Abstract
Background
We assessed the association between severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral load and hospital admission, intensive care unit (ICU) admission, and in-hospital mortality.
Methods
All SARS-CoV-2–positive persons with a combined nasopharyngeal and oropharyngeal swab that was collected between 17 March 2020 and 31 March 2021 in public health testing facilities were included.
Results
From 20 207 SARS-CoV-2–positive persons, 310 (1.5%) were hospitalized within 30 days. High viral loads (crossing point [Cp] <25) were associated with an increased risk of hospitalization as compared to low viral loads (Cp >30), adjusted for age and sex (adjusted odds ratio [aOR], 1.57 [95% confidence interval {CI}, 1.11–2.26]). The same association was seen for ICU admission (aOR, 7.06 [95% CI, 2.15–43.57]). The median [interquartile range] Cp value of the 17 patients who died in hospital was significantly lower compared to the 226 survivors (22.7 [3.4] vs 25.0 [5.2]).
Conclusions
Higher initial SARS-CoV-2 viral load is associated with an increased risk of hospital admission, ICU admission, and in-hospital mortality. Our findings emphasize the added value of reporting SARS-CoV-2 viral load or cycle threshold/Cp values to identify persons who are at the highest risk of adverse outcomes such as hospital or ICU admission and who therefore may benefit from more intensive monitoring or early initiation of antiviral therapy.
Keywords: COVID-19, hospital admission, mortality, SARS-CoV-2, viral load distribution
Higher initial SARS-CoV-2 viral load is associated with an increased risk of hospital admission, intensive care unit admission, and in-hospital mortality.
Molecular testing of respiratory samples by reverse-transcription polymerase chain reaction (RT-PCR) has been the primary method to diagnose severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Although SARS-CoV-2 RT-PCR results generally are reported in a qualitative manner (positive or negative), the quantitative test result (cycle threshold [Ct], or crossing point [Cp]) correlates with viral load, with low Ct/Cp values representing high viral loads.
To date, several studies have investigated the association between viral load with different clinical parameters and outcome measurements in patients with coronavirus disease 2019 (COVID-19). Although several of the largest studies so far found a relationship between higher SARS-CoV-2 viral load and in-hospital mortality, this association was lost after multivariate analysis in one study [1–5]. Others found no or even an inverse relationship between higher viral load and clinical symptoms, hospitalization, intensive care unit (ICU) admission, and overall mortality [6, 7]. Differences between the results of these studies may be explained by the setting of the studies; most were restricted to hospitalized patient populations and used the SARS-CoV-2 RT-PCR test result performed at admission to the hospital to study relations between viral load and clinical outcomes. Because the viral load is highest in the earliest (mostly presymptomatic) stages of the infection, followed by a gradual decline, the moment of testing in the infection phase will influence the viral load found in respiratory samples. Therefore, a SARS-CoV-2 RT-PCR test result performed at admission to the hospital may not provide a representative estimate of the SARS-CoV-2 viral load in the early stage of the infection, thereby explaining the inconsistent results found in these previous studies. Indeed, we recently demonstrated that median Cp values differ between tested populations; this difference was most pronounced in the first months of the pandemic [8]. Furthermore, if those with high risk of poor clinical outcome can be identified early in SARS-CoV-2 infection while symptoms are still mild, antiviral therapies could be targeted in those with the highest potential benefit. Only one study investigated mortality risk in persons testing positive in a community setting, revealing an association between low initial Ct value (high viral load) and mortality [9]. However, as all 1337 persons were included until 1 May 2020 when SARS-CoV-2 testing was not as established, heterogeneity within and between populations can be expected [8]. Indeed, the 12% overall mortality rate in the study suggests it not to have been a population representative of a public health test setting with short symptom duration and mild symptoms. Thus, the risk of hospitalization, ICU admission, and mortality in individuals with a high viral load in the early phase of SARS-CoV-2 infection is currently unknown.
In the Netherlands, as in many European countries, individuals with minor symptoms can have themselves tested at public health services free of charge. The majority of patients who present at these facilities are in the early stage of the infection (median of 2 days since onset of symptoms) [8]. In this study, we investigated whether initial SARS-CoV-2 viral loads of tests performed at a public health testing facility were associated with admission to regional hospitals and ICUs and in-hospital mortality.
