Abstract
Cu-exchanged chabazite is the catalyst of choice for NOx abatement in diesel vehicles aftertreatment systems via ammonia-assisted selective catalytic reduction (NH3–SCR). Herein, we exploit in situ X-ray absorption spectroscopy powered by wavelet transform analysis and machine learning-assisted fitting to assess the impact of the zeolite composition on NH3-mobilized Cu-complexes formed during the reduction and oxidation half-cycles in NH3–SCR at 200 °C. Comparatively analyzing well-characterized Cu-CHA catalysts, we show that the Si/Al ratio of the zeolite host affects the structure of mobile dicopper(II) complexes formed during the oxidation of the [CuI(NH3)2]+ complexes by O2. Al-rich zeolites promote a planar coordination motif with longer Cu–Cu interatomic distances, while at higher Si/Al values, a bent motif with shorter internuclear separations is also observed. This is paralleled by a more efficient oxidation at a given volumetric Cu density at lower Si/Al, beneficial for the NOx conversion under NH3–SCR conditions at 200 °C.
Cu-exchanged chabazite (Cu-CHA) currently is the catalyst of choice for the ammonia-assisted selective catalytic reduction (NH3-SCR) of harmful NOx in diesel vehicles aftertreatment systems.1−3 The NH3-SCR technology relies on the reaction of NO and NH3 in the presence of O2, to form N2 and H2O as benign products, according to the overall stoichiometry 4NH3 + 4NO + O2 → 4 N2 + 6 H2O.
Over the past decade, pervasive research efforts have led to a renewed mechanistic understanding of NH3–SCR over Cu-CHA, unveiling the atomic-scale structure of the main Cu-species involved.4−10 The reaction proceeds via CuII to CuI reduction, with release of the products. The catalytic cycle is then closed by reoxidation of CuI to CuII, requiring the activation of O2. The redox-active Cu ions in Cu-CHA are also known to dynamically respond to the gaseous feed across the relevant temperature window, switching between framework-coordinated and mobile configurations.2,11
After heating a Cu-CHA catalyst at temperatures above 400 °C and in the presence of O2, the Cu is present as a CuII species, docked to the lattice oxygen atoms in correspondence of the Al exchange sites (Z) in the six- and eight-membered rings (6r and 8r) of the CHA zeolite. Assuming a random Al distribution in the zeolite framework, the chemical identity and local structure are determined by the catalyst composition: low Si/Al and Cu/Al ratios favor bare Z2CuII species in 6r, while at higher Si/Al and Cu/Al ratios an extra-ligand is required for charge compensation, resulting in Z[CuIIOH] or oxygen-bridged multimeric Zx[CuxIIOy] species preferentially hosted in 8r.12−14 It is worthwhile to note that deviations from the random Al distribution, known to be promoted by specific synthesis approaches,15 could additionally influence the Cu-speciation in dehydrated Cu-CHA. Nonetheless, previous studies13,14,16 of the catalysts investigated herein point to a substantial agreement of Cu-speciation in the dehydrated state with the theoretical compositional phase diagram by Paolucci et al.,12 based on a random Al distribution in the zeolite framework subject to the Löwenstein’s rule.17
At 200 °C, the NH3-SCR reaction proceeds in a quasi-homogeneous fashion, over NH3-mobilized Cu-ions, yet electrostatically tethered to their framework exchange sites.6,18 Spectroscopic studies have been often performed by decoupling the reaction in the reduction and oxidation half-cycles, to facilitate the results interpretation. In the reduction half cycle exposure to a NO/NH3 mixture, results in the reduction of all Cu ions to form [CuI(NH3)2]+ complexes. In the oxidation half-cycle, these [CuI(NH3)2]+ complexes are exposed to O2, and the interaction of an O2 molecule with a pair of [CuI(NH3)2]+ leads to the formation of [Cu2(NH3)4O2]2+ complexes, which can be described as a μ-η2,η2-peroxo diamino dicopper(II).19−21
Paolucci et al. identified the volumetric Cu density as the key descriptor for the efficiency of the oxidation half cycle, validating that O2 activation requires a [CuI(NH3)2]+ pair, leading to a quadratic dependency of the NH3-SCR rate on Cu density at low Cu-loadings.6 Because the mobility of a [CuI(NH3)2]+ complex is limited to distances below approximately 9 Å from their anchoring point in the zeolite, a low Cu density implies a lower propensity for [CuI(NH3)2]+ pair formation, and a larger fraction of unreacted [CuI(NH3)2]+. As the O2 activation implies a change in the oxidation state of CuI to CuII, the fraction of unreacted [CuI(NH3)2]+ can be readily quantifiable by Cu K-edge X-ray Absorption Spectroscopy (XAS).
