Abstract
Context
Diabetes and cardiovascular diseases are common among men with Klinefelter syndrome (KS) and contribute to high morbidity and mortality.
Objective
To determine if cardiometabolic-related diagnoses are more prevalent among youth with KS than matched controls in a large population-based cohort.
Methods
Secondary data analysis of electronic health records from 6 pediatric institutions in the United States (PEDSnet). Patients included all youth with KS in the database (n = 1080) and 4497 youth without KS matched for sex, age (mean 13 years at last encounter), year of birth, race, ethnicity, insurance, site, and duration of care (mean 7 years). The main outcome measures were prevalence of 5 cardiometabolic-related outcomes: overweight/obesity, dyslipidemia, dysglycemia, hypertension, and liver dysfunction.
Results
The odds of overweight/obesity (OR 1.6; 95% CI 1.4-1.8), dyslipidemia (3.0; 2.2-3.9), and liver dysfunction (2.0; 1.6-2.5) were all higher in KS than in controls. Adjusting for covariates (obesity, testosterone treatment, and antipsychotic use) attenuated the effect of KS on these outcomes; however, boys with KS still had 45% greater odds of overweight/obesity (95% CI 1.2-1.7) and 70% greater odds of liver dysfunction (95% CI 1.3-2.2) than controls, and both dyslipidemia (1.6; 1.1-2.4) and dysglycemia (1.8; 1.1-3.2) were higher in KS but of borderline statistical significance when accounting for multiple comparisons. The odds of hypertension were not different between groups.
Conclusion
This large, population-based cohort of youth with KS had a higher odds of most cardiometabolic-related diagnoses than matched controls.
Keywords: cardiometabolic, diabetes, Klinefelter syndrome, sex chromosome aneuploidy, PEDSnet, population health
Klinefelter syndrome (KS) is a genetic condition in males who have an additional X chromosome. Population-based studies conducted in Europe have found higher morbidity and mortality in men with KS due to a variety of comorbidities, particularly diabetes and cardiovascular diseases (CVDs) (1-3). Features of metabolic syndrome, a diagnosis consisting of a constellation of conditions reflecting systemic insulin resistance that confer an increased risk for progression to type 2 diabetes and cardiac events, occur in up to 50% of men with KS (4). Central adiposity, dyslipidemia, and dysglycemia appear to be particularly prevalent, whereas hypertension does not (5). Although testosterone concentrations have an inverse relationship with the presence of metabolic syndrome, treatment with exogenous testosterone has not been shown to restore normal cardiometabolic function in KS (4, 6).
The data on cardiometabolic outcomes in youth with KS are scarce. Ross and colleagues have conducted the majority of published research in this area (7-9). Their work has shown that prepubertal boys with KS have greater adiposity, higher insulin resistance, and lower high-density lipoprotein (HDL) cholesterol than boys without KS, and that these metabolic syndrome features were associated with markers of testicular insufficiency. Two other studies have reported body fat percentage around 1 SD above average in adolescents and infants with KS (10, 11). To our knowledge, these small, single-center studies represent the only research on cardiometabolic disease risk in youth with KS.
Although the prevalence of KS is 1 in every 600 males, the diagnosis is often late or missed altogether, with less than 20% of boys with KS receiving a diagnosis before adulthood (12). This has resulted in the challenges faced by rare diseases communities, including decreased awareness and scarcity of quality research (12-15). Recruiting meaningful sample sizes and minimizing biases are particularly challenging for pediatric clinical research in genetic conditions like KS. Our current knowledge of cardiometabolic risk in KS relies primarily on single-center studies that are prone to ascertainment bias, limiting both generalizability and the evidence needed to develop care guidelines that promote better outcomes (15-17). Although there are some promising ongoing prospective and interventional studies in KS, the field is far behind what is needed to positively impact clinical care, and novel approaches are sorely needed. While epidemiological studies in the United States are challenged by the lack of a national healthcare system, innovative big health data resources have emerged and promise to have a significant impact on pediatric research. These sources provide the possibility of studying relatively large numbers of children with rare or underdiagnosed conditions such as KS.
Despite the preliminary pediatric cohort studies and convincing adult data, there has been no pediatric population–based evaluation of cardiometabolic health in KS. To address this gap, we utilized PEDSnet, a multicenter clinical data repository representing over 6 million children in the United States, to conduct a cross-sectional analysis from electronic health record (EHR) data on cardiometabolic risk outcomes in boys with and without KS. We hypothesized we would observe increased odds of cardiometabolic-related diagnoses in KS compared with matched controls.
Materials and Methods
Data Source
Data for this analysis were obtained from PEDSnet, a national clinical research network that aggregates EHR data from large pediatric health systems representing over 6 million children into a Common Data Model (https://pedsnet.org) (18, 19). The PEDSnet collaborative was developed to facilitate pediatric research across multiple nonprofit health systems with funding from the Patient-Centered Outcomes Research Institute. Deidentified EHR data from 6 participating pediatric organizations (Children’s Hospital Colorado, Children’s Hospital of Philadelphia, Nemours Children’s Health, Nationwide Children’s Hospital, St. Louis Children’s Hospital, and Seattle Children’s Hospital) were obtained from the PEDSnet Data Coordinating Center in November 2019.