METHODS
Setting, Study Design, and Participants
We performed a retrospective cohort study on data from persons with a positive SARS-CoV-2 RT-PCR result from combined nasopharyngeal (NP) and oropharyngeal (OP) swabs (first samples from unique persons only) that were analyzed between 17 March 2020 and 31 March 2021, using RT-PCR based on the presence of the E-gene in the regional public health laboratory Kennemerland, Haarlem, the Netherlands [10]. Cp values were calculated on Lightcycler 480 1.5.1 software (Roche Diagnostics, Basel, Switzerland). Only swabs derived from a public health testing facility in the region Kennemerland were included, as public health testing facilities employed a uniform sampling (combined NP/OP) and inclusion policy [8].
Data on hospital (and ICU) admission were collected from the 2 large teaching hospitals in the region Kennemerland. These 2 hospitals are the only public hospitals in the region and the primary referral centers for patients from this region. Only data on hospitalization (and/or ICU admission) within 30 days from the date of the first positive SARS-CoV-2 RT-PCR result were taken into account. Data on hospital mortality were collected from one large teaching hospital by assessing electronic patient files.
Statistical Analysis
Descriptive statistics were used to present the data: continuous variables were presented as median (interquartile range [IQR]), categorical variables were presented as numbers and percentages. Logistic regression analyses were used to analyze the relation between SARS-CoV-2 viral load and hospitalization, allowing additional adjustment for potentially confounding variables. Statistical analyses were performed with R and RStudio (R version 4.0.3) software using packages tidyverse and tidymodels. P values < .05 were considered significant.
RESULTS
A total of 20 207 individuals tested positive for SARS-CoV-2 in an RT-PCR test performed at a public health testing facility. Of these, 10 280 (50.9%) were female; median age was 40 (IQR, 29) years, and the median SARS-CoV-2 Cp value was 26.3 (IQR, 5.7).
Association Between Cp Value and Hospital Admission
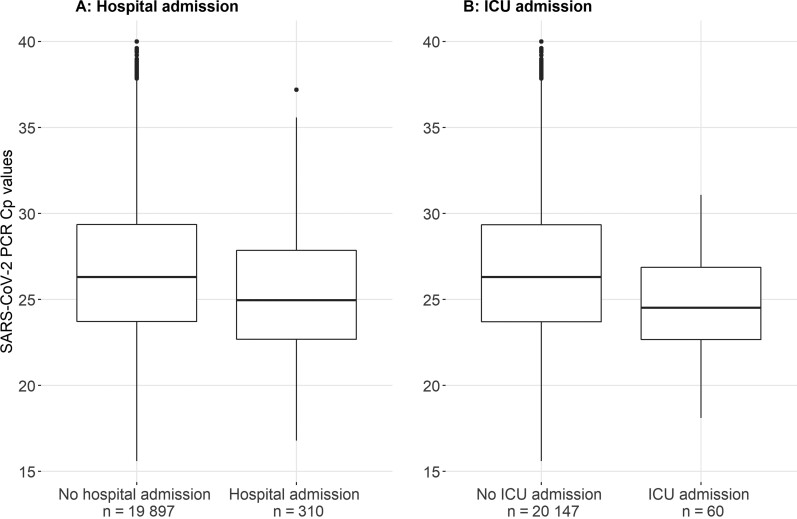
Of the 20 207 SARS-CoV-2–positive persons, 310 were hospitalized within 30 days of their positive SARS-CoV-2 RT-PCR test. Hospitalized patients were older (median age, 64.0 [IQR, 21.0] years vs 40.0 [IQR, 30.0] years; odds ratio [OR], 1.07 [95% confidence interval {CI}, 1.06–1.08]) and more often male (60.0% vs 49.0%; OR, 1.56 [95% CI, 1.25–1.97]) compared with nonhospitalized persons. Cp values at time of baseline measurement were considerably lower in those patients who were eventually hospitalized (median Cp value, 25.0 [IQR, 5.2] vs 26.3 [IQR, 5.6]; OR, 0.93 [95% CI, .90–.96]) as compared with persons who were not hospitalized (Figure 1). After adjusting for age and sex, the relation between initial SARS-CoV-2 viral load and hospitalization did not markedly change (adjusted OR [aOR], 0.96 [95% CI, .93–.99]).