In this scenario, the actual reaction takes place on mobile Cu species, and therefore, the stable position of framework-bound Cu ions seems less relevant for the NH3-SCR activity. However, the density and distribution of the framework Al atoms and Brønsted acid sites may influence the mobility of the [CuI(NH3)2]+ complexes, the stability of Cu pairs, and thus the formation of the [Cu2(NH3)4O2]2+ complexes. The potential implications for low-temperature NH3-SCR performance through such zeolite-host driven changes in [Cu2(NH3)4O2]2+ formation are still unexplored.
To determine the influence of the zeolite properties on the NH3-SCR reaction, we performed an in situ XAS study empowered by wavelet transform (WT) analysis and machine learning (ML)-assisted fitting of the extended X-ray absorption fine structure (EXAFS) spectra in order to interrogate three Cu-CHA catalysts with variable Si/Al ratios and Cu contents during the reduction and oxidation half-cycles in NH3-SCR at 200 °C. Cu content and Si/Al ratio in the catalysts are balanced in such a way that the Cu density in two of the three catalysts is equivalent, despite having different Si/Al ratios. The catalysts used in this study have a Si/Al ratio of 5, 15 and 29, with a Cu/Al ratio of 0.1, 0.5, and 0.6 (estimated Cu densities of 0.28, 0.44, and 0.28 Cu/Å3, respectively), denoted hereafter as “0.1_5”, “0.5_15”, and “0.6_29”. These catalysts have been characterized previously in depth after pretreatment in O2 at 400 °C.14 We use the 0.5_15 catalyst as a reference, because it was previously investigated under the same redox conditions.20 Additional details on the investigated catalysts, as well as on the experimental methods for in situ XAS data collection can be found in the SI, Section 1.
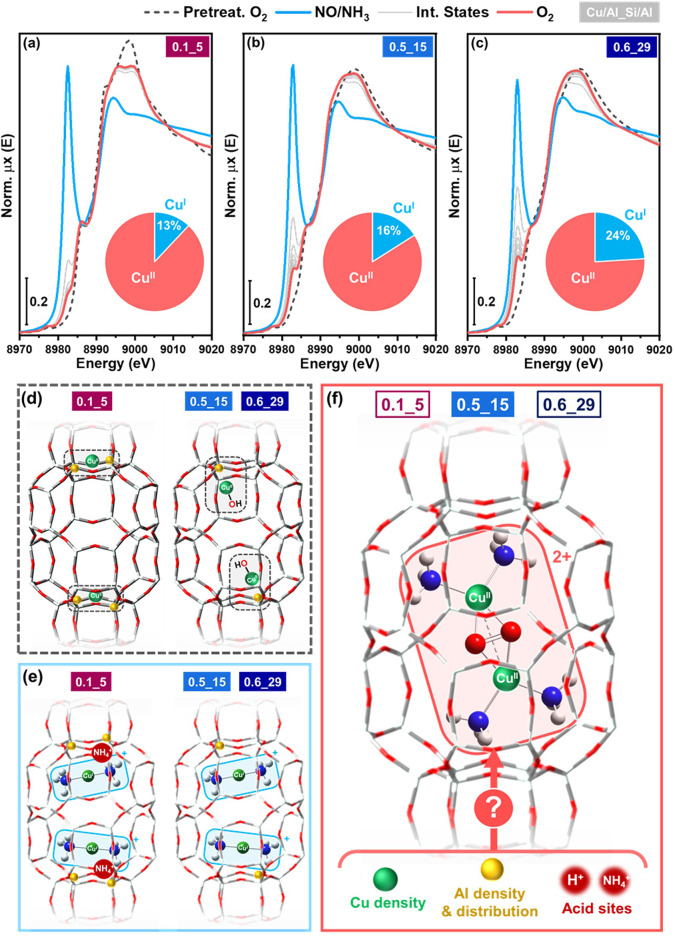
Figure 1a–c compares the Cu K-edge X-ray absorption near edge structure (XANES) spectra of the three catalysts measured after pretreatment in O2 at 400 °C and cooling down to 200 °C in the same gas feed, at the end of the reduction step in NO/NH3 and during the exposure of the reduced catalyst to O2, also collected under isothermal conditions at 200 °C. In parallel, Figure 1d–f illustrates the main envisaged Cu-species at each step in this process.
Figure 1.
In situ Cu K-edge XANES for (a) 0.1_5, (b) 0.5_15, and (c) 0.6_29 Cu-CHA catalysts collected at 200 °C after pretreatment in O2, at the end of the reduction step, during and after the oxidation step. Pie charts illustrate CuI/CuII percentages evaluated by XANES LCF at the end of the oxidation step for each catalyst; under the adopted experimental conditions, CuI and CuII correspond to [CuI(NH3)2]+ and [Cu2(NH3)4O2]2+ complexes, respectively. (d–f) Pictorial representation of the main Cu-species expected at each step as a function of the catalyst composition, with open questions on parameters influencing coordination motif in [Cu2(NH3)4O2]2+ complexes in part (f).
