Abstract
Background
Young people who inject drugs (PWID) have high hepatitis C virus (HCV) incidence and low treatment initiation rates. Novel, simplified care models need to be developed to engage, treat, and cure hard-to-reach patient populations, such as young PWID. We present final data from the randomized pilot clinical trial “HCV-Seek Test and Rapid Treatment” for curing HCV in young PWID.
Methods
Participants were recruited from the community and eligible if they were 18–29 years of age, HCV antibody-positive, treatment naive, and had injected drugs in the past 30 days. Participants were randomized 1:1 to “Rapid Treatment or Usual Care”. Participants randomized to Rapid Treatment received same-day medical evaluation, confirmatory and baseline laboratory testing, and a 7-day starter pack of sofosbuvir/velpatasvir at a syringe service program (SSP). Participants in “Usual Care” received same-day HCV confirmatory testing at the SSP and, if positive, facilitated referral to local providers. The primary endpoint was sustained virologic response at 12 weeks (SVR12) in HCV ribonucleic acid (RNA)+ participant.
Results
Forty-seven HCV antibody-positive participants were enrolled, and 25 participants had confirmed HCV and were included in the modified intention to treat analysis, with 9 of 14 (64%) of the Rapid Treatment arm and 1 of 11 (9.1%) of the Usual Care arm achieving a confirmed SVR12 (P = .01).
Conclusions
Among young HCV RNA+ PWID, significantly higher rates of cure were achieved using the Rapid Treatment model compared with facilitated referral. Providing easy access to HCV treatment for young PWID in low-threshold settings and initiating HCV treatment quickly appears to be a promising strategy for treating this hard-to-reach population.
Keywords: direct-acting antiviral, low threshold, minimal monitoring, pan-genotypic, simplified
Rapid Treatment intervention with same-day prescription and dispensation of pan-genotypic DAA therapy was feasible, appeared to be accepted by participants, and resulted in substantially higher rates of treatment initiation, treatment completion, and cure, compared with the standard of care.
The United States has been experiencing an injection drug use epidemic among young people for over a decade. Between 2002 and 2013, heroin use in the United States increased by 63%, with the largest increase (109%) in individuals aged 18–25 [1]. The rise in injection drug use has been accompanied by increases in incident hepatitis C virus (HCV) infection, as cases of acute HCV more than doubled between 2012 and 2019, and increased 8-fold among people age 20–29 [2].
The development of direct-acting antivirals (DAAs) with high efficacy and tolerability has revolutionized treatment of HCV [3, 4], leading to discussion of HCV elimination at both national and state levels [5, 6]. Complicating HCV elimination efforts are the concentration of infections in people who inject drugs (PWID) [7]. People who inject drugs face multiple barriers to successful treatment including mistrust within the medical system, explicit sobriety requirements from insurers, and a lengthy prior authorization process, which necessitates extensive medical workup before initiating treatment [8–10]. As a result, studies have consistently demonstrated low rates of HCV treatment engagement and treatment initiation in PWID [11–13]. Engaging and treating HCV in young PWID is further complicated by the common reluctance of this population to prioritize an infection that is unlikely to affect them for more than a decade [14]. Despite the difficulties of engaging PWID in HCV care, numerous studies have demonstrated PWID achieve high rates of sustained virologic response at 12 weeks (SVR12) [15, 16].
Overcoming the barriers to treat young PWID is essential: DAA therapy among young PWID has the potential to prevent incident infections, thereby reducing transmission, and potentially eliminate HCV spread entirely [17–19]. High rates of HCV treatment engagement of young PWID will not be possible without aggressively addressing barriers that complicate the treatment cascade and reducing the delay between HCV confirmation and treatment initiation. Programs need to reassess the need for extensive pretreatment laboratory testing that may require multiple visits, subjective treatment “readiness” assessments that may lead to delays or refusals to prescribe treatment, and lengthy insurance prior authorization process. To this end, simplified treatment modalities with minimal on treatment monitoring have been studied and implemented [20, 21]. We hypothesized that incorporating those strategies with rapid treatment initiation, and colocating care in a convenient low-threshold setting, could improve engagement and outcomes.
Our pilot randomized trial examined the safety, feasibility, and effectiveness of a rapid-treatment intervention co-located in a syringe service program (SSP), called HCV Seek, Test and Rapid Treatment (ST&RT), in young PWID.
METHODS
The ST&RT study is a randomized, open-label, pilot clinical trial comparing a Rapid Treatment model of HCV care delivered at an SSP to facilitated referral for HCV-infected PWID between ages 18 and 29. Participants who tested positive for HCV on rapid antibody testing were enrolled, had baseline laboratory tests drawn, and were randomized to either (1) Rapid Treatment initiation of the US Food and Drug Administration-approved fixed-dose pan-genotypic combination of sofosbuvir 400 mg and velpatasvir 100 mg with follow-up and medical monitoring at a community-based SSP or (2) Usual Care with onsite HCV care coordination at the SSP and referral to local HCV treatment providers.
