Abstract
Pyridine-2,6-dicarboxylic acid (dipicolinic acid [DPA]) constitutes approximately 10% of Bacillus subtilis spore dry weight and has been shown to play a significant role in the survival of B. subtilis spores exposed to wet heat and to 254-nm UV radiation in the laboratory. However, to date, no work has addressed the importance of DPA in the survival of spores exposed to environmentally relevant solar UV radiation. Air-dried films of spores containing DPA or lacking DPA due to a null mutation in the DPA synthetase operon dpaAB were assayed for their resistance to UV-C (254 nm), UV-B (290 to 320 nm), full-spectrum sunlight (290 to 400 nm), and sunlight from which the UV-B portion was filtered (325 to 400 nm). In all cases, air-dried DPA-less spores were significantly more UV sensitive than their isogenic DPA-containing counterparts. However, the degree of difference in UV resistance between the two strains was wavelength dependent, being greatest in response to radiation in the UV-B portion of the spectrum. In addition, the inactivation responses of DPA-containing and DPA-less spores also depended strongly upon whether spores were exposed to UV as air-dried films or in aqueous suspension. Spores lacking the gerA, gerB, and gerK nutrient germination pathways, and which therefore rely on chemical triggering of germination by the calcium chelate of DPA (Ca-DPA), were also more UV sensitive than wild-type spores to all wavelengths tested, suggesting that the Ca-DPA-mediated spore germination pathway may consist of a UV-sensitive component or components.
When starved for nutrients, Bacillus subtilis vegetative cells undergo cellular differentiation into dormant structures referred to as endospores, or more simply, spores (reviewed recently in references 23 and 32). In the dormant state, spores undergo no detectable metabolism and exhibit a higher degree of resistance to inactivation by various physical treatments, such as the following: wet and dry heat; UV and γ-radiation; chemical oxidants; and extreme desiccation, vacuum, and acceleration (reviewed recently in references 16, 17, 29, and 30). Upon encountering a favorable environment, i.e., the presence of nutrients, spores break their dormancy and germinate to resume vegetative growth (reviewed in references 3 and 7).
Spore resistance properties are often the result of multiple mechanisms whose effects are observed to be exerted at a number of developmental stages. One particularly well-studied example is spore UV resistance. Bacterial spores are 1 to 2 orders of magnitude more resistant to the lethal effects of irradiation by 254-nm UV than their vegetative counterparts (33; reviewed in reference 28), and spore UV resistance is the result of a complex set of molecular interactions which occur during sporulation, dormancy, and germination. DNA in the spore core is found in a complex with a group of small, acid-soluble spore proteins (called α/β-type SASP) that change the conformation of DNA from the B form to an A-like form and favor the formation of the spore-specific thymine dimer 5-thyminyl-5,6-dihydrothymine (spore photoproduct [SP]) upon UV irradiation of spores (reviewed in references 17, 29, and 30). SP accumulates in dormant UV-irradiated spores, and germinating spores repair SP by at least three DNA repair systems: the general nucleotide excision repair (NER) and recombination (Rec) systems encoded by genes designated uvr and rec, respectively, and an SP-specific repair enzyme called SP lyase encoded by the splB gene (reviewed in references 16, 17, and 29). The SASP, a SASP-specific germination protease (GPR), and SP lyase are all synthesized in the developing forespore compartment at stages II to III of sporulation as part of the EςF and EςG regulons, and all are packaged into the core of the dormant spore (5, 22, 34). SASP exert a dual function: during dormancy, SASP are DNA-binding proteins which alter spore DNA UV photochemistry to favor SP formation, and SASP are a major source of amino acids for the germinating spore via their proteolytic degradation initiated by GPR (reviewed in references 2, 29, and 30). During early germination, SP lyase causes direct reversal of SP to adjacent thymines in the DNA of UV-irradiated spores (11, 12). In addition, the presence of UV damage in spore DNA induces expression of the NER and Rec DNA repair systems during spore germination and outgrowth (27), which have also been shown to be important in repair of UV-induced spore DNA damage (13).
Another factor implicated in spore resistance properties and germination is the small molecule pyridine-2,6-dicarboxylic acid (dipicolinic acid [DPA]). The Ca2+ chelate of DPA (Ca-DPA) is a major constituent of the dormant spore core, accounting for approximately 10% of total spore dry weight (14, 15). The operon encoding the A and B subunits of DPA synthetase (called spoVFAB or dpaAB) is expressed as part of the EςK regulon in the mother cell compartment (1). DPA is synthesized in the mother cell and subsequently transported into the forespore by a currently unknown mechanism (1). DPA appears to be important in spore core dehydration and concomitant spore heat resistance, as spores of B. subtilis mutants lacking DPA due to null mutations in dpaAB have a lower core wet density and are sensitive to wet heat (19). However, DPA does not seem to play a role in the dry heat resistance of spores (19). In sharp contrast to its positive role in wet heat resistance and maintenance of dormancy, DPA apparently photosensitizes spores to 254-nm UV irradiation, as DPA-less spores are approximately 2.5-fold more UV-C resistant than isogenic spores containing DPA (19). Furthermore, it has been shown that in response to irradiation with 254-nm UV, DPA increases the quantum yield of SP in both spore DNA in vivo in complexes formed between α/β-type SASP and DNA in vitro (26).
