Abstract
Prevention of necrotic enteritis (NE), caused by Clostridium perfringens (C. perfringens), is one of the most important goals to improve the profitability of broiler chickens. This work aimed to compare the efficacy of 2 antibiotic alternatives including a postbiotic (dry feed additive and aqueous nonviable Lactobacillus (L.) species fermentation) and a probiotic (dry feed additive and aqueous Bacillus (B.) subtilis and B. lischeniformis mixture) with an antibiotic (amoxicillin in water) against NE. Four hundred, day-old broiler chicks were divided into 8 equal groups (Gs), n = 50 each (5 replicates; 10 each). Chickens of G1 (postbiotic dry-feed additive), G2 (postbiotic and antibiotic in drinking water), G3 (postbiotic dry and aqueous), G4 (probiotic dry-feed additive), G5 (probiotic and antibiotic in drinking water), G6 (probiotic dry and aqueous), and G7 (nontreated) were orally inoculated with a toxigenic C. perfringens type A on the d 19 to 21 of age and predisposed with 3X coccidial vaccine for induction of NE. However, chickens of G8 were kept nontreated or challenged. The severity of NE signs was markedly decreased in G3 in comparison with other challenged treatment groups, and the mortality rates were 22%, 10%, 16%, 22%, 12%, 20%, and 36% in Gs 1, 2, 3, 4, 5, 6, and 7, respectively. The best significant (P ≤ 0.05) feed conversion ratio was detected in G3 (1.51), G6 (1.54), and G2 and G8 (1.61). In addition, the European production efficiency factor was significantly (P ≤ 0.05) improved in G3 (279.33) and G2 (266.67), but it was decreased in G7 (177.33) when compared with G8 (339.33). An improvement in intestinal and hepatic pathology and liver function tests, as well as a significant (P ≤ 0.05) decrease in bacterial counts were observed in Gs 2, 5, 3, 6, 1, and 4, respectively in comparison with G7. Immunologically, the highest significant (P ≤ 0.05) hemagglutination inhibition antibody titers for Newcastle disease virus vaccine were in Gs 1 and 3 (6.4 log2). In conclusion, the combined feed and water postbiotic treatment demonstrated promising results in ameliorating the severity of NE and improving the hepatic and the immune status of broiler chickens when compared with the commonly used probiotic and antibiotic.
Key words: C. perfringens, broiler chicken, postbiotic, probiotic, antibiotic
INTRODUCTION
Necrotic enteritis (NE), induced by Clostridium perfringens (C. perfringens), has been unequivocally recognized as one of the most leading intestinal disease affecting chickens and turkeys. The disease is characterized by decreasing growth rate and poor feed conversion rate as a result of necrosis of the intestinal mucosa and cholangiohepatitis (Hofacre et al., 2018; Zahoor et al., 2018; Eraky and Abd El-Ghany, 2022). Several predisposing factors such as improper feeding and poor management practices (e.g., high stocking density and high-protein diets) can interrupt gut immune homeostasis and promote C. perfringens proliferation and NE infection (Drew et al., 2004; Wu et al., 2014). Additionally, coccidial infection has been implicated as the most important predisposing factor for enhancement of C. perfringens multiplication and consequent damage to the intestinal epithelial layer (Wu et al., 2014; Lee et al., 2018; Lu et al., 2020). Both clinical and subclinical forms of NE can significantly diminish profits in broiler's industry. A survey by Timbermont et al. (2011) estimated the annual global poultry industry cost of NE as close to $ 6 billion which included applied control measures and the cost of production losses.
After the discovery of NE in England during 1961 (Parish, 1961), many approaches were developed to control the disease. One of these approaches is the application of antibiotics which was used later on to increase the weight gain and feed efficiency as antibiotic growth promoters (AGPs) (Long and Truscott, 1976; Prescott et al., 1978, Durso and Cook, 2014). For instance, avoparcin, lincomycin, amoxicillin, tylosin, virginiamycin, and bacitracin were commonly used for both prevention and treatment of NE (Craven et al., 2001; McDevitt et al., 2006; Abd El-Hamid et al., 2015). Owing to the development of drug-resistant bacteria which consequently effects on public health, people started to reduce the use of antimicrobials in poultry production (Seal et al., 2013; Caly et al., 2015; Karavolias et al., 2018) and many countries have subsequently banned the administration of them in animal feed (Ajuwon, 2015; Kiu and Hall, 2018).
However, withdrawal of AGPs from commercial broiler farms dramatically increased the incidence of economically important diseases such as NE (Casewell et al., 2003; Van Immerseel et al., 2004; Sarson et al., 2009; Kumar et al., 2018). In Norway, a spike in NE cases was observed following withdrawal of avoparcin AGP. Although flocks raised in the conventional system had no cases of NE, 27% of drug-free flocks had clinical NE, and 49% had subclinical form of the disease (Gaucher et al., 2015). Strict biosecurity practices without application of AGPs can maintain production in some farms (Engster et al., 2002), but, it is difficult to rely on this approach for every farm. Therefore, AGPs alternatives are much needed to control NE and other diseases (Grave et al., 2004).
Several natural alternative feed additives have recently supported profitable production of safe poultry products without use of antibiotics. Probiotics, prebiotics, and synbiotics have been widely used for several years to enhance growth and prevent several enteric pathogens. Recently, postbiotics and paraprobiotics, as derivatives of probiotic cultures, have been used in humans, animals, and poultry to maintain the gut microbiome in healthy conditions and to prevent the attachment of pathogenic organisms, especially at the early life stages (Abd El-Ghany, 2020; Abd El-Hack et al., 2022).
The new term “postbiotics” that has recently been introduced into the poultry industry refers to the soluble factors (stabilized bacteria, cellular products, or metabolic byproducts) which are secreted by living bacteria or released after bacterial lysis (Loh et al., 2010; Cicenia et al., 2014; Klemashevich et al., 2014; Blacher et al., 2017; Johnson et al., 2019). These substances include enzymes, peptides, teichoic acids, peptidoglycan-derived muropeptides, polysaccharides, cell surface proteins, and organic acids (Abd El-Ghany, 2020). They are mainly derived from Lactobacillus, Bifidobacterium, Streptococcus, and Faecalibacterium species of bacteria (Konstantinov et al., 2008; Tsilingiri et al., 2012) and characterized by clear chemical structure, long shelf life, and safe usage and dosage. Postbiotics have antimicrobial, antioxidant, anti-inflammatory, immunomodulatory, hypocholesterolemic, antiproliferative, and hepatoprotective as well as growth promoter activities which lead to an improvement of the host's health (Aguilar-Toalá et al., 2018).
Till now, there is a scant information about application of postbiotics in the poultry sector for improving the health and maintaining the immune status of the host as well as controlling disease conditions, especially NE.
Therefore, this research aimed to compare the efficacies of a postbiotic, a probiotic and an antibiotic against experimental infection with C. perfringens in commercial broiler chickens.
MATERIALS AND METHODS
All experiments and procedures were complied with the general guidelines of the Cairo University-Institutional Animal Care and Use Committee (CU-IACUC), and all efforts were made to minimize suffering.
Birds and Design of Experimental Infection
Four hundred, day-old commercial broiler chickens (Ross-308) were purchased from NASCO Egypt poultry company, Alexandria. Birds were divided into 8 equal groups (Gs); each consisting of 50 birds (distributed into 5 replicates; 10 each), and they were floor-reared in separate clean and disinfected rooms for 35 d. Chickens were fed ad-libitum using basal broiler diets (corn-soybean based) that were formulated according to the nutrient requirements for broiler chickens (Ross 308) (Aviagen, 2019) and prepared to meet the National Research Council's Nutrient Requirements (NRC, 1994) of poultry. Nutrient content of ingredients were evaluated following the instructions of AOAC (AOAC, 2005) (Table 1). These diets were fed as crumbled pellets.
Table 1.
Ingredients’ percentage and calculated composition analysis of the experimental starter, grower and finisher diets (%, as- fed basis for Ross 308) (Aviagen, 2019).
| Ingredients, % | Starter (0–10 d) | Grower (11–21 d) | Finisher (22–35 d) |
|---|---|---|---|
| Yellow corn | 55.07 | 59.08 | 64.09 |
| Soybean meal (44%) | 33.5 | 29.4 | 24 |
| Corn gluten (60%) | 5 | 5 | 5 |
| Corn oil | 2 | 2.65 | 3.15 |
| Dicalcium phosphate | 1.73 | 1.6 | 1.5 |
| Lime stone | 1.35 | 1 | 1 |
| Salt | 0.4 | 0.4 | 0.4 |
| DL-methionine1 | 0.15 | 0.12 | 0.1 |
| HCl- lysine2 | 0.35 | 0.3 | 0.3 |
| Vitamins and minerals premix3 | 0.3 | 0.3 | 0.3 |
| Sodium bicarbonate | 0.1 | 0.1 | 0.1 |
| Choline chloride | 0.05 | 0.05 | 0.05 |
| Calculated composition | |||
| ME, Kcal/Kg diet | 3,010 | 3,105 | 3,200 |
| CP % | 23 | 21.5 | 19.5 |
| Ca % | 1 | 0.87 | 0.82 |
| Avail. P % | 0.47 | 0.44 | 0.41 |
| Methionine % | 0.56 | 0.51 | 0.47 |
| Lysine % | 1.44 | 1.29 | 1.14 |
| Meth.+Cyst. % | 0.93 | 0.86 | 0.78 |
| Na % | 0.20 | 0.20 | 0.20 |
Abbreviations: Av. (P), available phosphorous; CP, crude protein; ME, metabolizable energy; SBM, soybean meal.
