Abstract
The reports of vascular adverse events in the eye following COVID-19 vaccination are infrequent. We report the case of a healthy male who developed central retinal vein occlusion in his left eye three days following administration of the first dose of Covishield vaccine. As the underlying systemic and ocular risk factors were absent and laboratory investigations were normal, vein occlusion appeared to probably result from the vaccine. The patient developed retinal hemorrhages and non-perfusion ischemic areas all over the fundus. The macular edema was reduced with intravitreal triamcinolone acetonide, but the visual gain was not much, which appears to be due to the time lag in his initial presentation to the Ophthalmology Department. A close watch should be kept for ophthalmic adverse events to have an early intervention.
Keywords: retina and vaccine, post vaccination thromboembolism, vaccine adverse effects, covid vaccine, central retinal vein occlusion
Introduction
India is running the largest vaccination campaign against coronavirus disease 2019 (COVID-19), mainly with the Oxford-AstraZeneca COVID-19 vaccine (AZD1222; known locally as Covishield) and Covaxin (BBV152) vaccine. The COVID-19 vaccines can cause neurological, cardiovascular, otorhinolaryngological, and vascular adverse events attributed to thromboembolic, inflammatory, immunological, or anaphylactic mechanisms precipitated by the vaccine [1,2].
We report a case of a healthy young man who developed central retinal vein occlusion (CRVO) in the left eye (LE) within three days of receiving his first dose of the Covishield COVID-19 vaccine. CRVO is associated with risk factors such as raised intraocular pressure (IOP), hypertension, diabetes, cardiovascular disease, hypercoagulable state, inflammation, deranged lipid profile, and elevated serum homocysteine levels. These factors lead to a compression of the central retinal vein or obstruction in its blood flow from a thrombus or an embolus, causing ischemia, edema, and, finally, neovascularization [3]. Though an underlying or undiagnosed illness independent of vaccination may also be a causative factor, the emergence of complications within a short period from vaccination suggests the latter [4]. A search of the PubMed Index in different languages shows that few cases of CRVO after COVID-19 vaccination have been reported [4-7].
Case presentation
A 43-year-old healthy man developed sudden painless diminution of his vision in the LE three days after receiving the first dose of the Covishield COVID-19 vaccine (Batch No. 4121MC028; Expiry October 31, 2021). He had no history of smoking, ocular surgery, systemic illness, trauma, excessive physical exercise, or dehydration. He was diagnosed with CRVO in the LE at a separate clinic. Two months later, he presented to our ophthalmology outpatient department and tested negative on reverse transcriptase-polymerase chain reaction for COVID-19. His blood pressure was 130/90 mmHg. His laboratory investigation results were within reference ranges except for glycated hemoglobin, which was slightly raised (Table 1). His electrocardiogram and echocardiography study revealed no ischemia or any other significant findings.
Table 1. Laboratory investigations of the patient with reference ranges.
P, polymorphonuclear leucocytes; L, lymphocytes; M, monocytes; E, eosinophils, B: basophils
| Analyte | Patient’s Value | Reference Range |
| Hemoglobin | 14.3 gram/deciliters | 13-17 gram/deciliters |
| Blood sugar fasting | 67 milligrams/deciliters (slightly low) | 70-99 milligrams/deciliters |
| Blood sugar post prandial | 95 milligrams/deciliters (slightly low) | 120-180 milligrams/deciliters |
| Acetylated hemoglobin | 6.7% (slightly raised) | <6.5% |
| Serum glutamic oxaloacetic transaminase | 22 units/liter | <35 units/liter |
| Serum glutamic pyruvic transaminase | 32 units/liter | <45 units/liter |