DPA has also been implicated in the maintenance of spore dormancy, as DPA-less spores are rather unstable and tend to germinate spontaneously (19). Three genetic loci (gerA, gerB, and gerK) have been shown to be responsible for nutrient-induced germination of B. subtilis spores (8, 9, 25). Genetic and biochemical evidence suggests that these three loci likely encode the major receptor proteins that are responsible for recognizing a particular nutrient germinant(s) active on B. subtilis spores (8, 9, 20). This notion was recently strengthened by construction of a B. subtilis strain called FB72 containing deletion-insertion mutations inactivating all three ger operons (called here ΔgerA,B,K) (21). Spores of strain FB72 are unable to trigger germination in the presence of nutrients, but these mutant spores could germinate in a manner essentially identical to that of wild-type spores in response to an equimolar mixture of Ca2+ and DPA (Ca-DPA) (21). Thus, there appears to exist a DPA-dependent chemical spore germination pathway which operates in parallel to the three major nutrient-induced germination pathways (21).
We are interested in elucidating the role that DPA plays in spore resistance to environmental factors, particularly solar UV radiation (for recent reviews, see references 16 and 17). Most of the work on spore UV resistance has to date been performed using commercial low-pressure mercury lamps that emit predominantly monochromatic 254-nm UV-C. In contrast, solar UV radiation that reaches Earth's surface contains no 254-nm UV-C but spans approximately 290 to 400 nm (the UV-B and UV-A portions of the UV spectrum) (35). B. subtilis spores exposed to the UV-B and/or UV-A portions of the solar spectrum exhibit DNA repair responses distinct from those of UV-C-irradiated spores (37), likely due to wavelength-dependent differences in spore DNA photochemistry (31). The effects of DPA on spore resistance and SP quantum yield in vitro in response to laboratory 254-nm UV (19) suggested to us that DPA in spores may play a role in spore resistance to environmental UV, possibly through direct interaction with spore DNA. Thus, in this communication we describe experiments investigating the role of DPA in spore resistance to laboratory UV-C and also to UV wavelengths present in terrestrial solar radiation.
MATERIALS AND METHODS
Bacterial strains and cultural conditions.
The B. subtilis strains used in this study were all derivatives of strain 168 and were a generous gift from Peter Setlow, University of Connecticut Health Center. The wild-type strain used was PS832, a Trp+ revertant of 168. Strains FB72 (ΔgerA::spc ΔgerB::cat ΔgerK::erm) (referred to below as ΔgerA,B,K DPA+ spores) and FB108 (ΔgerA::spc ΔgerB::cat ΔgerK::erm ΔspoVF::tet) (referred to below as ΔgerA,B,K DPA− spores) were derived from PS832 as described previously (19, 21). Strains were sporulated and spores were purified as described previously (18); spores were assessed to be >95% free of growing or sporulating cells or germinated spores.
Sample preparation.
One million spores in 10 μl of water were spotted in triplicate on sterile glass microscope slides, and the spots were air dried at 37°C for 15 min. Spores were then exposed to different treatments of UV radiation as described in detail previously (24, 37), except as indicated below. After UV exposure, spores were recovered from the microscope slides as follows. Briefly, 0.1 ml of 10% (wt/vol) sterile polyvinyl alcohol (molecular weight, 30,000 to 70,000; Sigma Chemical Co., St. Louis, Mo.) was applied onto the dried spore spots and air dried at 37°C for 1.0 to 1.5 h. The resulting polyvinyl alcohol films containing the spores were then peeled from the microscope slides with a sterile scalpel and forceps (4), resuspended in 1 ml of a freshly prepared sterile solution of 60 mM DPA and 60 mM CaCl2 (pH 8), and germinated for 1 h at room temperature as previously described (21). Spores were then diluted serially 10-fold in phosphate-buffered saline (18) and plated on Luria-Bertani agar (6), and colonies were counted after overnight incubation at 37°C. The survival percentage of spores (%S) was calculated by the following equation: %S = (Nt/N0) × 100, where Nt and N0 stand for the numbers of CFU of the spores at the exposure time t and time zero, respectively. Spore UV resistances are expressed as the lethal dose of UV (in joules/square meter) required to inactivate 90% of the spore population (LD90) and are reported as averages ± standard deviations. Differences in LD90s were analyzed for statistical significance by analysis of variance (ANOVA). Differences with a P value of ≤0.05 were considered significant.
Artificial and solar UV exposures.