DL-methionine 99% feed grade China.
L-lysine 99% feed grade.
Vitamin and mineral premix (Hero mix) produced by Hero pharm and composed (per 3 kg) of vitamin A 12,000,000 IU, vitamin D3 2,500,000 IU, vitamin E 10,000 mg, vitamin K3 2,000 mg, vitamin B1 1,000 mg, vitamin B2 5,000 mg, vitamin B6 1,500 mg, vitamin B12 10 mg, niacin 30,000 mg, biotin 50 mg, folic acid 1,000 mg, pantothenic acid 10,000 mg, manganese 60,000 mg, zinc 50,000 mg, iron 30,000 mg, copper 4,000 mg, iodine 300 mg, selenium 100 mg, and cobalt 100 mg.
A coccidial vaccine “Fortegra” (MSD Animal Health, Inc. Company, New Cairo, Egypt) was used for triggering of a toxigenic C. perfringens type A (accession No. NCTC 8237/ATCC©13124). The experimental design, doses, and treatments are presented in the Table 2.
Table 2.
The experimental design and treatments.
 |
Treatments Used
A postbiotic, Culbac, (a nonviable Lactobacillus acidophilus species fermentation product; TransAgra International Inc., Storm Lake, Iowa) was used in doses of 1 kg/ton of starter and 500 g/ton of grower and finisher feed for the dry form and 4 mL/ L drinking water for the aqueous form as manufacture's recommendation. A probiotic powder (Immunobacteryne, Kronos Agro PC, Kyiv, Ukraine, Batch No: 01-05.110620.14) containing Bacillus (B). subtilis (2.5 × 1012 colony forming unit [CFU]/kg) and B. lischeniformis (2.5 × 1012 CFU/kg) was included as 200 g/ton feed and 1 g/ 5 L drinking water according to manufacturer's instructions. The used antibiotic was Amoxicillin 20% powder (Ghannam UCCMA, Cairo, Egypt, Batch No. 204/A/2/2018) and it was applied in a dose of 20 mg/kg body weight and 1 g/L drinking water as recommendation.
Birds were routinely vaccinated against Newcastle disease virus (NDV), infectious bronchitis virus (IBV), highly pathogenic avian influenza virus (HPAIV) (H5N1), and infectious bursal disease virus (IBDV) using NDV strain Hitchner B1 +IBV strain H120 (Cevac B1-L- Ceva sante animal, Budapest, Hungary, Batch No. 0103F352INKGA) through eye drop. Inactivated NDV +H5 (MEFLUVAC H5+ND, AIV (AI/Chickens/Egypt/2017/Rg H5N1 Classic and variant strains 2.2.1.1 and 2.2.1.2), with a dose of ≥108.5 (embryo infective dose fifty) EID50 and NDV (NDV/Chicken/Egypt/11478AF/2011 ND), with a dose of ≥108 EID50, MEVAC, Cairo, Egypt, Batch. No. 2004290101) were simultaneously used at 5 d of age. Moreover, an intermediate vaccine of IBDV (BG ARRIAH, Federal Centre for Animal Health [FGBI. ARRIAH], Simferopol, Russia, Batch No. 3. Dose: ≥ 3.5 EID50 of IBDV) was applied at 10-day-old via drinking water and the same vaccine was repeated at 17-day-old via eye drop. Finally, LaSota vaccine (Volvac LaSota MLV, ND vaccine (Boehringer Ingelheim, Mainz-Bingen, Germany, Lot No: 1909063A, Dose: ≥108 EID50) was used at 17-day-old via eye drop. Examination was done by using a digital camera (Leica EC3, Wetzlar, Germany) connected to a microscope (Leica DM500, Wetzlar, Germany) with software (Leica LAS, Wetzlar, Germany). method.
The Evaluation Parameters
-
1.
Clinical observation After experimental infection, clinically-observable signs, mortalities, and gross lesions were recorded daily for 14 d.
-
2.
Performance parameters Final body weight (FBWT), body weight gain (BWG), average feed intake (AFI), and feed conversion ratio (FCR) were recorded on weekly basis for 5 wk (1, 2, 3, 4, and 5-wk-old). Finally, the European production efficiency factor (EPEF) in all chicken groups was calculated.
-
3.
Macroscopic lesion scoring The criteria of scoring the intestinal NE lesions (from duodenum to ileum) in dead birds were previously recorded (Shojadoost et al., 2012). The scores were graded as 0 (No gross lesions), 1 (Thin or friable walls, or diffuse superficial but removable fibrin), 2 (Focal necrosis or ulceration, or nonremovable fibrin deposit; 1 to 5 foci), 3 (Focal necrosis or ulceration, or nonremovable fibrin deposit; 6 to 15 foci), 4 (Focal necrosis or ulceration, or nonremovable fibrin deposit; 16 or more foci), 5 (Patches of necrosis 2 to 3 cm long with variable foci), and 6 (Diffuse necrosis typical of field cases with variable, extensive foci). Chickens with lesion scores of 2 or more were identified as NE positive. After comparing scores of duodenum, jejunum, and ileum, the highest score was determined as the final NE lesion score for each chicken.
-
4.
Histopathological examination and lesion scoring Samples of 3 cm that obtained from jejunum (1 cm cut from the midpoint) and liver from each group (n = 5) were collected once at 35 d of age (2 wk postchallenge). Tissue specimens were removed, flushed with phosphate buffered saline (PBS, pH 7.4), and fixed in neutral buffered formaldehyde for 48 h. The fixed specimens were processed by the conventional paraffin embedding technique including dehydration through ascending grades of ethanol, clearing in 3 washes of xylene and melted paraffin, and finally embedding in paraffin wax at 65°C. Four, one-µm thick sections were stained by Hematoxylin and Eosin (H and E) as previously described (Bancroft and Layton, 2013). Examination was done by using a digital camera (Leica EC3, Wetzlar, Germany) connected to a microscope (Leica DM500, Wetzlar, Germany) with software (Leica LAS, Wetzlar, Germany). Semiquantitative scoring of intestinal and hepatic lesions was calculated (Gibson-Corley et al., 2013). Briefly, lesions in 10 fields from each slide for each bird were randomly chosen and averaged. Sample treatments were blinded for lesion scoring (Score scale: 0 = normal; 1 ≤ 25%; 2 = 26–50%; 3 = 51–75%; 4 = 76–100%).
-
5.
Reisolation of C. perfringens Intestinal samples from each group (n = 10) were collected once at 35- day-old and immediately plated. Ten-fold dilutions per each sample were prepared and 0.1 mL was spread (triplicate) on blood agar base containing 5% sheep blood supplemented with 100 mg/L neomycin sulfate for C. perfringens enumerations. The plates were anaerobically incubated at 37°C for 16 to 24 h. The α- and β-hemolytic colonies were counted as C. perfringens, and the presumptive colonies were randomly picked, Gram stained, and microscopically examined to confirm them as C. perfringens. Counts were expressed as log10 CFU/g of the intestinal contents (Dahiya et al., 2007).
-
6.
Intestinal bacterial coliform count Intestinal samples were collected and prepared as mentioned above. Samples were cultivated on MacConkey agar plates and aerobically incubated at 37°C for 24 h. Plates containing 30–300 bacterial colonies were selected to count. The final number was shown as log10 (CFU/g) (Huang et al., 2019).
-
7.
Immunological parameters for the detection of humoral immune response for NDV and AIV (H5) antibody titers using hemagglutination inhibition (HI) test Blood samples from each group (n = 6) were collected from the wing vein at 7, 14, 21, 28, and 35-day-old without anticoagulant for serum separation. Samples were centrifuged at 1435 g for 5 min at 4°C to obtain clear sera for hemagglutination inhibition (HI) test against NDV, and HPAI-H5N1. The hemagglutination (HA) units for both standard viral antigens (NDV [LaSota strain] and HPAI [H5N1]) were 8 log2. To detect HI titers for NDV and HPAI (H5N1), a total of 0.025 ml of each serum sample was double-fold serially diluted in phosphate buffer saline (PBS) across a plastic U-bottomed microliter plate. Four hemagglutinin units (HAU) of virus/ antigen were added in the same quantity to each well, allowed to rest for a minimum of 30 min at room temperature (i.e., approximately 25°C), and 0.050 mL of 0.5% (v/v) chicken's red blood cells (RBCs) was added to each well. After being gently mixed, RBCs were allowed to settle to a distinct button for approximately 40 min at room temperature. Positive and a negative control sera were applied in 2 rows. The HI titer was the highest dilution of serum causing complete inhibition of 4 HAU of antigen. The titers of HI were regarded as positive if there was inhibition at a serum dilution of 1/16 (4 log2 when expressed as the reciprocal) or more against 4 HAU of antigen (Office International des Epizooties OIE. Newcastle Disease Infection with Newcastle Disease Virus, 2021).