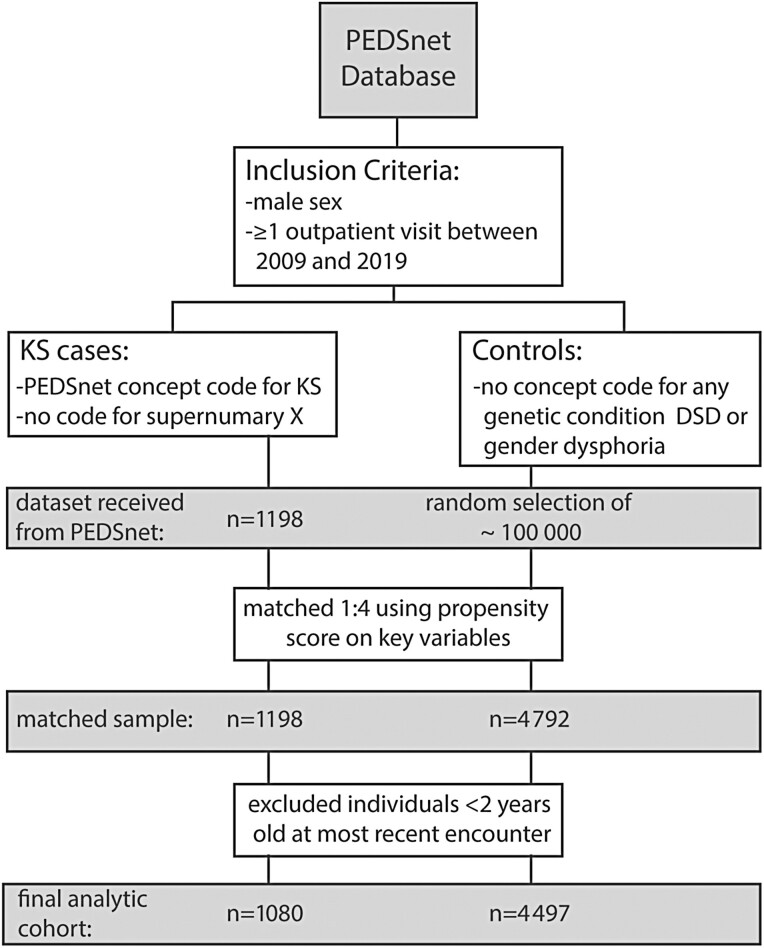
Inclusion criteria for both cases (KS) and controls included male sex and at least 1 outpatient encounter in the PEDSnet database between 2009 and 2019 at any age (Fig. 1). Cases were defined by a billing or problem list diagnosis code that mapped to KS (PEDSnet concept IDs: 4228490, 40420291, 4007591, or 4007592). Individuals with a diagnosis code for male supernumerary sex chromosome aneuploidy (48,XXYY, 48,XXXY, 49,XXXXY) were not included as these individuals often present with a more severe phenotype. A pool of 197039 potential controls without a diagnosis of any known genetic syndrome, disorder of sex development, or gender dysphoria was also obtained. The limited data set obtained included discrete data elements such as demographic variables, billing and problem list diagnoses, vital signs, and laboratory values. This study protocol was submitted to the local institutional review board and determined to be nonhuman subjects research (COMIRB #18-0887).
Figure 1.
Flow diagram for the study design and final analytic cohort.
Sample Matching
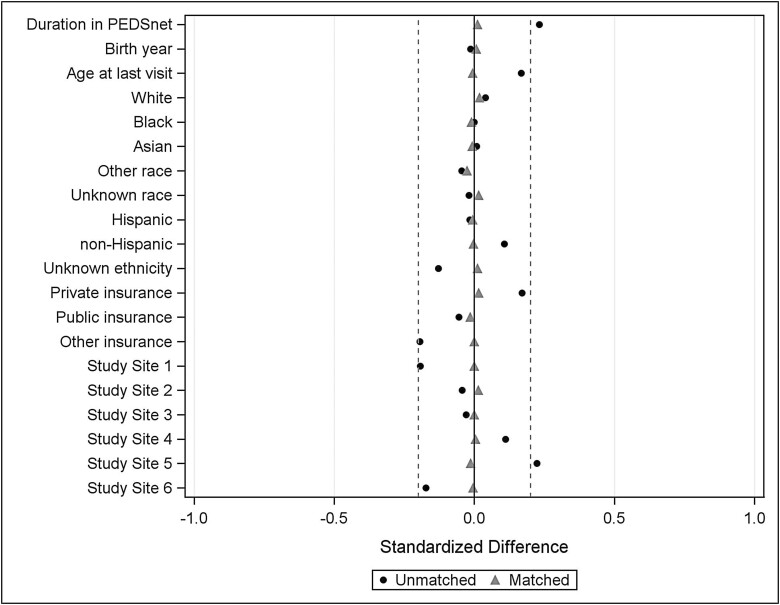
Control group subjects were significantly different from the cases on several demographic variables; therefore, propensity score matching was conducted to match each case with male controls based on 7 variables: PEDSnet site, race, ethnicity, payer status (public/private/other), year of birth, age at most recent encounter in PEDSnet, and duration in the PEDSnet database (time between first and last encounter; duration was set to 0 for individuals with a single encounter) (20, 21). Missing or unknown information for race and ethnicity was accommodated by including unknown as another category for the variable. Each case was matched to 4 controls on the propensity score using a greedy match algorithm and a caliper width of 0.10 (22). The balance of covariates between cases and control groups (ie, the similarity of the covariate distributions) was evaluated as a reduction in standardized mean difference, using a decision criterion of less than 0.20 to indicate that a covariate was balanced (Fig. 2) (23). For this analysis of cardiometabolic health outcomes, only patients >2 years of age were included to avoid perinatal conditions that would be unlikely to accurately reflect the cardiometabolic health conditions of interest. Patients who did not have a visit >2 years of age were excluded after matching; therefore, the final analytic cohort was not a precise 1:4 match.
Figure 2.
Standardized difference scores between cases and controls before (circles) and after (triangles) matching. After matching, the standardized difference was within ±0.2 (dashed lines), indicating balanced match.
Outcomes
The primary outcomes were the presence of the following 5 cardiometabolic risk conditions: overweight/obesity, dyslipidemia, dysglycemia, hypertension, and abnormal liver function. The presence of each of these conditions was determined by meeting the criteria defined in Table 1
Table 1.
Criteria and codes used for defining binary outcomes
| Outcome | Diagnoses, vital signs, or lab value criteria | SNOMED-CT or LOINC codes |
|---|---|---|
| Overweight/obesity | Increased BMI | 4849901 |
| Obesity | 414916001; 414915002 | |
| Overweight | 238131007 | |
| BMI >85th %ile if <18 yearsa (24) | 39156-5 | |
| BMI >25 if >18 yearsa | 39156-5 | |
| Dyslipidemia (25) | Dyslipidemia | 370992007 |
| Hyperlipidemia | 55822004 | |
| HDL <45 mg/dL if <18 yearsa | 2085-9 or 9833-5 | |
| HDL <40 mg/dL if >18 yearsa | 2085-9 or 9833-5 | |
| Triglycerides >100 mg/dL if <10 yearsa | 2571-8 | |
| Triglycerides >130 mg/dL if 10-19 yearsa | 2571-8 | |
| Triglycerides >150 mg/dL if >19 yearsa | 2571-8 | |
| LDL >100 mg/dL if <18 yearsa | 18262-6, 47213-4, 2089-1, 13457-7 | |
| LDL >130 mg/dL if >18 yearsa | 18262-6, 47213-4, 2089-1, 13457-7 | |
| Total cholesterol >200 mg/dLa | 2093-3 | |
| Dysglycemiab | Impaired glucose tolerance | 9414007 |
| Impaired fasting glycemia | 390951007 | |
| Diabetes mellitus type 2 | 44054006 | |
| Chronic hyperglycemia | 170765005 | |
| Dysglycemia | 426255005 | |
| Nondiabetic hyperglycemia | 700449008 | |
| Metabolic stress hyperglycemia | 237624007 | |
| Poor glycemic control | 237622006 | |
| Hyperglycemia due to diabetes mellitus | 822995009 | |
| Hemoglobin A1c >5.7%a | 4548-4 or 17856-6 | |
| Hypertension | Benign hypertension | 10725009 |
| Essential hypertension | 59621000 | |
| Diastolic hypertension | 48145000 | |
| Prehypertension | 702817009 | |
| Finding of increased blood pressure | 24184005 | |
| BP >90th%ile if <13 yearsa (26) | 8460-8, 8454-1, 8459-0, 8455-8, | |
| SBP >120 or DBP >80 if >13 yearsa | 8461-6, or 8453-3 | |
| Liver dysfunction | Alanine aminotransferase level abnormal | 166646003 |
| Nonalcoholic fatty liver | 197315008 | |
| Nonalcoholic steatohepatitis | 442685003 | |
| Increased aspartate transaminase level | 160931000119108 | |
| Aspartate aminotransferase level abnormal | 166668008 | |
| Elevated liver enzymes level | 707724006 (omit 707734002) | |
| AST or ALT >97.5 %ile if <17 yearsa (27) | 1920-8 or 1742-6 | |
| AST >35 if >17 yearsa (28) | 1920-8 | |
| ALT >33 if >17 yearsa (28) | 1742-6 |
a Required 2 or more separate events meeting these criteria to minimize abnormalities secondary to acute illness or spurious results.