Participants and Setting
Potential participants were referred to the ST&RT study from the Staying Safe Study [22], a community-based study recruiting young PWID to educational training intervention to prevent incident HCV infection. Screening for Staying Safe included rapid HCV antibody testing. Those who tested HCV antibody-positive were ineligible for the Staying Safe Study and were offered referral to the ST&RT study. The ST&RT research team evaluated the young PWID for eligibility, then consented and enrolled participants onsite. Eligible HCV antibody-positive participants were 18 to 29 years of age, spoke English, reported at least 1 illicit drug injection within the prior 30 days, and were HCV treatment naive. Pregnant women, individuals with known human immunodeficiency virus (HIV) or advanced liver disease (decompensated cirrhosis or hepatocellular carcinoma), and those currently engaged in hepatitis C treatment (defined as having at least 2 medical visits with a hepatitis C treatment provider in the last 6 months) were excluded. The study took place in New York City. Initial medical evaluations occurred within the Lower East Side Harm Reduction Center (LESHRC), a syringe service program, whereas research visits occurred either at the LESHRC, a satellite research office a short walk away, or over the phone (follow-up visits only).
The LESHRC site is a culturally diverse, community-based, nonprofit organization whose mission is to reduce the spread of HIV, HCV, and other drug-related harm among PWID and the community located in the Lower East Side of Manhattan in New York City. The site is a New York State Department of Health-authorized SSP offering services including injection equipment distribution, overdose prevention training and medication, onsite access to buprenorphine providers, HIV and HCV screening and treatment referral, case management, and other harm reduction services.
Study Design
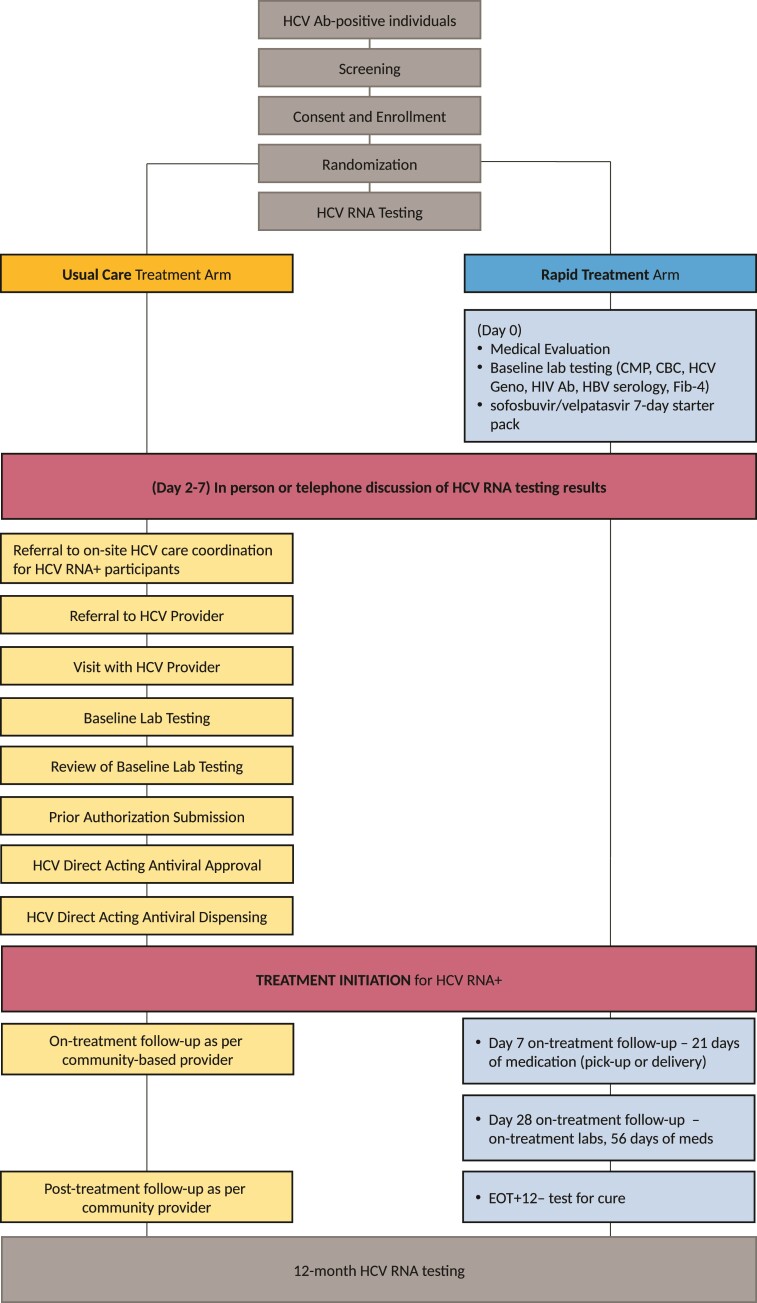
After signing an informed consent, enrolled participants underwent a baseline questionnaire, after which participants were randomized 1:1 to either the Rapid Treatment or Usual Care arm. Participants randomized to either arm had HCV ribonucleic acid (RNA) confirmatory testing drawn on site (Figure 1).
