Summary
Background
An increasing proportion of colorectal cancers (CRCs) are detected through screening due to the availability of organised population-based programmes. We aimed to analyse survival probabilities of patients with screen-detected CRC in European countries.
Methods
Data from CRC patients were obtained from 16 population-based cancer registries in nine European countries. We included patients with cancer diagnosed from the year organised CRC screening programmes were introduced until the most recent year with available data at the time of analysis, whose ages at diagnosis fell into the age groups targeted by screening. Patients were followed up with regards to vital status until 2016-2020 across the various countries. Overall and CRC-specific survival were analysed by mode of detection and stage at diagnosis for all countries combined and for each country separately using the Kaplan-Meier method.
Findings
We included data from 228 134 patients, of whom 134 597 (aged 60-69 years at diagnosis targeted by screening in all countries) were considered in analyses for all countries combined. 22·3% (38 080/134 597) of patients had cancer detected through screening. Most screen-detected cancers were found at stages I-II (65·6% [12 772/19 469 included in stage-specific analyses]), while the majority of non-screen-detected cancers were found at stages III-IV (56·4% [31 882/56 543 included in stage-specific analyses]). Five-year overall and CRC-specific survival rates for patients with screen-detected cancer were 83·4% (95% CI 82·9-83·9) and 89·2% (88·8-89·7), respectively; for patients with non-screen-detected cancer, they were much lower (57·5% [57·2-57·8] and 65·7% [65·4-66·1], respectively). The favourable survival of patients with screen-detected cancer was also seen within each stage – five-year overall survival rates for patients with screen-detected stage I, II, III, and IV cancers were 92.4% (95% CI 91·6-93·1), 87·9% (86·6-89·1), 80·7% (79·3-82·0), and 32·3 (29·4-35·2), respectively. These patterns were also consistently seen for each individual country.
Interpretation
Patients with cancer diagnosed at screening have a very favourable prognosis. In the rare case of detection of advanced stage cancer, survival probabilities are still much higher than those commonly reported for all patients regardless of mode of detection. Although these results cannot be taken to quantify screening effects, they provide useful and encouraging information for patients with screen-detected CRC and their physicians.
Funding
This study was supported in part by grants from the German Federal Ministry of Education and Research and the German Cancer Aid.
Keywords: Colorectal cancer, Survival, Screening, Europe
Research in context.
Evidence before this study
We searched in PubMed for articles reporting on survival of patients with screen-detected colorectal cancer (CRC) in European countries that were published up to January 2, 2022. We used the following search terms: “survival” AND (“colon cancer” OR “rectal cancer” OR “colorectal cancer”) AND “screen*” AND “Europe*”. Higher survival rates for patients with screen-detected cancer compared to patients with symptom-detected cancer have been reported in the context of pilot studies prior to introduction of population-based screening programmes and from a few regional and nationwide studies conducted during the first years of screening implementation. Given the increasing proportion of patients with cancer detected at screening, a comprehensive, up-to-date, Europe-wide survival analysis for this group of patients, especially by stage at diagnosis, is warranted.
Added value of this study
To the best of our knowledge, this is the first multi-country European study to provide detailed data on overall and CRC-specific survival probabilities of patients with screen-detected CRC, by stage at diagnosis.
Implications of all the available evidence
Although the data provided in this study cannot be taken to quantify screening effects, they can and should be used to inform patients, physicians, and the general population about the prognosis of patients with screen-detected CRC, who might otherwise feel discouraged by rather unfavourable estimates commonly available for all CRC patients irrespective of mode of detection. The data provided herein may further encourage the eligible population to make use of available screening options.
Alt-text: Unlabelled box
Introduction
Colorectal cancer (CRC) is the second most commonly diagnosed cancer and the second leading cause of cancer death in Europe, with nearly 520 000 new diagnoses and 245 000 related deaths in 2020.1 Five-year net survival has meanwhile reached levels above 60% in many European countries,2 with large variations by stage at diagnosis – from around 90% for patients diagnosed at stage I to just slightly over 10% for patients diagnosed with metastatic (stage IV) disease.3
Several CRC screening methods have been recommended for population-wide implementation, including faecal occult blood test (FOBT) (in particular faecal immunochemical test [FIT]), flexible sigmoidoscopy, and colonoscopy.4 In the past two decades, many European countries have launched programmes offering either one or multiple of these screening options,5 and an increasing proportion of CRC cases are detected by screening.6
Survival rates for screen-detected CRC patients are expected to be considerably higher than those commonly reported for all CRC patients combined due to the more favourable stage distribution of screen-detected cancers, but also within the same stage as a result of detection of less aggressive, more slowly progressing cancers.7 Furthermore, “lead time”, i.e. mere advancement of the time of diagnosis even if chances of cure are not increased, or overdiagnosis of cancers that would have never been detected in the absence of screening may additionally contribute to higher survival of patients with screen-detected cancer.
Although higher overall and stage-specific survival among patients with screen-detected CRC can therefore not be interpreted as reflecting screening benefits, it would still be most valuable for screen-detected CRC patients and their physicians to know about their survival probabilities to prevent them from being discouraged by overly pessimistic survival figures that are commonly available for all patients combined only, regardless of the mode of detection.2 The aim of this study was to provide overall and stage-specific survival rates for patients with screen- and non-screen-detected CRC in nine European countries with organised screening programmes.