b Excluded individuals with a diagnosis of type 1 diabetes mellitus.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; DBP, diastolic blood pressure; HDL, high density lipoprotein; LDL, low density lipoprotein; SBP systolic blood pressure.
for either a corresponding billing or problem list diagnosis or at least 2 instances of objective laboratory/visit data. A combination of coded diagnoses and objective data were used to increase the sensitivity of identifying a cardiometabolic-related diagnosis. Dates were not consistently associated with diagnoses, therefore the specific age at the time of first cardiometabolic-related diagnosis was not available. All diagnoses utilized SNOMED clinical terminology, including all child concepts of selected ancestor codes, which were then mapped to PEDSnet concept codes using Athena Observational Health Data Sciences and Informatics Vocabulary Repository. Body mass index (BMI) was calculated from recorded height and weight, and corresponding BMI percentiles and z-scores were obtained for all individuals under 20 years of age (24). Secondary outcomes included continuous variables of BMI z-scores, systolic and diastolic blood pressure, total cholesterol, HDL cholesterol, low-density lipoprotein (LDL) cholesterol, triglycerides, hemoglobin A1c, alanine transaminase (ALT), and aspartate transaminase (AST) taken from their most recent recorded measurement. A billing/problem list diagnosis of metabolic syndrome (SNOMED concept ID 237602007) and type 2 diabetes mellitus (SNOMED concept ID 44054002) were also explored as secondary outcomes.
Statistical Analysis
The prevalence of each of the cardiometabolic risk outcomes were compared between cases and controls using generalized estimating equations, which accounts for potential correlation introduced during the matching procedure. Bonferroni correction was used to account for the multiple comparisons of the 5 primary outcomes; therefore, P< .01 was considered statistically significant. Descriptive statistics including the prevalence of outcomes in the cases vs control groups, as well as OR and 95% CI were computed. Logistic regression with generalized estimating equations was performed, adjusted for age at most recent visit (all models), and overweight/obesity status, documentation of a testosterone prescription and documentation of an antipsychotic prescription (due to the known adverse cardiometabolic effects of many antipsychotic medications) to control for the contribution of these variables to the outcomes of interest. If an individual did not have an antipsychotic prescription or testosterone listed in their medical record, we assumed they did not receive either. Due to the clinical interest of evaluating the role of testosterone replacement on cardiometabolic health, we further assessed these outcomes within the KS group by testosterone treatment status. For continuous variables, data-cleaning measures included examination for outliers by visual and statistical inspection and removal of values that were determined to be biologically implausible for a pediatric population by clinician investigators (eg, weight <0.2 or > 300 kg, height <25 or >216 cm, etc.) Measures of central tendency for the most recently available measurements were compared between cases and controls. Finally, for visualization of the relationship of age and BMI, all available BMI measurements were used to create loess curves for the 25th, 50th, and 75th BMI percentiles by age for both KS and controls. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
Demographics for patients with KS (n = 1080) and their matched controls without KS (n = 4497) are shown in Table 2. The majority of the cohort was in their early teens at their most recent PEDSnet encounter and had been followed for an average duration of 5 years. The groups were well matched for site and sociodemographic variables. The majority of both cases and controls had a primary care type encounter (General Pediatrics, Adolescent Medicine). The most commonly coded diagnoses among all controls were well child visit, acute respiratory viruses (upper respiratory infection, cough, pharyngitis, otitis, rhinitis), constipation, and fever—consistent with what would be expected in a general pediatric population.
Table 2.
Demographics of the analytic sample
| Klinefelter Syndrome (n = 1080) |
Controls (n = 4497) | P value | |
|---|---|---|---|
| PEDSnet site | .93 | ||
| Site 1 | 87 (8.1) | 406 (9.0) | |
| Site 2 | 212 (19.6) | 871 (19.4) | |
| Site 3 | 107 (9.9) | 451 (10.0) | |
| Site 4 | 329 (30.5) | 1320 (29.4) | |
| Site 5 | 266 (24.6) | 1118 (24.9) | |
| Site 6 | 79 (7.3) | 331 (7.4) | |
| Race | .83 | ||
| Asian | 40 (3.7) | 145 (3.2) | |
| Black | 98 (9.1) | 439 (9.8) | |
| White | 755 (69.9) | 3100 (68.9) | |
| Other | 109 (10.1) | 479 (10.7) | |
| Unknown | 78 (7.2) | 334 (7.4) | |
| Ethnicity | .85 | ||
| Hispanic | 123 (11.4) | 490 (10.9) | |
| Non-Hispanic | 877 (81.2) | 3659 (81.4) | |
| Unknown | 80 (7.4) | 348 (7.7) | |
| Insurance type | .05 | ||
| Public | 347 (32.1) | 1386 (30.8) | |
| Private | 652 (60.4) | 2777 (61.8) | |
| Other | 64 (5.9) | 213 (4.7) | |
| Unknown | 17 (1.6) | 121 (2.7) | |
| Primary care encounter | 696 (64.4) | 2963 (65.9) | .37 |
| Age at first encounter (years) | 6.6 ± 5.8 | 6.3 ± 5.5 | .52 |
| Age at most recent encounter (years) | 13.5 ± 6.0 | 13.1 ± 5.2 | .02 |
| Duration in PEDSnet (years) | 7.0 ± 5.2 | 6.8 ± 5.4 | .27 |
| Age at first KS diagnosis (years) | 8.7 ± 5.9 | — | |
| Age at most recent KS diagnosis (years) | 12.3 ± 6.1 | — | |
| Testosterone prescription | 301 (27.9) | 15 (0.33) | <.001a |
| Age at first prescription | 15.1 (13.3-16.8) | 14.2 (13.5-15.8) | .39 |
| Antipsychotic medication prescription | 74 (6.9) | 124 (2.8) | <.001a |
Data are shown as n (%), mean ± standard deviation or median (25-75th %ile).
a Statistically significant at an alpha of .01.