-
8.
Liver function tests (aspartate aminotransferase [AST] and alanine aminotransferase [ALT]) Serum samples were collected at the end of experiment (35 d) and tested for liver functions in all chicken groups (Reitman and Frankel, 1957).
Statistical Analysis
Statistical calculations were made with the SPSS programming tool (IBM SPSS.20) (SPSS Inc., New York, USA) using one-way ANOVA followed by Duncan's multiple range tests. All significant deviations were based on P ≤ 0.05 (SPSS, 2011).
RESULTS
Clinical Observation of Signs, Mortalities, and Gross Lesions
Severe clinical signs of depression, off food, and bloody brownish diarrhea were observed in chickens of G7 (challenged-nontreated), 3 d post challenge with a toxigenic C. perfringens type A. However, the severity of these signs was markedly decreased in the treated chickens especially those of G3 (Culbac dry feed additive and aqueous in drinking water). The lowest mortality rate was recorded in G2 (Culbac aqueous and Amoxicillin in drinking water) as 10%, followed by G5 (Immunobacteryne and Amoxicillin in drinking water) as 12%, G3 (Culbac in both dry and aqueous forms) as 16%; G6 (Immunobacteryne in feed and drinking water) as 20%, G1 (Culbac dry feed additive) and G4 (Immunobacteryne feed additive) as 22% for both, compared to G7 (challenged-nontreated) as 36%. No mortality was observed in G8 (nonchallenged or treated).
The minimum intestinal lesion scores were 1 in Gs 2, 3, and 5 followed by score 2 in G6, 3 in G1, and 4 in G4 compared with 6 in G7. The score was 0 in G8. In addition, cholangiohepatitis lesion was the minimum in chickens of G3, G2, G5, and G6, respectively.
The Performance Parameters
Broiler chickens of the nonchallenged or treated G8 showed the highest significant (P ≤ 0.05) FBWT (1,904 g), followed by the Culbac dry and aqueous treated G3 (1,753 g) compared with the challenged-nontreated G7 (1,603 g). The best significant (P ≤ 0.05) FCR was in G3 (1.51), then the Immunobacteryne in feed and aqueous treated G6 (1.54), and G2 (Culbac aqueous and Amoxicillin in drinking water), and G8 (1.61) compared with all other Gs. Chickens of G8 revealed the highest significant (P ≤ 0.05) EPEF (339.33), followed by G3 (279.33), and G2 (266.67) compared with the lowest significant one in G7 (177.33) (P ≤ 0.05) (Table 3).
Table 3.
Performance parameters (initial BWT, final BWT [FBWT], body weight gain [BWG], average feed intake [AFI], feed conversion ratio [FCR], and European production efficiency factor [EPEF]) in all chicken groups.
| Item | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Initial BWT | 45.0 ± 0.0a | 45.0 ± 0.0a | 45.0 ± 0.0a | 45.0 ± 0.0a | 45.0 ± 0.0a | 45.0 ± 0.0a | 45.0 ± 0.0a | 45.0 ± 0.0a |
| FBWT | 1,571.05 ± 46.11c | 1,655.75 ± 36.16bc | 1,753.75 ± 34.34b | 1,649.5 ± 47.05bc | 1,659.25 ± 46.71bc | 1,676.25 ± 47.66bc | 1,603 ± 45.67c | 1,904 ± 39.79a |
| BWG | 1,526.05 ± 46.11c | 1,610.75 ± 36.16 bc | 1,708.75 ± 34.34b | 1,604.5 ± 47.05bc | 1,614.25 ± 46.71bc | 1,631.25 ± 47.66bc | 1,558 ± 45.67c | 1,859 ± 39.79a |
| AFI | 2,480.26 ± 6.07e | 2,564.56 ± 6.74c | 2,565.53 ± 6.24c | 2,641.11 ± 8.28b | 2,631.31 ± 5.58b | 2,475.04 ± 5.01e | 2,533.17 ± 6.43d | 2,967.35 ± 6.24a |
| FCR | 1.65 ± 0.05a | 1.61 ± 0.04ab | 1.51 ± 0.03b | 1.67 ± 0.05a | 1.66 ± 0.05a | 1.54 ± 0.04ab | 1.65 ± 0.05a | 1.61 ± 0.03ab |
| EPEF | 213.33 ± 14.948de | 266.67 ± 12.914bc | 279.33 ± 11.26b | 222.67 ± 17.91cd | 256.33 ± 23.975bcd | 248.67 ± 11.17bcd | 177.33 ± 3.93e | 339.33 ± 9.493a |
Different superscripts (a, b, c, d, e, f, g) within the same row are significantly different (P ≤ 0.05). Values are expressed as means ± standard error of the mean.
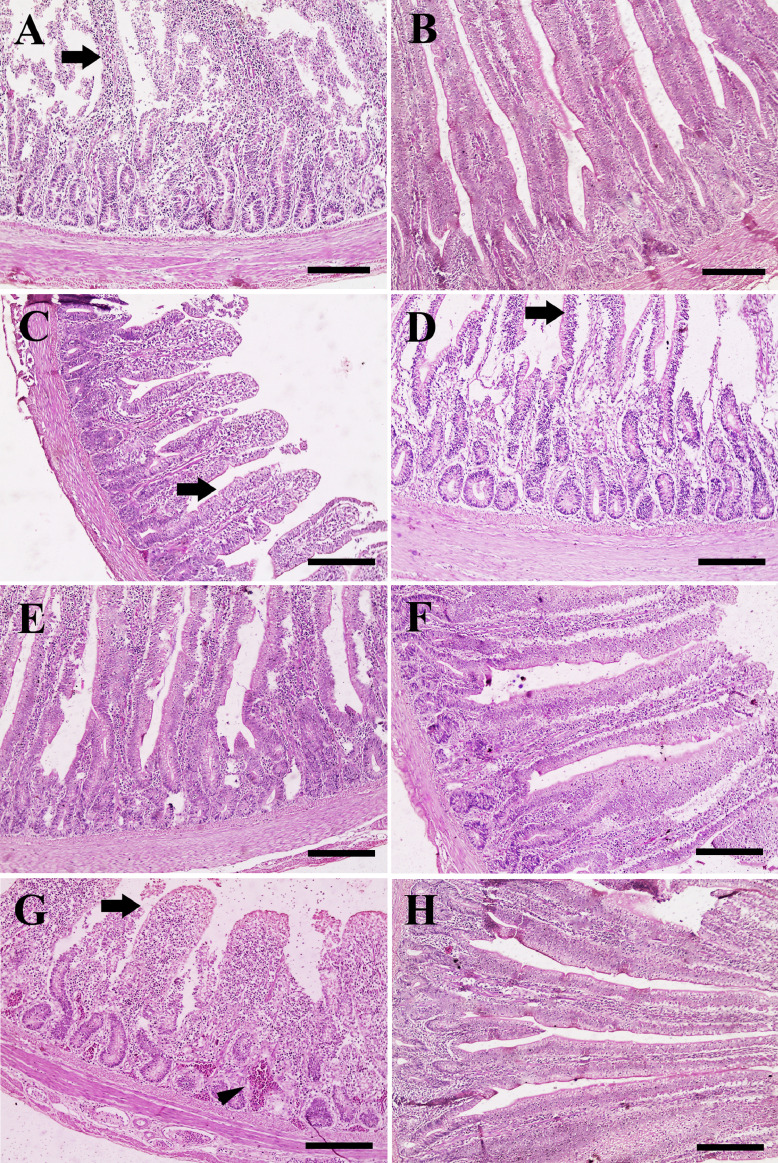
Histopathological Assessment of the Intestine and Liver
The intestine of G1 (Culbac dry feed additive) revealed necrotic villi with lymphocytic infiltration (Figure 1A), while Gs 2 (Culbac aqueous and Amoxicillin in drinking water), 5 (Immunobacteryne and Amoxicillin in drinking water), and 6 (Immunobacteryne in feed and drinking water) showed normal villi (Figure 1B, E, and F). Moreover, the intestine of G3 (Culbac in both dry and aqueous forms) had normal villi with slight degeneration (Figure 1C). Moderate degenerated villi with separated enterocytes was observed in G4 (Immunobacteryne feed additive) (Figure 1D), compared with severe submucosal congestion and degenerated villi in G7 (challenged-nontreated) (Figure 1G) and normal villi and enterocytes in G8 (Figure 1H). Regarding the intestinal histopathological lesion scoring (degeneration and congestion), Gs 2, 6, 5, 3, and 4, respectively showed significant improvement of lesions compared with G1, G7, and the nonchallenged or treated G8 (Table 4).