For all three catalysts, the XANES after pretreatment in O2 at 400 °C (dashed gray curves in Figure 1) shows the expected features for Z2CuII and Z[CuIIOH]/Zx[CuxIIOy] species, reflecting their different Cu/Al and Si/Al ratios.12−14 At the end of the reduction step, for each catalyst, the XANES indicates the formation of mobile [CuI(NH3)2]+ for all the Cu ions present. For the 0.1_5 composition, the reduction to [CuI(NH3)2]+ of the dominant Z2CuII species implies the formation of a new Brønsted acid site to maintain the charge balance in the zeolite. As NH3 is present in the applied reduction conditions, this results in the formation of NH4+ ions (Figure 1e).
During the oxidation step, the characteristic XANES features of CuII progressively develop, in line with the formation of [Cu2(NH3)4O2]2+ complexes.6,20 However, the spectral shape reached at the end of the oxidation step for the 0.1_5 catalyst differs from those observed for the 0.5_15 and 0.6_29 catalysts, especially in the white-line peak region. This indicates a different coordination motif for [Cu2(NH3)4O2]2+ in the Al-rich 0.1_5 catalyst, and therefore, we undertook a more in-depth analysis of the in situ XAS data.
Although the majority of the Cu ions are reoxidized to CuII after the oxidation step, from XANES linear combination fit (LCF) we found a residual amount of [CuI(NH3)2]+, determined by CHA Si/Al ratio, and ranging from 13% for Si/Al = 5, to 16% for Si/Al = 15 and 24% for Si/Al = 29 (see pie charts in Figure 1a-c and SI, Section 2 for details).
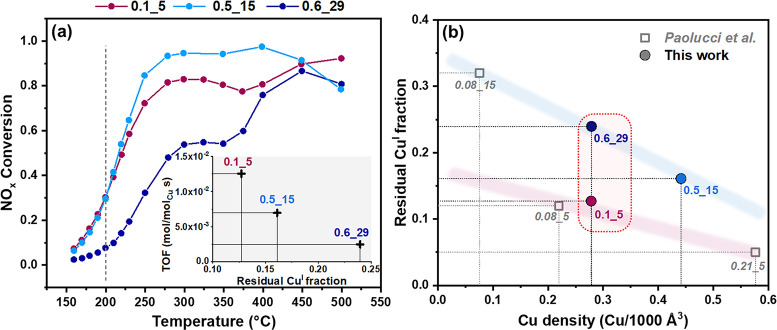
Figure 2a reports the NOx conversion in NH3-SCR for the three catalysts as a function of temperature. At 200 °C, 0.1_5 and 0.5_15 show almost equivalent conversion, which is instead lower for 0.6_29. The inset of Figure 2a correlates the turnover frequency (TOF), obtained by converting the measured NOx conversion at 200 °C to a rate constant based on a first order kinetic model, corresponding to an integral reactor analysis,22 and accounting for Cu wt % in the catalysts (SI, Table S1). Considering the increase in residual CuI as Si/Al increases observed from XANES LCF, the TOF at 200 °C follows the opposite trend: it is higher in 0.1_5 and it decreases in an approximately linear way for the 0.5_15 and 0.6_29 catalysts. This indicates that a more efficient oxidation of [CuI(NH3)2]+ by O2 activation is beneficial for the low-temperature NH3-SCR performance.
Figure 2.
(a) NOx conversion in the 150–500 °C temperature range for 0.1_5, 0.5_15, and 0.6_29. Amount of catalyst: 5 mg; feed gas: 500 ppm of NO, 533 ppm of NH3, 5% H2O, 10% O2 in N2; flow: 225 N mL/min. The bottom inset correlates residual CuI fractions evaluated by XANES LCF and turnover frequency (TOF) in mol/[molCu s] at 200 °C for the three catalysts. (b) Correlation between the same residual CuI fractions and the volumetric Cu density in Cu-CHA catalysts, comparing experimental values obtained in this work (colored full circles) and literature values by Paolucci et al.6 (empty gray squares).
We note that the difference in residual CuI fractions is not entirely determined by the Cu density, but that the Si/Al ratio also plays a role. Figure 2b compares Cu density with the residual CuI fraction for the three catalysts investigated in this study and for the data by Paolucci et al.6 It is worth noticing that the 0.1_5 and 0.6_29 catalysts show comparable Cu density, while the residual CuI fraction after oxidation is significantly lower for 0.1_5 than for 0.6_29. The data appear to branch based on the Si/Al ratio, with a different dependence on the Cu density for low and high Si/Al ratios (purple and blue shadowed areas in Figure 2b, for Si/Al = 5 and Si/Al = 15, 29, respectively). Overall, at comparable Cu density, for Si/Al = 5 a lower residual CuI fraction is observed than for higher Si/Al ratios. This behavior is reminiscent of the compositional effects on Cu-speciation after pretreatment in O2, when low Si/Al favors Z2CuII at the expense of Z[CuIIOH],12,13 although the oxidation step proceeds from mobile [CuI(NH3)2]+, spectroscopically indistinguishable over the compositional series.