In unadjusted models, males with KS had higher odds of meeting criteria for overweight/obesity, dyslipidemia, and liver dysfunction (Table 3). The odds of dysglycemia were also higher although did not meet the statistical threshold for significance with multiple comparisons, while the odds of hypertension were not significantly different. Nearly half of the sample with KS met criteria for 1 or more cardiometabolic outcomes, with 6% meeting criteria for 3 or more, representing a 2 times greater odds over controls. A diagnosis of metabolic syndrome (OR 12.7, 95% CI 5.4-30.0) and type 2 diabetes mellitus (OR 2.6, 1.1-6.0) were both greater in the KS group; however, the number of individuals with these diagnoses in this sample was small, so these results may not be reliable. Table 4 presents adjusted models that include potential explanatory variables. The presence of overweight/obesity was significantly associated with all outcomes and slightly attenuated the effect of KS on diagnoses of dyslipidemia and liver dysfunction, although they remained significant. Testosterone prescription was not associated with the outcomes of overweight/obesity, dysglycemia, hypertension, or liver dysfunction (P> .05 for all); however, it was significantly associated with dyslipidemia and mildly attenuated the effect of KS for that outcome (also see Table 5). A prescription for an antipsychotic medication was associated with increased odds of the cardiometabolic outcomes of interest except dysglycemia, again slightly attenuating the effect of KS but not impacting the overall result. When accounting for the predefined covariates in a combined model, KS continued to have an independent effect on obesity (P < .0001) and liver dysfunction (P = .0002), and a borderline effect on dyslipidemia (P = .023) and dysglycemia (P = .0326). These results were overall unchanged when the total number of outpatient visits were included in the model; however, due to collinearity with age this model is not presented.
Table 3.
Comparison of the prevalence of the primary cardiometabolic outcomes between Klinefelter syndrome (KS) and controls
| KS | Controls | OR (95% CI) | P value | |
|---|---|---|---|---|
| (n = 1080) | (n = 4497) | |||
| Overweight/obesity | 442 (40.9) | 1381 (30.7) | 1.56 (1.36-1.79) | <.0001a |
| Dyslipidemia | 88 (8.1) | 131 (2.9) | 2.96 (2.24-3.91) | <.0001a |
| Dysglycemia | 27 (2.5) | 64 (1.4) | 1.78 (1.13-2.78) | .0121 |
| Hypertension | 76 (7.0) | 319 (7.1) | 1.00 (0.77-1.29) | .9914 |
| Liver dysfunction | 134 (12.4) | 293 (6.5) | 2.03 (1.64-2.52) | <.0001a |
| Total no. of conditions | ||||
| 1 condition | 348 (32.2) | 1207 (26.8) | 1.29 (1.12-1.49) | .0006a |
| 2 conditions | 101 (9.4) | 274 (6.1) | 1.52 (1.19-1.93) | .0007a |
| 3 or more conditions | 67 (6.2) | 135 (3.0) | 1.96 (1.44-2.66) | <.0001 |
Data are shown as n (%) or unadjusted OR and 95% CI.
a Statistically significant at an alpha of .01.
Table 4.
Adjusted models for the primary cardiometabolic outcomes between KS and controls
| Outcome | Obesity | Testosterone Rx | Antipsychotic Rx | All covariatesa |
|---|---|---|---|---|
| Overweight/obesity | — | 1.52 (1.30-1.79)b | 1.50 (1.31-1.73)b | 1.49 (1.27-1.75)b |
| Dyslipidemia | 2.50 (1.85-3.37)b | 1.81 (1.22-2.67)b | 2.58 (1.93-3.44)b | 1.60 (1.07-2.40) |
| Dysglycemia | — | 1.81 (1.05-3.13) | 1.56 (0.98-2.48) | 1.84 (1.07-3.16) |
| Hypertension | 0.80 (0.61-1.06) | 1.02 (0.73-1.41) | 0.85 (0.64-1.12) | 0.84 (0.59-1.18) |
| Liver dysfunction | 1.78 (1.42-2.23)b | 1.98 (1.52-2.58)b | 1.77 (1.40-2.24) | 1.71 (1.30-2.25)b |
The models control for the addition of obesity, testosterone treatment, antipsychotic treatment, and the combined effect of all of those risk factors on cardiometabolic health. All of these models include the covariate age at most recent visit. Data are shown as OR and 95% CI.
Abbreviation: Rx, prescription.
a The outcomes of overweight/obesity and dysglycemia did not include overweight/obese in the model.
b Statistically significant at an alpha of 0.01.
Table 5.
Comparison of individuals with KS by testosterone treatment status
| No T | +T | OR (95% CI) | P value | |
|---|---|---|---|---|
| n = 779 | n = 301 | |||
| Overweight/obesity | 311 (39.9) | 131 (43.5) | 1.23 (0.89-1.70) | .2002 |
| Dyslipidemia | 32 (4.1) | 56 (18.6) | 3.03 (1.78-5.15) | <.0001a |
| Dysglycemia | 15 (1.9) | 12 (4.0) | 0.95 (0.41-2.23) | .9132 |
| Hypertension | 54 (6.9) | 22 (7.3) | 0.72 (0.42-1.30) | .2809 |
| Liver dysfunction | 92 (11.8) | 42 (14.0) | 1.03 (0.65-1.64) | .8921 |
Models include the covariate age at most recent visit. Data are shown as n (%) or unadjusted OR and 95% CI.
a Statistically significant at an alpha of .01.