Methods
Study design and data collection
In this longitudinal, international population-based study, data from CRC cases (ICD-10 codes C18-C20) were obtained from 16 population-based cancer registries in nine European countries (Belgium, Denmark, England, Ireland, the Netherlands, and Slovenia with nationwide data; and France, Italy, and Spain with regional data). Patients included in this analysis were diagnosed from the year organised CRC screening programmes were implemented up to the most recent year with available data at the time of analysis (up to 2014-2016 in most countries/regions), and were followed up with regards to vital status until December 2016–January 2020 across the various countries/regions (Table 1).
Table 1.
Numbers of colorectal cancer cases identified, excluded from, and included in the analysis.
| Country/region | Years of diagnosisa | Last follow-up date | Identified malignant casesb | Excluded cases |
Included cases (age group in years) | Cases included in stage-specific analysesd | ||
|---|---|---|---|---|---|---|---|---|
| Cases whose age at diagnosis was not in the age range of the population targeted by screening | Quality controlc | Cases with null survival | ||||||
| Belgiume | 2009-2016 | July 2018 | 66 051 | 31 779 | 0 | 26 | 34 246 (50-74) | 32 233 (94·1%) |
| Denmark | 2014-2018 | December 2018 | 22 579 | 8769 | 15 | 5 | 13 790 (50-74) | 11 167 (81·0%) |
| England | 2006-2015 | December 2018 | 335 991 | 202 870 | 169 | 1464 | 131 488 (60-74) | 48 241 (91·0%)f |
| France (5 regions) | - | - | 36 517 | 18 264 | 1 | 65 | 18 187 (50-74) | 17 569 (96·6%) |
| Burgundy | 2003-2016 | March 2019 | 10 295 | 5198 | 0 | 14 | 5083 (50-74) | 4949 (97·4%) |
| Calvados | 2004-2016 | March 2019 | 5160 | 2589 | 0 | 3 | 2568 (50-74) | 2458 (95·7%) |
| Doubs | 2008-2016 | March 2019 | 2773 | 1392 | 0 | 2 | 1379 (50-74) | 1336 (96·9%) |
| Finistere | 2004-2016 | April 2019 | 8469 | 4205 | 0 | 10 | 4254 (50-74) | 4112 (96·7%) |
| Isere | 2002-2016 | April 2019 | 9820 | 4880 | 1 | 36 | 4903 (50-74) | 4714 (96·1%) |
| Ireland | 2012-2016 | December 2018 | 12 848 | 9256 | 0 | 21 | 3571 (60-69) | 2857 (80·0%) |
| Italy (Turin) | 2003-2014 | December 2016g | 9523 | 7026 | 53 | 11 | 2433 (58-69) | -h |
| Netherlands | 2015i | February 2018 | 15 936 | 6469 | 0 | 6 | 9461 (60-75)j | 9324 (98·6%) |
| Slovenia | 2009-2015 | February 2019k | 10 513 | 5855 | 0 | 38 | 4620 (50-69) | 4387 (95·0%) |
| Spain (4 regions) | - | - | 25 081 | 14 695 | 11 | 37 | 10 338 (50-69) | 8594 (90·1%) |
| Basque Country | 2009-2015 | June 2019 | 14 974 | 8542 | 3 | 21 | 6413 (50-69) | 5237 (93·3%)l |
| Girona | 2013-2016 | December 2018 | 2280 | 1314 | 0 | 2 | 964 (50-69) | 870 (90·2%) |
| Murcia | 2006-2012 | January 2020 | 5866 | 3680 | 7 | 10 | 2169 (50-69) | 1970 (90·8%) |
| Tarragona | 2012-2014 | December 2016 | 1956 | 1159 | 1 | 4 | 792 (50-69) | 517 (65·3%) |
From the year screening was implemented up to the latest year with available data.
Malignant cases considered based on the international rules for reporting data on cancer incidence and survival – International Rules for Multiple primary cancers (ICD-O Third Edition). The exception was England which reports tumours with different morphology codes at the third digit level as multiple primary ones instead of using the IARC/IACR morphology groups.
Cases with negative survival, cases with missing, incomplete or inconsistent dates of diagnosis and follow-up/death, and cases with unknown data on sex and vital status were excluded.
Percentages shown in relation to the number of included cases.
In Belgium, screening was implemented on regional level. In Brussels and Wallonia, screening was introduced in 2009 and targeted individuals aged 50-74 years; in Flanders, it was introduced in 2013, was initially made available to those aged 66-74 years, and was gradually rolled out to include all individuals aged 56-74 years.
For England, data on stage were only available from 2012 onwards; therefore, patients diagnosed in 2006-2011 were not included in stage-specific analyses.
For Italy (Turin), the last follow-up date for disease-specific survival analyses was December 2015, as cause of death information was only available until that time point.
For Italy (Turin), data on stage were not available.
In the Netherlands, although the screening program started in 2014, data on mode of detection were only available for 2015.
In the Netherlands, although the screening program has been meanwhile extended to individuals aged 55-59, in 2015 only those whose age ranged from 60 to 75 years were invited.
For Slovenia, the last follow-up date for disease-specific survival analyses was December 2017, as cause of death information was only available until that time point.
For Spain (Basque Country), patients diagnosed in 2009 (>85% with unknown stage) were excluded from analyses of stage.
We collected the following patient- and tumour-level data: sex, age at diagnosis, date of diagnosis, mode of diagnosis (ie, screen- or non-screen-detected cancer), topography (ie, tumour site), tumour histology, stage at diagnosis (Union Internationale Contre le Cancer [UICC] TNM stage at the time of diagnosis), and date of and vital status at last contact (for Belgium, Denmark, England, Ireland, and the Netherlands, intervals in days between diagnosis and follow-up were provided instead of date of last contact). Cause of death information was also obtained from Denmark, England, Ireland, Italy (Turin), Slovenia, and Spain (Basque Country, Girona, and Tarragona). Data sources and relevant data quality indicators are provided in the appendix (pp 2-3).
