Summary
Patients with acutely decompensated cirrhosis have a dismal prognosis and frequently progress to acuteon-chronic liver failure, which is characterised by hepatic and extrahepatic organ failure(s). The pathomechanisms involved in decompensation and disease progression are still not well understood, and as specific disease-modifying treatments do not exist, research to identify novel therapeutic targets is of the utmost importance. This review amalgamates the latest knowledge on disease mechanisms that lead to tissue injury and extrahepatic organ failure – such as systemic inflammation, mitochondrial dysfunction, oxidative stress and metabolic changes – and marries these with the classical paradigms of acute decompensation to form a single paradigm. With this detailed breakdown of pathomechanisms, we identify areas for future research. Novel disease-modifying strategies that break the vicious cycle are urgently required to improve patient outcomes.
Keywords: Cirrhosis, Acute-on-chronic liver failure, ACLF, Tissue injury, PAMP, DAMP, Ascites, Variceal bleeding, Hepatic encephalopathy, Organ failure
Introduction
Acutely decompensated cirrhosis is characterised by the development of cirrhosis-related complications, such as ascites, hepatic encephalopathy, variceal bleeding or bacterial infections, and 30% of patients progress to extrahepatic organ failures and acute-on-chronic liver failure (ACLF). Mortality after 90 days remains high, ranging from 14% in acute decompensation to 50% in ACLF, mainly as there are insufficient therapies to stop disease progression. Acute decompensation is often caused by precipitants which induce a cascade of pathomechanistic processes, but in approximately 40% of cases the initiating event cannot be identified.1,2 This unpredictable disease onset, the variability of disease dynamics ranging from quick resolution of a “mere” acute decompensation to rapid progression to multi-organ failure and ACLF, as well as the various combinations of extrahepatic organ failures in patients with ACLF emphasise the complexity of the disease.3–5 After decades of research, most of these observations remain insufficiently explained. Pathomechanisms are generally multifaceted and increased research activity has resulted in high numbers of publications that require careful selection and interpretation. Traditional pathophysiological observations delineate portal hypertension with splanchnic and systemic vasodilation and hyperdynamic circulation as central mechanisms in theprocessofacutedecompensation.6 However,more recent findings introduce the concept that systemic inflammation, mitochondrial dysfunction, oxidative stress and metabolic changes can lead to tissue injury and extrahepatic organ failure.5,7 Marrying classical paradigms of acute decompensation with newer observations will be the key to comprehend the pathophysiology of decompensation.
Herein, we aim to delineate the central mechanisms of acutely decompensated cirrhosis and its progression to ACLF, thereby developing a pathophysiological paradigm to improve our understanding of disease phenotypes and to define fields of interest for future research activity.
The role of portal hypertension
Portal hypertension (PH) is a consequence of cirrhosis and an important determinant of its disease course and prognosis.8–10 In the compensated stage, the development of clinically significant portal hypertension (CSPH), defined by a hepatic venous pressure gradient (HVPG) of at least 10 mmHg, heralds the formation of portal-systemic collaterals and oesophageal or gastric varices and is per se associated with a worse prognosis.11 Decompensated cirrhosis is associated with the development of disease-related complications, such as ascites, variceal bleeding and hepatic encephalopathy, which require the HVPG to be elevated above 10 mmHg.9 Correcting PH has strong positive implications on a patients’ disease course and prognosis, thereby providing the rational for therapies that reduce portal pressure.12
Pathophysiology of portal hypertension in cirrhosis and its evolution with disease progression.
Haemodynamic factors
The interaction between blood flow (Q) and resistance (R) determines the pressure gradient in the portal venous system (ΔP). According to Ohm’s law, this relationship is expressed as: ΔP = Q x R in the context of haemodynamics.