Figure 1.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral load distribution of 20 207 unique patients who were tested at a public health testing facility. Data on SARS-CoV-2 viral load distribution are presented for patients who were (n = 310) or were not (n = 19 897) admitted to hospital within 30 days after their initial SARS-CoV-2 reverse-transcription polymerase chain reaction (RT-PCR) test (A), and for patients who were (n = 60) or were not (n = 20 147) admitted to the intensive care unit (ICU) within 30 days after their initial SARS-CoV-2 RT-PCR test (B). Data are presented as box-and-whisker plots with the central box covering the interquartile range with the median crossing point (Cp) value indicated by the line within the box. The whiskers extend to the minimum and maximum values within 1.5 interquartile ranges of the quartiles; more extreme values are plotted individually.
When patients were categorized in 3 SARS-CoV-2 viral load groups, the high viral load group (Cp <25) was associated with an increased risk of hospitalization compared to the low viral load group (Cp >30) (OR, 2.04 [95% CI, 1.46–2.93]). This association did not markedly change after adjusting for age and sex (aOR, 1.57 [95% CI, 1.11–2.26]) (Table 1). Analyzing the association of initial SARS-CoV-2 viral load and hospitalization in different age groups (Supplementary Table 1) showed a consistent association (although numbers per age group were limited) in all age groups. Additional data on the association between initial SARS-CoV-2 viral load and hospitalization over time (March 2020–March 2021) are presented in Supplementary Table 2. These show that the association was seen from October 2020 onward (when the majority of tests was performed).
Table 1.
Association Between Baseline Characteristics and Hospital Admission
| Baseline Characteristics | Total Population (N = 20 207) | Admission (n = 310) | No Admission (n = 19 897) | Univariable OR (95% CI) | P Value | Multivariable OR (95% CI) | P Value |
|---|---|---|---|---|---|---|---|
| Age, median (IQR) | 40.0 (29.0) | 64.0 (21.0) | 40.0 (30.0) | 1.07 (1.06–1.08) | <.001 | 1.07 (1.06–1.08) | <.001 |
| Male sex | 9927 (49.1) | 186 (60) | 9741 (49.0) | 1.56 (1.25–1.97) | <.001 | 1.50 (1.19–1.90) | <.001 |
| Cp value | |||||||
| >30 | 4050 (20.0) | 41 (13.2) | 4009 (20.1) | Ref | Ref | Ref | Ref |
| 25–30 | 8539 (42.3) | 113 (36.5) | 8426 (42.3) | 1.31 (.92–1.90) | .139 | 1.16 (.81–1.69) | .417 |
| <25 | 7618 (37.7) | 156 (50.3) | 7462 (37.5) | 2.04 (1.46–2.93) | <.001 | 1.57 (1.11–2.26) | .012 |
Data are presented as No. (%) unless otherwise indicated. ORs and related P values indicate the associations between baseline characteristics and hospital admission.
Abbreviations: CI, confidence interval; Cp, crossing point; IQR, interquartile range; OR, odds ratio.
For 7774 of the 20 207 persons, we had the first illness day available for analysis and found that time from symptom onset did not have a significant influence on the association between Cp value and hospital admission (Supplementary Table 3).
Association Between Cp Value and ICU Admission
Sixty patients were admitted to the ICU within 30 days of their initial positive SARS-CoV-2 RT-PCR test. Patients admitted to the ICU had lower initial SARS-CoV-2 Cp values (Figure 1) compared to patients who were not admitted to the ICU (median Cp value, 24.5 [IQR, 4.2] vs 26.3 [IQR, 5.7]; OR, 0.87 [95% CI, .81–.93]). In addition, patients admitted to the ICU were older (median age, 64.5 [IQR, 13.2] years vs 40.0 [IQR, 29.0] years; OR, 1.07 [95% CI, 1.06–1.09]), and more often male (65.0% vs 49.1%; OR, 1.93 [95% CI, 1.15–3.33]).
Adjusting the analysis on the relation between initial SARS-CoV-2 viral load and ICU admission for age and sex did not markedly change these results (aOR, 0.90 [95% CI, .84–.96]). The high viral load group (Cp <25) was associated with an increased risk of ICU admission compared to the low viral load group (Cp >30) (crude OR, 9.34 [95% CI, 2.85–57.55]), also after adjustment for age and sex (aOR, 7.06 [95% CI, 2.15–43.57]) (Table 2).