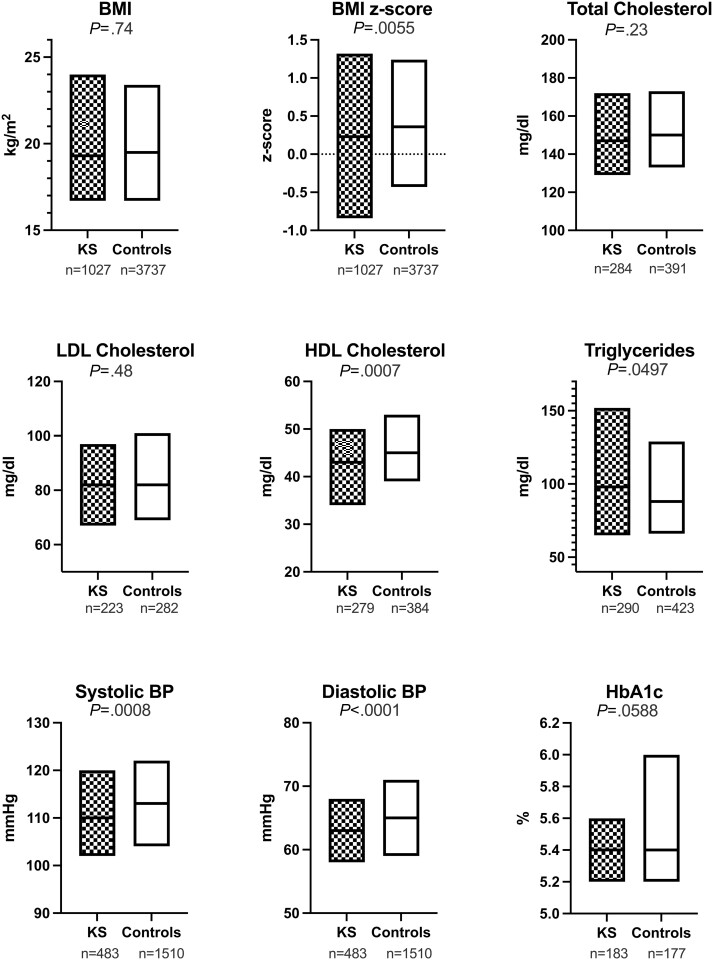
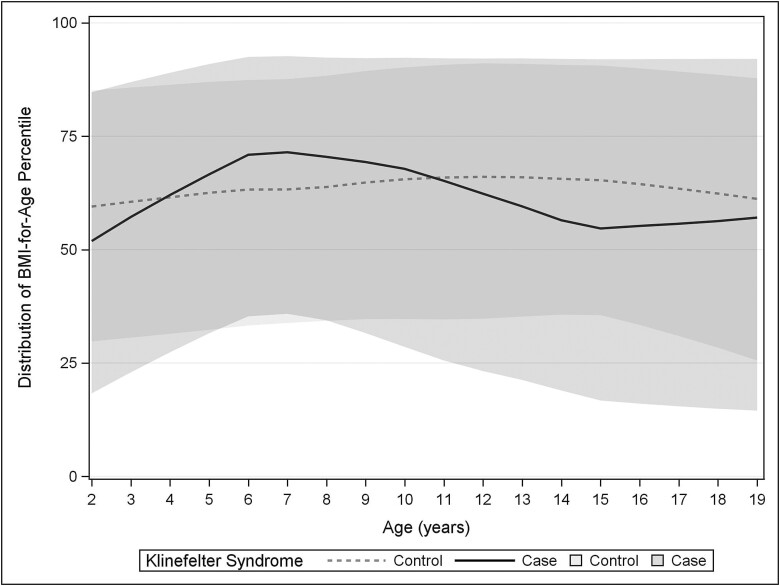
For continuous outcomes, data were more likely to be missing for controls than for cases. The numbers for available data, and median and interquartile range for all continuous outcomes are shown in Fig. 3. Absolute BMI was not significantly different between groups; however, BMI z-score was significantly lower in KS than in controls and the range was wider (Fig. 4). Systolic and diastolic blood pressures were significantly lower in KS than in controls, although the clinical relevance was minimal (2-3 mmHg). There were no differences in total or LDL cholesterol; HDL cholesterol was lower in KS and triglycerides were slightly higher.
Figure 3.
Box plots with median and interquartile range for Klinefelter Syndrome (black) vs controls (white) for all continuous variables. Values under each plot are the number of individuals out of the 1080 cases and 4497 controls for which data were available.
Figure 4.
Loess curves for all available BMI percentiles by age for KS cases (solid/dark) and controls (dashed/light) with the 50th percentile shown with a line and the shaded area representing the interquartile range (25th-75th percentiles).
Discussion
This population-based, cross-sectional study of pediatric patients with KS confirmed greater odds of cardiometabolic-related diagnoses compared with controls. Specifically, the odds of overweight/obesity, dyslipidemia, and liver dysfunction were higher in KS and the odds of dysglycemia were also greater, although of borderline statistical significance due to the low prevalence in both groups. The increased odds for these cardiometabolic-related diagnoses in KS remained after adjusting for known risk factors of CVD, implying an independent effect of KS on these outcomes. Importantly, the odds of hypertension were not higher in KS. This first ever population-based study of cardiometabolic health focused on in youth with KS supports previous smaller studies in pediatric KS cohorts as well as adult studies, and adds to the literature with a large clinical sample representative of the US population.
The assessment of overweight/obesity is arguably the most reliable outcome in this study. More than 85% of patients had at least 1 simultaneously documented height and weight available to allow calculation of BMI; therefore, we did not rely solely on the addition of overweight/obesity as a billing diagnosis or problem list condition. The finding of 40% of boys with KS meeting the criteria for overweight/obesity is concerning given the known association of childhood obesity with adult CVD (29). However, further exploration of BMI data in this cohort revealed that although there was a higher prevalence of overweight/obesity in KS, BMI and BMI z-scores were not higher and indeed the range of BMI was wider in KS across the pediatric age span. There is therefore a subset of boys with KS who have low BMIs, particularly in the toddler years and early adolescence, that warrant further investigation into different phenotypes in this population. Importantly, boys without overweight/obesity still had other cardiometabolic risk diagnoses; therefore, although prevention and mitigation of obesity are important, the absence of obesity is not entirely protective from the other cardiometabolic risk factors in KS.
The absolute prevalence of dyslipidemia (8.1%) and dysglycemia (2.5%) is lower than previously reported in clinical studies of youth with KS (4, 5, 7, 8, 30). Our prevalence rates are likely underestimated for both KS and controls due to reliance on discrete data elements in the EHR and the fact that not all patients were assessed for the outcomes in question. Males with KS were more likely than controls to have relevant laboratory assessments for these outcomes in the EHR, likely secondary to the assumed cardiometabolic risk in this population. Full cholesterol profiles and hemoglobin A1c are not routinely obtained in otherwise healthy children without known risk, which is supported by the large amount of missing data for these variables in our dataset. Therefore, it is likely that the true prevalence of these conditions is actually higher than we identified in this study. Nevertheless, the odds of dyslipidemia and dysglycemia were higher in KS, congruent with other studies that have reported a pattern of insulin resistance in KS (7, 31-33).
The literature on liver disease in KS is underwhelming. There are case reports of benign liver tumors and autoimmune hepatitis in individuals with KS (34-38). Fatty liver disease is assumed to be more prevalent in KS based on their higher rates of metabolic syndrome and findings in an XXY mouse model (39), but only a single abstract of original research reported liver dysfunction associated with obesity and total cholesterol in men with KS (40). In this study, we observed a 2-fold greater odds of liver dysfunction; however, actual values of AST and ALT were not different between KS and controls. This may be secondary to a minority of controls having had transaminases measured, and these may have been more likely to be measured during acute illness resulting in a temporary transaminitis rather than reflecting chronic liver disease. However, a recent testosterone intervention study in KS adults also found average liver enzymes were within the normal range (41). Further investigation of liver function and disease in KS is warranted.