Consequently, PH can be due to increased flow, increased vascular resistance, or a combination of both. Under physiological conditions, the total portal blood volume passes through the liver. Increased resistance across the diseased liver always initiates cirrhosis-related PH leading to prehepatic congestion. Increased blood flow through the portal system can only contribute significantly to PH when HVPG elevation is coupled with vascular endothelial growth factor (VEGF)-driven angiogenesis and porto-systemic collateral formation, which initiates increased portal blood flow. In decompensated cirrhosis, the amount of collateralised blood may exceed 90% of the total portal venous blood.13
Factors increasing hepatic vascular resistance
Vascular resistance is a direct function of vessel length and blood viscosity (mainly influenced by the haematocrit) and inversely related to the 4th power of the vessel diameter, which is equivalent to the sum of the cross-sectional area of sinusoids in the liver. This area is markedly reduced in cirrhosis because of pathophysiological alterations that can be divided into two main components. The first is a structural component that includes collagen deposition, liver fibrosis and nodule formation, which distort the liver vascular anatomy and increase liver stiffness, capillarisation of sinusoids and vascular occlusion. Vascular occlusion can be aggravated by microthrombi, which may be prevented by heparin or other anticoagulants.14 Together, this results in marked remodelling of the liver microcirculation. Microvascular thrombosis may further contribute to vascular remodelling by causing areas of parenchymal extinction and collapse, leading to further tissue injury which is aggravated by enhanced hepatic stellate cell (HSC) activation and deposition of extracellular matrix. The second is a dynamic component caused by myofibroblasts and the active contraction of activated HSCs around the sinusoids leading to increased hepatic vascular tone.15 The balance between structural and dynamic resistance follows the evolution from pre-CSPH to CSPH and decompensated cirrhosis. Whereas the former is dominated by the structural component, the dynamic component worsens with disease progression, and is more evident after decompensation. Moreover, factors triggering systemic inflammation might further enhance both the structural and dynamic components (Fig. 1).
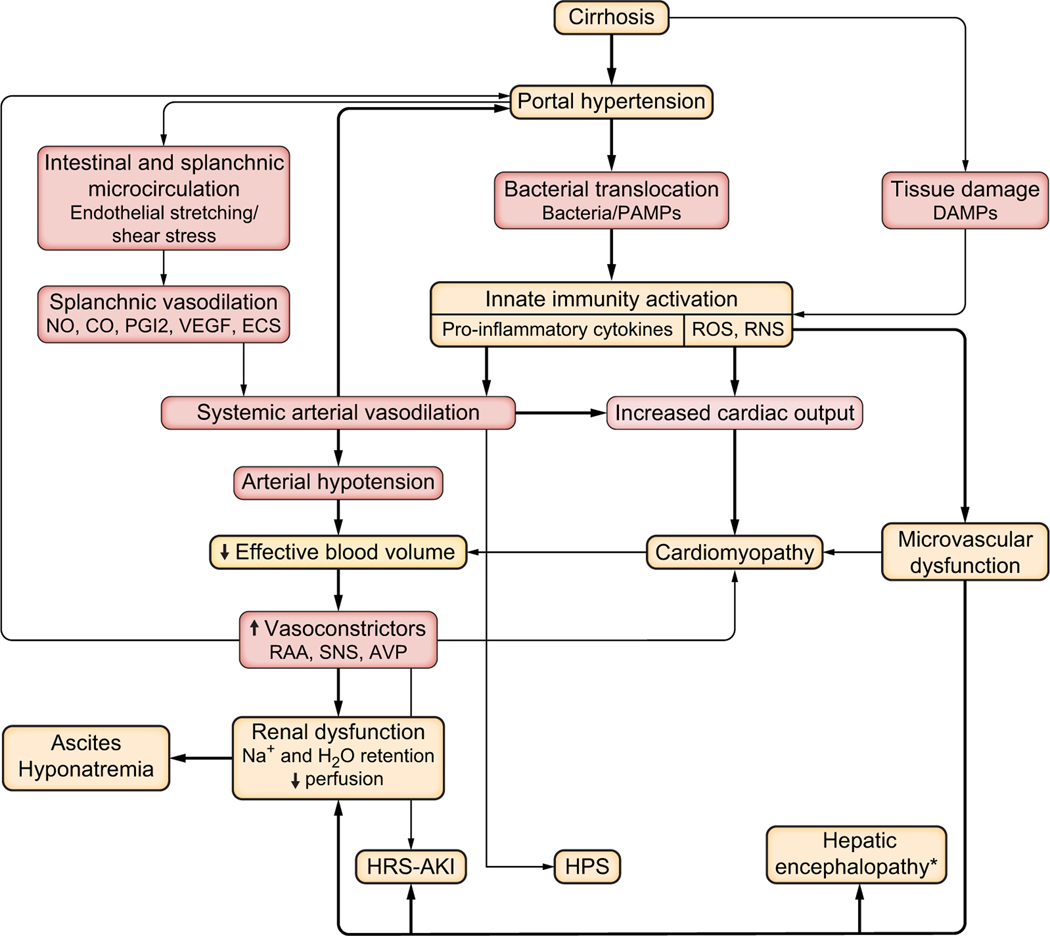
Fig. 1. Cardiocirculatory alteration in decompensated cirrhosis.