Table 2.
Association Between Baseline Characteristics and Intensive Care Unit Admission
| Baseline Characteristics | Total Population (N = 20 207) | ICU Admission (n = 60) | No ICU Admission (n = 20 147) | Univariable OR (95% CI) |
P Value | Multivariable OR (95% CI) | P Value |
|---|---|---|---|---|---|---|---|
| Age, median (IQR) | 40.0 (29.0) | 64.5 (13.2) | 40.0 (29.0) | 1.07 (1.06–1.09) | <.001 | 1.07 (1.06–1.09) | <.001 |
| Male sex | 9927 (49.1) | 39 (65.0) | 9888 (49.1) | 1.93 (1.15–3.33) | .016 | 1.84 (1.09–3.20) | .025 |
| Cp value | |||||||
| >30 | 4050 (20.0) | 2 (3.3) | 4048 (20.1) | Ref | Ref | Ref | Ref |
| 25–30 | 8539 (42.3) | 23 (38.3) | 8516 (42.3) | 5.47 (1.62–34.08) | .021 | 4.86 (1.43–30.33) | .032 |
| <25 | 7618 (37.7) | 35 (58.3) | 7583 (37.6) | 9.34 (2.85–57.55) | .002 | 7.06 (2.15–43.57) | .007 |
Data are presented as No. (%) unless otherwise indicated. ORs and related P values indicate the associations between baseline characteristics and ICU admission.
Abbreviations: CI, confidence interval; Cp, crossing point; ICU, intensive care unit; IQR, interquartile range; OR, odds ratio.
We had the first illness day available for analysis for 7774 of the 20 207 persons and found that time from symptom onset did not have a significant influence on the association between Cp value and ICU admission (Supplementary Table 4).
Association Between Cp Value and Clinical Parameters in Hospitalized Patients
For a subset of 243 of the 310 hospitalized patients, data were available on in-hospital mortality. Of these 243 hospitalized patients, the association of their initial SARS-CoV-2 Cp values with in-hospital mortality was analyzed. In total, 17 (7.0%) of these hospitalized patients died during their admission. The initial SARS-CoV-2 Cp value of these 17 deceased patients was significantly lower compared to the 226 survivors (median Cp value, 22.7 [IQR, 3.4] vs 25.0 [IQR, 5.2]; OR, 0.81 [95% CI, .68–.94]). In addition, these 17 patients were older (median age, 79.0 [IQR, 9.0] years vs 63.0 [IQR, 20.0] years; OR, 1.12 [95% CI, 1.07–1.20]) and had more often been admitted to the ICU (52.9% vs 16.8%; OR, 5.57 [95% CI, 2.01–15.73]) compared to the patients who survived. There were no significant differences in sex distribution (proportion of males: 70.6% vs 60.6%; OR, 1.69 [95% CI, .39–9.08]) or body mass index (BMI) (median, 29.4 [IQR, 3.6] kg/m2 vs 26.6 [IQR, 6.7] kg/m2; OR, 1.07 [95% CI, .92–1.23]) (Table 3).
Table 3.
Association Between Baseline Characteristics and In-Hospital Mortality Within a Subset of Admitted Patients (n = 243)
| Baseline Characteristics | Total Population (n = 243) | Died (n = 17) | Alive (n = 226) | Univariable OR (95% CI) |
P Value | Multivariable OR (95% CI) |
P Value |
|---|---|---|---|---|---|---|---|
| Age, median (IQR) | 65.0 (20.0) | 79.0 (9.0) | 63.0 (20.0) | 1.12 (1.07–1.20) | <.001 | 1.21 (1.10–1.37) | <.001 |
| Male sex | 149 (61.3) | 12 (70.6) | 137 (60.6) | 1.56 (.56–5.04) | .419 | 1.69 (.39–9.08) | .502 |
| Cp value, median (IQR) | 24.9 (5.2) | 22.7 (3.4) | 25.0 (5.2) | 0.81 (.68–.94) | .010 | 0.81 (.63–.99) | .062 |
| ICU admission | 47 (19.3) | 9 (52.9) | 38 (16.8) | 5.57 (2.01–15.73) | <.001 | 25.51 (5.22–184.76) | <.001 |
| BMI, kg/m2, median (IQR) | 27.0 (6.6) | 29.4 (3.6) | 26.6 (6.7) | 1.04 (.94–1.14) | .381 | 1.07 (.92–1.23) | .345 |
Data are presented as No. (%) unless otherwise indicated. ORs and related P values indicate the associations between baseline characteristics and in-hospital mortality.
