Abstract
Background
Blastomyces spp, the etiologic agents of blastomycosis, are endemic dimorphic fungi that require prolonged antifungal therapy, which can be complicated by adverse drug effects. Isavuconazonium sulphate (ISA) is a triazole with in vitro and in vivo activity against Blastomyces spp, but there is a paucity of clinical data supporting its use for treatment of blastomycosis.
Methods
This retrospective case series identified 14 patients with blastomycosis at least partially treated with ISA at the University of Wisconsin between 2015 and 2019. Treatment duration and outcomes were documented. In addition, 29 clinical isolates of Blastomyces spp between 2004 and 2017 were tested for minimum inhibitory concentrations against ISA and other antifungals.
Results
Fourteen patients were treated with a median of 255 days of ISA accounting for 68% of total therapy. Half (7 of 14) of the patients were immunocompromised, 11 of 14 (79%) were proven cases of blastomycosis, 7 of 14 (50%) had central nervous system (CNS) involvement, and 11 of 14 (79%) were cured. Antifungal susceptibility testing showed a consistently low minimum inhibitory concentration to ISA ≤ 0.015 mcg/mL.
Conclusions
This case series supports the efficacy and safety for ISA in the treatment of blastomycosis with or without CNS disseminated, especially when alternative triazoles cannot be used.
Keywords: Blastomyces dermatitidis, blastomycosis, isavuconazonium sulphate, susceptibility
This is the largest case series to date demonstrating the safety and efficacy of isavuconazonium sulphate to treat blastomycosis with prolonged therapy, and we report low minimum inhibitory concentrations of 29 clinical isolates of Blastomyces spp to isavuconazonium sulphate.
Blastomyces is a genus of thermally dimorphic fungi that cause infection in immunocompromised and immunocompetent patients. Among the 7 known species, Blastomyces dermatitidis and Blastomyces gilchristii are endemic to Wisconsin, USA [1]. The clinical presentation ranges from asymptomatic infection to acute respiratory distress syndrome (ARDS) and disseminated disease including verrucous skin lesions, osteomyelitis, and central nervous system (CNS) disease [1]. The Infectious Disease Society of America, the American Society of Transplantation, and the American Thoracic Society recommend amphotericin B is used initially for severe cases, those with CNS involvement, and immunocompromised patients followed by prolonged oral triazole therapy [2–4]. Itraconazole is preferred, but in cases with CNS disease voriconazole is typically used given its superior CNS penetration; however, fluconazole and itraconazole remain potential options [5].
As a class, triazoles are well tolerated. Adverse effects include hepatic toxicity, prolongation of the QT interval, and drug-drug interactions involving the hepatic CYP450 enzymes [6]. Itraconazole has a negative inotropic effect that can precipitate congestive heart failure [1]. Voriconazole has the highest rate of hepatic toxicity among the triazoles (30% compared to 10–20% with others), but it is also associated with skin photosensitivity, cutaneous malignancy, fluoride-associated osteitis, and transient photopsia [6, 7].
In March 2015, the US Food and Drug Administration approved isavuconazonium sulphate (ISA), the prodrug of isavuconazole, for the treatment of aspergillosis and mucormycosis. Isavuconazonium sulphate has in vivo, in vitro, and clinical efficacy against a broad spectrum of fungal pathogens including the endemic fungi [6–12]. However, there are no established breakpoints for resistance by the Clinical and Laboratory Standards Institute for Blastomyces spp, and previously published data have included few isolates of Blastomyces spp for susceptibility testing with ISA [9, 13, 14]. Although there is a paucity of clinical data to guide the use of ISA for blastomycosis, ISA has substantial benefits with once-daily dosing after appropriate loading doses, shortening of the QT interval, and less inhibition of the CYP34A enzyme with fewer drug interactions [6]. The goal of this retrospective case series is to investigate the safety and efficacy of ISA in patients with blastomycosis.
METHODS
Case Series Study Design
This retrospective case series of patients with blastomycosis treated with ISA at the University of Wisconsin Hospital and Clinics (UWHC) from 2015 to December 2019 focuses on the indications for ISA and long-term outcome data for a single center. Institutional Review Board approval was obtained. International Classification of Diseases, Ninth Revision (ICD-9)/ICD-10 codes were used to identify cases of blastomycosis and prescriptions for ISA, and adults (age ≥18 years old) that received at least 1 dose of ISA were included. All prescriptions of ISA at UWHC during the study period were cross-checked for cases of blastomycosis. Cases were excluded if blastomycosis was not the final diagnosis.