The thickness of the lines and arrows is proportional to the relevance of the pathophysiological mechanisms and clinical consequences. AKI, acute kidney injury; AVP, arginine-vasopressin; CO, carbon monoxide; DAMPs, danger-associated molecular patterns; ECS, endocannabinoids; HPS, hepatopulmonary syndrome; HRS, hepatorenal syndrome; NO, nitric oxide; PAMPs, pathogen-associated molecular patterns; PGI2; prostacyclin; RAA, renin-angiotensin-aldosterone system; RNS, reactive nitrogen species; ROS, reactive oxygen species; SNS, sympathetic nervous system. *Hepatic encephalopathy is not only caused by microvascular dysfunction but also by inflammation, hyperammonaemia and other factors.
HSC activation is central in the pathogenesis of portal hypertension since it is not only involved in the dynamic component but also in the structural component, as HSCs produce extracellular matrix and collagen, which leads to fibrosis. In addition, activated HSCs are contractile and represent the effector cells that increase resistance in response to liver sinusoidal endothelial cell de-differentiation, leading to endothelial dysfunction, a process secondary to liver injury, oxidative stress and inflammation.16 Increased liver resistance is aggravated by an imbalance between decreased endogenous vasodilators, mainly nitric oxide (NO), and increased endogenous vasoconstrictors, such as norepinephrine, angiotensin-2, endothelin and thromboxane A2.12 The dynamic resistance component can be targeted by vasodilators, such as NO donors or prazosin. However, these agents cannot be used alone in the clinical setting as they also worsen systemic vasodilation, thereby aggravating circulatory dysfunction and renal perfusion.17–19 Better results are obtained with statins and cGMP-activators, which upregulate hepatic NO release by overexpressing the transcription factor Kruppel-like factor 2 that regulates the expression of a wide variety of vasoprotective genes involved in controlling apoptosis, inflammation, oxidative stress, thrombosis and vasodilation. In addition, statins also inhibit RhoA-Rho-kinase activation, which further contributes to decrease liver resistance as RhoA-Rho-kinase activation is a major contributor to activated HSC contraction.20–22 Statins in particular have been shown to have anti-inflammatory and strong anti-fibrotic effects, thus modifying the structural component of increased liver resistance.23 Simvastatin administration improves survival in patients with decompensated cirrhosis (mostly Child-Pugh class B)24 and prevents lipopolysaccharide (LPS)-induced ACLF in preclinical models.20,25 Finally, fibrosis, circulatory changes and inflammation lead to increased liver stiffness, which through mechano-sensing pathways may worsen sinusoidal and perisinusoidal cell activation, further aggravating fibrogenesis.26
Factors modulating blood flow in the portal system
Changes in portal blood flow through the liver cause opposite variations in hepatic artery blood flow (“buffer response”), which tends to attenuate alterations in total hepatic blood flow.27 Under healthy conditions, portal blood inflow mainly changes after meals, which induce marked splanchnic vasodilation and subsequently increase portal vein inflow, which can also be achieved by volume expansion. In contrast, portal blood inflow is markedly decreased by stimulating adrenergic vasoconstriction, e.g. during hypovolemic shock and physical exercise or by the administration of splanchnic vasoconstrictors (terlipressin), inhibitors of glucagon secretion (somatostatin) or drugs that reduce cardiac output (beta-blockers).28 In advanced PH with high vascular resistance and elevated portosystemic shunting, the hepatic artery is maximally dilated.28 This is also relevant as it highlights that correcting splanchnic vasodilation in advanced cirrhosis may worsen liver perfusion and hepatic vascular tone, thus blunting the reduction of portal pressure.29