Figure 1.
Histopathological examination of chicken's intestine. (A) Intestine of chicken in G1 showing necrotic villi (arrow). (B) Intestine of chicken in G2 revealing normal villi. (C) Intestine of chicken in G3 revealing normal villi with slight degeneration (arrow). (D) Intestine of chicken in G4 showing moderate degenerated villi (arrow). (E) Intestine of chicken in G5 group revealing normal villi. (F) Intestine of chicken in G6 exposing normal villi. (G) Intestine of chicken in G7 exposing necrotic villi (arrow) and severe congestion (arrowhead). (H) Intestine of chicken in G8 revealing normal villi with normal enterocytes. Scale bar =200 μm.
Table 4.
Histopathological lesion scoring of intestine and liver in all chicken groups.
| Group | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|---|
| Intestine | Degeneration | 3.4 ± 0.22a | 0.2 ± 0.13ef | 1.6 ± 0.22c | 2.3 ± 0.21b | 1.1 ± 0.18cd | 0.7 ± 0.21de | 3.8 ± 0.13a | 0 ± 0f |
| Congestion | 3.2 ± 0.2a | 0 ± 0d | 1.2 ± 0.13c | 1.7 ± 0.15b | 1 ± 0.26c | 0.3 ± 0.15d | 3.5 ± 0.27a | 0 ± 0d | |
| Liver | Congestion | 3.4 ± 0.27a | 0.1 ± 0.1f | 1.2 ± 0.25c | 2.2 ± 0.2b | 0.8 ± 0.25cd | 0.5 ± 0.17de | 3.9 ± 0.1a | 0 ± 0f |
Means with different superscripts (a, b, c, d, e, f) within the same row are significantly different (P ≤ 0.05).
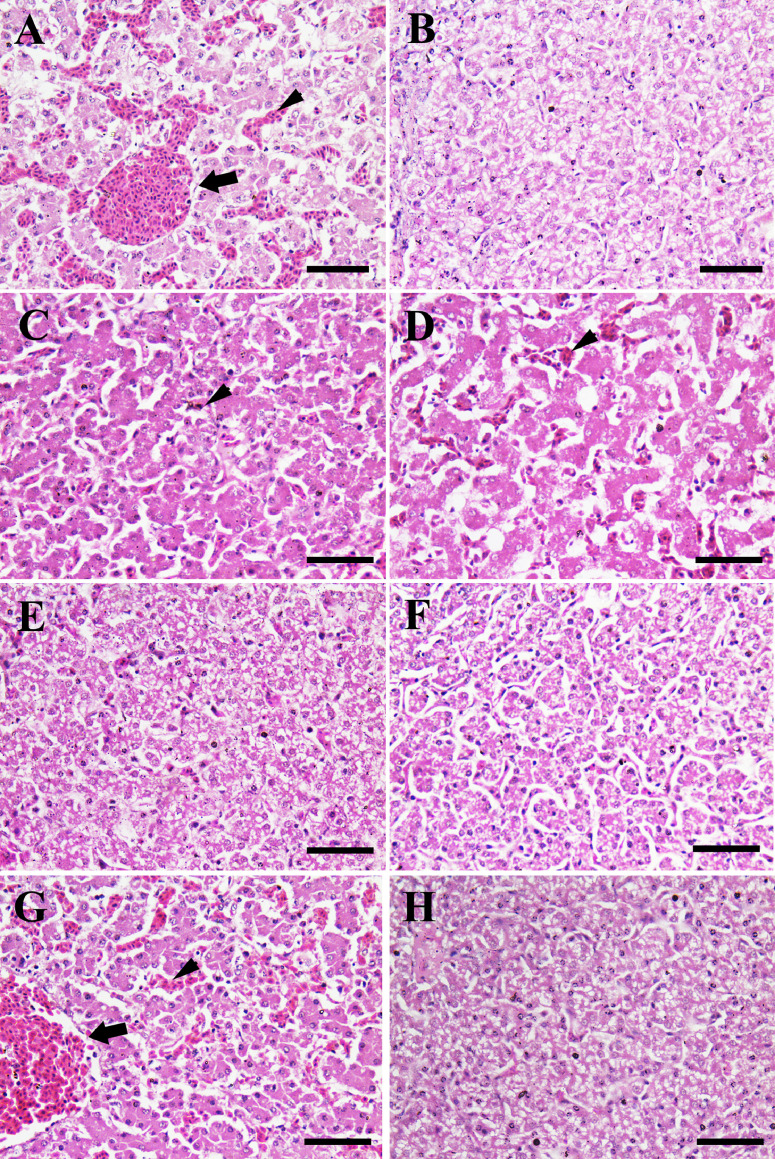
The histopathological examination of liver showed severe vascular and sinusoidal congestion and mild hemorrhage in G1 (Figure 2A), but normal hepatocytes in G2 (Figure 2B), normal hepatocytes without hemorrhage in G3 (Figure 2C), and normal hepatocytes with moderate sinusoid congestion in G4 (Figure 2D). In contrast, the liver revealed normal hepatocytes in in G5 and G6 (Figure 2E, F), severe congested central veins and sinusoids in G7 (Figure 2G) and normal hepatocytes in G8, (Figure 2H). The histopathological lesion scoring of liver indicated that Gs 2, 6, 5, 3, and 4, respectively had significant ameliorated hepatic congestion compared to G1, G7, and G8 (Table 4).
Figure 2.
Histopathological examination of chicken liver. (A) Liver of chicken in G1 showing congestion of central vein (arrow) and hepatic sinusoids (arrowhead). (B) Liver of chicken in G2 revealing normal hepatocytes with no congestion. (C) Liver of chicken in G3 revealing normal hepatocytes with slight congestion (arrowhead). (D) Liver of chicken in G4 showing normal hepatocytes with moderate congestion in the hepatic sinusoids (arrowhead). (E) Liver of chicken in G5 revealing normal hepatocytes. (F) Liver of chicken in G6 exposing normal hepatocytes. (G) Liver of chicken in G7 exposing central vein congestion (arrow) and congestion in the hepatic sinusoids (arrowhead). (H) Liver of chicken in G8 revealing normal hepatocytes. Scale bar =50 μm.
Intestinal Bacterial Count
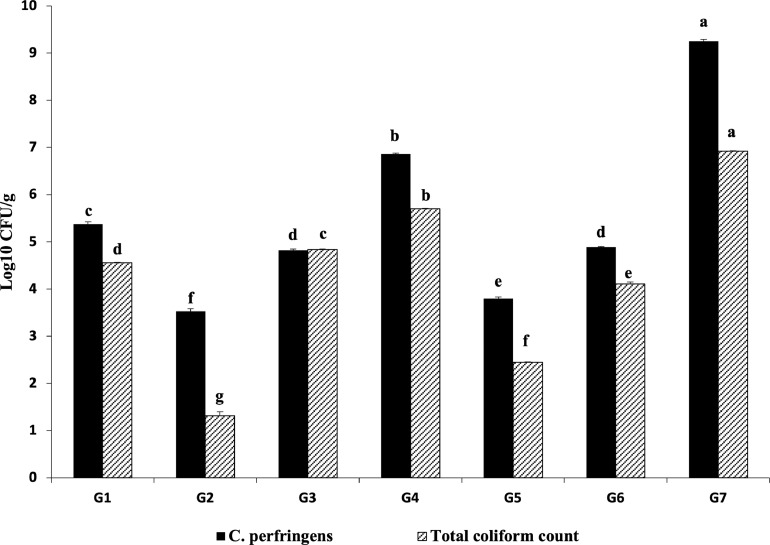
Both C. perfringens and total coliform counts were significantly (P ≤ 0.05) the lowest in the Culbac aqueous and Amoxicillin treated G2 (3.53 and 1.13 CFU/mL, respectively) and Immunobacteryne and Amoxicillin treated G5 (3.8 and 2.45 CFU/mL, respectively) compared with challenged-nontreated G7 (s 9.25 and 6.92 CFU/mL, respectively) and other Gs. Furthermore, G3, G6, G1, and G4, respectively showed significant (P ≤ 0.05) improvement in the count when compared with G7 (Figure 3). The bacterial counts in G8 showed nondetectable results.
Figure 3.
C. perfringens and total coliform counts (Log10 CFU/g). Different superscripts (a, b, c, d, e, f, g) within the same bar pattern, are significantly differ (P ≤ 0.05).