Figure 1.
Treatment model schematic, Usual Care, and Rapid Treatment Arms. Ab, antibody; CBC, complete blood count; CMP, comprehensive metabolic panel; DAA, direct-acting antiviral; EOT, end of treatment; Fib-4, Fibrosis-4; Geno, genotype; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; Lab, laboratory; RNA, ribonucleic acid.
Rapid Treatment Arm (Intervention)
In addition to onsite, same-day blood draw for HCV confirmatory testing, on the day of enrollment participants randomized to the Rapid Treatment arm had (1) an initial medical evaluation for HCV treatment with a HCV clinician, (2) standard-of-care baseline HCV laboratory testing including complete blood count, comprehensive metabolic panel, HCV genotype testing, hepatitis B serology, HIV antibody testing, and pregnancy testing (when applicable), and (3) were given a 1-week “Starter-Pack” (7-day supply) of pan-genotypic sofosbuvir 400 mg/velpatasvir 100 mg with instructions to not initiate therapy until contacted with their confirmatory results. Patient whose HCV RNA confirmatory testing returned positive were contacted and encouraged to start sofosbuvir/velpatasvir. Recommendation to initiate therapy occurred before HCV genotyping resulted. Patients whose HCV confirmatory testing was negative were told not to initiate therapy and instructed to return their Starter-Pack of medication. For those who initiated therapy, arrangements were made to dispense the rest of the study medication to complete the recommended 12-week course [20]. Study medications were packaged as subsequent 21-day and 56-day supplies, but flexible pickup and delivery arrangements were available, including weekly medication pickup at the syringe service program. Follow-up clinical laboratory testing was scheduled at 4 weeks of therapy (on-treatment monitoring and pick-up of final 56-day supply of sofosbuvir/velpatasvir), at end-of-treatment (research week 12), at 12 weeks posttreatment completion (sustained virologic response testing). All clinical visits (baseline and follow-up) occurred at the LESHRC, and staffing consisted of an HCV clinician (2 days per week) and a Rapid Treatment arm care coordinator who had phlebotomy training (3 days per week). When participants were unable to present for on-treatment laboratory testing, Rapid Treatment arm clinical staff prioritized getting “meds-in-hands” of the participants by whatever means, such as flexible pick-up dates or delivery from clinical staff (public transportation, bike, or scooter) to patients’ homes/desired location, to avoid gaps in medication treatment. Participants started on DAA therapy were encouraged to partake in a reinfection prevention training session, adapted from the Staying Safe HCV prevention intervention [23].
Usual Care (Control)
Usual Care participants were referred to the HCV patient navigator onsite within the SSP who was funded through the New York City Department of Health and Mental Hygiene, Check Hep C program [24] before HCV RNA test results. The Check Hep C program provides HCV RNA+ individuals supportive services, linkage to HCV care, accompaniment to medical appointments, adherence support, and medication access support at no cost. This program was already operational at the LESHRC site at the time of our study and had no relationship to the study intervention. Hepatitis C virus RNA results were conveyed to Usual Care participants either over the phone or in-person by study staff with results provided to Check Hep C navigators. The Check Hep C HCV patient navigator subsequently worked with participants to facilitate linkage and engagement in care at surrounding hospital-, clinic-, and/or harm reduction-based HCV treatment programs.
Study Assessment and Compensation
Research visits were conducted at baseline, 3, 6, 9, and 12 months by nonclinical research staff. Participants completed questionnaires eliciting sociodemographic characteristics, current and past injection and noninjection drug use, substance use disorder treatment, HIV and HCV risk behavior, healthcare utilization, and hepatitis C treatment linkage. In addition, HCV RNA and HIV antibody testing was conducted in all participants at each quarterly research visit. All laboratory testing in both arms, and medication (sofosbuvir/velpatasvir) provided to the Rapid Treatment arm, were paid for through the research study. Participants were compensated $60 per visit in both arms for quarterly research visits through 12 months. Participants were not compensated for engaging in clinical care or starting HCV treatment.