In advanced PH, the portal inflow increases due to splanchnic arteriolar vasodilation, mainly driven by VEGF-induced production of NO from the endothelial NO synthase (eNOS).30 Translocation of intestinal bacteria and/or bacterial products also enhance eNOS activation. Other vasodilators, such as carbon monoxide, glucagon, endocannabinoids and drugs acting as NO donors may aggravate splanchnic arterial vasodilation.12 Importantly, in advanced cirrhosis, and even more in decompensated patients, splanchnic arterial vasodilation initiates systemic haemodynamic alterations leading to reduced total peripheral resistance and systemic hypotension.12,31 The development of cirrhosis-associated complications during decompensation is linked to the systemic effects of PH which are discussed in the section on “cardiocirculatory dysfunction” (Fig. 1).
Other relevant factors modulating portal hypertension
VEGF-VEGF-receptor-2 (VEGFR2)-related angiogenesis plays a relevant role in PH, as it contributes to splanchnic vasodilation,32 the formation of portal-systemic collaterals,33 and the magnitude of the splenomegaly accompanying PH. Many preclinical studies have shown that inhibition of the VEGF-VEGFR2 pathway markedly attenuates the development of splanchnic vasodilation and portal-systemic collaterals in cirrhotic animal models.34 Similar effects have been observed after enhancing the release of endogenous antiangiogenic factors, such as pigment-derived endothelial factor and vasohibin-1,35,36 which may represent a safer way of modulating angiogenesis than direct VEGF inhibition, as they do not impair healthy blood vessel formation.37,38
Multiple studies have shown that systemic inflammation can precipitate ACLF and there are multiple triggers for inflammatory responses1,5,39,40 in decompensated cirrhosis that are reviewed in other sections of this article. However, data supporting the hypothesis that acute decompensation is mediated by an aggravation of PH are less convincing, although results from a recent observational study suggest that, individually, systemic inflammation and PH are major determinants in the progression of acute decompensation to ACLF.5 The presence of bacterial translocation was observed in about half of the patients with decompensated cirrhosis and ascites.41 However, significant differences in HVPG between those with and without bacterial translocation were lacking despite marked differences in pro-inflammatory cytokines. The only identifiable effect was shown on the degree of hepatic endothelial dysfunction, manifested by a greater HVPG increase after eating a test meal in patients with bacterial translocation.41,42 Studies assessing HVPG and systemic haemodynamics during spontaneous bacterial peritonitis (SBP) and shortly after its cure revealed no significant impact on HVPG despite a moderate reduction of the cardiac index after SBP resolution.43 A recent meta-analysis of studies assessing the effects of norfloxacin or rifaximin on HVPG disclosed no significant treatment effect on HVPG, except from a subgroup of patients receiving long-term antibiotic therapy in combination with non-selective beta-blockers.44 Altogether, data suggests only minor effects of bacterial translocation and bacterial infections on HVPG, which is compatible with the notion that acute decompensation and progression to ACLF are mainly mediated by pro-inflammatory factors rather than PH aggravation.5,45 On the contrary, there is evidence suggesting that systemic inflammation associated with obesity significantly increases HVPG in cirrhosis and the incidence of decompensation over long-term follow-up was higher in obese patients. In these patients, an intensive life-style intervention lowered HVPG and was accompanied by a decrease in pro-inflammatory cytokines.46,47
Systemic haemodynamics and cardiomyopathy
Another long-recognised feature of advanced cirrhosis is hyperdynamic circulation, a systemic haemodynamic abnormality primed by peripheral arterial vasodilation.48 Vasodilation is not ubiquitous, but mainly pertains to the splanchnic circulatory area as a consequence of PH,49 even though other regions, such as muscle and pulmonary circulations, are also involved.50,51
