Abstract
Background
Carotid artery stenosis is an important cause of stroke and transient ischemic attack. Correctly and rapidly identifying patients with symptomatic carotid artery stenosis is essential for adequate treatment with early cerebral revascularization. Doubts about the diagnostic value regarding the accuracy of duplex ultrasound (DUS) and the possibility of using DUS as the single diagnostic test before carotid revascularization are still debated.
Objectives
To estimate the accuracy of DUS in individuals with symptomatic carotid stenosis verified by either digital subtraction angiography (DSA), computed tomography angiography (CTA), or magnetic resonance angiography (MRA).
Search methods
We searched CRDTAS, CENTRAL, MEDLINE (Ovid), Embase (Ovid), ISI Web of Science, HTA, DARE, and LILACS up to 15 February 2021. We handsearched the reference lists of all included studies and other relevant publications and contacted experts in the field to identify additional studies or unpublished data.
Selection criteria
We included studies assessing DUS accuracy against an acceptable reference standard (DSA, MRA, or CTA) in symptomatic patients. We considered the classification of carotid stenosis with DUS defined with validated duplex velocity criteria, and the NASCET criteria for carotid stenosis measures on DSA, MRA, and CTA. We excluded studies that included < 70% of symptomatic patients; the time between the index test and the reference standard was longer than four weeks or not described, or that presented no objective criteria to estimate carotid stenosis.
Data collection and analysis
The review authors independently screened articles, extracted data, and assessed the risk of bias and applicability concerns using the QUADAS‐2 domain list. We extracted data with an effort to complete a 2 × 2 table (true positives, true negatives, false positives, and false negatives) for each of the different categories of carotid stenosis and reference standards. We produced forest plots and summary receiver operating characteristic (ROC) plots to summarize the data. Where meta‐analysis was possible, we used a bivariate meta‐analysis model.
Main results
We identified 25,087 unique studies, of which 22 were deemed eligible for inclusion (4957 carotid arteries). The risk of bias varied considerably across the studies, and studies were generally of moderate to low quality. We narratively described the results without meta‐analysis in seven studies in which the criteria used to determine stenosis were too different from the duplex velocity criteria proposed in our protocol or studies that provided insufficient data to complete a 2 × 2 table for at least in one category of stenosis. Nine studies (2770 carotid arteries) presented DUS versus DSA results for 70% to 99% carotid artery stenosis, and two (685 carotid arteries) presented results from DUS versus CTA in this category. Seven studies presented results for occlusion with DSA as the reference standard and three with CTA as the reference standard. Five studies compared DUS versus DSA for 50% to 99% carotid artery stenosis. Only one study presented results from 50% to 69% carotid artery stenosis.
For DUS versus DSA, for < 50% carotid artery stenosis, the summary sensitivity was 0.63 (95% confidence interval [CI] 0.48 to 0.76) and the summary specificity was 0.99 (95% CI 0.96 to 0.99); for the 50% to 69% range, only one study was included and meta‐analysis not performed; for the 50% to 99% range, the summary sensitivity was 0.97 (95% CI 0.95 to 0.98) and the summary specificity was 0.70 (95% CI 0.67 to 0.73); for the 70% to 99% range, the summary sensitivity was 0.85 (95% CI 0.77 to 0.91) and the summary specificity was 0.98 (95% CI 0.74 to 0.90); for occlusion, the summary sensitivity was 0.91 (95% CI 0.81 to 0.97) and the summary specificity was 0.95 (95% CI 0.76 to 0.99).
For sensitivity analyses, excluding studies in which participants were selected based on the presence of occlusion on DUS had an impact on specificity: 0.98 (95% CI 0.97 to 0.99). For DUS versus CTA, we found two studies in the range of 70% to 99%; the sensitivity varied from 0.57 to 0.94 and the specificity varied from 0.87 to 0.98. For occlusion, the summary sensitivity was 0.95 (95% CI 0.80 to 0.99) and the summary specificity was 0.91 (95% CI 0.09 to 0.99). For DUS versus MRA, there was one study with results for 50% to 99% carotid artery stenosis, with a sensitivity of 0.88 (95% CI 0.70 to 0.98) and specificity of 0.60 (95% CI 0.15 to 0.95); in the 70% to 99% range, two studies were included, with sensitivity that varied from 0.54 to 0.99 and specificity that varied from 0.78 to 0.89. We could perform only a few of the proposed sensitivity analyses because of the small number of studies included.
Authors' conclusions
This review provides evidence that the diagnostic accuracy of DUS is high, especially at discriminating between the presence or absence of significant carotid artery stenosis (< 50% or 50% to 99%). This evidence, plus its less invasive nature, supports the early use of DUS for the detection of carotid artery stenosis. The accuracy for 70% to 99% carotid artery stenosis and occlusion is high. Clinicians should exercise caution when using DUS as the single preoperative diagnostic method, and the limitations should be considered. There was little evidence of the accuracy of DUS when compared with CTA or MRA. The results of this review should be interpreted with caution because they are based on studies of low methodological quality, mainly due to the patient selection method. Methodological problems in participant inclusion criteria from the studies discussed above apparently influenced an overestimated estimate of prevalence values. Most of the studies included failed to precisely describe inclusion criteria and previous testing. Future diagnostic accuracy studies should include direct comparisons of the various modalities of diagnostic tests (mainly DUS, CTA, and MRA) for carotid artery stenosis since DSA is no longer considered to be the best method for diagnosing carotid stenosis and less invasive tests are now used as reference standards in clinical practice. Also, for future studies, the participant inclusion criteria require careful attention.
Plain language summary
How accurate is duplex ultrasound (DUS) imaging for diagnosing carotid artery stenosis in symptomatic patients?
Carotid artery stenosis (CAS) is a narrowing of the lumen (the inside space) of the carotid artery (usually due to cholesterol deposits called plaque). CAS is responsible for 8% of all strokes due to a blocked blood vessel (ischemic strokes) and is associated with a high chance of recurrence. In such circumstances, the treatment is to re‐establish adequate blood flow (by surgery or other approaches to open the artery) to prevent further neurologic episodes. Duplex ultrasound (DUS) can help identify the appropriate patients who will benefit from a more invasive treatment and those who should be with drugs alone.
What is the aim of this review?
To determine how accurate DUS is for diagnosing different grades of CAS in individuals with neurologic symptoms.
What was studied in the review?
DUS is used in clinical practice as the first test to detect carotid artery stenosis, usually with the result confirmed by other more expensive and invasive tests, such as computed tomography angiography (CTA), magnetic resonance angiography (MRA), or digital subtraction angiography (DSA). The advantage of DUS is that it is less expensive and helps to reduce the time required to select patients for treatment. We included studies assessing the accuracy of DUS compared with DSA, MRA, or CTA in patients with recent stroke symptoms. We grouped the results from studies that used approximately the same method and threshold to assess accuracy in the following categories of carotid artery stenosis: < 50%, 50% to 99%, 50% to 69%, 70% to 99%, and occlusion (blockage of the vessel).
What are the main results of this review?
This review included 22 studies (4957 carotid arteries tested). The searches were performed up to 15 February 2021. The results indicate the following: If DUS were to be used in a standardized cohort of 1000 patients:
For DUS versus DSA
< 50% CAS (4 studies, 1495 carotid arteries): Estimated 299 patients would have a DUS result indicating the presence of non‐significant CAS, of whom eight (2.7%) would be incorrectly classified. Of the 701 people with a result indicating that < 50% carotid stenosis is not present, 169 (24.1%) would be incorrectly classified.
50% to 99% CAS (5 studies, 1536 carotid arteries): Estimated 642 patients would have a DUS result indicating the presence of 50% to 99% CAS; of these, 147 (22.8%) would be incorrectly classified. Of the 358 people with a result indicating that 50% to 99% carotid stenosis is not present, 15 (4.2%) would be incorrectly classified.
70% to 99% CAS (9 studies, 2770 carotid arteries): Estimated 390 patients would have a DUS result indicating the presence of 70% to 99% CAS; of these, eight (2%) would be incorrectly classified. Of the 610 people with a result indicating that 70% to 99% carotid stenosis is not present, 68 (11.1%) would be incorrectly classified.
Occlusion (7 studies, 1212 carotid arteries): Estimated 205 patients would have a DUS result indicating carotid artery occlusion; of these, 41 (20%) would be incorrectly classified. Of the 795 people with a result indicating that carotid occlusion is not present, 16 (2%) would be incorrectly classified.
For DUS versus CTA
Occlusion (3 studies, 833 carotid arteries): An estimated 606 patients would have a DUS result indicating carotid artery occlusion; of these, 36 (6%) would be incorrectly classified. 394 people with a result indicating that carotid occlusion is not present, 30 (8%) would be incorrectly classified.
For DUS versus MRA
Meta‐analysis was not performed.
How reliable are the results of the studies in this review?
There were some problems with how the studies were conducted that could impair the correct estimates of the diagnostic accuracy. Many of the studies were of poor or unclear quality.
Who do the results of this review apply to?
The results are relevant for patients with neurologic symptoms who are suspected of having carotid artery stenosis.
What are the implications of this review?
The diagnostic accuracy of DUS is high, especially at discriminating between the presence or absence of significant carotid artery stenosis. This evidence, plus its less invasive nature, supports the early use of DUS for the detection of carotid artery stenosis.
Summary of findings
Summary of findings 1. Summary of findings table: Duplex ultrasound for diagnosing symptomatic carotid stenosis in the extracranial segments.
| Review question: | What is the diagnostic accuracy of duplex ultrasound for detecting symptomatic carotid stenosis? | ||||||
| Population | Symptomatic patients (sudden visual loss, hemispheric TIA, and ischemic stroke) with suspected carotid artery stenosis | ||||||
| Target condition | Carotid artery stenosis | ||||||
| Index test | Duplex ultrasound | ||||||
| Reference Standard | DSA in 19 studies (Anzidei 2012; Borisch 2003; Chua 2007; Colquhoun 1992; Cui 2018; D'Onofrio 2006; Bray 1995; Eliasziw 1995; Faught 1994; Golledge 1999; Hammond 2008; Hansen 1996; Heijenbrok‐Kal 2006; Huston 1993; Knudsen 2002; Link 1997; Lubezky 1998; Nederkoorn 2002; Wolfle 2002); MRA in three (Borisch 2003; D'Onofrio 2006; Das 2009); CTA in four (Barlinn 2016; Belsky 2000; Das 2009; Lubezky 1998) | ||||||
| Importance | Diagnostic accuracy of DUS to identify carotid artery stenosis in symptomatic patients can improve the path in defining the best treatment option | ||||||
| Included studies | We included 22 studies, with a total of 4957 carotid arteries, mean sample size of 126 carotid arteries, ranging from 24 to 1011; the mean age of participants was 66.3 years (range 53 to 72 years), and the median proportion of men was 70% of included participants. Eighteen prospective studies, two retrospective and, in two studies, it was unclear whether there was a prospective or retrospective design |
||||||
| Risk of bias and applicability concerns | Risk of bias varied considerably across the included studies; we considered nine studies as being at high risk of bias and one as having unclear concern in the patient selection domain, mostly due to failure to include all people with a negative screen or poorly reported patient selection methods; four studies were judged as having a high risk of bias in the index test domain, mostly because of no prespecified thresholds; two as being at high risk of bias and seven at unclear risk of bias in the reference standard domain, as the studies were not blinded or blinding was not described; and the risk of bias in the flow and timing domain was high in 14 studies because not all patients were included in the analysis and it was unclear in another two. Applicability concerns were generally low; six studies were judged as having high concern on the patient selection domain mostly because of previous testing. | ||||||
| Limitations | Seventeen studies were judged as having high risk of bias in at least one domain. The use of velocity criteria with prespecified thresholds and time we accepted between the index test and reference standard (four weeks) led to a lot of studies' exclusions. There were also a lack of data on some carotid stenosis categories and reference standards. | ||||||
| Reference Standard | Studies | Carotid arteries | Summary sensitivity (95% confidence interval) | Summary specificity (95% confidence interval) | Consequences in a cohort of 1000 | ||
| Prevalence of the range of stenosis (median) * | Implications * | Quality and Comments | |||||
| DSA | |||||||
| < 50% | 4 | 1495 | 0.63 (0.48 to 0.76) | 0.99 (0.96 to 0.99) | 0.46 | 460 out of 1000 patients will have < 50% carotid artery stenosis. Of these, 291 (63%) would be correctly diagnosed and receive appropriate clinical treatment and 169 (27%) would receive unnecessary further investigation with another imaging method. Other 532 patients would receive appropriate further investigation, and eight would have no other tests performed and miss a chance for the right diagnosis and the possibility of carotid revascularization . | Limited number of studies Risk of bias: High or unclear in most domains |
| 50‐99% | 5 | 1536 | 0.97 (0.95 to 0.98) | 0.70 (0.67 to 0.73) | 0.51 | 510 out of 1000 patients will have 50‐99% carotid artery stenosis. Of these, 495 (97%) would receive appropriate further investigation with another imaging method, and 15 (3%) would not have any other tests performed and would miss a chance to receive the right diagnosis and the possibility of carotid revascularization. Overall, 147 would receive unnecessary further investigation with another imaging method, and 343 would receive no further investigation and appropriate clinical treatment | Limited number of studies Risk of bias: High or unclear in most domains |
| 50‐69% | 1 | 313 | 0.28 (0.17 to 0.41) | 0.90 (0.85 to 0.93) | 0.19 | Meta‐analyses not conducted | |
| 70‐99% | 9 | 2770 | 0.85 (0.77 to 0.91) | 0.99 (0.96 to 0.99) | 0.45 | 451 out of 1000 patients will have 70‐99% carotid artery stenosis. Of these, 383 (85%) would receive appropriate carotid artery revascularization and 68 (15%) would miss or delay the chance to carotid revascularization. Another 8 would receive inappropriate carotid artery revascularization and 542 would receive appropriate clinical treatment. | Limited number of studies Risk of bias: Low risk in all domains in 2 studies |
| Occluded | 7 | 1212 | 0.91 (0.81 to 0.97) | 0.95 (0.99 to 0.76) | 0.18 | 180 out of 1000 patients will have carotid artery occlusion. Of these, 164 (91%) would receive appropriate clinical treatment. Another 41 would be false‐positive diagnosed with carotid occlusion and not have other tests performed, and miss a chance of the correct diagnosis and carotid revascularization. Other consequences would depend on the range of stenosis. | Limited number of studies Risk of bias: Low risk in all domains in 1 study Two studies only included patients with occlusion on DUS. Sensitivity analyses excluding them had impact on the results of specificity: 0.98 (95% CI: 0.97 to 0.99). |
| CTA | |||||||
| 70‐99% | 2 | 685 | Range: 0.57 to 0.94 | 0.87 to 0.98 | 0.18 | Meta‐analyses not conducted | |
| Occluded | 3 | 833 | 0.95 (0.80 to 0.99) | 0.91 (0.99 to 0.09) | 0.60 | 600 out of 1000 patients will have carotid artery occlusion. Of these, 570 (95%) would receive appropriate clinical treatment. Another 41 would be false‐positive diagnosed with carotid occlusion and not have other tests performed, and miss a chance of the correct diagnosis and carotid revascularization. Other consequences would depend on the range of stenosis | Limited number of studies Risk of bias: High or unclear in most domains 1 study only included patients with occlusion on DUS |
| MRA | |||||||
| 50‐99% | 1 | 31 | 0.88 (0.70 to 0.98) | 0.60 (0.15 to 0.95) | 0.84 | Meta‐analyses not conducted | |
| 70‐99% | 2 | 102 | Range: 0.54 to 0.99 | Range: 0.89 to 0.78 | 0.61 | Meta‐analyses not conducted | |
| CI: confidence interval; CTA: computed tomography angiography; DSA: digital subtraction angiography; DUS: duplex ultrasound; MRA: magnetic resonance angiography; TIA: transient Ischemic attack | |||||||
| * We calculated prevalence from the included studies by the reference standard. The prevalence values used to illustrate the review findings as absolute frequencies are the median from the included studies. | |||||||
| CAUTION: The results on this table should not be interpreted in isolation from the results of the individual included studies contributing to each summary test accuracy measure. These are reported in the main body of the text of the review. | |||||||
Background
Stroke is the third leading cause of death worldwide (Brott 2011; Flumignan 2017; Virani 2021), and probably the most important cause of long‐term disability (CDC 2001; Eliasziw 1994; Strong 2007). Approximately 15 million people have a stroke annually, of which 5 million die as a result of the event and another 5 million remain disabled (Mackay 2004). The estimated direct and indirect costs of care for stroke patients in the USA in 2017 were USD 49.8 billion (Virani 2021). Stroke is considered a devastating disease from the point of view of the patient and the health system.
There are two main categories of stroke: ischemic and hemorrhagic. Approximately 87% of all strokes are ischemic, the main causes of which are carotid artery stenosis, hypertension, and cardiac arrhythmia (Virani 2021). Carotid artery stenosis is responsible for approximately 8% of all strokes and occlusion is judged to be responsible for 3.5% (Flaherty 2013). Patients with carotid artery stenosis are at high risk of a new stroke episode (Easton 2009; Hillen 2003; Moore 1995). The estimated risk of recurrence after a first ischemic episode is 6.4% during the first two to three days, 19.5% within seven days, and 26.1% within 14 days after the initial neurologic event (Tsantilas 2015). In addition, the chances of dying from a subsequent stroke are much higher.
The most important reason for identifying individuals with symptomatic carotid stenosis is the chance to proceed with carotid artery revascularization to prevent a new ischemic episode of stroke or death (Morris 2017). The NASCET 1991 trial found that the two‐year risk of ipsilateral stroke for participants with 70% to 99% carotid stenosis was 26% in those undergoing clinical treatment and 9% in those treated surgically, and the risk was reduced from 22.2% to 15.7% after five years among participants with moderate stenosis (50% to 69%).
Carotid revascularization can be performed by conventional or endovascular surgical treatment and aims to re‐establish adequate blood flow by removing significant stenosis in the vessel. There is strong evidence that carotid endarterectomy should be performed within two weeks of the neurologic event, and urgent revascularization may be considered for stable individuals who have a limited area of infarction with a large penumbra (Fonseca 2021; Rerkasem 2020; Ricotta 2011; Rothwell 2004; Vasconcelos 2016). Important guidelines recommend carotid revascularization be performed as early as possible after the neurologic index event in patients with symptomatic carotid stenosis (≥ 50%) (ESVS Writing Group 2018; Hobson 2008; NICE 2017). The value of revascularization decreases over time: three months after the event, revascularization has no more benefit to the patient than it has to an asymptomatic patient (NCC‐CC 2008; Rothwell 2004). The diagnosis should be confirmed and the severity of extracranial carotid stenosis estimated to perform the correct treatment.
Duplex ultrasound (DUS) is a widely available, non‐invasive, and cost‐effective test, which is usually the test of choice for identifying carotid stenosis and characterizing the severity of the lesion. It is currently still used primarily as a screening and selection test for patients who will undergo more expensive and invasive tests, such as computed tomography angiography (CTA), magnetic resonance angiography (MRA), or digital subtraction angiography (DSA). This review seeks to establish the diagnostic value of DUS for the diagnosis of extracranial carotid stenosis in symptomatic patients. We aim to define whether an individual with symptomatic carotid stenosis should undergo carotid endarterectomy based on DUS alone. In addition, we assess whether DUS is accurate to identify carotid occlusion and patients with non‐significant carotid stenosis who should receive clinical management. This review also contributes to the best decision‐making when clinicians face patients who have an iodine allergy or kidney failure and cannot undergo CTA, MRA, or DSA but who would benefit from carotid revascularization.
Target condition being diagnosed
Carotid artery stenosis is an atherosclerotic lesion that narrows the carotid artery. The deposit of cholesterol plaques on the vessel walls leads to their narrowing and usually occurs in regions of bifurcations, branches, or curvatures, all places of flow disturbance. Although many factors related to the patient and the characteristics of the plaque are studied, the most important parameter in choosing the therapeutic option is still the degree of carotid artery stenosis.
Extracranial carotid artery stenosis can be clinically classified as mild (< 50%), moderate (50% to 69%), severe (70% to 99%), and occlusion (100%) (Grant 2003). Each threshold has an influence on treatment choices for the patient.
Individuals with sudden ipsilateral visual loss, transient ischemic attack (TIA), and ischemic stroke associated with significant (50% to 99%) carotid stenosis within 180 days are considered symptomatic and may require some type of revascularization procedure. Besides, symptomatic individuals with < 50% carotid artery stenosis should receive the best medical management available, and other sources of the stroke should be investigated. Patients with carotid artery occlusion should also receive medical management (ESVS Writing Group 2018; Flumignan 2017; Ricotta 2011).
Index test(s)
DUS is a widely available, low‐cost, truly non‐invasive technique; it is well tolerated by patients and thus ideal for screening and diagnosing atherosclerotic plaque. DUS presents high sensitivity and specificity for diagnosing internal carotid artery (ICA) stenosis in numerous studies, although the results can vary among laboratories and operators (ESVS Writing Group 2018; Souza 2005; Surur 2013; Ventura 2015; Wardlaw 2006a). Currently, DUS is the modality of choice for the initial evaluation of carotid artery disease (ESVS Writing Group 2018; Flumignan 2017; Ricotta 2011). DUS combines B‐mode ultrasonography for morphological images and pulse‐wave Doppler spectrum analysis for flow velocity measures. DUS usually evaluates anatomic images of cervical portions of the common carotid artery (CCA), ICA, and external carotid artery (ECA) and measures their blood flow velocity. DUS can directly measure the luminal diameter of the artery or stenotic section; but rather, its diagnosis relies on blood flow velocity as an indicator of the degree of stenosis.
In 1987, the first validated classification of stenosis based on objective velocity criteria, known as the ‘Strandness Criteria’, was published (Taylor 1987). Since then, different criteria for the classification of carotid stenosis have been developed, and there is still substantial variability from laboratory to laboratory. In 2003, the American Society of Radiology held a conference and standardized the ultrasound criteria to determine stenosis (Grant 2003). They recommended duplex velocity criteria (measurements of internal carotid artery [ICA] peak systolic velocity [PSV] and end‐diastolic velocity [EDV] as well as the ICA/common carotid artery [CCA] PSV ratio) and morphological characteristics (Table 2). The classification by Grant 2003 remains the most used and recommended criteria in clinical practice (AbuRahma 2008; AbuRahma 2011; ESVS Writing Group 2018; Ricotta 2011).
1. DUS criteria for internal carotid stenosis.
| Consensus panel based on Grant 2003 | ||||
| Degree of stenosis (%) | Primary parameters | Additional parameters | ||
| ICA PSV (cm/sec) | Plaque estimate (%)* | ICA/CCA PSV ratio | ICA EDV (cm/sec) | |
| Normal | < 125 | None | < 2.0 | < 40 |
| < 50% | < 125 | < 50 | < 2.0 | < 40 |
| 50% to 69% | 125 to 230 | ≥ 50 | 2.0 to 4.0 | 40 to 100 |
| ≥ 70% but less than near occlusion | > 230 | ≥ 50 | > 4.0 | > 100 |
| Near occlusion | High, low or undetectable | Visible | Variable | Variable |
| Total occlusion | Undetectable | Visible, no detectable lumen | Not applicable | Not applicable |
| *Plaque estimate (diameter reduction) based on DUS B‐mode and on additional color mode ultrasound | ||||
CCA: common carotid artery DUS: duplex ultrasound EDV: end diastolic velocity ICA: internal carotid artery PSV: peak systolic velocity
The disadvantages of DUS include limited visualization of the proximal CCA and distal ICA and technical difficulties related to the patient's physical condition (e.g. obesity, heart failure, postoperative status). Also, there are no widely acknowledged standardized criteria for pseudo‐occlusion on ultrasound (Fonseca 2021). Furthermore, contrast‐enhanced ultrasound is increasingly being used to evaluate patients with known or suspected atherosclerosis; it can help identify carotid plaque ulcerations, differentiate occlusion from pseudo occlusion, identify carotid dissection, and identify intraplaque neovascularization (Rafailidis 2017). With improved technology, the accuracy of this imaging test has increased significantly over time.
Clinical pathway
Evaluation of a patient with suspected symptomatic carotid stenosis should start with a complete history of the patient's comorbidity and risk factors for atherosclerotic disease. There should also be a physical examination because atherosclerotic carotid artery occlusive disease is a systemic disease (ESVS Writing Group 2018). The atherosclerotic carotid disease imaging diagnosis includes four tests: DUS, DSA, CTA, and MRA. These tests are used alone or in combination.
Patients who present with neurologic symptoms from non‐disabling stroke or TIA should undergo a non‐invasive diagnostic method in the initial evaluation (Brott 2011; ESVS Writing Group 2018; Flumignan 2017; NCC‐CC 2008; Ricotta 2011). Non‐invasive tests include DUS, CTA, and MRA. Patients presenting any degree of extracranial carotid stenosis should be treated with antiplatelet and lipid‐lowering therapy; carotid revascularization should be considered for those presenting significant stenosis (Brott 2011; ESVS Writing Group 2018; Hobson 2008; NICE 2017; Orrapin 2017; Ricotta 2011). Rapid imaging of the carotid artery is essential because there is a short time window for effective stroke prevention in patients presenting significant carotid artery stenosis. Although treatment is beneficial until 180 days after the first neurologic episode, current guidelines recommend that carotid intervention should be performed as soon as possible, ideally before 14 days (ESVS Writing Group 2018).
DSA was considered the gold standard to assess extracranial stenosis of carotid vessels, but it is an invasive method and carries a risk of morbidity or even mortality (ACAS 1995; Davies 1993; Hankey 1990). Its main limitations make this test unsuitable as a screening modality and rarely required for preoperative imaging (unless there are discrepancies on non‐invasive tests). CTA and MRA are replacing DSA. They usually use contrast agents and allow important additional evaluations of the aortic arch, supra‐aortic trunks, distal ICA, and intracranial vessels; this information is mandatory in stenting cases.
In clinical practice, the initial study is usually a bilateral carotid DUS to determine whether carotid stenosis contributes to the patient's symptoms (Brott 2011; ESVS Writing Group 2018; Ricotta 2011). After the first test, the treatment can be defined based solely on this initial test if it is reliable (Ricotta 2011). However, a second look by a different examiner or subsequent confirmation of results with DSA, CTA, or MRA for therapeutic programming is usual and recommended (ESVS Writing Group 2018).
The clinical pathway can vary depending on the center, and a recent guideline suggested that CTA is the most cost‐effective diagnostic method for patients at high risk of carotid artery stenosis in whom early revascularization could be performed (Kleindorfer 2021). Although current clinical guidelines recommend DUS as a first‐line imaging modality, studies have shown a significant misclassification rate before carotid endarterectomy (Collins 2005; Johnston 2001). Moreover, many authors draw attention to the low quality of the studies that have determined the accuracy of non‐invasive tests. Most guideline recommendations are based on old studies of questionable quality (Wardlaw 2006a).
Prior test(s)
In symptomatic patients (ischemic attack, amaurosis fugax, or ischemic stroke), DUS is recommended as the initial test because it is safe, inexpensive, and widely available. Therefore, individuals should not have any formal testing completed before DUS.
Role of index test(s)
DUS has been accepted by some investigators in qualified laboratories as a satisfactory method to determine the severity of carotid stenosis, being the basis of clinical decisions (Howard 2017). However, its use as the only imaging modality prior to performing carotid endarterectomy has been the subject of some controversy. In clinical practice, it is used primarily for screening and selecting patients for other non‐invasive and confirmatory tests, such as CTA or MRA.
Nevertheless, the accuracy of DUS remains a point of discussion (ESVS Writing Group 2018; Souza 2005; Surur 2013; Ventura 2015; Wardlaw 2006a).
Alternative test(s)
Digital subtraction angiography
Digital subtraction angiography was considered the gold standard against all other imaging modalities in individuals with extracranial cerebrovascular disease, even with its risks. Measurement of carotid stenosis is usually done using the NASCET 1991 method. The ECST 1998 method is avoided because it may overestimate carotid stenosis (Figure 1). The cut‐off points of 50% and 70% carotid artery stenosis with the NASCET method have been shown to be equivalent to approximately 75% and 85% for the ECST method, respectively (Nicolaides 1996). The major DSA limitations that make it inappropriate as a screening modality include its cost and associated risks, specifically of stroke and death. Studies have reported a 4% risk of TIA or minor stroke, a 1% risk of major stroke, and even a small (1%) risk of death (Davies 1993; Hankey 1990). Given its invasive characteristics, DSA has now been replaced by other effective, non‐invasive diagnostic methods, and DSA should be reserved for patients in whom non‐invasive imaging methods are contraindicated or inconclusive. In this review, we will consider the NASCET method for determining carotid stenosis by using DSA compared with DUS (Figure 1).
1.
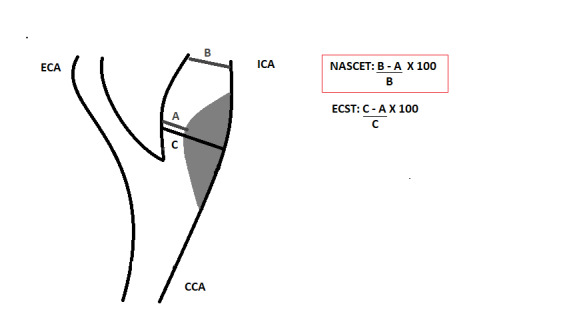
Longitudinal view of carotid bifurcation with methods of measuring carotid stenosis at angiography
A : narrowest ICA diameter B : normal distal cervical ICA diameter C : estimated original diameter at the site of the most stenosis CCA : common carotid artery ECA : external carotid artery ECST : European Carotid Surgery Trial ICA : internal carotid artery NASCET : North American Symptomatic Carotid Endarterectomy Trial
Contrast‐enhanced magnetic resonance angiography
MRA is another option to provide images of the carotid artery by different techniques, with or without contrast enhancement. The sensitivity of contrast‐enhanced MRA is high, and contrast should be used in all examinations for carotid stenosis diagnosis. Essentially, MRA uses the information of a powerful magnetic field, radiofrequency waves, and a computer program to create highly detailed imaging from different human tissues, including vessels and blood. In a systematic review of published studies on DUS and MRA, using DSA as the gold standard, MRA was found to be both sensitive and specific for detecting carotid stenosis, with a pooled sensitivity of 95% and a pooled specificity of 90% for the diagnosis of 70% to 99% carotid artery stenosis (Nederkoorn 2003). The classification of stenosis is according to the NASCET method (Figure 1).
The notable strengths of contrast‐enhanced MRA are its relative insensitivity to arterial calcification and lack of exposure to ionizing radiation. The limitations of contrast‐enhanced MRA include overestimation of stenosis, the inability to discriminate between subtotal and complete arterial occlusion, and the risk of nephrogenic systemic fibrosis when patients with pre‐existing renal dysfunction are exposed to high doses of gadolinium (Brott 2011). Furthermore, a substantial fraction of patients cannot be examined, such as patients who have claustrophobia, extreme obesity, or incompatible implanted devices such as pacemakers or defibrillators, and MRA is not a readily available method.
Computed tomography angiography
CTA is a validated tool for non‐invasive assessment of the degree of carotid artery stenosis (Daolio 2019; Duddalwar 2004). The rapid acquisition of spiral CTA images allows excellent timing with contrast administration and provides quality images that are less susceptible than MRA to overestimating the severity of carotid stenosis. As with MRA, CTA provides anatomic imaging from the aortic arch through the circle of Willis and the brain parenchyma, with multiplanar reconstruction and analysis allowing evaluation of even very tortuous vessels. Vessel wall imaging is an advantage of CTA and MRA over DSA because the latter detects only the flow (i.e. the contrast in blood). The classification of stenosis is measured according to the NASCET method (Figure 1). However, there are acknowledged drawbacks to CTA, such as the need for intravenous contrast and potential contrast nephrotoxicity, the ionizing radiation dosage, and calcification artefacts.
Rationale
Symptomatic patients with extracranial carotid stenosis should be evaluated rapidly and revascularization planned. If symptomatic patients undergo endarterectomy based on DUS alone, they will not be exposed to ionizing radiation or potentially nephrotoxic contrast materials. In addition, it will be much more cost‐effective to the health system. It must also be considered that there are many places where access to DSA, CTA, or MRA is limited, a factor that could delay treatment, whereas DUS is widely available in hospitals around the world. On the other hand, the decision of the best path to identify carotid stenosis should consider the risk of missing a potentially treatable stenosis (i.e. false‐negative result), which could lead the patient to a new and potentially worse ischemic episode, and the risk of performing surgery unnecessarily based on a false‐positive result.
Doubts about the diagnostic value regarding DUS have previously been published (Moore 1995), and other authors have also questioned its value (Collins 2005). Currently, various guidelines suggest performing DUS as the first diagnostic method, with additional imaging required when DUS is non‐diagnostic (ESVS Writing Group 2018; NCC‐CC 2008; Ricotta 2011). Others suggest that two non‐invasive methods should be performed before endarterectomy and, if only DUS is to be performed, then it should be repeated with a second operator to confirm the result (ESVS Writing Group 2018).
The complexity of diagnostic tests associated with significant variability in the estimates of their accuracy in the literature and studies without standardization of methodology increases the difficulty of standardizing the best diagnostic path for patients with neurologic symptoms suspected of carotid stenosis. Recommendations from different societies are often based on individual studies and old reviews. Knowing the limitations and accuracy of DUS in these patients and evaluating the methodology applied for these determinations play a fundamental role in decision‐making in clinical practice (ESVS Writing Group 2018; NCC‐CC 2008; Ricotta 2011). Understanding diagnostic tests goes beyond knowing their accuracy: it requires identifying their risks, benefits, consequences, and the correct interpretation of results to offer the best therapeutic planning to the patient.
Objectives
To assess the accuracy of DUS in symptomatic patients (sudden visual loss, hemispheric TIA, and ischemic stroke) with suspected extracranial carotid artery stenosis verified by DSA, MRA, or CTA.
Secondary objectives
We planned to assess and evaluate in subgroup analyses any method that could improve accuracy in addition to duplex: microbubble contrast, Power Doppler or similar, and color mode. However, due to the lack of data on contrast and Power Doppler, we only performed subgroup analysis for the color resource in the 70% to 99% range of carotid artery stenosis.
Methods
Criteria for considering studies for this review
Types of studies
We included cross‐sectional or diagnostic test accuracy (DTA) cohort studies assessing DUS against an acceptable reference standard (DSA, MRA, or CTA). We included both prospective and retrospective studies. We included both blinded and non‐blinded studies and investigated the effect of excluding non‐blinded studies by means of sensitivity analyses. We considered a study to be blinded if the examiner of one method did not know the result of the other test. Case reports and case‐control studies were not considered eligible for inclusion because they often overestimate the accuracy that a test has in clinical practice (Rutjes 2005). We excluded studies with an excessively long period (more than four weeks) of time between the index and reference tests, due to changes in the patient’s stenosis and risk of clinical degradation over time and the definition of a symptomatic patient (NASCET 1991). The timing of revascularization of symptomatic internal carotid artery stenosis has been changing over the years. It is still accepted that treatment is beneficial until 180 days after the first neurological episode, but current guidelines already recommend that carotid intervention should be performed as soon as possible, ideally before 14 days (ESVS Writing Group 2018). Therefore, we found four weeks between tests a reasonable time for carotid imaging.
Participants
Symptomatic patients with suspected carotid artery stenosis. Individuals with sudden visual loss, hemispheric TIA, and ischemic stroke associated with carotid stenosis are considered symptomatic (Rothwell 2004). We accepted studies in which at least 70% of included participants were symptomatic.
We excluded participants who did not receive DUS, those for whom the time between the index test(s) and the alternative test(s) was too long (more than four weeks), or those who had had a disabling stroke (modified Rankin Score ≥ 3) because the presence of a severe neurological impairment is known to limit the accuracy of diagnostic techniques (Bonita 1988; Rankin 1957).
Index tests
For DUS, we considered B‐mode identification and velocity‐based estimation of carotid stenosis with or without additional resources (e.g. microbubble contrast, Power Doppler or similar, and color mode). We considered the classification of carotid stenosis with DUS defined with validated duplex velocity criteria (measurements of ICA PSV, EDV, and the ICA/CCA PSV ratio) and morphological characteristics. We used the velocity criteria statement and the parameter priorities of Grant 2003 (Table 2).
Target conditions
Extracranial carotid stenosis can be clinically classified as mild (< 50%), moderate (50% to 69%), severe (70% to 99%), and occlusion (100%) (NASCET 1991). The data from studies should be consistent with this definition or conversion should be possible. Symptomatic carotid stenosis is defined as when an individual presents with sudden ipsilateral visual loss, hemispheric TIA, or ischemic stroke within three months associated with carotid stenosis (Rothwell 2004).
Reference standards
We accepted DSA, MRA, or CTA as reference standards. Due to risks associated with its use, DSA is no longer routinely performed for diagnosis in many centers (ESVS Writing Group 2018). However, until the end of the 20th century, catheter‐based angiography was the test used to measure carotid stenosis in the majority of carotid endarterectomy trials. Carotid stenosis should be classified according to the NASCET method (or conversion should be possible) (Figure 1). As current guidelines support the investigation of carotid stenosis with less invasive methods such as MRA and CTA, we also accepted any of these as standard reference methods, and we presented the results separately.
Search methods for identification of studies
Electronic searches
On 15 February 2021, the Cochrane Stroke Group Information Specialist searched the following electronic databases combining topic‐related and DUS terms:
Cochrane Register of Diagnostic Test Accuracy Studies (CRDTAS); the full list of the databases, journals, and conference proceedings that have been searched, as well as the search strategies used, are described in the 'Specialised register' section on Cochrane Stroke's website;
Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library, latest issue) (Appendix 1);
MEDLINE Ovid (from 1946 to present) (Appendix 2);
Embase Ovid (from 1974 to present) (Appendix 3);
ISI Web of Science: Science Citation Index Expanded (SCI‐EXPANDED) and Conference Proceedings Citation Index‐Science (CPCI‐S) (from 1900 to present) (Appendix 4);
Database of Abstracts of Reviews of Effects (DARE) (Appendix 5);
Health Technology Assessment (HTA) Database and International HTA Database; database.inahta.org (Appendix 5);
Latin American and Caribbean Health Science Information (LILACS) and Índice Bibliográfico Español de Ciencias de la Salud (IBECS) (from 1982 to present) (Appendix 6).
We developed the MEDLINE search strategy with the help of the Cochrane Stroke Group Information Specialist, and we adapted it for the other databases, where necessary Appendix 2).
Searching other resources
We searched the following trial registries (15 February 2021) for details of ongoing and unpublished trials:
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov) (Appendix 7);
World Health Organization International Clinical Trials Registry Platform (ictrptest.azurewebsites.net/Default.aspx).
We checked the bibliographies of the included trials for additional references to relevant studies and used the Science Citation Index Cited Reference Search for forward tracking of important articles. We also contacted specialists in the field, manufacturers, and the authors of the included studies for any unpublished data.
Data collection and analysis
Selection of studies
Three review authors (NC, LCUN, and RLGF) independently screened and applied the selection criteria to the titles and abstracts identified as a result of our search strategy. We excluded duplicates and studies that did not meet the inclusion criteria. We retrieved the full‐text articles for reports deemed relevant, and two review authors (NC and RLGF) independently assessed the full‐text articles for inclusion or exclusion, and identified and recorded the reasons for exclusion. Any disagreements were resolved through discussion with the author team (JCCBS, CDQF, RS, LCUN, and VV). We included studies as of 1980 because that was when DUS technology began to be applied in clinical practice.
Data extraction and management
Two review authors (NC and RLGF) independently extracted the data from the included studies using a standard form. Any disagreements were resolved by discussion until consensus was established. When necessary, a third review author was consulted (LCUN). When necessary, we contacted the study authors for missing data. We sent data requests to study authors of studies not included in meta‐analyses before excluding a study due to insufficient data. We collected data on details of the included study (authors, study origin, year and language of publication, study design); characteristics of participants (age and gender); index test and definition of criteria used to determine the grade of stenosis; tests carried out prior to the index test; reference standard and definition of criteria used to determine the grade of stenosis; and numerical results (number of true positives, false positives, true negatives, and false negatives). When possible, we extracted 2 × 2 data directly. Alternatively, we reconstructed 2 × 2 tables by entering data on sensitivity, specificity, the total number of participants, and the proportion of diseased participants in the Review Manager 5 diagnostic accuracy calculator (RevMan 2020). We also extracted details of test threshold(s) used for interpretation of the results and the data on the technical aspects of DUS and the reference standards.
Assessment of methodological quality
We adopted the Quality Assessment of Diagnostic Accuracy Studies (QUADAS‐2) tool to assess the methodological quality of the included studies (Whiting 2006; Whiting 2011). Any disagreements were resolved by discussion; if disagreement persisted, all review authors were consulted. We presented the outcome data of the methodological quality assessment in Table 3 summarizing the number of studies with low, high, or unclear risk of bias for each of the four domains (patient selection, index test(s), reference standard, and flow and timing). We used Review Manager 5 to construct methodological quality summary graphs (RevMan 2020). We planned to conduct sensitivity analyses excluding studies at high risk of bias. We considered the overall risk of bias of an included study as low if there was no high‐risk judgement in the four main domains: patient selection, index test, reference standard, and flow and timing.