Variables Collected
Variables collected on each case included age, sex, ethnicity, comorbid conditions (diabetes, chronic kidney disease, chronic lung disease, chronic liver disease), immunosuppression (stem cell transplant (SCT), solid organ transplant (SOT), human immunodeficiency virus (HIV)/acquired immune deficiency syndrome (AIDS), inherited severe immunodeficiency, sustained neutropenia defined as absolute neutrophil count <500 cells/mL for 10 days, prednisone or equivalent steroid use of 0.3 mg/kg for 3 weeks, tumor necrosis factor-alpha antagonists, alemtuzumab, antithymocyte globulin, cyclosporine, tacrolimus, and rejection of solid organ transplant within 90 days of diagnosis. Information on diagnosis included cultures, pathology, relevant imaging, and indirect microbiologic data (eg, Blastomyces and Histoplasma antigens and serology). Duration of antifungal therapies counted in days, indications for ISA (eg, adverse drug effect, drug-drug interactions), and serum level of ISA were recorded. Follow-up records were searched for toxicities attributed to ISA, total duration of infectious disease follow-up, and outcomes.
Definitions
Disseminated disease was defined as pulmonary disease with extrapulmonary disease (eg, skin, bone, joint, CNS). Acute presentation was defined as time from onset of symptoms to diagnosis <1 month, subacute as 2–3 months, and chronic as >3 months. Severity of illness was defined as mild if the patient was treated in the outpatient setting, moderate if any amount of inpatient care was required, and severe if critical care was required.
Proven cases of blastomycosis were defined as illness consistent with endemic mycosis plus either culture of Blastomyces spp (eg, affected site, blood) or histopathologic evidence of Blastomyces spp. Probable cases were defined as illness consistent with endemic mycosis plus mycological evidence of infection not included as a proven case (eg, detectable antibodies and antigens from a clinical specimen). Cure was defined as completion of therapy after resolution of symptoms, improved imaging, and serial Blastomyces serum or urine antigens.
Susceptibility Testing Methods
Clinical isolates of Blastomyces spp collected at the University of Wisconsin Hospital between 2004 and 2017 were tested for antifungal susceptibility retrospectively. They were cultured at 35°C for 10–15 days on potato dextrose agar (Oxoid, Hampshire, England) to induce conidial formation. Conidia were then suspended in Roswell Park Memorial Institute (RPMI) 1640 medium (Sigma-Aldrich, St. Louis, MO) at 1.0 × 105 conidia/mL and 200 µL was added to 96-well plates containing the antifungals. Five antifungals were tested: itraconazole, voriconazole, and isavuconazole (Santa Cruz Biotechnology, Santa Cruz, CA), posaconazole (Selleck Chemicals, Houston, TX), and amphotericin B (AMRESCO, Solon, OH). Stock solutions were prepared in dimethyl sulfoxide (Sigma-Aldrich) except for amphotericin B, which was in sterile water. Drugs were diluted into RPMI medium via serial doubling dilutions, with concentrations ranging from 0.015 to 8.0 µg/mL except for itraconazole, which had concentrations ranging from 0.13 to 64 µg/mL.
Patient Consent Statement
This study was approved by the Institutional Review Board of the University of Wisconsin. Informed consent was waived because only deidentified information was used.
RESULTS
From 2015 to 2019 we identified 14 patients (Table 1), there were 6 females and 8 males, the median age was 53 years (range 21–69), and there were 11 whites, 2 African Americans, and 1 patient of Hmong ethnicity. Half of the patients (7 of 14, 50%) were immunocompromised including 6 SOT recipients (4 renal, 1 heart, 1 bilateral lung) and 1 patient on infliximab for Crohn’s disease. The most common comorbidities were chronic kidney disease (6 of 14, 43%), diabetes mellitus (5 of 14, 36%), and chronic lung disease (3 of 14, 21%; 1 chronic obstructive pulmonary disease, 1 cystic fibrosis after bilateral lung transplant, and 1 restrictive lung disease due to obesity). No patients with SCT, HIV/AIDS, inherited severe immunodeficiency, sustained neutropenia, significant prednisone exposure, or liver cirrhosis were identified.
Table 1.