Additionally, we summarised relevant characteristics of the organised screening programmes implemented in the included countries, notably screening test, year of programme initiation, target age group, screening interval, coverage, and participation (appendix pp 4-5). These data were obtained from Europe-, nation-, and region-wide screening reports (appendix pp 4-5, 21).
This study was approved by the Ethics Committee of the Medical Faculty of the University of Heidelberg (S-84/2019).
Statistical analyses
In this analysis, cases whose ages at diagnosis were not in the age range of the population targeted by screening, cases with missing data on sex, vital status, missing or inconsistent dates of diagnosis and follow-up/death (ie, date of last follow-up/death preceding date of diagnosis) or null survival (same date of diagnosis and death) were excluded (Table 1). Furthermore, cases with missing TNM staging data were excluded from stage-specific analyses. For England (years of diagnosis 2006-2011), Turin, Italy (all years), and the Basque Country, Spain (year 2009), TNM staging data were missing for more than 85% of the cases; therefore, these countries (years of diagnosis) were not considered in analyses of stage. Data were analysed for all countries combined and for each country individually. In analyses where data from all countries were pooled, only patients aged between 60 and 69 years at diagnosis were included, as this was the target group common to all screening programmes in the included countries (appendix pp 4-5).
First, we analysed demographic and tumour characteristics of CRC cases, namely sex, age at diagnosis, tumour location (proximal colon [caecum to transverse colon], distal colon [splenic flexure to sigmoid colon], rectum [rectosigmoid junction and rectum], and overlapping or unspecific location), and stage at diagnosis, according to mode of detection. Differences between screen- and non-screen-detected cases were analysed through chi-square test.
We subsequently assessed overall survival for screen-detected, non-screen-detected, and all CRC patients combined. Survival time was defined as the difference in days between the date of diagnosis and the date of death (deceased patients) or was censored at the date of last follow-up.
For England, Ireland, Italy (Turin), Slovenia, and Spain (Basque Country, Girona, and Tarragona), for which cause of death information was available, CRC-specific survival was also assessed. Survival time was censored at the date of death from causes other than CRC; and deceased cases with unknown cause of death were excluded (appendix p 1). CRC-specific survival analyses were not done for Denmark because cause of death information was missing for a large proportion of cases (34% of deceased patients with screen-detected cancer and 23% with non-screen-detected cancer).
Survival was estimated using the Kaplan-Meier method, and three- and five-year survival rates and 95% confidence intervals (CIs) were calculated – for all CRC cases and screen- and non-screen-detected cases separately – by sex, age at diagnosis, tumour location, and stage at diagnosis. Survival curves up to five years after diagnosis were plotted according to mode of detection and stage at diagnosis. For Denmark and the Netherlands, survival was only analysed up to four and three years after diagnosis, respectively, given the recent implementation of screening and lack of data for later follow-up times.
We abstained from statistically quantifying potential differences in survival between patients with screen- and non-screen-detected cancer (eg, through Cox proportional-hazards models), because this study was not conceived, and its results should not be used, to quantify screening effects. The purpose, instead, is to inform patients with screen-detected (and non-screen-detected) cancer about their survival probabilities, which may be very different from the ones that are commonly available to them (ie, for all patients combined regardless of mode of detection).
All analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC, USA). An alpha level of 0.05 was set for statistical tests.
Role of the funding source
The sponsor had no role in the study design, data collection, data analysis, interpretation of data, writing of the report, or the decision to submit the paper for publication.
Results
In total, we included 228 134 CRC cases, of whom 134 597 (aged 60-69 years at diagnosis targeted by screening in all countries) were considered in analyses for all countries combined (Tables 1 and 2). Demographic and tumour characteristics are shown for all patients regardless of mode of detection and separately for patients with screen- and non-screen-detected cancer in Table 2 (all countries combined) and appendix (pp 6-10) (each country separately). The majority of patients were male (62·0% [83 444/134 597]), had cancer in the distal colon or rectum (68·6% [92 396/134 597]), and had cancer detected outside of screening (77·7% [104 517/134 597]); about half of the cancers were diagnosed at advanced stages III or IV (50·8% [38 579/76 012 included in stage-specific analyses]) (Table 2). Across countries, we observed similar proportions of male/female patients to that seen for all countries combined, however, the distribution of cancers by subsite and stage varied considerably. Also, there were large inter-country differences in the proportion of patients with screen-detected CRC (11·3-40·7%) (appendix pp 6-10).
Table 2.
Characteristics of colorectal cancer patients diagnosed in 9 European countries at ages 60-69 years, by mode of detection.a
| Characteristic | All cases | Screen-detected cases | Non-screen-detected cases | p-value |
|---|---|---|---|---|
| Total | 134 597 | 30 080 (22.3%) | 104 517 (77.7%) | - |
| Sex | ||||
| Male | 83 444 (62·0%) | 19 723 (66·6%) | 63 721 (61·0%) | <0.0001 |
| Female | 51 153 (38·0%) | 10 357 (34·4%) | 40 796 (39·0%) | |
| Age at diagnosis (years) | ||||
| 60-64 | 59 761 (44·4%) | 13 475 (44·8%) | 46 286 (44·3%) | 0.12 |
| 65-69 | 74 836 (55·6%) | 16 605 (55·2%) | 58 231 (55·7%) | |
| Tumour location | ||||
| Proximal colon | 37 168 (27·6%) | 6709 (22·3%) | 30 459 (29·1%) | <0.0001 |
| Distal colon | 42 831 (31·8%) | 12 641 (42·0%) | 30 190 (28·9%) | |
| Rectum | 49 565 (36·8%) | 10 254 (34·1%) | 39 311 (37·6%) | |
| Overlapping or unspecific | 5033 (3·7%) | 476 (1·6%) | 4557 (4·4%) | |
| Stage at diagnosisb | ||||
| I | 18 911 (24·9%) | 8380 (43·0%) | 10 531 (18·6%) | <0.0001 |
| II | 18 522 (24·4%) | 4392 (22·6%) | 14 130 (25·0%) | |
| III | 21 681 (28·5%) | 5221 (26·8%) | 16 460 (29·1%) | |
| IV | 16 898 (22·2%) | 1476 (7·6%) | 15 422 (27·3%) |
The specific years for which data were included for each country/region are provided in Table 1.