2. QUADAS‐2 'Risk of bias' and applicability judgements.
| Quality Assessment of Diagnostic Accuracy Studies‐2 ( QUADAS‐2) | |||
| Patient Selection | A. Risk of bias | Signaling question 1: was a consecutive or random sample of patients enrolled? |
Yes: it is described that the sample was consecutive or a random sample. No: it is described that the sample was not consecutive or a random sample. Unclear: the method of sampling is ambiguous. |
| Signaling question 2: was a case‐control design avoided? |
Yes No |
||
| Signaling question 3: did the study avoid inappropriate exclusions? |
Yes: the study included all symptomatic patients. No: the study excluded patients with neurological symptoms. Unclear: the study's exclusion criteria allow for inappropriate exclusions. |
||
| Could the selection of participants have introduced bias? |
RISK: High Low Unclear |
||
| B. Concerns regarding applicability | We will include individuals with symptomatic carotid stenosis (i.e. those with sudden visual loss, hemispheric TIA, and ischemic stroke within 3 months associated with carotid stenosis). Patients may or may not have been previously tested. We will describe included participants (symptoms, prior testing, presentation, intended use of index test, and setting). |
||
| Is there concern that the included participants do not match the review question? |
CONCERN: High Low Unclear |
||
| Index tests(s) | A. Risk of bias | Index test: DUS, i.e. B‐mode identification (morphological analysis) and velocity‐based estimation of carotid artery stenosis with or without color mode We will describe the index test and how it was conducted and interpreted. |
|
| Signaling question 1: were the index test (DUS) results interpreted without knowledge of the results of the reference standard? |
Yes: it is described that the index test was performed and interpreted in a blind manner. No: the results of the reference standard were known to the DUS operator. Unclear: it is not reported. |
||
| Signaling question 2: if a threshold was used, was it prespecified? We will use the velocity criteria statement reported in Grant 2003 . |
Yes: the threshold used to define positive stenosis was prespecified. No: threshold was not described or was determined after analyzing the results. Unclear: the threshold that was used to define positive stenosis and how it was chosen is unclear. |
||
| Could the conduct or interpretation of the index test have introduced bias? |
RISK: High Low Unclear |
||
| B. Concerns regarding applicability | Is there concern that the index test, its conduct, or interpretation differ from the review question? |
CONCERN: High Low Unclear |
|
| Reference standard | A. Risk of bias | Due to risks associated with its use, DSA is no longer routinely performed in many centers. We will therefore accept as reference standards any one of the following: DSA, MRA, or CTA. We will describe the reference standard test and how it was conducted and interpreted. |
|
| Signaling question 1: is the reference standard likely to correctly classify the target condition? Does the study report that either standards DSA, MRA, or CTA was performed for all participants? Are the reference standard results reported as NASCET 1991 method or is conversion possible ( Figure 1 )? |
Yes: reference standard was described and performed for all included participants. No: the test was not performed in all included participants. Unclear: it is not described if the test was performed to all included participants. |
||
| Signaling question 2: were the reference standard results interpreted without knowledge of the results of the index test? Was the person classifying the reference standard results unaware of the DUS results? |
Yes: the person performing the reference standard test results was unaware of the DUS test results. No: the person performing the reference standard test results was aware of the DUS test results. Unclear: not reported |
||
| Could the reference standard, its conduct, or its interpretation have introduced bias? |
RISK: High: the reference standard was not read blind to the index test, or participants received the reference standard according to the results of the index test. Low: all included participants received the reference standard, and it was performed in a blind manner. Unclear: not reported |
||
| B. Concerns regarding applicability | Is there concern that the target condition as defined by the reference standard does not match the review question? |
CONCERN: High Low Unclear |
|
| Flow and timing | A. Risk of bias | We will describe any participants who did not receive the index test(s) and/or reference standard or who were excluded from the 2 x 2 table. | |
| We will describe the time interval and any interventions between index test(s) and reference standard. | |||
| Signaling question 1: was there an appropriate interval between index test and reference standard? |
Yes: the time interval between DUS and reference standard was less than 4 weeks. No: the time interval between DUS and reference standard was more than 4 weeks. Unclear: the time interval between DUS and reference standard was not reported or reported as median time. |
||
| Did all patients receive the same reference standard? |
Yes No Unclear: not reported |
||
| Were all patients included in the analysis? |
Yes No Unclear: not reported |
||
| Could the patient flow have introduced bias? |
RISK: High Low Unclear |
||
CEMRA: contrast‐enhanced magnetic resonance angiography CTA: computed tomography angiography DSA: digital subtraction angiography DUS: duplex ultrasound MRA: magnetic resonance angiography TIA: transient ischemic attack
Statistical analysis and data synthesis
We performed the analyses following Chapter 10 of the Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy (Macaskill 2010). We primarily used Cochrane's Review Manager 5 software for baseline analyses (RevMan 2020); we used R software (R Project 2018) for additional analyses and plots, when necessary. Since all included studies reported data using the carotid arteries as the unit of analysis, we also considered the number of carotid arteries as our unit of analysis submitted to both the index test (DUS) and the reference standard. Carotid stenosis should be classified according to the NASCET method (or conversion should be possible) in the reference standard tests, and we adopted the threshold described by Grant 2003 (Table 2) to the index test.
We extracted or derived data from the included studies for each imaging test and each range of carotid stenosis and generated 2 × 2 contingency tables of true‐positive cases, false‐positive cases, false‐negative cases, and true‐negative cases. We considered severe (70% to 99%) and moderate (50% to 69%) carotid artery stenosis as positive and analyzed each of these ranges separately; we also analyzed < 50% carotid stenosis and carotid occlusion. If more than one test was used as a reference test, we constructed a 2 × 2 table for each one, comparing it with DUS. We calculated sensitivity and specificity with 95% confidence intervals (CIs) for each test in each study. We used forest plots to display the sensitivity and specificity estimates measured in each study and to illustrate the variation in estimates among studies.
When at least three studies were evaluating the same range of stenosis and the same reference standard and reported consistent test accuracy estimates, we pooled sensitivity and specificity using the bivariate random‐effects method. This method is recommended for studies using the same positivity threshold (Reitsma 2005). In the bivariate model, the combination of two normally distributed outcomes, the logit‐transformed sensitivities and specificities, while acknowledging the possible correlation between them, leads to the bivariate normal distribution. The parameters of the bivariate model are estimated in a single model to incorporate the possible correlation between sensitivities and specificities. We calculated the Chi 2 test for equality to assess the heterogeneity of sensitivity and specificity among studies. We also calculated Spearman’s correlation coefficient to investigate the presence of the threshold effect (correlation between sensitivity and specificity estimates), considering a correlation coefficient of ‐0.60 as indicative of the threshold effect. From the bivariate model, we used Review Manager 5 (RevMan 2020), to plot estimates of sensitivity and specificity from each study and to generate summary receiver operating characteristic (sROC) curves. We calculated a 95% confidence region and a prediction region around the summary estimates from the parameters of the bivariate model and added it to the plot to illustrate the precision in which the estimate was combined (region of an average) and to illustrate the probable range of values that would be expected in 95% of future studies. The combined estimates of likelihood ratios and diagnostic odds ratios (DOR) were obtained by using the Zwindermann & Bossuyt procedure (Zwinderman 2008). This procedure uses the adjustment parameters of the bivariate model to generate sensitivity samples and false positive rates and calculate the 95% CI. In this case, we use the number of 10,000 iterations.
All analyses were performed with the aid of the 'mada' package (Doebler 2017) implemented in the R program (R Project 2018).
We summarized findings with absolute values on 1000 tested participants with the estimated number of false positives (undue treatment) and false negatives (missing appropriate treatment).
Investigations of heterogeneity
We performed meta‐regression analyses to explore potential sources of heterogeneity among the studies by adding one covariate at a time to the bivariate model. A P value less than 0.05 was considered to indicate a significant effect. For the categorical covariates that influence the heterogeneity in the sensitivity and specificity estimates, we performed subgroup analyses if the number of studies made it meaningful to add parameters to the models.
In the protocol, we planned to investigate the potential sources of heterogeneity of the generation of technology; characteristics of the participant population (age and gender); additional ultrasound resources (color mode and Power Doppler, or similar); use of contrast‐enhanced DUS (microbubbles) versus DUS; and time of publication. As evident from the forest and ROC plots, there was considerable between‐study heterogeneity in the test accuracy estimates. However, due to the small number of studies, it was not possible to perform all the planned analyses. We added mean age of the participant population and the prevalence of the disease as covariates in each comparison to analyze potential sources of heterogeneity. We were unable to perform meta‐regression analyses for the participants’ gender, generation of technology, and use of contrast‐enhanced DUS (microbubbles) due to a lack of data.
Sensitivity analyses
We intended to conduct several sensitivity analyses to compare the diagnostic accuracy by investigating the effect of excluding studies at high risk of bias and, in particular, non‐blinded studies. Due to the lack of suitable data (small number of studies in each category), sensitivity analyses were limited. It was possible to perform a sensitivity analysis to examine the impact of blinding for all carotid stenosis ranges for the DUS versus DSA comparison, except for < 50% carotid artery stenosis. We did not conduct sensitivity analyses by excluding studies at high risk of bias because there were insufficient data in each category of stenosis. We considered the overall risk of bias of an included study as low if there was no high‐risk judgement in the four main domains: patient selection, index test, reference standard, and flow and timing. For the 70% to 99% range of carotid artery stenosis, we included studies with similar thresholds, but not exactly the prespecified ones. Hence, we decided to perform a sensitivity analysis excluding all studies that did not exactly use the speed parameter as specified in Table 2. We also performed sensitivity analyses in the occlusion category by excluding Hammond 2008 and Lubezky 1998 , which only included patients that had already been diagnosed with carotid artery occlusion on DUS and, therefore, had no false negative test and low rates of specificity.
Assessment of reporting bias
We did not assess reporting bias because the relevant methods are not well developed for systematic reviews of DTA studies.
Results
Results of the search
Excluded studies
The results of the literature searches are outlined in Figure 2 . We initially identified 36,419 studies and removed 11,332 duplicates. Hence, 25,087 records remained for possible eligibility. After reading the title and the abstract of these records, we excluded 24,434 of them, as they did not meet the inclusion criteria, leaving 653 full‐text studies for eligibility assessment. After the full‐text evaluation, we excluded a further 289 articles that were not relevant to this review, and we excluded another 342 with one or more of the following reasons.
2.
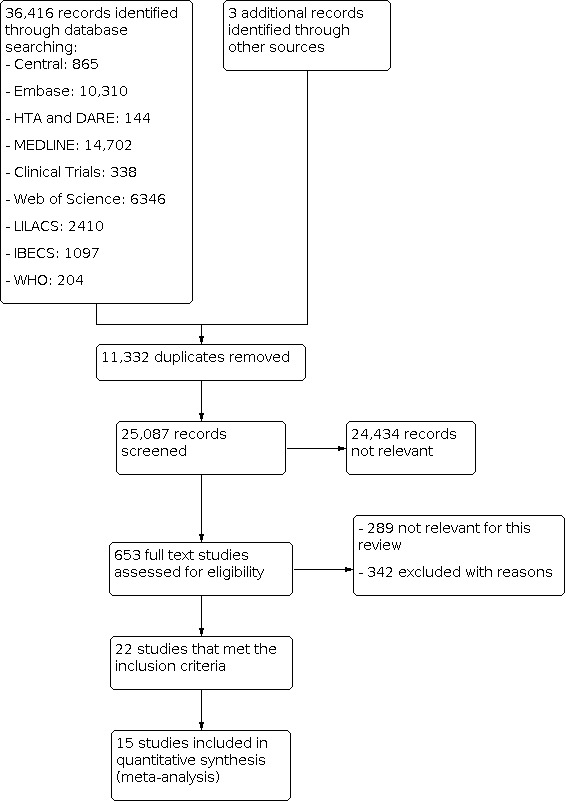
Study flow diagram
Studies did not assess or did not provide data on DUS accuracy for symptomatic carotid stenosis, even though it was performed (19).
Less than 70% of the participants included were symptomatic (75).
Studies did not define the proportion of symptomatic patients (132).
Preliminary paper of DUS technique described (subjective visual impression of the degree of stenosis) or no objective criteria to estimate stenosis (55).
Time between the index test and the alternative test was not specified or was more than four weeks (58).
Accuracy was determined by comparison with the surgical specimen (2).
Case‐control design (1).
Included studies
We included 22 studies that met our prespecified inclusion criteria. These studies had a total of 4957 carotid arteries, with a mean sample size of 126, ranging from 24 to 1011. The mean age of participants was 66.3 years (range 53 to 72 years), and the mean proportion of men was 70% of included participants. Five studies did not provide the participants’ demographic details (D'Onofrio 2006; Eliasziw 1995; Faught 1994; Hammond 2008; Knudsen 2002), and Chua 2007 described the male‐to‐female sex ratio as 2.9:1. From these 22 included studies, 15 were conducted in Europe (5 in Germany, 3 in the UK, 2 in the Netherlands, 2 in Italy, 1 in Denmark, 1 in France, and 1 in Sweden), 2 in Asia (1 in China and 1 in Singapore), 3 in North America (Faught 1994; Huston 1993 in the USA, and Eliasziw 1995 included patients from 50 North American centers), and 2 in Israel. We present a summary of the characteristics of the included studies in Table 4 .
3. Summary table of included studies.
| Name | Study location | Ultrasound technology | Microbubbles contrast | Reference Standard | Quantitative analysis | DUS threshold | Carotids included | Number of participants | Mean age |
| Anzidei 2012 | Italy | Aplio XV device (Toshiba Medical Systems, Japan) or Mylab 70 (Esaote Biomedica, Genoa, Italy) | No | DSA | < 50%, ≥ 50‐99% and occlusion | NASCET + PSV 125‐130 to ≥ 50% | 335 | 170 | 69 |
| Barlinn 2016 | Germany | Aplio MX Toshiba‐ SSA‐780a System®, Toshiba Medical Systems, Germany | No | CTA | < 70%, ≥ 70‐99% and occlusion | (DEGUM criteria) ≥ 50%: ≥ 200 cm/s and ≥ 70%: ≥ 300 cm/s Table 11 |
593 | 303 | 72 |
| Belsky 2000 | Israel | Acuson 128, Acuson, Mountain View CA | No | CTA | < 70%, ≥ 70‐99% and occlusion | 70–99%: PSV ≥ 250 and EDV ≥ 100 | 92 | 46 | 70 |
| Borisch 2003 | Germany | Sonoline Elegra 5.0 system (Siemens) | No | DSA, MRA | < 70%, ≥ 70‐99% and occlusion to DSA. < 70% and ≥ 70% for MRA | ≥ 70%: PSV ≥ 250 /Ratio (r)ICA/CCA > 3 | 71 | 39 | 67.4 |
| Chua 2007 | Singapore | Diasonics Spectra (Diasonics Inc, Milpatas, California) | No | DSA | No | PSV ICA/ICCA ≥ 1.5 to 50% and ≥ 3.1 to ≥ 70% | 188 | 94 | 64 |
| Colquhoun 1992 | UK | Acuson 128 duplex scanner | No | DSA | No | PSV ≥ 120: ≥ 50% and PSV ≥ 250 for ≥ 80% | 53 | 50 | 53 |
| Cui 2018 | China | Esaote North America, Inc., Indianapolis, IN, USA | No | DSA | No | Table 2 ; Grant 2003 | 216 | 54 | 63 |
| D'Onofrio 2006 | Italy | Sequoia 512, Acuson/Siemens | No | DSA/MRA | ≥ 50‐99% and ≥ 70‐99% | ≥ 60% PSV ≥ 130 and EDV ≥ 40. ≥ 80% PSV ≥ 250 and EDV ≥ 100 | 41 DSA / 31 MRA | 32 | Not described |
| Das 2009 | Germany | GE Vivid 7 | No | CTA/MRA | No | (DEGUM criteria) ≥ 50%: ≥ 200 cm/s and ≥ 70%: ≥ 300 cm/s Table 11 |
30 | 15 | 69 |
| Bray 1995 | France | Not specified | No | DSA | No | ≥ 50%: PSV ≥ 130 cm/s. ≥ 70%: PSV ≥ 250 cm/s | 128 | 64 | 62 |
| Eliasziw 1995 | North America | Not specified | No | DSA | < 70%, ≥ 70‐99% | ≥ 70%: PSV ≥ 250 | 1011 | 1011 | Not described |
| Faught 1994 | United States | QUAD I Angiodynograph (Quantum Medical Systems, Issaquah, Wash.) until the latter part of 1989, after which the Quantum 2000 (Quantum Medical Systerns) | No | DSA | < 50%, ≥ 50‐99%, < 70% and ≥ 70‐99% | PSV < 110 cm/s for stenosis 0‐29%/PSV 111‐130 cm/s for stenosis 30‐49%/PSV > 130 cm/s, EDV ≥ 100 for stenosis 50‐69%/PSV ≥ 230 cm/s, EDV ≥ 100 cm/s for stenosis 70‐99% | 770 | 405 | Not described |
| Golledge 1999 | UK | Ultramark 9, HDI, Advanced Technology Laboratories, Wash | No | DSA | ≥ 70‐99% | EDV ≥ 90 cm/s | 100 | 50 | 71 |
| Hammond 2008 | UK | Acuson 128 XP10 | No | DSA | Occlusion | No flow | 24 | 30 | Not described |
| Hansen 1996 | Sweden | Acuson XP 10 | No | DSA | No | y = 0.54.e 0.021 x (y = PSV ICA and x = the degree of stenosis expressed as the diameter reduction in %) | 162 | 81 | 68 |
| Heijenbrok‐Kal 2006 | Netherlands | Ultramark 9 HDI or HDI 3000 (311 participants). Diasonics Master Series (39 participants) | No | DSA | < 50%, ≥ 50%, 50‐69%, ≥ 70%, near occlusion and occlusion | Table 2 ; Grant 2003 | 313 | 350 | 67 |
| Huston 1993 | United States | Acuson 128 | No | DSA | < 50%, ≥ 50‐99% and occlusion | PSV < 125 cm/s = < 50% PSV ≥ 125 cm/s and EDV < 135 cm/s = 50‐79% PSV ≥ 125 cm/s and EDV ≥ 135 cm/s ≥ 80% |
77 | 50 | 67 |
| Knudsen 2002 | Denmark | Siemens Sonoline Elegra | No | DSA | No | ≥ 70%: PSV ≥ 150 cm/s, EDV ≥ 90 cm/s and PSV ratio ≥ 2.8 | 129 | 65 | Not described |
| Link 1997 | Germany | Philips P700 | No | DSA | 70‐99% and occlusion | PSV ≥ 200 cm/s | 56 | 28 | 63 |
| Lubezky 1998 | Israel | HD13000 system | No | CTA, DSA | Occlusion | No flow | 148 CTA/54 DSA | 148 | 70 |
| Nederkoorn 2002 | Netherlands | Not specified | No | DSA | < 70%, ≥ 70‐99% and occlusion | 0‐29%: PSV < 150 cm/s 30‐49%: PSV 150‐190 cm/s 50‐69% PSV 190‐270 cm/s 70‐99%: PSV ≥ 270 cm/s Occlusion: no flow |
313 | 350 | 67 |
| Wolfle 2002 | Germany | Siemens (Elegra) | No | DSA | ≥ 70‐99% | 70‐99%: PSV ≥ 230 cm/s and EDV ≥ 70 cm/s | 94 | 47 | 68 |
CCA: common carotid artery CTA: computed tomography angiography DEGUM: The German Society of Ultrasound in Medicine DSA: digital subtraction angiography EDV: end diastolic velocity ICA: internal carotid artery MRA: magnetic resonance angiography NASCET: North American Symptomatic Carotid Endaterectomy Trial PSV: peak systolic velocity
Eighteen studies used a prospective method for participant recruitment (Anzidei 2012; Borisch 2003; Bray 1995; Chua 2007; Colquhoun 1992; Cui 2018; Das 2009; D'Onofrio 2006; Eliasziw 1995; Hammond 2008; Hansen 1996; Heijenbrok‐Kal 2006; Huston 1993; Knudsen 2002; Link 1997; Nederkoorn 2002; Wolfle 2002; Golledge 1999), two used a retrospective method (Barlinn 2016; Belsky 2000), and in two studies it was unclear whether there was a prospective or retrospective design (Faught 1994; Lubezky 1998).
An effort was made to group the results from studies into clinically relevant categories described in Grant 2003 that serve as the basis for treatment decisions and were prespecified in Table 2 in our protocol (Cassola 2018). Details on the reported cut‐offs are presented in the Characteristics of included studies tables. When the criteria used to determine stenosis were too different from the duplex velocity criteria proposed in our protocol or when there was insufficient data to complete a 2 × 2 table for at least one category of stenosis, we described the results narratively without meta‐analysis. Seven studies, therefore, were not included in our quantitative analysis, and were described only narratively (Bray 1995; Chua 2007; Colquhoun 1992; Cui 2018; Das 2009; Hansen 1996; Knudsen 2002).
We focussed our review on symptomatic participants, but we also considered for inclusion studies with up to 30% of asymptomatic participants, we included six studies with mixed populations: Bray 1995 (18% presenting carotid bruit); Colquhoun 1992 (12% presenting non‐specific complaints); Faught 1994 (23%, authors did not describe why these patients were included); Hansen 1996 (11%, this study included only patients before carotid endarterectomy and asymptomatic patients previously undergone an endarterectomy on the symptomatic side, were operated on because of a contralateral asymptomatic severe stenosis); Lubezky 1998 (22%, this study evaluated only occlusion, had an unclear design); and Wolfle 2002 (27%, authors did not describe why these patients were included).
Chua 2007 was a prospective study of 188 carotid arteries in which the authors compared DUS and DSA. However, the calculated data on sensitivity and specificity were based on the ICA/CCA PSV ratio criterion (PSV ratio 3.1 for ≥ 70% ICA stenosis).
Colquhoun 1992 compared DUS to DSA in 53 carotid arteries, but the criteria to determine stenosis on DSA was ECST, and conversion to NASCET was not possible with the available data.
Cui 2018 was a prospective study that compared DUS and DSA in 54 participants but classified stenosis in the ICA and CCA, counting four vessels in each participant and presenting the results grouped. In this way, each participant was counted twice in the analysis.
Das 2009 was a prospective study of 30 internal carotid arteries that compared DUS to MRA and CTA. However, it provided a graphical representation of the results, and it was impossible to extract them into a 2 × 2 table with individual data.
Bray 1995 was a prospective study that compared DUS to DSA in 128 carotid arteries but provided insufficient data to complete a 2 × 2 table with results from each category of stenosis.
Hansen 1996 was a prospective study of 162 arteries comparing DUS with DSA. The degree of stenosis on DSA was calculated by measuring the smallest diameter in the stenotic zone compared with the diameter of the normal CCA proximal to the stenosis. It was not possible to convert this into the NASCET grade of stenosis.
Knudsen 2002 was a prospective study of 129 arteries comparing DUS to DSA. However, the threshold used to classify a ≥ 70% ICA stenosis was PSV ≥ 150 cm/s, EDV ≥ 90 cm/s, and ICA/CCA PSV ratio ≥ 2.8, which we considered too different from our pre‐established thresholds.
Nineteen studies used DSA as the reference standard (Anzidei 2012; Borisch 2003; Bray 1995; Chua 2007; Colquhoun 1992; Cui 2018; D'Onofrio 2006; Eliasziw 1995; Faught 1994; Golledge 1999; Hammond 2008; Hansen 1996; Heijenbrok‐Kal 2006; Huston 1993; Knudsen 2002; Link 1997; Lubezky 1998; Nederkoorn 2002; Wolfle 2002). Two of these studies also presented a comparison between DUS and MRA (Borisch 2003; D'Onofrio 2006), and Lubezky 1998 also presented results from DUS versus CTA. A total of four studies compared DUS and CTA (Barlinn 2016; Belsky 2000; Das 2009; Lubezky 1998), and three presented a comparison of DUS versus MRA (Borisch 2003; Das 2009; D'Onofrio 2006). There were insufficient data to perform a meta‐analysis of MRA as the reference standard (only two studies included). From the sixteen studies included in the quantitative analysis, when possible, we extracted data and completed a 2 × 2 table for each of the categories we proposed. We were able to include the most studies in the 70% to 99% carotid artery stenosis as well as occlusion categories. Nine studies (2770 carotid arteries) presented DUS versus DSA results for 70% to 99% carotid artery stenosis (Borisch 2003; D'Onofrio 2006; Eliasziw 1995; Faught 1994; Golledge 1999; Heijenbrok‐Kal 2006; Link 1997; Nederkoorn 2002; Wolfle 2002), and two studies presented results from DUS versus CTA for 685 carotid arteries (Barlinn 2016; Belsky 2000). Seven studies presented results for occlusion with DSA as the reference standard (Anzidei 2012; Borisch 2003; Hammond 2008; Heijenbrok‐Kal 2006; Huston 1993; Link 1997; Lubezky 1998; Nederkoorn 2002). Only Heijenbrok‐Kal 2006 presented 50% to 69% carotid stenosis results; therefore, it was impossible to perform a meta‐analysis.
The list and details of the included studies are presented in the Characteristics of included studies tables.
Methodological quality of included studies
Risk of bias varied considerably across the included studies. We summarized the results of the methodological quality of the included studies in Figure 3 and Figure 4.
3.
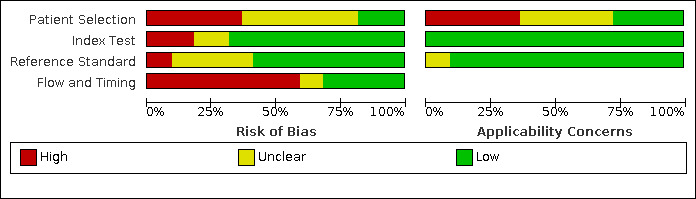
Risk of bias and applicability concerns graph: review authors' judgements about each domain presented as percentages across included studies
4.
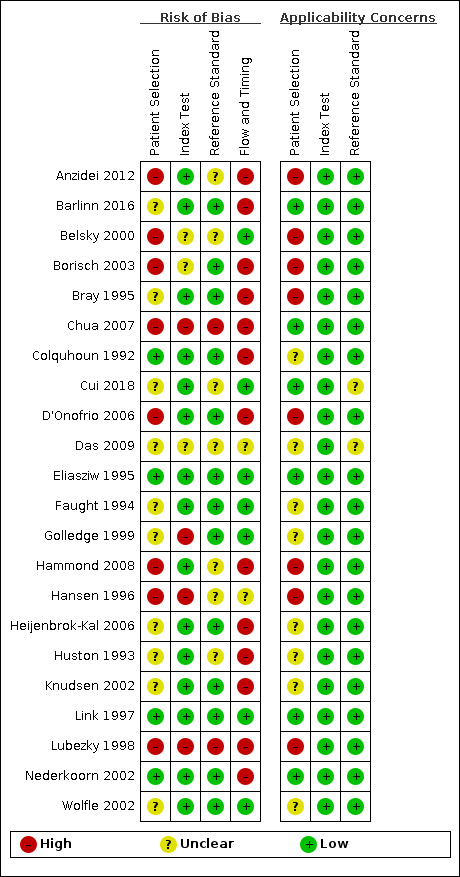
Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study
Only Eliasziw 1995 and Link 1997 were judged as being at low risk of bias in all domains. Chua 2007 and Lubezky 1998 were classified as having high risk of bias in all domains.
Patient selection
In terms of risk of bias, 10 studies were judged as being unclear regarding patient selection (Barlinn 2016; Bray 1995; Cui 2018; Das 2009; Faught 1994; Golledge 1999; Heijenbrok‐Kal 2006; Huston 1993; Knudsen 2002; Wolfle 2002), mostly because the recruitment method and the sampling procedures were unclear. Eight studies were considered as being at high risk of bias (Anzidei 2012; Belsky 2000; Borisch 2003; Chua 2007; D'Onofrio 2006; Hammond 2008; Hansen 1996; Lubezky 1998). The primary potential source of bias in this domain was the failure to include all people with a negative screen. Mainly because they only enrolled patients with known disease, selected participants based on previous examinations or participants already referred to the institution for preoperative evaluation can result in greater estimates of diagnostic accuracy. Another potential source of bias was the exclusion of difficult‐to‐diagnose patients (i.e. extensive calcified carotid plaques). Anzidei 2012 only included patients with > 30% carotid artery stenosis on DUS. Belsky 2000 selected patients who were candidates for carotid endarterectomy of either one or both ICA. Borisch 2003 included patients referred for preoperative imaging. Chua 2007 excluded occlusion of one or both ICA and atypical flow patterns within vessels, such as low velocities in near‐occlusion, and extensive calcified plaques resulting in long segments of acoustic shadowing. D'Onofrio 2006 only included patients with ultrasonographic findings of > 50% carotid artery stenosis. Hammond 2008 only evaluated patients with an apparent carotid occlusion on DUS. Hansen 1996 only included patients already with a planned carotid endarterectomy. Lubezky 1998 only included patients with carotid occlusion diagnosed by DUS. Only four studies were at low risk of bias in this domain (Colquhoun 1992; Eliasziw 1995; Link 1997; Nederkoorn 2002).
Index test
Four studies were at high risk of bias based on the judgements made about the index test because all of them had no prespecified thresholds (Chua 2007; Golledge 1999; Hansen 1996; Lubezky 1998). We judged three studies as having unclear risk of bias for the index test (Belsky 2000; Borisch 2003; Das 2009), and all other included studies as low risk of bias.
Reference standard
We judged Chua 2007 and Lubezky 1998 as having high risk of bias in the reference standard domain because the study personnel was not blinded to the results from DUS. We judged seven studies as being at unclear risk of bias for this domain (Anzidei 2012; Belsky 2000; Cui 2018; Das 2009; Hammond 2008; Hansen 1996; Huston 1993), mostly because we did not know if the result of the reference standard was interpreted without the knowledge of the result of DUS. All other included studies were judged as having low risk of bias.
Flow and timing
Fourteen studies had a methodological concern due to flow and timing. In 11 of them, not all participants were included in the analysis (Anzidei 2012; Barlinn 2016; Borisch 2003; Bray 1995; Chua 2007; Colquhoun 1992; Hammond 2008; Heijenbrok‐Kal 2006; Huston 1993; Knudsen 2002; Nederkoorn 2002), and in two studies the participants did not receive the same reference standard (D'Onofrio 2006; Lubezky 1998). We judged two studies as having unclear risk of bias for this domain (Das 2009; Hansen 1996), and all other included studies as low risk of bias.
Applicability concerns
We analyzed the applicability concerns regarding patient selection, index test, and reference standard. Five included studies were judged as having low concern in all three domains (Barlinn 2016; Chua 2007; Eliasziw 1995; Link 1997; Nederkoorn 2002;).
Patient selection was the domain where most issues were found. Only six studies were judged as being of low concern (Barlinn 2016; Chua 2007; Cui 2018; Eliasziw 1995; Link 1997; Nederkoorn 2002); eight studies were judged as being of unclear concern (Colquhoun 1992; Das 2009; Faught 1994; Golledge 1999; Heijenbrok‐Kal 2006; Huston 1993; Knudsen 2002; Wolfle 2002); and eight studies were judged as being of high concern (Anzidei 2012; Belsky 2000; Borisch 2003; Bray 1995; D'Onofrio 2006; Hammond 2008; Hansen 1996; Lubezky 1998), mostly because of prior testing used for patient selection or because they described the included population as patients referred to surgery or referred to DSA.
There were no studies whose authors declared a conflict of interest.
Findings
The findings are collected in Table 1 .
We were able to formally compare five ranges of stenosis for DUS versus DSA (< 50%, 50% to 69%, 50% to 99%, 70% to 99%, and occlusion), and occlusion for DUS versus CTA. We did not perform meta‐analyses of studies in carotid artery stenosis categories and different reference standards for which two or fewer studies were included.
Duplex ultrasound versus digital subtraction angiography
Carotid artery stenosis of < 50%
For this category, there were four studies involving 1495 carotid arteries in 975 participants (Anzidei 2012; Faught 1994; Heijenbrok‐Kal 2006; Huston 1993). Sensitivity varied from 0.36 to 0.76, and specificity varied from 0.96 to 0.98 (Figure 5). Using the bivariate model, we estimated a summary sensitivity of 0.63 (95% CI 0.48 to 0.76) and a summary specificity of 0.99 (95% CI 0.97 to 1.0). The summary receiver operating plot (sROC) along with the summary point is illustrated in Figure 6 . The prevalence of < 50% carotid artery stenosis ranged from 14% to 70%.
5.
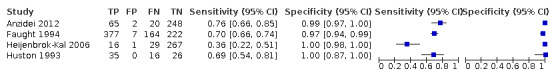
Forest plot of paired sensitivity and specificity estimated for studies assessing < 50% carotid artery stenosis with DSA as reference standard
6.
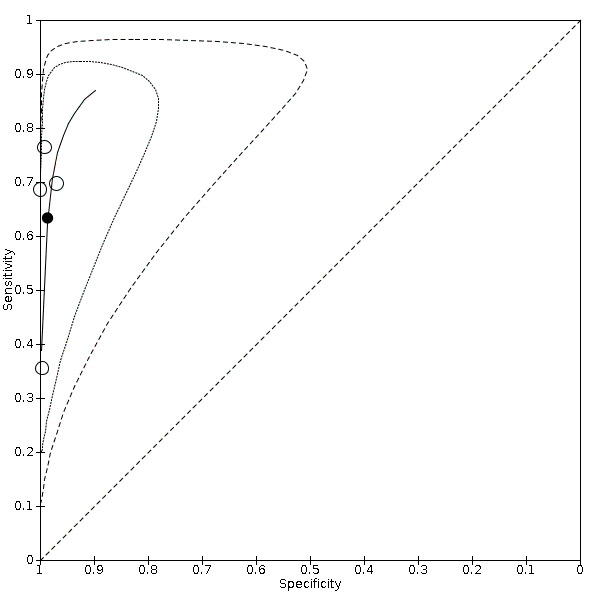
Summary ROC Plot of studies assessing < 50% carotid artery stenosis with DSA as reference standard
A meta‐regression analysis showed that the year of publication, the participants' age, and disease prevalence did not impact the accuracy estimates (sensitivity and specificity) (Table 5). All studies had a prospective design. There were not enough studies to perform subgroup analysis.
4. Meta‐regression analysis for < 50% carotid artery stenosis.
| Covariate | P value of sensitivity | P value of specificity |
| Prevalence | 0.237 | 0.015 |
| Year of publication | 0.830 | 0.069 |
| Participants age | 0.033 | 0.952 |
Carotid artery stenosis of 50% to 69%
Only one study with 330 participants (313 analyzed) and 313 carotid arteries was included (Heijenbrok‐Kal 2006). Sensitivity in this study was 0.28 (95% CI 0.17 to 0.41) and specificity was 0.9 (95% CI: 0.85 to 0.93). We did not perform a meta‐analysis on this combination, for reasons outlined above.
Carotid artery stenosis of 50% to 99%
We included five studies (1536 carotid arteries in 1007 participants) in this stenosis range (Anzidei 2012; D'Onofrio 2006; Faught 1994; Heijenbrok‐Kal 2006; Huston 1993). Sensitivity varied from 0.95 to 0.99 and specificity from 0.67 to 0.76 (Figure 7). Using the bivariate model, we estimated a summary sensitivity of 0.97 (95% CI 0.95 to 0.98) and a summary specificity of 0.70 (95% CI 0.67 to 0.73).The sROC plot along with the summary point is illustrated in Figure 8. The prevalence of 50% to 99% carotid artery stenosis ranged from 19% to 72% in the included studies.
7.
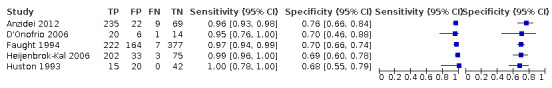
Forest plot of paired sensitivity and specificity estimated for studies assessing 50‐99% carotid artery stenosis with DSA as reference standard
8.
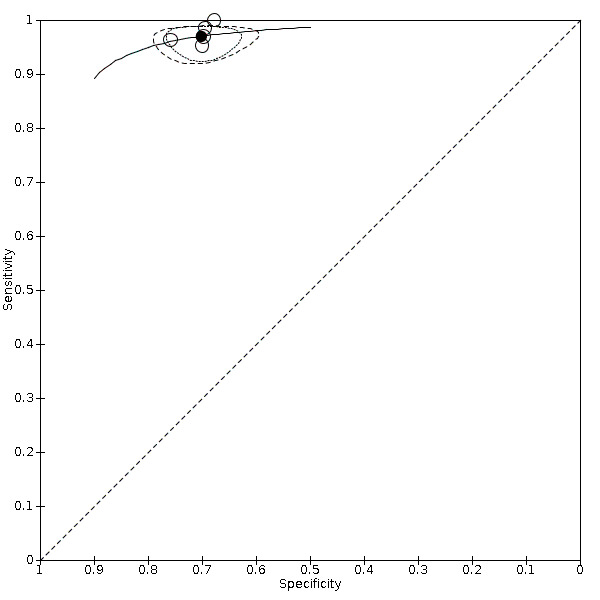
Summary ROC Plot of studies assessing 50‐99% carotid artery stenosis with DSA as reference standard
A meta‐regression analysis showed that the year of publication, the participants' age, and disease prevalence did not impact the accuracy estimates (sensitivity and specificity) (Table 6). All studies, except Anzidei 2012, had a prospective design.
5. Meta‐regression analysis for 50‐99% carotid artery stenosis.
| Covariate | P value of sensitivity | P value of specificity |
| Prevalence | 0.959 | 0.377 |
| Year of publication | 0.786 | 0.346 |
| Participants age | 0.231 | 0.308 |
We performed sensitivity analyses for lack of blinding of the index test interpreters to reference standard results or vice versa by excluding two non‐blinded studies (Anzidei 2012; Huston 1993). We found no impact on the results based on the likelihood ratio test (Chi2 = 1.00, P = 0.61).
Carotid artery stenosis of 70% to 99%
We were able to include the most studies for carotid artery stenosis of 70% to 99%, namely 2770 carotid arteries (in 2312 participants) from nine studies (Borisch 2003; D'Onofrio 2006; Eliasziw 1995; Faught 1994; Golledge 1999; Heijenbrok‐Kal 2006; Link 1997; Nederkoorn 2002; Wolfle 2002) and 2770 carotid arteries were included in analysis. Our prespecified threshold for classifying this range of stenosis was an ICA PSV of 230 cm/s, but we accepted Borisch 2003, D'Onofrio 2006, and Eliasziw 1995 all of which used an ICA PSV of 250 cm/s, Nederkoorn 2002 that used an ICA PSV of 270 cm/s, and Link 1997 that used an ICA PSV of 200 cm/s because we considered all of them similar thresholds.
Sensitivity varied from 0.68 to 0.97 and specificity varied from 0.67 to 0.97 (Figure 9). Using the bivariate model, the summary sensitivity was 0.85 (95% CI 0.77 to 0.91) and the summary specificity was 0.98 (5% CI 0.74 to 0.90). The sROC plot along with the summary point is illustrated in Figure 10. The prevalence of 70% to 99% carotid artery stenosis ranged from 17% to 72% in included studies.
9.
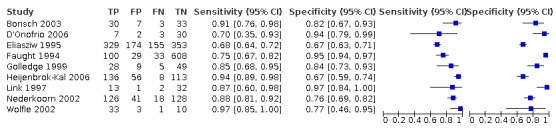
Forest plot of paired sensitivity and specificity estimated for studies assessing 70‐99% carotid artery stenosis with DSA as reference standard
10.
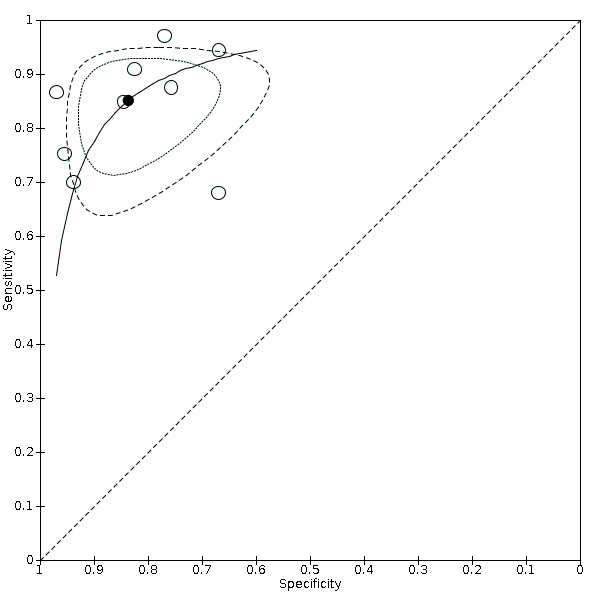
Summary ROC Plot of studies assessing 70‐99% carotid artery stenosis with DSA as reference standard
A meta‐regression analysis to explore heterogeneity showed that prevalence (Chi2 = 12.32, P = 0.00211) and year of publication (Chi2 = 14.57, P = 0.000684) impacted accuracy estimates, with a trend for higher estimates of summary specificity for higher prevalence and a trend for higher estimates of summary sensitivity for more recent publications. The participants’ age did not impact the estimates of accuracy (Table 7).
6. Meta‐regression analysis for 70‐99% carotid artery stenosis.
| Covariate | P value of sensitivity | P value of specificity |
| Prevalence | 0.080 | < 0.005 |
| Year of publication | < 0.005 | 0.229 |
| Participants age | 0.899 | 0.422 |
We performed sensitivity analyses for lack of blinding of the index test interpreters to reference standard results or vice versa by excluding one non‐blinded study (Borisch 2003). We found no impact on the results (likelihood ratio test: Chi2 = 0.45, P = 0.80).
A sensitivity analysis restricted to studies that used the exact velocity criteria described in our protocol (PSV ≥ 230 c/s) resulted in a summary sensitivity of 0.90 (95% CI 0.77 to 0.96) and a summary specificity of 0.83 (95% CI 0.59 to 0.95) (Faught 1994; Heijenbrok‐Kal 2006; Wolfle 2002), but we found no impact on the results (likelihood ratio test: Chi2 = 1.62, P = 0.45).
Subgroup analysis for additional ultrasound resources (only the color resource was analyzed) showed no statistically significant difference between summary sensitivity or specificity (likelihood ratio test: Chi2 = 4.26, P = 0.12).
Carotid artery occlusion
Seven studies (1212 carotid arteries in 1165 participants) compared DUS and DSA for carotid artery occlusion (Anzidei 2012; Borisch 2003; Hammond 2008; Heijenbrok‐Kal 2006; Huston 1993; Link 1997; Lubezky 1998; Nederkoorn 2002). Sensitivity varied from 0.62 to 0.99, and specificity varied from 0.07 to 0.99 (Figure 11). We estimated a summary sensitivity of 0.91 (95% CI 0.81 to 0.97) and a summary specificity of 0.95 (95% CI 0.76 to 0.99). The sROC plot along with the summary point is illustrated in Figure 12. The prevalence of carotid artery occlusion ranged from 14% to 95%. A meta‐regression analysis showed that the year of publication and participants’ age did not impact the estimates of accuracy (sensitivity and specificity), but it did show a trend for higher summary specificity for higher prevalence (Chi2 = 18.91, P < 0.001) (Table 8). Link 1997 had a retrospective design; all other studies had a prospective design.
11.
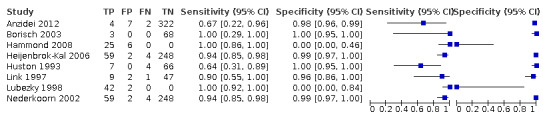
Forest plot of paired sensitivity and specificity estimated for studies assessing carotid artery occlusion with DSA as reference standard
12.
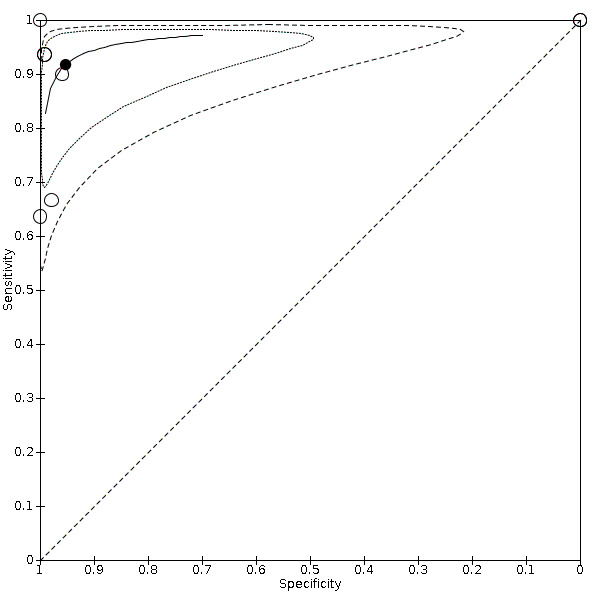
Summary ROC Plot of studies assessing carotid artery occlusion with DSA as reference standard
7. Meta‐regression analysis for carotid artery occlusion.
| Covariate | P value of sensitivity | P value of specificity |
| Prevalence | 0.016 | < 0.005 |
| Year of publication | 0.608 | 0.714 |
| Participants age | 0.817 | 0.405 |
We performed sensitivity analyses for lack of blinding of the index test interpreters to reference standard results or vice versa by excluding five non‐blinded studies (Anzidei 2012; Borisch 2003; Hammond 2008; Huston 1993; Lubezky 1998). We found a significant impact on the estimates of sensitivity (likelihood ratio test: Chi2 = 7.75, P = 0.020).The summary sensitivity estimate was 0.93 (95% CI 0.87 to 0.96).