Clinical Characteristics of Patients With Blastomycosis
| Patient | Age (Years); Sex | History | Immunocompromised | Blastomycosis Presentation (Severity, Acuity, Pulmonary Imaging) | Proven/Probable: Culture or Histopathology | Dissemination: Organ Involvement |
|---|---|---|---|---|---|---|
| 1 | 47; Female | Diabetes mellitus | No | Moderate, acute, miliary nodules | Proven: BAL cultured Blastomyces dermatitidis | Yes: pulmonary, CNS |
| 2 | 69; Male | ESRD, OHTx 14 years prior on MMF, tacrolimus | Yes | Moderate, acute, cavitary nodule | Proven: transbronchial biopsy Necrotizing granulomas with BBB yeast culture grew B dermatitidis | Yes: pulmonary, CNS |
| 3 | 53; Female | No comorbidities | No | Mild, chronic, nodules | Proven: skin biopsy with BBB and grew B dermatitidis | Yes: pulmonary, cutaneous |
| 4 | 48; Male | CKD, LURTx 4 years prior on MMF, tacrolimus, prednisone | Yes | Moderate, acute, nodules | Proven: brain biopsy with filamentous fungi and grew B dermatitidis | Yes: pulmonary, CNS, possible prostate |
| 5 | 46; Female | CKD, DBDRTx 3 years prior on azathioprine, prednisone | Yes | Moderate, acute, nodules | Proven: BAL with BBB and grew B dermatitidis | No: isolated pulmonary |
| 6 | 57; Male | Diabetes mellitus, COPD | No | Moderate, chronic, ground-glass opacities | Proven: vertebral bone bx with BBB and grew B dermatitidis | Yes: pulmonary, bone |
| 7 | 53; Male | Diabetes mellitus, CKD, DBDRTx 4 months prior on MMF, tacrolimus, prednisone | Yes | Severe, acute, miliary nodules | Proven: skin biopsy, synovial fluid, BAL with BBB and grew B dermatitidis | Yes: pulmonary, CNS, cutaneous, joint |
| 8 | 66; Male | Diabetes mellitus, restrictive lung disease | No | Mild, chronic, nodules | Probable: skin biopsy with yeast not definitive for Blastomyces spp | Yes: pulmonary, probable cutaneous |
| 9 | 57; Male | Diabetes mellitus, CKD, DCDRTx 1 year prior on MMF, prednisone, belatacept | Yes | Severe, subacute, consolidation with effusion | Proven: skin with BBB and grew B dermatitidis | Yes: pulmonary, probable CNS, cutaneous |
| 10 | 62; Female | Crohn’s Disease on infliximab | Yes | Moderate, acute, consolidation | Probable: transbronchial biopsy with granulomas, no yeast | No: isolated pulmonary |
| 11 | 26; Male | No comorbidities | No | Moderate, chronic, Consolidation | Proven: BAL, transbronchial biopsy, NP mass Bx with BBB and grew B dermatitidis | Yes: pulmonary, CNS, cutaneous, bone |
| 12 | 38; Male | No comorbidities | No | Moderate, acute, cavitary nodule | Proven: BAL with BBB and grew B dermatitidis | Yes: pulmonary, probable CNS |
| 13 | 21; Female | No comorbidities | No | Mild, acute, ground-glass opacities | Probable: no culture or histopathology | No: isolated pulmonary |
| 14 | 53; Female | ESRD, Cystic Fibrosis, DBDBLTx 6 year prior on MMF, cyclosporine, prednisone | Yes | Moderate, acute, consolidation | Proven: BAL with BBB and grew B dermatitidis | No: isolated pulmonary |
Abbreviations: BAL, bronchoalveolar lavage; BBB, broad-based budding yeast; bx, biopsy; CNS, central nervous system; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DBDBLTx, donor after brain death bilateral lung transplantation; DBDRTx, donor after brain death renal transplantation; DCDRTx, donor after cardiac death renal transplantation; ESRD, end-stage renal disease; LURTx, live unrelated renal transplantation; MMF, mycophenolate mofetil; NP, nasopharyngeal; OHTx, orthotopic heart transplantation.
Solid Organ Transplant Recipients
There was a high proportion of SOT recipients (6 of 14, 43%) in this series. One underwent T-cell lymphocyte depletion with antithymocyte globulin; none received alemtuzumab. All SOT recipients were on ≥2 agents for maintenance immunosuppression with mycophenolate mofetil, tacrolimus, and prednisone being the most common (Table 1). None were treated for allograft rejection in the 90 days preceding diagnosis of blastomycosis. The median time from transplant to blastomycosis diagnosis was 3.5 years (range, 4 months to 14 years). Concomitant opportunistic infections occurred in 1 patient: BK viremia at time of blastomycosis diagnosis and disseminated Mycobacterium tuberculosis 14 months later.