The UV irradiation conditions were performed as described previously (24, 37). Spores were subjected to artificial UV-C radiation by using a UV-C lamp producing predominantly 254-nm UV radiation or a UV-B lamp modified as described previously (37) to emit 290- to 320-nm UV-B radiation with peak emission at 302 nm (lamp models UVS-11 and UVM-57, respectively; UV Products, Inc., San Gabriel, Calif.). UV dosimetry was performed using a UVX radiometer (UV Products) fitted with the appropriate UV-C, UV-B, or UV-A sensors. Spores were directly exposed to sunlight on clear days during the daily period when maximal solar intensity occurred (between solar 10:00 a.m. and 2:00 p.m.), which was calculated for the longitude of Tucson, Ariz. (111°2′W), by using the Voyager II computer program (Carina Software, San Leandro, Calif.). Spores exposed to full-spectrum sunlight were covered by a single layer of Saran wrap (Dow Products), which transmits essentially all solar UV (37), and spores exposed to solar UV-A were covered by a 1.25-cm (0.5-inch)-thick glass plate that completely blocks UV wavelengths shorter than 325 nm, as determined previously (37). Since DPA-less spores are known to be heat sensitive (19) and temperatures higher than 70°C were routinely recorded during solar exposures, microscope slides containing spore samples exposed to sunlight were placed on a cooling platform with circulating ice water which maintained the temperature at the microscope slide surface at ≤20°C, as measured with a surface contact thermometer.
RESULTS
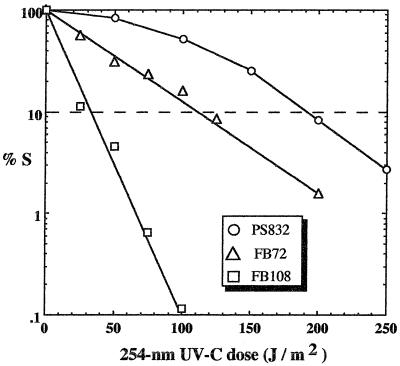
In order to assess the possible importance of DPA in B. subtilis spore resistance to UV radiation, spores of strains PS832, FB72, and FB108 were subjected to artificial UV-C and UV-B in the laboratory, to full-spectrum sunlight, and to sunlight from which the UV-B component had been filtered (UV-A sunlight). Spore resistance was expressed as the LD90. Typical inactivation curves for air-dried spores of strains PS832, FB72, and FB108 in response to 254-nm UV-C are depicted in Fig. 1. It was observed that the wild-type strain PS832 exhibited a dose-response curve characteristic of bacterial spores, consisting of a shoulder extending to 20 to 30% survival, followed by logarithmic inactivation kinetics thereafter. In contrast, strains FB72 and FB108 exhibited strict logarithmic inactivation kinetics with no apparent shoulder (Fig. 1). The inactivation curves for the three strains in response to UV-B and full-spectrum sunlight were observed to follow the same general shapes (data not shown). In response to UV-A sunlight, all three strains exhibited a shoulder to approximately 50% survival, followed by logarithmic inactivation (data not shown).
FIG. 1.
Typical inactivation curves of spores of B. subtilis strains PS832 (wild type), FB72 (ΔgerA,B,K DPA+), and FB108 (ΔgerA,B,K DPA−) irradiated as air-dried films with 254-nm UV-C. The LD90 is denoted by the horizontal dashed line.
Artificial UV-C radiation.
Survival of air-dried films of spores exposed to 254-nm UV-C was determined in three separate experiments (Table 1). The results indicated that inactivation of the gerA, gerB, and gerK operons rendered air-dried FB72 spores twofold more sensitive to UV-C than the wild-type parental strain PS832 and that further inactivation of DPA synthetase in strain FB108 rendered air-dried spores sixfold more UV-C sensitive than wild-type spores. The differences in LD90s for all three strains were significant by ANOVA. The lower apparent LD90s of strains FB72 and FB108 relative to those of wild-type spores cannot be explained simply by lower germination efficiency of these spores due the ΔgerA,B,K mutations, as unirradiated spores germinated efficiently in response to Ca-DPA (data not shown). While spores of strain FB108 (ΔgerA,B,K DPA−) were very sensitive to UV-C with an LD90 of 31 ± 5.3 J/m2, these spores were still nearly 10-fold more UV-C resistant than spores lacking the two major spore DNA repair systems, NER and SP lyase, which demonstrated an LD90 of 3.5 J/m2 (37).
TABLE 1.