We performed sensitivity analyses excluding Hammond 2008 and Lubezky 1998, which only included patients that had already been diagnosed with carotid artery occlusion on DUS and, therefore, had no false negative test and low rates of specificity. There was a significant impact on the results of specificity (likelihood ratio test: Chi2 = 16.44, P < 0.001). The summary estimate for specificity was 0.98 (95% CI 0.97 to 0.99).
See Appendix 8 for characteristics of the studies not included in meta analysis.
Duplex ultrasound versus computed tomography angiography
We included three studies for DUS versus CTA analyses: Barlinn 2016 and Belsky 2000 provided data for 70% to 99% carotid artery stenosis as well as for occlusion; Lubezky 1998 only included carotid arteries with occlusion.
Carotid artery stenosis of 70% to 99%
For this range of stenosis, two studies (685 carotid arteries in 354 participants) fulfilled our prespecified criteria (Barlinn 2016; Belsky 2000). Sensitivity varied from 0.57 to 0.94 and specificity varied from 0.87 to 0.98 (Figure 13). The prevalence of 70% to 99% carotid artery stenosis ranged from 1% to 34%.
13.

Forest plot of paired sensitivity and specificity estimated for studies assessing 70‐99% carotid artery stenosis with CTA as reference standard
Carotid artery occlusion
Three studies (833 carotid arteries in 499 participants) compared DUS and CTA for carotid artery occlusion (Barlinn 2016; Belsky 2000; Lubezky 1998). Sensitivity varied from 0.85 to 1.0 and specificity varied from 0.04 to 1.0 (Figure 14). The prevalence of carotid artery occlusion ranged from 4% to 91%. Using the bivariate model, the estimated summary sensitivity was 0.95 (95% CI 0.79 to 0.99) and the summary specificity was 0.91 (95% CI 0.09 to 0.99). The sROC plot along with the summary point is illustrated in Figure 15. The prevalence of 70% to 99% carotid artery stenosis ranged from 17% to 72%. There were not enough studies to perform subgroup analysis.
14.

Forest plot of paired sensitivity and specificity estimated for studies assessing carotid artery occlusion with CTA as reference standard
15.
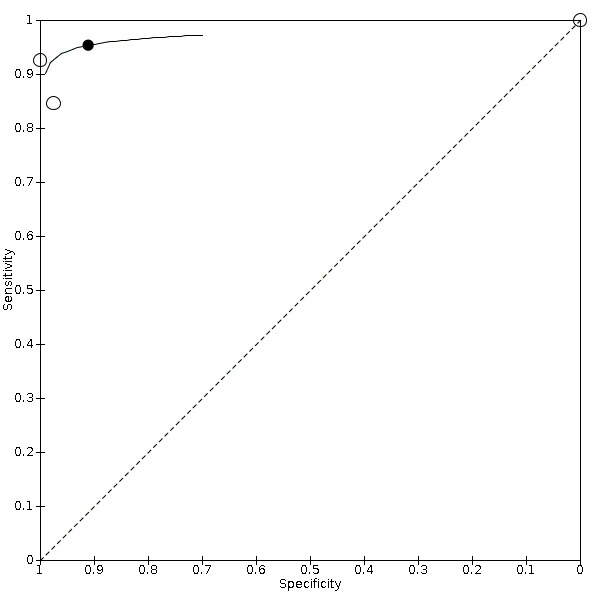
Summary ROC Plot of studies assessing carotid artery occlusion with CTA as reference standard
Das 2009 also compared DUS with CTA, with a correlation coefficient (r) between CTA and DUS of 0.84 (95% CI 0.69 to 0.92). However, as stated above, the study provided results of comparisons between tests in graphic format and data extraction was not possible. Therefore, we could not establish sensitivity and specificity, even in a narrative form.
Duplex ultrasound versus contrast‐enhanced magnetic resonance angiography
Two studies (102 carotid arteries) were included in analyses of DUS versus MRA (Borisch 2003; D'Onofrio 2006).
Carotid artery stenosis of 50% to 99%
Only D'Onofrio 2006 presented results for 50% to 99% carotid artery stenosis,with a sensitivity of 0.88 (95% CI 0.70 to 0.98) and a specificity of 0.60 (95% CI 0.15 to 0.95). The prevalence was 42%.
Carotid artery stenosis of 70% to 99%
Borisch 2003 and D'Onofrio 2006 provided data for 70% to 99% carotid artery stenosis. The sensitivity varied from 0.54 to 0.99 and specificity varied from 0.78 to 0.89 (Figure 16). The prevalence was 43% to 80%.
16.

Forest plot of paired sensitivity and specificity estimated for studies assessing 70‐99% carotid artery stenosis with MRA as reference standard
Borisch 2003 used DUS, MRA, and DSA to examine both carotid artery bifurcations from 39 consecutive participants (7 women and 32 men; age range 41 to 80 years; mean age, 67.4 ± 8.4 years) with clinically suspected symptomatic carotid artery stenoses that were referred for preoperative imaging. In this study, four radiologists evaluated the results, and the findings are presented with pooled data (4 observers × 71 vessels = 284 evaluations). We included the DSA results in the meta‐analysis since the authors reported the stenosis measurements from four radiologists (inflated the numbers in the 2 × 2 table); the results were divided by four to reflect the actual number of participants. But there were not enough studies included with MRA as the reference standard. For detecting 70% to 99% carotid stenosis, the reported sensitivity was 100% and specificity was 81.4%. Total agreement between MRA and DUS was achieved in 80% of evaluations (227 of 284). The results of other categories of stenosis are not presented.
D'Onofrio 2006 examined 21 participants with DUS, MRA, and DSA, including 41 carotid arteries in the analysis (1 participant had previous endarterectomy). The authors divided the carotid stenosis into four categories: < 39% (insignificant), 40% to 59% (borderline lesion), 60% to 79% (significant lesion), and 80% to 99% (very significant lesion). We chose to include in the analysis the results from 60% to 99% in our category of 50% to 99% carotid artery stenosis and the results from 80% to 99% in our category of 70% to 99% carotid artery stenosis because the velocities used for detecting these parameters are similar to those we propose (Appendix 9). The authors found poor agreement between DUS and MRA because DUS overestimated measurements in the lower stenosis categories while MRA overestimated the higher ones. For detecting 70% to 99% carotid artery stenosis, the reported sensitivity was 58.8% and the specificity was 88.8%. For detecting 50% to 99% carotid artery stenosis, the sensitivity was 88.5% and the specificity was 50%.
Das 2009 included 15 participants with symptomatic stenosis of the ICA. All participants underwent CTA, MRA, and DUS. The authors used the DEGUM criteria to classify carotid stenosis (Appendix 9). The correlation coefficient (r) between CTA and MRA was 0.83 (95% CI 0.68 to 0.92) and the correlation coefficient (r) between DUS and MRA was 0.83 (95% CI 0.66 to 0.91). The study provided results of comparisons between tests in graphic format and data extraction was not possible. Therefore, we could not establish sensitivity and specificity, even in a narrative form.
Discussion
Summary of main results
We aimed to assess the diagnostic accuracy of DUS for diagnosing carotid artery stenosis in symptomatic patients compared with DSA, MRA, or CTA as reference standards. The main results are shown in the Table 1. We included 22 studies (4957 carotid arteries). We did not include seven studies in our quantitative analysis, only describing them narratively (Chua 2007; Colquhoun 1992; Cui 2018; Das 2009; Bray 1995; Hansen 1996; Knudsen 2002). We included 15 studies in the quantitative analysis with results grouped into the categories we proposed in our protocol (Cassola 2018).
The following is a summary of the results for which statistical significance could be determined (we pre‐set the test consequence graphic as suggested by Whiting 2018):
For DUS versus DSA
< 50% carotid artery stenosis (four studies, 1495 carotid arteries): The estimated summary sensitivity of DUS was 0.63 (95% CI: 0.48 to 0.76) and the estimated summary specificity was 0.99 (95% CI: 0.96 to 0.99). In a hypothetical cohort of 1000 patients (median prevalence of included studies 46%), 460 patients would have < 50% carotid artery stenosis. Of these, 291 (63%) would be correctly diagnosed and receive appropriate clinical treatment and 169 (27%) would receive unnecessary further investigation with another imaging method. Other 532 patients would receive appropriate further investigation, and eight would have no other tests performed and miss a chance for the right diagnosis and the possibility of carotid revascularization (Figure 17).
50% to 99% carotid artery stenosis (five studies, 1536 carotid arteries): The estimated summary sensitivity of DUS was 0.97 (95% CI 0.95 to 0.98) and the estimated summary specificity was 0.70 (95% CI 0.67 to 0.73). In a hypothetical cohort of 1000 patients (median prevalence of included studies 51%), 510 patients would have 50% to 99% carotid artery stenosis. Of these, 495 (97%) would receive appropriate further investigation with another imaging method, and 15 (3%) would not have any other tests performed and would miss a chance to receive the right diagnosis and the possibility of carotid revascularization. Overall, 147 would receive unnecessary further investigation with another imaging method, and 343 would receive no further investigation and appropriate clinical treatment (Figure 18);
70% to 99% carotid artery stenosis (nine studies, 2770 carotid arteries): The estimated summary sensitivity of DUS was 0.85 (95% CI 0.77 to 0.91) and the estimated summary specificity was 0.98 (95% CI 0.74 to 0.90). In a hypothetical cohort of 1000 patients (median prevalence of included studies 45%), 451 patients would have 70% to 99% carotid artery stenosis. Of these, 383 (85%) would receive appropriate carotid artery revascularization and 68 (15%) would miss or delay the chance to carotid revascularization. Another 8 would receive inappropriate carotid artery revascularization and 542 would receive appropriate clinical treatment. (Figure 19);
occlusion (seven studies, 1212 carotid arteries): estimated summary sensitivity of DUS was 0.91 (95% CI: 0.81 to 0.97) and the estimated summary specificity was 0.95 (95% CI: 0.76 to 0.99), respectively. In a hypothetical cohort of 1000 patients (median prevalence of included studies was 18%), 180 will have carotid artery occlusion. Of these, 164 (91%) would receive appropriate clinical treatment. Another 41 would be false‐positive diagnosed with carotid occlusion and not have other tests performed, and miss a chance of the correct diagnosis and carotid revascularization. Other consequences would depend on the range of stenosis (Figure 20);
17.
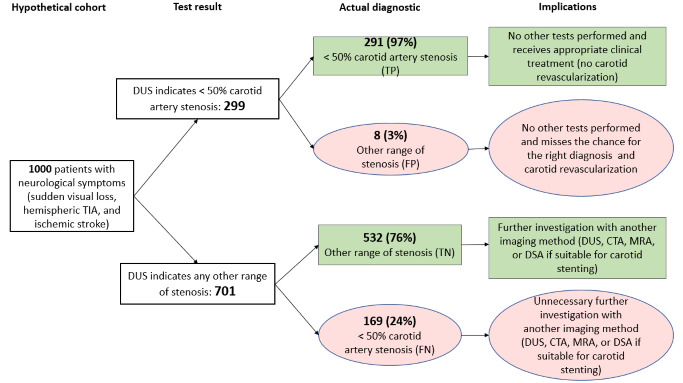
DSA < 50%: Hypothetical cohort of 1000 symptomatic patients assessed for carotid artery stenosis. We considered pretest probability the median prevalence of the included studies (0.46). tp: true positive; fp: false positive; tn: true negative; fn: false negative
18.
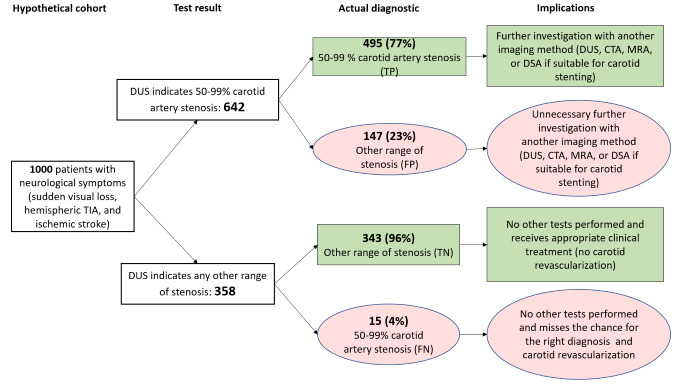
DSA 50‐99%: Hypothetical cohort of 1000 symptomatic patients assessed for carotid artery stenosis. We considered pretest probability the median prevalence of the included studies (0.51). tp: true positive; fp: false positive; tn: true negative; fn: false negative
19.
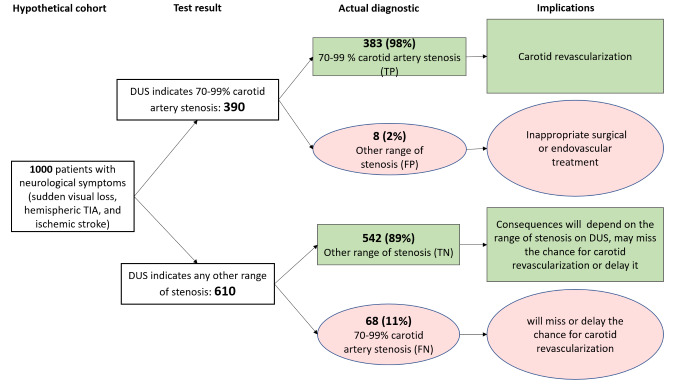
DSA 70‐99%: Hypothetical cohort of 1000 symptomatic patients assessed for carotid artery stenosis. We considered pretest probability the median prevalence of the included studies (0.45). tp: true positive; fp: false positive; tn: true negative; fn: false negative
20.
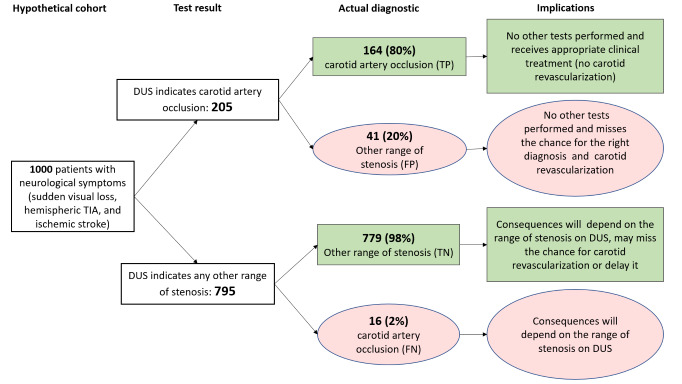
DSA Occlusion: Hypothetical cohort of 1000 symptomatic patients assessed for carotid artery stenosis. We considered pretest probability the median prevalence of the included studies (0.18). tp: true positive; fp: false positive; tn: true negative; fn: false negative
For DUS versus CTA
occlusion (three studies, 833 carotid arteries): The estimated summary sensitivity of DUS was 0.95 (95% CI 0.80 to 0.99) and the estimated summary specificity was 0.91 (95% CI 0.09 to 0.99). In a hypothetical cohort of 1000 patients (median prevalence of included studies was 60%), 600 patients would have carotid artery occlusion. Of these, 570 (95%) would receive appropriate clinical treatment. Another 41 would be false‐positive diagnosed with carotid occlusion and not have other tests performed, and miss a chance of the correct diagnosis and carotid revascularization. Other consequences would depend on the range of stenosis (Figure 21).
21.
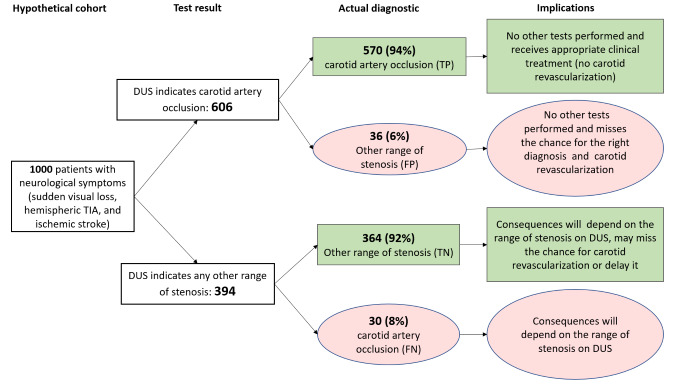
CTA occlusion: Hypothetical cohort of 1000 symptomatic patients assessed for carotid artery stenosis. We considered pretest probability the median prevalence of the included studies (0.60). tp: true positive; fp: false positive; tn: true negative; fn: false negative
For DUS versus MRA, we found data in two categories, but there were not enough data for meta‐analysis.
Strengths and weaknesses of the review
The strength of this review is that we adhered to the recommended review methods and performed an extensive search of the literature without language restrictions. Therefore, we reviewed a large number of publications. We followed the standard recommendations of the Cochrane DTA (methods.cochrane.org/sdt/) and our previously published protocol (Cassola 2018) to avoid bias in the review process.
The use of velocity criteria with prespecified thresholds is both a strength and a weakness of this review. We used the most common validated criteria currently being used in most centers (Table 2). In 2011, the Society for Vascular Surgery® (SVS) and the European Society for Vascular Surgery in 2017 included the same criteria in their published guidelines (ESVS Writing Group 2018; Ricotta 2011). In 2014, the Intersocietal Accreditation Commission (IAC) endorsed the same criteria (IAC 2014), but in May 2021, it published some suggested changes for the ≥ 50% carotid artery stenosis criteria (Gornik 2021). Unfortunately, we had to exclude many studies because their thresholds were too different from those proposed in our protocol or did not describe thresholds. We believe that assessing the accuracy of DUS without a prespecified threshold would lead to unrealistic estimates of accuracy and even more heterogeneity among studies because the same velocity criteria can be used to classify carotid artery stenosis of 50% or 70% depending on the center performing DUS. Higher velocity criteria tend to decrease sensitivity and increase specificity. Therefore, different cut‐off velocity thresholds should achieve different estimates of sensitivity and specificity. We provided separate summary estimates of sensitivity and specificity for each proposed category of stenosis.
One significant limitation of our review concerns the issue of reproducibility. Most of the studies did not provide information regarding DUS operator experience, so we could not include any analysis on this characteristic. The diagnostic test interpretation is operator dependent, and multiple factors can affect the accuracy of the measurements, including the correct examination protocol and conditions inherent to the patients, such as hemodynamic factors and the presence of collateral flow through the circle of Willis or the ophthalmic artery.
Another issue is that we used DSA, MRA, or CTA as the reference standard. DSA is still considered the gold‐standard test for carotid artery stenosis. Still, in current practice, its use for diagnostic purposes has been largely supplanted by non‐invasive angiographic modalities (CTA, MRA). Thus, we decided to include CTA and MRA as reference standards. A diagnostic accuracy study should include all patients suspected of having the target condition and for whom the test would be considered (Leeflang 2009). The ideal population in this review would be all patients with neurologic symptoms (stroke, TIA, or sudden visual loss). We expected that recent studies comparing DUS and DSA would not include all symptomatic patients due to the invasive characteristic of DSA and the risk of complications. Therefore, we accepted CTA and MRA as reference tests to include studies in which all symptomatic patients were evaluated. It was frustrating to find little evidence comparing DUS to these less invasive diagnostic tests that are more frequently used in clinical practice. We also recognize that interobserver variation exists for all the reference tests, but with an acceptable agreement (Bucek 2007; Lenhart 2002; Saba 2008).
The estimates of a diagnostic accuracy test (i.e. sensitivity and specificity) are not fixed properties. They describe the behavior of a test under specific conditions, and they typically change at different segments of the disease spectrum and with varying disease prevalence (Leeflang 2012). Therefore, accuracy reviews should consider using the test in clinical practice when defining the population of interest to be studied. Methodological problems in patient inclusion criteria from the studies discussed above apparently influenced an overestimated estimate of prevalence values. For example, we found a median prevalence of 51% for 50% to 99% stenosis and 45% for 70% to 99% stenosis, well above what we see in clinical practice. It is difficult to quantify the effect of this on the systematic review results. The literature provides conflicting results of prevalence effects on DTA estimates (Leeflang 2009; Whiting 2004). In our review, the meta‐regression analyses adding prevalence as a covariate did not impact the accuracy estimates for carotid artery stenosis < 50% and 50% to 99%. However, there was increased specificity with higher prevalence in the 70% to 99% category. In the occlusion category, studies that included only patients with occlusion diagnosed by DUS had a significant impact, reducing the specificity estimates.
We found few studies from each category of stenosis. Many studies provided limited information about the mechanism of enrolling participants into the study. Many of them only included patients with known diseases, and many of them were retrospective. Because DUS is the recommended initial diagnostic test for assessing carotid stenosis, it is likely that some prospective studies and probably all retrospective studies focussed on participants who showed some degree of disease based on an initial assessment with DUS and who received further investigation with more invasive or expensive techniques. A sensitivity analysis would be possible if the included studies provided enough information from previous examinations that had been performed.
In 2006, a systematic review was carried out to compare noninvasive imaging in the diagnosis of symptomatic carotid (Wardlaw 2006a; Wardlaw 2006b) and already discussed the lack of quality evidence over carotid artery diagnostic methods and the need for well‐designed studies on this topic. One main difference was that our review evaluated DUS versus CTA and MRA also as gold standards (besides DSA). Sadly in our review, we found that many years later, we still have poor‐quality studies regarding the accuracy of noninvasive diagnostic imaging of the carotid artery.
The time we accepted between the index test and reference standard (four weeks) is another crucial factor in this review: we excluded some studies that exceeded this interval. We know that there is a progression of the plaque, especially the evolution of the patient's condition, with a risk of new neurologic events shortly after the first symptoms. Currently, it is already considered that the patient should be treated early after a cerebral ischemic event, ideally within an interval of up to 2 weeks, with a reduction in the relative risk with surgical treatment after this period (ESVS Writing Group 2018; Vasconcelos 2016). Considering that this review dealt with symptomatic patients, we believe that the interval of up to four weeks for the complete diagnostic investigation of the patient was a reasonable period.
Finally, it was impossible to perform meta‐analysis for all ranges of stenosis and all reference standards proposed due to the small number of studies contributing to this data.
Applicability of findings to the review question
The findings of this review apply to patients presenting neurologic symptoms and suspected carotid artery stenosis. However, the results cannot be considered definitive because of the small number of included studies in each stenosis category. Many of the included studies were at high or unclear risk of bias and there was heterogeneity among the studies. Using the QUADAS‐2 tool, many studies included in the primary analyses had limitations related to patient selection either because of unclear patient selection methods or the authors had selected only patients with known disease (previous test performed). We also have concerns regarding flow and timing: many studies did not include all patients in the analysis, or the patients did not receive the same reference standard.
The 'generation of technology' is not a surrogate for 'date of publication' because a recent publication can use an old DUS device. However, the year of publication can indirectly correlate with the 'generation of technology'. We could not assess the generation of technology for this review version, but we evaluated the implication of the year of publication with meta‐regression. Most of the included studies did not report the assessor's proficiency, and, therefore, we could not assess this evidence.
Authors' conclusions
Implications for practice.
Ultrasound is undoubtedly an exam with a crucial role in the diagnosis of patients with symptomatic carotid stenosis. Understanding its complexity and limitations helps better fit it into clinical practice and offer the most cost‐effective treatment to the patient. The findings of this review provide evidence that DUS is accurate at discriminating between the presence or absence of significant carotid artery stenosis (< 50% or 50% to 99%). Therefore, there is evidence to support the use of DUS as the first choice modality for the detection of carotid stenosis. Evidence suggests that no further imaging may be necessary to detect the presence of carotid artery stenosis in cases of DUS detecting > 50% carotid stenosis, given the high value of sensitivity for this category. Nonetheless, if the result is < 50% and clinical suspicion of carotid stenosis is high, another diagnostic test could add clinical information.
The results of this review indicate that DUS sensitivity and specificity for 70% to 99% carotid artery stenosis are high, but clinicians should exercise caution in using DUS as the single preoperative diagnostic method. It could be applicable, especially in centers that do not have immediate access to more sophisticated vascular imaging techniques, and the appropriate treatment time window would be lost. Our results showed that in a cohort of 1000 patients (with a high prevalence of carotid stenosis), 8 would receive inappropriate carotid revascularization treatment. When there is lower prevalence of the disease, this number can increase. Proceeding with additional diagnostic tests could improve the accuracy of the carotid stenosis diagnostic, however we could not assess the accuracy of the DUS as a confirmatory test after a first positive test.
The values of sensitivity and specificity for detecting occlusion of the carotid artery are high. However, the quality of the included studies is low and the consequences of a false‐positive result are severe, often leading the patient to miss the chance of carotid revascularization. Therefore, there appears to be a good case for a confirmatory test for these patients.
We found little evidence regarding the accuracy estimates of DUS versus MRA or CTA as reference standards.
The low methodological quality of the studies may reduce the reliability of the conclusion. There were many studies at high risk of bias, and most of them had concerns regarding their applicability, mainly due to the patient selection domain. Therefore, clinicians will have to decide whether additional imaging is necessary after DUS bearing in mind the time when this imaging is performed, and the potential benefits of performing a surgical treatment within a short time.
Implications for research.
Regardless of the positive findings of this review, more studies with high methodological quality of DUS accuracy would improve clinical decisions in patients with symptomatic carotid stenosis. In future studies, study selection criteria require careful attention: appropriate inclusion and exclusion criteria, and a standardized and replicable threshold to determine carotid stenosis. We recognize the challenge of performing a DTA study including all patients with neurologic symptoms. Frequently, smaller centers receive these symptomatic patients. There should be criteria for their referral to specialized centers where more invasive or expensive tests such as DSA, CTA, or MRA are available. Thus, future studies could consider assessing the accuracy of DUS as a confirmatory test in patients previously diagnosed with carotid stenosis based on initial tests.
Although DSA was considered the gold standard for diagnosing carotid artery stenosis, due to the risks related to the procedure, in current clinical practice it is usually reserved for select situations. This change in practice may be the main reason most studies fail to include patients who cover the entire spectrum of neurologic disease. Those patients receiving DSA usually have already been tested with a less invasive technique (usually DUS). Therefore, the validation of the accuracy of DUS using DSA as a reference test can be difficult or impossible because of ethical concerns. Future studies should also include comparisons of DUS versus CTA or MRA because these are the diagnostic tests performed in the clinical practice pathway (the 'new gold standards'). In particular, the criteria regarding patients with 50% to 69% carotid artery stenosis requires attention to determine the potentiality of using DUS to identify this situation accurately.
History
Protocol first published: Issue 11, 2018
Notes
Parts of the methods section of this protocol are based on a standard template established by Cochrane.
Acknowledgements
We thank Joshua David Cheyne, Cochrane Stroke Information Specialist, for his assistance with the electronic searches. We thank Cochrane Stroke, Cochrane Brazil, and the Division of Vascular and Endovascular Surgery, Universidade Federal de Sao Paulo, Brazil for their support.
Appendices
Appendix 1. CENTRAL search strategy
IDSearchHits #1MeSH descriptor: [Carotid Arteries] this term only682 #2MeSH descriptor: [Carotid Artery, Common] this term only207 #3MeSH descriptor: [Carotid Artery, External] this term only12 #4MeSH descriptor: [Carotid Artery, Internal] this term only205 #5{or #1‐#4}1067 #6MeSH descriptor: [Arteriosclerosis] this term only957 #7MeSH descriptor: [Atherosclerosis] this term only1094 #8MeSH descriptor: [Constriction, Pathologic] this term only640 #9{or #6‐#8}2667 #10#5 and #9200 #11MeSH descriptor: [Carotid Artery Diseases] this term only454 #12MeSH descriptor: [Carotid Artery Thrombosis] this term only18 #13MeSH descriptor: [Carotid Stenosis] this term only608 #14MeSH descriptor: [Carotid Artery Injuries] explode all trees20 #15((carotid near/5 (steno* or thrombo* or disease* or arter* or atherosclero* or atheroma* or narrow* or plaque* or occlus* or occlud* or constrict* or emboli* or block*))):ti,ab,kw4908 #16{or #10‐#15}4908 #17MeSH descriptor: [Ultrasonography] this term only4610 #18MeSH descriptor: [Ultrasonography, Doppler] this term only560 #19MeSH descriptor: [Ultrasonography, Doppler, Duplex] explode all trees875 #20MeSH descriptor: [Ultrasonography, Doppler, Pulsed] this term only67 #21(((duplex or color or colour or doppler) near/3 (ultrasound or ultrasonograph* or ultrasonic* or scan*))):ti,ab,kw (Word variations have been searched)4088 #22(((duplex or color or colour or doppler) near/3 (sonograph* or echograph* or echosound or echoscop* or echogram* or sonogram* or doptone))):ti,ab,kw (Word variations have been searched)880 #23((CDUS or DUS)):ti,ab,kw (Word variations have been searched)343 #24{OR #17‐#23}9262 #25#16 and #24812
Appendix 2. MEDLINE Ovid search strategy
1. carotid arteries/ or exp carotid artery, common/
2. arteriosclerosis/ or atherosclerosis/
3. constriction, pathologic/
4. 2 or 3
5. 1 and 4
6. carotid artery diseases/ or carotid artery thrombosis/ or carotid stenosis/
7. exp carotid artery injuries/
8. (carotid adj5 (steno$ or thrombo$ or disease$ or arter$ or atherosclero$ or atheroma$ or narrow$ or plaque$ or occlus$ or occlud$ or constrict$ or emboli$ or block$)).tw.
9. 5 or 6 or 7 or 8
10. Ultrasonography/
11. ultrasonography, doppler/ or ultrasonography, doppler, duplex/ or ultrasonography, doppler, color/ or ultrasonography, doppler, pulsed/
12. (duplex or color or doppler).tw.
13. (ultrasound or ultrasonograph$ or ultrasonic$ or scan$).tw.
14. (sonograph$ or echograph$ or echosound or echoscop$ or echogram$ or sonogram$ or doptone).tw.
15. (CDUS or DUS).tw.
16. 10 or 11 or 12 or 14 or 15
17. 9 and 16
Appendix 3. Embase Ovid search strategy
1. carotid artery/ or exp common carotid artery/ or external carotid artery/ 2. arteriosclerosis/ or arteriolosclerosis/ or atherosclerosis/ or atheroma/ or atheromatosis/ or atherosclerotic plaque/ or brain atherosclerosis/ or carotid atherosclerosis/ 3. ligation/ 4. 2 or 3 5. 1 and 4 6. exp carotid artery obstruction/ or carotid artery disease/ 7. carotid artery injury/ or carotid atherosclerosis/ 8. (carotid adj5 (steno$ or thrombo$ or disease$ or arter$ or atherosclero$ or atheroma$ or narrow$ or plaque$ or occlus$ or occlud$ or constrict$ or emboli$ or block$)).tw. 9. or/5‐8 10. contrast‐enhanced ultrasound/ or interventional ultrasonography/ 11. doppler flowmetry/ or color doppler flowmetry/ 12. doppler ultrasonography/ or duplex doppler ultrasonography/ or pulsed doppler ultrasonography/ 13. ((duplex or colo?r or doppler) adj3 (ultrasound or ultrasonograph$ or ultrasonic$ or scan$)).tw. 14. ((duplex or colo?r or doppler) adj3 (sonograph$ or echograph$ or echosound or echoscop$ or echogram$ or sonogram$ or doptone)).tw. 15. (CDUS or DUS).tw. 16. or/10‐15 17. 9 and 16 18. (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) 19. 17 not 18
Appendix 4. ISI Web of Science search strategy
# 5 #4 AND #1 Indexes=SCI‐EXPANDED, CPCI‐S Timespan=1900‐2019 # 4 #3 OR #2 Indexes=SCI‐EXPANDED, CPCI‐S Timespan=1900‐2019 # 3 TS=((duplex or color or colour or doppler) NEAR/3 (sonograph* or echograph* or echosound or echoscop* or echogram* or sonogram* or doptone)) Indexes=SCI‐EXPANDED, CPCI‐S Timespan=1900‐2019 # 2 TS=((duplex or color or colour or doppler) NEAR/3 (ultrasound or ultrasonograph* or ultrasonic* or scan*)) Indexes=SCI‐EXPANDED, CPCI‐S Timespan=1900‐2019 # 1 TS=(carotid NEAR/5 (steno* or thrombo* or disease* or arter* or atherosclero* or atheroma* or narrow* or plaque* or occlus* or occlud* or constrict* or emboli* or block*)) Indexes=SCI‐EXPANDED, CPCI‐S Timespan=1900‐2019
Appendix 5. HTA and DARE search strategy
1MeSH DESCRIPTOR Carotid Arteries IN DARE,HTA40 2MeSH DESCRIPTOR Carotid Artery, Common EXPLODE ALL TREES IN DARE,HTA17 3MeSH DESCRIPTOR Carotid Artery Diseases EXPLODE ALL TREES199 4MeSH DESCRIPTOR Carotid Artery Injuries EXPLODE ALL TREES7 5(carotid):TI AND (steno* or thrombo* or disease* or arter* or atherosclero* or atheroma* or narrow* or plaque* or occlus* or occlud* or constrict* or emboli* or block*):TI154 6#1 OR #2 OR #3 OR #4 OR #5241 7MeSH DESCRIPTOR Ultrasonography IN DARE,HTA154 8MeSH DESCRIPTOR Ultrasonography, Doppler IN DARE,HTA56 9MeSH DESCRIPTOR Ultrasonography, Doppler, Duplex EXPLODE ALL TREES IN DARE,HTA41 10MeSH DESCRIPTOR Ultrasonography, Doppler, Pulsed IN DARE,HTA1 11(duplex or colour or color or doppler):TI AND (ultrasound or ultrasonograph* or ultrasonic* or scan*):TI OR (sonograph* or echograph* or echosound or echoscop$ or echogram* or sonogram* or doptone):TI52 12#7 OR #8 OR #9 OR #10 OR #11278 13#6 AND #1214
Appendix 6. LILACS and IBECS search strategy
((mh: (carotid arteries) OR mh: (arterias carótidas) OR mh: (artérias carótidas) OR (arter* carotid)) OR (mh: (carotid artery diseases) OR mh: (enfermedades de las arterias carótidas) OR mh: (doenças das artérias carótidas) OR (arter* disease* carotid) OR (arter* disease* common carotid) OR (arter* disease* external carotid) OR (arter* disease* internal carotid) OR (atherosclerotic disease* carotid) OR (carotid arter* disorder*) OR (carotid atherosclerotic disease*) OR (carotid atheroscleros*) OR (aterosclerosis de la carótida) OR (aterosclerose carotídea) OR (aterosclerose da carótida) OR (c10.228.140.300.200*) OR (c14.907.253.123*)) OR (mh: (constriction, pathologic) OR mh: (constricción patológica) OR mh: (constrição patológica) OR (constriction* pathologic*) OR (stenos*) OR (stricture*) OR (estenos*) OR (estrechamiento patológico) OR (estreitamento patológico)) OR (mh: (carotid artery injuries) OR mh: (traumatismos de las arterias carótidas) OR mh: (lesões das artérias carótidas) OR (carotid arteriopath* traumatic) OR (carotid false aneurysm*) OR (injur* carotid artery) OR (artery trauma carotid) OR (pseudoaneurysm carotid) OR (seudoaneurisma de la carótida) OR (pseudoaneurisma de la carótida) OR (lesiones de las arterias carótidas) OR (pseudoaneurisma carotídeo) OR (traumatismos das artérias carótidas) OR (c10.228.140.300.200.345*) OR (c10.228.140.300.350.500*) OR (c10.900.250.300*) OR (c14.907.253.123.345*) OR (c14.907.253.535.500*) OR (c26.915.200.200*)) OR (mh: (atherosclerosis) OR mh: (aterosclerosis) OR mh: (aterosclerose) OR (atheroscleroses) OR (atherogenesis) OR (aterosclerosis de la carótida) OR (aterosclerose carotídea) OR (aterosclerose da carótida)) OR (mh: (carotid artery thrombosis) OR mh: (trombosis de las arterias carótidas) OR mh: (trombose das artérias carótidas) OR (carotid thrombosis) OR (common carotid artery thrombosis) OR (external carotid artery thrombosis) OR (internal carotid artery thrombosis) OR (carotid arter* thrombos*) OR (trombosis arterial carotídea) OR (c10.228.140.300.200.355*) OR (c14.907.253.123.355*) OR (c14.907.253.566.206*) OR (c14.907.355.590.213.206*))) AND ((mh: (ultrasonography) OR mh: (ultrasonografía) OR mh: (ultrassonografia) OR (computer echotomography) OR (diagnos* ultrasonic) OR (diagnos* ultrasound*) OR (ultrasonic tomography) OR (ultrasound imaging*) OR (imaging ultrasonic) OR (medical sonography) OR (echography) OR (echotomography computer) OR (sonography medical) OR (echotomography) OR (ecografía) OR (ecotomografía por computador) OR (sonografía médica) OR (ecografía médica) OR (tomografía ultrasonica) OR (diagnóstico por ultrasonido) OR (imagen ultrasónica) OR (imagen ultrasonográfica) OR (imagen de ultrasonido) OR (imagen por ultrasonido) OR (ecotomografía) OR (ecografia) OR (ecotomografia por computador) OR (sonografia médica) OR (ecografia médica) OR (tomografia ultrassônica) OR (diagnóstico por ultrassom) OR (imagem ultrassônica) OR (imagem ultrassonográfica) OR (imagem de ultrassom) OR (imagem por ultrassom) OR (ecotomografia)) OR (mh: (ultrasonography, doppler, duplex) OR mh: (ultrasonografía doppler dúplex) OR mh: (ultrassonografia doppler dupla) OR (doppler duplex ultrasonography) OR (ultrasonografía doppler doble) OR (ultrasonografía dúplex‐doppler)) OR (mh: (ultrasonography, doppler, color) OR mh: (ultrasonografía doppler en color) OR mh: (ultrassonografia doppler em cores) OR (color doppler ultrasonography)) OR (mh: (diagnostic imaging) OR mh: (diagnóstico por imagen) OR mh: (diagnóstico por imagem) OR (imaging diagnostic) OR (imaging medical) OR (imagen clínica) OR (diagnóstico por imageamento) OR (imageamento diagnóstico) OR (imageamento clínico) OR (imageamento médico) OR (imagens clínicas) OR (imageologia clínica) OR (imageologia médica) OR (imagiologia clínica) OR (imagiologia médica) OR (radiodiagnóstico) OR (e01.370.350*) OR (vs3.003.001.006.005.001*))) AND (instance:"regional") AND (db:("LILACS" OR "IBECS"))
Appendix 7. ClinicalTrials.gov search strategy
( Ultrasound OR ultrasonography ) AND ( carotid artery OR carotid stenosis ) [DISEASE]
Appendix 8. Studies not included in a meta‐analysis
Chua 2007 included 114 symptomatic patients that had undergone DUS and DSA. The objective was to evaluate optimal ultrasonographic criteria for the determination of ICA stenosis of more than or equal to 50%, 60%, and 70%. The authors evaluated the following velocity criteria: ICA PSV, CCA PSV, ICA EDV, CCA EDV, the ICA/CCA PSV ratio, the ICA PSV/CCA EDV ratio, and the ICA/CCA EDV ratio. They found that the ICA/CCA PSV ratio and the ICA PSV/CCA EDV ratio performed superiorly to the other velocity criteria. The data provided are from the area under the ROC curves; the sensitivity and specificity from each criterion were not provided. The ICA/CCA RSV ratio results were for ≥ 50% (ratio of 1.5) sensitivity, specificity, and accuracy: 100%, 85%, and 93%, respectively. For ≥ 60% (ratio of 2.6), sensitivity, specificity, and accuracy were 100%, 94%, and 97%, respectively. For ≥ 70% (ratio of 3.1), sensitivity, specificity, and accuracy were 100%, 91%, and 95%, respectively. The ICA PSV/CCA EDV ratio is rarely used and there are few data in the literature. The authors found the following: for ≥ 50%, a ratio of 3.5 has sensitivity, specificity, and accuracy of 100%, 58%, and 93%, respectively. For both ≥ 60% and ≥ 70%, the same ratio of 10.3 achieved 100% sensitivity, 96% specificity, and 91% accuracy. We were unable to include this study in our analysis because the data from PSV criteria were not provided and the ICA/CCA PSV ratio was too different from our prespecified criteria.
Colquhoun 1992 included 52 patients (99 carotid arteries) referred for DSA by their clinicians. The authors compared DUS versus DSA in different sites of the carotid artery (six segments) – proximal CCA, distal CCA, bulb, proximal ICA, distal ICA, and ECA – and divided stenosis into six grades (no disease, 0% to 24%, 25% to 49%, 50% to 74%, 75% to 99%, and occlusion). They presented the results in tables comparing grades of stenosis for each of the different segments separately. The agreement in grading for each vessel segment was 74.5%, and the sensitivity and specificity could be calculated for each segment separately. In the proximal ICA (segment 4), the estimated sensitivity and specificity for ≥ 50% and ≥ 75% ICA stenosis were 100% and 92%, respectively. This study was not included in the analysis because the authors classified carotid stenosis according to ECST –"DSA measurement of stenosis: the degree of stenosis for each area was assessed using calipers to measure the width of the lumen at the site of maximal stenosis and expressing this as a percentage of the true lumen, predicted by extrapolation from above and below the stenosis". Moreover, we could not convert the ranges of stenosis from the study to the NASCET classification. Some authors believe that because the results from measurements in NASCET and ECST models are approximately linear (Rothwell 1994), the conversion could be made. Because the results are given in ranges and the literature differs on the best mathematical model (Eliasziw 1994a), we were unable to perform such a conversion for this study.
Cui 2018 included 54 patients and examined four vessels – the bilateral CCA and the bilateral ICA – with DUS and DSA. Thus, the results were presented pooled (216 vessels). It was not possible to separate the CCA and ICA results, and therefore we did not include the results from this study in the analysis. The diagnostic accuracy of DUS found in this study, based on the diagnostic results of DSA, was 74.1% (160/216 vessels). For 1% to 49% stenosis, the sensitivity and specificity were 78.2% and 79.1%, respectively. For 50% to 69% stenosis, the sensitivity and specificity were 50% and 93.4%, respectively. For 70% to 99% stenosis, the sensitivity and specificity were 100% and 98.1%, respectively.
Bray 1995 included 64 patients with cerebrovascular disease referred to DSA who had undergone DUS in the 24h prior to DSA. Patients were submitted both to standard duplex sonography and colour Doppler sonographic imaging (CDI); all images were examined separately by two randomly selected radiologists. The authors found high concordance between radiologists in DSA evaluation. This study also assessed stenosis by diameter and area and compared the two ultrasonic methods. The results were the same in carotid artery stenoses > 70%; for the other grades, CDI performed better than standard duplex sonography. For combined hemodynamic (velocity criteria) and morphological data on carotid stenosis, no significant differences were found between techniques. For ≥ 70% carotid artery stenosis, sensitivity and specificity were 85% and 97%, respectively, for standard duplex sonography, and 85% and 96%, respectively, for CDI. In minor stenoses, the accuracy was slightly lower: 83% and 84% for CDI and standard duplex sonography, respectively. The velocity criteria described are very similar to those we prespecified in our protocol, but the data presented were insufficient to complete a 2 × 2 table for any category.