Study Outcomes
The primary outcome of the study was the percentage of participants achieving an SVR12 with treatment within 12 months of study enrollment. Patients who failed to complete at least 1 of the final 2 quarterly research visits (at 9 and 12 months) were considered lost to follow-up unless they already had a confirmed SVR12. Secondary outcomes included achievement of steps along the HCV treatment cascade including referral to HCV clinician, attendance of a clinical visit, completion of baseline laboratory testing, DAA treatment initiation, and cure. An additional secondary outcome included time to treatment initiation. Attendance of clinical visit was defined as presenting to a single visit with a HCV treatment provider, and HCV treatment initiation was defined as participant self-reporting ingestion of at least 1 dose of DAA therapy.
Statistical Analysis
A sample size of 54 HCV RNA+ young PWID was sought to be able to detect a statistically significant difference at the alpha level of .05 if 78% of Rapid Treatment arm participants and 41% of Usual Care arm participants achieved SVR12 with 80% power. Participant’s baseline characteristics and outcomes were reported as frequencies with percentages or means with standard deviations. Comparisons between the study arms were analyzed with use of χ2, Fisher's exact, or Wilcoxon ranked-sum tests where appropriate.
All analyses were performed in a modified intention-to-treat population where participants who were HCV RNA− at baseline were not analyzed for HCV-treatment outcomes, but all HCV RNA+ individuals were analyzed with the group in which they were randomized. The 95% confidence intervals (CIs) were obtained using 2-sided t test, with a P values of .05 or less were considered statistically significant. Kaplan-Meier curve and hazard ratio (HR) were used to compare the time to treatment initiation and time to sustained virologic response in the 2 study arms. Analysis was performed using SPSS version 25.
Patient Consent
All participants provided written informed consent, and the study was conducted in accordance with Good Clinical Practice and the ethical principles that originated in the Declaration of Helsinki. The study design was approved by the Weill Cornell Medicine and NYU School of Medicine institutional review boards.
RESULTS
Between March 2018 and March 2020, 47 HCV antibody-positive young PWID were screened for eligibility, 39 participants of which were eligible, enrolled, underwent randomization (19 to Rapid Treatment arm and 20 to Usual Care arm). Enrollment was terminated early due restrictions on in-person research during the first wave of severe acute respiratory syndrome coronavirus 2 in New York City during the Spring of 2020. Of those enrolled, 13 participants were HCV RNA−, and 1 participant was found to be HCV treatment experienced and excluded postrandomization. Twenty-five participants (14 Rapid Treatment arm and 11 Usual Care arm) were included in the modified intention-to-treat analysis. Overall, the 2 arms were balanced with regards to baseline characteristics (Table 1). In the Rapid Treatment arm, 7 (50%) of the participants had genotype 1 virus, the mean Fibrosis-4 was 0.53 (range, 0.12–3.70), and no patients were coinfected with hepatitis B (Supplementary Table).
Table 1.
Baseline Characteristics of Study Participants
| Characteristic | Baseline | Baseline | Baseline | |
|---|---|---|---|---|
| RNA-Positive Rapid Treatment | RNA-Positive Usual Care | RNA Negative | Full Sample | |
| (n = 14) | (n = 11) | (n = 13) | (n = 38) | |
| Mean age in years (SD) | 26.07 (3.54) | 25.36 (2.66) | 26.92 (2.87) | 26.16 (3.06) |
| Female gender | 3 (21.4%) | 3 (27.3%) | 4 (30.8%) | 10 (26.3%) |
| Race/ethnicity | ||||
| Non-Hispanic white | 6 (42.9%) | 6 (54.5%) | 9 (30.8%) | 21 (55.3%) |
| Non-Hispanic black | 1 (7.1%) | 0 (0.0%) | 0 (0.0%) | 1 (2.6%) |
| Hispanic | 6 (42.9%) | 3 (27.3%) | 4 (30.8%) | 13 (34.2%) |
| Other | 1 (7.1%) | 2 (18.2%) | 0 (0.0%) | 3 (7.9%) |
| Homeless, ever in last 90 days | 2 (14.3%) | 4 (36.4%) | 7 (53.8%) | 13 (34.2%) |
| Insurance | ||||
| Public Insurance, Medicaid, or Medicare | 12 (85.7%) | 11 (100.0%) | 11 (84.6%) | 34 (89.5%) |
| Commercial insurance | 0 (0%) | 0 (0%) | 0 (0.0%) | 0 (0%) |
| Uninsured | 2 (14.3%) | 0 (0.0%) | 2 (15.4%) | 4 (10.5%) |
| Recent incarceration, last 90 days | 3 (21.4%) | 2 (18.2%) | 0 (0.0%) | 5 (13.2%) |
| Injection days in last 30 says, mean (SD) | 18.21 (11.64) | 16.91 (13.48) | 17.08 (11.91) | 17.45 (11.96) |
| Medication for opioid use disorder, last 90 days | 7 (50.0%) | 6 (54.5%) | ||
| Methadone | 6 (42.9%) | 6 (54.5%) | 8 (61.5%) | 20 (52.6%) |
| Buprenorphine | 1 (7.1%) | 0 (0.0%) | 0 (0.0%) | 1 (2.6%) |
| Drug injection, past 90 days | ||||
| Heroin | 14 (100.0%) | 10 (90.9%) | 13 (100.0%) | 37 (97.4%) |
| Cocaine/crack | 9 (64.3%) | 4 (36.4%) | 9 (69.2%) | 22 (57.9%) |
| Fentanyl | 7 (50.0%) | 2 (18.2%) | 6 (46.2%) | 15 (39.5%) |
| Methamphetamine | 1 (7.1%) | 2 (18.2%) | 0 (0.0%) | 3 (7.9%) |
| Speedball (heroin and cocaine) | 6 (42.9%) | 3 (27.3%) | 5 (38.5%) | 14 (36.8%) |
| Other | 0 (0.0%) | 1 (9.1%) | 2 (15.4%) | 3 (7.9%) |
| Previously known HCV positive status | 8 (57.1%) | 6 (54.5%) | 5 (38.5%) | 19 (50.0%) |
| Previously sought HCV care, self-report | 4 (28.6%) | 3 (27.3%) | 2 (15.4%) | 9 (23.7%) |
Abbreviations: HCV, hepatitis C virus; RNA, ribonucleic acid; SD, standard deviation.