Resistance of B. subtilis spores to UV radiation
| Treatment | LD90a
|
||
|---|---|---|---|
| PS832 (wild type) | FB72 (DPA+) | FB108 (DPA−) | |
| UV-C (J/m2) | 193 ± 4.8 | 91.3 ± 24 | 31 ± 5.3 |
| UV-C in aqueous suspension (J/m2) | 205 ± 7.1 | 124 ± 24.7 | 252 ± 11.3 |
| Laboratory UV-B (kJ/m2) | 66.2 ± 10.4 | 21.4 ± 4.3 | 3.0 ± 1.2 |
| Laboratory UV-B in aqueous suspension (kJ/m2) | 62.7 ± 1.9 | 91 ± 0.0 | 26.7 ± 1.2 |
| Full-spectrum sunlight (UV-B + -A) (kJ/m2)b | 101.5 ± 5.1 | 50.5 ± 6.1 | 12.5 ± 2.0 |
| UV-A sunlight (kJ/m2)c | 399.4 ± 32.3 | 666.7 ± 73.7 | 209.0 ± 13.9 |
Three trials were conducted with each strain, except for UV-C in suspension (two trials). Numbers represent averages ± standard deviations. All spore preparations were irradiated as air-dried films unless otherwise indicated.
Measurements were made with the UV-B probe.
Measurements were made with the UV-A probe.
The results obtained with spores of FB72 and FB108 indicated that DPA-less FB108 spores were threefold more sensitive to UV-C than were isogenic FB72 spores containing DPA (Table 1; Fig. 1), and the observed difference in UV-C resistance between FB72 and FB108 spores was significant by ANOVA (P = 0.013). These results surprised us, as they were in apparent contradiction to previous work indicating that DPA-less spores were more resistant to 254-nm UV-C than spores containing DPA and, further, that sporulation of DPA-less strains in sporulation medium containing DPA resulted in partial restoration of spore UV-C sensitivity (19, 26). In previous experiments (19, 26), however, spores were treated with UV-C in aqueous suspension, not as air-dried films. We therefore repeated the UV-C experiments using the same UV-C lamp and the same batches of spores, but in aqueous suspension as described previously (18, 19, 26). All differences in LD90s among spores of the three strains irradiated with UV-C in suspension were significant by ANOVA. When irradiated with UV-C in aqueous suspension, DPA-less FB108 spores were observed to be twofold more resistant to UV-C than isogenic DPA-containing FB72 spores (significant at P = 0.022 by ANOVA) (Fig. 2), in agreement with previous observations (19). Comparison of the LD90 of DPA-less FB108 spores irradiated in the wet state and the LD90 of those irradiated in the dry state revealed a highly significant (P < 0.001) eightfold difference in their UV-C sensitivities (Fig. 2). In contrast, the UV-C resistance of spores of wild-type strain PS832 and ΔgerA,B,K DPA+ strain FB72 did not differ significantly with respect to whether the spores were irradiated in the dry state or in suspension (Fig. 2). It therefore appears that the resistance of DPA-less FB108 spores to 254-nm UV-C is strongly dependent upon whether the spores are irradiated in aqueous suspension or as air-dried films.
FIG. 2.
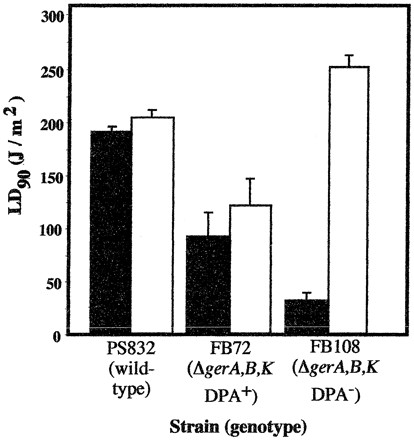
Spore resistance of strains PS832, FB72, and FB108 irradiated with 254-nm UV-C as air-dried films (solid bars) or in aqueous suspension (open bars). Data are averages ± standard deviations of three independent experiments.
Artificial UV-B radiation.
In order to investigate spore resistance to environmentally relevant UV wavelengths, air-dried spore films were exposed to radiation from a commercial UV-B lamp as described in Materials and Methods. The LD90s from three separate experiments each performed on spores of strains PS832, FB72, and FB108 were determined to be significantly different by ANOVA. As was observed with artificial UV-C radiation, the same general trend in spore UV-B sensitivity was observed for air-dried spores (Table 1). Air-dried spores of FB72 and FB108 were 3- and 22-fold more UV-B sensitive, respectively, than were spores of the wild-type parental strain PS832 (Table 1). Furthermore, air-dried spores of the DPA-less strain FB108 were sevenfold more UV-B sensitive than isogenic DPA-containing spores of strain FB72 (Table 1). Notably, air-dried ΔgerA,B,K DPA− spores of FB108 were observed to be extremely sensitive to UV-B radiation (LD90 = 3 kJ/m2), and their level of UV-B sensitivity was comparable to that exhibited by B. subtilis spores lacking the major DNA repair pathways for UV resistance (NER and SP lyase) (37). Thus, DPA appears to be a major UV-B photoprotective compound in air-dried B. subtilis spores.