Hansen 1996 included 81 patients (162 arteries) that were examined with DUS and DSA. Stenosis on DUS was graded from the peak systolic velocity according to the equation: y = 0.54 * e 0.021 * x , where y is the peak systolic velocity in the ICA in m/s and x is the degree of stenosis expressed as the diameter reduction in percentage. In addition, a new equation was created for comparison with this original one. Carotid stenosis was determined by using DUS: diameter reduction in percent = [{(b × a) / b } × 100], where a is the smallest diameter in the stenotic zone and b is the diameter of the normal CCA proximal to the stenosis. The authors presented the comparison of DUS velocity criteria and DSA graphically; hence, we were unable to extract data (too many overlapping points). The authors discussed whether DSA is necessary for preoperative evaluation. They found that DSA was recommended in 14 of the 162 arteries examined (8.5%): in 11 of these arteries, DUS showed occlusion of the ICA (DSA confirmed occlusion in 10), and for the other three arteries the reason was poor DUS quality. The authors concluded that DSA did not change management in any of the patients. Although this study fulfilled the participant inclusion criteria, the methods used to determine stenosis based on DUS and DSA were too different from those prespecified in our protocol for quantitative analysis. Moreover, the authors did not provide sufficient data to establish values of sensitivity and specificity, even in a narrative form.
Knudsen 2002 is a short report that included 65 patients with suspected symptomatic high‐grade ICA (unclear how these patients were recruited); all patients underwent DUS and DSA. The authors found an overall agreement of 88%; for detecting ≥ 70% carotid artery stenosis, the reported sensitivity was 94% and the specificity was 86%. For detecting < 70% carotid artery stenosis, the calculated sensitivity was 86.6% and the specificity was 97.1%. We did not include this study in the meta‐analysis because ≥ 70% carotid artery stenosis was characterised by an ICA PSV ≥ 150 cm/s, an ICA EDV ≥ 90 cm/s, and a ICA/CCA PSV ratio > 2.8. These values are too different from those prespecified in our protocol.
Appendix 9. Carotid stenosis threshold criteria from included studies
Criteria used by Bray 1995
| Standard duplex | Stenosis % | Colour Doppler | |
| Systolic velocity cm/s | Spectral analysis | ||
| > 250 | ± Negative low frequencies of high energy | 70‐100 | Marked colour fading. Severe lumen narrowing. ± post‐stenosis flow reversal. ± mosaic pattern |
| > 130 | ± Low frequency of high energy | 50‐69 | Moderate color fading. Moderate lumen narrowing. ± mosaic pattern |
| 110‐130 | ± Minimal spectral broadening | 30‐49 | Laminar flow or color fading during systole |
| < 110 | Normal spectrum | 0‐29 | Laminar flow. Red flow in systole and diastole |
Criteria used by Eliasziw 1995
| Stenosis category (%) | ICPSV (v) cm/s | ICPFC (f) kHz | Ratio (r) ICA/CCA |
| < 70 | < 250 | < 8 | < 3 |
| 70‐79 | 250 ≤ v < 375 | 8 ≤ f < 12 | 3 ≤ r < 4,5 |
| 80‐89 | 375 ≤ v < 500 | 12 ≤ f < 16 | 4,5 ≤ r < 6 |
| 90‐99 | ≥ 500 | ≥ 16 | ≥ 6 |
Footnotes
CCA: common carotid artery f: frequency ICA: internal carotid artery ICPFC: internal carotid peak frequency change ICPSV: internal carotid peak systolic velocity r: ratio v: velocity
Criteria used by Arbeille 1995
| Diameter stenosis category | Peak systolic velocity | End diastolic velocity | Systolic velocity ratio (ICA/CCA) | Diastolic velocity ratio (ICA/CCA) |
| 0% | 110 cm/s | 40 cm/s | 1.8 | 2.6 |
| 1%‐39% | 110 cm/s | 40 cm/s | 1.8 | 2.6 |
| 40%‐59% | 130 cm/s | 40 cm/s | 1.8 | 2.6 |
| 60%‐79% | 130 cm/s | 40 cm/s | 1.8 | 2.6 |
| 80%‐99% | 250 cm/s | 100 cm/s | 2.5 | 5.5 |
| 100% | N/A | N/A | N/A | N/A |
Footnotes
CCA: common carotid artery ICA: internal carotid artery N/A: not applicable
Criteria adapted and translated from Arning 2010
| Defined by NASCET (% +‐ 5%) | 10% | 20‐40% | 50% | 60% | 70% | 80% | 90% | occlusion |
| Defined by ECST (% +‐ 5%) | 45% | 50‐60% | 70% | 75% | 80% | 90% | 95% | occlusion |
| Main criteria (1‐5) | ||||||||
| 1. B‐mode image | +++ | + | ||||||
| 2. Color Duplex image | + | +++ | + | + | + | + | + | +++ |
| 3. Intrastenotic PSV (cm/s) | ~200 | ~250 | ~300 | 350‐400 | 100‐500 | ‐ | ||
| 4. Post‐stenotic PSV (cm/s) | > 50 | < 50 | < 30 | ‐ | ||||
| 5. Collaterals and precursors (periorbital arteries/ACA) | (+) | ++ | +++ | +++ | ||||
| Supplementary criteria (6‐10) | ||||||||
| 6. Pre‐stenotic EDV reduction | (+) | ++ | +++ | +++ | ||||
| 7. Post‐stenotic flow disturbances | + | + | ++ | +++ | (+) | |||
| 8. Intrastenotic EDV (cm/s) | < 100 | < 100 | > 100 | > 100 | ||||
| 9. Perivascular tissue vibration | (+) | ++ | ++ | |||||
| 10. PSV ratio (ICA/CCA) | ≥ 2 | > 2 | ≥ 4 | ≥ 4 | ||||
Footnotes
Criteria 1: non‐stenosing plaques (up to 10% according to NASCET) will be shown in the B‐image, documentation of width, length and morphology of vessel wall changes Criteria 2: evidence of the minor stenosis (local aliasing effect) in contrast to the non‐stenosing plaque, illustration of the direction of flow in the case of moderate and severe stenoses and evidence of vascular occlusion Criteria 3: apply to stenoses with a length of 1‐2 cm and only limited in multi‐vessel processes Criteria 4: measurement at the most distal extracranial position outside the zone with jet stream and flow disturbances Criteria 5: possibly only one of the collateral connections affected; if an extracranial examination is carried out alone, the value of the findings is lower and requires careful interpretation because of frequent anatomical variants Criteria 6: indirect stenosis criteria, in the case of high‐grade, hemodynamically relevant stenosis (≥ 70% according to NASCET), the flow volume decreases; this leads to a decrease in the flow velocity if the vessel cross‐section remains constant. Diastolic reduction of the flow velocity (increased pulsatility) recognizable, while the systolic flow velocity still remains the same Criteria 7: not always pathological; should only be considered relevant for stenosis diagnosis together with other stenosis criteria Criteria 8: when PSV cannot be measured with sufficient accuracy, EDV maximum flow velocity can be used here as an additional criterion Criteria 9: the 'confetti' symbol is only recognizable when the PRF is set low Criteria 10: is useful for the assessment of carotid artery tandem stenosis, hyperfusion and primary (constitutional) narrow vessels ACA: anterior cerebral artery CCA: common carotid artery DEGUM: Deutsche Gesellshaft für Ultraschall in der Medizin ECST: European Carotid Surgery Trial EDV: end diastolic velocity ICA: internal carotid artery NASCET: North American Symptomatic Carotid Endaterectomy Trial PSV: peak systolic velocity
Data
Presented below are all the data for all of the tests entered into the review.
Tests. Data tables by test.
| Test | No. of studies | No. of participants |
|---|---|---|
| 1 DSA < 50% | 4 | 1495 |
| 2 DSA 50‐99% | 5 | 1536 |
| 3 DSA 50‐69% | 1 | 313 |
| 4 DSA 70‐99% | 9 | 2708 |
| 5 DSA Occlusion | 8 | 1243 |
| 6 CTA 70%‐99% | 2 | 685 |
| 7 CTA Occlusion | 3 | 833 |
| 8 MRA 70‐99% | 2 | 102 |
| 9 MRA 50‐99% | 1 | 31 |
1. Test.
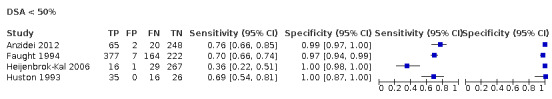
DSA < 50%
2. Test.
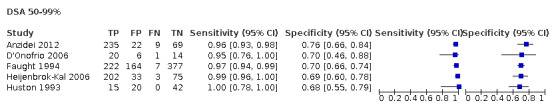
DSA 50‐99%
3. Test.

DSA 50‐69%
4. Test.
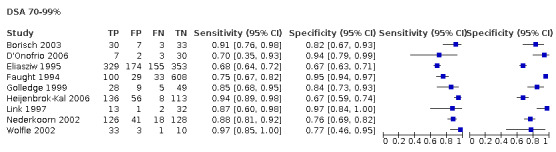
DSA 70‐99%
5. Test.
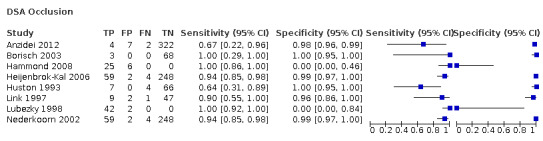
DSA Occlusion
6. Test.

CTA 70%‐99%
7. Test.

CTA Occlusion
8. Test.

MRA 70‐99%
9. Test.

MRA 50‐99%
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Anzidei 2012.
| Study characteristics | |||
| Patient Sampling | 416 symptomatic patients (amaurosis fugax, stroke, transient ischemic attack (TIA) or reversible ischemic neurological deficit) and suspected carotid artery stenosis underwent DUS. Only patients with stenoses on DUS > 30% with irregular atheroma, were sent for combined evaluation with CTA and MRA and only those with an indication for treatment after theses studies underwent DSA Exclusion criteria: Patients that had contraindications to MR, CT or DSA or had already undergone surgical or endovascular treatment for carotid stenosis |
||
| Patient characteristics and setting | 170 patients (included in analysis), 108 male/62 female, mean age 69 ± 6.5 (range 62‐90 years) risk factors: 17 diabetes mellitus, 34 hypertension, 13 dyslipidemia, 18 current smoker, 11 former smoker Only patients with known disease |
||
| Index tests |
DUS Image production: Aplio XV or Mylab 70 with dedicated software for the vascular study and a 5‐12 MHz multiband linear transducer Contrast: No Criteria used to determine grade of stenosis: quoted "The degree of stenosis was determined. Measure of the residual lumen at the point of maximum narrowing and peak velocity (125–130 cm/s) were visualized at the level of the stenosis." |
||
| Target condition and reference standard(s) |
Reference standard: DSA Target condition: symptomatic carotid stenoses Criteria used to determine grade of stenosis: NASCET criteria Complications: There were 11 cases (6.5%) of complications following the DSA procedure (one cerebral ischemia, four pseudoaneurysms and six hematomas at the puncture site); eight (4%) patients suffered moderate‐to‐severe adverse reactions to the iodinated contrast agent. |
||
| Flow and timing | 416 with symptomatic carotid stenoses underwent DUS. 205 patients underwent CTA and MRA and 170 patients underwent DSA (only those that were treated). In two cases, MRA examination was not considered of diagnostic quality due to motion artefacts. Interval for performing all four techniques was 7 ± 3 days. |
||
| Comparative | |||
| Methods |
Study design: Prospective accuracy cohort study. Unclear whether consecutive recruitment Study location: Italy Year and language of publication: Published in 2011 in English and Italian Study period: May 2006 and May 2010 Participants enrolled: 416 patients underwent DUS; 205 patients underwent CTA and MRA; 170 patients underwent DSA (underwent treatment). Carotids included in analyses: 335 |
||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Barlinn 2016.
| Study characteristics | |||
| Patient Sampling | Retrospectively evaluated 346 consecutive patients with acute cerebral ischemia who were admitted to a tertiary stroke center. Patients were eligible if their diagnostic workup included DUS and CTA performed within 5 days of each other Exclusion criteria: quote "Patients who underwent acute revascularization therapy of the extracranial ICA prior to completion of both diagnostic studies were excluded from our analysis". |
||
| Patient characteristics and setting | All patients were symptomatic, 346 patients with acute Ischemic stroke (n = 284) or transient ischemic attack (n = 62) 303 acute cerebral Ischemic patients included in analyses Mean age, 72 ± 12 years. 58% men and 42% women; median baseline National Institutes of Health Stroke Scale score, 4 [IQR 7] No information on risk factor |
||
| Index tests |
DUS . Image production: Duplex ultrasonography (Toshiba Aplio MX SSA‐780a System®, Toshiba Medical Systems, Germany) with a 7.5–10‐MHz linear array transducer was used for examinations of the extracranial carotid arteries. Contrast: No Criteria used to determine grade of stenosis: DEGUM ultrasound criteria (Appendix 9Arning 2010) |
||
| Target condition and reference standard(s) |
Reference standard: CTA Target condition: assessment of extracranial ICA steno‐occlusive disease in patients with acute cerebral ischemia Criteria used to determine grade of stenosis: NASCET criteria Complications: Not described |
||
| Flow and timing | 43 patients (12%) were not eligible for the final analysis due to the following reasons: ultrasonographic assessment after acute revascularization therapy, n = 13; elapsed time between DUS and CTA > 5 days, n = 23; and only one vascular imaging modality assessable (e.g. streak artefacts from dental implants on CTA), n = 7. The median elapsed time between DUS and CTA was 1 (IQR, 2) day |
||
| Comparative | |||
| Methods |
Study design: Retrospective design; participants identified retrospectively based on availability of complete records, if their diagnostic workup included DUS and CTA performed within 5 days Study location: Germany Year and language of publication: Published in 2016 in English Study period: from January 2012 to December 2012 Participants enrolled: 303 patients Carotids included in analyses: 593 DUS and CTA carotid artery pairs available for comparison |
||
| Notes | Only included retrospectively patients that had undergone the index and the reference tests; unclear if DUS results were used to select patients to CTA | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Belsky 2000.
| Study characteristics | |||
| Patient Sampling | 46 randomly chosen patients with symptoms of transient hemispheric attacks or unilateral visual disturbances were enrolled. No further details of patient sampling and recruitment were reported. The patients initially underwent duplex sonography due to suspected cerebral vascular disease. Patients who were candidates for carotid endarterectomy of either one or both the internal carotid arteries were then sent for CTA. Exclusion criteria: Not described |
||
| Patient characteristics and setting | All patients were symptomatic. 92 internal carotid arteries from 46 patients. 30 men and 16 women, ranging from 16 to 80 years of age (median, 70 years) No other patient characteristics were described. |
||
| Index tests | DUS Image production: Imaging was conducted by color DUS with either a 7‐MHz linear array transducer (Acuson 128, Acuson, Mountain View CA) or a broadband 5–12 MHz linear array transducer (ATL 3000 HDI, Advanced Technology Laboratories, Bothell WA) Contrast: No Criteria used to determine grade of stenosis: The degree of stenosis found on duplex sonography was categorized as mild, moderate and severe, according to the peak systolic and end diastolic velocities (PSV and EDV) on the internal carotid artery, measured in cms; mild stenosis, 0–29%: PSV < 125 and EDV < 40; moderate stenosis: PSV ≥ 125 and EDV ≥ 40; severe stenosis, 70–99%: PSV ≥ 250 and EDV ≥ 100. Occlusion was determined when flow through the internal carotid artery could not be registered by color DUS. |
||
| Target condition and reference standard(s) |
Reference standard: CTA Target condition: assessment of extracranial ICA steno‐occlusive disease in patients with acute cerebral ischemia Criteria used to determine grade of stenosis: NASCET criteria Complications: Not described |
||
| Flow and timing | Only patients with both tests (index and reference standard) were included. There were no exclusions described. CTA examinations were performed up to 1 month post‐duplex ultrasound. |
||
| Comparative | |||
| Methods |
Study design: Retrospective design; participants identified retrospectively based on availability of complete records Study location: Israel Year and language of publication: Published in 2000 in English Study period: between January 1996 and December 1998 Participants enrolled: 46 patients Carotids included in analyses: 92 |
||
| Notes | Only patients who were candidates for carotid endarterectomy of either one or both the internal carotid arteries were then sent for CTA. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Borisch 2003.
| Study characteristics | |||
| Patient Sampling | 39 consecutive patients with clinically suspected symptomatic carotid artery stenoses, referred to our institution for preoperative imaging, were included. Exclusion criteria: Patients with known contraindications for contrast‐enhanced MR angiography or DSA |
||
| Patient characteristics and setting | All patients were symptomatic: amaurosis fugax (n = 10), single or recurrent transient Ischemic attack (n = 14), and stroke in the previous 8 weeks (n = 15) 39 patients included. 7 women and 32 men; age range, 41–80 years; mean age, 67.4 ± 8.4 years) Risk factors not described |
||
| Index tests |
DUS Image production: Sonoline Elegra 5.0 system (Siemens), with a 7.5‐MHz linear array transducer Contrast: No Criteria used to determine grade of stenosis: previously published criteria described in Eliasziw 1995; Appendix 9 |
||
| Target condition and reference standard(s) |
Reference standard: DSA and MRA Target condition: assessment of clinically suspected symptomatic carotid artery stenoses Criteria used to determine grade of stenosis: NASCET criteria Complications: No neurologic events occurred |
||
| Flow and timing | Both carotid artery bifurcations were examined with contrast‐enhanced MR angiography, duplex sonography, and selective DSA within 10 days. 7 carotids were excluded from statistical analysis: in 3 patients, the carotid artery could not be visualized selectively on DSA images; in 4 patients, the insonation of the stenosis was not possible because of sonographic attenuation due to echogenic plaque in the artery wall. |
||
| Comparative | |||
| Methods |
Study design: Prospective, consecutive, accuracy cohort study Study location: Germany Year and language of publication: Published in 2003 in English Study period: August 1999 and July 2002 Participants enrolled: 39 Carotids included in analyses: 71 |
||
| Notes | The data presented refer to numbers that are pooled data (4 observers × 71 vessels = 284 evaluations) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Bray 1995.
| Study characteristics | |||
| Patient Sampling | Patientes referred to the radiology department for DSA of the supra‐aortic vessels were consecutively included. Exclusion criteria not described |
||
| Patient characteristics and setting | 64 patients, 53 men and 11 women, mean age 62.6 +‐ 13.5 years 28 had an established stroke, 19 had only transient Ischemic attacks (TIA). There were 12 with a cervical bruit, and 5 had a cerebral hemorrhage. Risk factors not described |
||
| Index tests |
DUS Image production: The device has not been specified but performed with a 7 MHz linear probe. Contrast: No Criteria used to determine grade of stenosis: Appendix 9 |
||
| Target condition and reference standard(s) |
Reference standard: DSA Target condition: suspected for carotid disease patients Criteria used to determine grade of stenosis: quantitative measurement of the ratio between the diameters of the residual lumen of the stenosis and the presumably normal carotid artery was made, usually 4 cm beyond the carotid bulb. Complications: Not described |
||
| Flow and timing | 7 other patients were excluded from the consecutive series because of incomplete or delayed investigations. DUS were performed in the 24 h prior to angiography. Did not describe prior testing, but included only patients referred to DSA of supra‐aortic vessels |
||
| Comparative | |||
| Methods |
Study design: Prospective, consecutive, accuracy cohort study Study location: France Year and language of publication: Published in 1995 in English. Study period: Not described Participants enrolled: 64 patients. Carotids included in analyses: 128 |
||
| Notes | Did not describe whether patients had undergone any screening imaging test before being included in the study | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Chua 2007.
| Study characteristics | |||
| Patient Sampling | Evaluated 114 patients who presented within 120 days of the onset of ischemic symptoms (transient ischemic attack or non‐disabling stroke), 20 were excluded Exclusion criteria: Patients with cardiac embolism and prior ipsilateral carotid endarterectomy were excluded. Occlusion of one or both ICA and atypical flow patterns within vessels, such as low velocities in near‐occlusion, and extensive calcified plaques resulting in long segments of acoustic shadowing |
||
| Patient characteristics and setting | 94 patients ranged from 53 to 76 years (mean, 64; standard deviation, 8.8). The male‐to‐female sex ratio was 2.9:1 No information on risk factors |
||
| Index tests |
DUS Image production: Diasonics Spectra (Diasonics Inc, Milpatas, California) using a 7.5‐MHz transducer Contrast: No Criteria used to determine grade of stenosis: ROC curve analysis |
||
| Target condition and reference standard(s) |
Reference standard: DSA Target condition: evaluate optimal criteria for determination of ICA stenosis in symptomatic patients Criteria used to determine grade of stenosis: NASCET criteria Complications: None |
||
| Flow and timing | 20 were excluded from the study because of the occlusion of one or both ICA and atypical flow patterns within vessels, such as low velocities in near‐occlusion, and extensive calcified plaques resulting in long segments of acoustic shadowing. Carotid duplex ultrasonography and DSA within 1 month of each other |
||
| Comparative | |||
| Methods |
Study design: Prospective, not consecutive, accuracy cohort study Study location: Singapore Year and language of publication: Published in 2007 in English Study period: January 1995 to December 2003 Participants enrolled: 94 Carotids included in analyses: 188 |
||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | No | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Colquhoun 1992.
| Study characteristics | |||
| Patient Sampling | Patients included were referred by their clinicians for DSA examination of the carotid arteries, and then the ultrasound examinations were performed. Did not mention if previous tests were performed before inclusion |
||
| Patient characteristics and setting | 42 (84%) of patients included were symptomatic: 27 were referred with transient ischemic attacks, two with amaurosis fugax, 15 with stroke and six with non‐specific complaints. 50 patients, 30 men, mean age 58 years (44‐70) and 20 women, mean age 53 years (38‐68) Risk factors not described Included patients were referred for DSA, but previous testing was not described. |
||
| Index tests |
DUS Image production: Acuson 128 duplex scanner with color flow mapping using a 5 MHz small parts linear array probe (L538) Contrast: No Criteria used to determine grade of stenosis: When possible, the percentage diameter stenosis was measured directly from the image with electronic caliper. Peak systolic velocity of 120 cm/s in the internal carotid artery was taken to indicate > 50% diameter stenosis, and > 250 cm/s with diastolic velocities > 100 cm/s was taken to indicate > 80% diameter stenosis. |
||
| Target condition and reference standard(s) |
Reference standard: DSA Target condition: suspected for carotid disease patients Criteria used to determine grade of stenosis: The degree of stenosis for each area was assessed using calipers to measure the width of the lumen at the site of maximal stenosis and expressing this as a percentage of the true lumen, predicted by extrapolation from above and below the stenosis. Complications: No complications were recorded. |
||
| Flow and timing |
Exclusions: Two patients did not attend for ultrasound and in one patient the DSA image from one side was spoilt by a swallowing artefact. Results were therefore obtained from 99 carotid arteries. Also excluded from analysis: 46 of the 99 carotid arteries that were successfully scanned, but no abnormality was detected by either DSA or ultrasound Exams were performed within four weeks. |
||
| Comparative | |||
| Methods |
Study design: Prospective, consecutive, accuracy cohort study Study location: Newcastle, UK Year and language of publication: Published in 1992 in English Study period: Not described Participants enrolled: 50 patients Carotids included in analyses: 53 |
||
| Notes | Excluded from analysis: carotids in which either DSA and DUS found no abnormalities | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Cui 2018.
| Study characteristics | |||
| Patient Sampling | 54 patients presenting with carotid stenosis were admitted to the First Affiliated Hospital of Xinxiang Medical University (Xinxiang, China). Unclear how they were recruited | ||
| Patient characteristics and setting | All patients where carotid stenosis was suspected to arise from vascular diseases in the head and neck. In addition, all patients had experienced transient Ischemic attack and other neurological symptoms. The patients consisted of 32 males and 22 females aged between 37 and 82 years, with a mean age of 63.06 ± 13.21 years. Risk factors not described |
||
| Index tests |
DUS Image production: A Color Doppler Ultrasography (CDUS) diagnostic instrument (Esaote North America, Inc., Indianapolis, IN USA) with a probe frequency range of 5‐12 MHz Contrast: No Criteria used to determine grade of stenosis: Grant 2003 |
||
| Target condition and reference standard(s) |
Reference standard: DSA Target condition: patients where carotid stenosis was suspected to arise from vascular diseases in the head and neck Criteria used to determine grade of stenosis: NASCET criteria Complications: Not described |
||
| Flow and timing | No patients were excluded from analysis. All patients underwent CDUS, CE MRA and DSA examinations within 1 week of diagnosis. |
||
| Comparative | |||
| Methods |
Study design: Prospective cohort study Study location: China Year and language of publication: Published in 2017 in English Study period: from January 2012 to January 2014 Participants enrolled: 54 patients. Carotids included in analyses: 216 |
||
| Notes | Considered 216 carotid arteries because investigators counted the common carotid artery and the internal carotid artery as separated vessels for analysis Did not describe whether patients had undergone any screening imaging test before being included in the study |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
D'Onofrio 2006.
| Study characteristics | |||
| Patient Sampling | 32 consecutive patients with symptoms of carotid artery disease (transient ischemic attack, minor disabling ischemic stroke, or amaurosis fugax) and ultrasonographic findings of stenosis > 50% of the internal carotid artery. In 21 cases, the carotid bifurcation was studied with CDUS, CE‐MRA and DSA and in 11 cases with Doppler US and CE‐MRA only. Exclusion criteria: Not described |
||
| Patient characteristics and setting | All patients were symptomatic, all with proven carotid stenosis on DUS. Not described |
||
| Index tests |
DUS Image production: Doppler US was carried out by the same operator on the same ultrasound unit (Sequoia 512, Acuson/Siemens, Germany) Contrast: No Criteria used to determine grade of stenosis: described in Arbeille 1995 (Appendix 9) |
||
| Target condition and reference standard(s) |
Reference standard: DSA and MRA Target condition: patients with symptoms of carotid artery disease Criteria used to determine grade of stenosis: NASCET criteria Complications: Not described |
||
| Flow and timing | 1 carotid was not included in analysis because of previous endarterectomy. Unclear why only 31 arteries included in comparison between DUS and MRA All exams were performed within 1 week. |
||
| Comparative | |||
| Methods |
Study design: Prospective, consecutive, accuracy cohort study Study location: Italy Year and language of publication: Published in 2005 in English and Italian Study period: Not described Participants enrolled: 32 patients Carotids included in analyses: 41 for comparison between DUS and DSA and 31 for comparison between DUS and MRA |
||
| Notes | Did not describe whether patients had undergone any screening imaging test before being included in the study | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Das 2009.
| Study characteristics | |||
| Patient Sampling | 15 patients with symptomatic stenosis of the internal carotid artery were included in this study. All patients underwent one Dual‐Source CTA, MRA and DUS of the supra‐aortic arteries, to assess the degree of stenosis of the internal carotid | ||
| Patient characteristics and setting | 15 symptomatic patients were included, 12 men and 3 women Average age was 69 years (range 53–79 years) Risk factors not described |
||
| Index tests |
DUS Image production: GE Vivid 7 (GE Healthcare, Milwaukee, USA) with a 7 Mhz probe Contrast: No Criteria used to determine grade of stenosis: DEGUM criteria (Appendix 9) |
||
| Target condition and reference standard(s) |
Reference standard: CTA and MRA Target condition: symptomatic stenosis of the internal carotid Criteria used to determine grade of stenosis: NASCET criteria Complications: No |
||
| Flow and timing | All three investigations were made within a week. | ||
| Comparative | |||
| Methods |
Study design: Prospective study, unclear whether consecutive recruitment Study location: Germany Year and language of publication: Published in 2009 in English Study period: between April 2007 and March 2008 Participants enrolled: 15 patients Carotids included in analyses: 30 |
||
| Notes | Did not describe whether patients had undergone any screening imaging test before being included in the study | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Eliasziw 1995.
| Study characteristics | |||
| Patient Sampling | The study used data collected from the first 3 years of NASCET; 1360 patients were recruited from 50 academic centers across North America. A total of 1011 patients had complete ultrasonographic data. Exclusion criteria: data incomplete or unavailable, ischemic event attributable to a cardiac source of embolism, age over 80 years, presence of significant intracranial vascular disease, and life‐threatening or other disabling conditions |
||
| Patient characteristics and setting | All patients were symptomatic; other characteristics of the included patients were not described. | ||
| Index tests |
DUS Image production: the ultrasound device was not specified; the vast majority of the transducers used were in the 5‐MHz range. Contrast: No Criteria used to determine grade of stenosis: described in Appendix 9 |
||
| Target condition and reference standard(s) |
Reference standard: DSA Target condition: Symptomatic patients with severe (70% to 99%) carotid stenoses Criteria used to determine grade of stenosis: NASCET criteria Complications: stroke rate from angiography was 0.78% |
||
| Flow and timing | Ultrasonography was performed concurrently to the angiogram. | ||
| Comparative | |||
| Methods |
Study design: Cross‐sectional study; participants consecutively enrolled Study location: Multicenter in North America Year and language of publication: Published in 1995 in English Study period: from January 1988 through February 1991 Participants enrolled: 1011 patients Carotids included in analyses: 1011 carotids |
||
| Notes | Ultrasonography was performed concurrently to the angiogram but was not used in the decision‐making process for entering patients into the study. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Faught 1994.
| Study characteristics | |||
| Patient Sampling | Patients from 2 non‐invasive vascular laboratories affiliated with the Southern Illinois University School of Medicine. Did not specify recruitment. Exclusion criteria: inadequate arteriograms and patients with ICA occlusions |
||
| Patient characteristics and setting | 77% of the patients included were symptomatic; no other characteristics described | ||
| Index tests |
DUS Image production: quote "Duplex examinations were performed with a QUAD I Angiodynograph (Quantum Medical Systems, Issaquah, Wash.) until the latter part of 1989, after which the Quantum 2000 (Quantum Medical Systerns)". Contrast: No Criteria used to determine grade of stenosis: ROC curve analysis |
||
| Target condition and reference standard(s) |
Reference standard: DSA Target condition: suspected carotid disease patients Criteria used to determine grade of stenosis: NASCET Complications: Not described |
||
| Flow and timing | Arteriograms were performed within 1 month. | ||
| Comparative | |||
| Methods |
Study design: Unclear whether prospective design. Unclear whether consecutive recruitment Study location: Chicago, USA Year and language of publication: Published in 1994 in English Study period: from January 1, 1989 through October 30, 1992 Participants enrolled: 405 patients Carotids included in analyses: 770 arteries |
||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Golledge 1999.
| Study characteristics | |||
| Patient Sampling | Selected symptomatic patients admitted to the hospital | ||
| Patient characteristics and setting | 50 patients, all symptomatic 62% men and 38% women. Median age 71 years (range from 47 to 84 years) |
||
| Index tests |
DUS Image production: duplex scan imaging was performed with a 5‐MHz probe (angle of insonation, 60 degrees; Ultramark 9, HDI, Advanced Technology Laboratories, Wash.). Contrast: No Criteria used to determine grade of stenosis: ROC curve analysis |
||
| Target condition and reference standard(s) |
Reference standard: DSA Target condition: carotid artery stenosis Criteria used to determine grade of stenosis: NASCET Complications: No significant complications |
||
| Flow and timing | DSA was performed within 24 hours from DUS. | ||
| Comparative | |||
| Methods |
Study design: Unclear whether prospective design Study location: UK Year and language of publication: Published in 1999 in English Study period: June 1996 to June 1997 Participants enrolled: 50 patients Carotids included in analyses: 100 arteries |
||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Hammond 2008.
| Study characteristics | |||
| Patient Sampling |
Inclusion criteria: patients with non‐disabling neurology symptoms and an apparent carotid occlusion on DUS Exclusion criteria: patients failing to undergo all exams within 14 days; patients who refused to provide informed consent |
||
| Patient characteristics and setting | Not described | ||
| Index tests |
DUS Image production: Acuson 128 XP10 US machine 7 MHz linear array transducer Contrast: yes. Contrast agent: Levovist (Schering, UK) Criteria used to determine grade of stenosis: quote "Vessels were characterised as definitely occluded (if no flow was seen anywhere within the cervical ICA) or definitely patent (if a flow channel was seen throughout the cervical ICA)". |
||
| Target condition and reference standard(s) |
Reference standard: DSA Target condition: carotid occlusion Criteria used to determine grade of stenosis: quote "Vessels were characterised as definitely occluded if no contrast could be identified in the line of the cervical ICA or if there was significant discontinuity with backfilling of the siphon and distal ICA from the intracranial vessels". Complications: Not described |
||
| Flow and timing | DUS, DSA and MRA were performed within 14 days. Quote "Nineteen vessels were excluded on the basis of incomplete imaging due to patient failure to attend (8 vessels), claustrophobia and inability to tolerate MRA (10 vessels), or refusal to consent to DSA (1 vessel). A further 9 vessels were excluded because there was a delay of > 14 days in completing of their imaging." A confident diagnosis could not be made in 5/31 (16%) vessels, so 24 vessels included in analysis |
||
| Comparative | |||
| Methods |
Study design: Prospective accuracy study. Unclear whether consecutive recruitment Study location: UK Year and language of publication: Published in 2008 in English Study period: Between April 2001 and August 2004 Participants enrolled: 30 patients Carotids included in analyses: 24 arteries |
||
| Notes | Only evaluated patients with the diagnosis of occlusion or pseudocollusion | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Hansen 1996.
| Study characteristics | |||
| Patient Sampling | Patients with a planned carotid endarterectomy sent to be examined with DUS and DSA; unclear where the patients were selected and exams performed previously Exclusion criteria not described |
||
| Patient characteristics and setting | 81 consecutive patients (22 women and 59 men), aged 49‐83 years (mean 68 years) 89% symptomatic patients: 28 patients (34.5%) had had minor strokes, 32 (39.5%) transient ischemic attacks (TIA), and 12 (15%) amaurosis fugax. 9 (11%); those previously undergoing an endarterectomy on the symptomatic side, were operated on because of a contralateral asymptomatic severe stenosis. |
||
| Index tests |
DUS Image production: Acuson XP 10 (Acuson, Mountain View, CA, U.S.A.), using either a 7 MHz B‐mode real‐time linear scanner including a 5 MHz pulsed and color‐coded Doppler, or a 5 MHz B‐mode real‐time linear scanner including a 3.5 MHz pulsed and color‐coded Doppler Contrast: No Criteria used to determine grade of stenosis: ROC curve analysis |
||
| Target condition and reference standard(s) |
Reference standard: DSA Target condition: internal carotid stenosis Criteria used to determine grade of stenosis: quote "Diameter reduction in percent = (b ‐ a)/b * 100. Where a is the smallest diameter in the stenotic zone, and b is the diameter of the normal CCA proximal to the stenosis." Complications: Not described |
||
| Flow and timing | All exams were performed within 1 month | ||
| Comparative | |||
| Methods |
Study design: Prospective, consecutive, accuracy cohort study Study location: Sweden Year and language of publication: Published in 1996 in English Study period: Not described Participants enrolled: 81 patients Carotids included in analyses: 162 arteries |
||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Heijenbrok‐Kal 2006.
| Study characteristics | |||
| Patient Sampling | Patients with amaurosis fugax, transient ischemic attack, or minor stroke from three hospitals. Unclear whether other exams were performed previously Occlusions or pseudo‐occlusions were excluded. |
||
| Patient characteristics and setting | 350 symptomatic patients 76% male (266) and 24% female (84) Mean age of 67 years (range, 39–88 years) |
||
| Index tests |
DUS Image production: for 311 patients: Ultramark 9 HDI or HDI 3000 (Advanced Technology Laboratories, Bothell, Wash.); for 39 patients: Diasonics Master Series (GE Medical Systems, Milwaukee, Wis.) Contrast: No Criteria used to determine grade of stenosis: PSV of 230 cm/sec for the diagnosis of 70%–99% stenosis and 125 cm/sec for the diagnosis of 50%–99% stenosis |
||
| Target condition and reference standard(s) |
Reference standard: DSA Target condition: carotid artery stenosis Criteria used to determine grade of stenosis: NASCET Complications: Not described |
||
| Flow and timing | 313 patients included in analysis (had both examinations). 323 patients were evaluated with DSA and 330 patients with duplex US. Quote "Values were missing owing to the following reasons: Sometimes it was not feasible to perform both examinations before surgery, some patients withdrew from the study after having undergone one examination, and the examination was not always correctly performed according to our study protocol. Also, occasionally, the PSV was not measured when duplex US was performed. Finally, in seven patients, it was impossible to measure the degree of stenosis because of poor image quality and the poor reliability of the DSA findings". DUS and DSA were performed within 4 weeks. |
||
| Comparative | |||
| Methods |
Study design: Prospective diagnostic study Study location: Netherlands Year and language of publication: Published in 2006 in English Study period: January 1997 through January 2000 Participants enrolled: 313 patients Carotids included in analyses: 313 arteries |
||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Huston 1993.
| Study characteristics | |||
| Patient Sampling | 52 consecutive patients referred for cerebral angiography were selected, but 2 patients were excluded because they did not complete the MRA examination (claustrophobia and severe back pain). Unclear whether other exams were performed previously |
||
| Patient characteristics and setting | 50 symptomatic patients (symptoms of hemispheric ischemia) 35 men and 15 women aged 42‐82 years. The median age was 67. |
||
| Index tests |
DUS Image production: Acuson 128; Acuson, Mountain View, Calif. Contrast: No Criteria used to determine grade of stenosis: stenosis 1‐49% ‐ PSV < 125 cm/s; stenosis 50‐79% ‐ PSV ≥ 125 cm/s and EDV ≤ 135 cm/s; 80‐99% ‐ PSV ≥ 125 cm/s and EDV ≥ 135 cm/s |
||
| Target condition and reference standard(s) |
Reference standard: DSA Target condition: patients with symptoms of carotid artery disease referred to DSA Criteria used to determine grade of stenosis: NASCET Complications: Not described |
||
| Flow and timing | 52 patients were included, but 2 did not complete MRA examination and were excluded. 98 carotid bifurcations were evaluated by DSA, but statistical analysis was limited to the 77 arteries that had DUS and DSA performed within 2 weeks. | ||
| Comparative | |||
| Methods |
Study design: Prospective accuracy cohort study Study location: USA Year and language of publication: Published in 1993 in English Study period: Not described Participants enrolled: 52 patients Carotids included in analyses: 77 arteries |
||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Knudsen 2002.
| Study characteristics | |||
| Patient Sampling | Included patients with suspected symptomatic high‐grade ICA stenosis but did not specify how they suspected it was high‐grade stenosis | ||
| Patient characteristics and setting | 65 symptomatic patients Characteristics not described Unclear if there were previous exams performed |
||
| Index tests |
DUS Image production: Siemens Sonoline Elegra, Siemens Medical System, Washington Contrast: No Criteria used to determine grade of stenosis:stenosis ≥ 70% stenosis was characterized by an ICA PSV ≥ 150 cm/s, an ICA end diastolic velocity ≥ 90 cm/s, and a PSV ratio ≥ 2.8. No flow indicated occlusion. |
||
| Target condition and reference standard(s) |
Reference standard: DSA Target condition: symptomatic high‐grade ICA stenosis Criteria used to determine grade of stenosis: NASCET Complications: Not described |
||
| Flow and timing | DSA and DUS performed within 2 days 65 patients and 129 arteries were included in analysis; unclear why 1 artery was excluded |
||
| Comparative | |||
| Methods |
Study design: Prospective, consecutive, accuracy cohort study Study location: Denmark Year and language of publication: Published in 2002 in English Study period: a 12‐month period 1998 to 1999 Participants enrolled: 65 patients Carotids included in analyses: 129 arteries |
||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Link 1997.
| Study characteristics | |||
| Patient Sampling | Patients were evaluated for symptomatic cerebrovascular disease and agreed to all tree examinations (DUS, DSA and CTA). Exclusion criteria not described |
||
| Patient characteristics and setting | 28 patients included, 10 women (35.7%) and 18 men (64.3%). All patients were symptomatic. Age: 46‐77 years old (mean 63 years) |
||
| Index tests |
DUS Image production: Philips P700. Color‐coded duplex sonography was performed with 5‐ and 7.5‐MHz linear‐array transducers Contrast: No Criteria used to determine grade of stenosis: Carotid stenosis were classified as mild (0‐29%) when PSV was less than 100 cm/s; moderate (30‐69%) when PSV ranged from 100 cm/s to 200cm/s; and severe (70‐99%) when PSV was greater than 200 cm/s |
||
| Target condition and reference standard(s) |
Reference standard: DSA Target condition: symptomatic carotid stenosis Criteria used to determine grade of stenosis: NASCET Complications: Not described |
||
| Flow and timing | All three examinations were performed within 48 hours. All arteries included in analysis |
||
| Comparative | |||
| Methods |
Study design: Prospective, consecutive, accuracy cohort study Study location: Germany Year and language of publication: Published in 1996 in English Study period: Not described Participants enrolled: 28 patients Carotids included in analyses: 56 arteries |
||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Lubezky 1998.
| Study characteristics | |||
| Patient Sampling | The study included patients with carotid occlusion diagnosed by CDUS. Exclusion criteria: quote "Patients in whom the duplex scan was equivocal, with poor visualization of the ICA, were excluded from the study. Only cases in which a technically satisfactory duplex scanning was obtained were included". |
||
| Patient characteristics and setting | 148 patients diagnosed with carotid occlusion. 102 male (70%) and 46 female (30%) Age: ranged from 43 to 89 years old (average age was 70 years) 22% were asymptomatic and 88% were symptomatic. Other risk factors for atherosclerosis: 99 patients (67%) had hypertension, 85 (58%) were current or past smokers, 64 (44%) had a history of ischemic heart disease, 56 (38%) had hypercholesterolemia, 44 (30%) had diabetes, and 38 patients (26%) had peripheral vascular disease. Five patients (3%) had a history of neck radiation. |
||
| Index tests |
DUS Image production: HD13000 system (Advanced Technology Laboratories, Bothell, WA, USA) using a linear 5‐10 MHz, 38 mm transducer Contrast: No Criteria used to determine occlusion: Not specified, only occlusion included |
||
| Target condition and reference standard(s) |
Reference standard: CTA and DSA Target condition: carotid artery occlusion Criteria used to determine grade of stenosis: only occlusion included Complications: Not described |
||
| Flow and timing | Maximum time interval between studies was 30 days. | ||
| Comparative | |||
| Methods |
Study design: Unclear Study location: Israel Year and language of publication: Published in 1998 in English Study period: From 1995 to 1997 Participants enrolled: 148 patients Carotids included in analyses: 148 arteries compared with CTA and 54 arteries compared with DSA |
||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | High risk | ||
Nederkoorn 2002.