Clinical Manifestations
The majority of patients presented with acute blastomycosis (9 of 14, 64%; subacute 1 of 14, 7%; and chronic 4 of 14, 29%). Likewise, most SOT recipients had acute blastomycosis (5 of 6, 83%; subacute 1 of 6, 17%; chronic 0 of 6). Blastomycosis was classified as mild in 3 of 14 (21%), moderate in 9 of 14 (64%), and severe in 2 of 14 (14%). The 2 patients with severe blastomycosis who required intensive care unit level care were SOT recipients and neither was diagnosed with ARDS.
Pneumonia was the most common manifestation occurring in 10 of 14 (71%), and all had pulmonary findings on imaging. Isolated pulmonary disease occurred in 4 of 10 (29%), confirmed extrapulmonary dissemination occurred in 8 of 14 (64%) (CNS 5 of 8, 63%; cutaneous 4 of 8, 50%; bone 2 of 8, 25%; joint 1 of 8, 13%), and 2 had probable disseminated disease (CNS and cutaneous).
Five of the 7 patients treated for CNS dissemination had proven CNS dissemination with neurologic symptoms at presentation, 3 of 5 (60%) had findings on brain magnetic resonance imaging (MRI), and 4 of 5 (80%) had cerebral spinal fluid (CSF) concerning for CNS involvement. Two patients with probable CNS involvement had no neurologic symptoms and equivocal brain MRI or CSF sampling (Table 2).
Table 2.
Details of CNS Dissemination
| Patient | CNS Symptoms | Brain MRI Findings | CSF Studies (WBC Cell/µL, Glucose mg/dL, Protein mg/dL) | CSF Blastomyces Antigen (ng/mL) |
|---|---|---|---|---|
| 1 | Headache, nausea, vomiting | Normal | WBC 1, glucose 79, protein 12 | 0.28 |
| 2 | Encephalopathy | Normal | WBC 242 (94% PMNs), glucose 106, protein 190 | Not done |
| 4 | Headache, nausea, vomiting, gait disturbance | Enhancing lesion in cerebellum | WBC 2410 (93% lymphocytes), glucose 47, protein 226 | Not done |
| 7 | Encephalopathy | Diffuse punctate lesions | WBC 73 (97% lymphocytes), glucose 97, protein 266 | Negative |
| 9 | None | Normal | WBC <1, glucose 102, protein 17 | Positive below quantification |
| 11 | Headache, cranial nerve 6 palsy diplopia | Posterior Nasopharyngeal mass extending through clivus involving cavernous sinus | WBC 4, glucose 71, protein 30 | Negative |
| 12 | None | Enhancing lesions in occipital lobe and peritonsillar region | None | None |
Abbreviations: CNS, central nervous system; CSF, cerebral spinal fluid; MRI, magnetic resonance imaging; PMN, polymorphonuclear leukocytes; WBC, white blood cell count.
Diagnosis of Blastomycosis
Most patients had proven blastomycosis (11 of 14, 78%), and the diagnosis was most frequently confirmed with bronchoalveolar lavage (6 of 11, 55%). Other confirmatory samples included transbronchial biopsy, skin punch biopsy, bone biopsy, synovial fluid culture, brain biopsy, and nasopharyngeal mass biopsy. Of the proven cases, 10 of 11 (91%) had positive serum and urine antigens, patient 6 had negative serum and urine antigens but confirmatory vertebral bone biopsy. None of the CSF cultures grew Blastomyces spp.
Of the 3 patients with probable blastomycosis, patient 8 had an indeterminate skin punch biopsy, patient 10 had detectable Blastomyces and Histoplasma urine antigens, and patient 13 had detectable Blastomyces serum antigen. One patient had confirmed CNS dissemination and possible prostatic blastomycosis but no prostate biopsy performed.
Treatment
Most patients (78%; 11 of 14) were initially treated with liposomal amphotericin B (LAMB) for a median duration of 14 days (range, 7–46 days), and patients 7 and 14 were treated with a second course of LAMB. Patients 3, 8, and 13 were immunocompetent with mild disease and were not treated with LAMB. After LAMB, if given, 9 patients were treated with itraconazole for a median duration of 16 days (range, 2–186 days), 8 patients were treated with voriconazole for a median duration of 92 days (range, 28–413 days), and 1 patient was treated with posaconazole for 39 days. The most common reason for transitioning to ISA was an adverse drug effect from alternative triazoles in 8 of 14 (57%) patients and only 2 of 14 (14%) for concerning drug-drug interactions (Table 3).