An immediate consequence of peripheral arterial vasodilation is the reduction in effective arterial blood volume. This evokes a series of compensatory events: on one side, baroreceptor reflexes activate vasoconstrictor and sodium and water retaining systems, such as the renin-aldosterone axis, sympathetic nervous system, and secretion of arginine-vasopressin;52 on the other, total blood volume expansion secondary to renal sodium retention along with β1-adrenoceptor stimulation promote an increase in cardiac output. Alongside this haemodynamic imbalance, the heart contributes to the development of effective hypovolemia. In advanced stages of decompensation, the heart still sustains an enhanced cardiac output, although this becomes insufficient to meet with the needs of systemic circulation following disease progression or stressful situations. Impaired cardiac chronotropic and inotropic responses to sympathetic nervous system stimulation account for this dysfunction.53 This is especially evident in patients developing hepatorenal syndrome type 1 or 2 (now termed HRS-AKI and HRS-NAKI),54 where a relative reduction of cardiac output evolves.55 These abnormalities are likely the expression of cirrhotic cardiomyopathy, which is defined as chronic cardiac dysfunction in the absence of any other cardiac disease, characterised by blunted contractile responsiveness to stress, altered diastolic relaxation, and electrophysiological abnormalities, the most common being prolongation of the Q-T interval that, interestingly, is normalised by beta-blockers.56
Compensatory mechanisms succeed in attenuating the effects of peripheral arterial vasodilation in earlier disease stages, e.g. in pre-ascitic cirrhosis and the first phase of the ascitic stage, but ultimately fail.48 As a result, decompensated cirrhosis is characterised by a sustained reduction of effective arterial blood volume and its consequences.
Microcirculation
Changes in the hepatic microcirculation during the evolution of cirrhosis and the transition to decompensation and ACLF have been described. Systemic vascular abnormalities in decompensated cirrhosis also involve the microcirculation, which is essential to ensure tissue oxygenation and remove carbon dioxide and other waste products. Although systemic microcirculatory abnormalities are less well defined than those of the intrahepatic circulation in patients with cirrhosis, evidence for these abnormalities is emerging.57 The assessment of sublingual microcirculation in patients with cirrhosis, with and without sepsis, showed that microvascular perfusion was impaired in decompensated patients due to vasoconstriction.58 Similarly, a significantly increased post-occlusive hyperaemic response, which indicates microvascular dysfunction, and reduced flow in the peripheral microcirculation seem to be typical in this disease stage.59–61 These abnormalities, which suggest precapillary shunt formation, reflect what is known about critically ill patients with sepsis, where microcirculatory dysfunction is associated with worse clinical outcomes and organ failure.62,63 Likewise, a link between altered microcirculation, severity of cirrhosis and organ failure was also reported for patients with decompensated cirrhosis.64
Molecular mechanisms that promote and aggravate cardiovascular dysfunction
As previously discussed, PH plays a pivotal role in the development of cardiovascular dysfunction in cirrhosis. Furthermore, the pro-inflammatory and pro-oxidant milieu of decompensated cirrhosis are now considered a major cause of diffuse endothelial activation and microvascular dysfunction, which are the background for arterial vasodilation, multi-organ dysfunction and failure. In experimental models of cirrhosis, systemic inflammation induced by abnormal translocation of bacteria and bacterial products (pathogen-associated molecular patterns [PAMPs]) enhance eNOS activity in mesenteric arterioles65 and the expression of both eNOS and inducible nitric oxide synthase (iNOS) in aortic walls, an effect blunted by norfloxacin administration.66 The subsequent release of NO and other vasodilators represents the main mechanism promoting arterial vasodilation.