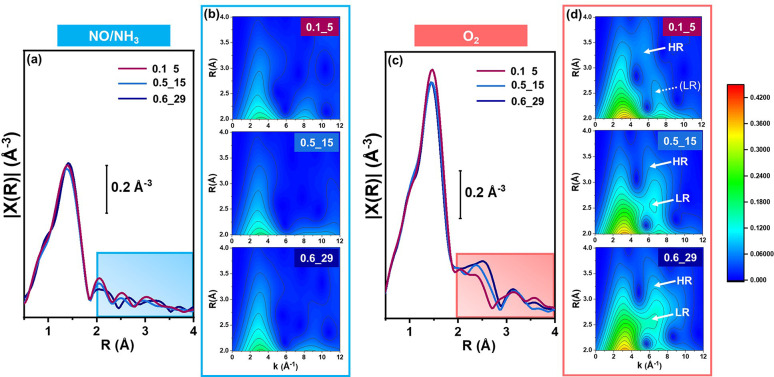
To correlate quantitatively the structural differences in the CHA zeolites with the observed trends in oxidation efficiency, we compared the EXAFS of the three catalysts at the end of both the reduction and oxidation steps (Figure 3). Together with the conventional Fourier transform (FT)-EXAFS representation, we exploited a WT-based analysis23,24 to unambiguously identify possible Cu–Cu scattering contributions possessing a characteristic lobe centered at ca. 7 Å–1 along the k direction14,20,25−28 (SI, Section 3 for details).
Figure 3.
Snapshots of local coordination environment of Cu ions by EXAFS, comparing 0.1_5, 0.5_15 and 0.6_29 at the end of the (a, b) reduction and (c, d) oxidation steps. (a, c) Magnitude of FT-EXAFS spectra, obtained by Fourier transforming k2χ(k) spectra in the 2.4–12.0 Å–1 range. (b, d) Corresponding EXAFS-WT maps, magnified in the 2–4 Å range and plotted using a common intensity scale. Low-R (LR) and high-R (HR) features in the k-range diagnostic of Cu–Cu scattering are highlighted by white arrows in part (d).
EXAFS confirms that after the reduction step all the Cu is present as [CuI(NH3)2]+ complexes, without any detectable dependence on the Cu and Al contents in the catalyst (Figure 3a,b). In all cases, unstructured FT-EXAFS features are observed beyond the first-shell peak originating from Cu–N single scattering. In parallel, the WT maps only shows a low-k lobe assigned to multiple scattering paths involving the two N atoms of the NH3 ligands. EXAFS fits based on the [CuI(NH3)2]+ structure confirmed this picture (SI, Section 4.2).
In all cases, the FT-EXAFS shows a first-shell peak with the same intensity at the end of the oxidation step, indicating 4-fold coordinated CuII ions. In the R-range within 2–4 Å, a broad peak centered at 2.5 Å grows in intensity in the order 0.1_5 < 0.5_15 < 0.6_29, accompanied by further variations in the 3–4 Å range (Figure 3c). The WT maps show a low-k lobe originating from multiple scattering contributions from low-Z (O and N) neighbors for all catalysts (see Figure 3d and SI, Figure S5b for the corresponding ΦR(k)density power functions), with an intensity and R-space location that is only slightly affected by the catalyst composition. In the high-k range characteristic of Cu–Cu scattering, we observe two local maxima along the R direction at ca. 2.5 and 3.2 Å (LR and HR labels in Figure 3d, respectively), pointing to an EXAFS-resolvable bimodal distribution of Cu–Cu interatomic distances. The LR feature becomes more intense relative to the HR feature as the Si/Al ratio increases; the HR feature is also inherently weaker due to the dampening of the EXAFS signal as R increases.
Taking into account phase correction, the HR feature is compatible with the DFT-optimized geometry of quasi-planar μ-η2,η2-peroxo diamino dicopper(II) with intranuclear separation of 3.40 Å, as reported for 0.5_15 in our previous work.20 However, the conventional EXAFS refinement solely based on this model revealed a local lack of fit at ca. 2.5 Å (SI, Section 4.3). This corresponds to the LR feature becoming more visible for 0.6_29 in the WT maps.
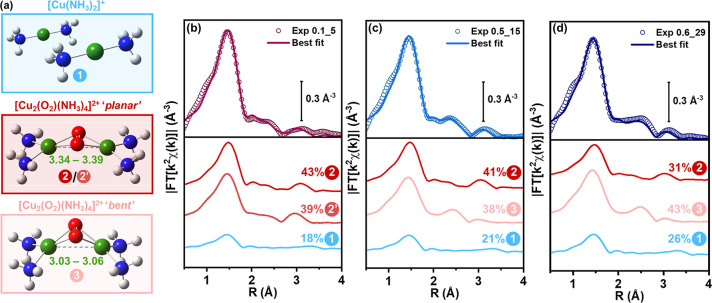
To further understand the emerging structural complexity, we employed a novel ML-assisted EXAFS analysis approach29−31 to build up a robust three-component fitting model. The model accounts for a minor presence of [CuI(NH3)2]+ (Figure 4a, 1) and for a bimodal distribution of the Cu–Cu distances in μ–η2,η2-peroxo diamino dicopper(II). On the basis of the WT analysis, the latter is described by combining contributions from the already proposed quasi-planar configuration (Figure 4a, 2/2′) and a “bent” motif (Figure 4a, 3) for the same complex, refined through ML-based optimization of the Cu–O–Cu angle (see SI, Section 5 for a detailed description of the methodology and a complete report on fitting results).