Hemagglutination Inhibition (HI) Test for NDV and HPAI V (H5N1)
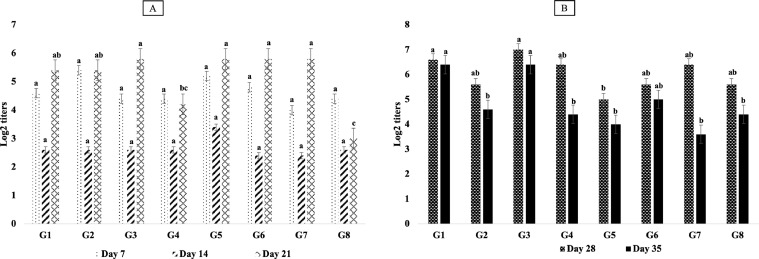
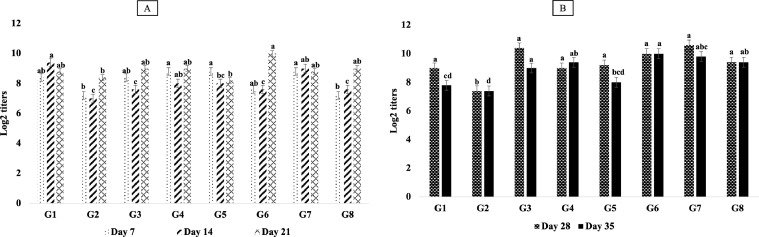
Regarding the HI results of NDV, there were no significant (P > 0.05) differences among all Gs on the d 7th, and 14th of age. However, G3, G5, and G7 showed the highest significant (P ≤ 0.05) different results (5.8 log2) on d 21st of age, and also G3 and G1 had the highest significant (P ≤ 0.05) titers (6.6 log2 and 6.4 log2, respectively) on d 28th and 35th of age in comparison with other Gs (Figure 4). The results of HI titers for HPAIV (H5N1) indicated highest significant (P ≤ 0.05) values in G4, G5, and G7 (8.8 log2) on a d 7th of age, while these titers were the greatest in G3 and G6 (9.8 log2 and 10 log2, respectively) on a d 35th of age in comparison with others (Figure 5).
Figure 4.
Results of HI test detecting humoral antibody response (Log2 titers) for Newcastle disease (A: HI titers for all chicken groups on 7-, 14-, and 21-dayold; B: HI titers for all chicken groups on 28- and 35-day-old). Different superscripts (a, b, c, d) within the same bar pattern, are significantly differ (P ≤ 0.05).
Figure 5.
Results of HI test detecting humoral antibody response (Log2 titers) for HPAI-H5N1 (A: HI titers for all chicken groups on 7-, 14-, and 21-day-old; B: HI titers for all chicken groups on 28- and 35-day-old). Different superscripts (a, b, c, d) within the same bar pattern, are significantly differ (P ≤ 0.05).
Liver Function Tests
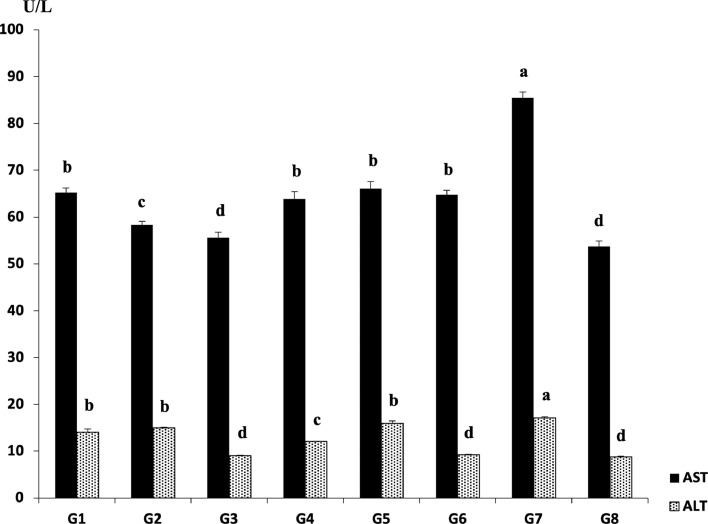
The results of AST and ALT are summarized in Figure 6. The best significantly (P ≤ 0.05) AST values were in the nonchallenged or treated G8 (53.71 U/L) and the Culbac dry and aqueous treated G3 (55.62 U/L) in comparison with other Gs. In addition, G2, G4, G6, G1, and G5, respectively showed significant (P ≤ 0.05) improvement of AST when compared with G7 (85.47 U/L). The results of ALT were most alleviated significantly (P ≤ 0.05) in G3 (9.03 U/L), G6 (9.26 U/L), G4 (12.11 U/L), G1 (14.06 U/L), G2 (15.01 U/L), and G5 (15.98 U/L) as compared with G7 (17.09 U/L).
Figure 6.
Results of liver function tests (AST and ALT) (U/L). Different superscripts (a, b, c, d) within the same bar pattern, are significantly differ (P ≤ 0.05) (Reitman and Frankel, 1957).
DISCUSSION
Several nonantimicrobial strategies or AGPs alternatives (e.g., plant extracts, essential oils, organic acids, prebiotics, probiotics, synbiotics, and postbiotics) have delivered encouraging outcomes in controlling NE and minimizing acquired antimicrobial resistance of C. perfringens (Gadde et al., 2017; Hofacre et al., 2018). However, the protective efficacies of these therapeutics against NE were inconsistent and variable (Dahiya et al., 2006; Thanh et al., 2009; Caly et al., 2015; Agunos et al., 2019; Johnson et al., 2019; Abd El-Hack et al., 2022; Shojadoost et al., 2022; Swaggerty et al., 2022) and the threat of live probiotics proliferating mechanisms of antimicrobial resistance remains largely untested, but stabilized postbiotics would not carry this threat. Accordingly, the current study was designed to compare the efficacies of 3 antimicrobials (a postbiotic, a probiotic, and an antibiotic) for controlling C. perfringens experimental infection in commercial broiler chickens.
Postbiotic or probiotic aqueous plus antibiotic (G2 or G5, respectively) as well as postbiotic dry and aqueous (G3) significantly (P ≤ 0.05) diminished the clinical disease, mortality rate, and the intestinal lesion scores compared with challenged-nontreated chickens (G7). These results strongly indicated an improvement in chickens performance especially in the Culbac dry and aqueous treated G3 which had the 2nd significant (P ≤ 0.05) highest FBWT and EPEF following G8 (nontreated, nonchallenged) side by side with the best significant (P ≤ 0.05) FCR. It has been observed that postbiotics feed additives could improve the health and growth performance in broilers by promoting their immune status, growth gene expression, and gut health (improved intestinal villi, limited population of Enterobacteriaceae and fecal pH, with increasing populations of lactic acid bacteria) (Thanh et al., 2009; Loh et al., 2010; Rosyidah et al., 2011; Kareem et al., 2016, 2017). Recent studies concluded that dietary supplementation of postbiotics produced from L. plantarum increased FBWT, BWG, FCR, intestinal villus height, immune response, some intestinal mRNA gene expression, and beneficial bacterial population in the cecum, but reduced Enterococcus (E.) and Escherichia coli (E. coli) populations in heat-stressed broilers (Humam et al., 2020). In the same way, Johnson et al. (2019) tested a postbiotic (fermented product containing organic acids produced from a cocktail of probiotic strains: Pediococcus acidilactici, L. reuteri, E. faecium, and L. acidophilus [Flock Vitality]) against C. perfringens challenge in chickens and concluded that the used postbiotic reduced the lesion scores, mortality, and C. perfringens counts, but increased the BWG. Decreasing the mortality rate and the intestinal lesion scores following C. perfringens challenge were the main modulating features of some microencapsulated blend of organic acids (citric and sorbic), botanicals (thymol and vanillin), and various probiotics and phytobiotic natural compounds (Hussein et al., 2020; Swaggerty et al., 2022).
Here, C. perfringens and total coliform counts were significantly (P ≤ 0.05) lower in chickens of G2 followed by G5 (both exposed to traditional antibiotic, Amoxicillin plus Culbac and Immunobacteryne, respectively) compared to G7 and other Gs. In addition, G3, G6, G1, and G4 showed significant (P ≤ 0.05) improved count when compared with G7. However, the postbiotic, Culbac, in G1, G2, and G3 induced more significant (P ≤ 0.05) improvement in the total coliform count than probiotics. This improvement may be attributed to the more direct and the faster action of postbiotics than probiotics. Postbiotics contain several antimicrobial components including bacteriocins (short-chain fatty acids, peptides, and proteins) and organic acids which can minimize the pH of the gut, prevent the growth of pathogens, and consequently induce a positive influence on poultry health (Aguilar-Toalá et al., 2018). Several researches reported that postbiotics produced by Lactobacillus species have multiple health benefits and an inhibitory effect on different gut pathogens such as E. coli, Salmonella typhimurium, Listeria monocytogenes, and vancomycin-resistant Enterococcus and they recommended using of postbiotics as potential alternatives to antibiotics (Thanh et al., 2009, 2010; Van Thu et al., 2011; Tsilingiri et al., 2012; Choe et al., 2013; Cicenia et al., 2014; Kareem et al., 2014; Klemashevich et al., 2014).