Abbreviations: BMI, body mass index; CI, confidence interval; Cp, crossing point; ICU, intensive care unit; IQR, interquartile range; OR, odds ratio.
Adjusting the analysis on the relation between initial SARS-CoV-2 viral load and in-hospital mortality for age, sex, ICU admission, and BMI did not markedly change these results (aOR, 0.81 [95% CI, .63–.99]).
When the 243 patients who were included in this study (and for whom data were available on mortality) were compared to all other COVID-19–hospitalized patients from the participating hospitals (n = 1.209) during the study period, the study patients were significantly younger (median age, 65.0 [IQR, 21.0] vs 68.0 [IQR, 22.0]; OR, 0.99 [95% CI, .98–.99]) and showed a lower in-hospital mortality rate (proportion in-hospital mortality: 7.0% vs 12.4%; OR, 0.53 [95% CI, .30–.87]) (Supplementary Table 5).
DISCUSSION
In this study of 20 207 SARS-CoV-2–positive persons who were tested in public health testing facilities, we found that lower SARS-CoV-2 RT-PCR Cp values (indicating higher viral load) were associated with admission to regional hospital wards (Cp <25 group compared to the low viral load group [Cp >30]) (aOR, 1.57 [95% CI, 1.11–2.26]) as well as to the ICU (aOR, 7.06 [95% CI, 2.15–43.57]). In addition, higher initial SARS-CoV-2 viral load was associated with in-hospital mortality.
Although several studies have investigated the association between viral load with different clinical parameters and outcome measurements in COVID-19 patients, a consensus has not been reached [11]. Some studies showed an association of higher SARS-CoV-2 viral load and hospital admission, disease severity, or mortality, where other studies found no or an inverse association [6, 7]. However, few studies analyzed >500 persons, the majority of them included only hospitalized patients, and almost none of them took into account that the moment of testing in the infection phase probably will influence the viral load found in respiratory samples [11].
Our study is unique, as we have a large study population (20 207 SARS-CoV-2–positive persons), who were tested with a homogeneous sampling method. By only including tests performed by the public health service, we tried to remove heterogeneity of inclusion criteria, although the testing policy changed slightly during the study period (Supplementary Text). As additional analysis showed that the association of higher viral load with hospital admission was stable over time (Supplementary Table 2), we do not believe that the changes in testing policy have substantially affected our results. Furthermore, we anticipated one of the problems that occur when studying SARS-CoV-2 viral loads in respiratory samples: the lack of comparability of Ct or Cp values derived from different laboratories, as these are assay- and method-specific [12]. All samples in our study were analyzed in the same laboratory, using the same method.
One other distinguishing feature of our study is the study population of persons identified by the public health service, where individuals can have themselves tested with minor symptoms. As the majority of patients who present at these facilities are in their early stage of the infection (median, 2 days since start onset symptoms), the initial SARS-CoV-2 viral load assessed in these patients is more likely to represent a value close to the individual peak viral load compared to samples taken at a later stage of the disease, which is common in studies that included hospitalized patients only [8, 11].
As has been postulated based on other viral infections, higher SARS-CoV-2 viral load may lead to increased disease severity and could by itself be a negative prognostic value for the course of the disease. Higher viral loads after infection could be the result of high inoculum doses and indeed, a dose-dependent effect of SARS inocula on morbidity and mortality was found in BALB/c mice [13, 14]. One may suggest that patients with higher SARS-CoV-2 inocula have higher initial viral loads, increasing the risk of hospital and ICU admission. Alternatively, one could postulate that higher initial viral loads are not necessarily caused by higher inocula, but rather by a reduced capability to clear the initial viral infection, leading to higher loads in the early stage of infection. Further studies should focus on this distinction. Of note, our study included persons before large-scale vaccination programs were in place, thus eliminating vaccination status as a cause for the correlation between higher viral loads and poor outcomes. Vaccination began in January 2021 with residents of nursing homes, some high-risk groups, and nursing personal. As our study focusses on the tests performed within public health testing facilities (where mainly noninstitutionalized persons are tested), we think that vaccination had minor influence on our results.