There were 6860 (8·3%) cases with unknown stage. For Italy, data on stage were not available; for England and the Basque Country (Spain), only from 2012 and 2010 onwards, respectively.
In comparison with non-screen-detected cases, screen-detected cases were more often male (66·6% [19 723/30 080] vs. 61·0% [63 721/104 517], p<0·0001), had cancer more often detected in the distal colon (42·0% [12 641/30 080] vs. 28·9% [30 190/104 517]) and less often in the proximal colon (22·3% [6709/30 080] vs. 29·1% [30 459/104 517]) – p<0·0001, and had cancer much more frequently detected at stage I (43·0% [8380/19 469] vs. 18·6% [10 531/56 543) and much less frequently detected at stage IV (7·6% [1476/19 469] vs. 27·3% [15 422/56 543) – p<0·0001 (Table 2). These patterns were also consistently seen for each country separately (appendix pp 6-10).
Median follow-up times for all cases combined and screen- and non-screen-detected cases separately, by country, are presented in the appendix (pp 11-13).
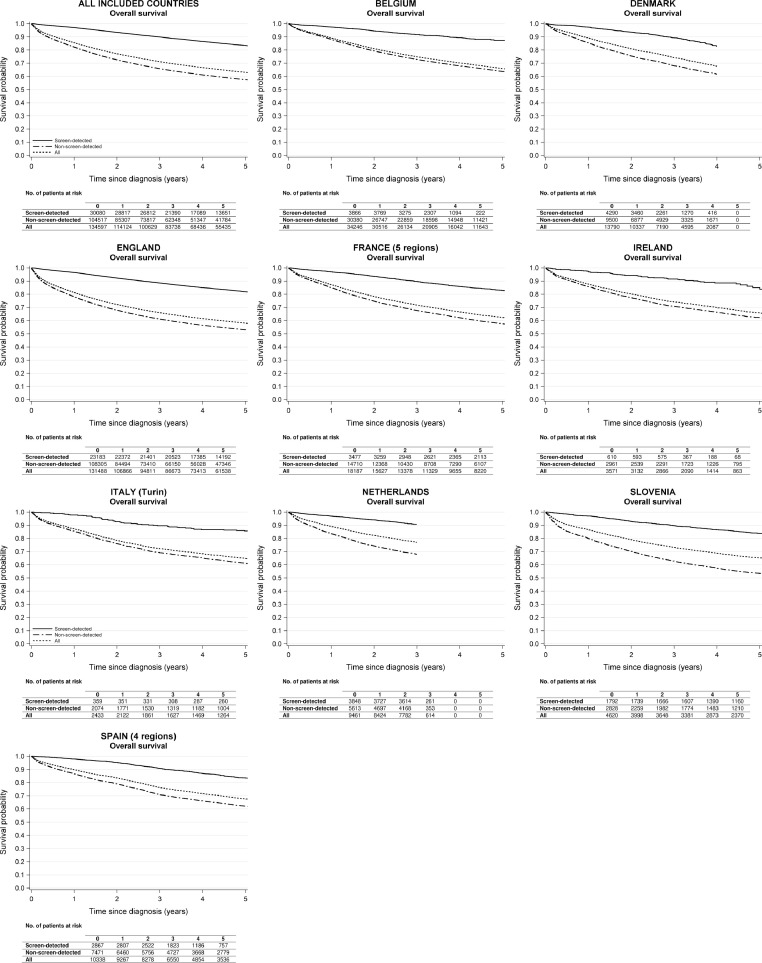
In all countries, screen-detected CRC patients had substantially higher overall survival than patients with non-screen-detected cancer and all patients combined (Figure 1; appendix pp 14-17). Analysing the data for all countries together, three-year overall survival for patients with screen-detected cancer, non-screen-detected cancer, and all patients combined was 89·9% (95% CI 89·6-90·3), 65·8% (95% CI 65·5-66·1), and 71·1% (70·9-71·4%), respectively. Five years after diagnosis, overall survival for screen-detected CRC patients was still very high (83·4% [95% CI 82·9-83·9%]), and much higher than for patients with non-screen-detected cancer (57·5% [95% CI 57·2-57·8]) and all CRC patients combined (63·1% [95% CI 62·8-63·4%]).
Figure 1.
Overall survival of colorectal cancer patients by mode of detection and country.
For all countries combined, only data from patients aged 60-69 years at diagnosis were included as this was the target group of screening across all included countries. The specific years and ages at diagnosis included for each country/region are shown in Table 1.