The percentage of participants achieving SVR12 within 1 year of study enrollment was significantly higher in the Rapid Treatment arm (64.3%) compared with the Usual Care arm (9.1%) (Table 2). Of the 4 Rapid Treatment arm participants who did not have SVR12 or confirmed treatment failure, 1 decided against treatment, 1 was incarcerated within 1 week and remained incarcerated for the remainder of the study period, and 2 had an on-treatment response (viral load undetectable) and completed therapy but did not return for SVR12 testing (Supplementary Figure). There were no severe adverse effects in either arm.
Table 2.
Clinical Outcomes at 12 Months in the Rapid Treatment and Usual Care Arms
| Rapid Treatment (n = 14) | Usual Care (n = 11) | P Value | |
|---|---|---|---|
| SVR achieved during study by 12 months (intention-to-treat) | |||
| Yes | 9 (64.3%) | 1 (9.1%) | .01 |
| No | 5 (35.7%) | 10 (90.9%) | |
| Continued viremia | 2 | 10 | |
| Pending viral load testing | 3a | 0 | |
| Clinical outcome of those who initiated DAA therapy | (n = 13) | (n = 3) | |
| SVR | 9/13 (69.2%) | 1/3 (33.3%) | .52 |
| Treatment failure | 1/13 (7.7%) | 2/3 (66.7%) | .07 |
| Unknown | 3/13 (23.1%)a | 0/3 (0.0%) | |
Abbreviations: DAA, direct-acting antiviral; SVR, sustained virologic response.
One patient incarcerated within 7 days of initiating DAA therapy (remained incarcerated through remainder of study window); 2 patients without SVR12 testing, both of whom had undetectable viral load on treatment and confirmed treatment completion.
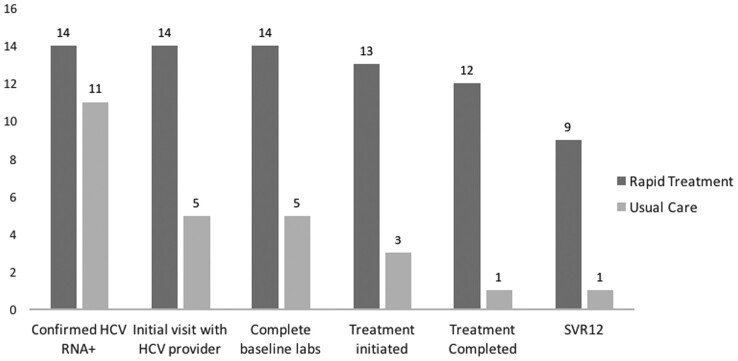
The percentage of participants who advanced along the care cascade was significantly higher at each step for the Rapid Treatment arm compared with the Usual Care arm from initial HCV clinical visit (14 of 14 [100%] versus 5 of 11 [45.4%], P < .05), completion of baseline laboratory testing (14 of 14 [100%] versus 5 of 11 [45.5%], P < .05), treatment initiation (13 of 14 [92.9%] versus 3 of 11 [27.3%], P < .05), and treatment completed (12 of 14 [85.7%] versus 1 of 11 [9.1%], P < .05) (Figure 2). Of those who initiated, treatment completion was achieved in 12 of 13 (92.3%) of the Rapid Treatment arm compared with 1 of 3 (33.3%) in the Usual Care arm. Of the participants who initiated DAA therapy, treatment failure (defined as a detectable HCV RNA occurring after treatment completion, but before 12 weeks posttreatment) was seen in 1 of 13 (7.7%) of the Rapid Treatment arm and 2 of 3 (66.7%) in the Usual Care arm.