Our study has a few notable limitations. The data on hospital (and ICU) admission of our study population were collected from the 2 large teaching hospitals in our region that largely cover the adherence area of the regional public health laboratory Kennemerland (where the tests were performed). It cannot, however, be excluded that (ICU) hospitalization data of some of the included patients were missed if they were admitted to other hospitals in adjacent regions. However, we do not believe that this will have influenced our main results because the chance of admission to a hospital in another region is not likely to be related to the initial SARS-CoV-2 viral load of a particular patient and would only have resulted in nondifferential misclassification of our outcome measurement. Although this is the largest study to connect viral load with increased risk of mortality, the number of in-hospital deaths in our study population was low. However, obtaining larger study sizes is hampered by the fact that a uniform PCR technique should be used, and sufficient inclusions are needed before vaccination programmes and variants emerged as potential confounders. Finally, including only patients who were able to have themselves tested at public health service testing facilities may have resulted in a healthy selection of all SARS-CoV-2–positive patients, as patients were able to make an appointment and present themselves to the public healthcare facility. Even though this generally took place after a mean of 2 days, patients who got very ill or needed to be admitted to the hospital may not have been included. The data presented in Supplementary Table 3 seem to confirm a healthy selection of the patients, as the hospitalized study patients were significantly younger and showed lower in-hospital mortality rates compared to hospitalized patients not included in our study. This should be taken into account when interpreting the findings presented in this study.
In conclusion, our findings emphasize the added value of reporting SARS-CoV-2 viral load based on Cp values in identifying persons who are at highest risk of adverse outcomes such as hospital or ICU admission and who therefore may benefit from more intensive monitoring or early antiviral therapies. However, the interpretation of a single Ct or Cp value in less standardized settings should still be performed cautiously as it may be affected by sample collection, the time between the start of symptoms and sampling, and the lack of comparability of Ct or Cp values derived from different laboratories, as these are assay- and method-specific [12].
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Acknowledgments
We would like to thank all physicians, nurses, public health testing personnel, laboratory technicians, and administration personnel who have worked to provide severe acute respiratory syndrome coronavirus 2 testing for a large number of individuals, making it possible to perform these analyses.
Contributor Information
Dennis Souverein, Regional Public Health Laboratory Kennemerland, Haarlem, The Netherlands.
Karlijn van Stralen, Spaarne Gasthuis, Hoofddorp/Haarlem, The Netherlands.
Steven van Lelyveld, Spaarne Gasthuis, Hoofddorp/Haarlem, The Netherlands.
Claudia van Gemeren, Spaarne Gasthuis, Hoofddorp/Haarlem, The Netherlands.
Milly Haverkort, Public Health Service Kennemerland, Haarlem, The Netherlands.
Dominic Snijders, Spaarne Gasthuis, Hoofddorp/Haarlem, The Netherlands.
Robin Soetekouw, Spaarne Gasthuis, Hoofddorp/Haarlem, The Netherlands.
Erik Kapteijns, Rode Kruis Ziekenhuis, Beverwijk, The Netherlands.
Evelien de Jong, Rode Kruis Ziekenhuis, Beverwijk, The Netherlands.
Gonneke Hermanides, Rode Kruis Ziekenhuis, Beverwijk, The Netherlands.
Sem Aronson, Regional Public Health Laboratory Kennemerland, Haarlem, The Netherlands; Spaarne Gasthuis, Hoofddorp/Haarlem, The Netherlands.
Bjorn Herpers, Regional Public Health Laboratory Kennemerland, Haarlem, The Netherlands.
Jeroen den Boer, Regional Public Health Laboratory Kennemerland, Haarlem, The Netherlands.
Alex Wagemakers, Regional Public Health Laboratory Kennemerland, Haarlem, The Netherlands.
Sjoerd Euser, Regional Public Health Laboratory Kennemerland, Haarlem, The Netherlands.