The small intestine of chickens acts as a potential barrier for separation of the internal and external environment of the body. It is relatively short, highly specialized in the digestion and absorption of nutrients, and its absorption area mainly depends on the surface area of the intestinal villi. The structure of the small intestine provides important information on the health status of the digestive tract (Teirlynck et al., 2009). In the current study, the histopathology of C. perfringens experimentally infected chickens revealed severe submucosal congestion and degeneration of intestinal villi in G7; however, these histopathological effects were significantly improved in chickens of Gs 2, 6, and 5, respectively and showed significant comparable minimal lesions in chickens of G3. This result may indicate beneficial effects of both postbiotics and probiotics on the absorptive surface of the jejunum. Such improvement in the morphological parameters of the intestinal mucosa increases the absorption rate as a result of the competition for resources and ecological niche between lactic acid bacteria (LAB) and pathogenic microorganisms (Shojadoost et al., 2022). This competition is mainly based on the inhibitory effect of volatile fatty acids and bacteriocins produced by LAB on the growth of pathogenic bacteria (Vieco-Saiz et al., 2019). Here, the use of a probiotic in feed (G4) moderately decreased enterocyte degeneration; while the use of a postbiotic in feed (G1)-induced severe villus necrosis and lymphocytic infiltration. This result indicates that feed treatment with either probiotic or postbiotic had very low or no effect on the intestinal histopathology of chickens experimentally infected with C. perfringens.
Interestingly, the liver of chickens in Gs 2, 6, 5, and 3, respectively demonstrated normal histology which indicates that the combination of the antibiotic and the postbiotic or the probiotic in drinking water, or the combination of the dry and aqueous formula of the postbiotic or the probiotic are effective in restoring the drawbacks of NE on liver. However, chickens that received only feed treatment with the probiotic and the postbiotic showed moderate and severe vascular sinusoidal congestion with mild hemorrhages. This effect may indicate the low ability of these treatments to alleviate liver lesions despite their prolonged use.
Monitoring of liver enzymes such as AST and ALT is essential for evaluating the function and viability of the liver. Accordingly, the significant (P ≤ 0.05) increase in the activity levels of both AST and ALT reflects the hepatic damage and biliary stasis induced by C. perfringens toxins (Fraser et al., 1991; Allam et al., 2013). The greater improvement in the liver function tests in G3 as compared with the antibiotic treated chicken's points to the ameliorating effects of postbiotics on the damaged hepatocytes of C. perfringens challenged chickens (Llanco et al., 2012; Abd El-Hamid et al., 2015).
For the control of NE, another benefit of postbiotics is the immunological enhancement. In this work, significant HI titers were observed for NDV of both G3 (from the 3rd wk of age till the end of experiment) and G1 (at the last 2 wk of age) and also for HPAI-H5N1 of G3, G4, and G6, respectively (from the 3rd wk of age till the end of experiment). Johnson et al. (2019) proved that postbiotics could modulate the immune response in C. perfringens challenged chickens in terms of enhancement of innate immunity, reduction of the proinflammatory reaction, and generation of a homeostatic-like response. To the best of our knowledge, the proper function of the mucosa-associated immune system relies on the presence of intestinal bacteria. Bacteriocins as antibacterial compounds produced by LAB enhance the intestinal immune system, improve the resistance to pathogenic bacterial colonization, and maintain specific homeostasis in the gastrointestinal tract (Cheng et al., 2014; Madej and Bednarczyk, 2015; Asgari et al., 2016). Additionally, Lactobacillus bacteria or their metabolic byproducts can increase the number of intraepithelial lymphocytes and immunoglobulin A-producing cells in the intestinal tract which lead to development of intestinal resistance and increased the secretion of interleukin (IL-6) (Salah et al., 2012; Jayaraman et al., 2013; Rajput et al., 2013; Ruiz et al., 2015; Khalique et al., 2020). Shojadoost et al. (2022) reported that inoculation of 4 different species of Lactobacilli in broiler chickens reduced NE severity, significantly modulated the broilers’ immune responses by alteration of interferon -γ (IL)-1β, -2, -12p35, -17, and transforming growth factor beta gene transcription in the intestine, enhanced production of CD8 + T cells and B cells in the cecal tonsil, and modulated the intestinal microbiota composition.
Conclusion
A combination of an aqueous postbiotic (Culbac) and antibiotic (Amoxicillin) induced the best ameliorating effect on NE in broiler chickens. In comparison with probiotic and antibiotic, the combined feed and water treatment of a postbiotic “Culbac” gave more promising results for controlling NE in terms of enhancing the humoral immune response and the hepatic health of C. perfringens experimentally infected broiler chickens.
DISCLOSURES
The authors reported no potential conflict of interest.
REFERENCES
- Abd El-Ghany W.A. Paraprobiotics and postbiotics: contemporary and promising natural antibiotics alternatives and their applications in the poultry field. Open Vet. J. 2020;10:323–330. doi: 10.4314/ovj.v10i3.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.E., Elbestawy A.R., El-Shall N.A., Saad A.M., Salem H.M., et al. Necrotic enteritis in broiler chickens: disease characteristics and prevention using organic antibiotic alternatives – a comprehensive review. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Hamid H.S., Ellakany H.F., Bekhit A.A., Elbestawy A.R., Bedewae S. Clinical and laboratory studies on chicken isolates of Clostridium Perfringens in El-Behera, Egypt. J. World's Poult. Res. 2015;5:21–28. [Google Scholar]
- Aguilar-Toalá J., Garcia-Varela R., Garcia H., Mata-Haro V., González-Córdova A., Vallejo-Cordoba B., Hernández-Mendoza A. Postbiotics: an evolving term within the functional foods field. Trends Food Sci. Technol. 2018;75:105–114. [Google Scholar]
- Agunos A., Deckert A., Le ´ger D., Gow S., Carson C. Antimicrobials used for the therapy of necrotic enteritis and coccidiosis in broiler chickens and turkeys in Canada, farm surveillance results (2013–2017) Avian Dis. 2019;63:433–445. doi: 10.1637/11971-091718-Reg.1. [DOI] [PubMed] [Google Scholar]
- Ajuwon K.M. Toward a better understanding of mechanisms of probiotics and prebiotics action in poultry species. J. Appl. Poult. Res. 2015;25:277–283. [Google Scholar]
- Allam H.H., Nahad A.G., Abdullaha S.H., Dina M.M. Immuno-biochemical and pathological studies on necrotic enteritis in Pekin duckling with trial of treatment. J. Mansoura Vet. Med. 2013;XV:211–226. [Google Scholar]
- AOAC. 2005. Official Methods of Analysis of the Association of Analytical Chemists International: Gaithersburg, MD, USA.
