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCoul E D, Bedrosian J C, Akselrod O, Anand V K, Schwartz T H. Preservation of multidimensional quality of life after endoscopic pituitary adenoma resection. J Neurosurg. 2015;123(03):813–820. doi: 10.3171/2014.11.JNS14559. [DOI] [PubMed] [Google Scholar]
- 15.Alobid I, Enseñat J, Mariño-Sánchez F et al. Expanded endonasal approach using vascularized septal flap reconstruction for skull base tumors has a negative impact on sinonasal symptoms and quality of life. Am J Rhinol Allergy. 2013;27(05):426–431. doi: 10.2500/ajra.2013.27.3932. [DOI] [PubMed] [Google Scholar]
- 16.Shapira Galitz Y, Halperin D, Bavnik Y, Warman M. Sino-nasal outcome test-22: translation, cross-cultural adaptation, and validation in Hebrew-speaking patients. Otolaryngol Head Neck Surg. 2016;154(05):951–956. doi: 10.1177/0194599816629378. [DOI] [PubMed] [Google Scholar]
- 17.Wengier A, Ram Z, Warshavsky A, Margalit N, Fliss D M, Abergel A. Endoscopic skull base reconstruction with the nasoseptal flap: complications and risk factors. Eur Arch Otorhinolaryngol. 2019;276(09):2491–2498. doi: 10.1007/s00405-019-05531-4. [DOI] [PubMed] [Google Scholar]
- 18.Phillips K M, Hoehle L P, Caradonna D S, Gray S T, Sedaghat A R. Minimal clinically important difference for the 22-item Sinonasal Outcome Test in medically managed patients with chronic rhinosinusitis. Clin Otolaryngol. 2018;43(05):1328–1334. doi: 10.1111/coa.13177. [DOI] [PubMed] [Google Scholar]
- 19.Levy M J, Jäger H R, Powell M, Matharu M S, Meeran K, Goadsby P J. Pituitary volume and headache: size is not everything. Arch Neurol. 2004;61(05):721–725. doi: 10.1001/archneur.61.5.721. [DOI] [PubMed] [Google Scholar]
- 20.Gondim J A, de Almeida J P, de Albuquerque L A, Schops M, Gomes E, Ferraz T. Headache associated with pituitary tumors. J Headache Pain. 2009;10(01):15–20. doi: 10.1007/s10194-008-0084-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levy M J, Matharu M, Goadsby P J. Chronic headache and pituitary tumors. Curr Pain Headache Rep. 2008;12(01):74–78. doi: 10.1007/s11916-008-0014-5. [DOI] [PubMed] [Google Scholar]
- 22.Leopold D A, Hummel T, Schwob J E, Hong S C, Knecht M, Kobal G.Anterior distribution of human olfactory epithelium Laryngoscope 2000110(3 Pt 1):417–421. [DOI] [PubMed] [Google Scholar]
- 23.Alam S, Li C, Bradburn K H, Zhao K, Lee T S. Impact of middle turbinectomy on airflow to the olfactory cleft: a computational fluid dynamics study. Am J Rhinol Allergy. 2019;33(03):263–268. doi: 10.1177/1945892418816841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mariano F C, Hamerschmidt R, Soares C MC, Moreira A T. The middle turbinate resection and its repercussion in olfaction with the University of Pennsylvania Smell Identification Test (UPSIT) Int Arch Otorhinolaryngol. 2018;22(03):280–283. doi: 10.1055/s-0037-1608679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akkoca Ö, Tüzüner A, Ünlü C E, Şimşek G, Kaytez S K, Uğurlu G A. Comparison of the effects of 2 surgical techniques used in the treatment of concha bullosa on olfactory functions. Ear Nose Throat J. 2020;99(07):437–441. doi: 10.1177/0145561319881061. [DOI] [PubMed] [Google Scholar]
- 26.Soler Z M, Hwang P H, Mace J, Smith T L. Outcomes after middle turbinate resection: revisiting a controversial topic. Laryngoscope. 2010;120(04):832–837. doi: 10.1002/lary.20812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Delarestaghi M M, Rajaeih S, Firouzabadi F D et al. Evaluation of the effect of endoscopic partial middle turbinectomy surgery on the quality of life of patients with chronic rhinosinusitis and nasal polyps. Rhinology. 2020;58(03):208–212. doi: 10.4193/Rhin19.258. [DOI] [PubMed] [Google Scholar]
- 28.de Carvalho A CM, Dolci R LL, Rickli J CKet al. Evaluation of olfactory function in patients undergoing endoscopic skull base surgery with nasoseptal flap Braz J Otorhinolaryngol 2020S1808-8694(20):30052–30055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Little A S, Kelly D F, Milligan J et al. Comparison of sinonasal quality of life and health status in patients undergoing microscopic and endoscopic transsphenoidal surgery for pituitary lesions: a prospective cohort study. J Neurosurg. 2015;123(03):799–807. doi: 10.3171/2014.10.JNS14921. [DOI] [PubMed] [Google Scholar]