| Study characteristics | |||
| Patient Sampling | 350 consecutive symptomatic patients suspected of having carotid artery stenosis were included. Not described if any previous exams were performed Exclusion criteria: patients with contraindications for MRA |
||
| Patient characteristics and setting | 350 patients included, 76% male and 24% female. Mean age 67 years (range from 39‐88 years) 100% of the patients were symptomatic. 4% had previous carotid endarterectomy. Other risk factors for atherosclerosis: 49% had hypertension; 15% had diabetes; 51% had cardiac history (angina, myocardial infarction, heart failure, or bypass surgery or PTA); 23% had peripheral arterial disease; 49% were current smokers and 34% were ex‐smokers. | ||
| Index tests |
DUS Image production: Not described Contrast: No Criteria used to determine stenosis: Carotid stenosis were classified as mild (0‐29%) when PSV was less than 150 cm/s; mild‐to‐moderate (30‐49%) when PSV ranged from 150 cm/s to < 190 cm/s; moderate (50‐69%) when PSV ranged from 190 cm/s to < 270 cm/s; severe (70‐99%) when PSV was greater than 270 cm/s; and occlusion when no flow was detected. |
||
| Target condition and reference standard(s) |
Reference standard: DSA Target condition: carotid artery stenosis Criteria used to determine grade of stenosis: NASCET criteria Complications: 1.4% minor stroke, 0.3% major stroke and 0.6% mortality |
||
| Flow and timing | From the 350 patients, 323 DSA, 330 DUS, and 295 MRA were included in the analysis. 313 arteries were included in DUS versus DSA analysis. Missing data were caused by: impossibility of performing all three exams before surgery, patients withdrawn, tests not performed according to the study protocol, or poor quality of images of some MRA and DSA. Patients underwent DUS, MRA, and DSA examination within a maximum of 4 weeks. |
||
| Comparative | |||
| Methods |
Study design: Prospective, consecutive, accuracy cohort study Study location: University Medical Center Utrecht, University Medical Center Rotterdam, and Enschede Medical Center (Netherlands) Year and language of publication: Published in 2002 in English Study period: January 1997 to November 2000 Participants enrolled: 350 patients Carotids included in analyses: 313 arteries |
||
| Notes | Only the symptomatic side was included in the analysis. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Wolfle 2002.
| Study characteristics | |||
| Patient Sampling | 47 patients were included with suspected severe (≥ 70%) carotid stenosis; unclear how patients were selected Exclusion criteria: patients with claustrophobia or contraindications for MRA |
||
| Patient characteristics and setting | 47 patients included; 34 (72%) were symptomatic and 13 (28%) were asymptomatic 37 (79%) male and 10 (21%) female The median age was 68 years (ranging from 46‐84 years). |
||
| Index tests |
DUS Image production: Siemens (Elegra) with a 7,5MHz linear scan Contrast: No Criteria used to determine stenosis: Carotid stenosis was classified as severe (70‐99%) when PSV was greater than 230 cm/s and EDV was greater than 70 cm/s. |
||
| Target condition and reference standard(s) |
Reference standard: DSA Target condition: ≥ 70% carotid artery stenosis Criteria used to determine grade of stenosis: NASCET criteria Complications: 2.1% stroke rate |
||
| Flow and timing | Median time between exams was 2.8 days (SD 2.17) | ||
| Comparative | |||
| Methods |
Study design: Prospective, not consecutive, accuracy study Study location: Germany Year and language of publication: Published in 2002 in German Study period: Not described Participants enrolled: 47 patients Carotids included in analyses: 94 arteries |
||
| Notes | Patients were only included with proven carotid stenosis. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
CCA: common carotid artery CDUS: color duplex ultrasound CE: contrast enhanced CTA: computed tomography angiography DEGUM: The German Society of Ultrasound in Medicine DSA: digital subtraction angiography DUS: duplex ultrasound EDV: end diastolic velocity ICA: internal carotid artery IQR: interquartile range MRA: magnetic resonance angiography MR: magnetic resonance NASCET: North American Symptomatic Carotid Endaterectomy Trial PSV: peak systolic velocity PTA: percutaneous transluminal angioplasty ROC:receiver operating curve SD: standard deviation TIA: transient ischemic attack US: ultrasound
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| AbuRahma 1995 | Study did not define the proportion of symptomatic patients and time between index test and alternative test was not specified |
| AbuRahma 1997 | Less than 70% of the patients included were symptomatic and time between index test and alternative test was not specified |
| AbuRahma 1998 | Less than 70% of the patients included were symptomatic |
| AbuRahma 2011 | Less than 70% of the patients included were symptomatic |
| Ackerstaff 1982 | Less than 70% of the patients included were symptomatic |
| Ackroyd 1984 | Study did not define the proportion of symptomatic patients and time between index test and alternative test was not specified |
| Adiga 1984 | Study did not provide enough data for construction of a 2 x 2 table and the method of calculating the degree of stenosis |
| Alexandrov 1993 | Study did not define the proportion of symptomatic patients and time between index test and alternatives tests was not specified |
| Alexandrov 1997a | Study did not define the proportion of symptomatic patients and time between index test and alternatives tests was not specified |
| Alexandrov 1997b | Study did not define the proportion of symptomatic patients |
| Alves 1982 | Time between index test and alternative test was not specified |
| Alves 1983 | Less than 70% of the patients included were symptomatic, time between index test and alternative test was not specified, and the study did not provide enough information about the method of calculating the degree of stenosis |
| Ammar 2017 | Retrospective study that did not provide any suitable test comparison. The object of the study was if additional imaging studies (over DUS) were necessary for treatment planning |
| Anderson 1983 | Time between index test and alternative test was not specified. An experimental study about the US method; the quantification of stenosis was based on subjective visual impression |
| Anderson 2000 | The DUS examinations were not standardized and there was no description of time between examinations |
| Appleberg 1982 | Study did not define the proportion of symptomatic patients and time between index test and alternatives tests was not specified |
| Arbeille 1984 | Study did not define the proportion of symptomatic patients and the degree of stenosis was determined by a subjective visual impression of the Doppler spectrum analysis |
| Arbeille 1997 | Only DUS was assessed; there was no comparison with CTA or DSA or MRA |
| Archie 1981 | Study did not define the proportion of symptomatic patients and time between index test and alternatives tests was not specified |
| Arous 2019 | Study did not define the proportion of symptomatic patients and time between index test and alternative test was more than four weeks |
| Auffray‐Calvier 1996 | Comparision on MRA and DSA. DUS was performed, but there were no data on DUS accuracy |
| Azieva 2016 | No suitable diagnostic accuracy data |
| Back 2000 | Less than 70% of the patients included were symptomatic |
| Back 2003 | No direct comparison between DUS and MRA or DSA. The study compared MRA and DSA after inconclusive duplex scan |
| Bain 1998 | Time between index test and alternative test was more than four weeks |
| Ballard 1994 | Less than 70% of the patients included were symptomatic and time between index test and alternative test was more than four weeks |
| Ballard 1997 | Less than 70% of the patients included were symptomatic |
| Ballotta 1999 | Less than 70% of the patients included were symptomatic |
| Bandyk 1985 | Time between index test and alternative test was not specified and the degree of stenosis was determined by a subjective visual impression of the Doppler spectrum analysis |
| Barlinn 2018 | Less than 70% of the patients included were symptomatic |
| Barnes 1976 | Study did not define the proportion of symptomatic patients |
| Barnes 1982 | Preliminary paper of DUS technique; no objective criteria to estimate stenosis described (subjective visual impression of the degree of stenosis). Time between index test and alternative test was not specified |
| Barry 1987 | Less than 70% of the patients included were symptomatic |
| Bartylla 1997 | Study did not define the proportion of symptomatic patients and time between index test and alternatives tests was not specified |
| Baskett 1976 | Preliminary paper on DUS technique. Most of the included population were healthy volunteers |
| Beckett 1990 | Study did not define the proportion of symptomatic patients and time between index test and alternatives tests was not specified |
| Beebe 1999 | Study did not define the proportion of symptomatic patients.Time between index test and alternative test was more than four weeks |
| Beer 1983 | Preliminary paper of DUS technique; no objective criteria to estimate stenosis described (subjective visual impression of the degree of stenosis) |
| Beer 1986 | Preliminary paper of DUS technique; no objective criteria to estimate stenosis described (subjective visual impression of the degree of stenosis) |
| Benhamou 1984 | Preliminary paper of DUS technique; no objective criteria to estimate stenosis described (subjective visual impression of the degree of stenosis) and compared DUS results with postoperative endarterectomy specimens |
| Berger 1983 | Preliminary paper of DUS technique; no objective criteria to estimate stenosis described (subjective visual impression of the degree of stenosis) and index test was transvenous digital subtraction angiography. Study did not define the proportion of symptomatic patients |
| Berman 1995 | Time between index test and alternatives tests was not specified |
| Berry 1980 | Preliminary paper of DUS technique; no objective criteria to estimate stenosis described (subjective visual impression of the degree of stenosis). Time between index test and alternative test was not specified |
| Beutler 1985 | Study did not define the proportion of symptomatic patients and the degree of stenosis was determined by a subjective visual impression of the Doppler spectrum analysis |
| Biasi 1998 | Less than 70% of the patients included were symptomatic |
| Binaghi 2001 | Study did not define the proportion of symptomatic patients |
| Birmpili 2018 | Study did not define the proportion of symptomatic patients |
| Blackshear 1984 | No direct comparison of DUS and DSA. Compared systolic peak frequency on DUS with pressure gradient measured at operation |
| Blackshear 1985 | Study did not define the proportion of symptomatic patients |
| Blackshear 1987 | Study did not define the proportion of symptomatic patients |
| Bladin 1995 | Accuracy of DUS was not assessed |
| Blasberg 1982 | Time between index test and alternative test was more than four weeks and the study did not define the proportion of symptomatic patients |
| Bloch 1979 | Preliminary paper of DUS technique; no objective criteria to estimate stenosis described ("Audible Doppler sounds from the flowmeter were distributed to a speaker and to a stereo tape recorder. A lateral projection image of the common carotid artery and its major branches was produced with this device"). |
| Boccalon 1985 | Study did not define the proportion of symptomatic patients |
| Bone 1976 | Preliminary paper of DUS technique; no objective criteria to estimate stenosis described and time between index test and alternative test was not specified |
| Bone 1988 | Time between index test and alternative test was not specified |
| Bonig 2000 | Time between index test and alternative test was not specified |
| Boyko 2018 | DUS and other angiographic modalities were performed within 6 months |
| Boyle 1995 | Time between index test and alternative test was more than four weeks. Accuracy of duplex was assessed compared with operative findings |
| Branas 1994 | Study did not define the proportion of symptomatic patients |
| Braun 2008 | Time between index test and alternative test was more than four weeks and the study did not define the proportion of symptomatic patients |
| Browman 1995 | Study did not define the proportion of symptomatic patients |
| Bucek 2006 | Study did not define the proportion of symptomatic patients |
| Buijs 1993 | Time between index test and alternative test was more than four weeks and less than 70% of the patients included were symptomatic |
| Bulger 2005 | Time between index test and alternative test was more than four weeks and less than 70% of the patients included were symptomatic |
| Busse 1974 | Preliminary paper of DUS technique; no objective criteria to estimate stenosis described |
| Busuttil 1996 | Less than 70% of the patients included were symptomatic |
| Caes 1987 | Preliminary paper of DUS technique; no objective criteria to estimate stenosis described (subjective visual impression of the degree of stenosis). Time between index test and alternative test was not specified |
| Cape 1984 | Study did not define the proportion of symptomatic patients |
| Cappetti 1996 | Study did not define the proportion of symptomatic patients |
| Carnicelli 2013 | Study did not define the proportion of symptomatic patients and patients were included if they underwent CTA within 6 months of a DUS |
| Carpenter 1995 | Study did not define the proportion of symptomatic patients |
| Carpenter 1996 | Study did not define the proportion of symptomatic patients |
| Carroll 1989 | Time between index test and alternative test was not specified |
| Chaix 1985 | Subjective criteria to estimate stenosis on DUS and the proportion of symptomatic patients were not specified |
| Chan 1982 | Study did not define the proportion of symptomatic patients and time between index test and alternatives tests was not specified |
| Chang 1995 | Study did not define the proportion of symptomatic patients and time between exams was up to 2 months. Another sample of patients was included and time between exams was up to 6 months |
| Chang 2002 | Less than 70% of the patients included were symptomatic |
| Chen 1997 | Less than 70% of the patients included were symptomatic |
| Chen 1998 | Study did not define the proportion of symptomatic patients |
| Chervu 1994 | Less than 70% of the patients included were symptomatic |
| Chowdhury 2011 | The exact criteria for determination of the degree of stenosis was not specified |
| Clevert 2006 | Less than 70% of the patients included were symptomatic |
| Clevert 2007 | Less than 70% of the patients included were symptomatic |
| Colhoun 1984 | Study did not define the proportion of symptomatic patients |
| Collins 2005 | Time between index test and alternative test was not specified |
| Colon 1979 | Preliminary paper of DUS technique; no objective criteria to estimate stenosis described and the study did not define the proportion of symptomatic patients |
| Connolly 1985 | Time between index test and alternative test was more than four weeks and the study did not define the proportion of symptomatic patients |
| Cooperberg 1992 | Study did not define the proportion of symptomatic patients |
| Corti 1998 | Less than 70% of the patients included were symptomatic (stroke, amayrosis fugax, transient ischemic attack). The exact criteria for determination of the degree of stenosis was not specified |
| Criswell 1998 | Study did not define the proportion of symptomatic patients |
| Crummy 1979 | Preliminary paper of DUS technique; no objective criteria to estimate stenosis described and time between index test and alternative test was not specified |
| Csanyi 1993 | Study did not define the proportion of symptomatic patients and the average time between DSA was 24.3 + 21.0 days |
| Curley 1998 | Time between index test and alternative test was not specified |
| Daiss 1984 | Time between index test and alternative test was not specified |
| Dalotto 1985 | Preliminary paper of DUS technique; no objective criteria to estimate stenosis described and did not define the proportion of symptomatic patients |
| Daolio 2019 | Time between index test and alternative test was more than four weeks and less than 70% of the patients included were symptomatic |
| Dawson 1991 | Less than 70% of the patients included were symptomatic |
| Dawson 1993 | Less than 70% of the patients included were symptomatic |
| Dean 2005 | The exact criteria for determination of the degree of stenosis was not specified |
| De la Cruz Cosme 2017 | The exact criteria for determination of the degree of stenosis was not specified |
| De Monti 2003 | Study did not define the proportion of symptomatic patients |
| Dharmasaroja 2018 | Time between index test and alternative test was more than four weeks and the study did not define the proportion of symptomatic patients |
| Dilley 1986 | Study did not define the proportion of symptomatic patients |
| Dinkel 2001 | Time between index test and alternative test was not specified and it stated that most of the participants had symptomatic cerebrovascular disease, but the proportion was not described |
| Dippel 1999 | Time between index test and alternative test was more than four weeks |
| Dix 2000 | Study did not define the proportion of symptomatic patients |
| Doyle 2012 | Study did not define the proportion of symptomatic patients |
| Doyle 2014 | Study did not define the proportion of symptomatic patients |
| Drevet 1997 | Less than 70% of the patients included were symptomatic |
| Eckmann 1990 | Less than 70% of the patients included were symptomatic |
| Ellis 1996 | Time between index test and alternative test was more than four weeks and the study did not define the proportion of symptomatic patients |
| Elmore 1998 | Study did not define the proportion of symptomatic patients |
| El‐Saden 2001 | Time between index test and alternative test was more than four weeks |
| Engelhardt 2005 | Less than 70% of the patients included were symptomatic |
| Erdoes 1996 | Study did not sufficiently provide data for 2 × 2 table production |
| Erickson 1989 | Time between index test and alternative test was more than four weeks and the study did not define the proportion of symptomatic patients |
| Felber 1985 | Time between index test and alternative test was more than four weeks and no the criteria used to estimate stenosis was not described |
| Fell 1981 | Time between index test and alternative test was more than four weeks and the study did not define the proportion of symptomatic patients |
| Filis 2002 | Less than 70% of the patients included were symptomatic |
| Fillinger 1996 | Time between index test and alternative test was more than four weeks and the study did not define the proportion of symptomatic patients |
| Finkenzeller 2008 | Time between index test and alternative test was not specified |
| Fischer 1985 | Preliminary paper of DUS technique; no objective criteria to estimate stenosis described and study did not define the proportion of symptomatic patients |
| Fischer 1985a | Preliminary paper of DUS technique; no objective criteria to estimate stenosis described and study did not define the proportion of symptomatic patients |
| Fix 1984 | Preliminary paper of DUS technique; no objective criteria to estimate stenosis described |
| Flanigan 1985 | The exact criteria for determination of the degree of stenosis was not specified |
| Fragata 2006 | Time between index test and alternative test was more than four weeks |
| French‐Sherry 2016 | Study did not define the proportion of symptomatic patients |
| Friese 2001 | Study did not define the proportion of symptomatic patients |
| Fujimoto 2006 | Study did not define the proportion of symptomatic patients |
| Furst 1993 | Time accepted between index test and alternative test was more than four weeks |
| Furst 1999 | Case‐control design |
| Geidel 1991 | Time between index test and alternative test was not specified |
| Geuder 1989 | Time between index test and alternative test was not specified and the exact criteria for determination of the degree of stenosis was not specified |
| Giraldi 1986 | Evaluated patients with occlusion of the internal carotid artery for information on the collateral circles (Willis and pre‐Willis) |
| Glover 1984 | Less than 70% of the patients included were symptomatic |
| Gmelin 1985 | Time between index test and alternative test was more than four weeks and the study did not define the proportion of symptomatic patients |
| Golledge 1996 | Time accepted between index test and alternative test was more than four weeks |
| Goodson 1987 | Time between index test and alternative test was not specified and the exact criteria for determination of the degree of stenosis was not specified |
| Gortler 1994 | Accuracy was determined by comparison with the surgical specimen |
| Grajo 2007 | Time accepted between index test and alternative test was more than four weeks |
| Grant 1999 | Less than 70% of the patients included were symptomatic |
| Grant 2000 | Time between index test and alternative test was more than four weeks and the study did not define the proportion of symptomatic patients |
| Griewing 1996 | Less than 70% of the patients included were symptomatic |
| Griffiths 1998 | Time between index test and alternative test was not specified and the exact criteria for determination of the degree of stenosis was not specified. |
| Griffiths 2001 | Time between index test and alternative test was not specified and the exact criteria for determination of the degree of stenosis was not specified |
| Hames 1981 | Preliminary paper of DUS technique; no objective criteria to estimate stenosis described |
| Hames 1985 | Preliminary paper of DUS technique; no objective criteria to estimate stenosis described |
| Harward 1986 | Time between index test and alternative test was more than four weeks and the study did not define the proportion of symptomatic patients |
| Hathout 2005 | The average time interval between sonography and arteriography was 2 months and the study did not define the proportion of symptomatic patients |
| Hathout 2015 | Time between index test and alternative test was more than four weeks and the study did not define the proportion of symptomatic patients |
| Herring 1984 | Study did not define the proportion of symptomatic patients |
| Hetzel 1993 | Time between index test and alternative test was more than four weeks and the study did not define the proportion of symptomatic patients |
| Hjelmgren 2018 | Evaluated non‐stenotic carotid plaques |
| Hobson 1980 | Preliminary paper of DUS technique; no objective criteria to estimate stenosis described |
| Honish 2005 | Time between index test and alternative test was not specified |
| Horrocks 1979 | Preliminary paper of DUS technique; no objective criteria to estimate stenosis described |
| Howard 1991 | Study did not define the proportion of symptomatic patients |
| Humphrey 1990 | Time between index test and alternative test was not specified |
| Hunink 1993 | Study did not define the proportion of symptomatic patients |
| Huston 1998 | Study did not define the proportion of symptomatic patients |
| Huston 2000 | Study did not define the proportion of symptomatic patients |
| Hutchison 1985 | Preliminary paper of DUS technique; no objective criteria to estimate stenosis described. Time between index test and alternative test was more than four weeks and the study did not define the proportion of symptomatic patients |
| Hwang 2002 | Study did not define the proportion of symptomatic patients |
| Hwang 2003 | Study did not define the proportion of symptomatic patients |
| Hwang 2003a | Time between index test and alternative test was more than four weeks and the study did not define the proportion of symptomatic patients |
| Jackson 1985 | Less than 70% of the patients included were symptomatic |
| Jackson 1998 | Less than 70% of the patients included were symptomatic |
| Jacobs 1985 | Study did not define the proportion of symptomatic patients |
| Jogestrand 2002 | Time between index test and alternative test was more than four weeks and study did not provide enough data for construction of a 2 x 2 table |
| Johnson 2000 | Time between index test and alternative test was not specified |
| Johnston 1982 | Less than 70% of the patients included were symptomatic |
| Johnston 1985 | Study did not define the proportion of symptomatic patients |
| Johnston 2001 | Less than 70% of the patients included were symptomatic |
| Jones 1982 | Preliminary paper of DUS technique; no objective criteria to estimate stenosis described |
| Juhel 1983 | Preliminary paper of DUS technique; no objective criteria to estimate stenosis described |
| Jung 2000 | Less than 70% of the patients included were symptomatic |
| Jung 2002 | Less than 70% of the patients included were symptomatic |
| Kagawa 1996 | Less than 70% of the patients included were symptomatic |
| Keberle 2001 | Study did not define the proportion of symptomatic patients |
| Keller 1978 | Preliminary paper of DUS technique; no objective criteria to estimate stenosis described and the study did not define the proportion of symptomatic patients |
| Kim 2016 | Time between index test and alternative test was more than four weeks |
| Kim 2018 | Time between index test and alternative test was more than four weeks |
| Kirsch 1994 | Study did not define the proportion of symptomatic patients |
| Knox 1982 | Study did not define the proportion of symptomatic patients and time accepted between index test and alternative test was more than four weeks |
| Koga 1983 | Less than 70% of the patients included were symptomatic |
| Koga 2001 | Study did not define the proportion of symptomatic patients |
| Korteweg 2008 | Time between index test and alternative test was more than four weeks |
| Krappel 2002 | Study did not define the proportion of symptomatic patients |
| Krasinski 2009 | Only included subjects without hemodynamically significant carotid stenosis and did not describe if they are symptomatic or asymptomatic. The objective was to evaluate potential spatial differences in carotid atherosclerosis measured using 3D MR and US |
| Kreske 1999 | Study did not define the proportion of symptomatic patients |
| Kuhn 1981 | Preliminary paper of DUS technique; no objective criteria to estimate stenosis described and study did not define the proportion of symptomatic patients |
| Kuhn 1984 | Less than 70% of the patients included were symptomatic |
| Labropoulos 1997 | Study did not define the proportion of symptomatic patients |
| Langlois 1983 | Study did not define the proportion of symptomatic patients |
| Lee 1992 | Study did not define the proportion of symptomatic patients |
| Lee 1996 | Time between index test and alternative test was more than four weeks |
| Lefemine 1986 | Preliminary paper of DUS technique and the study did not supply information on accuracy data |
| Leonardo 2003 | Less than 70% of the patients included were symptomatic |
| Levien 1985 | Time between index test and alternative test was not specified |
| Lewis 1980 | Preliminary paper of DUS technique; no objective criteria to estimate stenosis described |
| Lewis 2002 | Time between index test and alternative test was more than four weeks and study did not provide enough data for construction of a 2 x 2 table |
| Lindegaard 1984 | Time between index test and alternative test was not specified |
| Link 1997a | Time between index test and alternative test was not specified |
| Long 2001 | Less than 70% of the patients included were symptomatic |
| Lovelock 2003 | Study did not define the proportion of symptomatic patients |
| Ludwig 1984 | Preliminary paper of DUS technique; no objective criteria to estimate stenosis described. Time between index test and alternative test was not described and the study did not define the proportion of symptomatic patients |
| Lusby 1981 | Preliminary paper of DUS technique; no objective criteria to estimate stenosis described (subjective visual impression of the degree of stenosis). Time between index test and alternative test was not specified |
| Macharzina 2018 | Less than 70% of the patients included were symptomatic |
| Macheers 1986 | Study did not define the proportion of symptomatic patients |
| MacKenzie 2002 | Study did not define the proportion of symptomatic patients |
| Makaryus 2009 | Less than 70% of the patients included were symptomatic |
| Manga 1986 | Study did not define the proportion of symptomatic patients |
| Mansour 1995 | Less than 70% of the patients included were symptomatic |
| Marshall 1988 | Less than 70% of the patients included were symptomatic |
| Martin‐Conejero 2007 | Less than 70% of the patients included were symptomatic |
| Matos 2014 | Less than 70% of the patients included were symptomatic and time between index test and alternative test was more than four weeks |
| Mattle 1991 | Time between index test and alternative test was not specified and did not define the proportion of symptomatic patients |
| Mattos 1992 | Study did not define the proportion of symptomatic patients |
| Mattos 1994 | Less than 70% of the patients included were symptomatic |
| Matz 2017 | Time between index test and alternative test was not specified and did not define the proportion of symptomatic patients |
| McLaren 1996 | Study did not define the proportion of symptomatic patients |
| Mitchell 1991 | Time between index test and alternative test was not specified and did not define the proportion of symptomatic patients |
| Mittl 1994 | Time between index test and alternative test was not specified |
| Modaresi 1999 | Study did not define the proportion of symptomatic patients |
| Moll 2000 | Time between index test and alternative test was not specified and did not define the proportion of symptomatic patients |
| Moll 2001 | Time between index test and alternative test was not specified and did not define the proportion of symptomatic patients |
| Moneta 1993 | Study did not define the proportion of symptomatic patients |
| Moore 1986 | Study did not define the proportion of symptomatic patients |
| Moore 1988 | Preliminary paper of DUS technique; no objective criteria to estimate stenosis described. The study did not define the proportion of symptomatic patients |
| Muller 2015 | Study did not define the proportion of symptomatic patients |
| Murie 1984 | Preliminary paper of DUS technique; no objective criteria to estimate stenosis described. Time between index test and alternative test was more than four weeks |
| Muto 1996 | Less than 70% of the patients included were symptomatic |
| Neale 1994 | Study did not define the proportion of symptomatic patients |
| Neff 2005 | Study did not define the proportion of symptomatic patients |
| Neschis 2001 | Study did not define the proportion of symptomatic patients |
| New 2001 | Study did not define the proportion of symptomatic patients and time between index test and alternative test was more than four weeks |
| Nichtweiss 1987 | Time between index test and alternative test was not specified and the method of calculating the carotid stenosis was not described |
| Nonent 2004 | Less than 70% of the patients included were symptomatic |
| Nonent 2011 | Study did not define the proportion of symptomatic patients |
| Nordal 1993 | Time between index test and alternative test was not specified and the method of calculating the carotid stenosis was not described |
| Norrving 1981 | Preliminary paper of DUS technique; no objective criteria to estimate stenosis described. Time between index test and alternative test was not specified |
| Norrving 1985 | Study did not define the proportion of symptomatic patients and time between index test and alternative test was not specified |
| Nowak 2007 | Same patients from Jogestrand 2002. Time between index test and alternative test was more than four weeks |
| O'Callaghan 2011 | Study did not define the proportion of symptomatic patients |
| O'Leary 1987 | Study did not define the proportion of symptomatic patients |
| Ohm 2005 | Less than 70% of the patients included were symptomatic |
| Orgles 1999 | Study did not define the proportion of symptomatic patients |
| Paciaroni 2003 | The exact criteria for determination of the degree of stenosis was not specified |
| Padayachee 1982 | Less than 70% of the patients included were symptomatic |
| Padayachee 1997 | Study did not define the proportion of symptomatic patients |
| Paivansalo 1996 | Time between index test and alternative test was more than four weeks |
| Patel 1995 | Less than 70% of the patients included were symptomatic ("There were 74 symptomatic carotid bifurcations (42%)") |
| Patel 2002 | Time accepted between index test and alternative test was more than four weeks ("The median time lapse between DUS and the other three imaging techniques was 33 days (range 27 to 185 days)") |
| Pelz 2015 | Less than 70% of the patients included were symptomatic |
| Petisco 2015 | Less than 70% of the patients included were symptomatic |
| Pfister 2009 | Study did not define the proportion of symptomatic patients |
| Poindexter 1991 | Less than 70% of the patients included were symptomatic |
| Polak 1989 | Study did not define the proportion of symptomatic patients |
| Polak 1992 | Less than 70% of the patients included were symptomatic |
| Polak 1993 | MRA and DUS were used in combination. There was no DUS alone accuracy data and time between index test and alternative test was more than four weeks |
| Portilla 2010 | Study did not define the proportion of symptomatic patients |
| Puzich 1986 | Preliminary paper of DUS technique; no objective criteria to estimate stenosis described |
| Py 2001 | Time between index test and alternative test was more than four weeks |
| Qureshi 2001 | Study did not define the proportion of symptomatic patients |
| Ratliff 1985 | Study did not define the proportion of symptomatic patients |
| Ricotta 1987 | Study did not define the proportion of symptomatic patients |
| Riles 1992 | Study did not define the proportion of symptomatic patients |
| Rodrigus 1995 | Study did not define the proportion of symptomatic patients |
| Saba 2008 | Study did not define the proportion of symptomatic patients |
| Saba 2010 | Study did not define the proportion of symptomatic patients |
| Sabeti 2004 | Less than 70% of the patients included were symptomatic |
| Saia 1981 | Preliminary paper of DUS technique; no objective criteria to estimate stenosis described. Study did not define the proportion of symptomatic patients |
| Samarzija 2018 | Study did not define the proportion of symptomatic patients and time between index test and alternative test was more than four weeks |
| Sameshima 1999 | Study did not provide the method of calculating the degree of stenosis and time between index test and alternative test was not specified |
| Saouaf 1998 | Less than 70% of the patients included were symptomatic |
| Satiani 1988 | Study did not define the proportion of symptomatic patients |
| Savic 2010 | Time between index test and alternative test was not specified |
| Senant 1984 | Preliminary paper of DUS technique; no objective criteria to estimate stenosis described |
| Serfaty 2000 | Less than 70% of the patients included were symptomatic |
| Shaalan 2008 | Less than 70% of the patients included were symptomatic |
| Shakhnovich 2010 | Study did not define the proportion of symptomatic patients |
| Sillesen 1988 | Preliminary paper of DUS technique; no objective criteria to estimate stenosis described. Study did not define the proportion of symptomatic patients |
| Sillesen 1991 | Less than 70% of the patients included were symptomatic |
| Sitzer 1993 | Criteria to determine carotid stenosis was not based on velocity criteria and time between tests was not described |
| Slovut 2010 | Study did not define the proportion of symptomatic patients |
| Soulez 1999 | Study did not define the proportion of symptomatic patients |
| Srinivasan 1995 | Time between index test and alternative test was not specified |
| Staikov 2000 | Time between index test and alternative test was more than four weeks |
| Staikov 2002 | Study did not define the proportion of symptomatic patients |
| Staikov 2004 | Time between index test and alternative test was more than four weeks |
| Stavenow 1987 | Less than 70% of the patients included were symptomatic |
| Stefanini 2012 | Time between index test and alternative test was more than four weeks |
| Steger 1995 | Less than 70% of the patients included were symptomatic |
| Steinke 1990 | Less than 70% of the patients included were symptomatic |
| Steinke 1997 | Less than 70% of the patients included were symptomatic |
| Sumner 1979 | Preliminary paper of DUS technique; no objective criteria to estimate stenosis described |
| Sumner 1982 | Less than 70% of the patients included were symptomatic |
| Tarnawski 1990 | Validation of MRA technique using a pulsatile phantom and in vivo healthy asymptomatic subjects |
| Tateishi 2013 | Study did not define the proportion of symptomatic patients |
| Tian 2016 | Study did not define the proportion of symptomatic patients |
| Titi 2007 | Time between index test and alternative test was not specified |
| Tokunaga 2016 | Time between index test and alternative test was not specified |
| Tola 2004 | Asymptomatic patients |
| Torvaldsen 1985 | Less than 70% of the patients included were symptomatic |
| Tschammler 1991 | Study did not define the proportion of symptomatic patients and time between index test and alternative test was not specified |
| Turnipseed 1982 | Preliminary paper of DUS technique; no objective criteria to estimate stenosis described. |
| Turnipseed 1993a | Time between index test and alternative test was not specified |
| Utz 1983 | Study did not define the proportion of symptomatic patients |
| Vaisman 1986 | Study did not define the proportion of symptomatic patients and time between index test and alternative test was not specified |
| Van Prehn 2008 | Study did not define the proportion of symptomatic patients |
| Vit 2003 | Study did not define the proportion of symptomatic patients |
| Von Arbin 1983 | Preliminary paper of DUS technique; no objective criteria to estimate stenosis described. Study did not define the proportion of symptomatic patients |
| Wardlaw 2005 | Time between index test and alternative test was not specified |
| Weaver 1980 | Preliminary paper of DUS technique; no objective criteria to estimate stenosis described (subjective visual impression of the degree of stenosis). Study did not define the proportion of symptomatic patients |
| Weaver 1980a | Preliminary paper of DUS technique; no objective criteria to estimate stenosis described (subjective visual impression of the degree of stenosis). Study did not define the proportion of symptomatic patients |
| Weintraub 1985 | Preliminary paper of DUS technique; no objective criteria to estimate stenosis described and compared DUS results with postoperative endarterectomy specimens. Study did not provide enough data for construction of a 2 x 2 table |
| Wessels 2004 | Less than 70% of the patients included were symptomatic |
| Wetzner 1984 | Study did not define the proportion of symptomatic patients |
| Wikstrom 2002 | Study did not define the proportion of symptomatic patients and time between index test and alternative test was not specified |
| Wilkerson 1991 | Less than 70% of the patients included were symptomatic |
| Wilterdink 1996 | Study did not define the proportion of symptomatic patients and time between index test and alternative test was not specified |
| Winkelaar 1999 | Study did not define the proportion of symptomatic patients |
| Withers 1990 | Study did not define the proportion of symptomatic patients and time between index test and alternative test was not specified |
| Wolverson 1983 | Preliminary paper of DUS technique; no objective criteria to estimate stenosis described |
| Wolverson 1985 | Preliminary paper of DUS technique; no objective criteria to estimate stenosis described |
| Worthy 1997 | Time between index test and alternative test was not specified |
| Yiu‐Tong 1985 | Less than 70% of the patients included were symptomatic |
| Young 1992 | Time between index test and alternative test was not specified |
| Young 1994 | Did not use a valid method for determining the degree of stenosis on DSA. (quote: "We have relied on experienced radiologists reporting their visual impression of the degree of stenosis present, as we believe that this is the method most commonly used in routine clinical practice.") |
| Yurdakul 2004 | Study did not define the proportion of symptomatic patients and time between index test and alternative test was not specified |
| Yurdakul 2004a | Asymptomatic patients |
| Zananiri 1993 | Asymptomatic patients |
| Zanette 1982 | Preliminary paper of DUS technique; no objective criteria to estimate stenosis described |
| Zanette 1987 | Preliminary paper of DUS technique; no objective criteria to estimate stenosis described |
| Zierler 1990 | Asymptomatic patients |
| Zorzon 1987 | Study did not define the proportion of symptomatic patients |
| Zwicker 1987 | Less than 70% of the patients included were symptomatic |
| Zwiebel 1983 | Less than 70% of the patients included were symptomatic |
| Zwiebel 1985 | Less than 70% of the patients included were symptomatic |
CTA: computed tomography angiography DSA: digital subtraction angiography MR: magnetic resonance MRA: magnetic resonance angiography US: ultrasound
Differences between protocol and review
The International Network of Agencies for Health Technology Assessment has taken responsibility for the HTA database, previously managed by the Centre for Reviews and Dissemination (CRD). This was previously done by the Centre for Reviews and Dissemination (CRD).
We aimed only to include participants with symptomatic carotid stenosis but accepted studies in which at least 70% of included participants were symptomatic.
We removed from QUADAS‐2 the question ‘Was the person conducting the test (DUS) sufficiently trained?’ in the index test domain.
We were unable to perform all the sensitivity and subgroup analyses and meta‐regressions we had originally planned and detailed in the protocol, due to the small number of included studies.
Sensitivity analyses have been added to explore the effects of excluding studies that only included patients with known occlusion on DUS.
Two authors joined the review team after the protocol was published (LCUN and NCJ) and contributed to the final version of the review.
Contributions of authors
NC, JCCBS, RS, VV, and RLGF designed the review, registered the review title, and contributed to the design of the review. NC, CDQF, and RLGF developed the search strategies, with additional input from Joshua David Cheyne, Cochrane Stroke Information Specialist. NC, CDQF, and LCUN screened papers against eligibility criteria and appraised the quality of the papers. NC, RLGF, and NCJ extracted study data and performed and interpreted analyses. NC wrote the first draft of the review with contributions from RLGF. All authors reviewed and approved the protocol content prior to submission.
Sources of support
Internal sources
No sources of support provided
External sources
-
Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil
This study was financed in part by CAPES, finance code 001.
Declarations of interest
NC: none known.
JCCBS: none known.
CDQF: none known.
RS: none known.
VV: none known.
NCJ: none known.
LCUN: none known.
RLGF: none known.