Spores of PS832, FB72, and FB108 were also subjected to UV-B irradiation in aqueous suspension (Table 1). As seen with UV-C (Fig. 1), wild-type spores of strain PS832 exhibited essentially the same LD90 when exposed to UV-B in suspension and in the air-dried state (Table 1). In contrast, spores of FB72 (ΔgerA,B,K DPA+) and FB108 (ΔgerA,B,K DPA−) were four- and ninefold, respectively, more resistant to UV-B when irradiated in suspension than in the dry state (Table 1). In addition, in aqueous suspension, FB108 spores were 3.4-fold more UV-B sensitive than spores of FB72 (Fig. 2), opposite to the result obtained with 254-nm UV-C (Fig. 1). Thus, the resistance of spores to laboratory UV-C and UV-B is highly dependent upon (i) the presence or absence of DPA within the spore, (ii) the hydration environment in which spores are irradiated (wet or dry), and (iii) the UV wavelength(s) used.
Full-spectrum sunlight.
In order to investigate the role of DPA in spore resistance to solar UV, on three separate days (1, 8, and 11 March 2000), air-dried spores of strains PS832, FB72, and FB108 were exposed to full-spectrum solar radiation containing both UV-B and UV-A wavelengths (Table 1). The differences in LD90s obtained for the three strains were all significant by ANOVA. In air-dried spores, the same pattern of spore UV resistance was observed with full-spectrum sunlight as with artificial UV-C and UV-B, in that spores of FB72 and FB108 were two- and eightfold more sensitive to full-spectrum sunlight, respectively, than were wild-type PS832 spores, and FB108 spores were fourfold more sensitive than FB72 spores. Again, ΔgerA,B,K DPA− spores of FB108 were observed to be very sensitive to full-spectrum solar radiation, approaching the level of sensitivity exhibited by spores lacking the NER and SP lyase DNA repair pathways (37).
UV-A sunlight.
On clear days between 20 March 2000 and 24 May 2000, spores of strains PS832, FB72, and FB108 were exposed to sunlight lacking the UV-B component (i.e., UV-A sunlight), as described in Materials and Methods and previously (24, 31, 37). In response to UV-A sunlight, the differences in LD90s among the three strains were much less dramatic than the differences seen with full-spectrum solar UV; FB72 spores were only 1.7-fold more resistant than wild-type spores to UV-A sunlight, and FB108 spores were twofold more sensitive than wild-type PS832 spores (Table 1). Spores of FB72 (ΔgerA,B,K DPA+) were threefold more resistant to solar UV-A than were their isogenic DPA-less counterparts FB108 (ΔgerA,B,K DPA−) (Table 1). Solar irradiation of spores suspended in phosphate-buffered saline was not performed, as control of long-term exposure of spores to solar UV radiation in suspension using our system was found to be technically problematic.
DISCUSSION
DPA has long been known to be a major component of the spore core, but the role of DPA in spore resistance properties has been the subject of some controversy over the past several decades (reviewed in references 28 and 29). Only recently, with the availability of well-characterized mutant B. subtilis strains which produce nutrient-germination-defective and DPA-less spores (1, 19, 21), has it become possible to systematically investigate the role of DPA in spore dormancy, resistance, and germination. Ideally, it would have been desirable to study the UV resistance properties of DPA-less spores in the absence of additional deletions in the gerA, gerB, and gerK operons due to the necessity of germinating these spores with Ca-DPA. This rather artificial complication was necessary, however, given the extreme instability of GerABK+, DPA-less spores (19). Recently, Paidhungat et al. (19) characterized several resistance properties of spores of B. subtilis strains FB72 and FB108 to understand the role of DPA in spore survival upon exposure to several different physical stresses imposed in the laboratory. They found that DPA provided significant spore resistance to wet heat but not to dry heat and that the presence of DPA in spores lowered spore resistance to 254-nm UV (19), presumably by acting as a photosensitizing agent (26). As part of ongoing studies aimed at bridging the gap between our understanding of spore resistance and longevity in the laboratory and our understanding of them in the environment (reviewed in references 16 and 17), we investigated the role of DPA in the survival of spores upon exposure to UV radiation from both artificial UV sources in the laboratory and solar UV in the field.
Air-dried spores.
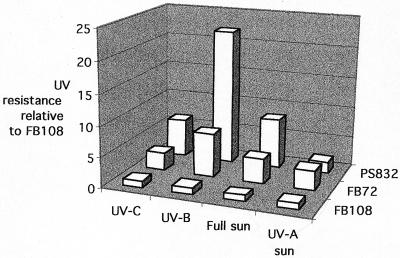
In accordance with standard techniques developed for solar dosimetry studies using bacterial spores (4, 10, 36) and our previous work (24, 31, 37), we used primarily air-dried films of spores as the experimental system, due to several technical advantages which have been reviewed recently (16, 17). The data from our experiments exploring the role of DPA in the UV resistance of air-dried B. subtilis spores are summarized in Fig. 3. From examination of the summary data, at least two obvious trends can be observed.
FIG. 3.