- Asgari F., Madjd Z., Falak R., Bahar M.A., Nasrabadi M.H., Raiani M., Shekarabi M. Probiotic feeding affects T cell populations in blood and lymphoid organs in chickens. Beneficial Microbes. 2016;(7):669–675. doi: 10.3920/BM2016.0014. [DOI] [PubMed] [Google Scholar]
- Aviagen . Aviagen; west virginia, USA: 2019. Broiler (Ross 308) Nutrition Specifications; pp. 1–10. [Google Scholar]
- Bancroft J.D., Layton C. In: Theory and Practice of Histological Techniques. 7th ed. Suvarna SK, Layton C, Bancroft JD, editors. ElSevier, Churchill Livingstone; Pennsylvania: 2013. The hematoxylin and eosin; pp. 179–220. editors. [Google Scholar]
- Blacher E., Levy M., Tatirovsky E., Elinav E. Microbiome-modulated metabolites at the interface of host immunity. J. Immunol. 2017;198:572–580. doi: 10.4049/jimmunol.1601247. [DOI] [PubMed] [Google Scholar]
- Caly D.L., D'Inca R., Auclair E., Drider D. Alternatives to antibiotics to prevent necrotic enteritis in broiler chickens: a microbiologist's perspective. Front. Microbiol. 2015;6:1336. doi: 10.3389/fmicb.2015.01336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casewell M., Friis C., Marco E., McMullin P., Phillips I. The European ban on growth-promoting antibiotics and emerging consequences for human and animal health. J. Antimicrob. Chemother. 2003;52:159–161. doi: 10.1093/jac/dkg313. [DOI] [PubMed] [Google Scholar]
- Cheng G., Hao H., Xie S., Wang X., Dai M., Huang L., Yuan Z. Antibiotic alternatives: the substitution of antibiotics in animal husbandry? Front. Microbiol. 2014;5:69–83. doi: 10.3389/fmicb.2014.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe D.W., Foo H.L., Loh T.C., Hair-Bejo M., Awis Q.S. Inhibitory property of metabolite combinations produced from Lactobacillus plantarum strains. Pertanika J. Trop. Agric. Sci. 2013;36:79–88. [Google Scholar]
- Cicenia A., Scirocco A., Carabotti M., Pallotta L., Marignani M., Severi C. Postbiotic activities of Lactobacilli-derived factors. J. Clin. Gastroent. 2014;48:S18–S22. doi: 10.1097/MCG.0000000000000231. [DOI] [PubMed] [Google Scholar]
- Craven S.E., Stern N.J., Bailey J.S., Cox N.A. Incidence of Clostridium perfringens in broiler chickens and their environment during production and processing. Avian Dis. 2001;45:887–896. [PubMed] [Google Scholar]
- Dahiya J., Wilkie D., Van Kessel A., Drew M. Potential strategies for controlling necrotic enteritis in broiler chickens in post-antibiotic era. Anim. Feed Sci. Tech. 2006;129:60–88. [Google Scholar]
- Dahiya J., Hoehler D., Van Kessel A., Drew M. Effect of different dietary methionine sources on intestinal microbial populations in broiler chickens. Poult. Sci. 2007;86:2358–2366. doi: 10.3382/ps.2007-00133. [DOI] [PubMed] [Google Scholar]
- Drew M., Syed N., Goldade B., Laarveld B., Van Kessel A. Effects of dietary protein source and level on intestinal populations of Clostridium perfringens in broiler chickens. Poult. Sci. 2004;83:414–420. doi: 10.1093/ps/83.3.414. [DOI] [PubMed] [Google Scholar]
- Durso L.M., Cook K.L. Impacts of antibiotic use in agriculture: what are the benefits and risks? Curr. Opin. Microbiol. 2014;19:37–44. doi: 10.1016/j.mib.2014.05.019. [DOI] [PubMed] [Google Scholar]
- Engster H.M., Marvil D., Stewart-Brown B. The effect of withdrawing growth promoting antibiotics from broiler chickens: a long-term commercial industry study. J. Appl. Poult. Res. 2002;11:431–436. [Google Scholar]
- Eraky R.D., Abd El-Ghany W.A. Genetic characterization, antibiogram pattern, and pathogenicity of Clostridium perfringens isolated from broiler chickens with necrotic enteritis. J. Indonesian Trop. Anim. Agric. 2022;47:1–16. [Google Scholar]
- Fraser M., Bergeron A., Mays A., Aiello E.S. 7th ed. Merck & Co., Inc.; Michigan, USA: 1991. The Merck Veterinary Manual. [Google Scholar]
- Gadde U., Kim W.H., Oh S.T., Lillehoj H.S. Alternatives to antibiotics for maximizing growth performance and feed efficiency in poultry: a review. Anim. Health Res. Rev. 2017;18:26–45. doi: 10.1017/S1466252316000207. [DOI] [PubMed] [Google Scholar]
- Gaucher M.L., Quessy S., Letellier A., Arsenault J., Boulianne M. Impact of a drug-free program on broiler chicken growth performances, gut health, Clostridium perfringens and Campylobacter jejuni occurrences at the farm level. Poult. Sci. 2015;94:1791–1801. doi: 10.3382/ps/pev142. [DOI] [PubMed] [Google Scholar]
- Gibson-Corley K.N., Olivier A.K., Meyerholz D.K. Principles for valid histopathologic scoring in research. Vet. Pathol. 2013;50:1007–1015. doi: 10.1177/0300985813485099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grave K., Kaldhusdal M., Kruse H., Harr L.M.F., Flatlandsmo K. What has happened in Norway after the ban of avoparcin? Consumption of antimicrobials by poultry. Prev. Vet. Med. 2004;62:59–72. doi: 10.1016/j.prevetmed.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Hofacre C.L., Smith J.A., Mathis G.F. An optimist's view on limiting necrotic enteritis and maintaining broiler gut health and performance in today's marketing, food safety, and regulatory climate. Poult. Sci. 2018;97:1929–1933. doi: 10.3382/ps/pey082. [DOI] [PubMed] [Google Scholar]
- Huang L., Luo L., Zhang Ys., Wang Z., Xia Z. Effects of the dietary probiotic, Enterococcus faecium NCIMB11181, on the intestinal barrier and system immune status in Escherichia coli O78-challenged broiler chickens. Probiot, Antimicrob. Proteins. 2019;11:946–956. doi: 10.1007/s12602-018-9434-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humam A.M., Loh T.C., Foo H.L., Izuddin W.I., Awad E.A., Idrus Z., Samsudin A.A., Mustapha N.M. Dietary supplementation of postbiotics mitigates adverse impacts of heat stress on antioxidant enzyme activity, total antioxidant, lipid peroxidation, physiological stress indicators, lipid profile and meat quality in broilers. Animals. 2020;10:982–1003. doi: 10.3390/ani10060982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein E.O.S., Ahmed S.H., Abudabos A.M., Suliman G.M., Abd El-Hack M.E., Swelum A.A., Alowaimer A.N. Ameliorative effects of antibiotic-, probiotic- and phytobiotic-supplemented diets on the performance, intestinal health, carcass traits, and meat quality of Clostridium perfringens-infected broilers. Animals. 2020;10:669. doi: 10.3390/ani10040669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman S., Thangavel G., Kurian H., Mani R., Mukkalil R., Chirakkal H. Bacillus subtilis PB6 improves intestinal health of broiler chickens challenged with Clostridium perfringens-induced necrotic enteritis. Poult. Sci. 2013;92:370–374. doi: 10.3382/ps.2012-02528. [DOI] [PubMed] [Google Scholar]
- Johnson C.N., Kogut M.H., Genovese K., He H., Kazemi S., Arsenault R.J. Administration of a postbiotic causes immunomodulatory responses in broiler gut and reduces disease pathogenesis following challenge. Microorganisms. 2019;7:268. doi: 10.3390/microorganisms7080268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karavolias J., Salois M.J., Baker K.T., Watkins K. Raised without antibiotics: impact on animal welfare and implications for food policy. Transl. Anim. Sci. 2018;2:337–348. doi: 10.1093/tas/txy016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kareem K.Y., Foo H.L., Loh T.C., Ooi M.F., Samsudin A.A. Inhibitory activity of postbiotic produced by strains of Lactobacillus plantarum using reconstituted media supplemented with inulin. Gut Pathog. 2014;6:23–30. doi: 10.1186/1757-4749-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kareem K.Y., Loh T.C., Foo H.L., Akit H., Samsudin A.A. Effects of dietary postbiotic and inulin on growth performance, IGF1 and GHR mRNA expression, faecal microbiota and volatile fatty acids in broilers. BMC Vet. Res. 2016;12:163–173. doi: 10.1186/s12917-016-0790-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kareem K.Y., Loh T.C., Foo H.L., Asmara S.A., Akit H. Influence of postbiotic RG14 and inulin combination on cecal microbiota, organic acid concentration, and cytokine expression in broiler chickens. Poult. Sci. 2017;96:966–975. doi: 10.3382/ps/pew362. [DOI] [PubMed] [Google Scholar]
- Khalique A., Zeng D., Shoaib M., Wang H., Qing X., Rajput D.S., Pan K., Ni X. Probiotics mitigating subclinical necrotic enteritis (SNE) as potential alternatives to antibiotics in poultry. AMB Expr. 2020;10:50. doi: 10.1186/s13568-020-00989-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiu R., Hall L.J. An update on the human and animal enteric pathogen Clostridium perfringens. Emerg. Microbes Infect. 2018;7:1–15. doi: 10.1038/s41426-018-0144-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemashevich C., Wu C., Howsmon D., Alaniz R.C., Lee K., Jayaraman A. Rational identification of diet-derived postbiotics for improving intestinal microbiota function. Curr. Opin. Biotech. 2014;26:85–90. doi: 10.1016/j.copbio.2013.10.006. [DOI] [PubMed] [Google Scholar]