New
References
References to studies included in this review
Anzidei 2012 {published data only}
- Anzidei M, Napoli A, Zaccagna F, Di Paolo P, Saba L, Cavallo Marincola B, et al. Diagnostic accuracy of colour Doppler ultrasonography, CT angiography and blood-pool-enhanced MR angiography in assessing carotid stenosis: a comparative study with DSA in 170 patients. La Radiologia Medica 2012;117(1):54-71. [DOI] [PubMed] [Google Scholar]
Barlinn 2016 {published data only}
- Barlinn K, Floegel T, Kitzler HH, Kepplinger J, Siepmann T, Pallesen L, et al. Diagnostic accuracy of CT-angiography for internal carotid artery stenosis - comparison with a revisited multiparametric ultrasound approach. International Journal of Stroke 2015;10 Suppl 2:303. [Google Scholar]
- Barlinn K, Floegel T, Kitzler HH, Kepplinger J, Siepmann T, Pallesen L, et al. Diagnostic accuracy of CT-angiography for internal carotid artery stenosis - comparison with a revisited multiparametric ultrasound approach. Stroke 2015;46 Suppl 1:MP21. [Google Scholar]
- Barlinn K, Floegel T, Kitzler HH, Kepplinger J, Siepmann T, Pallesen L-P, et al. Multi-parametric ultrasound criteria for internal carotid artery disease - comparison with CT angiography. Neuroradiology 2016;58(9):845-51. [DOI] [PubMed] [Google Scholar]
Belsky 2000 {published data only}
- Belsky M, Gaitini D, Goldsher D, Hoffman A, Daitzchman M. Color-coded duplex ultrasound compared to CT angiography for detection and quantification of carotid artery stenosis. European Journal of Ultrasound 2000;12(1):49-60. [DOI] [PubMed] [Google Scholar]
Borisch 2003 {published data only}
- Borisch I, Horn M, Butz B, Zorger N, Draganski B, Hoelscher T, et al. Preoperative evaluation of carotid artery stenosis: comparison of contrast-enhanced MR angiography and duplex sonography with digital subtraction angiography. American Journal of Neuroradiology 2003;24(6):1117-22. [PMC free article] [PubMed] [Google Scholar]
Bray 1995 {published data only}
- Bray JM, Galland F, Lhoste P, Nicolau S, Dubas F, Emile J, et al. Colour Doppler and duplex sonography and angiography of the carotid artery bifurcations. Prospective, double-blind study. Neuroradiology 1995;37(3):219-24. [DOI: 10.1007/BF01578261] [DOI] [PubMed] [Google Scholar]
Chua 2007 {published data only}
- Chua H-C, Sitoh Y-Y, Earnest A, Venketasubramanian N. Detection of internal carotid artery stenosis with duplex velocity criteria using receiver operating characteristic analysis. Annals of the Academy of Medicine Singapore 2007;36(4):247-52. [PubMed] [Google Scholar]
- Chua HC, Venketasubramanian N, Teo BC, Sitoh YY. A comparative study of duplex ultrasonography versus angiography in detection of extracranial carotid stenosis in Singapore. Stroke 2004;35(6):E257. [Google Scholar]
Colquhoun 1992 {published data only}
- Colquhoun I, Oates CP, Martin K, Hall K, Whittingham TA. The assessment of carotid and vertebral arteries - a comparison of cfm duplex ultrasound with intravenous digital subtraction angiography. British Journal of Radiology 1992;65(780):1069-74. [DOI] [PubMed] [Google Scholar]
Cui 2018 {published data only}
- Cui H, Yan R, Zhai Z, Ren J, Li Z, Li Q, et al. Comparative analysis of 3D time-resolved contrast-enhanced magnetic resonance angiography, color doppler ultrasound and digital subtraction angiography in symptomatic carotid stenosis. Experimental and Therapeutic Medicine 2018;15(2):1654-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
D'Onofrio 2006 {published data only}
- D'Onofrio M, Mansueto G, Faccioli N, Guarise A, Tamellini P, Bogina G, et al. Doppler ultrasound and contrast-enhanced magnetic resonance angiography in assessing carotid artery stenosis. La Radiologia Medica 2006;111(1):93-103. [DOI: 10.1007/s11547-006-0010-y] [DOI] [PubMed] [Google Scholar]
Das 2009 {published data only}
- Das M, Muhlenbruch G, Mahnken AH, Krings T, Langer S, Schaaf M, et al. Dual source CT angiography of carotid stenoses in comparison to MR angiography and duplex sonography. Gefasschirurgie 2009;14(6):500-4. [Google Scholar]
Eliasziw 1995 {published data only}
- Eliasziw M, Rankin RN, Fox AJ, Haynes RB, Barnett HJ. Accuracy and prognostic consequences of ultrasonography in identifying severe carotid artery stenosis. North American Symptomatic Carotid Endarterectomy Trial (NASCET) Group. Stroke 1995;26(10):1747-52. [DOI: 10.1161/01.str.26.10.1747] [DOI] [PubMed] [Google Scholar]
Faught 1994 {published data only}
- Faught WE, Mattos MA, Vanbemmelen PS, Hodgson KJ, Barkmeier LD, Ramsey DE, et al. Color-flow duplex scanning of carotid arteries - new velocity criteria-based on receiver operator characteristic analysis for threshold stenoses used in the symptomatic and asymptomatic carotid trials. Journal of Vascular Surgery 1994;19(5):818-28. [DOI: 10.1016/s0741-5214(94)70006-0] [DOI] [PubMed] [Google Scholar]
Golledge 1999 {published data only}
- Golledge J, Ellis M, Sabharwal T, Sikdar T, Davies AH, Greenhalgh RM. Selection of patients for carotid endarterectomy. Journal of Vascular Surgery 1999;30(1):122-30. [DOI: 10.1016/s0741-5214(99)70184-9] [DOI] [PubMed] [Google Scholar]
Hammond 2008 {published data only}
- Hammond CJ, McPherson SJ, Patel JV, Gough MJ. Assessment of apparent internal carotid occlusion on ultrasound: prospective comparison of contrast-enhanced ultrasound, magnetic resonance angiography and digital subtraction angiography. European Journal of Vascular and Endovascular Surgery 2008;35(4):405-12. [DOI: 10.1016/j.ejvs.2007.12.008] [DOI] [PubMed] [Google Scholar]
Hansen 1996 {published data only}
- Hansen F, Bergqvist D, Lindblad B, Lindh M, Matzsch T, Lanne T. Accuracy of duplex sonography before carotid endarterectomy - a comparison with angiography. European Journal of Vascular and Endovascular Surgery 1996;12(3):331-6. [DOI: 10.1016/s1078-5884(96)80252-8] [DOI] [PubMed] [Google Scholar]
Heijenbrok‐Kal 2006 {published data only}
- Heijenbrok-Kal MH, Buskens E, Nederkoorn PJ, Van der Graaf Y, Hunink MGM. Optimal peak systolic velocity threshold at duplex us for determining the need for carotid endarterectomy: a decision analytic approach. Radiology 2006;238(2):480-8. [DOI: 10.1148/radiol.2381041078] [DOI] [PubMed] [Google Scholar]
Huston 1993 {published data only}
- Huston J 3rd, Lewis BD, Wiebers DO, Meyer FB, Riederer SJ, Weaver AL. Carotid artery: prospective blinded comparison of two-dimensional time-of-flight MR angiography with conventional angiography and duplex US. Radiology 1993;186(2):339-44. [DOI: 10.1148/radiology.186.2.8421731] [DOI] [PubMed] [Google Scholar]
Knudsen 2002 {published data only}
- Knudsen L, Johansen A, Justesen P, Jorgensen HB. Accuracy of duplex scan of internal carotid arteries. European Journal of Vascular and Endovascular Surgery 2002;24(1):86-7. [DOI: 10.1053/ejvs.2002.1659] [DOI] [PubMed] [Google Scholar]
Link 1997 {published data only}
- Link J, Brossmann J, Penselin V, Gluer CC, Heller M. Common carotid artery bifurcation: preliminary results of CT angiography and color-coded duplex sonography compared with digital subtraction angiography. American Journal of Roentgenology 1997;168(2):361-5. [DOI: 10.2214/ajr.168.2.9016207] [DOI] [PubMed] [Google Scholar]
Lubezky 1998 {published data only}
- Lubezky N, Fajer S, Barmeir E, Karmeli R. Duplex scanning and CT angiography in the diagnosis of carotid artery occlusion: a prospective study. European Journal of Vascular and Endovascular Surgery 1998;16(2):133-6. [DOI: 10.1016/s1078-5884(98)80154-8] [DOI] [PubMed] [Google Scholar]
Nederkoorn 2002 {published data only}
- Nederkoorn PJ, Kappelle LJ, Van der Graaf Y, Hunink MG, Eikelboom BC, Mall WP. Duplex ultrasound, MR angiography and conventional angiography in carotid artery disease: interpretation of separate test results and their combinations. Stroke 2001;32(1):335. [Google Scholar]
- Nederkoorn PJ, Mali WP, Eikelboom BC, Elgersma OE, Buskens E, Hunink MG, et al. Preoperative diagnosis of carotid artery stenosis: accuracy of noninvasive testing. Stroke 2002;33(8):2003-8. [DOI: 10.1161/01.str.0000021900.58396.44] [DOI] [PubMed] [Google Scholar]
- Nederkoorn PJ, Mali WPTHM, Elgersma OEH, Buskens E, Hunink MGM, Eikelboom BC, et al. Non-invasive imaging in symptomatic A-carotis stenosis: adequate diagnostic value and cost-effectiveness with the combination of duplex and magnetic resonance angiography [Non-invasieve beeldvorming bij symptomatische A-carotisstenose: goedediagnostische waarde en kosteneffectiviteit bij de combinatie vanduplexonderzoek en magnetischeresonantieangiografie (MRA)]. Nederlands Tijdschrift voor Geneeskunde 2003;147(8):345-50. [Google Scholar]
Wolfle 2002 {published data only}
- Wolfle KD, Schnur C, Pfadenhauer K, Bruijnen H, Bohndorf K, Loeprecht H. MR-angiography and duplex-ultrasonography: predictive reliability for angiographically determined internal carotid artery stenosis >/= 70-99%. Zentralblatt fur Chirurgie 2002;127(2):81-8. [DOI: 10.1055/s-2002-22031] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
AbuRahma 1995 {published data only}
- AbuRahma AF, Richmond BK, Robinson PA, Khan S, Pollack JA, Alberts S. Effect of contralateral severe stenosis or carotid occlusion on duplex criteria of ipsilateral stenoses: comparative study of various duplex parameters. Journal of Vascular Surgery 1995;22(6):751-2. [DOI] [PubMed] [Google Scholar]
AbuRahma 1997 {published data only}
- AbuRahma AF, Pollack JA, Robinson PA, Mullins D. The reliability of color duplex ultrasound in diagnosing total carotid artery occlusion. American Journal of Surgery 1997;174(2):185-7. [DOI] [PubMed] [Google Scholar]
AbuRahma 1998 {published data only}
- AbuRahma AF, Robinson PA, Strickler DL, Alberts S, Young L. Proposed new duplex classification for threshold stenoses used in various symptomatic and asymptomatic carotid endarterectomy trials. Annals of Vascular Surgery 1998;12(4):349-58. [DOI] [PubMed] [Google Scholar]
AbuRahma 2011 {published data only}
- AbuRahma AF, Srivastava M, Stone PA, Mousa AY, Jain A, Dean LS, et al. Critical appraisal of the carotid duplex consensus criteria in the diagnosis of carotid artery stenosis. Journal of Vascular Surgery 2011;53(1):53-60. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ackerstaff 1982 {published data only}
- Ackerstaff RA, Eikelboom BC, Hoeneveld H, Moll FL, Devries AR. The accuracy of ultrasonic duplex scanning in carotid-artery disease. Clinical Neurology and Neurosurgery 1982;84(4):213-20. [DOI] [PubMed] [Google Scholar]
Ackroyd 1984 {published data only}
- Ackroyd N, Lane R, Dart L, Appleberg M. Colour-coded carotid Doppler imaging: an angiographic comparison of 324 bifurcations. Australian and New Zealand Journal of Surgery 1984;54(6):509-17. [DOI] [PubMed] [Google Scholar]
Adiga 1984 {published data only}
- Adiga KR, Fresso SJ, Nayden J. Noninvasive methods in the diagnosis of extracranial carotid artery disease: a correlation with carotid arteriography in eighty patients. Angiology 1984;35(6):331-40. [DOI] [PubMed] [Google Scholar]
Alexandrov 1993 {published data only}
- Alexandrov AV, Bladin CF, Maggisano R, Norris JW. Measuring carotid stenosis - time for a reappraisal. Stroke 1993;24(9):1292-6. [DOI] [PubMed] [Google Scholar]
Alexandrov 1997a {published data only}
- Alexandrov AV, Brodie DS, McLean A, Hamilton P, Murphy J, Burns PN. Correlation of peak systolic velocity and angiographic measurement of carotid stenosis revisited. Stroke 1997;28(2):339-42. [DOI] [PubMed] [Google Scholar]
Alexandrov 1997b {published data only}
- Alexandrov AV, Vital D, Brodie DS, Hamilton P, Grotta JC. Grading carotid stenosis with ultrasound - an interlaboratory comparison. Stroke 1997;28(6):1208-10. [DOI] [PubMed] [Google Scholar]
Alves 1982 {published data only}
- Alves AM, Sterensis JB. Correlation between ultrasonic Doppler arterial scanning of the extracranial circulation and cerebral angiography. Vascular Surgery 1982;16(2):106-10. [Google Scholar]
Alves 1983 {published data only}
- Alves AM, Sterensis JB. Noninvasive versus invasive evaluation of the extracranial circulation in patients with suspected cerebral vascular disease. Angiology 1983;34(3):183-91. [DOI] [PubMed] [Google Scholar]
Ammar 2017 {published data only}
- Ammar CP, Helmer SD, Ammar AD. Carotid duplex ultrasonography: additional imaging is rarely necessary for appropriate treatment planning for carotid artery disease. American Surgeon 2017;83(4):377-80. [PubMed] [Google Scholar]
Anderson 1983 {published data only}
- Anderson DC, Loewenson R, Yock D, Farber R, Larson D, Bromer M. B-mode, real-time carotid ultrasonic imaging. Correlation with angiography. Archives of Neurology 1983;40(8):484-8. [DOI] [PubMed] [Google Scholar]
Anderson 2000 {published data only}
- Anderson GB, Ashforth R, Steinke DE, Ferdinandy R, Findlay JM. CT angiography for the detection and characterization of carotid artery bifurcation disease. Stroke 2000;31(9):2168-74. [DOI] [PubMed] [Google Scholar]
Appleberg 1982 {published data only}
- Appleberg M, Chambers JL, Lane R. A comparison of non-invasive methods in the assessment of extracranial carotid artery disease. Australian and New Zealand Journal of Surgery 1982;52(3):258-64. [DOI] [PubMed] [Google Scholar]
Arbeille 1984 {published data only}
- Arbeille P, Lapierre F, Patat F, Benhamou AC, Alison D, Dusorbier C, et al. Evaluation of the degree of carotid stenosis by spectral analysis of the Doppler signal. Comparison of the results of spectral analysis, angiography and anatomo-pathology. Archives des Maladies du Coeur et des Vaisseaux 1984;77(10):1097-107. [PubMed] [Google Scholar]
Arbeille 1997 {published data only}
- Arbeille P, Bouin-Pineau M, Chambrier P, Boulay J, Porcher M, Tondereau G, et al. A comparative study of the Doppler methods for evaluation of the degree of carotid artery stenosis. Continuous, pulsed, color Doppler. Archives des Maladies du Coeur et des Vaisseaux 1997;90(1):41-50. [PubMed] [Google Scholar]
Archie 1981 {published data only}
- Archie JP Jr. A simple, non-dimensional, normalized common carotid Doppler velocity wave-form index that identifies patients with carotid stenosis. Stroke 1981;12(3):322-4. [DOI] [PubMed] [Google Scholar]
Arous 2019 {published data only}
- Arous EJ, Judelson DR, Malka KT, Wyman AS, Simons JP, Aiello FA, et al. Carotid duplex velocity criteria recommended by the Society of Radiologists in Ultrasound and endorsed by the Intersocietal Accreditation Commission lack predictive ability for identifying high-grade carotid artery stenosis. Annals of Vascular Surgery 2019;61:227-32. [DOI] [PubMed] [Google Scholar]
Auffray‐Calvier 1996 {published data only}
- Auffray-Calvier E, De Kersaint-Gilly A, Desal HA, Viarouge MP, Havet T. Can carotid stenosis be operated without arteriography? Contribution of MR and helical CT angiography. Journal d'Echographie et de Medecine par Ultrasons 1996;17(4-5):243-55. [Google Scholar]
Azieva 2016 {published data only}
- Azieva AZ, Dudanov IP, Kozlov KL, Ordynets SV, Abuazab BS, Stafeeva IV, et al. Bilateral stenoses of carotids. The surgical treatment in acute period of ischemic stroke in patients of different age groups. Advances in Gerontology 2016;29(5):737-41. [PubMed] [Google Scholar]
Back 2000 {published data only}
- Back MR, Wilson JS, Rushing G, Stordahl N, Linden C, Johnson BL, et al. Magnetic resonance angiography is an accurate imaging adjunct to duplex ultrasound scan in patient selection for carotid endarterectomy. Journal of Vascular Surgery 2000;32(3):429-38. [DOI] [PubMed] [Google Scholar]
Back 2003 {published data only}
- Back MR, Rogers GA, Wilson JS, Johnson BL, Shames ML, Bandyk DF. Magnetic resonance angiography minimizes need for arteriography after inadequate carotid duplex ultrasound scanning. Journal of Vascular Surgery 2003;38(3):422-31. [DOI] [PubMed] [Google Scholar]
Bain 1998 {published data only}
- Bain DJ, Fergie N, Quin RO, Greene M. Role of arteriography in the selection of patients for carotid endarterectomy. British Journal of Surgery 1998;85(6):768-70. [DOI] [PubMed] [Google Scholar]
Ballard 1994 {published data only}
- Ballard JL, Fleig K, Delange M, Killeen JD. The diagnostic-accuracy of duplex ultrasonography for evaluating carotid bifurcation. American Journal of Surgery 1994;168(2):123-6. [DOI] [PubMed] [Google Scholar]
Ballard 1997 {published data only}
- Ballard JL, Deiparine MK, Bergan JJ, Bunt TJ, Killeen JD, Smith LL. Cost-effective evaluation and treatment for carotid disease. Archives of Surgery 1997;132(3):268-71. [DOI] [PubMed] [Google Scholar]
Ballotta 1999 {published data only}
- Ballotta E, Da Giau G, Abbruzzese E, Saladini M, Renon L, Scannapieco G, et al. Carotid endarterectomy without angiography: can clinical evaluation and duplex ultrasonographic scanning alone replace traditional arteriography for carotid surgery workup? A prospective study. Surgery 1999;126(1):20-7. [DOI] [PubMed] [Google Scholar]
Bandyk 1985 {published data only}
- Bandyk DF, Levine AW, Pohl L, Towne JB. Classification of carotid bifurcation disease using quantitative Doppler spectrum analysis. Archives of Surgery 1985;120(3):306-14. [DOI] [PubMed] [Google Scholar]
Barlinn 2018 {published data only}
- Barlinn K, Rickmann H, Kitzler H, Krogias C, Strohm H, Abramyuk A, et al. Validation of multiparametric ultrasonography criteria with digital subtraction angiography in carotid artery disease: a prospective multicenter study [Validierung von multiparametrischen ultraschallkriterien zur graduierung von karotisstenosen mit der digitalen subtraktionsangiografie: eine prospektive multizentrische studie]. Ultraschall in der Medizin 2018;39(5):535-43. [DOI] [PubMed] [Google Scholar]
Barnes 1976 {published data only}
- Barnes RW, Bone GE, Reinertson JE, Hokanson DE, Strandness DE. Doppler ultrasonic flow analysis and imaging in carotid-artery disease. European Surgical Research 1976;8:25-6. [Google Scholar]
Barnes 1982 {published data only}
- Barnes RW, Rittgers SE, Putney WW. Real-time Doppler spectrum analysis: predictive value in defining operable carotid artery disease. Archives of Surgery 1982;117(1):52-7. [DOI] [PubMed] [Google Scholar]
Barry 1987 {published data only}
- Barry R, Nel CJC. Comparison of duplex doppler scanning with contrast angiography in carotid-artery disease. South African Medical Journal 1987;72(12):851-2. [PubMed] [Google Scholar]
Bartylla 1997 {published data only}
- Bartylla K, Muller M, Hagen T. Quantifying carotid stenosis: a comparison on 8 angiographical methods and continuous-wave Doppler sonography. Klinische Neuroradiologie 1997;7(4):200-4. [Google Scholar]
Baskett 1976 {published data only}
- Baskett JJ, Beasley MG, Gosling RG, Hyams DE. Proceedings: the assessment and management of carotid arterial disease using spectral analysis of the Doppler signal. British Journal of Radiology 1976;49(584):731. [PubMed] [Google Scholar]
Beckett 1990 {published data only}
- Beckett WW, Davis PC, Hoffman JC. Duplex Doppler sonography of the carotid-artery - false positive results in an artery contralateral to an artery with marked stenosis. American Journal of Roentgenology 1990;155(5):1091-5. [DOI] [PubMed] [Google Scholar]
Beebe 1999 {published data only}
- Beebe HG, Salles-Cunha SX, Scissons RP, Dosick SM, Whalen RC, Gale SS, et al. Carotid arterial ultrasound scan imaging: a direct approach to stenosis measurement. Journal of Vascular Surgery 1999;29(5):838-44. [DOI] [PubMed] [Google Scholar]
Beer 1983 {published data only}
- Beer G, Romanoff H, Gordon R, Katz G, Haskell L, Anner H. Noninvasive tests for carotid artery occlusive disease. Harefuah 1983;104(11-12):495-8. [PubMed] [Google Scholar]
Beer 1986 {published data only}
- Beer G, Romanoff H. Non invasive testing in carotid-artery occlusive disease - the value of direct doppler ultrasonography. International Angiology 1986;5(1):27-31. [PubMed] [Google Scholar]
Benhamou 1984 {published data only}
- Benhamou AC, Dutreix JL, Genre O, Arbeille P, Marchal C, Pourcelot L, et al. Validation of quantitative and qualitative data of real-time carotid echotomography in comparison with standard Doppler tests, arteriography and anatomopathology. On 59 carotid endarterectomies. Journal des Maladies Vasculaires 1984;9(3):185-94. [PubMed] [Google Scholar]
Berger 1983 {published data only}
- Berger G, Sprugel W, Seyferth W. Diagnosis of extracranial carotid diseases. Doppler-echo flow and transvenous digital subtraction angiography. Deutsche Medizinische Wochenschrift 1983;108(3):86-93. [DOI] [PubMed] [Google Scholar]
Berman 1995 {published data only}
- Berman SS, Devine JJ, Erdoes LS, Hunter GC. Distinguishing carotid-artery pseudo-occlusion with color-flow doppler. Stroke 1995;26(3):434-8. [DOI] [PubMed] [Google Scholar]
Berry 1980 {published data only}
- Berry SM, O'Donnell JA, Hobson RW 2nd. Capabilities and limitations of pulsed Doppler sonography in carotid imaging. Journal of Clinical Ultrasound 1980;8(5):405-12. [DOI] [PubMed] [Google Scholar]
Beutler 1985 {published data only}
- Beutler J, Wrangell U. Doppler sonography and digital subtraction angiography of the arteries of the neck compared with angiography. Aktuelle Neurologie 1985;12(3):94-7. [Google Scholar]
Biasi 1998 {published data only}
- Biasi GM, Mingazzini PM, Baronio L, Piglionica MR, Ferrari SA, Elatrozy TS, et al. Carotid plaque characterization using digital image processing and its potential in future studies of carotid endarterectomy and angioplasty. Journal of Endovascular Surgery 1998;5(3):240-6. [DOI] [PubMed] [Google Scholar]
Binaghi 2001 {published data only}
- Binaghi S, Maeder P, Uske A, Meuwly JY, Devuyst G, Meuli RA. Three-dimensional computed tomography angiography and magnetic resonance angiography of carotid bifurcation stenosis. European Neurology 2001;46(1):25-34. [DOI] [PubMed] [Google Scholar]
Birmpili 2018 {published data only}
- Birmpili P, Porter L, Shaikh U, Torella F. Comparison of measurement and grading of carotid stenosis with computed tomography angiography and Doppler ultrasound. Annals of Vascular Surgery 2018;51:217-24. [DOI] [PubMed] [Google Scholar]
Blackshear 1984 {published data only}
- Blackshear WM Jr, Lamb SL, Kollipara VS, Anderson JD, Murtagh FR, Shah CP, et al. Correlation of hemodynamically significant internal carotid stenosis with pulsed Doppler frequency analysis. Annals of Surgery 1984;199(4):475-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Blackshear 1985 {published data only}
- Blackshear Jr WM, Lamb S, Murtagh R. Pulsed Doppler ultrasonic arteriography and spectrum analysis for quantitating carotid occlusive disease. Journal of Cardiovascular Ultrasonography 1985;4(2):105-12. [Google Scholar]
Blackshear 1987 {published data only}
- Blackshear WM, Seifert KB, Lamb S, Kollipara VS, Murtagh FR, Shah CP, et al. Pulsed Doppler frequency and carotid stenosis. Journal of Surgical Research 1987;42(2):179-84. [DOI] [PubMed] [Google Scholar]
Bladin 1995 {published data only}
- Bladin CF, Alexandrov AV, Murphy J, Maggisano R, Norris JW. Carotid stenosis index - a new method of measuring internal carotid-artery stenosis. Stroke 1995;26(2):230-4. [DOI] [PubMed] [Google Scholar]
Blasberg 1982 {published data only}
- Blasberg DJ. Duplex sonography for carotid-artery disease - an accurate technique. American Journal of Neuroradiology 1982;3(6):609-14. [PMC free article] [PubMed] [Google Scholar]
Bloch 1979 {published data only}
- Bloch S, Baltaxe HA, Shoumaker RD. Reliability of doppler scanning of the carotid bifurcation: angiographic correlation. Radiology 1979;132(3):687-91. [DOI] [PubMed] [Google Scholar]
Boccalon 1985 {published data only}
- Boccalon H, Ginestet MC, Soules C, Puel P. Exploration of the cervical arteries within the framework of an angiology service. Our experience using Doppler spectral analysis. Journal des Maladies Vasculaires 1985;10(4):315-20. [PubMed] [Google Scholar]
Bone 1976 {published data only}
- Bone GE, Barnes RW. Clinical implications of the Doppler cerebrovascular examination: a correlation with angiography. Stroke 1976;7(3):271-4. [DOI] [PubMed] [Google Scholar]
Bone 1988 {published data only}
- Bone G, Ladurner G, Karas I, Grobovschek M, Punzengruber J. Evaluation of the carotid bifurcation by duplex ultrasound, intravenous and intra-arterial digital subtraction angiography. European Neurology 1988;28(2):73-6. [DOI] [PubMed] [Google Scholar]
Bonig 2000 {published data only}
- Bonig L, Weder B, Schott D, Keel A, Nguyen T, Zaunbauer W. Prediction of angiographic carotid artery stenosis indexes by colour Doppler-assisted duplex imaging. A critical appraisal of the parameters used. European Journal of Neurology 2000;7(2):183-90. [DOI] [PubMed] [Google Scholar]
Boyko 2018 {published data only}
- Boyko M, Kalashyan H, Becher H, Romanchuk H, Saqqur M, Rempel JL, et al. Comparison of carotid Doppler ultrasound to other angiographic modalities in the measurement of carotid artery stenosis. Journal of Neuroimaging 2018;28(6):683-7. [DOI] [PubMed] [Google Scholar]
Boyle 1995 {published data only}
- Boyle MJ, Wolinski AP, Grimley RP. Accuracy 0f duplex versus angiography in patients undergoing carotid surgery. Journal of the Royal Society of Medicine 1995;88(1):20-3. [PMC free article] [PubMed] [Google Scholar]
Branas 1994 {published data only}
- Branas CC, Weingarten MS, Czeredarczuk M, Schafer PF. Examination of carotid arteries with quantitative color Doppler flow imaging. Journal of Ultrasound in Medicine 1994;13(2):121-7. [DOI] [PubMed] [Google Scholar]
Braun 2008 {published data only}
- Braun RM, Bertino RE, Milbrandt J, Bray M. Ultrasound imaging of carotid artery stenosis: application of the society of radiologists in ultrasound consensus criteria to a single institution clinical practice. Ultrasound Quarterly 2008;24(3):161-6. [DOI] [PubMed] [Google Scholar]
Browman 1995 {published data only}
- Browman MW, Cooperberg PL, Harrison PB, Marsh JI, Mallek N. Duplex ultrasonography criteria for internal carotid stenosis of more than 70-percent diameter - angiographic correlation and receiver operating characteristic curve analysis. Canadian Association of Radiologists Journal 1995;46(4):291-5. [PubMed] [Google Scholar]
Bucek 2006 {published data only}
- Bucek RA, Puchner S, Haumer M, Rand T, Sabeti S, Minar E, et al. Grading of internal carotid artery stenosis: comparative analysis of different flow velocity criteria and multidetector computed tomographic angiography. Journal of Endovascular Therapy 2006;13(2):182-9. [DOI] [PubMed] [Google Scholar]
Buijs 1993 {published data only}
- Buijs PC, Klop RBJ, Eikelboom BC, Mali WPTM, Bakker CJG, Beek FJA, et al. Carotid bifurcation imaging - magnetic-resonance angiography compared to conventional angiography and doppler ultrasound. European Journal of Vascular Surgery 1993;7(3):245-51. [DOI] [PubMed] [Google Scholar]
Bulger 2005 {published data only}
- Bulger CM, Gao W, Jacobs C, McCarthy WJ. Beyond the categories: a formula-driven prediction of carotid stenosis. Journal for Vascular Ultrasound 2005;29(1):15-20. [Google Scholar]
Busse 1974 {published data only}
- Busse R, Sperling W, Korner H, Bauer RD, Pasch T, Vonderem J. Applicability of ultrasonic doppler flowmetry to detection of carotid-artery stenosis. Zeitschrift fur Kardiologie 1974;63(8):755-67. [PubMed] [Google Scholar]
Busuttil 1996 {published data only}
- Busuttil SJ, Franklin DP, Youkey JR, Elmore JR. Carotid duplex overestimation of stenosis due to severe contralateral disease. American Journal of Surgery 1996;172(2):144-7. [DOI] [PubMed] [Google Scholar]
Caes 1987 {published data only}
- Caes F, Vierendeels T, Janssens-Willem E, Cham B, Welch W. Comparison of auscultation, continuous wave Doppler imaging, intravenous digital subtraction angiography and conventional angiography in diagnosis of carotid artery disease. Angiology 1987;38(11):799-806. [DOI] [PubMed] [Google Scholar]
Cape 1984 {published data only}
- Cape CA, DeSaussure RL, Nixon J. Carotid ultrasonography in carotid artery disease. Southern Medical Journal 1984;77(2):183-6. [DOI] [PubMed] [Google Scholar]
Cappetti 1996 {published data only}
- Cappetti P, Giannoni C, Casi F, Caponi A, Ciaccio B. Carotid vessels studied by Doppler color echography and digital angiography. Results of meta analysis. Minerva Cardioangiologica 1996;44(7-8):373-6. [PubMed] [Google Scholar]
Carnicelli 2013 {published data only}
- Carnicelli AP, Stone JJ, Doyle A, Chowdhry AK, Mix D, Ellis J, et al. Cross-sectional area for the calculation of carotid artery stenosis on computed tomographic angiography. Journal of Vascular Surgery 2013;58(3):659-65. [DOI] [PubMed] [Google Scholar]
Carpenter 1995 {published data only}
- Carpenter JP, Lexa FJ, Davis JT. Determination of sixty percent or greater carotid artery stenosis by duplex Doppler ultrasonography. Journal of Vascular Surgery 1995;22(6):697-705. [DOI] [PubMed] [Google Scholar]
Carpenter 1996 {published data only}
- Carpenter JP, Lexa FJ, Davis JT. Determination of duplex Doppler ultrasound criteria appropriate to the North American Symptomatic Carotid Endarterectomy Trial. Stroke 1996;27(4):695-9. [DOI] [PubMed] [Google Scholar]
Carroll 1989 {published data only}
- Carroll BA. Duplex sonography in patients with hemispheric symptoms. Journal of Ultrasound in Medicine 1989;8(10):535-40. [DOI] [PubMed] [Google Scholar]
Chaix 1985 {published data only}
- Chaix AF, Begon F, Chabassiere D, Goubault F, Hurmic A, De Verbizier G, et al. Detection and quantification of carotid atheroma. Respective value of vascular ultrasonography, spectrum analysis and digital intravenous angiography. Annales de Cardiologie et d'Angeiologie 1985;34(7):479-84. [PubMed] [Google Scholar]
Chan 1982 {published data only}
- Chan A, Beach KW, Langlois Y, Roederer GO, Strandness DE. Evaluation of extra-cranial carotid-artery disease by ultrasonic duplex scanning - a clinical perspective. Australian and New Zealand Journal of Surgery 1982;52(6):562-9. [DOI] [PubMed] [Google Scholar]
Chang 1995 {published data only}
- Chang YJ, Golby AJ, Albers GW. Detection of carotid stenosis - from NASCET results to clinical practice. Stroke 1995;26(8):1325-8. [DOI] [PubMed] [Google Scholar]
Chang 2002 {published data only}
- Chang JB, Stein TA, Hajee M, Diamond A, Lustrin E, Warshall C. Computed tomography in the decision for carotid endarterectomy. International Journal of Angiology 2002;11(4):203-6. [Google Scholar]
Chen 1997 {published data only}
- Chen JC, Salvian AJ, Taylor DC, Teal PA, Marotta TR, Hsiang YN. Can duplex ultrasonography select appropriate patients for carotid endarterectomy? European Journal of Vascular and Endovascular Surgery 1997;14(6):451-6. [DOI] [PubMed] [Google Scholar]
Chen 1998 {published data only}
- Chen JC, Salvian AJ, Taylor DC, Teal PA, Marotta TR, Hsiang YN. Predictive ability of duplex ultrasonography for internal carotid artery stenosis of 70%-99%: a comparative study. Annals of Vascular Surgery 1998;12(3):244-7. [DOI] [PubMed] [Google Scholar]
Chervu 1994 {published data only}
- Chervu A, Moore WS. Carotid endarterectomy without arteriography. Annals of Vascular Surgery 1994;8(3):296-302. [DOI] [PubMed] [Google Scholar]
Chowdhury 2011 {published data only}
- Chowdhury RN, Rahman KM, Khan SU, Sarker R, Nabi S, Siddiqui MR, et al. Digital subtraction angiogram (DSA) is superior to duplex ultrasound (USD) in diagnosis of extracranial carotid stenosis - a comparative study. Journal of Medicine 2011;12(1):12-6. [Google Scholar]
Clevert 2006 {published data only}
- Clevert D-A, Johnson T, Michaely H, Jung EM, Flach PM, Strautz TI, et al. High-grade stenoses of the internal carotid artery: comparison of high-resolution contrast enhanced 3D MRA, duplex sonography and power Doppler imaging. European Journal of Radiology 2006;60(3):379-86. [DOI] [PubMed] [Google Scholar]
Clevert 2007 {published data only}
- Clevert D-A, Johnson T, Jung EM, Flach PM, Strautz TI, Ritter G, et al. Color Doppler, power Doppler and B-flow ultrasound in the assessment of ICA stenosis: comparison with 64-MD-CT angiography. European Radiology 2007;17(8):2149-59. [DOI] [PubMed] [Google Scholar]
Colhoun 1984 {published data only}
- Colhoun E, Macerlean D. Carotid-artery imaging using duplex scanning and bidirectional arteriography - a comparison. British Journal of Radiology 1984;57(677):450. [DOI] [PubMed] [Google Scholar]
Collins 2005 {published data only}
- Collins P, McKay I, Rajagoplan S, Bachoo P, Robb O, Brittenden J. Is carotid duplex scanning sufficient as the sole investigation prior to carotid endarterectomy? British Journal of Radiology 2005;78(935):1034-7. [16249605] [DOI] [PubMed] [Google Scholar]
Colon 1979 {published data only}
- Colon EJ, De Weerd JP, Notermans SL, Vingerhoets HM. Reliability of Doppler sonography in extracranial cerebro-vascular stenosis. Clinical Neurology and Neurosurgery 1979;81(2):108-13. [DOI] [PubMed] [Google Scholar]
Connolly 1985 {published data only}
- Connolly JE, Brownell DA, Levine EF, McCart PM. Accuracy and indications of diagnostic studies for extracranial carotid disease. Archives of Surgery 1985;120(11):1229-32. [DOI] [PubMed] [Google Scholar]
Cooperberg 1992 {published data only}
- Cooperberg E. Ultrasound doppler spectral-analysis in the diagnosis of occlusive lesions of the carotid arteries. Ultrasound in Medicine and Biology 1992;18(4):421-5. [DOI] [PubMed] [Google Scholar]
Corti 1998 {published data only}
- Corti R, Ferrari C, Roberti M, Alerci M, Pedrazzi PL, Gallino A. Spiral computed tomography - a novel diagnostic approach for investigation of the extracranial cerebral arteries and its complementary role in duplex ultrasonography. Circulation 1998;98(10):984-9. [DOI] [PubMed] [Google Scholar]
Criswell 1998 {published data only}
- Criswell BK, Langsfeld M, Tullis MJ, Marek J. Evaluating institutional variability of duplex scanning in the detection of carotid artery stenosis. American Journal of Surgery 1998;176(6):591-6. [DOI] [PubMed] [Google Scholar]
Crummy 1979 {published data only}
- Crummy AB, Zwiebel WJ, Barriga P, Strother CM, Sackett JF, Turnipseed WD, et al. Doppler evaluation of extracranial cerebrovascular disease. American Journal of Roentgenology 1979;132(1):91-3. [DOI] [PubMed] [Google Scholar]
Csanyi 1993 {published data only}
- Csanyi A, Egervari A, Poharnok L. Duplex ultrasonography in the study of carotid and vertebral artery stenosis (comparison with intra-arterial digitalized subtraction angiography). Orvosi Hetilap 1993;134(49):2691-6. [PubMed] [Google Scholar]
Curley 1998 {published data only}
- Curley PJ, Norrie L, Nicholson A, Galloway JMD, Wilkinson ARW. Accuracy of carotid duplex is laboratory specific and must be determined by internal audit. European Journal of Vascular and Endovascular Surgery 1998;15(6):511-4. [DOI] [PubMed] [Google Scholar]
Daiss 1984 {published data only}
- Daiss W, Diener HC, Thron A, Rosenberg M. Diagnosis of stenoses and occlusions of extracranial arteries. Comparison of Doppler sonography, duplex scan, and cerebral angiography. Deutsche Medizinische Wochenschrift 1984;109(42):1595-9. [DOI] [PubMed] [Google Scholar]
Dalotto 1985 {published data only}
- Dalotto C, Pelz DM, Rankin RN. A comparison of angiography, intravenous digital subtraction angiography and duplex ultrasound in the diagnosis of carotid-artery atherosclerosis. Journal of the Canadian Association of Radiologists 1985;36(3):200-8. [PubMed] [Google Scholar]
Daolio 2019 {published data only (unpublished sought but not used)}
- Daolio RM, Cassola N, Flumignan C, Nakano L, Guedes H, Amorim J, et al. Accuracy of vascular ultrasound compared with computed tomography angiography for extracranial carotid stenosis imaging. Journal of Vascular Surgery 2019;69(6):E239-40. [DOI: 10.1016/j.jvs.2019.04.356] [DOI] [Google Scholar]
Dawson 1991 {published data only}
- Dawson DL, Zierler RE, Kohler TR. Role of arteriography in the preoperative evaluation of carotid-artery disease. American Journal of Surgery 1991;161(5):619-24. [DOI] [PubMed] [Google Scholar]
Dawson 1993 {published data only}
- Dawson DL, Zierler RE, Strandness DE, Clowes AW, Kohler TR. The role of duplex scanning and arteriography before carotid endarterectomy - a prospective-study. Journal of Vascular Surgery 1993;18(4):673-83. [PubMed] [Google Scholar]
Dean 2005 {published data only}
- Dean N, Lari H, Saqqur M, Amir N, Khan K, Mouradian M, et al. Reliability of carotid doppler performed in a dedicated stroke prevention clinic. Canadian Journal of Neurological Sciences 2005;32(3):327-31. [DOI] [PubMed] [Google Scholar]
De la Cruz Cosme 2017 {published data only}
- De la Cruz Cosme C, Dawid Milner MS, Ojeda Burgos G, Gallardo Tur A, Marquez Martinez M, Segura T. Validation of a basic neurosonology laboratory for detecting cervical carotid artery stenosis. Neurologia 2017;34(6):367-75. [DOI] [PubMed] [Google Scholar]
De Monti 2003 {published data only}
- De Monti M, Ghilardi G, Caverni L, Ceriani L, Soldi S, Massaro F, et al. Multidetector helical angio CT oblique reconstructions orthogonal to internal carotid artery for preoperative evaluation of stenosis. A prospective study of comparison with color Doppler US, digital subtraction angiography and intraoperative data. Minerva Cardioangiologica 2003;51(4):373-85. [PubMed] [Google Scholar]
Dharmasaroja 2018 {published data only}
- Dharmasaroja PA, Uransilp N, Watcharakorn A, Piyabhan P. Accuracy of carotid duplex criteria in diagnosis of significant carotid stenosis in Asian patients. Journal of Stroke and Cerebrovascular Diseases 2018;27(3):778-82. [DOI] [PubMed] [Google Scholar]
Dilley 1986 {published data only}
- Dilley RB, Bernstein EF. A comparison of B-mode real-time imaging and arteriography in the intraoperative assessment of carotid endarterectomy. Journal of Vascular Surgery 1986;4(5):457-63. [PubMed] [Google Scholar]
Dinkel 2001 {published data only}
- Dinkel HP, Moll R, Debus S. Colour flow Doppler ultrasound of the carotid bifurcation: can it replace routine angiography before carotid endarterectomy? British Journal of Radiology 2001;74(883):590-4. [DOI] [PubMed] [Google Scholar]
Dippel 1999 {published data only}
- Dippel DWJ, De Kinkelder A, Bakker SLM, Van Kooten F, Van Overhagen H, Koudstaal PJ. The diagnostic value of colour duplex ultrasound for symptomatic carotid stenosis in clinical practice. Neuroradiology 1999;41(1):1-8. [DOI] [PubMed] [Google Scholar]
Dix 2000 {published data only}
- Dix J, Skrocki J. Evaluation of carotid stenosis by angiography: potential bias toward overestimated measurements introduced by prior interpretation of Doppler sonograms. American Journal of Neuroradiology 2000;21(4):639-42. [PMC free article] [PubMed] [Google Scholar]
Doyle 2012 {published data only}
- Doyle AJ, Stone JJ, Carnicelli A, Hislop SJ, Singh MJ, Kim J, et al. CT angiography-derived duplex ultrasound velocity criteria in patients with carotid artery stenosis. Journal of Vascular Surgery 2012;55(6):92. [DOI] [PubMed] [Google Scholar]
Doyle 2014 {published data only}
- Doyle AJ, Stone JJ, Carnicelli AP, Chandra A, Gillespie DL. CT angiography-derived duplex ultrasound velocity criteria in patients with carotid artery stenosis. Annals of Vascular Surgery 2014;28(5):1219-26. [DOI] [PubMed] [Google Scholar]
Drevet 1997 {published data only}
- Drevet D, Russier S, Age B, Lepine PM, Zabot JM, Joffre P. Evaluation of Doppler ultrasound, helical CT, magnetic resonance angiography in detection of atherosclerotic carotid bifurcation with arteriographic comparison. Journal of Radiology 1997;78(12):1271-7. [PubMed] [Google Scholar]
Eckmann 1990 {published data only}
- Eckmann A, Schmiedt W, Storket S, Kuhn FP. Diagnosis of atherosclerotic lesions of the extracranial carotid artery using duplex sonography and IA-DSA. A prospective pathologically and anatomically controlled study. RoFo: Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin 1990;153(1):48-55. [DOI] [PubMed] [Google Scholar]
Ellis 1996 {published data only}
- Ellis PK, Kelly BE, Bennett D, McIlrath EM. A comparison of intra-arterial digital subtraction angiography with doppler sonography in the assessment of carotid arterial stenosis. Ulster Medical Journal 1996;65(2):137-41. [PMC free article] [PubMed] [Google Scholar]
Elmore 1998 {published data only}
- Elmore JR, Franklin DP, Thomas DD, Youkey JR. Carotid endarterectomy: the mandate for high quality duplex. Annals of Vascular Surgery 1998;12(2):156-62. [DOI] [PubMed] [Google Scholar]
El‐Saden 2001 {published data only}
- El-Saden SM, Grant EG, Hathout GM, Zimmerman PT, Cohen SN, Baker JD. Imaging of the internal carotid artery: the dilemma of total versus near total occlusion. Radiology 2001;221(2):301-8. [DOI] [PubMed] [Google Scholar]
Engelhardt 2005 {published data only}
- Engelhardt M, Bruijnen H, Schnur C, Pfadenhauer K, Bohndorf K, Wolfle KD. Duplex scanning criteria for selection of patients for internal carotid artery endarterectomy. VASA: Zeitschrift fur Gefasskrankheiten 2005;34(1):36-40. [DOI] [PubMed] [Google Scholar]
Erdoes 1996 {published data only}
- Erdoes LS, Marek JM, Mills JL, Berman SS, Whitehill T, Hunter GC, et al. The relative contributions of carotid duplex scanning, magnetic resonance angiography, and cerebral arteriography to clinical decisionmaking: a prospective study in patients with carotid occlusive disease. Journal of Vascular Surgery 1996;23(5):950-6. [DOI] [PubMed] [Google Scholar]