The absence of hypertension in KS is reported in many studies, even in the presence of other metabolic syndrome features. In the general population, men have a higher prevalence of hypertension than women (42). Whether the sex difference in hypertension is genetic, hormonal, or environmental is unknown, but the potential protective effect of 2 X chromosomes and/or higher estrogen levels could be further explored by studying males with KS. For example, one of the mechanisms proposed for biological sex difference in hypertension is the protective effect of the adipocytokine adiponectin, with males having lower levels than females and lower levels often found in individuals with metabolic syndrome (43-45). Normal adiponectin levels have been reported in men with KS despite their poor cardiometabolic profiles, which would be congruent with a protective effect of adiponectin against hypertension (4). Inclusion of KS as a model in investigating sex differences in hypertension may elucidate underlying mechanisms.
Finally, with the exception of dyslipidemia, testosterone treatment did not have a positive or negative association with cardiometabolic outcomes, which is congruent with adult literature (46, 47). The impact on dyslipidemia may be secondary to an HDL-lowering effect of androgens through increasing hepatic lipase activity (48). However, the literature is mixed on the effect of testosterone treatment on HDL in hypogonadal populations, with several studies finding no change in HDL with testosterone treatment and others reporting any HDL decline is only in the HDL3c subfraction, which is not associated with anti-atherogenic properties (49-51). In sum, results from this study are congruent with prior work suggesting that, with the exception of body composition (9, 11, 52), there is not a clear impact of testosterone treatment on cardiometabolic risk in KS. On the contrary, a prescription for an antipsychotic medication was associated with greater odds of most cardiometabolic-related diagnoses, although KS remained at greater risk even after considering the known side-effects of antipsychotics.
There are several unique strengths of this study. This is the largest cohort of youth with KS, representing >1000 patients with KS seeking clinical care at 6 pediatric centers throughout the United States. This design minimizes the bias inherent in single-center studies that rely on recruitment and voluntary enrollment and increases generalizability. Secondly, we were able to compare with a well-matched group representing the typical pediatric population receiving care at these same institutions. Finally, EHR data depend on medical documentation, which is regarded as more accurate that self-reported data and allowed for quantitative analyses of parameters such as BMI and laboratory values. However, there are limitations of this work as well. First, the diagnosis of KS was unable to be confirmed as genetic test results are not discrete data elements in the EHR, and the diagnosis of KS has not been specifically validated in this database. Another limitation of using EHR data is probable errors of commission and omission for the outcomes we assessed. While we do not anticipate the rates of these errors would differ between cases and controls, clinicians may have management and diagnosis bias in favor of recognizing cardiometabolic conditions in KS. There was also substantial missing data for our continuous outcomes, which reflect both care practices (obtaining these labs are not standard of care for typical pediatric patients) and discrete data elements available in the EHR (eg, labs obtained at outside facilities are often not imported into the EHR). Although we have included some potential explanatory variables (ie, prescription data), using EHR data also limit our ability to evaluate other variables that may contribute to cardiometabolic risk in KS, such as diet, physical activity, sleep, family history, estrogen levels, or aberrant metabolic pathways, and this warrants additional research. Finally, the pediatric centers participating in PEDSnet are tertiary care referral centers, and it is possible the boys with KS receiving care at these institutions are not representative of the pediatric KS population as a whole. Despite these limitations, these data represent a large, national clinic sample of youth with KS rather than small convenience samples from prior studies, emphasizing the clinical significance of cardiometabolic differences in this population. These results are an important contribution to our understanding of the associated cardiometabolic impacts of KS that can inform clinical counseling and management in pediatric and adolescent care.
In conclusion, this population-based study of 1080 youth with KS demonstrates higher odds of early CVD risk markers including overweight/obesity, dyslipidemia, and liver dysfunction compared with matched controls. Testosterone treatment does not reduce these odds, and other models suggest that this increased CVD risk in KS youth remains even after accounting for obesity status and medication use. These data are congruent with epidemiological studies in adults and smaller clinical pediatric studies. Counseling and screening for cardiometabolic risk conditions should be part of routine clinical care for boys with KS. Additional research is needed to determine the pathophysiology, longitudinal trajectory, and effective interventions for cardiometabolic-related conditions in youth with KS.
Acknowledgments
This project was developed during the Subspecialists Clinical Outcomes REsearch (SCORE) fellowship supported by the Department of Pediatrics and Section of Endocrinology under the mentorship of Amanda Dempsey, MD, PhD, MPH. The research in this publication was conducted using PEDSnet, a Pediatric Learning Health System, and includes data from the following PEDSnet institutions: Children’s Hospital of Philadelphia, Children’s Hospital Colorado, Seattle Children’s Hospital, Nemours Children’s Health, St. Louis Children’s Hospital, and Nationwide Children’s Hospital. PEDSnet is a Partner Network Clinical Data Research Network in PCORnet, the National Patient Centered Clinical Research Network, an initiative funded by the Patient Centered Outcomes Research Institute (PCORI). The authors would like to acknowledge contributions from additional SCORE leadership, Elizabeth Juarez-Colunga, PhD, Jacob Thomas, Angela Moss, Hanieh Razzaghi, PEDSnet and all PEDSnet site contributors.
Glossary
Abbreviations
- ALT
alanine transaminase
- AST
aspartate transaminase
- BMI
body mass index
- CVD
cardiovascular disease
- EHR
electronic health record
- HDL
high-density lipoprotein
- KS
Klinefelter syndrome
- LDL
low-density lipoprotein
Contributor Information
Shanlee M Davis, Department of Pediatrics, University of Colorado School of Medicine, Aurora, CO 80045, USA; eXtraOrdinarY Kids Clinic, Children’s Hospital Colorado, Aurora, CO 80045, USA.
Natalie J Nokoff, Department of Pediatrics, University of Colorado School of Medicine, Aurora, CO 80045, USA.
Anna Furniss, Adult & Child Consortium for Health Outcomes Research and Delivery Science (ACCORDS), University of Colorado School of Medicine, Aurora, CO 80045, USA.
Laura Pyle, Department of Pediatrics, University of Colorado School of Medicine, Aurora, CO 80045, USA; Department of Biostatistics and Informatics, University of Colorado School of Public Health, Aurora, CO 80045, USA.
Anna Valentine, Department of Pediatrics, University of Colorado School of Medicine, Aurora, CO 80045, USA.
Patricia Fechner, Department of Endocrinology, Seattle Children’s Hospital, Seattle, WA 98105, USA.
Chijioke Ikomi, Division of Endocrinology, Nemours Children’s Health, Wilmington, DE 19803, USA.