Figure 2.
Hepatitis C virus (HCV) care cascade by treatment strategy. Labs, laboratory tests; RNA, ribonucleic acid; SVR, sustained virologic response.
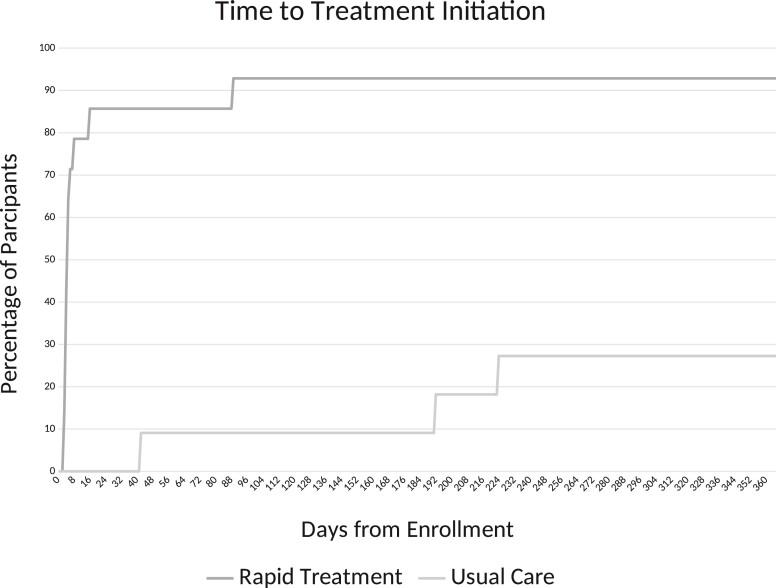
The median time from enrollment to treatment initiation was significantly faster in the Rapid Treatment arm 5 days (range, 3–89 days) versus 192 days (range, 42–224 days) in the Usual Care arm (HR = 9.58; 95% CI, 1.17 to 78.22) (Figure 3). In the Rapid Treatment arm, the median time from resulted positive RNA test to treatment initiation was 1 day (range, 0–81 days).
Figure 3.
Time to treatment initiation among participants by treatment strategy.
DISCUSSION
This study demonstrates evidence in support of a rapid-, simplified-HCV diagnosis and treatment initiation model in low-threshold settings to better engage young people who inject drugs. Same day medical evaluation, and rapid direct antiviral therapy initiation upon HCV RNA confirmation was well accepted and resulted in significantly higher rates of treatment initiation and cure in the Rapid Treatment arm than in the active control arm of patient navigation.
The Rapid Treatment HCV treatment model used a streamlined approach focused on treating a communicable disease as soon as the diagnosis is made, expanding on care delivery models used in other infections such as gonorrhea, chlamydia, syphilis, and HIV [25]. In the study, participants were able to initiate HCV DAA therapy as fast as within 3 days of engagement, delayed only by the turnaround time of commercial laboratory HCV RNA testing. The time from treatment engagement to DAA treatment initiation could be further shortened (potentially to same-day) with the incorporation of point-of-care HCV RNA or antigen testing, or a faster turnaround for laboratory-based HCV RNA testing, but neither was available in the United States at the time of this study.
We did not observe an increased risk of treatment failure in the Rapid Treatment arm. A potential reason for this was the deliberate prioritization of the Rapid Treatment arm of getting subsequent weeks of DAA therapy in the hands of participants resulting in high treatment completion rates (12 of 13 participants). The high rates of treatment completion are important given the potential concern that Rapid Treatment could lead to low rates of treatment completion and subsequent development of HCV resistance. It is unfortunate that we did not collect robust data on medication adherence or the frequency, or site, in which medication was distributed (onsite pick at the SSP versus delivery to patient). However, the focus on meds-in-hands in the Rapid Treatment arm probably contrasted with the Usual Care model, which often relies on specialty pharmacies arranging multiple deliveries of DAA therapy to patients, even those who lack reliable phone service or homes to which to deliver [26].
There are several limits to the generalizability of this study. First, sofosbuvir/velpatasvir and laboratory testing was provided by the study for the intervention arm, alleviating barriers of insurance status and DAA authorization to test the strategy of rapid treatment initiation. The vast majority of our study participants had active insurance (89.5%); however, this may not be representative across all of the United States where insurance, specifically Medicaid, programs may be less expansive. Insurance authorization for DAA therapy continues to be a significant barrier to getting DAA therapy into the hands of HCV-infected individuals in a timely manner [27–29]. Until delays between medical evaluation and treatment initiation are addressed, the most marginalized patient populations—such as young PWID—are unlikely to achieve HCV cure at high rates. In addition, limiting enrollment to HCV treatment-naive individuals between the ages of 18–29 years of age, thus limiting the likelihood of advanced fibrosis or cirrhosis, allowed the use of a uniform treatment regimen for all participants. The potential expansion of this model is likely possible in other populations (eg, older populations or treatment experienced), but a singular treatment approach/agent as used in the study may not be possible. Finally, this study was conducted at a single site with a small sample size. Larger studies are needed to confirm our findings and to be able to address other important concerns like the risk of reinfection in this population.