Erickson 1989 {published data only}
- Erickson SJ, Mewissen MW, Foley WD, Lawson TL, Middleton WD, Quiroz FA, et al. Stenosis of the internal carotid artery: assessment using color Doppler imaging compared with angiography. American Journal of Roentgenology 1989;152(6):1299-305. [DOI] [PubMed] [Google Scholar]
Felber 1985 {published data only}
- Felber S, Aichner F, Homma H. The value of Doppler sonographic and duplex sonographic examinations of the extracranial carotid system as performed by technical assistants. Ultraschall in der Medizin 1985;6(5):282-5. [DOI] [PubMed] [Google Scholar]
Fell 1981 {published data only}
- Fell G, Phillips DJ, Chikos PM, Harley JD, Thiele BL, Strandness DE. Ultrasonic duplex scanning for disease of the carotid-artery. Circulation 1981;64(6):1191-5. [DOI] [PubMed] [Google Scholar]
Filis 2002 {published data only}
- Filis KA, Arko FR, Johnson BL, Pipinos II, Harris EJ, Olcott C 4th, et al. Duplex ultrasound criteria for defining the severity of carotid stenosis. Annals of Vascular Surgery 2002;16(4):413-21. [DOI] [PubMed] [Google Scholar]
Fillinger 1996 {published data only}
- Fillinger MF, Baker RJ, Zwolak RM, Musson A, Lenz JE, Mott J, et al. Carotid duplex criteria for a 60% or greater angiographic stenosis: variation according to equipment. Journal of Vascular Surgery 1996;24(5):856-64. [DOI] [PubMed] [Google Scholar]
Finkenzeller 2008 {published data only}
- Finkenzeller T, Tacke J, Clevert D-A, Jung W, Kubale R, Schreyer A, et al. Quantification of extracranial ICA stenoses with vessel ultrasound by CCDS and B-flow in comparison to 64-slice multidector CTA, contrast-enhanced MRA and DSA. Ultraschall in der Medizin 2008;29(3):294-301. [DOI] [PubMed] [Google Scholar]
Fischer 1985 {published data only}
- Fischer M, Stegmann T, Becker H, Luska G, Sturm J, Alexander K. Doppler frequency spectrum analysis and digital subtraction angiography in carotid artery disease. Thoracic and Cardiovascular Surgeon 1985;33(5):304-7. [DOI] [PubMed] [Google Scholar]
Fischer 1985a {published data only}
- Fischer GG, Anderson DC, Farber R, Lebow S. Prediction of carotid disease by ultrasound and digital subtraction angiography. Archives of Neurology 1985;42(3):224-7. [DOI] [PubMed] [Google Scholar]
Fix 1984 {published data only}
- Fix CH. Noninvasive study of carotid disease - a comparison study of pulsed doppler ultrasonic arteriography to duplex scanning technique. Medical Ultrasound 1984;8(1):8-14. [Google Scholar]
Flanigan 1985 {published data only}
- Flanigan DP, Schuler JJ, Vogel M, Borozan PG, Gray B, Sobinsky KR. The role of carotid duplex scanning in surgical decision making. Journal of Vascular Surgery 1985;2(1):15-25. [PubMed] [Google Scholar]
Fragata 2006 {published data only}
- Fragata I, Galo S, Manita M, Ferreira S, Reis J. Prevalence of carotid artery disease in an ischemic stroke population: role of Doppler ultrasonography. Acta Medica Portuguesa 2006;19(6):446-50. [PubMed] [Google Scholar]
French‐Sherry 2016 {published data only}
- French-Sherry E, Burns K. Combination of duplex ultrasound data improves positive predictive value for carotid stenoses greater than 70 percent. Journal for Vascular Ultrasound 2016;40(1):9-13. [Google Scholar]
Friese 2001 {published data only}
- Friese S, Krapf H, Fetter M, Klose U, Skalej M, Kuker W. Ultrasonography and contrast-enhanced MRA in ICA-stenosis: is conventional angiography obsolete? Journal of Neurology 2001;248(6):506-13. [DOI] [PubMed] [Google Scholar]
Fujimoto 2006 {published data only}
- Fujimoto S, Toyoda K, Kishikawa K, Inoue T, Yasumori K, Ibayashi S, et al. Accuracy of conventional plus transoral carotid ultrasonography in distinguishing pseudo-occlusion from total occlusion of the internal carotid artery. Cerebrovascular Diseases 2006;22(2-3):170-6. [DOI] [PubMed] [Google Scholar]
Furst 1993 {published data only}
- Furst G, Kahn T, Sitzer M, Fischer H, Hofer M, Fehlings T, et al. Quantification of extracranial stenoses of the carotid-artery - MR angiography and doppler sonography vs intra-arterial angiography. Fortschritte Auf Dem Gebiete Der Rontgenstrahlen Und Der Neuen Bildgebenden Verfahren 1993;159(4):368-74. [DOI] [PubMed] [Google Scholar]
Furst 1999 {published data only}
- Furst G, Saleh A, Wenserski F, Malms J, Cohnen M, Aulich A, et al. Reliability and validity of noninvasive imaging of internal carotid artery pseudo-occlusion. Stroke 1999;30(7):1444-9. [DOI: 10.1161/01.str.30.7.1444] [DOI] [PubMed] [Google Scholar]
Geidel 1991 {published data only}
- Geidel M, Deckert F, Neumann T, Wehr M. The correlation of duplex sonography and intra-arterial DSA in degenerative vascular diseases in the supra-aortic region. Zeitschrift fur die gesamte innere Medizin und ihre Grenzgebiete 1991;46(6):195-7. [PubMed] [Google Scholar]
Geuder 1989 {published data only}
- Geuder JW, Lamparello PJ, Riles TS, Giangola G, Imparato AM. Is duplex scanning sufficient evaluation before carotid endarterectomy? Journal of Vascular Surgery 1989;9(2):193-201. [PubMed] [Google Scholar]
Giraldi 1986 {published data only}
- Giraldi C, Parenti G, Canapicchi R. Collateral circles in carotid artery occlusion. A comparative study between CW doppler and contrast angiography. Journal of Nuclear Medicine and Allied Sciences 1986;30(2-3):125-8. [PubMed] [Google Scholar]
Glover 1984 {published data only}
- Glover JL, Bendick PJ, Jackson VP, Becker GJ, Dilley RS, Holden RW. Duplex ultrasonography, digital subtraction angiography, and conventional angiography in assessing carotid atherosclerosis. Archives of Surgery 1984;119(6):664-9. [DOI] [PubMed] [Google Scholar]
Gmelin 1985 {published data only}
- Gmelin E, Borgis CJ, Kummer D, Tanzer B, Weiss HD. A comparison of doppler ultrasound, intravenous DSA and conventional film angiography in the diagnosis of stenosing lesions at the carotid bifurcation. Fortschritte Auf Dem Gebiete Der Rontgenstrahlen Und Der Nuklearmedizin 1985;142(1):52-6. [DOI] [PubMed] [Google Scholar]
Golledge 1996 {published data only}
- Golledge J, Wright R, Pugh N, Lane IF. Colour-coded duplex assessment alone before carotid endarterectomy. British Journal of Surgery 1996;83(9):1234-7. [PubMed] [Google Scholar]
Goodson 1987 {published data only}
- Goodson SF, Flanigan DP, Bishara RA, Schuler JJ, Kikta MJ, Meyer JP. Can carotid duplex scanning supplant arteriography in patients with focal carotid territory symptoms? Journal of Vascular Surgery 1987;5(4):551-7. [PubMed] [Google Scholar]
Gortler 1994 {published data only}
- Gortler M, Kleiser B, Widder B, Friedrich JM, Wallner B, Weidenmaier W, et al. Assessment of duplex sonography, intravenous DSA, and MR angiography (2D and 3D time-of-flight, 'black blood' spin-echo technique) in evaluating high-grade carotid artery stenosis. Neurology Psychiatry and Brain Research 1994;2(3):183-7. [Google Scholar]
Grajo 2007 {published data only}
- Grajo JR, Barr RG. Duplex Doppler sonography of the carotid artery: velocity measurements in an artery with contralateral stenosis. Ultrasound Quarterly 2007;23(3):199-202. [DOI] [PubMed] [Google Scholar]
Grant 1999 {published data only}
- Grant EG, Duerinckx AJ, El Saden S, Melany ML, Hathout G, Zimmerman P, et al. Doppler sonographic parameters for detection of carotid stenosis: Is there an optimum method for their selection? American Journal of Roentgenology 1999;172(4):1123-9. [DOI] [PubMed] [Google Scholar]
Grant 2000 {published data only}
- Grant EG, Duerinckx AJ, El Saden SM, Melany ML, Hathout GM, Zimmerman PT, et al. Ability to use duplex US to quantify internal carotid arterial stenoses: fact or fiction? Radiology 2000;214(1):247-52. [DOI] [PubMed] [Google Scholar]
Griewing 1996 {published data only}
- Griewing B, Morgenstern C, Driesner F, Kallwellis G, Walker ML, Kessler C. Cerebrovascular disease assessed by color-flow and power Doppler ultrasonography - comparison with digital subtraction angiography in internal carotid artery stenosis. Stroke 1996;27(1):95-100. [DOI] [PubMed] [Google Scholar]
Griffiths 1998 {published data only}
- Griffiths GD, Razzaq R, Ashleigh R, Farrell A, Charlesworth D. Variability in measurement of internal carotid stenosis by arch angiography and duplex ultrasonography. British Journal of Surgery 1998;85:94. [DOI] [PubMed] [Google Scholar]
Griffiths 2001 {published data only}
- Griffiths GD, Razzaq R, Farrell A, Ashleigh R, Charlesworth D. Variability in measurement of internal carotid artery stenosis by arch angiography and duplex ultrasonography - time for a reappraisal? European Journal of Vascular and Endovascular Surgery 2001;21(2):130-6. [DOI] [PubMed] [Google Scholar]
Hames 1981 {published data only}
- Hames TK, Humphries KN, Powell TV, McLellan DL. Comparison of angiography with continuous wave Doppler ultrasound in the assessment of extracranial arterial disease. Journal of Neurology, Neurosurgery and Psychiatry 1981;44(8):661-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hames 1985 {published data only}
- Hames TK, Humphries KN, Ratliff DA, Birch SJ, Gazzard VM, Chant AD. The validation of duplex scanning and continuous wave Doppler imaging: a comparison with conventional angiography. Ultrasound in Medicine & Biology 1985;11(6):827-34. [DOI] [PubMed] [Google Scholar]
Harward 1986 {published data only}
- Harward TR, Bernstein EF, Fronek A. Continuous-wave versus range-gated pulsed Doppler power frequency spectrum analysis in the detection of carotid arterial occlusive disease. Annals of Surgery 1986;204(1):32-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hathout 2005 {published data only}
- Hathout GM, Fink JR, El-Saden SM, Grant EG. Sonographic NASCET index: a new doppler parameter for assessment of internal carotid artery stenosis. American Journal of Neuroradiology 2005;26(1):68-75. [PMC free article] [PubMed] [Google Scholar]
Hathout 2015 {published data only}
- Hathout G, Nayak N, Abdulla A, Huang J. The revised sonographic NASCET Index: a new hemodynamic parameter for the assessment of internal carotid artery stenosis. Ultraschall in der Medizin 2015;36(4):362-8. [DOI] [PubMed] [Google Scholar]
Herring 1984 {published data only}
- Herring MB, Russ M, Benge C, Gardner AL. A comparison of doppler flow measurements, ultrasonic and arteriographic imaging in the evaluation of stenoses of the carotid-artery. Surgery Gynecology & Obstetrics 1984;159(1):67-9. [PubMed] [Google Scholar]
Hetzel 1993 {published data only}
- Hetzel A, Eckenweber B, Trummer B, Wernz M, Vonreutern GM. Color-coded duplex sonography of pre-occlusive carotid stenoses. Ultraschall in der Medizin 1993;14(5):240-6. [DOI] [PubMed] [Google Scholar]
Hjelmgren 2018 {published data only}
- Hjelmgren O, Schmidt C, Johansson L, Bergstrom GML. Comparison between magnetic resonance imaging and B-mode ultrasound in detecting and estimating the extent of human carotid atherosclerosis. Clinical Physiology and Functional Imaging 2018;38(2):296-303. [DOI] [PubMed] [Google Scholar]
Hobson 1980 {published data only}
- Hobson RW 2nd, Berry SM, Katocs AS Jr, O'Donnell JA, Jamil Z, Savitsky JP. Comparison of pulsed Doppler and real-time B-mode echo arteriography for noninvasive imaging of the extracranial carotid arteries. Surgery 1980;87(3):286-93. [PubMed] [Google Scholar]
Honish 2005 {published data only}
- Honish C, Sadanand V, Fladeland D, Chow V, Pirouzmand F. The reliability of ultrasound measurements of carotid stenosis compared to MRA and DSA. Canadian Journal of Neurological Sciences 2005;32(4):465-71. [DOI] [PubMed] [Google Scholar]
Horrocks 1979 {published data only}
- Horrocks M, Roberts VC, Cotton LT. Doppler ultrasound in the evaluation of carotid-artery stenosis - comparative-study. British Journal of Radiology 1979;52(618):515-6. [Google Scholar]
Howard 1991 {published data only}
- Howard G, Chambless LE, Baker WH, Ricotta JJ, Jones AM, O'Leary D, et al. A multicenter validation study of Doppler ultrasound versus angiography. Journal of Stroke and Cerebrovascular Diseases 1991;1(4):166-73. [DOI] [PubMed] [Google Scholar]
Humphrey 1990 {published data only}
- Humphrey P, Sandercock P, Slattery J. A simple method to improve the accuracy of non-invasive ultrasound in selecting TIA patients for cerebral angiography. Journal of Neurology, Neurosurgery and Psychiatry 1990;53(11):966-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hunink 1993 {published data only}
- Hunink MG, Polak JF, Barlan MM, O'Leary DH. Detection and quantification of carotid artery stenosis: efficacy of various Doppler velocity parameters. American Journal of Roentgenology 1993;160(3):619-25. [DOI] [PubMed] [Google Scholar]
Huston 1998 {published data only}
- Huston J, Nichols DA, Luetmer PH, Rydberg CH, Lewis BD, Meyer FB, et al. MR angiographic and sonographic indications for endarterectomy. American Journal of Neuroradiology 1998;19(2):309-15. [PMC free article] [PubMed] [Google Scholar]
Huston 2000 {published data only}
- Huston J, James EM, Brown RD, Lefsrud D, Ilstrup DM, Robertson EF, et al. Redefined duplex ultrasonographic criteria for diagnosis of carotid artery stenosis. Mayo Clinic Proceedings 2000;75(11):1133-40. [DOI] [PubMed] [Google Scholar]
Hutchison 1985 {published data only}
- Hutchison KJ, Potemkowski AP. Carotid-artery assessment with continuous wave doppler ultrasound, comparison of 2 methods with angiography. Angiology 1985;36(11):785-91. [DOI] [PubMed] [Google Scholar]
Hwang 2002 {published data only}
- Hwang C-S, Shau W-Y, Tegeler CH. Doppler velocity criteria based on receiver operating characteristic analysis for the detection of threshold carotid stenoses. Journal of Neuroimaging 2002;12(2):124-30. [DOI] [PubMed] [Google Scholar]
Hwang 2003 {published data only}
- Hwang C-S, Liao K-M, Lee J-H, Tegeler CH. Measurement of carotid stenosis: comparisons between duplex and different angiographic grading methods. Journal of Neuroimaging 2003;13(2):133-9. [PubMed] [Google Scholar]
Hwang 2003a {published data only}
- Hwang C-S, Liao K-M, Tegeler CH. A multiple regression model of combined duplex criteria for detecting threshold carotid stenosis and predicting the exact degree of carotid stenosis. Journal of Neuroimaging 2003;13(4):324-9. [PubMed] [Google Scholar]
Jackson 1985 {published data only}
- Jackson VP, Kuehn DS, Bendick PJ, Becker GJ, Holden RW, Dilley RS. Duplex carotid sonography: correlation with digital subtraction angiography and conventional angiography. Journal of Ultrasound in Medicine 1985;4(5):239-49. [DOI] [PubMed] [Google Scholar]
Jackson 1998 {published data only}
- Jackson MR, Chang AS, Robles HA, Gillespie DL, Olsen SB, Kaiser WJ, et al. Determination of 60% or greater carotid stenosis: a prospective comparison of magnetic resonance angiography and duplex ultrasound with conventional angiography. Annals of Vascular Surgery 1998;12(3):236-43. [DOI] [PubMed] [Google Scholar]
Jacobs 1985 {published data only}
- Jacobs NM, Grant EG, Schellinger D, Byrd MC, Richardson JD, Cohan SL. Duplex carotid sonography - criteria for stenosis, accuracy, and pitfalls. Radiology 1985;154(2):385-91. [DOI] [PubMed] [Google Scholar]
Jogestrand 2002 {published data only}
- Jogestrand T, Lindqvist M, Nowak J, Swedish Quality Board for Carotid Surgery. Diagnostic performance of duplex ultrasonography in the detection of high grade internal carotid artery stenosis. European Journal of Vascular and Endovascular Surgery 2002;23(6):510-8. [DOI] [PubMed] [Google Scholar]
Johnson 2000 {published data only}
- Johnson MB, Wilkinson ID, Wattam J, Venables GS, Griffiths PD. Comparison of Doppler ultrasound, magnetic resonance angiographic techniques and catheter angiography in evaluation of carotid stenosis. Clinical Radiology 2000;55(12):912-20. [DOI] [PubMed] [Google Scholar]
Johnston 1982 {published data only}
- Johnston KW, Brown PM, Kassam M. Problems of carotid Doppler scanning which can be overcome by using frequency analysis. Stroke 1982;13(5):660-6. [DOI] [PubMed] [Google Scholar]
Johnston 1985 {published data only}
- Johnston KW, Haynes RB, Douville Y, Lally ME, Brown PM, Cobbold RS. Accuracy of carotid Doppler peak frequency analysis: results determined by receiver operating characteristic curves and likelihood ratios. Journal of Vascular Surgery 1985;2(4):515-23. [PubMed] [Google Scholar]
Johnston 2001 {published data only}
- Johnston DC, Goldstein LB. Clinical carotid endarterectomy decision making: noninvasive vascular imaging versus angiography. Neurology 2001;56(8):1009-15. [PMID: ] [DOI] [PubMed] [Google Scholar]
Jones 1982 {published data only}
- Jones AM, Biller J, Cowley AR, Howard G, Toole JF, McKinney WM. Extra-cranial carotid-artery arteriosclerosis - diagnosis with continuous-wave doppler and real-time ultrasound studies. Archives of Neurology 1982;39(7):393-4. [DOI] [PubMed] [Google Scholar]
Juhel 1983 {published data only}
- Juhel P, Leroux C. Ultrasonic study of the carotid artery bifurcation. Comparative study with arteriography (50 cases). Archives des Maladies du Coeur et des Vaisseaux 1983;76(12):1437-44. [PubMed] [Google Scholar]
Jung 2000 {published data only}
- Jung EM, Butter F, Rupp N. Diagnosis of pre-occlusive stenosis of the internal carotid artery by power mode ultrasound. RoFo: Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin 2000;172(7):636-40. [DOI] [PubMed] [Google Scholar]
Jung 2002 {published data only}
- Jung EM, Kubale R, Clevert DA, Lutz R, Rupp N. B-flow and contrast medium-enhanced power Doppler (Optison(R)) - preoperative diagnosis of high-grade stenosis of the internal carotid artery. RoFo: Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin 2002;174(1):62-9. [DOI] [PubMed] [Google Scholar]
Kagawa 1996 {published data only}
- Kagawa R, Moritake K, Shima T, Okada Y. Validity of B-mode ultrasonographic findings in patients undergoing carotid endarterectomy in comparison with angiographic and clinicopathologic features. Stroke 1996;27(4):700-5. [DOI] [PubMed] [Google Scholar]
Keberle 2001 {published data only}
- Keberle M, Jenett M, Wittenberg G, Kessler C, Beissert M, Hahn D. Comparison of 3D power doppler ultrasound, color doppler ultrasound and digital subtraction angiography in carotid stenosis. RoFo: Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin 2001;173(2):133-8. [DOI] [PubMed] [Google Scholar]
Keller 1978 {published data only}
- Keller HM, Schubiger O, Krayenbuhl C, Zumstein B. Cerebrovascular Doppler examination and cerebral angiography - alternative or complementary? Neuroradiology 1978;16:140-4. [DOI] [PubMed] [Google Scholar]
Kim 2016 {published data only}
- Kim AH, Augustin G, Kim H, Shevitz AJ, Morrow KL, Trivonovich MR, et al. Comparison of two duplex ultrasound diagnostic criteria for carotid stenosis with computed tomography angiography. Journal of Vascular Surgery 2016;64(3):864-5. [Google Scholar]
Kim 2018 {published data only}
- Kim AH, Augustin G, Shevitz A, Kim H, Trivonovich MR, Powell AR, et al. Carotid Consensus Panel duplex criteria can replace modified University of Washington criteria without affecting accuracy. Vascular Medicine 2018;23(2):126-33. [DOI] [PubMed] [Google Scholar]
Kirsch 1994 {published data only}
- Kirsch JD, Wagner LR, James EM, Charboneau JW, Nichols DA, Meyer FB, et al. Carotid-artery occlusion - positive predictive value of duplex sonography compared with arteriography. Journal of Vascular Surgery 1994;19(4):642-9. [DOI] [PubMed] [Google Scholar]
Knox 1982 {published data only}
- Knox RA, Breslau PJ, Strandness DE Jr. A simple parameter for accurate detection of severe carotid disease. British Journal of Surgery 1982;69(4):230-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Koga 1983 {published data only}
- Koga H, Nishimura S, Kawano T, Ono H, Mori K, Yokoyama H. Digital subtraction angiography and ultrasound sonography for detection of occlusive carotid lesions in the neck. Neurologia Medico-Chirurgica 1983;23(9):705-10. [DOI] [PubMed] [Google Scholar]
Koga 2001 {published data only}
- Koga M, Kimura K, Minematsu K, Yamaguchi T. Diagnosis of internal carotid artery stenosis greater than 70% with power Doppler duplex sonography. American Journal of Neuroradiology 2001;22(2):413-7. [PMC free article] [PubMed] [Google Scholar]
Korteweg 2008 {published data only}
- Korteweg MA, Kerkhoff H, Bakker J, Elgersma OEH. Efficacy of patient selection strategies for carotid endarterectomy by contrast-enhanced MRA on a 1 T machine and duplex ultrasound in a regional hospital. Clinical Radiology 2008;63(2):174-83. [DOI] [PubMed] [Google Scholar]
Krappel 2002 {published data only}
- Krappel FA, Bauer E, Harland U. Can TOF MRA replace duplex and Doppler sonography in preoperative assessment of the carotid arteries? A prospective comparison and review of the literature. Zeitschrift fur Orthopadie und ihre Grenzgebiete 2002;140(4):435-9. [DOI] [PubMed] [Google Scholar]
Krasinski 2009 {published data only}
- Krasinski A, Chiu B, Fenster A, Parraga G. Magnetic resonance imaging and three-dimensional ultrasound of carotid atherosclerosis: mapping regional differences. Journal of Magnetic Resonance Imaging 2009;29(4):901-8. [DOI] [PubMed] [Google Scholar]
Kreske 1999 {published data only}
- Kreske ED, Wolk SW, Shanley CJ, Lampman RM, Knake JE, Lange LA, et al. Duplex ultrasonography to predict internal carotid artery stenoses exceeding 50% and 70% as defined by NASCET: the need for multiple criteria. Vascular Surgery 1999;33(5):497-506. [Google Scholar]
Kuhn 1981 {published data only}
- Kuhn FP, Kramer G, Gunther R, Thelen M. B-scan sonography of the carotid artery. Technique, results and indications (author's translation). RoFo: Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin 1981;135(4):407-11. [DOI] [PubMed] [Google Scholar]
Kuhn 1984 {published data only}
- Kuhn FP, Kramer G, Bauer R, Klotter HJ. 10-MHz-B-scan sonography of the cervical carotid arteries. RoFo: Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin 1984;140(6):677-83. [DOI] [PubMed] [Google Scholar]
Labropoulos 1997 {published data only}
- Labropoulos N, Androulakis A, Allan R, Giannoukas AD, Touloupakis E, Tegos T, et al. Value of color flow duplex imaging in detection of subtotal and total internal carotid artery occlusion. Vascular Surgery 1997;31(6):775-9. [Google Scholar]
Langlois 1983 {published data only}
- Langlois Y, Roederer GO, Chan A, Phillips DJ, Beach KW, Martin D, et al. Evaluating carotid artery disease. The concordance between pulsed Doppler/spectrum analysis and angiography. Ultrasound in Medicine & Biology 1983;9(1):51-63. [DOI] [PubMed] [Google Scholar]
Lee 1992 {published data only}
- Lee TH, Ryu SJ, Chen ST, Tseng KJ. Comparison between carotid duplex sonography and angiography in the diagnosis of extracranial internal carotid artery occlusion. Journal of the Formosan Medical Association 1992;91(6):575-9. [PubMed] [Google Scholar]
Lee 1996 {published data only}
- Lee DH, Gao FQ, Rankin RN, Pelz DM, Fox AJ. Duplex and color Doppler flow sonography of occlusion and near occlusion of the carotid artery. American Journal of Neuroradiology 1996;17(7):1267-74. [PMC free article] [PubMed] [Google Scholar]
Lefemine 1986 {published data only}
- Lefemine AA, Broach J, Woolley TW. Comparison of arteriography and noninvasive techniques for the diagnosis of carotid artery disease. A statistical analysis of 140 patients. American Surgeon 1986;52(10):526-31. [PubMed] [Google Scholar]
Leonardo 2003 {published data only}
- Leonardo G, Crescenzi B, Cotrufo R, Tecame S, De Santo LS, Della Corte A, et al. Improvement in accuracy of diagnosis of carotid artery stenosis with duplex ultrasound scanning with combined use of linear array 7.5 MHz and convex array 3.5 MHz probes: validation versus 489 arteriographic procedures. Journal of Vascular Surgery 2003;37(6):1240-7. [DOI] [PubMed] [Google Scholar]
Levien 1985 {published data only}
- Levien LJ, Voll CL, Lithgow-Jolly P, Fritz VU. The value of noninvasive investigation in the diagnosis of total occlusion of the internal carotid artery. Stroke 1985;16(6):945-9. [DOI] [PubMed] [Google Scholar]
Lewis 1980 {published data only}
- Lewis RR, Beasley MG, Gosling RG. Detection of disease at the carotid bifurcation using ultrasound - including an imaging system. Journal of the Royal Society of Medicine 1980;73(3):172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lewis 2002 {published data only}
- Lewis SC, Wardlaw JM. Which Doppler velocity is best for assessing suitability for carotid endarterectomy? European Journal of Ultrasound 2002;15(1-2):9-20. [DOI] [PubMed] [Google Scholar]
Lindegaard 1984 {published data only}
- Lindegaard KF, Bakke SJ, Grip A, Nornes H. Pulsed Doppler techniques for measuring instantaneous maximum and mean flow velocities in carotid arteries. Ultrasound in Medicine & Biology 1984;10(4):419-26. [DOI] [PubMed] [Google Scholar]
Link 1997a {published data only}
- Link J, MullerHulsbeck S, Wesner F, Hopfner M, Schwarzenberg H, Heller M. The value of 3D-CT angiography and duplex sonography in comparison to arteriography in carotid artery stenoses. Rofo: Fortschritte Auf Dem Gebiet Der Rontgenstrahlen Und Der Bildgebenden Verfahren 1997;166(1):30-5. [DOI] [PubMed] [Google Scholar]
Long 2001 {published data only}
- Long SM, Kern JA, Fiser SM, Kaza AK, Cassada DC, Miller BT, et al. Carotid arteriography impacts carotid stenosis management. Vascular Surgery 2001;35(4):251-7. [DOI] [PubMed] [Google Scholar]
Lovelock 2003 {published data only}
- Lovelock C, Mitchell P, Brown J, Campbell D, Field P, Parsons M, et al. Is Doppler ultrasound sufficient as the sole investigation before carotid endarterectomy? Journal of Clinical Neurosciences 2003;10(4):420-4. [DOI] [PubMed] [Google Scholar]
Ludwig 1984 {published data only}
- Ludwig M, Harder T, Trubestein G. Two-dimensional ultrasonics - a non-invasive method for the diagnosis of arteriosclerotic changes in the region of the supra-aortic branches. Deutsche Medizinische Wochenschrift 1984;109(49):1878-80. [DOI] [PubMed] [Google Scholar]
Lusby 1981 {published data only}
- Lusby RJ, Machleder HI, Jeans W, Skidmore R, Woodcock JP, Clifford PC, et al. Vessel wall and blood flow dynamics in arterial disease. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 1981;294(1072):231-9. [DOI: 10.1098/rstb.1981.0102] [DOI] [PubMed] [Google Scholar]
Macharzina 2018 {published data only}
- Macharzina RR, Kocher S, Messe SR, Rutkowski T, Hoffmann F, Vogt M, et al. 4-dimensionally guided 3-dimensional color-Doppler ultrasonography quantifies carotid artery stenosis with high reproducibility and accuracy. JACC: Cardiovascular Imaging 2018;11(3):386-96. [DOI] [PubMed] [Google Scholar]
Macheers 1986 {published data only}
- Macheers SK, Kerstein MD. Ultrasonic imaging in the diagnosis of carotid vascular disease with attention to operated upon nonangiographic lesions. American Surgeon 1986;52(10):532-5. [PubMed] [Google Scholar]
MacKenzie 2002 {published data only}
- MacKenzie KS, French-Sherry E, Burns K, Pooley T, Bassiouny HS. B-mode ultrasound measurement of carotid bifurcation stenoses: is it reliable? Vascular and Endovascular Surgery 2002;36(2):123-35. [DOI] [PubMed] [Google Scholar]
Makaryus 2009 {published data only}
- Makaryus AN, Phillips LM, Wright P, Freeman J, Green SJ, Ong L, et al. Mandatory diagnostic angiography for carotid artery stenosis prior to carotid artery intervention. Journal of Interventional Cardiology 2009;22(1):16-21. [DOI] [PubMed] [Google Scholar]
Manga 1986 {published data only}
- Manga P, Dhurandhar RW, Stockard B. Doppler frequency ratio and peak frequency in the assessment of carotid artery disease: a comparative study with angiography. Ultrasound in Medicine & Biology 1986;12(7):573-6. [DOI] [PubMed] [Google Scholar]
Mansour 1995 {published data only}
- Mansour MA, Mattos MA, Hood DB, Hodgson KJ, Barkmeier LD, Ramsey DE, et al. Detection of total occlusion, string sign, and preocclusive stenosis of the internal carotid-artery by color-flow duplex scanning. American Journal Surgery 1995;170(2):154-8. [DOI] [PubMed] [Google Scholar]
Marshall 1988 {published data only}
- Marshall WG Jr, Kouchoukos NT, Murphy SF, Pelate C. Carotid endarterectomy based on duplex scanning without preoperative arteriography. Circulation 1988;78(3 Pt 2):I1-5. [PubMed] [Google Scholar]
Martin‐Conejero 2007 {published data only}
- Martin-Conejero A, Sanchez-Hervas L, Monux-Ducaju G, Reina-Gutierrez T, Morata-Barrado PC, Serrano-Hernando FJ. Validation of Doppler ultrasound as the only diagnosis of extracranial carotid stenosis. Angiologia 2007;59(3):217-24. [Google Scholar]
Matos 2014 {published data only}
- Matos JM, Barshes NR, Mccoy S, Pisimisis G, Felkai D, Kougias P, et al. Validating common carotid stenosis by duplex ultrasound with carotid angiogram or computed tomography scan. Journal of Vascular Surgery 2014;59(2):435-9. [DOI] [PubMed] [Google Scholar]
Mattle 1991 {published data only}
- Mattle HP, Kent KC, Edelman RR, Atkinson DJ, Skillman JJ. Evaluation of the extracranial carotid arteries - correlation of magnetic-resonance angiography, duplex ultrasonography, and conventional angiography. Journal of Vascular Surgery 1991;13(6):838-45. [PubMed] [Google Scholar]
Mattos 1992 {published data only}
- Mattos MA, Hodgson KJ, Ramsey DE, Barkmeier LD, Sumner DS. Identifying total carotid occlusion with color flow duplex scanning. European Journal of Vascular Surgery 1992;6(2):204-10. [DOI] [PubMed] [Google Scholar]
Mattos 1994 {published data only}
- Mattos MA, Hodgson KJ, Faught WE, Mansour A, Barkmeier LD, Ramsey DE, et al. Carotid endarterectomy without angiography - is color-flow duplex scanning sufficient. Surgery 1994;116(4):776-83. [PubMed] [Google Scholar]
Matz 2017 {published data only}
- Matz O, Nikoubashman O, Rajkumar P, Keuler A, Wiesmann M, Schulz JB, et al. Grading of proximal internal carotid artery (ICA) stenosis by Doppler/duplex ultrasound (DUS) and computed tomographic angiography (CTA): correlation and inter-rater reliability in real-life practice. Acta Neurologica Belgica 2017;117(1):183-8. [DOI] [PubMed] [Google Scholar]
McLaren 1996 {published data only}
- McLaren JT, Donaghue CC, Drezner AD. Accuracy of carotid duplex examination to predict proximal and intrathoracic lesions. American Journal of Surgery 1996;172(2):149-50. [DOI] [PubMed] [Google Scholar]
Mitchell 1991 {published data only}
- Mitchell P, Gibson R, Thomson K. Duplex ultrasound assessment of extra cranial carotid artery disease: its introduction into a radiology department. Australasian Radiology 1991;35(1):40-3. [DOI] [PubMed] [Google Scholar]
Mittl 1994 {published data only}
- Mittl RL, Broderick M, Carpenter JP, Goldberg HI, Listrud J, Mishkin MM, et al. Blinded-reader comparison of magnetic-resonance angiography and duplex ultrasonography for carotid-artery bifurcation stenosis. Stroke 1994;25(1):4-10. [DOI] [PubMed] [Google Scholar]
Modaresi 1999 {published data only}
- Modaresi KB, Cox TCS, Summers PE, Jarosz JM, Verma H, Taylor PR, et al. Comparison of intra-arterial digital subtraction angiography, magnetic resonance angiography and duplex ultrasonography for measuring carotid artery stenosis. British Journal of Surgery 1999;86(11):1422-6. [DOI] [PubMed] [Google Scholar]
Moll 2000 {published data only}
- Moll R, Fieger M, Knupffer J, Schindler G. Which imaging in the diagnosis of common carotid artery bifurcation disease? Value of color flow Doppler, CT angiography vs digital subtraction angiography. Clinical Neuroradiology 2000;10(2):68-75. [Google Scholar]
Moll 2001 {published data only}
- Moll R, Dinkel HP. Value of the CT angiography in the diagnosis of common carotid artery bifurcation disease: CT angiography versus digital subtraction angiography and color flow Doppler. European Journal of Radiology 2001;39(3):155-62. [DOI] [PubMed] [Google Scholar]
Moneta 1993 {published data only}
- Moneta GL, Edwards JM, Chitwood RW, Taylor LM Jr, Lee RW, Cummings CA, et al. Correlation of North American Symptomatic Carotid Endarterectomy Trial (NASCET) angiographic definition of 70% to 99% internal carotid artery stenosis with duplex scanning. Journal of Vascular Surgery 1993;17(1):152-9. [DOI] [PubMed] [Google Scholar]
Moore 1986 {published data only}
- Moore DJ, Miles RD, Ohgi S, Landholt G, Call PJ, Kolm P, et al. Relative accuracy of the diagnostic components of noninvasive carotid arterial tests: a comparison of pulsed Doppler arteriography and spectrum analysis. Journal of Vascular Surgery 1986;3(3):502-6. [DOI] [PubMed] [Google Scholar]
Moore 1988 {published data only}
- Moore WS, Ziomek S, Quinones-Baldrich WJ, Machleder HI, Busuttil RW, Baker JD. Can clinical evaluation and noninvasive testing substitute for arteriography in the evaluation of carotid artery disease? Annals of Surgery 1988;208(1):91-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Muller 2015 {published data only}
- Muller M, Agten CA, Osterreich M, Hoffmann M. Assessing internal carotid artery stenosis with a semiautomated computed tomography angiography tool and duplex ultrasound. Journal of Vascular Surgery 2015;61(6):1449-56. [DOI] [PubMed] [Google Scholar]
Murie 1984 {published data only}
- Murie JA, Quin RO, Forrest H, Sheldon CD. Pulsed Doppler imaging and spectrum analysis for detection of carotid artery disease. Angiology 1984;35(4):215-21. [DOI] [PubMed] [Google Scholar]
Muto 1996 {published data only}
- Muto PM, Welch HJ, Mackey WC, O'Donnell TF. Evaluation of carotid artery stenosis: is duplex ultrasonography sufficient? Journal of Vascular Surgery 1996;24(1):17-24. [DOI] [PubMed] [Google Scholar]
Neale 1994 {published data only}
- Neale ML, Chambers JL, Kelly AT, Connard S, Lawton MA, Roche J, et al. Reappraisal of duplex criteria to assess significant carotid stenosis with special reference to reports from the North American Symptomatic Carotid Endarterectomy Trial and the European Carotid Surgery Trial. Journal of Vascular Surgery 1994;20(4):642-9. [DOI] [PubMed] [Google Scholar]
Neff 2005 {published data only}
- Neff KW, Kilian AK, Meairs S, Duber C. Correlation of duplex sonographic stenosis grading by means of cross-sectionally determined and MRI-based blood volume quantification in unilateral stenosis of the internal carotid artery. Rofo: Fortschritte Auf Dem Gebiet Der Rontgenstrahlen Und Der Bildgebenden Verfahren 2005;177(7):992-9. [DOI] [PubMed] [Google Scholar]
Neschis 2001 {published data only}
- Neschis DG, Lexa FJ, Davis JT, Carpenter JP. Duplex criteria for determination of 50% or greater carotid stenosis. Journal of Ultrasound in Medicine 2001;20(3):207-15. [DOI] [PubMed] [Google Scholar]
New 2001 {published data only}
- New G, Roubin GS, Oetgen ME, Lawrence EJ, Iyer SS, Moussa I, et al. Validity of duplex ultrasound as a diagnostic modality for internal carotid artery disease. Catheterization and Cardiovascular Interventions 2001;52(1):9-15. [DOI] [PubMed] [Google Scholar]
Nichtweiss 1987 {published data only}
- Nichtweiss M, Wiegand C, Wuppermann T. Possibility of errors in conventional Doppler sonography of the carotid arteries. Deutsche Medizinische Wochenschrift 1987;112(47):1807-11. [DOI] [PubMed] [Google Scholar]
Nonent 2004 {published data only}
- Nonent M, Serfaty JM, Nighoghossian N, Rouhart F, Derex L, Rotaru C, et al. Concordance rate differences of 3 noninvasive imaging techniques to measure carotid stenosis in clinical routine practice: results of the CARMEDAS multicenter study. Stroke 2004;35(3):682-6. [DOI] [PubMed] [Google Scholar]
Nonent 2011 {published data only}
- Nonent M, Ben Salem D, Serfaty J-M, Buthion V, Pasco-Papon A, Rotaru C, et al. Overestimation of moderate carotid stenosis assessed by both Doppler US and contrast enhanced 3D-MR angiography in the CARMEDAS study. Journal of Neuroradiology 2011;38(3):148-55. [DOI] [PubMed] [Google Scholar]
Nordal 1993 {published data only}
- Nordal HJ, Dullerud R, Amthor KF. Grading stenoses of the internal carotid-artery by estimation of blood velocity with pulsed Doppler ultrasound. European Neurology 1993;33(1):38-43. [DOI] [PubMed] [Google Scholar]
Norrving 1981 {published data only}
- Norrving B, Cronqvist S. Doppler examination of the carotid arteries. A comparative study with angiography. Acta Neurologica Scandinavica 1981;64(4):241-52. [DOI] [PubMed] [Google Scholar]
Norrving 1985 {published data only}
- Norrving B, Jungqvist G, Olivecrona H, Cronqvist S, Nilsson B. Non-invasive detection of carotid bifurcation disease by continuous-wave Doppler with spectral analysis. Acta Neurologica Scandinavica 1985;72(2):203-9. [DOI] [PubMed] [Google Scholar]
Nowak 2007 {published data only}
- Nowak J, Jogestrand T. Duplex ultrasonography is an efficient diagnostic tool for the detection of moderate to severe internal carotid artery stenosis. Clinical Physiology and Functional Imaging 2007;27(3):144-7. [DOI] [PubMed] [Google Scholar]
O'Callaghan 2011 {published data only}
- O'Callaghan M, Crotty G, Mok T, Harrington H. Concordance between carotid doppler ultrasound and computerised tomographic angiography in predicting the need for surgery in carotid artery stenosis. European Journal of Neurology 2011;18:565. [Google Scholar]
O'Leary 1987 {published data only}
- O'Leary DH, Bryan FA, Goodison MW, Rifkin MD, Gramiak R, Ball M, et al. Measurement variability of carotid atherosclerosis: real-time (B-mode) ultrasonography and angiography. Stroke 1987;18(6):1011-7. [DOI] [PubMed] [Google Scholar]
Ohm 2005 {published data only}
- Ohm C, Bendick PJ, Monash J, Bove PG, Brown OW, Long GW, et al. Diagnosis of total internal carotid occlusions with duplex ultrasound and ultrasound contrast. Vascular and Endovascular Surgery 2005;39(3):237-43. [DOI] [PubMed] [Google Scholar]
Orgles 1999 {published data only}
- Orgles CS, Chakraverty S, Gopichandran TD. Carotid stenosis in the real world - can Doppler ultrasound replace angiography in a district general hospital setting? Clinical Radiology 1999;54(10):655-8. [DOI] [PubMed] [Google Scholar]
Paciaroni 2003 {published data only}
- Paciaroni M, Caso V, Cardaioli G, Corea F, Milia P, Venti M, et al. Is ultrasound examination sufficient in the evaluation of patients with internal carotid artery severe stenosis or occlusion? Cerebrovascular Diseases 2003;15(3):173-6. [DOI] [PubMed] [Google Scholar]
Padayachee 1982 {published data only}
- Padayachee TS, Lewis RR, Gosling RG. Detection of carotid bifurcation disease: comparison of ultrasound tests with angiography. British Journal of Surgery 1982;69(4):218-22. [DOI] [PubMed] [Google Scholar]
Padayachee 1997 {published data only}
- Padayachee TS, Cox TCS, Modaresi KB, Colchester ACF, Taylor PR. The measurement of internal carotid artery stenosis: comparison of duplex with digital subtraction angiography. European Journal of Vascular and Endovascular Surgery 1997;13(2):180-5. [DOI] [PubMed] [Google Scholar]
Paivansalo 1996 {published data only}
- Paivansalo M, Leinonen S, Turunen J, Tikkakoski T, Suramo I. Quantification of carotid artery stenosis with various Doppler velocity parameters. Fortschritte Auf Dem Gebiete Der Rontgenstrahlen Und Der Neuen Bildgebenden Verfahren 1996;164(2):108-13. [DOI] [PubMed] [Google Scholar]
Patel 1995 {published data only}
- Patel MR, Kuntz KM, Klufas RA, Kim D, Kramer J, Polak JF, et al. Preoperative assessment of the carotid bifurcation. Can magnetic resonance angiography and duplex ultrasonography replace contrast arteriography? Stroke 1995;26(10):1753-8. [DOI] [PubMed] [Google Scholar]
Patel 2002 {published data only}
- Patel SG, Collie DA, Wardlaw JM, Lewis SC, Wright AR, Gibson RJ, et al. Outcome, observer reliability, and patient preferences if CTA, MRA, or Doppler ultrasound were used, individually or together, instead of digital subtraction angiography before carotid endarterectomy. Journal of Neurology, Neurosurgery and Psychiatry 2002;73(1):21-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pelz 2015 {published data only}
- Pelz JO, Weinreich A, Fritzsch D, Saur D. Quantification of internal carotid artery stenosis with 3D ultrasound angiography. Ultraschall in der Medizin 2015;36(5):487-93. [DOI] [PubMed] [Google Scholar]
Petisco 2015 {published data only}
- Petisco ACG, Saleh MH, Jesus CA, Metzger PB, Dourado MS. Doppler ultrasonography of carotid arteries: velocity criteria validated by arteriography [Doppler ecografia das artérias carótidas: critérios de velocidade validados pela arteriografia]. Arquivos Brasileiros de Cardiologia 2015;28(1):17-21. [Google Scholar]
Pfister 2009 {published data only}
- Pfister K, Rennert J, Greiner B, Jung W, Stehr A, Gossmann H, et al. Pre-surgical evaluation of ICA-stenosis using 3D power Doppler, 3D color coded Doppler sonography, 3D B-flow and contrast enhanced B-flow in correlation to CTA/MRA: first clinical results. Clinical Hemorheology and Microcirculation 2009;41(2):103-16. [DOI] [PubMed] [Google Scholar]
Poindexter 1991 {published data only}
- Poindexter JM, Shah PM, Clauss RH, Babu SC. The clinical utility of carotid duplex scanning. Journal of Cardiovascular Surgery 1991;32(1):64-8. [PubMed] [Google Scholar]
Polak 1989 {published data only}
- Polak JF, Dobkin GR, O'Leary DH, Wang AM, Cutler SS. Internal carotid artery stenosis: accuracy and reproducibility of color-Doppler-assisted duplex imaging. Radiology 1989;173(3):793-8. [DOI] [PubMed] [Google Scholar]