Table 3.
Treatment Durations of Patients With Blastomycosis
| Patient | Duration of Therapy (Days) | ISA Indication | Outcome | ||||
|---|---|---|---|---|---|---|---|
| LAMB | ISA | ITRA | VORI | Total | |||
| 1 | 21 | 219 | 0 | 28 | 268 | ADE (VORI: elevated liver enzymes, nausea, vomiting) | Ongoing antigen positivity, moved out of state |
| 2 | 46 | 61 | 0 | 36 | 143 | ADE (VORI: CNS toxicity) | Death unrelated (hemorrhagic stroke) |
| 3 | 0 | 31 | 186 | 0 | 217 | ADE (ITRA: hypertension, bradycardia) | Cure |
| 4 | 7 | 318 | 0 | 175 | 500 | ADE (VORI: rash, myalgia) | Cure |
| 5 | 9 | 270 | 120 | 0 | 399 | Subtherapeutic ITRA, ADE (POSA: drug fever) | Cure |
| 6 | 30 | 379 | 6 | 0 | 415 | Prolonged QT, ADE (ITRA: nausea, vomiting) | Cure |
| 7 | 28; 7 | 430 | 16 | 413 | 859 | DDI (VORI and Rifampin) | Ongoing antigen positivity, continued on ISA |
| 8 | 0 | 193 | 0 | 0 | 193 | Prolonged QT, heart failure | Cure |
| 9 | 14 | 241 | 4 | 119 | 378 | ADE (VORI: peripheral neuropathy) | Cure |
| 10 | 7 | 374 | 2 | 0 | 383 | ADE (ITRA: nausea, vomiting) | Cure |
| 11 | 7 | 203 | 61 | 174 | 445 | Subtherapeutic VORI | Cure |
| 12 | 19 | 298 | 0 | 61 | 378 | ADE (VORI: CNS toxicity) | Cure |
| 13 | 0 | 151 | 6 | 64 | 221 | ADE (ITRA/VORI: nausea, vomiting) | Cure |
| 14 | 13; 14 | 385 | 29 | 0 | 414 | Prolonged QT, progression on ITRA, DDI (Warfarin) | Cure |
Abbreviations: ADE, adverse drug effect; DDI, drug-drug interaction; ISA, isavuconazonium sulphate; ITRA, itraconazole; POSA, posaconazole; LAMB, liposomal amphotericin B; VORI, voriconazole.
When patients were transitioned to ISA, it was well tolerated and was used for a median duration of 255 days (range, 31–430 days). Three patients had possible adverse drug reactions attributed to ISA. Patient 1 had nausea after 219 days of ISA, which improved when switched back to voriconazole. Patient 4 was switched from voriconazole to ISA for rash and myalgia, with rash recurring on ISA, but dermatology eventually diagnosed eczema rather than drug reaction. Patient 8 developed elevation of alanine transaminase and aspartate transaminase 2–3 times upper limit of normal, which improved with discontinuation of therapy. Two patients had short courses of ISA: patient 2 died from intracerebral hemorrhage after 61 days of ISA, and patient 3 only received 31 days of ISA after approximately 6 months of itraconazole (Table 3).
Five patients had ISA serum level monitoring during their therapy with 16 total levels measured (median, level 3.6 mcg/mL; range, 1.1–40 mcg/mL). Patient 7 had only 1 level measured at 40 mcg/mL and had no apparent toxicity.
Outcomes
Most patients completed treatment and were cured (11 of 14, 79%) after a median of 270 days of isavuconazole (range, 31–385 days) and median of 383 days of total therapy (range, 193–500 days). Of the 11 cured patients, 7 of 11 (64%) required at least 12 months of therapy (eg, immunocompromised or CNS dissemination) and received a median of 298 days of ISA (range, 203–385 days) accounting for a median of 79% of their therapy. The other 4 of 11 patients received a median of 172 days of ISA (range, 31–379) accounting for a median of 80% of their therapy. There was only 1 death during therapy. Patient 2 had a fatal intraparenchymal hemorrhage approximately 4 months into therapy for disease with CNS dissemination. Patient 7 continued to have detectable Blastomyces urine antigen for 2.5 years after his initial diagnosis and was continued on ISA. Patient 1 transferred care to another institution before therapy could be completed but had detectable Blastomyces urine antigen on last follow-up. All patients were followed by an infectious disease specialist for a median duration of 12.5 months (range, 3–54 months). Of those cured, the median follow-up with this specialist after cessation of triazoles was 2 months (interquartile range, 0–10 months).