Figure 4.
(a) Molecular models for the structural components included in the Machine Learning (ML)-assisted EXAFS fitting model: 1 [CuI(NH3)2]+, 2 “planar”, and 3 “bent” motifs for μ-η2,η2-peroxo diamino dicopper(II); when relevant, characteristic EXAFS-derived ranges for Cu–Cu interatomic distances are reported, in Å. (b–d) Comparison between magnitudes of experimental (colored circles) and best fit (thick lines) FT-EXAFS spectra at the end of the oxidation step for (b) 0.1_5, (c) 0.5_15, and (d) 0.6_29 (see SI, Figure S13 for the corresponding imaginary parts). The scaled components for Cu-species 1, 2/2′, 3 are also reported, vertically translated for the sake of clarity, together with percentages of each component over total Cu refined by ML-EXAFS fitting.
Figure 4b–d compares the corresponding experimental and best-fit EXAFS spectra for the three catalysts, highlighting the individual scattering contributions and refined percentage for each Cu-species. The percentages of residual [CuI(NH3)2]+ agree well with those from XANES LCF, revealing the same trend in the oxidation efficiency as a function of the Si/Al ratio. The previously observed lack of fit at ca. 2.5 Å is now resolved for the whole set of samples.
The fit confirms that two distinct μ-η2,η2-peroxo diamino dicopper(II) coordination motifs are required to fully model the EXAFS of 0.5_15 and 0.6_29, which is reflected in a different Cu–Cu distance for the bent (3.03–3.06 Å) and planar complex (3.34–3.39 Å). Interestingly, for 0.1_5 the three-component fit restitutes Cu–Cu distances of 3.38 and 3.39 Å, not distinguishable within the accuracy of EXAFS analysis and both pointing to the same planar motif (2/2′ labels in Figure 4b). The bent motif is instead progressively favored as the Si/Al ratio increases, becoming the major structural component for the 0.6_29 sample.
ML-EXAFS fitting ensures a quantitative understanding of the subtle differences observed in the XANES spectral shape as well as in the high-R portion of WT maps, where the LR/HR features fairly match the optimized Cu–Cu distances for planar and bent μ-η2,η2-peroxo diamino dicopper(II). The adopted approach allows refining, in principle, any structure starting from an initially guessed molecular complex with significantly different interatomic distances and angles. These results therefore highlight the potential of in situ XAS combined with integrated WT and ML-assisted EXAFS analysis.
Summarizing, the XANES and EXAFS results for the Cu-CHA catalysts consistently show, that the Si/Al ratio of the zeolite host affects the structure of mobile dicopper(II) complexes formed during the oxidation of the [CuI(NH3)2]+ complexes by O2. Diffuse Reflectance UV–vis spectroscopy, qualitatively responsive to the structure of Cu-oxo species, further supports these results (see SI, Section 6). Therefore, we suggest that the diverse electrostatic landscape and the higher Brønsted acid site density in Al-rich zeolites could trigger host–guest interactions promoting a longer internuclear separation in the planar μ-η2,η2-peroxo diamino dicopper(II) motif. Plausibly, the NH3 ligands in the dicopper(II) complex could participate into H-bonding interactions with ZH sites, as well as ZNH4 and ZNH4nNH3 associations, most likely formed at the reduction step in the presence of NH3 at 200 °C,32 triggering the observed elongation effect in Cu–Cu distances. Importantly, this is accompanied by a more efficient oxidation at a given volumetric Cu density, which is beneficial for the NOx conversion under NH3-SCR conditions at 200 °C. With this respect, Al-rich zeolites could favor dynamic Cu ion exchange between nearby sites, thus enhancing the mobility of [CuI(NH3)2]+ complexes beyond the limit dictated by electrostatic tethering to the initial exchange sites. A similar mechanism based on H+/H2O-aided diffusion of CuI was proposed in the context of continuous partial oxidation of methane to methanol over Cu-CHA, although in this case ZNH4 is suggested to hinder the exchange pathway.33 Overall, the findings obtained in this work provide a robust experimental basis for further theoretical modeling of low-temperature NH3-SCR mechanism, aimed at validating these hypotheses within a quasi-homogeneous Cu-catalyzed oxidation half-cycle actively driven by the compositional characteristics of the zeolitic host.