Young people who inject drugs have the highest incidence of acute HCV of any age group, which is associated with higher risk injection practices, and are likely to have the highest rates of transmitting their HCV infection to others. The potential impact of treating and curing young people of their HCV has a dual impact: providing an individual health benefit and providing treatment as prevention. Further research is required to determine whether this theoretical impact is true, especially through the incorporation of longitudinal follow-up for reinfection.
CONCLUSIONS
In conclusion, a Rapid Treatment intervention with same-day prescription and dispensation of pan-genotypic DAA therapy was feasible and accepted by participants and resulted in substantially higher rates of treatment initiation, treatment completion, and cure, compared with the standard of care. Young PWID constitute a significant percentage of HCV incident infections, and HCV elimination will not be possible without improvement in programs focused on HCV prevention and treatment. By removing or shortening unnecessary delays in treatment initiation, rapid treatment initiation of HCV DAA appeared safe and effective in young PWID.
Supplementary Material
Acknowledgments
Financial support. This work was funded through a research grant to Weill Cornell Medicine from Gilead Sciences Inc. (Grant IN-US-342-4456). Additional funding was provided by the National Institute of Drug Abuse (Grants R01DA041298, R01DA041501, and K01DA048172), National Institute of Mental Health (Grant T32MH073553), and the National Center for Advancing Translational Sciences (Grant UL1TR002384). We received referral assistance from the Staying Safe Intervention: Preventing HCV Among Youth Opioid Injectors (Grant R01DA041501).
Potential conflicts of interest. B. E., S. N. K., and K. M. M. have received research grants to their respective institutions from Gilead Sciences Inc. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Benjamin Eckhardt, Division of Infectious Diseases and Immunology, New York University School of Medicine, New York, New York, USA.
Shashi N Kapadia, Division of Infectious Diseases, Weill Cornell Medicine, New York, New York, USA; Department of Population Health Sciences, Weill Cornell Medicine, New York, New York, USA.
Pedro Mateu-Gelabert, CUNY Graduate School of Public Health and Health Policy, New York, New York, USA.
Melinda Pai, Division of Infectious Diseases, Weill Cornell Medicine, New York, New York, USA.
Chunki Fong, CUNY Graduate School of Public Health and Health Policy, New York, New York, USA.
Yesenia Aponte-Melendez, CUNY Graduate School of Public Health and Health Policy, New York, New York, USA.
Kristen M Marks, Division of Infectious Diseases, Weill Cornell Medicine, New York, New York, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. Jones CM, Logan J, Gladden RM, Bohm MK, Author MPH. Vital signs: demographic and substance use trends among heroin users—United States, 2002–2013. MMWR Morb Mortal Wkly Rep 2015; 64:719. [PMC free article] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention . Viral hepatitis surveillance report. 2019. Available at: https://www.cdc.gov/hepatitis/statistics/SurveillanceRpts.htm. Accessed 4 December 2021.
- 3. Hoofnagle JH, Sherker AH. Therapy for hepatitis C–the costs of success. N Engl JMed 2014; 370:1552–3. [DOI] [PubMed] [Google Scholar]
- 4. Falade-Nwulia O, Suarez-Cuervo C, Nelson DR, Fried MW, Segal JB, Sulkowski MS. Oral direct-acting agent therapy for hepatitis C virus infection: a systematic review. Ann Intern Med 2017; 166:637–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Global Hepatitis Report 2017: World Health Organization; 2017.. Available at http://apps.who.int/iris. Accessed 27 July 2021.
- 6. Biden J. A Proclamation on National Hepatitis Testing Day, 2021. Available at: https://www.whitehouse.gov/briefing-room/presidential-actions/2021/05/18/a-proclamation-on-national-hepatitis-testing-day-2021/. Accessed 27 July 2021.