Antifungal Susceptibility Testing
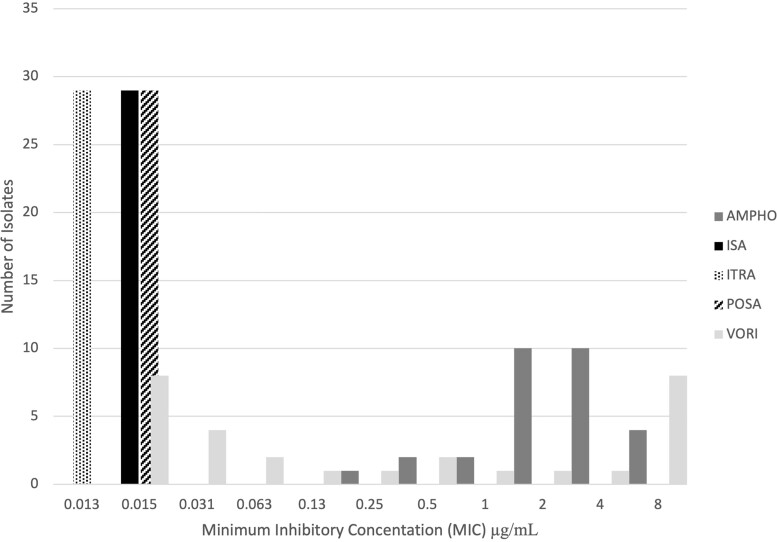
Twenty-nine clinical isolates were available for antifungal susceptibility testing. Most were B dermatitidis (24 of 29, 83%), and B gilchristii was the only other species (5 of 29, 17%). The minimum inhibitory concentrations (MICs) of ISA, posaconazole, and itraconazole were at or below their most dilute concentrations of 0.015 µg/mL, 0.015 µg/mL, and 0.13 µg/mL, respectively. The MICs of amphotericin B ranged from 0.25 µg/mL to ≥8.0 µg/mL with a peak at 4 µg/mL. The MICs of voriconazole were distributed in a bimodal fashion with 2 local maxima at ≥8.0 µg/mL and 0.015 µg/mL (Figure 1). There was no appreciable difference in MIC comparing B dermatitidis and B gilchristii isolates. The B dermatitidis isolate from case patient 14 was included in susceptibility testing and had an ISA MIC <0.015 µg/mL, itraconazole <0.013, posaconazole <0.015, voriconazole <0.015, and amphotericin B of 2.
Figure 1.
Isavuconazonium susceptibility against Blastomyces. Blastomyces spp antifungal susceptibility testing. Antifungals are abbreviated as follows: AMPHO, amphotericin B; ISA, isavuconazonium sulfate; ITRA, itraconazole; POSA, posaconazole; VORI, voriconazole.
DISCUSSION
We present the largest series of blastomycosis treated with ISA with long-term, follow-up data available to assess tolerability and cure, with a median of 255 days of ISA use accounting for 68% of total therapy on average, 11 of 14 (79%) classified as cure, minimal adverse reactions (change in therapy in 1 patient), and 1 death during treatment thought to be unrelated to infection or treatment course. Other case series have only included 3 cases each of blastomycosis treated with ISA, and Thompson et al [8] documented only 1 case using ISA for more than 1 week [15].
Although most isolates tested for antifungal susceptibility do not represent the cases in this series, the results are supportive of ISA use in blastomycosis. Prior publications have shown low ISA MICs for Blastomyces spp including 6 isolates with MIC ranging from 0.5 to 4 mcg/mL [9], 3 isolates with MIC ranging from 0.0004 to 0.0008 µg/mL [13], and 9 isolates ranging from 0.125 to 0.5 µg/mL [14]. We show the ISA MICs of 29 isolates to be 0.015 mcg/mL or less. This is supportive of a low MIC epidemiological cutoff value.
Currently, ISA is only used for blastomycosis after considering itraconazole or voriconazole. However, given the efficacy and low ISA MIC in this series, there are reasons to expand the use of ISA as first-line therapy, particularly among immunocompromised patients who require 12 months of therapy and are at increased risk for drug-drug interactions, QT lengthening therapies, and adverse effects. Not only does ISA have less hepatic toxicity, but it allows for once-daily dosing with or without food, high bioavailability, and QT shortening instead of prolongation. In addition, intravenous ISA does not contain B-cyclodextrin, which can accumulate in persons with renal insufficiency [6]. Posaconazole has also been used to treat disseminated blastomycosis, but its dosing interval depends on the formulation used and it is associated with QT prolongation [16].