| Blood urea | 34 milligrams/deciliters | 15-40 milligrams/deciliters |
| Serum creatinine | 1.0 milligrams/deciliters | 0.6-1.1 milligrams/deciliters |
| Total leucocyte count | 6.98 x 103/microliters | 4-10 x 103/microliters |
| Differential leucocyte count | P63 L30 M4 E3 B0 | |
| Platelet count | 187 x 103/microliters | 150-400 x 103/microliters |
| Bleeding time | 3 minutes | 2-5 minutes |
| Clotting time | 4 minutes | 3-6 minutes |
| Prothrombin time | 11.5 seconds | 11-13.5 seconds |
| Activated partial thromboplastin time | 25 seconds | 21-35 seconds |
| Blood lipid profile | ||
| High-density lipoproteins | 39 milligrams/deciliters | 40-60 milligrams/deciliters |
| Triglycerides | 140 milligrams/deciliters | <150 milligrams/deciliters |
| Serum homocysteine | 14 micromoles/Liters | 5-15 micromoles/Liters |
| C-reactive protein | 2.1 milligrams/Liters | <5 milligrams/Liters |
| D-dimer | 200 nanograms/milliliters | <500 nanograms/milliliters |
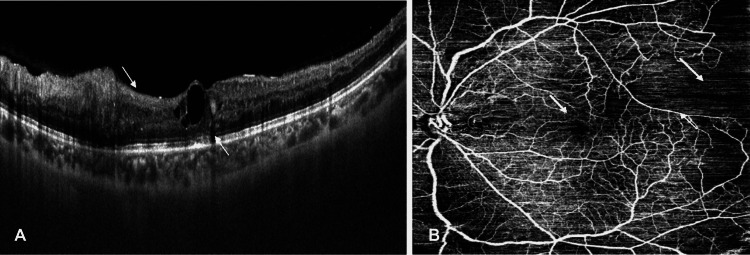
The ocular examination showed that he had best-corrected visual acuity (BCVA) of logarithm of the minimum angle of resolution (LogMAR) +0.2 (20/32, 6/9) in the right eye (RE) and LogMAR +1.5 (20/630, 6/190) in the LE. The pupils in the LE reacted sluggishly to the light reflex test. His IOP was 16 mmHg in both eyes. The anterior chamber had normal depth and open angles. There was no neovascularization of the iris or angle. The fundus examination showed optic disc edema, dilated tortuous veins, dot-blot and flame-shaped hemorrhages across the fundus, and blunted foveal reflex in the LE. Findings in the RE were unremarkable. The optical coherence tomography (OCT) in the LE showed disorganization of the inner retinal layers, disruption of the inner segment-outer segment junction, and distorted foveal contour with large cystic spaces. The central foveal thickness (CFT) measured 854 microns. The foveal contour was normal in the RE, and the CFT measured 242 microns. The OCT angiography showed an enlarged distorted foveal avascular zone (FAZ) and capillary non-perfusion in a 12-mm x 12-mm scan in the LE (Figure 1) and normal-sized circular FAZ in the RE.
Figure 1. Optical coherence tomography scan of the left eye (arrows show disorganized retinal inner layers and disruption of inner segment-outer segment junction). (B) Optical coherence tomography angiography scan of the left eye (arrows show enlarged foveal avascular zone and capillary non-perfusion).
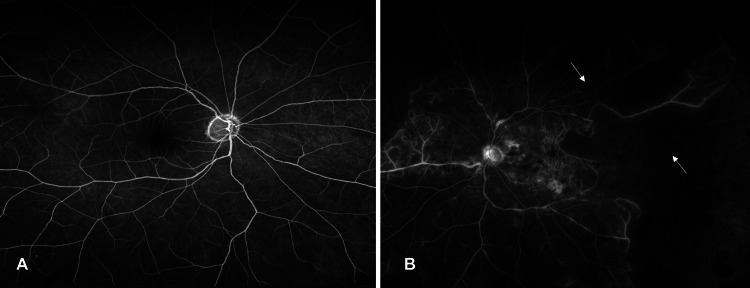
The fundus fluorescein angiography (FFA) revealed that the LE had delayed venous filling, blocked fluorescence from retinal hemorrhages, and capillary non-perfusion areas extending through 360 degrees in the peripheral fundus but no neovascularization of disc or elsewhere (Figure 2). The FFA of the RE was normal. The flash electroretinogram showed the scotopic and photopic amplitudes of the b-wave at 230 and 110 microvolts in RE and 80 and 50 microvolts in LE, respectively.
Figure 2. (A) Fundus fluorescein angiography of the right eye. (B) Fundus fluorescein angiography of the left eye (arrows show blocked fluorescence and capillary non-perfusion).
We established a diagnosis of ischemic CRVO and cystoid macular edema (CME) in the LE [3]. The RE was unremarkable. He was given an intravitreal injection of triamcinolone acetonide (4 mg/0.1 ml) in the LE for CME. One month later, the LE had BCVA of LogMAR +1.2 (20/320, 6/95), and the CFT measured 652 micrometers. The patient presented again six months after the initial episode. He had BCVA of LogMAR +1.0 (20/200, 6/60). His fundus examination showed that hemorrhages and exudates had resolved. The CFT had reduced to 320 micrometers. The FFA showed capillary non-perfusion areas extending through 360 degrees in the peripheral fundus and one neovascularization elsewhere (NVE) in the superonasal quadrant. A targeted laser was performed for ischemic areas and the NVE.