Brianna Magnusen, Institute for Informatics, Washington University School of Medicine in St. Louis, St. Louis, MO 63110, USA.
Leena Nahata, Center for Biobehavioral Health, Abigail Wexner Research Institute, Columbus, OH 43215, USA; Division of Endocrinology, Nationwide Children’s Hospital, Columbus, OH 43215, USA.
Maria G Vogiatzi, Department of Pediatrics, Children’s Hospital of Philadelphia, Philadelphia, PA 19104, USA.
Amanda Dempsey, Department of Pediatrics, University of Colorado School of Medicine, Aurora, CO 80045, USA; Adult & Child Consortium for Health Outcomes Research and Delivery Science (ACCORDS), University of Colorado School of Medicine, Aurora, CO 80045, USA; Merck and Company, Wales, PA 19454, USA.
Financial Support
This work was supported in part by National Institutes of Health (NIH)/Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) K23HD092588 and R03HD102773 (S.M.D.), National Institute of Health (NIH)/National Health, Lung, and Blood Institute (NHLBI) K23HL151868 (N.J.N.), Doris Duke Foundation (S.M.D., N.J.N.), Contents are the authors’ sole responsibility and do not necessarily represent views of the funders. The funders had no role in the design and conduct of the study.
Disclosure Summary
N.J.N. conducts research and is a consultant for Neurocrine Biosciences and previously consulted for Antares Pharma, Inc. A.D. is now employed by Merck and Co; research was completed prior to this employment. The other authors have indicated that they have no financial relationships relevant to this article to disclose.
Data Availability
The datasets generated during the current study are not publicly available but are available from the corresponding author on reasonable request. Restrictions apply to the availability of the data analyzed during this study as it was used under license with PEDSnet. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References
- 1. Bojesen A, Gravholt CH. Morbidity and mortality in Klinefelter syndrome (47,XXY). Acta Paediatr 2011;100(6):807-813. [DOI] [PubMed] [Google Scholar]
- 2. Swerdlow AJ, Hermon C, Jacobs PA, et al. Mortality and cancer incidence in persons with numerical sex chromosome abnormalities: a cohort study. Ann Hum Genet. 2001;65(Pt 2):17717788-1771188. [DOI] [PubMed] [Google Scholar]
- 3. Falhammar H, Claahsen-van der Grinten H, Reisch N, et al. Health status in 1040 adults with disorders of sex development (DSD): a European multicenter study. Endocr Connect. 2018;7(3):466478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bojesen A, Kristensen K, Birkebaek NH, et al. The metabolic syndrome is frequent in Klinefelter’s syndrome and is associated with abdominal obesity and hypogonadism. Diabetes Care. 2006;29(7):1591-1598. [DOI] [PubMed] [Google Scholar]
- 5. Salzano A, Arcopinto M, Marra AM, et al. Klinefelter syndrome, cardiovascular system, and thromboembolic disease: review of literature and clinical perspectives. Eur J Endocrinol. 2016;175(1):R27-R40. [DOI] [PubMed] [Google Scholar]
- 6. Pasquali D, Arcopinto M, Renzullo A, et al. Cardiovascular abnormalities in Klinefelter syndrome. Int J Cardiol. 2013;168(2):754-759. [DOI] [PubMed] [Google Scholar]
- 7. Bardsley MZ, Falkner B, Kowal K, Ross JL. Insulin resistance and metabolic syndrome in prepubertal boys with Klinefelter syndrome. Acta Paediatr. 2011;100(6):866-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Davis S, Lahlou N, Bardsley M, et al. Gonadal function is associated with cardiometabolic health in pre-pubertal boys with Klinefelter syndrome. Andrology. 2016;4(6):1169-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Davis SM, Cox-Martin MG, Bardsley MZ, Kowal K, Zeitler PS, Ross JL. Effects of Oxandrolone on cardiometabolic health in boys with Klinefelter syndrome: a randomized controlled trial. J Clin Endocrinol Metab. 2017;102(1):176-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aksglaede L, Molgaard C, Skakkebaek NE, Juul A. Normal bone mineral content but unfavourable muscle/fat ratio in Klinefelter syndrome . Arch Dis Child. 2008;93(1):30-34. [DOI] [PubMed] [Google Scholar]
- 11. Davis SM, Reynolds RM, Dabelea DM, Zeitler PS, Tartaglia NR. Testosterone treatment in infants with 47,XXY: effects on body composition. J Endocr Soc. 2019;3(12):2276-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bojesen A, Juul S, Gravholt CH. Prenatal and postnatal prevalence of Klinefelter syndrome: a national registry study. J Clin Endocrinol Metab. 2003;88(2):622-626. [DOI] [PubMed] [Google Scholar]
- 13. Abramsky L, Chapple J. 47,XXY (Klinefelter syndrome) and 47,XYY: estimated rates of an indication for postnatal diagnosis with implications for prenatal counselling. Prenat Diagn. 1997;17(4):363-368. [DOI] [PubMed] [Google Scholar]
- 14. Berglund A, Viuff MH, Skakkebaek A, Chang S, Stochholm K, Gravholt CH. Changes in the cohort composition of turner syndrome and severe non-diagnosis of Klinefelter, 47,XXX and 47,XYY syndrome: a nationwide cohort study. Orphanet J Rare Dis. 2019;14(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nieschlag E, Ferlin A, Gravholt CH, et al. The Klinefelter syndrome: current management and research challenges. Andrology. 2016;4(3):545-549. [DOI] [PubMed] [Google Scholar]
- 16. Brun JL, Gangbo F, Wen ZQ, et al. Prenatal diagnosis and management of sex chromosome aneuploidy: a report on 98 cases. Prenat Diagn. 2004;24(3):213-218. [DOI] [PubMed] [Google Scholar]
- 17. Jaramillo C, Nyquist C, Riggan KA, Egginton J, Phelan S, Allyse M. Delivering the diagnosis of sex chromosome aneuploidy: experiences and preferences of parents and individuals. Clin Pediatr (Phila). 2019;58(3):336-342. [DOI] [PubMed] [Google Scholar]
- 18. Forrest CB, Margolis P, Seid M, Colletti RB. PEDSnet: how a prototype pediatric learning health system is being expanded into a national network. Health Aff (Millwood). 2014;33(7):1171-1177. [DOI] [PubMed] [Google Scholar]
- 19. Khare R, Utidjian L, Ruth BJ, et al. A longitudinal analysis of data quality in a large pediatric data research network. J Am Med Inform Assoc. 2017;24(6):1072-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mitra R, Reiter JP.A comparison of two methods of estimating propensity scores after multiple imputation. Stat Methods Med Res. 2016;25(1):188-204. [DOI] [PubMed] [Google Scholar]
- 21. Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39(1):33-38. [Google Scholar]
- 22. Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39(1):33-38. [Google Scholar]