Diagnosis of CNS blastomycosis is challenging because CSF culture is insensitive, and neuroimaging findings are nonspecific [5, 17]. This was seen in this series with no growth of Blastomyces spp on CSF culture. Voriconazole has been preferred for CNS disease because it has better blood-brain barrier penetration than itraconazole [5]. However, susceptibility testing found that 10 of 29 (34%) Blastomyces isolates had high (≥2 µg/mL) MICs to voriconazole. A study by Schmitt-Hoffmann et al [18] using a rat model demonstrated that the accumulation of ISA in brain tissue is similar to voriconazole, suggesting that ISA may be efficacious for CNS fungal disease [18]. In other mouse models, ISA was shown to reduce fungal burden of CNS candidiasis and burden of cryptococcal meningitis when compared with fluconazole [10, 11]. A retrospective study of a subset of patients with CNS fungal disease initially included in clinical trials evaluating the efficacy of ISA showed 42-day survival of 80.6%, 84-day survival of 69.4%, and complete or partial clinical response of 58.3% at end of treatment [12]. These were similar outcomes compared with trials of voriconazole in CNS fungal disease [19]. The study by Schmitt-Hoffman et al [18] also found that bone had lower levels of ISA accumulation. Nevertheless, in our study, patients 6 and 11 with osteomyelitis were effectively treated with a long course of ISA.
A prior series of blastomycosis at UWHC included 19 (17.9%) SOT recipients whereas this current study included 6 (43%) SOT recipients [15]. Although UWHC remains a large transplant center in an endemic area for blastomycosis, the comparative overrepresentation in this series is likely due to the prolonged duration of treatment these complex patients require, putting them at increased risk for adverse drug effects from other triazoles, particularly the 4 of 6 with CNS disease who could not tolerate voriconazole. Drug-drug interactions with calcineurin inhibitors was not documented as a reason for use of ISA.
This study is strengthened by the inclusion of 7 patients with CNS dissemination, 6 SOT recipients, therapeutic drug levels, documented adverse drug effects, median of 12.5 months of infectious disease follow-up, and 29 clinical isolates with antifungal susceptibility testing.
Limitations to this study are the inherent retrospective design and small sample size; however, this is the largest study to date to assess the role of isavuconazonium sulphate for continuation of treatment of blastomycosis after adverse drug reactions or drug-drug interactions with other triazoles. Short duration of follow-up after cessation of triazole therapy limits detection of relapse, but this is uncommon in blastomycosis. Survival bias systematically favors good outcomes in these patients because ISA was used as a second-line therapy in all but patient 8, but patients were included regardless of outcome. In addition, a significant limitation due to the design is a lack of standardization in initial therapy, and the duration of LAMB among hospitalized cases ranged from 7 to 46 days. A future study could compare ISA versus itraconazole or voriconazole for either disseminated disease not involving the CNS or disease involving the CNS, respectively, to more accurately compare treatment efficacy.
CONCLUSIONS
This descriptive retrospective case series reviewed 14 cases of proven or probable blastomycosis treated at UWHC between 2015 and 2019 with isavuconazonium sulphate for a median of 255 days, and 11 of 14 patients were classified as cured. In addition, it includes 29 clinical isolates with isavuconazole MICs of ≤0.015 µg/mL. These data support the use of isavuconazonium sulphate for blastomycosis in immunocompromised and immunocompetent patients with adverse drug reactions or drug-drug interactions with other triazoles. There are good reasons to study isavuconazonium sulphate versus an alternative triazole therapy in a prospective trial.
Acknowledgments
M. J. S. thanks the coauthors for guidance and mentoring with this work.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Contributor Information
Michael J Scolarici, Department of Medicine, Division of Infectious Diseases, University of Wisconsin, Madison, Wisconsin, USA.
Coleton King, Wisconsin State Laboratory of Hygiene, Madison, Wisconsin, USA.
Alana Sterkel, Wisconsin State Laboratory of Hygiene, Madison, Wisconsin, USA; Department of Pathology and Laboratory Medicine, University of Wisconsin, Madison, Wisconsin, USA.
Jeannina Smith, Department of Medicine, Division of Infectious Diseases, University of Wisconsin, Madison, Wisconsin, USA.
Gregory Gauthier, Department of Medicine, Division of Infectious Diseases, University of Wisconsin, Madison, Wisconsin, USA.