Discussion
The vascular adverse events in the eye following COVID-19 vaccination have been observed infrequently [2]. Sonawane et al. reported two cases of ischemic CRVO that presented three to four days after the second dose of the COVID-19 vaccine (Covishield, AZD1222, ChAdOX 1) [5]. The first case was a 50-year-old man with uncontrolled diabetes and deranged renal functions whose RE had ischemic CRVO, and CME and LE showed mild non-proliferative diabetic retinopathy changes. Their second case was a 43-year-old woman with no systemic disease who presented with an elevated erythrocyte sedimentation rate of 49 mm/hour, C-reactive protein of 14.6 mg/L (reference range: <5 mg/L), a rheumatoid factor of 11 IU/mL (reference range: <8 IU/mL) and d-dimer of 6077.4 ng/mL (reference range, <500 ng/mL). The involved RE did not show CME at presentation [5]. Bialasiewicz et al. described a case of an otherwise healthy 50-year-old man with ischemic CRVO presenting with sudden, painful diminution of vision immediately after the second dose of the COVID-19 vaccine (BioNTech/Pfizer lot number EP6017) [6]. Endo et al. a case of reported a 52-year-old healthy man who developed non-ischemic CRVO 15 days after receiving the first dose of the vaccine (Pfizer-BioNTech) [7]. Ikegami et al. reported a case of combined central retinal artery and vein occlusion in the RE two days after the second dose of the vaccine (mRNA-1273) in a 54-year-old hypothyroid woman [4]. In both cases, various laboratory tests on coagulation, antibody, lipid, and glucose profiles were within reference ranges [4,7].
AstraZeneca's COVID-19 vaccine has been associated with an increased risk of blood clots in combination with low levels of blood platelets (a percentage of risk being 0.0007%) [8]. Even after the first dose of vaccination with ChAdOX 1 (Covishield), Simpson et al. reported a high incidence of adverse effects (1.13 cases per 100,000 vaccinations) such as immune thrombocytopenic purpura and arterial thromboembolic and hemorrhagic events [9]. While CRVO in patients over 65 years of age is associated with systemic vascular conditions such as diabetes or hypertension, younger patients usually have an underlying hypercoagulable or inflammatory etiology [3]. Our patient was a healthy young man who developed CRVO following the first dose of the Covishield vaccine. The appearance of CRVO shortly after vaccination suggests an association between the two events.
Conclusions
While rare, ocular events can occur following the administration of the COVID-19 vaccine. This case described an otherwise healthy man who developed sight-threatening complications following the first dose of the Covishield vaccine. Vigilance for ophthalmic adverse events following vaccine administration is required for early intervention.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study
References
- 1.Deep vein thrombosis (DVT) occurring shortly after the second dose of mRNA SARS-CoV-2 vaccine. Carli G, Nichele I, Ruggeri M, Barra S, Tosetto A. Intern Emerg Med. 2021;16:803–804. doi: 10.1007/s11739-021-02685-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Case Series Drug Analysis Print Name: COVID-19 Vaccine AstraZeneca analysis. [ Apr; 2021 ];https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/997110/FOI_21-435_PDF_Attachment_2.pdf 2021
- 3.Kakkar P, Goel S, Kumar A, Kishore A. Retina Medical & Surgical Management. First Edition. New Delhi, India: Jaypee Brothers; 2018. Retinal vein occlusion; pp. 264–280. [Google Scholar]
- 4.Combined central retinal artery and vein occlusion shortly after mRNA-SARS-CoV-2 vaccination. Ikegami Y, Numaga J, Okano N, Fukuda S, Yamamoto H, Terada Y. QJM. 2022;114:884–885. doi: 10.1093/qjmed/hcab287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Central retinal vein occlusion post-COVID-19 vaccination. Sonawane NJ, Yadav D, Kota AR, Singh HV. Indian J Ophthalmol. 2022;70:308–309. doi: 10.4103/ijo.IJO_1757_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Central retinal vein occlusion occurring immediately after 2nd dose of mRNA SARS-CoV-2 vaccine. Bialasiewicz AA, Farah-Diab MS, Mebarki HT. Int Ophthalmol. 2021;41:3889–3892. doi: 10.1007/s10792-021-01971-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Central retinal vein occlusion after mRNA SARS-CoV-2 vaccination: a case report. Endo B, Bahamon S, Martínez-Pulgarín DF. Indian J Ophthalmol. 2021;69:2865–2866. doi: 10.4103/ijo.IJO_1477_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.AstraZeneca’s COVID-19 vaccine: EMA finds possible link to very rare cases of unusual blood clots with low blood platelets. [ May; 2021 ];https://www.ema.europa.eu/en/news/astrazenecas-covid-19-vaccine-ema-finds-possible-link-very-rare-cases-unusual-blood-clots-low-blood 7 April. 2021 9:2021. [Google Scholar]
- 9.First-dose ChAdOx1 and BNT162b2 COVID-19 vaccines and thrombocytopenic, thromboembolic and hemorrhagic events in Scotland. Simpson CR, Shi T, Vasileiou E, et al. Nat Med. 2021;27:1290–1297. doi: 10.1038/s41591-021-01408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]