- Konstantinov S.R., Smidt H., de Vos W.M., Bruijns S.C., Singh S.K., Valence F., et al. S layer protein aof Lactobacillus acidophilus NCFM regulates immature dendritic cell and T cell functions. Proc. Natl. Acad. Sci. U. S. A. 2008;105:19474–19479. doi: 10.1073/pnas.0810305105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Chen C., Indugu N., Werlang G.O., Singh M., Kim W.K., Thippareddi H. Effect of antibiotic withdrawal in feed on chicken gut microbial dynamics, immunity, growth performance and prevalence of foodborne pathogens. PLoS ONE. 2018;13 doi: 10.1371/journal.pone.0192450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Lee S., Gadde U., Oh S., Lee S., Lillehoj H. Allium hookeri supplementation improves intestinal immune response against necrotic enteritis in young broiler chickens. Poult. Sci. 2018;97:1899–1908. doi: 10.3382/ps/pey031. [DOI] [PubMed] [Google Scholar]
- Llanco L.A., Viviane N., Ferreira A.J., Avilacampos M.J. Toxinotyping and antimicrobial susceptibility of Clostridium perfringens isolated from broiler chickens with necrotic enteritis. Int. J. Microbiol. Res. 2012;4:290–294. [Google Scholar]
- Loh T.C., Thanh N.T., Foo H.L., Hair-Bejo M., Azhar B.K. Feeding of different levels of metabolite combinations produced by Lactobacillus plantarum on growth performance, fecal microflora, volatile fatty acids and villi height in broilers. Anim. Sci. J. 2010;81:205–214. doi: 10.1111/j.1740-0929.2009.00701.x. [DOI] [PubMed] [Google Scholar]
- Long J.R., Truscott R.B. Necrotic enteritis in broiler chickens. III. Reproduction of the disease. Can. J. Comp. Med. 1976;40:53–59. [PMC free article] [PubMed] [Google Scholar]
- Lu M., Li R.W., Zhao H., Yan X., Lillehoj H.S., Sun Z., et al. Effects of Eimeria maxima and Clostridium perfringens infections on cecal microbial composition and the possible correlation with body weight gain in broiler chickens. Res. Vet. Sci. 2020;132:142–149. doi: 10.1016/j.rvsc.2020.05.013. [DOI] [PubMed] [Google Scholar]
- Madej J.P., Bednarczyk M. Effect of in ovo-delivered prebiotics and synbiotics on the morphology and specific immune cell composition in the gut-associated lymphoid tissue. Poult. Sci. 2015;94:1209–1219. doi: 10.3382/ps/pev291. [DOI] [PubMed] [Google Scholar]
- McDevitt R.M., Brooker J.D., Acamovic T., Sparks N.H.C. Necrotic enteritis; a continuing challenge for the poultry industry. Worlds. Poult. Sci. J. 2006;62:221–247. [Google Scholar]
- NRC . National Academies Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Manual of Standards for Diagnostic Tests and Vaccines for Terrestrial Animals (Mammals, Birds and Bees), Chapter 10.9. OIE - Terrestrial Animal Health Code; Paris, France: 2021. Office International des Epizooties (OIE). Newcastle Disease (Infection with Newcastle Disease Virus) pp. 10–11. [Google Scholar]
- Parish W.E. Necrotic enteritis in the fowl (Gallus gallus domesticus). I. Histopathology of the disease and isolation of a strain of Clostridium welchii. J. Comp. Pathol. 1961;71:377–393. http://www.ncbi.nlm.nih.gov/pubmed/14483884 [PubMed] [Google Scholar]
- Prescott J.F., Sivendra R., Barnum D.A. The use of bacitracin in the prevention and treatment of experimentally-induced necrotic enteritis in the chicken. Can. Vet. J. 1978;19:181–183. [PMC free article] [PubMed] [Google Scholar]
- Rajput I.R., Li L.Y., Xin X., Wu B.B., Juan Z.L., Cui Z.W., Li W.F. Effect of Saccharomyces boulardii and Bacillus subtilis B10 on intestinal ultrastructure modulation and mucosal immunity development mechanism in broiler chickens. Poult. Sci. 2013;92:956–965. doi: 10.3382/ps.2012-02845. [DOI] [PubMed] [Google Scholar]
- Reitman S., Frankel F. A colorimetric method for determination of serum glutamic oxaloacetic and glutamic pyruvic transaminases. J. Am. Clin. Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- Rosyidah M.R., Loh T.C., Foo H.L., Cheng X.F., Bejo M.H. Effect of feeding metabolites and acidifier on growth performance, faecal characteristics and microflora in broiler chickens. J. Anim. Vet. Adv. 2011;10:2758–2764. [Google Scholar]
- Ruiz R., Peinado M.J., Aranda-Olmedo I., Abecia L., Suárez-Pereira E., Ortiz Mellet C., Fernández J.M.G., Rubio L.A. Effects of feed additives on ileal mucosa–associated microbiota composition of broiler chickens. J. Anim. Sci. 2015;93:3410–3420. doi: 10.2527/jas.2015-8905. [DOI] [PubMed] [Google Scholar]
- Salah R.B., Trabelsi I., Mansour R.B., Lassoued S., Chouayekh H., Bejar S. A new Lactobacillus plantarum strain, TN8, from the gastro intestinal tract of poultry induces high cytokine production. Anaerobe. 2012;18:436–444. doi: 10.1016/j.anaerobe.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Sarson A.J., Wang Y., Kang Z., Dowd S.E., Lu Y., Yu H., Han Y., Zhou H., Gong J. Gene expression profiling within the spleen of Clostridium perfringens-challenged broilers fed antibiotic-medicated and non-medicated diets. BMC Genomics. 2009;10:260. doi: 10.1186/1471-2164-10-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal B.S., Lillehoj H.S., Donovan D.M., Gay C.G. Alternatives to antibiotics: A symposium on the challenges and solutions for animal production. Anim. Health Res. Rev. 2013;14:78–87. doi: 10.1017/S1466252313000030. [DOI] [PubMed] [Google Scholar]
- Shojadoost B., Vince A.R., Prescott J.F. The successful experimental induction of necrotic enteritis in chickens by Clostridium perfringens: a critical review. Vet. Res. 2012;43:74. doi: 10.1186/1297-9716-43-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shojadoost B., Alizadeh M., Boodhoo N., Astill J., Karimi S.H., Doost J.S., Taha‑Abdelaziz K., Kulkarni R., Sharif S. Effects of treatment with Lactobacilli on necrotic enteritis in broiler chickens [e-pub ahead of print] Probiotics Antimicro. Prot. 2022 doi: 10.1007/s12602-021-09901-5. accessed January 19, 2022. [DOI] [PubMed] [Google Scholar]
- SPSS, IBM SPSS statistics for Windows, In: Version 20, 2011, IBM Corp.; New York, USA.
- Swaggerty C.L., Byrd J.A., Arsenault R.J., Perry F., Johnson C.N., Genovese K.J., He H., Kogut M.H., Piva A., Grilli E. A blend of microencapsulated organic acids and botanicals reduces necrotic enteritis via specific signaling pathways in broilers. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanh N.T., Loh T.C., Foo H.L., Hair-Bejo M., Azhar B.K. Effects of feeding metabolite combinations produced by Lactobacillus plantarum on growth performance, fecal microbial population, small intestine villus height and fecal volatile fatty acids in broilers. Br. Poult. Sci. 2009;50:298–306. doi: 10.1080/00071660902873947. [DOI] [PubMed] [Google Scholar]
- Thanh N.T., Loh T.C., Foo H.L., Bejo M.H., Azhar B.K. Inhibitory activity of metabolites produced by strains of Lactobacillus plantarum isolated from Malaysian fermented food. Int. J. Probiotics Prebiotics. 2010;5:37–43. [Google Scholar]
- Teirlynck E., Bjerrum L., Eeckhaut V., Huygebaert G., Pasmans F., Haesebrouck F., Dewulf J., Ducatelle R., Van Immerseel F. The cereal type in feed influences gut wall morphology and intestinal immune cell infiltration in broiler chickens. Br. J. Nutr. 2009;102:1453–1461. doi: 10.1017/S0007114509990407. [DOI] [PubMed] [Google Scholar]
- Timbermont L., Haesebrouck F., Ducatelle R., Van Immerseel F. Necrotic enteritis in broilers: an updated review on the pathogenesis. Avian Pathol. 2011;40:341–347. doi: 10.1080/03079457.2011.590967. [DOI] [PubMed] [Google Scholar]
- Tsilingiri K., Barbosa T., Penna G., Caprioli F., Sonzogni A., Viale G., Rescigno M. Probiotic and postbiotic activity in health and disease: comparison on a novel polarised ex-vivo organ culture model. Gut. 2012;61:1007–1015. doi: 10.1136/gutjnl-2011-300971. [DOI] [PubMed] [Google Scholar]
- Van Immerseel F., De Buck J., Pasmans F., Huyghebaert G., Haesebrouck F., Ducatelle R. Clostridium perfringens in poultry: an emerging threat for animal and public health. Avian Pathol. 2004;33:537–549. doi: 10.1080/03079450400013162. [DOI] [PubMed] [Google Scholar]
- Van Thu T., Foo H.L., Loh T.C., Bejo M.H. Inhibitory activity and organic acid concentrations of metabolite combinations produced by various strains of Lactobacillus plantarum. Afr. J. Biotechnol. 2011;10:1359–1363. http://www.academicjournals.org/AJB [Google Scholar]
- Vieco-Saiz N., Belguesmia Y., Raspoet R., Auclair E., Gancel F., Kempf I., Drider D. Benefits and inputs from lactic acid bacteria and their bacteriocins as alternatives to antibiotic growth promoters during food-animal production. Front. Microbiol. 2019;10:1–17. doi: 10.3389/fmicb.2019.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S-B., Stanley D., Rodgers N., Swick R.A., Moore R.J. Two necrotic enteritis predisposing factors, dietary fishmeal and Eimeria infection, induce large changes in the caecal microbiota of broiler chickens. Vet. Microbiol. 2014;169:188–197. doi: 10.1016/j.vetmic.2014.01.007. [DOI] [PubMed] [Google Scholar]
- Zahoor I., Ghayas A., Basheer A. Genetics and genomics of susceptibility and immune response to necrotic enteritis in chicken: a review. Mol. Biol. Rep. 2018;45:31–37. doi: 10.1007/s11033-017-4138-8. [DOI] [PubMed] [Google Scholar]