Acknowledgments
The authors acknowledge funding from project n. 2017KKP5Z PRIN-2017 MOSCATo (Cutting-edge X-ray methods and models for the understanding of surface site reactivity in heterogeneous catalysts and sensors); Horizon 2020 Excellence Science ERC-Synergy program 2019-CUBE: “Unravelling the secrets of Cu-based catalysts for C–H activation” (grant agreement no. 856446); European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement no. 955839 (CHASS). This work reflects only the authors’ view, and the Agency is not responsible for any use that can be made of the information it contains. The European Synchrotron Radiation Facility (ESRF, Grenoble, France) is acknowledged for beamtime allocation on the BM23 beamline. The authors are grateful to T. Selleri for help during the XAS experiments, as well as to P. N. Vennestrøm and A. Molokova for insightful discussions.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jpclett.2c01107.
Details on compositional characteristics, evaluation of Cu density and synthesis methods for the investigated Cu-CHA catalysts. Experimental methods for in situ XAS and UV–vis spectroscopies. Additional information about methods, models and supplementary results for the employed XAS data analysis strategies: XANES LCF, EXAFS WT, conventional EXAFS fitting, and ML-assisted EXAFS fitting. Complementary in situ UV–vis spectroscopy results (PDF)
Author Present Address
⊥ A.M.: Interface Science Department, Fritz Haber Institute, Faradayweg 4-6, 14195 Berlin, Germany
Author Present Address
¶ C.N.: Politecnico di Milano, Laboratory of Catalysis and Catalytic Processes, Department of Energy, via La Masa, 34, 20156 Milano, Italy
The authors declare no competing financial interest.
Supplementary Material
References
- Beale A. M.; Gao F.; Lezcano-Gonzalez I.; Peden C. H. F.; Szanyi J. Recent Advances in Automotive Catalysis for NOx Emission Control by Small-Pore Microporous Materials. Chem. Soc. Rev. 2015, 44, 7371–7405. 10.1039/C5CS00108K. [DOI] [PubMed] [Google Scholar]
- Borfecchia E.; Beato P.; Svelle S.; Olsbye U.; Lamberti C.; Bordiga S. Cu-CHA - A Model System for Applied Selective Redox Catalysis. Chem. Soc. Rev. 2018, 47, 8097–8133. 10.1039/C8CS00373D. [DOI] [PubMed] [Google Scholar]
- Gao F.; Peden C. H. F. Recent Progress in Atomic-Level Understanding of Cu/SSZ-13 Selective Catalytic Reduction Catalysts. Catalysts 2018, 8, 140. 10.3390/catal8040140. [DOI] [Google Scholar]
- Janssens T. V. W.; Falsig H.; Lundegaard L. F.; Vennestrøm P. N. R.; Rasmussen S. B.; Moses P. G.; Giordanino F.; Borfecchia E.; Lomachenko K. A.; Lamberti C.; Bordiga S.; Godiksen A.; Mossin S.; Beato P. A Consistent Reaction Scheme for the Selective Catalytic Reduction of Nitrogen Oxides with Ammonia. ACS Catal. 2015, 5, 2832–2845. 10.1021/cs501673g. [DOI] [Google Scholar]
- Lomachenko K. A.; Borfecchia E.; Negri C.; Berlier G.; Lamberti C.; Beato P.; Falsig H.; Bordiga S. The Cu-CHA deNOx Catalyst in Action: Temperature-Dependent NH3-Assisted Selective Catalytic Reduction Monitored by Operando XAS and XES. J. Am. Chem. Soc. 2016, 138, 12025–12028. 10.1021/jacs.6b06809. [DOI] [PubMed] [Google Scholar]
- Paolucci C.; Khurana I.; Parekh A. A.; Li S. C.; Shih A. J.; Li H.; Di Iorio J. R.; Albarracin-Caballero J. D.; Yezerets A.; Miller J. T.; Delgass W. N.; Ribeiro F. H.; Schneider W. F.; Gounder R. Dynamic Multinuclear Sites Formed by Mobilized Copper Ions in NOx Selective Catalytic Reduction. Science 2017, 357, 898–903. 10.1126/science.aan5630. [DOI] [PubMed] [Google Scholar]
- Marberger A.; Petrov A. W.; Steiger P.; Elsener M.; Krocher O.; Nachtegaal M.; Ferri D. Time-Resolved Copper Speciation During Selective Catalytic Reduction of NO on Cu-SSZ-13. Nat. Catal. 2018, 1, 221–227. 10.1038/s41929-018-0032-6. [DOI] [Google Scholar]