- 23. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083-3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000;314(314):1-27. [PubMed] [Google Scholar]
- 25. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596-e646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Flynn JT, Kaelber DC, Baker-Smith CM, et al. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140(3):e20173035. [DOI] [PubMed] [Google Scholar]
- 27. Bussler S, Vogel M, Pietzner D, et al. New pediatric percentiles of liver enzyme serum levels (alanine aminotransferase, aspartate aminotransferase, gamma-glutamyltransferase): effects of age, sex, body mass index, and pubertal stage. Hepatology. 2018;68(4):1319-1330. [DOI] [PubMed] [Google Scholar]
- 28. Kwo PY, Cohen SM, Lim JK. ACG clinical guideline: evaluation of abnormal liver chemistries. Am J Gastroenterol. 2017;112(1):18-35. [DOI] [PubMed] [Google Scholar]
- 29. Umer A, Kelley GA, Cottrell LE, Giacobbi P Jr., Innes KE, Lilly CL. Childhood obesity and adult cardiovascular disease risk factors: a systematic review with meta-analysis. BMC Public Health. 2017;17(1):683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Davis SM, DeKlotz S, Nadeau KJ, Kelsey MM, Zeitler PS, Tartaglia NR. High prevalence of cardiometabolic risk features in adolescents with 47,XXY/Klinefelter syndrome. Am J Med Genet C Semin Med Genet. 2020;184(2):327-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Andersen NH, Bojesen A, Kristensen K, et al. Left ventricular dysfunction in Klinefelter syndrome is associated to insulin resistance, abdominal adiposity and hypogonadism. Clin Endocrinol. 2008;69(5):785-791. [DOI] [PubMed] [Google Scholar]
- 32. Gravholt CH, Andersen NH, Conway GS, et al. Clinical practice guidelines for the care of girls and women with Turner syndrome: proceedings from the 2016 Cincinnati International Turner Syndrome Meeting. Eur J Endocrinol. 2017;177(3):G1-G70. [DOI] [PubMed] [Google Scholar]
- 33. Ishikawa T, Yamaguchi K, Kondo Y, Takenaka A, Fujisawa M. Metabolic syndrome in men with Klinefelter’s syndrome. Urology. 2008;71(6):1109-1113. [DOI] [PubMed] [Google Scholar]
- 34. Beuers U, Richter WO, Ritter MM, Wiebecke B, Schwandt P. Klinefelter’s syndrome and liver adenoma. J Clin Gastroenterol. 1991;13(2):214-216. [DOI] [PubMed] [Google Scholar]
- 35. Santarelli L, Gabrielli M, Orefice R, et al. Association between Klinefelter syndrome and focal nodular hyperplasia. J Clin Gastroenterol. 2003;37(2):189-191. [DOI] [PubMed] [Google Scholar]
- 36. Sasaki N, Yamauchi K, Sato R, Masuda T, Sawai T, Inoue H. Klinefelter’s syndrome associated with systemic lupus erythematosus and autoimmune hepatitis. Mod Rheumatol. 2006;16(5):305-308. [DOI] [PubMed] [Google Scholar]
- 37. Tian Y, Wang C, Liu JX, Wang HH. Primary biliary cirrhosis-related autoimmune hemolytic anemia: three case reports and review of the literature. Case Rep Gastroenterol. 2009;3(2):240-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tsuruta S, Kimura N, Ishido K, et al. Calcifying nested stromal epithelial tumor of the liver in a patient with Klinefelter syndrome: a case report and review of the literature. World J Surg Oncol. 2018;16(1):227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chen X, Williams-Burris SM, McClusky R, et al. The sex chromosome trisomy mouse model of XXY and XYY: metabolism and motor performance. Biol Sex Differ. 2013;4(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jensen A, Bojesen A, Gravholt C. Mild liver dysfunction in Klinefelter syndrome is associated with abdominal obesity and elevated total cholesterol. 2009:P2-P768. [DOI] [PubMed]
- 41. Kabilan A, Skakkebaek A, Chang S, Gravholt CH. Evaluation of the efficacy of transdermal and injection testosterone therapy in Klinefelter syndrome: a real-life study. J Endocr Soc. 2021;5(6):bvab062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ramirez LA, Sullivan JC. Sex differences in hypertension: where we have been and where we are going. Am J Hypertens. 2018;31(12):1247-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ohashi K, Ouchi N, Matsuzawa Y. Adiponectin and hypertension. Am J Hypertens. 2011;24(3):263-269. [DOI] [PubMed] [Google Scholar]
- 44. Adamczak M. Decreased plasma adiponectin concentration in patients with essential hypertension. Am J Hypertens. 2003;16(1):72-75. [DOI] [PubMed] [Google Scholar]
- 45. Ohman-Hanson RA, Cree-Green M, Kelsey MM, et al. Ethnic and sex differences in adiponectin: from childhood to adulthood. J Clin Endocrinol Metab. 2016;101(12):4808-4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ehrhart MD, Guthrie IR, Qeadan F, Burge MR. Metabolic effects of androgen-associated body mass in Klinefelter syndrome. Arch Med (Oviedo). 2018;10(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pizzocaro A, Vena W, Condorelli R, et al. Testosterone treatment in male patients with Klinefelter syndrome: a systematic review and meta-analysis. J Endocrinol Invest. 2020;43(12):1675-1687. [DOI] [PubMed] [Google Scholar]
- 48. Herbst KL, Amory JK, Brunzell JD, Chansky HA, Bremner WJ. Testosterone administration to men increases hepatic lipase activity and decreases HDL and LDL size in 3 wk. Am J Physiol Endocrinol Metab. 2003;284(6):E1112-E1118. [DOI] [PubMed] [Google Scholar]
- 49. Bolu E, Sonmez A, Tapan S, et al. HDL cholesterol subfractions and the effect of testosterone replacement in hypogonadism. Horm Metab Res. 2013;45(6):443-448. [DOI] [PubMed] [Google Scholar]
- 50. Shabsigh R, Katz M, Yan G, Makhsida N. Cardiovascular issues in hypogonadism and testosterone therapy. Am J Cardiol. 2005;96(12B):67M-72M. [DOI] [PubMed] [Google Scholar]
- 51. Tan KC, Shiu SW, Pang RW, Kung AW. Effects of testosterone replacement on HDL subfractions and apolipoprotein A-I containing lipoproteins. Clin Endocrinol. 1998;48(2):187-194. [PubMed] [Google Scholar]
- 52. Host C, Bojesen A, Erlandsen M, et al. A placebo-controlled randomized study with testosterone in Klinefelter syndrome: beneficial effects on body composition. Endocr Connect. 2019;8(9):1250-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during the current study are not publicly available but are available from the corresponding author on reasonable request. Restrictions apply to the availability of the data analyzed during this study as it was used under license with PEDSnet. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.