Summary of UV resistance data for air-dried spores of B. subtilis strains PS832 (wild type), FB72 (ΔgerA,B,K DPA+), and FB108 (ΔgerA,B,K DPA−). The data for each type of UV exposure were normalized to data of strain FB108 for comparison.
First, spores containing DPA (strains FB72 and PS832) were consistently more UV resistant than DPA-less spores of strain FB108. This effect held true for all UV wavelengths tested extending from 254 to 400 nm and was most pronounced when spores were exposed to artificial UV-B radiation in the laboratory (280 to 320 nm, with a peak at 302 nm), where PS832 and FB72 spores were 22- and 7-fold more UV resistant, respectively, than DPA-less FB108 spores (Fig. 3). Interestingly, the effect of DPA was least pronounced in response to solar radiation lacking the UV-B component (i.e., UV-A sunlight), where PS832 and FB72 spores were only 1.9- and 3.2-fold more UV resistant, respectively, than were FB108 spores (Fig. 3). Thus, the data strongly indicate that DPA exerts a profound UV photoprotective effect on air-dried spores, especially in response to UV-B wavelengths. Although the precise mechanism of this effect is at present unknown, it is interesting that the absorption spectra (Amax = 270 nm) of both DPA and Ca-DPA extend into the UV-B region to approximately 295 nm (data not shown), thus overlapping with the solar UV wavelengths which are most energetic and biologically relevant in terms of nucleic acid and protein photoabsorption.
Second, comparison of the UV resistance data between wild-type PS832 spores and spores of isogenic strain FB72 revealed that deletion of the gerA, gerB, and gerK nutrient germination pathways rendered FB72 spores more sensitive to UV-C, UV-B, and full-spectrum sunlight by a factor of 2 to 3; again, the difference in UV resistance was most pronounced in response to UV-B wavelengths (Fig. 3). By what mechanism could mutational inactivation of the three major nutrient germination pathways lead to a UV-sensitive spore phenotype? It has been shown that spores lacking the gerA, gerB, and gerK nutrient germination pathways can still respond to Ca-DPA itself as a chemical germinant (21). The precise molecular details of the nonnutrient Ca-DPA germination pathway are at present lacking, but it is known that the Ca-DPA pathway differs from nutrient-triggered germination pathways in that decoating of spores abolishes the Ca-DPA pathway but not the nutrient-triggered pathways (21). The evidence presented here suggests an additional difference: some component(s) of the Ca-DPA germination pathway also appears to be selectively inactivated by UV in air-dried spores, especially by UV-B wavelengths extending from 254 to 320 nm (Fig. 3). This effect does not appear to extend into the UV-A wavelengths (>325 nm), as FB72 spores were actually twofold more resistant to UV-A than were spores of the wild-type parental strain PS832 (Fig. 3).
UV resistance of dry spores versus spores in suspension.
Another surprising result which emerged from the present study concerned the sharp differences in the pattern of UV resistance observed among spores of strain PS832, FB72, and FB108, depending upon whether they were irradiated as air-dried films or in aqueous suspension. The UV-C resistances of spores of wild-type strain PS832 and strain FB72 (ΔgerA,B,K DPA+) did not differ significantly with respect to whether the spores were irradiated in the wet or dry state, while spores of FB108 (ΔgerA,B,K DPA−) were eightfold more UV-C resistant in suspension than in the dry state (Fig. 2). The results indicate that while DPA appears to photosensitize spores in aqueous suspension to UV-C, in agreement with previous results (19), DPA in contrast appears to be photoprotective in spores in the air-dried state. These results imply that the environment within the spore core differs substantially when spores are in the air-dried state versus when they are in aqueous suspension. In support of this notion is the observation that DPA exerts a profound effect on the resistance of spores to wet, but not dry, heat (19).
In response to environmentally relevant UV-B wavelengths, only wild-type PS832 spores showed no significant difference in their UV resistance between the wet and dry states (Table 1). In contrast, spores of both strains FB72 and FB108 were significantly more resistant to UV-B (four- and ninefold, respectively) when irradiated in suspension than when irradiated in the dry state (Table 1). In addition, because the UV-B resistance of spores in suspension was not reduced by deletion of the gerA, gerB, and gerK nutrient germination operons, it thus appears that the DPA-dependent germination pathway is not damaged by UV-B radiation when spores are irradiated in suspension. As discussed above, these results imply not only that DPA serves a direct function in spore resistance to environmentally relevant UV wavelengths but also that the postulated DPA-dependent germination pathway is affected differentially by UV-B depending upon the hydration state of the spore. At present, it is unknown whether the DPA-dependent germination pathway is relevant to spores in the environment.
In summary, exposure to solar UV radiation is one of the most important factors limiting bacterial spore longevity in the environment. In this study, we have shown that DPA is a major UV resistance factor in spores, especially when air-dried spores on surfaces are exposed to environmentally relevant UV-B wavelengths. The role of DPA in spore resistance has not yet been completely elucidated but appears to be complex, involving both DPA itself and the DPA-dependent germination pathway in spores.