- Chen L.; Janssens T. V. W.; Vennestrøm P. N. R.; Jansson J.; Skoglundh M.; Grönbeck H. A Complete Multisite Reaction Mechanism for Low-Temperature NH3-SCR over Cu-CHA. ACS Catal. 2020, 10, 5646–5656. 10.1021/acscatal.0c00440. [DOI] [Google Scholar]
- Hu W.; Selleri T.; Gramigni F.; Fenes E.; Rout K. R.; Liu S.; Nova I.; Chen D.; Gao X.; Tronconi E. On the Redox Mechanism of Low-Temperature NH3-SCR over Cu-CHA: A Combined Experimental and Theoretical Study of the Reduction Half Cycle. Angew. Chem., Int. Ed. 2021, 60, 7197–7204. 10.1002/anie.202014926. [DOI] [PubMed] [Google Scholar]
- Feng Y.; Wang X.; Janssens T. V. W.; Vennestrøm P. N. R.; Jansson J.; Skoglundh M.; Grönbeck H. First-Principles Microkinetic Model for Low-Temperature NH3-Assisted Selective Catalytic Reduction of NO over Cu-CHA. ACS Catal. 2021, 11, 14395–14407. 10.1021/acscatal.1c03973. [DOI] [Google Scholar]
- Krishna S. H.; Jones C. B.; Miller J. T.; Ribeiro F. H.; Gounder R. Combining Kinetics and Operando Spectroscopy to Interrogate the Mechanism and Active Site Requirements of NOx Selective Catalytic Reduction with NH3 on Cu-Zeolites. J. Phys. Chem. Lett. 2020, 11, 5029–5036. 10.1021/acs.jpclett.0c00903. [DOI] [PubMed] [Google Scholar]
- Paolucci C.; Parekh A. A.; Khurana I.; Di Iorio J. R.; Li H.; Albarracin Caballero J. D.; Shih A. J.; Anggara T.; Delgass W. N.; Miller J. T.; Ribeiro F. H.; Gounder R.; Schneider W. F. Catalysis in a Cage: Condition-Dependent Speciation and Dynamics of Exchanged Cu Cations in SSZ-13 Zeolites. J. Am. Chem. Soc. 2016, 138, 6028–6048. 10.1021/jacs.6b02651. [DOI] [PubMed] [Google Scholar]
- Martini A.; Borfecchia E.; Lomachenko K. A.; Pankin I. A.; Negri C.; Berlier G.; Beato P.; Falsig H.; Bordiga S.; Lamberti C. Composition-Driven Cu-Speciation and Reducibility in Cu-CHA Zeolite Catalysts: A Multivariate XAS/FTIR Approach to Complexity. Chem. Sci. 2017, 8, 6836–6851. 10.1039/C7SC02266B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negri C.; Martini A.; Deplano G.; Lomachenko K. A.; Janssens T. V. W.; Borfecchia E.; Berlier G.; Bordiga S. Investigating the Role of Cu-Oxo Species in Cu-Nitrate Formation over Cu-CHA Catalysts. Phys. Chem. Chem. Phys. 2021, 23, 18322–18337. 10.1039/D1CP01754C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Iorio J. R.; Gounder R. Controlling the Isolation and Pairing of Aluminum in Chabazite Zeolites Using Mixtures of Organic and Inorganic Structure-Directing Agents. Chem. Mater. 2016, 28, 2236–2247. 10.1021/acs.chemmater.6b00181. [DOI] [Google Scholar]
- Pappas D. K.; Borfecchia E.; Dyballa M.; Pankin I. A.; Lomachenko K. A.; Martini A.; Signorile M.; Teketel S.; Arstad B.; Berlier G.; Lamberti C.; Bordiga S.; Olsbye U.; Lillerud K. P.; Svelle S.; Beato P. Methane to Methanol: Structure Activity Relationships for Cu-CHA. J. Am. Chem. Soc. 2017, 139, 14961–14975. 10.1021/jacs.7b06472. [DOI] [PubMed] [Google Scholar]
- Löwenstein W. The Distribution of Aluminum in the Tetrahedra of Silicates and Aluminates. Am. Mineral. 1954, 39, 92–96. [Google Scholar]
- Gao F.; Mei D.; Wang Y.; Szanyi J.; Peden C. H. F. Selective Catalytic Reduction over Cu/SSZ-13: Linking Homo- and Heterogeneous Catalysis. J. Am. Chem. Soc. 2017, 139, 4935–4942. 10.1021/jacs.7b01128. [DOI] [PubMed] [Google Scholar]
- Chen L.; Falsig H.; Janssens T. V. W.; Grönbeck H. Activation of Oxygen on (NH3CuNH3)+ in NH3-SCR over Cu-CHA. J. Catal. 2018, 358, 179–186. 10.1016/j.jcat.2017.12.009. [DOI] [Google Scholar]