- 7. Trickey A, Fraser H, Lim AG, et al. The contribution of injection drug use to hepatitis C virus transmission globally, regionally, and at country level: a modelling study. Lancet Gastroenterol Hepatol 2019; 4:435–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Edlin BR, Kresina TF, Raymond DB, et al. Overcoming barriers to prevention, care, and treatment of hepatitis C in illicit drug users. Clin Infect Dis 2005; 40(Suppl 5):S276–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Do A, Mittal Y, Liapakis A, et al. Drug authorization for sofosbuvir/ledipasvir (harvoni) for chronic HCV infection in a real-world cohort: a new barrier in the HCV care cascade. PLoS One 2015; 10:e0135645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lo Re V 3rd, Gowda C, Urick PN, et al. Disparities in absolute denial of modern hepatitis C therapy by type of insurance. Clin Gastroenterol Hepatol 2016; 14:1035–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yehia BR, Schranz AJ, Umscheid CA, Lo Re V 3rd. The treatment cascade for chronic hepatitis C virus infection in the United States: a systematic review and meta-analysis. PLoS One 2014; 9:e101554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Morris MD, Mirzazadeh A, Evans JL, et al. Treatment cascade for hepatitis C virus in young adult people who inject drugs in San Francisco: low number treated. Drug Alcohol Depend 2019; 198:133–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tsui JI, Miller CM, Scott JD, Corcorran MA, Dombrowski JC, Glick SN. Hepatitis C continuum of care and utilization of healthcare and harm reduction services among persons who inject drugs in Seattle. Drug Alcohol Depend 2019; 195:114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jordan AE, Masson CL, Mateu-Gelabert P, et al. Perceptions of drug users regarding hepatitis C screening and care: a qualitative study. Harm Reduct J 2013; 10:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hajarizadeh B, Cunningham EB, Reid H, Law M, Dore GJ, Grebely J. Direct-acting antiviral treatment for hepatitis C among people who use or inject drugs: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2018; 3:754–67. [DOI] [PubMed] [Google Scholar]
- 16. Eckhardt B, Mateu-Gelabert P, Aponte-Melendez Y, et al. Accessible hepatitis C care for people who inject drugs. JAMA Intern Med 2022; 182:494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Edlin BR, Winkelstein ER. Can hepatitis C be eradicated in the United States? Antiviral Res 2014; 110:79–93. [DOI] [PubMed] [Google Scholar]
- 18. Grebely J, Dore GJ. Can hepatitis C virus infection be eradicated in people who inject drugs? Antiviral Res 2014; 104:62–72. [DOI] [PubMed] [Google Scholar]
- 19. Burki T. Elimination on the agenda for hepatitis C. Lancet Infect Dis 2014; 14:452–3. [DOI] [PubMed] [Google Scholar]
- 20. AASLD. American Association for the Study of Liver Diseases . HCV guidance: recommendations for treatment, management, and treating hepatitis C - simplified HCV treatment for treatment-naive adults without cirrhosis. Available at: hcvguidelines.org/treatment-naive/simplified-treatment. Accessed 5 December 2021.
- 21. Solomon S, Wagner-Cardoso S, Smeaton L, et al. A minimal monitoring approach for the treatment of hepatitis C virus infection (ACTG A5360 [MINMON]): a phase 4, open-label, single-arm trial. 2022. [DOI] [PMC free article] [PubMed]
- 22. ClinicalTrials.gov . Identifier NCT03418636, The staying safe intervention (Ssafe). Available at: https://clinicaltrials.gov/ct2/show/NCT03418636. Accessed 27 July 2021.
- 23. Mateu-Gelabert P, Gwadz MV, Guarino H, et al. The staying safe intervention: training people who inject drugs in strategies to avoid injection-related HCV and HIV infection. AIDS Educ Prev 2014; 26:144–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ford MM, Johnson N, Desai P, Rude E, Laraque F. From care to cure: demonstrating a model of clinical patient navigation for hepatitis C care and treatment in high-need patients. Clin Infect Dis 2017; 64:685–91. [DOI] [PubMed] [Google Scholar]
- 25. Pilcher CD, Ospina-Norvell C, Dasgupta A, et al. The effect of same-day observed initiation of antiretroviral therapy on HIV viral load and treatment outcomes in a US public health setting. J Acquir Immune Defic Syndr 2017; 74:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gowda C, Lott S, Grigorian M, et al. Absolute insurer denial of direct-acting antiviral therapy for hepatitis C: a national specialty pharmacy cohort study. Open Forum Infect Dis 2018; 5:ofy076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Canary LA, Klevens RM, Holmberg SD. Limited access to new hepatitis C virus treatment under state Medicaid programs. Ann Intern Med 2015; 163:226–8. [DOI] [PubMed] [Google Scholar]
- 28. Kapadia SN, Jeng PJ, Schackman BR, Bao Y. State Medicaid hepatitis C treatment eligibility criteria and use of direct-acting antivirals. Clin Infect Dis 2018; 66:1618–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Behrends CN, Gutkind S, Deming R, Fluegge KR, Bresnahan MP, Schackman BR. Impact of removing Medicaid fee-for-service hepatitis C virus (HCV) treatment restrictions on HCV provider experience with Medicaid managed care organizations in New York City. J Urban Heal 2021; 98:563–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.