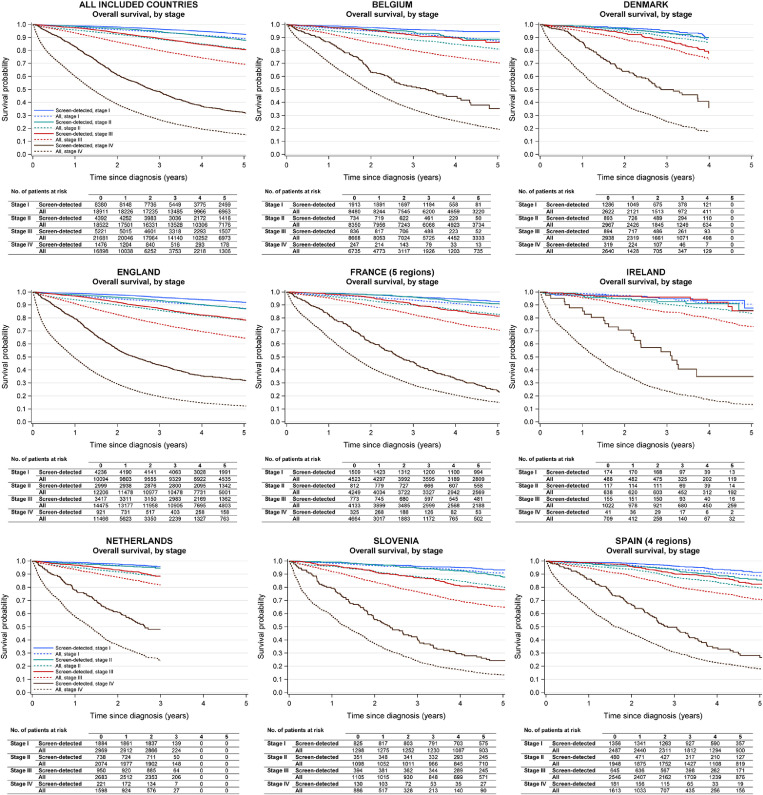
Overall survival estimates according to stage at diagnosis are shown in Figure 2 and in the appendix (pp 14-17). In analyses of all countries together, five-year overall survival for patients with stage I cancer was 92·4% (95% CI 91·6-93·1) (screen-detected), 86·7% (95% CI 86·0-87·4) (non-screen-detected), and 89·1% (95% CI 88·6-89·6) (all patients); and for patients with stage II cancer 87·9% (95% CI 86·6-89·1) (screen-detected), 79·2 % (95% CI 78·5-80·0) (non-screen-detected), and 81·2% (95% CI 80·5-81·8) (all patients). For screen-detected CRC patients, five-year overall survival was also rather high even with diagnosis of stage III cancer (80·7% [95% CI 79·3-82·0]); the corresponding figures for patients with non-screen-detected cancer and all patients combined were 66·2% (95% CI 65·3-66·9) and 69·4% (95% CI 68·7-70·1) only. Further, in the rare case of detection of metastatic (stage IV) cancer through screening, patients still had 48·4% (95% CI 45·7-51·1) probability of survival three years after diagnosis and 32·3% (95% CI 29·4-35·2) five years after diagnosis (compared to 3-year survival of 24·5% [95% CI 23·9-25·2] and 26·6% [95% 25·9-27·3] and 5-year survival of 13·9% [95% CI 13·3-14·5] and 15·4% [95% CI 14·8-16·0] for non-screen-detected and all patients combined, respectively).
Figure 2.
Overall survival of screen-detected colorectal cancer patients, and all colorectal cancer patients regardless of mode of detection, by disease stage and country.
For all countries combined, only data from patients aged 60-69 years at diagnosis were included as this was the target group of screening across all included countries. The specific years and ages at diagnosis included for each country/region are shown in Table 1.
Survival rates by sex, age, and tumour location are also presented in the appendix (pp 14-17). Overall, survival probabilities were slightly higher in women than in men, in patients detected at younger than at older ages, and in patients with a cancer located in the distal colon than in those with proximal colon cancer or rectal cancer.
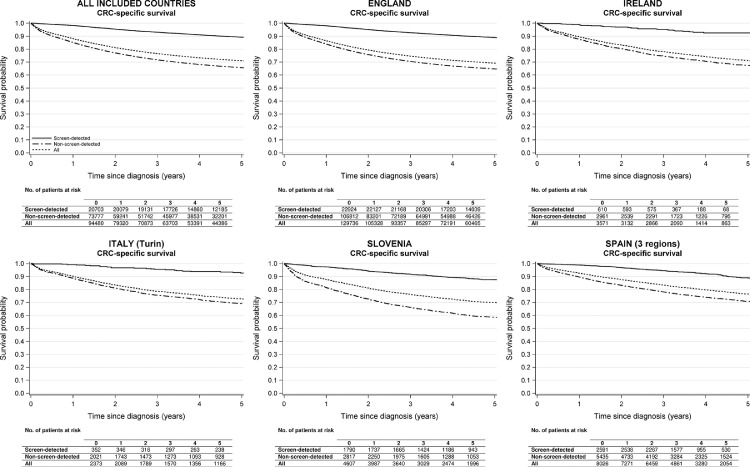
CRC-specific survival estimates are shown in Figures 3 and 4 and in the appendix (pp 18-20). Looking at the data from all countries together, CRC-specific survival five years after diagnosis was 89·2% (95% CI 88·8-89·7) for patients with screen-detected cancer and was as low as 65·7% (95% CI 65·4-66·1) and 71·1% (95% CI 70·7-71·4) for non-screen-detected and all patients combined, respectively. CRC-specific survival patterns by sex, age, stage, and tumour location were in line with those described for overall survival.
Figure 3.
Disease-specific survival of colorectal cancer patients by mode of detection and country.
For all countries combined, only data from patients aged 60-69 years at diagnosis were included as this was the target group of screening across all included countries. The specific years and ages at diagnosis included for each country/region are shown in Table 1.