- Negri C.; Selleri T.; Borfecchia E.; Martini A.; Lomachenko K. A.; Janssens T. V. W.; Cutini M.; Bordiga S.; Berlier G. Structure and Reactivity of Oxygen-Bridged Diamino Dicopper(II) Complexes in Cu-Ion-Exchanged Chabazite Catalyst for NH3-Mediated Selective Catalytic Reduction. J. Am. Chem. Soc. 2020, 142, 15884–15896. 10.1021/jacs.0c06270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda A.; Shionoya H.; Hotta Y.; Takewaki T.; Sawabe K.; Satsuma A. Spectroscopic Evidence of Efficient Generation of Dicopper Intermediate in Selective Catalytic Reduction of NO over Cu-Ion-Exchanged Zeolites. ACS Catal. 2020, 10, 12333–12339. 10.1021/acscatal.0c03425. [DOI] [Google Scholar]
- Levenspiel O.Chemical Reaction Engineering, 3rd ed.; Wiley: New York, 1999. [Google Scholar]
- Funke H.; Scheinost A. C.; Chukalina M. Wavelet Analysis of Extended X-Ray Absorption Fine Structure Data. Phys. Rev. B 2005, 71, 094110. 10.1103/PhysRevB.71.094110. [DOI] [Google Scholar]
- Timoshenko J.; Kuzmin A. Wavelet Data Analysis of EXAFS Spectra. Comput. Phys. Commun. 2009, 180, 920–925. 10.1016/j.cpc.2008.12.020. [DOI] [Google Scholar]
- Pankin I. A.; Martini A.; Lomachenko K. A.; Soldatov A. V.; Bordiga S.; Borfecchia E. Identifying Cu-Oxo Species in Cu-Zeolites by XAS: A Theoretical Survey by DFT-Assisted XANES Simulation and EXAFS Wavelet Transform. Catal. Today 2020, 345, 125–135. 10.1016/j.cattod.2019.09.032. [DOI] [Google Scholar]
- Sushkevich V. L.; Safonova O. V.; Palagin D.; Newton M. A.; van Bokhoven J. A. Structure of Copper Sites in Zeolites Examined by Fourier and Wavelet Transform Analysis of EXAFS. Chem. Sci. 2020, 11, 5299–5312. 10.1039/D0SC01472A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini A.; Signorile M.; Negri C.; Kvande K.; Lomachenko K. A.; Svelle S.; Beato P.; Berlier G.; Borfecchia E.; Bordiga S. EXAFS Wavelet Transform Analysis of Cu-MOR Zeolites for the Direct Methane to Methanol Conversion. Phys. Chem. Chem. Phys. 2020, 22, 18950–18963. 10.1039/D0CP01257B. [DOI] [PubMed] [Google Scholar]
- Deplano G.; Martini A.; Signorile M.; Borfecchia E.; Crocellà V.; Svelle S.; Bordiga S. Copper Pairing in the Mordenite Framework as a Function of the CuI/CuII Speciation. Angew. Chem., Int. Ed. 2021, 60, 25891–25896. 10.1002/anie.202109705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini A.; Guda S. A.; Guda A. A.; Smolentsev G.; Algasov A.; Usoltsev O.; Soldatov M. A.; Bugaev A.; Rusalev Y.; Lamberti C.; Soldatov A. V. PyFitit: The Software for Quantitative Analysis of XANES Spectra Using Machine-Learning Algorithms. Comput. Phys. Commun. 2020, 250, 107064. 10.1016/j.cpc.2019.107064. [DOI] [Google Scholar]
- Martini A.; Bugaev A. L.; Guda S. A.; Guda A. A.; Priola E.; Borfecchia E.; Smolders S.; Janssens K.; De Vos D.; Soldatov A. V. Revisiting the Extended X-ray Absorption Fine Structure Fitting Procedure through a Machine Learning-Based Approach. J. Phys. Chem. A 2021, 125, 7080–7091. 10.1021/acs.jpca.1c03746. [DOI] [PubMed] [Google Scholar]
- Guda A. A.; Guda S. A.; Martini A.; Kravtsova A. N.; Algasov A.; Bugaev A.; Kubrin S. P.; Guda L. V.; Šot P.; van Bokhoven J. A.; Copéret C.; Soldatov A. V. Understanding X-Ray Absorption Spectra by Means of Descriptors and Machine Learning Algorithms. NPJ. Comput. Mater. 2021, 7, 203. 10.1038/s41524-021-00664-9. [DOI] [Google Scholar]
- Giordanino F.; Borfecchia E.; Lomachenko K. A.; Lazzarini A.; Agostini G.; Gallo E.; Soldatov A. V.; Beato P.; Bordiga S.; Lamberti C. Interaction of NH3 with Cu-SSZ-13 Catalyst: A Complementary FTIR, XANES, and XES Study. J. Phys. Chem. Lett. 2014, 5, 1552–1559. 10.1021/jz500241m. [DOI] [PubMed] [Google Scholar]
- Dinh K. T.; Sullivan M. M.; Narsimhan K.; Serna P.; Meyer R. J.; Dincă M.; Román-Leshkov Y. Continuous Partial Oxidation of Methane to Methanol Catalyzed by Diffusion-Paired Copper Dimers in Copper-Exchanged Zeolites. J. Am. Chem. Soc. 2019, 141, 11641–11650. 10.1021/jacs.9b04906. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.