Christopher Saddler, Department of Medicine, Division of Infectious Diseases, University of Wisconsin, Madison, Wisconsin, USA.
References
- 1. McBride JA, Gauthier GM, Klein BS. Clinical manifestations and treatment of blastomycosis. Clin Chest Med 2017; 38:435–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chapman SW, Dismukes WE, Proia LA, et al. Clinical practice guidelines for the management of blastomycosis: 2008 update by the Infectious Diseases Society of America. Clin Infect Dis 2008; 46:1801–12. [DOI] [PubMed] [Google Scholar]
- 3. Miller R, Assi M. AST infectious diseases community of practice. Endemic fungal infections in solid organ transplant recipients-guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant 2019; 33:e13553. [DOI] [PubMed] [Google Scholar]
- 4. Limper AH, Knox KS, Sarosi GA, et al. An official American Thoracic Society statement: treatment of fungal infections in adult pulmonary and critical care patients. Am J Respir Crit Care Med 2011; 183:96–128. [DOI] [PubMed] [Google Scholar]
- 5. Ta M, Flowers SA, Rogers PD. The role of voriconazole in the treatment of central nervous system blastomycosis. Ann Pharmacother 2009; 43:1696–700. [DOI] [PubMed] [Google Scholar]
- 6. Nett JE, Andes DR. Antifungal agents: spectrum of activity, pharmacology, and clinical indications. Infect Dis Clin North Am 2016; 30:51–83. [DOI] [PubMed] [Google Scholar]
- 7. Maertens JA, Raad II, Marr KA, et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. Lancet Lond Engl 2016; 387:760–9. [DOI] [PubMed] [Google Scholar]
- 8. Thompson GR, Rendon A, Ribeiro Dos Santos R, et al. Isavuconazole treatment of cryptococcosis and dimorphic mycoses. Clin Infect Dis 2016; 63:356–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. González GM. In vitro activities of isavuconazole against opportunistic filamentous and dimorphic fungi. Med Mycol 2009; 47:71–6. [DOI] [PubMed] [Google Scholar]
- 10. Wiederhold NP, Kovanda L, Najvar LK, et al. Isavuconazole is effective for the treatment of experimental cryptococcal meningitis. Antimicrob Agents Chemother 2016; 60:5600–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Majithiya J, Sharp A, Parmar A, Denning DW, Warn PA. Efficacy of isavuconazole, voriconazole and fluconazole in temporarily neutropenic murine models of disseminated Candida tropicalis and Candida krusei. J Antimicrob Chemother 2009; 63:161–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schwartz S, Cornely OA, Hamed K, et al. Isavuconazole for the treatment of patients with invasive fungal diseases involving the central nervous system. Med Mycol 2020; 58:417–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yamazaki T, Inagaki Y, Fujii T, et al. In vitro activity of isavuconazole against 140 reference fungal strains and 165 clinically isolated yeasts from Japan. Int J Antimicrob Agents 2010; 36:324–31. [DOI] [PubMed] [Google Scholar]
- 14. Dukik K, Al-Hatmi AMS, Curfs-Breuker I, Faro D, de Hoog S, Meis JF. Antifungal susceptibility of emerging dimorphic pathogens in the family ajellomycetaceae. Antimicrob Agents Chemother 2018; 62:e01886-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McBride JA, Sterkel AK, Matkovic E, Broman AT, Gibbons-Burgener SN, Gauthier GM. Clinical manifestations and outcomes in immunocompetent and immunocompromised patients with blastomycosis. Clin Infect Dis 2021; 72:1594–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Proia LA, Harnisch DO. Successful use of posaconazole for treatment of blastomycosis. Antimicrob Agents Chemother 2012; 56:4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bariola JR, Perry P, Pappas PG, et al. Blastomycosis of the central nervous system: a multicenter review of diagnosis and treatment in the modern era. Clin Infect Dis 2010; 50:797–804. [DOI] [PubMed] [Google Scholar]
- 18. Schmitt-Hoffmann A-H, Kato K, Townsend R, et al. Tissue distribution and elimination of isavuconazole following single and repeat oral-dose administration of isavuconazonium sulfate to rats. Antimicrob Agents Chemother 2017; 61:e01292-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schwartz S, Reisman A, Troke PF. The efficacy of voriconazole in the treatment of 192 fungal central nervous system infections: a retrospective analysis. Infection 2011; 39:201–10. [DOI] [PubMed] [Google Scholar]