Notes
Author contributions. S. E., S. A., B. H., A. W., and D. S. participated in conceptualization. S. E., S. A., K. S., S. L., C. G., D. S., R. S., E. K., E. J., G. H., B. H., A. W., and D. S. contributed to data collection. S. E., A. W., and D. S. wrote the original draft. S. A., C. G., K. S., and S. L. contributed in reviewing and editing the manuscript. S. E., K. S., S. A., A. W., and D. S. performed data curation. S. E. and D. S. contributed to data analysis. All authors critically reviewed and approved the final version.
Data availability. The data underlying this article will be shared on reasonable request to the corresponding author.
Patient consent. The Medical Ethical Committee of the Amsterdam University Medical Center approved this study on 19 January 2021 (study number 2021.0170). Prior to this, the Institutional Review Board of the Spaarne Gasthuis, Hoofddorp/Haarlem, the Netherlands, approved the study (study number 2021.0046). The data were anonymized after collection and analyzed under code. Procedures were done in accordance with the General Data Protection Regulation and Good Clinical Practice standards. Patient consent was waived by the ethics committee of the Amsterdam University Medical Center.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Magleby R, Westblade LF, Trzebucki A, et al. Impact of severe acute respiratory syndrome coronavirus 2 viral load on risk of intubation and mortality among hospitalized patients with coronavirus disease 2019. Clin Infect Dis 2021; 73:e4197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pujadas E, Chaudhry F, McBride R, et al. SARS-CoV-2 viral load predicts COVID-19 mortality. Lancet Respir Med 2020; 8:e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Westblade LF, Brar G, Pinheiro LC, et al. SARS-CoV-2 viral load predicts mortality in patients with and without cancer who are hospitalized with COVID-19. Cancer Cell 2020; 38:661–71.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boyapati A, Wipperman MF, Ehmann PJ, et al. Baseline severe acute respiratory syndrome viral load is associated with coronavirus disease 2019 severity and clinical outcomes: post hoc analyses of a phase 2/3 trial. J Infect Dis 2021; 224:1830–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maltezou HC, Raftopoulos V, Vorou R, et al. Association between upper respiratory tract viral load, comorbidities, disease severity, and outcome of patients with SARS-CoV-2 infection. J Infect Dis 2021; 223:1132–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yilmaz A, Marklund E, Andersson M, et al. Upper respiratory tract levels of severe acute respiratory syndrome coronavirus 2 RNA and duration of viral RNA shedding do not differ between patients with mild and severe/critical coronavirus disease 2019. J Infect Dis 2021; 23:15–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Argyropoulos KV, Serrano A, Hu J, et al. Association of initial viral load in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) patients with outcome and symptoms. Am J Pathol 2020; 190:1881–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Euser S, Aronson S, Manders I, et al. SARS-CoV-2 viral-load distribution reveals that viral loads increase with age: a retrospective cross-sectional cohort study [manuscript published online ahead of print 8 September 2021]. Int J Epidemiol 2021. doi:10.1093/ije/dyab145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Waudby-West R, Parcell BJ, Palmer CNA, Bell S, Chalmers JD, Siddiqui MK. The association between SARS-CoV-2 RT-PCR cycle threshold and mortality in a community cohort. Eur Respir J 2021; 58:2100360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill 2020; 25:2000045. Erratum in: Euro Surveill 2020; 25(14). Erratum in: Euro Surveill 2020; 25(30). Erratum in: Euro Surveill 2021; 26(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Trunfio M, Venuti F, Alladio F, et al. Diagnostic SARS-CoV-2 cycle threshold value predicts disease severity, survival, and six-month sequelae in COVID-19 symptomatic patients. Viruses 2021; 13:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rhoads D, Peaper DR, She RC, et al. College of American pathologists (CAP) microbiology committee perspective: caution must be used in interpreting the cycle threshold (Ct) value. Clin Infect Dis 2021; 72:e685–6. [DOI] [PubMed] [Google Scholar]
- 13. Spinelli MA, Glidden DV, Gennatas ED, et al. Importance of non-pharmaceutical interventions in lowering the viral inoculum to reduce susceptibility to infection by SARS-CoV-2 and potentially disease severity. Lancet Infect Dis 2021; 21:e296–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Roberts A, Deming D, Paddock CD, et al. A mouse-adapted SARS-coronavirus causes disease and mortality in BALB/c mice. PLoS Pathog 2007; 3:e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.