ACKNOWLEDGMENTS
We thank Madan Paidhungat and Peter Setlow for generous donation of the strains used in this study, for communicating results prior to publication, and for critical reading of the manuscript.
This work was supported by a grant (USDA-Hatch ARZT-126753-02-H-116) from the Arizona Agriculture Experimental Station to W.L.N.
REFERENCES
- 1.Daniel R A, Errington J. Cloning, DNA sequence, functional analysis and transcriptional regulation of the genes encoding dipicolinic acid synthetase required for sporulation in Bacillus subtilis. J Mol Biol. 1993;232:468–483. doi: 10.1006/jmbi.1993.1403. [DOI] [PubMed] [Google Scholar]
- 2.Driks A, Setlow P. Morphogenesis and properties of the bacterial spore. In: Brun Y, Shimkets L, editors. Prokaryotic development. Washington, D.C.: American Society for Microbiology; 1999. pp. 191–218. [Google Scholar]
- 3.Johnstone K. The trigger mechanism of spore germination: current concepts. J Appl Bacteriol Symp Suppl. 1994;76:17S–24S. doi: 10.1111/j.1365-2672.1994.tb04354.x. [DOI] [PubMed] [Google Scholar]
- 4.Lindberg C, Horneck G. Action spectra for survival and spore photoproduct formation of Bacillus subtilis irradiated with short-wavelength (200–300 nm) UV at atmospheric pressure and in vacuo. J Photochem Photobiol B Biol. 1991;11:69–80. doi: 10.1016/1011-1344(91)80269-n. [DOI] [PubMed] [Google Scholar]
- 5.Mason J M, Hackett R H, Setlow P. Regulation of expression of genes coding for small, acid-soluble proteins of Bacillus subtilis spores: studies using lacZ gene fusions. J Bacteriol. 1988;170:239–244. doi: 10.1128/jb.170.1.239-244.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 7.Moir A, Kemp E H, Robinson C, Corfe B M. The genetic analysis of bacterial spore germination. J Appl Bacteriol Symp Suppl. 1994;76:9S–16S. [PubMed] [Google Scholar]
- 8.Moir A, Lafferty E, Smith D A. Genetic analysis of spore germination mutants of Bacillus subtilis 168: the correlation of phenotype and map location. J Gen Microbiol. 1979;111:165–180. doi: 10.1099/00221287-111-1-165. [DOI] [PubMed] [Google Scholar]
- 9.Moir A, Smith D A. The genetics of bacterial spore germination. Annu Rev Microbiol. 1990;44:531–553. doi: 10.1146/annurev.mi.44.100190.002531. [DOI] [PubMed] [Google Scholar]
- 10.Munakata N. Killing and mutagenic action of sunlight upon Bacillus subtilis spores: a dosimetric system. Mutat Res. 1981;82:263–268. doi: 10.1016/0027-5107(81)90155-x. [DOI] [PubMed] [Google Scholar]
- 11.Munakata N, Rupert C S. Genetically controlled removal of “spore photoproduct” from deoxyribonucleic acid of ultraviolet-irradiated Bacillus subtilis spores. J Bacteriol. 1972;111:192–198. doi: 10.1128/jb.111.1.192-198.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munakata N, Rupert C S. Dark repair of DNA containing “spore photoproduct” in Bacillus subtilis. Mol Gen Genet. 1974;130:239–250. doi: 10.1007/BF00268802. [DOI] [PubMed] [Google Scholar]
- 13.Munakata N, Rupert C S. Effects of DNA polymerase-defective and recombination-defective mutations on the ultraviolet sensitivity of Bacillus subtilis spores. Mutat Res. 1975;27:157–169. doi: 10.1016/0027-5107(75)90075-5. [DOI] [PubMed] [Google Scholar]
- 14.Murrell W G. The biochemistry of the bacterial spore. Adv Microb Physiol. 1967;1:133–251. [Google Scholar]
- 15.Murrell W G, Warth A D. Composition and heat resistance of bacterial spores. In: Campbell L L, Halvorson H O, editors. Spores III. Washington, D.C.: American Society for Microbiology; 1965. pp. 1–24. [Google Scholar]
- 16.Nicholson W L, Fajardo-Cavazos P. DNA repair and the ultraviolet radiation resistance of bacterial spores: from the laboratory to the environment. Recent Res Dev Microbiol. 1997;1:125–140. [Google Scholar]
- 17.Nicholson W L, Munakata N, Horneck G, Melosh H J, Setlow P. Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol Mol Biol Rev. 2000;64:548–572. doi: 10.1128/mmbr.64.3.548-572.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nicholson W L, Setlow P. Sporulation, germination, and outgrowth. In: Harwood C R, Cutting S M, editors. Molecular biological methods for Bacillus. Chichester, England: John Wiley & Sons; 1990. pp. 391–450. [Google Scholar]