Polak 1992 {published data only}
- Polak JF, Bajakian RL, O'Leary DH, Anderson MR, Donaldson MC, Jolesz FA. Detection of internal carotid-artery stenosis - comparison of MR angiography, color doppler sonography, and arteriography. Radiology 1992;182(1):35-40. [DOI] [PubMed] [Google Scholar]
Polak 1993 {published data only}
- Polak JF, Kalina P, Donaldson MC, O'Leary DH, Whittemore AD, Mannick JA. Carotid endarterectomy - preoperative evaluation of candidates with combined doppler sonography and MR angiography - work in progress. Radiology 1993;186(2):333-8. [DOI] [PubMed] [Google Scholar]
Portilla 2010 {published data only}
- Portilla Cuenca JC, Ramirez-Moreno JM, Fernandez de Alarcon L, Garcia Castanon I, Caballero Munoz M, Serrano Cabrera A, et al. Validation of supra-aortic trunks ultrasound in the diagnosis of atherosclerotic disease of the internal carotid artery. Comparison of the results with angiography. Neurologia 2010;25(6):357-63. [PubMed] [Google Scholar]
Puzich 1986 {published data only}
- Puzich R, Becker E, Hegerl U, Rossdeutscher R, Banzer D, Hepp W. Diagnostics of extracranial carotid stenoses - comparison of CW-doppler sonography and intravenous digital subtraction angiography. European Archives of Psychiatry and Clinical Neuroscience 1986;235(4):210-3. [DOI] [PubMed] [Google Scholar]
Py 2001 {published data only}
- Py MO, Andre C, Azevedo FS, Domingues RC, Salomao RF. Internal carotid artery stenosis: comparison of duplex scan and magnetic resonance angiography with digital subtraction angiography. Arquivos de Neuro-Psiquiatria 2001;59(3-B):665-71. [DOI] [PubMed] [Google Scholar]
Qureshi 2001 {published data only}
- Qureshi AI, Suri MF, Ali Z, Kim SH, Fessler RD, Ringer AJ, et al. Role of conventional angiography in evaluation of patients with carotid artery stenosis demonstrated by Doppler ultrasound in general practice. Stroke 2001;32(10):2287-91. [DOI] [PubMed] [Google Scholar]
Ratliff 1985 {published data only}
- Ratliff DA, Hames TK, Humphries KN, Birch S, Chant ADB. The reliability of doppler ultrasound techniques in the assessment of carotid disease. Angiology 1985;36(6):333-40. [DOI] [PubMed] [Google Scholar]
Ricotta 1987 {published data only}
- Ricotta JJ, Bryan FA, Bond MG, Kurtz A, O'Leary DH, Raines JK, et al. Multicenter validation study of real-time (B-mode) ultrasound, arteriography, and pathologic examination. Journal of Vascular Surgery 1987;6(5):512-20. [PubMed] [Google Scholar]
Riles 1992 {published data only}
- Riles TS, Eidelman EM, Litt AW, Pinto RS, Oldford F, Schwartzenberg GW. Comparison of magnetic-resonance angiography, conventional angiography, and duplex scanning. Stroke 1992;23(3):341-6. [DOI] [PubMed] [Google Scholar]
Rodrigus 1995 {published data only}
- Rodrigus IE, De Maeseneer MG, Van Schil PE, Pickut BA, D'Archambeau OC, Vanmaele RG. Colour duplex scanning versus angiography: a retrospective assessment of carotid stenosis. Cardiovascular Surgery 1995;3(2):213-7. [DOI] [PubMed] [Google Scholar]
Saba 2008 {published data only}
- Saba L, Sanfilippo R, Montisci R, Mallarini G. Agreement between multidetector-row CT angiography and ultrasound echo-color Doppler in the evaluation of carotid artery stenosis. Cerebrovascular Diseases 2008;26(5):525-32. [DOI] [PubMed] [Google Scholar]
Saba 2010 {published data only}
- Saba L, Sanfilippo R, Montisci R, Mallarini G. Correlation between US-PSV and MDCTA in the quantification of carotid artery stenosis. European Journal of Radiology 2010;74(1):99-103. [DOI] [PubMed] [Google Scholar]
Sabeti 2004 {published data only}
- Sabeti S, Schillinger M, Mlekusch W, Willfort A, Haumer M, Nachtmann T, et al. Quantification of internal carotid artery stenosis with duplex US: comparative analysis of different flow velocity criteria. Radiology 2004;232(2):431-9. [DOI] [PubMed] [Google Scholar]
Saia 1981 {published data only}
- Saia A, Manzoni S, De Zanche L, Meneghetti G, Tonin P. Carotid Doppler examination: a correlation with angiography. Italian Journal of Neurological Sciences 1981;2(1):73-6. [DOI] [PubMed] [Google Scholar]
Samarzija 2018 {published data only}
- Samarzija K, Milosevic P, Jurjevic Z, Erdeljac E. Grading of carotid artery stenosis with computed tomography angiography: whether to use the narrowest diameter or the cross-sectional area. Insights into Imaging 2018;9(4):527-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sameshima 1999 {published data only}
- Sameshima T, Futami S, Morita Y, Yokogami K, Miyahara S, Sameshima Y, et al. Clinical usefulness of and problems with three-dimensional CT angiography for the evaluation of arteriosclerotic stenosis of the carotid artery: comparison with conventional angiography, MRA, and ultrasound sonography. Surgical Neurology 1999;51(3):301-9. [DOI] [PubMed] [Google Scholar]
Saouaf 1998 {published data only}
- Saouaf R, Grassi CJ, Hartnell GG, Wheeler H, Suojanen JN. Complete MR angiography and Doppler ultrasound as the sole imaging modalities prior to carotid endarterectomy. Clinical Radiology 1998;53(8):579-86. [DOI] [PubMed] [Google Scholar]
Satiani 1988 {published data only}
- Satiani B, Porter R, Biggers K, Burns R. Evaluation of the color coded doppler ultrasound in detecting carotid bifurcation disease. Journal of Cardiovascular Surgery 1988;29(2):196-200. [PubMed] [Google Scholar]
Savic 2010 {published data only}
- Savic ZN, Davidovic LB, Sagic DZ, Brajovic MD, Popovic SS. Correlation of color Doppler with multidetector CT angiography findings in carotid artery stenosis. Scientific World Journal 2010;10(101131163):1818-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Senant 1984 {published data only}
- Senant J, Menard JF. Comparison of the Doppler examination and arteriography in 209 cases of carotid stenosis and thrombosis. Revue d'Electroencéphalographie et de Neurophysiologie Clinique 1984;14(2):161-4. [DOI] [PubMed] [Google Scholar]
Serfaty 2000 {published data only}
- Serfaty JM, Chirossel P, Chevallier JM, Ecochard R, Froment JC, Douek PC. Accuracy of three-dimensional gadolinium-enhanced MR angiography in the assessment of extracranial carotid artery disease. American Journal of Roentgenology 2000;175(2):455-63. [DOI] [PubMed] [Google Scholar]
Shaalan 2008 {published data only}
- Shaalan WE, Wahlgren CM, Desai T, Piano G, Skelly C, Bassiouny HS. Reappraisal of velocity criteria for carotid bulb/internal carotid artery stenosis utilizing high-resolution B-mode ultrasound validated with computed tomography angiography. Journal of Vascular Surgery 2008;48(1):103-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shakhnovich 2010 {published data only}
- Shakhnovich I, Kiser D, Satiani B. Importance of validation of accuracy of duplex ultrasonography in identifying moderate and severe carotid artery stenosis. Vascular and Endovascular Surgery 2010;44(6):483-8. [DOI] [PubMed] [Google Scholar]
Sillesen 1988 {published data only}
- Sillesen H, Neergaard K, Buchardt Hansen HJ. Quantitative assessment of carotid artery disease by continuous wave and pulsed multigated Doppler - prospective comparison with angiography. Ultrasound in Medicine & Biology 1988;14(8):641-8. [DOI] [PubMed] [Google Scholar]
Sillesen 1991 {published data only}
- Sillesen HH, Just SR, Hansen L, Schroeder TV. Diagnosis of carotid artery sclerosis with Doppler ultrasound. Description of the method and a prospective comparison with arteriography. Ugeskrift for Laeger 1991;153(49):3483-5. [PubMed] [Google Scholar]
Sitzer 1993 {published data only}
- Sitzer M, Furst G, Fischer H, Siebler M, Fehlings T, Kleinschmidt A, et al. Between-method correlation in quantifying internal carotid stenosis. Stroke 1993;24:1513–8. [DOI] [PubMed] [Google Scholar]
Slovut 2010 {published data only}
- Slovut DP, Romero JM, Hannon KM, Dick J, Jaff MR. Detection of common carotid artery stenosis using duplex ultrasonography: a validation study with computed tomographic angiography. Journal of Vascular Surgery 2010;51(1):65-70. [DOI] [PubMed] [Google Scholar]
Soulez 1999 {published data only}
- Soulez G, Therasse E, Robillard P, Fontaine A, Denbow N, Bourgouin P, et al. The value of internal carotid systolic velocity ratio for assessing carotid artery stenosis with Doppler sonography. American Journal of Roentgenology 1999;172(1):207-12. [DOI] [PubMed] [Google Scholar]
Srinivasan 1995 {published data only}
- Srinivasan J, Mayberg MR, Weiss DG, Eskridge J. Duplex accuracy compared with angiography in the Veterans Affairs Cooperative Studies Trial for Symptomatic Carotid Stenosis. Neurosurgery 1995;36(4):648-55. [DOI] [PubMed] [Google Scholar]
Staikov 2000 {published data only}
- Staikov IN, Arnold M, Mattle HP, Remonda L, Sturzenegger M, Baumgartner RW, et al. Comparison of the ECST, CC, and NASCET grading methods and ultrasound for assessing carotid stenosis. European Carotid Surgery Trial. North American Symptomatic Carotid Endarterectomy Trial. Journal of Neurology 2000;247(9):681-6. [DOI] [PubMed] [Google Scholar]
Staikov 2002 {published data only}
- Staikov IN, Nedeltchev K, Arnold M, Remonda L, Schroth G, Sturzenegger M, et al. Duplex sonographic criteria for measuring carotid stenoses. Journal of Clinical Ultrasound 2002;30(5):275-81. [DOI] [PubMed] [Google Scholar]
Staikov 2004 {published data only}
- Staikov I, Arnold M, Mattle H, Schroth G, Remonda L, Sturzenegger M, et al. Doppler ultrasound criteria for measuring carotid stenosis. Acta Medica Bulgarica 2004;31(2):3-13. [Google Scholar]
Stavenow 1987 {published data only}
- Stavenow L, Bjerre P, Lindgarde F. Experiences of duplex ultrasonography of carotid arteries performed by clinician - correlation to angiography. Acta Medica Scandinavica 1987;222(1):31-6. [DOI] [PubMed] [Google Scholar]
Stefanini 2012 {published data only}
- Stefanini M, Gaspari E, Boi L, Del Giudice C, Mastrangeli R, Nucera F, et al. Correlation between US-PSV and 64-Row MDCTA with advanced vessel analysis in the quantification of 50-70% carotid artery stenosis. International Journal of Vascular Medicine 2012;2012(101542608):928638. [DOI] [PMC free article] [PubMed] [Google Scholar]
Steger 1995 {published data only}
- Steger W, Vogl TJ, Rausch M, Pegios W, Schedel H, Heinzinger K. CT angiography in carotid stenosis. Diagnostic value compared to color-coded duplex ultrasonography and MR angiography. RoFo: Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin 1995;162(5):373-80. [DOI] [PubMed] [Google Scholar]
Steinke 1990 {published data only}
- Steinke W, Kloetzsch C, Hennerici M. Carotid-artery disease assessed by color doppler flow imaging - correlation with standard doppler sonography and angiography. American Journal of Roentgenology 1990;154(5):1061-8. [DOI] [PubMed] [Google Scholar]
Steinke 1997 {published data only}
- Steinke W, Ries S, Artemis N, Schwartz A, Hennerici M. Power Doppler imaging of carotid artery stenosis - comparison with color Doppler flow imaging and angiography. Stroke 1997;28(10):1981-7. [DOI] [PubMed] [Google Scholar]
Sumner 1979 {published data only}
- Sumner DS, Russell JB, Ramsey DE, Hajjar WM, Miles RD. Noninvasive diagnosis of extracranial carotid arterial disease: a prospective evaluation of pulsed-Doppler imaging and oculoplethysmography. Archives of Surgery 1979;114(11):1222-9. [DOI] [PubMed] [Google Scholar]
Sumner 1982 {published data only}
- Sumner DS, Russell JB, Miles RD. Are noninvasive tests sufficiently accurate to identify patients in need of carotid arteriography? Surgery 1982;91(6):700-6. [PubMed] [Google Scholar]
Tarnawski 1990 {published data only}
- Tarnawski M, Padayachee S, West DJ, Graves MJ, Ayton VT, Taylor MG, et al. The measurement of time-averaged flow by magnetic-resonance-imaging using continuous acquisition in the carotid arteries and its comparison with doppler ultrasound. Clinical Physics and Physiological Measurement 1990;11(1):27-36. [DOI] [PubMed] [Google Scholar]
Tateishi 2013 {published data only}
- Tateishi Y, Tsujino A, Hamabe J, Tasaki O, Morikawa M, Horie N, et al. Measurement of carotid stenosis using duplex ultrasonography with a microconvex array transducer: a validation with cerebral angiography. Journal of Stroke and Cerebrovascular Diseases 2013;22(8):e360-5. [DOI] [PubMed] [Google Scholar]
Tian 2016 {published data only}
- Tian Y, Ye Z, Wu J, Ma J, Zheng C, Fan X, et al. Prospective comparison contrast-enhanced ultrasound, and digital subtraction angiography in assessment of extracranial internal carotid artery stenosis. Acta Medica Mediterranea 2016;32(2):427-30. [Google Scholar]
Titi 2007 {published data only}
- Titi M, George C, Bhattacharya D, Rahi A, Woodhead PM, Stevenson WJ, et al. Comparison of carotid Doppler ultrasound and computerised tomographic angiography in the evaluation of carotid artery stenosis. Surgeon 2007;5(3):132-6. [DOI] [PubMed] [Google Scholar]
Tokunaga 2016 {published data only}
- Tokunaga K, Koga M, Yoshimura S, Arihiro S, Suzuki R, Nagatsuka K, et al. Optimal peak systolic velocity thresholds for predicting internal carotid artery stenosis greater than or equal to 50%, 60%, 70%, and 80%. Journal of Stroke and Cerebrovascular Diseases 2016;25(4):921-6. [DOI] [PubMed] [Google Scholar]
Tola 2004 {published data only}
- Tola M, Yurdakul M, Cumhur T. Combined use of color duplex ultrasonography and B-flow imaging for evaluation of patients with carotid artery stenosis. American Journal of Neuroradiology 2004;25(10):1856-60. [PMC free article] [PubMed] [Google Scholar]
Torvaldsen 1985 {published data only}
- Torvaldsen S, McCauley J, Patel AS, Chakera TM. Assessment of cervical carotid artery disease. A comparison between the Doppler "Echoflow" and conventional angiography. Medical Journal of Australia 1985;142(10):542-5. [PubMed] [Google Scholar]
Tschammler 1991 {published data only}
- Tschammler A, Landwehr P, Hohmann M, Moll R, Wittenberg G, Lackner K. Color-coded duplex sonography of the extracranial arteries supplying the brain: diagnostic significance and sources of error in comparison with i.a. DSA. RoFo: Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin 1991;155(5):452-9. [DOI] [PubMed] [Google Scholar]
Turnipseed 1982 {published data only}
- Turnipseed WD, Sackett JF, Strother CM, Crummy AB, Mistretta CA. A comparison of standard cerebral arteriography with noninvasive Doppler imaging and intravenous angiography. Archives of Surgery 1982;117(4):419-21. [DOI] [PubMed] [Google Scholar]
Turnipseed 1993a {published data only}
- Turnipseed WD, Kennell TW, Turski PA, Acher CW, Hoch JR. Combined use of duplex imaging and magnetic resonance angiography for evaluation of patients with symptomatic ipsilateral high-grade carotid stenosis. Journal of Vascular Surgery 1993;17(5):832-40. [DOI] [PubMed] [Google Scholar]
Utz 1983 {published data only}
- Utz F, Furst H, Becker HM. Non-invasive diagnosis of severe extra-cranial stenoses of the internal carotid-artery - validity of doppler ultrasonography. Langenbeck's Archives of Surgery 1983;361:943. [Google Scholar]
Vaisman 1986 {published data only}
- Vaisman U, Wojciechowski M. Carotid-artery disease - new criteria for evaluation by sonographic duplex scanning. Radiology 1986;158(1):253-5. [DOI] [PubMed] [Google Scholar]
Van Prehn 2008 {published data only}
- Van Prehn J, Muhs BE, Pramanik B, Ollenschleger M, Rockman CB, Cayne NS, et al. Multidimensional characterization of carotid artery stenosis using CT imaging: a comparison with ultrasound grading and peak flow measurement. European Journal of Vascular and Endovascular Surgery 2008;36(3):267-72. [DOI] [PubMed] [Google Scholar]
Vit 2003 {published data only}
- Vit A, De Candia A, Piccoli G, Como G, Pelizzo F, Bazzaocchi M. Color-Doppler sonography vs CT-angiography in discriminating carotid atherosclerotic plaques for surgical treatment. A prospective study. La Radiologia Medica 2003;106(4):382-90. [PubMed] [Google Scholar]
Von Arbin 1983 {published data only}
- Von Arbin M, Britton M, De Faire U, Gustafsson P. Non invasive assessment of the internal carotid artery in stroke patients. Scandinavian Journal of Clinical and Laboratory Investigation 1983;43(4):275-83. [PubMed] [Google Scholar]
Wardlaw 2005 {published data only}
- Wardlaw JM, Lewis S. Carotid stenosis measurement on colour Doppler ultrasound: agreement of ECST, NASCET and CCA methods applied to ultrasound with intra-arterial angiographic stenosis measurement. European Journal of Radiology 2005;56(2):205-11. [DOI] [PubMed] [Google Scholar]
Weaver 1980 {published data only}
- Weaver RG, Ball MR, Howard G, McKinney HG, Jones AM, Toole JF. Continuous wave doppler ultrasound and arteriogram for carotid bifurcation atherosclerosis. American Journal of Neuroradiology 1980;1(1):115-6. [Google Scholar]
Weaver 1980a {published data only}
- Weaver RG, Howard G, McKinney WM, Ball MR, Jones AM, Toole JF. Comparison of doppler ultrasonography with arteriography of the carotid-artery bifurcation. Stroke 1980;11(4):402-4. [DOI] [PubMed] [Google Scholar]
Weintraub 1985 {published data only}
- Weintraub MI, Lambert D, Rothman AL. Carotid ultrasonography - the new "gold standard". Surgical and angiographic correlation. Angiology 1985;36(1):19-22. [DOI] [PubMed] [Google Scholar]
Wessels 2004 {published data only}
- Wessels T, Harrer JU, Stetter S, Mull M, Klotzsch C. Three-dimensional assessment of extracranial Doppler sonography in carotid artery stenosis compared with digital subtraction angiography. Stroke 2004;35(8):1847-51. [DOI] [PubMed] [Google Scholar]
Wetzner 1984 {published data only}
- Wetzner SM, Kiser LC, Bezreh JS. Duplex ultrasound imaging: vascular applications. Radiology 1984;150(2):507-14. [DOI] [PubMed] [Google Scholar]
Wikstrom 2002 {published data only}
- Wikstrom J, Johansson LO, Rossitti S, Karacagil S, Ahlstrom H. High-resolution carotid artery MRA. Comparison with fast dynamic acquisition and duplex ultrasound scanning. Acta Radiologica 2002;43(3):256-61. [DOI] [PubMed] [Google Scholar]
Wilkerson 1991 {published data only}
- Wilkerson DK, Keller I, Mezrich R, Schroder WB, Sebok D, Gronlundjacobs J, et al. The comparative evaluation of 3-dimensional magnetic-resonance for carotid-artery disease. Journal of Vascular Surgery 1991;14(6):803-11. [DOI] [PubMed] [Google Scholar]
Wilterdink 1996 {published data only}
- Wilterdink JL, Feldmann E, Easton JD, Ward R. Performance of carotid ultrasound in evaluation candidates for carotid endarterectomy is optimized by an approach based on clinical outcome rather than accuracy. Stroke 1996;27(6):1094-8. [DOI] [PubMed] [Google Scholar]
Winkelaar 1999 {published data only}
- Winkelaar GB, Chen JC, Salvian AJ, Taylor DC, Teal PA, Hsiang YN. New duplex ultrasound scan criteria for managing symptomatic 50% or greater carotid stenosis. Journal of Vascular Surgery 1999;29(6):986-93. [DOI] [PubMed] [Google Scholar]
Withers 1990 {published data only}
- Withers CE, Gosink BB, Keightley AM, Casola G, Lee AA, Vansonnenberg E, et al. Duplex carotid sonography - peak systolic velocity in quantifying internal carotid-artery stenosis. Journal of Ultrasound in Medicine 1990;9(6):345-9. [DOI] [PubMed] [Google Scholar]
Wolverson 1983 {published data only}
- Wolverson MK, Heiberg E, Sundaram M, Tantanasirviongse S, Shields JB. Carotid atherosclerosis: high-resolution real-time sonography correlated with angiography. American Journal of Roentgenology 1983;140(2):355-61. [DOI] [PubMed] [Google Scholar]
Wolverson 1985 {published data only}
- Wolverson MK, Heiberg E, Tantana S, Pilla TJ. Intravenous DSA and duplex sonography as screening examinations for carotid disease - comparison in 102 vessels. American Journal of Neuroradiology 1985;6(4):569-73. [PMC free article] [PubMed] [Google Scholar]
Worthy 1997 {published data only}
- Worthy SA, Henderson J, Griffiths PD, Oates CP, Gholkar A. The role of duplex sonography and angiography in the investigation of carotid artery disease. Neuroradiology 1997;39(2):122-6. [DOI] [PubMed] [Google Scholar]
Yiu‐Tong 1985 {published data only}
- Yiu-Tong C, Yuk-Keung L, Fu-Li C. A study of cerebrovascular disease by Doppler ultrasound. Chinese Medical Journal 1985;35(1):34-40. [Google Scholar]
Young 1992 {published data only}
- Young N, Soo YS, Fischer P. Comparison of duplex ultrasound with angiography in assessment of carotid bifurcation disease. Australasian Radiology 1992;36(1):54-8. [DOI] [PubMed] [Google Scholar]
Young 1994 {published data only}
- Young GR, Humphrey PR, Shaw MD, Nixon TE, Smith ET. Comparison of magnetic resonance angiography, duplex ultrasound, and digital subtraction angiography in assessment of extracranial internal carotid artery stenosis. Journal of Neurology, Neurosurgery and Psychiatry 1994;57(12):1466-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yurdakul 2004 {published data only}
- Yurdakul M, Tola M, Cumhur T. B-flow imaging of internal carotid artery stenosis: comparison with power Doppler imaging and digital subtraction angiography. Journal of Clinical Ultrasound 2004;32(5):243-8. [DOI] [PubMed] [Google Scholar]
Yurdakul 2004a {published data only}
- Yurdakul M, Tola M, Ozdemir E, Isiksalan ON, Cumhur T. Center specific duplex Doppler threshold values in carotid artery stenosis. Tanisal ve Girisimsel Radyoloji: Tibbi Goruntuleme ve Girisimsel Radyoloji Dernegi yayin organi 2004;10(2):167-72. [PubMed] [Google Scholar]
Zananiri 1993 {published data only}
- Zananiri FV, Jackson PC, Halliwell M, Harris RA, Hayward JK, Davies ER, et al. A comparative study of velocity measurements in major blood vessels using magnetic-resonance imaging and doppler ultrasound. British Journal of Radiology 1993;66(792):1128-33. [DOI] [PubMed] [Google Scholar]
Zanette 1982 {published data only}
- Zanette E, Bozzao L, Pantano P, Antonelli M, Bernardi S, Buttinelli C, et al. The combined use of radionuclide angiography and Doppler-sonography in the screening of carotid lesions. Rivista di Patologia Nervosa e Mentale 1982;102(4):145-54. [PubMed] [Google Scholar]
Zanette 1987 {published data only}
- Zanette E, Bozzao L, Buttinelli C, Mariottini A, Pappata S, Lenzi GL. High resolution real-time B-mode echotomography in the diagnosis of extracranial carotid lesions. Comparison with traditional angiography. Acta Neurochirurgica 1987;84(1-2):43-7. [DOI] [PubMed] [Google Scholar]
Zierler 1990 {published data only}
- Zierler RE, Kohler TR, Strandness DE. Duplex scanning of normal or minimally diseased carotid arteries - correlation with arteriography and clinical outcome. Journal of Vascular Surgery 1990;12(4):447-55. [PubMed] [Google Scholar]
Zorzon 1987 {published data only}
- Zorzon M, Carraro N, Pasqua M, Monti F, Mase G. Reliability of Doppler ultrasonography in relation to computerized digital angiography in patients with carotid bruit. Rivista di Neurologia 1987;57(6):342-5. [PubMed] [Google Scholar]
Zwicker 1987 {published data only}
- Zwicker C, Huben H, Langer M, Fiegler W. Combined use of duplex sonography and CW Doppler in diseases of the carotid arteries. Rontgen-Blatter; Zeitschrift fur Rontgen-Technik und Medizinisch-Wissenschaftliche Photographie 1987;40(10):311-4. [PubMed] [Google Scholar]
Zwiebel 1983 {published data only}
- Zwiebel WJ, Austin CW, Sackett JF, Strother CM. Correlation of high-resolution, b-mode and continuous-wave doppler sonography with arteriography in the diagnosis of carotid stenosis. Radiology 1983;149(2):523-32. [DOI] [PubMed] [Google Scholar]
Zwiebel 1985 {published data only}
- Zwiebel WJ, Strother CM, Austin CW, Sackett JF. Comparison of ultrasound and IV-DSA for carotid evaluation. Stroke 1985;16(4):633-43. [DOI] [PubMed] [Google Scholar]
Additional references
AbuRahma 2008
- AbuRahma AF, Abu-Halimah S, Bensenhaver J, Dean LS, Keiffer T, Emmett M, et al. Optimal carotid duplex velocity criteria for defining the severity of carotid in-stent restenosis. Journal of Vascular Surgery 2008;48(3):589-94. [PMID: ] [DOI] [PubMed] [Google Scholar]
ACAS 1995
- Walker MD, Marler JR, Goldstein M, Grady PA, Toole JF, Baker WH, et al. Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA 1995;273(18):1421-8. [PMID: ] [PubMed] [Google Scholar]
Arbeille 1995
- Arbeille P, Desombre C, Aesh B, Philippot M, Lapierre F. Quantification and assessment of carotid-artery lesions - degree of stenosis and plaque volume. Journal of Clinical Ultrasound 1995;23(2):113-24. [DOI: 10.1002/jcu.1870230206] [DOI] [PubMed] [Google Scholar]
Arning 2010
- Arning C, Widder B, Von Reutern GM, Stiegler H, Görtler M. Revision of DEGUM ultrasound criteria for grading internal carotid artery stenoses and transfer to NASCET measurement [Revision der DEGUM-kriterien und transfer in NASCET-stenosierungsgrade]. Ultraschall in der Medizin 2010;31(3):251-7. [DOI: 10.1055/s-0029-1245336] [DOI] [PubMed] [Google Scholar]
Bonita 1988
- Bonita R, Beaglehole R. Recovery of motor function after stroke. Stroke 1988;19(12):1497-500. [PMID: ] [DOI] [PubMed] [Google Scholar]
Brott 2011
- Brott TG, Halperin JL, Abbara S, Bacharach JM, Barr JD, Bush RL, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American Stroke Association, American Association of Neuroscience Nurses, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Congress of Neurological Surgeons, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of NeuroInterventional Surgery, Society for Vascular Medicine, and Society for Vascular Surgery. Circulation 2011;124(4):489-532. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bucek 2007
- Bucek RA, Puchner S, Haumer M, Reiter M, Minar E, Lammer J. CTA quantification of internal carotid artery stenosis: application of luminal area vs. luminal diameter measurements and assessment of inter-observer variability. Journal of Neuroimaging 2007;17(3):219-26. [DOI: 10.1111/j.1552-6569.2007.00124] [DOI] [PubMed] [Google Scholar]
CDC 2001
- Centers for Disease Control and Prevention (CDC). Prevalence of disabilities and associated health conditions among adults — United States, 1999. Morbidity and Mortality Weekly Report 2001;50(7):120-5. [PMID: ] [PubMed] [Google Scholar]
Davies 1993
- Davies KN, Humphrey PR. Complications of cerebral angiography in patients with symptomatic carotid territory ischaemia screened by carotid ultrasound. Journal of Neurology, Neurosurgery and Psychiatry 1993;56(9):967-72. [PMID: ] [DOI] [PMC free article] [PubMed]
Doebler 2017 [Computer program]
- MADA: Meta-Analysis of Diagnostic Accuracy. Doebler P, Version R package version 0.5.8. 2017. Available from CRAN.R-project.org/package=mada.
Duddalwar 2004
- Duddalwar VA. Multislice CT angiography: a practical guide to CT angiography in vascular imaging and intervention. British Journal of Radiology 2004;77 Spec No 1:S27-38. [PMID: ] [DOI] [PubMed] [Google Scholar]
Easton 2009
- Easton JD, Saver JL, Albers GW, Alberts MJ, Chaturvedi S, Feldmann E, et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. Stroke 2009;40(6):2276-93. [PMID: ] [DOI] [PubMed] [Google Scholar]
ECST 1998
- European Carotid Surgery Trialists' Collaborative Group. Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet 1991;351(9113):1379-87. [PMID: 9593407]] [PubMed] [Google Scholar]
Eliasziw 1994
- Eliasziw M, Streifler JY, Fox AJ, Hachinski VC, Ferguson GG, Barnett HJ. Significance of plaque ulceration in symptomatic patients with high-grade carotid stenosis. North American Symptomatic Carotid Endarterectomy Trial. Stroke 1994;25(2):304-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Eliasziw 1994a
- Eliasziw M, Smith RF, Singh N, Holdsworth DW, Fox AJ, Barnett HJ. Further comments on the measurement of carotid stenosis from angiograms. North American Symptomatic Carotid Endarterectomy Trial (NASCET) Group. Stroke 1994;25(12):2445-9. [DOI] [PubMed] [Google Scholar]
ESVS Writing Group 2018
- Naylor AR, Ricco JB, De Borst GJ, Debus S, De Haro J, Halliday A, et al. Editor's choice: Management of atherosclerotic carotid and vertebral artery disease: 2017 Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS). European Journal of Vascular and Endovascular Surgery 2018;55(1):3-81. [PMID: ] [DOI] [PubMed] [Google Scholar]
Flaherty 2013
- Flaherty ML, Kissela B, Khoury JC, Alwell K, Moomaw CJ, Woo D, et al. Carotid artery stenosis as a cause of stroke. Neuroepidemiology 2013;40(1):36-41. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Flumignan 2017
- Flumignan CDQ, Flumignan RLG, Navarro TP. Extracranial carotid stenosis: evidence based review [Estenose de carotida extracraniana: revisao baseada em evidencias]. Revista do Colegio Brasileiro de Cirurgioes 2017;44(3):293-301. [PMID: ] [DOI] [PubMed] [Google Scholar]
Fonseca 2021
- Fonseca AC, Merwick Á, Dennis M, Ferrari J, Ferro JM, Kelly P, et al. European Stroke Organisation (ESO) guidelines on management of transient ischaemic attack. European Stroke Journal 2021;6(2):V. [DOI: 10.1177/23969873211027003] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Gornik 2021
- Gornik HL, Rundek T, Gardener H, Benenati JF, Dahiya N, Hamburg NM, et al. Optimization of duplex velocity criteria for diagnosis of internal carotid artery (ICA) stenosis: a report of the Intersocietal Accreditation Commission (IAC) Vascular Testing Division Carotid Diagnostic Criteria Committee. Vascular Medicine 2021;26(5):515-25. [DOI: 10.1177/1358863X211011253] [DOI] [PMC free article] [PubMed] [Google Scholar]
Grant 2003
- Grant EG, Benson CB, Moneta GL, Alexandrov AV, Baker JD, Bluth EI, et al. Carotid artery stenosis: gray-scale and Doppler US diagnosis — Society of Radiologists in Ultrasound Consensus Conference. Radiology 2003;229(2):340-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hankey 1990
- Hankey GJ, Warlow CP, Sellar RJ. Cerebral angiographic risk in mild cerebrovascular disease. Stroke 1990;21(2):209-22. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hillen 2003
- Hillen T, Coshall C, Tilling K, Rudd AG, McGovern R, Wolfe CD. Cause of stroke recurrence is multifactorial: patterns, risk factors, and outcomes of stroke recurrence in the South London Stroke Register. Stroke 2003;34(6):1457-63. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hobson 2008
- Hobson RW 2nd, Mackey WC, Ascher E, Murad MH, Calligaro KD, Comerota AJ, et al. Management of atherosclerotic carotid artery disease: clinical practice guidelines of the Society for Vascular Surgery. Journal of Vascular Surgery 2008;48(2):480-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Howard 2017
- Howard VJ, Meschia JF, Lal BK, Turan TN, Roubin GS, Brown RD Jr, et al. Carotid revascularization and medical management for asymptomatic carotid stenosis: protocol of the CREST-2 clinical trials. International Journal of Stroke 2017;12(7):770-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kleindorfer 2021
- Kleindorfer DO, Towfighi A, Chaturvedi S, Cockroft KM, Gutierrez J, Lombardi-Hill D, et al. Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke 2021;52(7):e364-e467. [DOI: 10.1161/STR.0000000000000375] [DOI] [PubMed] [Google Scholar]
Leeflang 2009
- Leeflang MM, Bossuyt PM, Irwig L. Diagnostic test accuracy may vary with prevalence: implications for evidence-based diagnosis. Journal of Clinical Epidemiology 2009;62(1):5-12. [DOI: 10.1016/j.jclinepi.2008.04.007] [DOI] [PubMed] [Google Scholar]
Leeflang 2012
- Leeflang MMG, Deeks JJ, Rutjes AWS, Reitsma JB, Bossuyt PMM. Bivariate meta-analysis of predictive values of diagnostic tests can be an alternative to bivariate meta-analysis of sensitivity and specificity. Journal of Clinical Epidemiology 2012;65(10):1088–97. [DOI] [PubMed] [Google Scholar]
Lenhart 2002
- Lenhart M, Framme N, Völk M, Strotzer M, Manke C, Nitz WR, et al. Time-resolved contrast-enhanced magnetic resonance angiography of the carotid arteries: diagnostic accuracy and inter-observer variability compared with selective catheter angiography. Investigative Radiology 2002;37(10):535-41. [DOI: 10.1097/00004424-200210000-00001] [DOI] [PubMed] [Google Scholar]
Macaskill 2010
- Macaskill P, Gatsonis C, Deeks JJ, Harbord RM, Takwoingi Y. Chapter 10: Analysing and presenting results. In: Deeks JJ, Bossuyt PM, Gatsonis C (editors). Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 1.0.0. The Cochrane Collaboration, 2013. Available from srdta.cochrane.org.
Mackay 2004
- Mackay J, Mensah G. The atlas of heart disease and stroke. apps.who.int/iris/bitstream/10665/43007/1/9241562768.pdf (accessed 27 August 2018).
Moore 1995
- Moore WS, Barnett HJ, Beebe HG, Bernstein EF, Brener BJ, Brott T, et al. Guidelines for carotid endarterectomy. A multidisciplinary consensus statement from the Ad Hoc Committee, American Heart Association. Circulation 1995;91(2):566-79. [PMID: ] [DOI] [PubMed] [Google Scholar]
Morris 2017
- Morris DR, Ayabe K, Inoue T, Sakai N, Bulbulia R, Halliday A, et al. Evidence-based carotid interventions for stroke prevention: state-of-the-art review. Journal of Atherosclerosis and Thrombosis 2017;24(4):373-87. [PMID: ] [DOI] [PMC free article] [PubMed]
NASCET 1991
- Barnett HJM, Taylor DW, Haynes RB, Sackett DL, Peerless SJ, North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. New England Journal of Medicine 1991;325(7):445-53. [PMID: ] [DOI] [PubMed] [Google Scholar]
NCC‐CC 2008
- National Collaborating Centre for Chronic Conditions. Stroke: National Clinical Guideline for Diagnosis and Initial Management of Acute Stroke and Transient Ischaemic Attack (TIA). Royal College of Physicians. www.ncbi.nlm.nih.gov/pubmedhealth/PMH0009998/pdf/PubMedHealth_PMH0009998.pdf (accessed 22 July 2018). [PMID: ] [PubMed]
Nederkoorn 2003
- Nederkoorn PJ, Van der Graaf Y, Hunink MG. Duplex ultrasound and magnetic resonance angiography compared with digital subtraction angiography in carotid artery stenosis: a systematic review.. Stroke 2003;34(5):1324-32. [DOI: 10.1161/01.STR.0000068367.08991.A2.] [DOI] [PubMed] [Google Scholar]
NICE 2017
- National Institute for Health and Care Excellence (NICE). Stroke and transient ischaemic attack in over 16s: diagnosis and initial management. www.nice.org.uk/guidance/CG68 (accessed 22 July 2018). [PubMed]
Nicolaides 1996
- Nicolaides AN, Shifrin EG, Bradbury A, Dhanjil S, Griffin M, Belcaro G, et al. Angiographic and duplex grading of internal carotid stenosis: can we overcome the confusion? Journal of Endovascular Surgery 1996;3(2):158-65. [PMID: ] [DOI] [PubMed] [Google Scholar]
Orrapin 2017
- Orrapin S, Rerkasem K. Carotid endarterectomy for symptomatic carotid stenosis. Cochrane Database of Systematic Reviews 2017, Issue 6. Art. No: CD001081. [DOI: 10.1002/14651858.CD001081.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rafailidis 2017
- Rafailidis V, Charitanti A, Tegos T, Destanis E, Chryssogonidis I. Contrast-enhanced ultrasound of the carotid system: a review of the current literature. Journal of Ultrasound 2017;20(2):97-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rankin 1957
- Rankin J. Cerebral vascular accidents in patients over the age of 60. I. General considerations. Scottish Medical Journal 1957;2(4):127-36. [PMID: ] [DOI] [PubMed] [Google Scholar]
Reitsma 2005
- Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. Journal of Clinical Epidemiology 2005;58(10):982-90. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rerkasem 2020
- Rerkasem A, Orrapin S, Howard DPJ, Rerkasem K. Carotid endarterectomy for symptomatic carotid stenosis.. Cochrane Database of Systematic Reviews 2020, Issue 9. Art. No: CD001081. [DOI: 10.1002/14651858.CD001081.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2020 [Computer program]
- The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.4. The Cochrane Collaboration, 2020.
Ricotta 2011
- Ricotta JJ, Aburahma A, Ascher E, Eskandari M, Faries P, Lal BK, Society for Vascular Surgery. Updated Society for Vascular Surgery guidelines for management of extracranial carotid disease: executive summary. Journal of Vascular Surgery 2011;54:832–6. [DOI: 10.1016/j.jvs.2011.07.004] [DOI] [PubMed] [Google Scholar]
Rothwell 1994
- Rothwell PM, Gibson RJ, Slattery J, Sellar RJ, Warlow CP. Equivalence of measurements of carotid stenosis. A comparison of three methods on 1001 angiograms. European Carotid Surgery Trialists' Collaborative Group. Stroke 1994;25(12):2435-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rothwell 2004
- Rothwell PM, Eliasziw M, Gutnikov SA, Warlow CP, Barnett HJ. Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery. Lancet 2004;363(9413):915-24. [PMID: ] [DOI] [PubMed] [Google Scholar]
R Project 2018 [Computer program]
- R Foundation for Statistical Computing R Project for Statistical Computing. Version 3.5.1. Vienna, Austria: R Foundation for Statistical Computing, 2018. Available at www.R-project.org.
Rutjes 2005
- Rutjes AW, Reitsma JB, Vandenbroucke JP, Glas AS, Bossuyt PM. Case-control and two-gate designs in diagnostic accuracy studies. Clinical Chemistry 2005;51(8):1335-41. [PMID: ] [DOI] [PubMed] [Google Scholar]
Souza 2005
- Souza LV, Castro CC, Cerri GG. Evaluation of carotid atherosclerosis by ultrasound and magnetic resonance imaging [Avaliação da aterosclerose carotídea por intermédio de ultra-sonografia e ressonância magnética]. Radiologia Brasileira 2005;38(2):81-94. [DOI: 10.1590/S0100-39842005000200003] [DOI] [Google Scholar]
Strong 2007
- Kathleen S, Colin M, Ruth B. Preventing stroke: saving lives around the world. Lancet Neurology 2007;6(2):182-7. [DOI: 10.1016/S1474-4422(07)70031-5] [DOI] [PubMed] [Google Scholar]
Surur 2013
- Surur AM, Buccolini TV, Londero HF, Marangoni MA, Allende NJ. Noninvasive assessment of carotid stenosis in relation to atherosclerosis: correlation between color Doppler ultrasound and magnetic resonance angiography with gadolinium [Valoración no invasiva de la estenosis carotídea de causa aterosclerótica: correlación entre la ecografía Doppler color y la angiografía por resonancia magnética con gadolinio]. Revista Argentina de Radiología 2013;77(4):267-74. [DOI: 10.7811/rarv77n4a04] [DOI] [Google Scholar]
Taylor 1987
- Taylor DC, Strandness DE. Carotid artery duplex scanning. Journal of Clinical Ultrasound 1987;15(9):635–44. [DOI] [PubMed] [Google Scholar]
Tsantilas 2015
- Tsantilas P, Kuhnl A, Kallmayer M, Knappich C, Schmid S, Kuetchou A, et al. Stroke risk in the early period after carotid related symptoms: a systematic review. Journal of Cardiovascular Surgery 2015;56:845–52. [PubMed] [Google Scholar]
Vasconcelos 2016
- Vasconcelos V, Cassola N, Da Silva EM, Baptista-Silva JC. Immediate versus delayed treatment for recently symptomatic carotid artery stenosis. Cochrane Database of Systematic Reviews 2016, Issue 9. Art. No: CD011401. [DOI: 10.1002/14651858.CD011401.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ventura 2015
- Ventura CA, Silva ES, Cerri GG, Leao PP, Tachibana A, Chammas MC. Can contrast-enhanced ultrasound with second-generation contrast agents replace computed tomography angiography for distinguishing between occlusion and pseudo-occlusion of the internal carotid artery? Clinics 2015;70(1):1-6. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Virani 2021
- Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, et al. Heart disease and stroke statistics — 2021 Update. A report from the American Heart Association. Circulation 2021;143(8):e254–e743. [DOI: 10.1161/CIR.0000000000000950] [DOI] [PubMed] [Google Scholar]
Wardlaw 2006a
- Wardlaw JM, Chappell FM, Stevenson M, De Nigris E, Thomas S, Gillard J, et al. Accurate, practical and cost-effective assessment of carotid stenosis in the UK. Health Technology Assessment 2006;10(30):iii-iv, ix-x, 1-182. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wardlaw 2006b
- Wardlaw JM, Chappell FM, Best JJ, Wartolowska K, Berry E. Non-invasive imaging compared with intra-arterial angiography in the diagnosis of symptomatic carotid stenosis: a meta-analysis. Lancet 2006;367(9521):1503-12. [DOI: 10.1016/S0140-6736(06)68650-9] [DOI] [PubMed] [Google Scholar]
Whiting 2004
- Whiting P, Rutjes AWS, Reitsma JB, Glas AS, Bossuyt PMM, Kleijnen J. Sources of variation and bias in studies of diagnostic accuracy: a systematic review. Annals of Internal Medicine 2004;140(3):189–202. [DOI] [PubMed] [Google Scholar]
Whiting 2006
- Whiting PF, Weswood ME, Rutjes AW, Reitsma JB, Bossuyt PN, Kleijnen J. Evaluation of QUADAS, a tool for the quality assessment of diagnostic accuracy studies. BMC Medical Research Methodology 2006;6:9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Whiting 2011
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of Internal Medicine 2011;155(8):529-36. [PMID: ] [DOI] [PubMed] [Google Scholar]
Whiting 2018
- Whiting P, Davenport C. Understanding test accuracy research: a test consequence graphic. Diagnostic and Prognostic Research 2018;2:2. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zwinderman 2008
- Zwinderman AH, Bossuyt PM. We should not pool diagnostic likelihood ratios in systematic reviews. Statistics in Medicine 2008;27(5):687-97. [DOI: 10.1002/sim.2992] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Cassola 2018
- Cassola N, Baptista‐Silva JCC, Flumignan CDQ, Sesso R, Vasconcelos V, Flumignan RLG. Duplex ultrasound for diagnosing symptomatic carotid stenosis in the extracranial segments. Cochrane Database of Systematic Reviews 2018, Issue 11. Art. No: CD013172. [DOI: 10.1002/14651858.CD013172] [DOI] [PMC free article] [PubMed] [Google Scholar]