CRC, colorectal cancer.
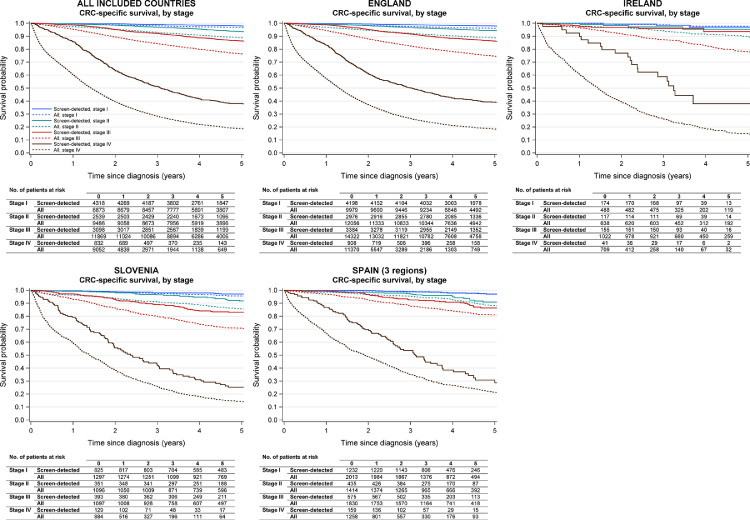
Figure 4.
Disease-specific survival of colorectal cancer patients, and all colorectal cancer patients regardless of mode of detection, by disease stage and country.
For all countries combined, only data from patients aged 60-69 years at diagnosis were included as this was the target group of screening across all included countries. The specific years and ages at diagnosis included for each country/region are shown in Table 1.
CRC, colorectal cancer.
The abovementioned patterns of survival according to mode of detection and stage at diagnosis were also consistently seen across all countries (Figure 1, Figure 2, Figure 3, Figure 4, appendix pp 14-20).
Discussion
In this international population-based study, we provided overall and disease-specific survival probabilities for screen- and non-screen-detected CRC patients, and all CRC patients irrespective of mode of detection, for nine European countries that have introduced organised population-based CRC screening programmes. Survival rates for patients with screen-detected cancer were much higher than those found for patients with non-screen-detected cancer and all patients combined, and this pattern was consistently seen for all countries and within each disease stage.
Survival probabilities for patients with screen-detected CRC have been previously reported in the context of pilot studies prior to implementation of population-based screening programmes8,9 and in a few regional and nationwide studies conducted during the first years of screening roll out, mostly in the early 2000s.7,10, 11, 12, 13, 14, 15 These studies reported five-year overall survival (patients aged 50-69, 50-74, or 50-79) of around 80% or above, ie, close to or within the range of our findings.
In our study, besides presenting more up-to-date survival probabilities for patients with screen-detected cancer, we provide the first Europe-wide analysis – according to stage at diagnosis – in the era of organised population-based programmes.
It is important to stress that this study was not designed to show or prove potential benefits of screening on CRC burden; these have been consistently shown elsewhere by substantial effects on CRC incidence and mortality.16, 17, 18, 19, 20, 21, 22 In fact, the higher survival of screen-detected cases may partly reflect lead-time bias (mere advancement of diagnosis through screening without improving the chances of prolonged life), length-time bias (higher proportions of slowly growing and less aggressive tumours among screen-detected cases), or overdiagnosis bias (a sort of length-time bias, in which a tumour that would have never caused symptoms or death is found at screening).23 Length-time bias may indeed help explain the higher survival even within each stage for patients with screen-detected cancer than for patients with non-screen-detected cancer. Besides, residual lead-time bias, potentially not fully accounted for by the rather crude classification of stage, might have also played a role; yet a previous study has shown that the higher survival of patients with screen-detected cancer remained even after adjustment for tumour size and number of affected lymph nodes.7 Moreover, patients undergoing screening might also be more likely to adhere to therapy and behave overall more health conscious (eg, have a healthier lifestyle),5 potentially influencing prognosis and, to a certain extent, contributing to the observed disparities in survival by mode of detection, particularly for patients with stage III and IV cancers.
Irrespective of the causes for the very favourable prognosis of patients with screen-detected cancer, our data show the actual survival probabilities for this increasing group of patients and are thus of high clinical relevance. These data may not only prevent screen-detected CRC patients from being discouraged by unfavourable survival estimates commonly available for all patients regardless of mode of detection, but also encourage the general eligible population to make use of available screening options.
We also observed that survival of patients with cancer located in the distal part of the colon was overall higher than that of patients with proximal colon cancer. This observation may be explained, to a large extent, by a more favourable stage distribution of cancers located in the distal than in the proximal colon, as well by distinct molecular features between subsites.22,24, 25, 26
Despite the overall very high survival for patients with screen-detected cancer, we still observed some variability across countries in total and stage-specific survival, which might in part reflect disparities in provision of cancer care (eg, adjuvant and palliative therapy). Comparisons between countries should, however, be made with caution given the different years and age groups included, which reflect the variety of screening strategies in the included countries.22 There are also differences in the primary screening tests available that need to be kept in mind – in Belgium, both guaiac-based FOBT (gFOBT) and FIT were used; in England, gFOBT; in France, gFOBT up to 2014 and FIT from 2015 on; in Italy, flexible sigmoidoscopy and FIT; in the other included countries, FIT.22 These differences in screening strategies might in part help explain the observed variations in stage and subsite distribution of cancers across countries. For example, the Netherlands and Slovenia, with FIT-based programmes and comparatively high participation rates, are among the countries with the most favourable stage distribution and the highest share of distal CRCs, which are more often found at screening. Besides, when comparing the data for all patients combined, one also needs to take into account that the share of screen-detected cancers varied substantially across countries (overall higher for countries with FIT-based programmes and higher participation rates). For these reasons, we did not place much focus on comparing results between countries and, instead, pointed to the overall patterns.