- 19.Paidhungat M, Setlow B, Driks A, Setlow P. Characterization of spores of Bacillus subtilis which lack dipicolinic acid. J Bacteriol. 2000;182:5505–5512. doi: 10.1128/jb.182.19.5505-5512.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paidhungat M, Setlow P. Isolation and characterization of mutations in Bacillus subtilis that allow spore germination in the novel germinant d-alanine. J Bacteriol. 1999;181:3341–3350. doi: 10.1128/jb.181.11.3341-3350.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paidhungat M, Setlow P. Role of Ger proteins in nutrient and nonnutrient triggering of spore germination in Bacillus subtilis. J Bacteriol. 2000;182:2513–2519. doi: 10.1128/jb.182.9.2513-2519.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pedraza-Reyes M, Gutiérrez-Corona F, Nicholson W L. Temporal regulation and forespore-specific expression of the spore photoproduct lyase gene by sigma-G RNA polymerase during Bacillus subtilis sporulation. J Bacteriol. 1994;176:3983–3991. doi: 10.1128/jb.176.13.3983-3991.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piggot P J, Moran C P Jr, Youngman P, editors. Regulation of bacterial differentiation. Washington, D.C.: American Society for Microbiology; 1994. [Google Scholar]
- 24.Riesenman P J, Nicholson W L. Role of the spore coat layers in Bacillus subtilis spore resistance to hydrogen peroxide, artificial UV-C, UV-B, and solar UV radiation. Appl Environ Microbiol. 2000;66:620–626. doi: 10.1128/aem.66.2.620-626.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sammons R L, Moir A, Smith D A. Isolation and properties of spore germination mutants of Bacillus subtilis 168 deficient in the initiation of germination. J Gen Microbiol. 1981;124:229–241. [Google Scholar]
- 26.Setlow B, Setlow P. Dipicolinic acid greatly enhances production of spore photoproduct in bacterial spores upon UV irradiation. Appl Environ Microbiol. 1993;59:640–643. doi: 10.1128/aem.59.2.640-643.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Setlow B, Setlow P. Role of DNA repair in Bacillus subtilis spore resistance. J Bacteriol. 1996;178:3486–3495. doi: 10.1128/jb.178.12.3486-3495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Setlow P. Resistance of bacterial spores to ultraviolet light. Comments Mol Cell Biophys. 1988;5:253–264. [Google Scholar]
- 29.Setlow P. Mechanisms for the prevention of damage to DNA in spores of Bacillus species. Annu Rev Microbiol. 1995;49:29–54. doi: 10.1146/annurev.mi.49.100195.000333. [DOI] [PubMed] [Google Scholar]
- 30.Setlow P. Bacterial spore resistance. In: Storz G, Hengge-Aronis R, editors. Bacterial stress responses. Washington, D.C.: American Society for Microbiology; 1999. pp. 217–233. [Google Scholar]
- 31.Slieman T A, Nicholson W L. Artificial and solar UV radiation induces strand breaks and cyclobutane pyrimidine dimers in Bacillus subtilis spore DNA. Appl Environ Microbiol. 2000;66:199–205. doi: 10.1128/aem.66.1.199-205.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stragier P, Losick R. Molecular genetics of sporulation in Bacillus subtilis. Annu Rev Genet. 1996;30:297–341. doi: 10.1146/annurev.genet.30.1.297. [DOI] [PubMed] [Google Scholar]
- 33.Stuy J H. Studies on the mechanism of radiation inactivation of microorganisms. III. Inactivation of germinating spores of Bacillus cereus. Biochim Biophys Acta. 1956;22:241–246. doi: 10.1016/0006-3002(56)90146-9. [DOI] [PubMed] [Google Scholar]
- 34.Sussman M D, Setlow P. Cloning, nucleotide sequence, and regulation of the Bacillus subtilis gpr gene, which codes for the protease that initiates degradation of small, acid-soluble proteins during spore germination. J Bacteriol. 1991;173:291–300. doi: 10.1128/jb.173.1.291-300.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Urbach F, Gange R W, editors. The biological effects of ultraviolet A radiation. New York, N.Y: Praeger Publishers; 1986. [Google Scholar]
- 36.Wang T-C V. A simple convenient biological dosimeter for monitoring solar UV-B radiation. Biochem Biophys Res Commun. 1991;177:48–53. doi: 10.1016/0006-291x(91)91946-a. [DOI] [PubMed] [Google Scholar]
- 37.Xue Y, Nicholson W L. The two major spore DNA repair pathways, nucleotide excision repair and spore photoproduct lyase, are sufficient for the resistance of Bacillus subtilis spores to artificial UV-C and UV-B but not to solar radiation. Appl Environ Microbiol. 1996;62:2221–2227. doi: 10.1128/aem.62.7.2221-2227.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]