This study has several strengths and limitations. To our knowledge, this is the first multi-country population-based study from Europe providing overall and CRC-specific survival estimates for screen-detected CRC patients separately. To do so, we used high-quality cancer registry data with high completeness levels of stage at diagnosis (> 90% for most registries), which allowed us to conduct detailed survival analyses according to stage. As far as limitations are concerned, besides the inclusion of different years and age groups across countries, the very recent implementation of screening in Denmark and the Netherlands limited us from providing data on survival five years after diagnosis for patients diagnosed in these two countries; and for several countries, over 50% of patients were followed-up for less than five years. Also, the low numbers of patients with screen-detected cancers with long follow-up time in some countries, particularly Ireland, led to estimates of survival with large confidence intervals (especially in stage-specific analyses). Furthermore, there were inter-country differences in registration of mode of detection. Specifically, in France, Ireland, and the Netherlands, data were obtained from patients’ medical records instead of linkage with screening databases from the organised programmes and may be more prone to misclassification. Finally, the lack of information regarding interval cancers did not allow us to provide separate survival probabilities for patients with cancer detected after a negative test/ follow-up colonoscopy and before the next test was due.
It is also worth mentioning that the data shown in this study are for patients with cancer diagnosed in nine (high-income) European countries and are likely to be very different from those in other countries or regions. In particular, the lower levels of health care provision, disease diagnosis, and treatment in low-income countries are expected to lead to lower survival probabilities than those reported herein.2
In summary, we found that patients with screen-detected CRC have a very favourable prognosis in European countries. Even in the rare case of detection of cancer at advanced stage through screening, the survival probabilities are much higher than those reported for patients with non-screen-detected cancer and for all CRC patients combined. These data are essential to appropriately inform patients, physicians, and the general population about the survival probabilities after a screening-based CRC diagnosis.
Contributors
HB and RC conceived the study. RC conducted the literature search. HDS, NVD, MCN, JC, A-MB, VB, GL, A-SW, MCari, MR, PD, FP, PMW, CS, SR, VEPPL, MAGE, ST, TZ, ALdMM, RM-G, MP, JG, MCaru, AS-G and M-DC prepared the national and regional databases. RC carried out the analysis and drafted the manuscript. All authors contributed to the interpretation of the results and critically revised the manuscript. RC and HB directly accessed and verified the raw data and take responsibility for the integrity and accuracy of the analyses. All authors had full access to all the data reported in the study and accept responsibility to submit the paper for publication.
Data sharing statement
Summary statistical data will be available from the corresponding author upon reasonable request with the permission of the contributing cancer registries.
Declaration of interests
HDS and NVD are employed by the Belgian Cancer Registry, which is financed by regional and federal authorities for collecting data regarding new cancer diagnoses and cancer screening in Belgium, and for disseminating associated epidemiological parameters.
Acknowledgements
We are thankful to all cancer registries and their staff for the efforts in collecting and preparing the data for this study. Specifically, Belgian Cancer Registry (BCR), Danish Cancer Registry, Danish Colorectal Cancer Group Database, Danish Quality Database for Colon Cancer Screening, National Cancer Registration and Analysis Service (NCRAS) – Public Health England (data provided under the Open Government Licence: https://doi.org/10.25503/wd5j-e989), Digestive Cancer Registry of Burgundy, Digestive tumors registry of Calvados, Cancer Registry of Doubs, Digestive tumors registry of Finistere, Cancer registry of Isere, National Cancer Registry Ireland, Piedmont Cancer Registry, Netherlands Cancer Registry (IKNL), Slovenian Cancer Registry, Basque Cancer Registry, Girona Cancer Registry, Murcia Cancer Registry and Tarragona Cancer Registry. The centers for cancer screening responsible for the colorectal cancer screening programs in Flanders (Centrum voor Kankeropsporing, CvKO), Wallonia (Centre Communautaire de Référence, CCR) and Brussels (Brussels Prevention, Bruprev) provided BCR with data on colorectal cancer detection mode within existing data flows and legal frameworks. For their tasks regarding colorectal cancer screening, CvKO, CCR, Bruprev and BCR receive funding from the respective regional authorities.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lanepe.2022.100458.
Appendix. Supplementary materials
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023–1075. doi: 10.1016/S0140-6736(17)33326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Araghi M, Arnold M, Rutherford MJ, et al. Colon and rectal cancer survival in seven high-income countries 2010–2014: variation by age and stage at diagnosis (the ICBP SURVMARK-2 project) Gut. 2021;70(1):114–126. doi: 10.1136/gutjnl-2020-320625. [DOI] [PubMed] [Google Scholar]
- 4.Schreuders EH, Ruco A, Rabeneck L, et al. Colorectal cancer screening: a global overview of existing programmes. Gut. 2015;64(10):1637–1649. doi: 10.1136/gutjnl-2014-309086. [DOI] [PubMed] [Google Scholar]
- 5.Cardoso R, Guo F, Heisser T, Hoffmeister M, Brenner H. Utilisation of colorectal cancer screening tests in European countries by type of screening offer: results from the european health interview survey. Cancers (Basel) 2020;12(6) doi: 10.3390/cancers12061409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cardoso R, Guo F, Heisser T, et al., Proportion and stage distribution of screen-detected and non-screen-detected colorectal cancer in nine European countries: an international, population-based study. Lancet Gastroenterol Hepatol, 2022; published online May 10, 10.1016/S2468-1253(22)00084-X. [DOI] [PubMed]
- 7.Brenner H, Jansen L, Ulrich A, Chang-Claude J, Hoffmeister M. Survival of patients with symptom- and screening-detected colorectal cancer. Oncotarget. 2016;7(28):44695–44704. doi: 10.18632/oncotarget.9412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pande R, Froggatt P, Baragwanath P, Harmston C. Survival outcome of patients with screening versus symptomatically detected colorectal cancers. Colorectal Dis. 2013;15(1):74–79. doi: 10.1111/j.1463-1318.2012.03120.x. [DOI] [PubMed] [Google Scholar]
- 9.Lindebjerg J, Osler M, Bisgaard C. Colorectal cancers detected through screening are associated with lower stages and improved survival. Dan Med J. 2014;61(1):A4758. [PubMed] [Google Scholar]
- 10.Gill MD, Bramble MG, Hull MA, et al. Screen-detected colorectal cancers are associated with an improved outcome compared with stage-matched interval cancers. Br J Cancer. 2014;111(11):2076–2081. doi: 10.1038/bjc.2014.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parente F, Vailati C, Boemo C, et al. Improved 5-year survival of patients with immunochemical faecal blood test-screen-detected colorectal cancer versus non-screening cancers in northern Italy. Dig Liver Dis. 2015;47(1):68–72. doi: 10.1016/j.dld.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 12.Idigoras Rubio I, Arana-Arri E, Portillo Villares I, et al. Participation in a population-based screening for colorectal cancer using the faecal immunochemical test decreases mortality in 5 years. Eur J Gastroenterol Hepatol. 2019;31(2):197–204. doi: 10.1097/MEG.0000000000001338. [DOI] [PubMed] [Google Scholar]
- 13.Spolverato G, Capelli G, Battagello J, et al. More favorable short and long-term outcomes for screen-detected colorectal cancer patients. Front Oncol. 2021;11:446. doi: 10.3389/fonc.2021.620644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ibáñez-Sanz G, Milà N, Vidal C, et al. Positive impact of a faecal-based screening programme on colorectal cancer mortality risk. PLoS One. 2021;16(6) doi: 10.1371/journal.pone.0253369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tepeš B, Mlakar DN, Stefanovič M, Štabuc B, Grazio SF, Zakotnik JM. The impact of 6 years of the national colorectal cancer screening program on colorectal cancer incidence and 5-year survival. Eur J Cancer Prev. 2021;30(4):304–310. doi: 10.1097/CEJ.0000000000000628. [DOI] [PubMed] [Google Scholar]
- 16.Hewitson P, Glasziou P, Watson E, Towler B, Irwig L. Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (hemoccult): an update. Am J Gastroenterol. 2008;103(6):1541–1549. doi: 10.1111/j.1572-0241.2008.01875.x. [DOI] [PubMed] [Google Scholar]
- 17.Brenner H, Stock C, Hoffmeister M. Effect of screening sigmoidoscopy and screening colonoscopy on colorectal cancer incidence and mortality: systematic review and meta-analysis of randomised controlled trials and observational studies. BMJ. 2014;348:g2467. doi: 10.1136/bmj.g2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atkin W, Wooldrage K, Parkin DM, et al. Long term effects of once-only flexible sigmoidoscopy screening after 17 years of follow-up: the UK Flexible Sigmoidoscopy Screening randomised controlled trial. Lancet. 2017;389(10076):1299–1311. doi: 10.1016/S0140-6736(17)30396-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holme Ø, Løberg M, Kalager M, et al. Long-term effectiveness of sigmoidoscopy screening on colorectal cancer incidence and mortality in women and men: a randomized trial. Ann Intern Med. 2018;168(11):775–782. doi: 10.7326/M17-1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller EA, Pinsky PF, Schoen RE, Prorok PC, Church TR. Effect of flexible sigmoidoscopy screening on colorectal cancer incidence and mortality: long-term follow-up of the randomised US PLCO cancer screening trial. Lancet Gastroenterol Hepatol. 2019;4(2):101–110. doi: 10.1016/S2468-1253(18)30358-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Senore C, Riggi E, Armaroli P, et al. Long-term follow-up of the italian flexible sigmoidoscopy screening trial. Ann Intern Med. 2022;175(1):36–45. doi: 10.7326/M21-0977. [DOI] [PubMed] [Google Scholar]
- 22.Cardoso R, Guo F, Heisser T, et al. Colorectal cancer incidence, mortality, and stage distribution in European countries in the colorectal cancer screening era: an international population-based study. Lancet Oncol. 2021;22(7):1002–1013. doi: 10.1016/S1470-2045(21)00199-6. [DOI] [PubMed] [Google Scholar]
- 23.IARC Colorectal cancer screening. IARC Handb Cancer Prev. 2019;17:1–300. [Google Scholar]
- 24.Missiaglia E, Jacobs B, D'Ario G, et al. Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. Ann Oncol. 2014;25(10):1995–2001. doi: 10.1093/annonc/mdu275. [DOI] [PubMed] [Google Scholar]
- 25.Huyghe JR, Harrison TA, Bien SA, et al. Genetic architectures of proximal and distal colorectal cancer are partly distinct. Gut. 2021;70(7):1325–1334. doi: 10.1136/gutjnl-2020-321534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffmeister M, Bläker H, Jansen L, et al. Colonoscopy and reduction of colorectal cancer risk by molecular tumor subtypes: a population-based case-control study. Am J Gastroenterol. 2020;115(12):2007–2016. doi: 10.14309/ajg.0000000000000819. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.