Exposure to mediators and vasoactive substances, especially to increased levels of angiotensin II and norepinephrine, is involved in the development and aggravation of cardiomyopathy during decompensation.67 There is also convincing experimental evidence that left ventricular contractility is associated with tumour necrosis factor-α (TNFα)-induced activation of the NF-κB-iNOS pathway, oxidative damage and altered β-receptor signalling.68 Evidence for a relationship between the degree of an altered cardiodynamic state and systemic inflammation, as assessed by measuring circulating IL-6 and −8 and soluble IL-33 receptor, has also been provided in humans. Interestingly, IL-6 levels independently predicted the occurrence of fatal ACLF over a median follow-up of 3 years.69–71
Consequences of cardiovascular dysfunction – complications and organ dysfunctions
Reduced effective blood volume, cardiomyopathy and microvascular and endothelial dysfunction represent the pathophysiological background of many complications of decompensated cirrhosis, a situation where compensatory mechanisms are no longer effective (Fig. 1). In the presence of sinusoidal PH, total blood volume expansion secondary to retention of sodium leads to ascites formation52 and enhanced water reabsorption, which is responsible for dilutional hyponatremia.72 Reduced concentrations of serum albumin, which is the most abundant protein in the extracellular fluid and is responsible for about 70–80% of the plasma oncotic pressure,73 are common in advanced cirrhosis and have an adverse prognostic impact.74 Hypoalbuminemia is traditionally considered to facilitate ascites formation by reducing plasma colloid-osmotic pressure. However, fluid partition between intra- and extra-vascular spaces is regulated by the colloid-osmotic pressure gradient between these compartments, which is not substantially altered in cirrhosis with ascites,75 bringing into question the exact role of hypoalbuminemia in ascites formation.
Other complications of decompensated cirrhosis, such as microvascular disturbances contributing to the development of hepatic encephalopathy,76 a reduced cardiovascular responsiveness to vasoconstrictors favouring the occurrence of shock,77 or pulmonary circulation abnormalities, which potentially lead to hepatopulmonary syndrome,51,78 might aggravate the disease course and culminate into ACLF.
PAMPs, damage-associated molecular patterns (DAMPs) derived from the diseased liver, and pro-inflammatory molecules reach the kidney structures via glomerular filtration and peritubular capillaries. The sustained exposure of epithelial tubular cells to these inflammatory mediators primes a cascade of events, such as oxidative burst and TNFα secretion into the luminal fluid, ultimately leading to tubular damage and apoptosis. The consequent excessive sodium chloride delivery to the macula densa promotes glomerular vasoconstriction. These mechanisms have been described and hypothesised in sepsis-induced renal failure,79 but are likely present in patients with decompensated cirrhosis. Indeed, evidence for the upregulation of renal Toll-like receptor-4 (TLR4) has been provided not only in patients with an acute deterioration of cirrhosis and renal dysfunction, but also, to a lesser extent, in those without renal impairment.80 Furthermore, norfloxacin administration attenuates an increase in renal protein expression of TLR4, NF-κB, and caspase-3 in rats with advanced liver fibrosis or cirrhosis induced by bile duct ligation, so that their increased susceptibility to LPS-induced renal damage was prevented.81 Coherent with these findings, the absence of proteinuria and haematuria do not rule out the presence of renal lesions in patients with cirrhosis and renal impairment.82
It is conceivable that the more intense the systemic inflammation, the faster the transition from functional forms of AKI to acute tubular necrosis (ATN-AKI). This may explain why biomarkers of renal tubular damage, such as urinary neutrophil gelatinase-associated lipocalin, IL-18, kidney injury molecule-1 and liver-type fatty acid binding protein, better discriminate HRS-AKI from ATN-AKI in patients without bacterial infection than in those with sepsis-induced HRS-AKI, where full-blown systemic inflammation is expected (77–79). This evidence indicates that HRS-AKI can no longer be considered purely functional and implies that refined diagnostic and therapeutic approaches are warranted.
The role of inflammation in decompensated cirrhosis
Characterising inflammation in decompensated cirrhosis
Inflammation is a first-line general response of the innate immune system to danger signals from pathogens or from damaged cells. In normal homeostasis, inflammation is rapidly induced and later resolved in a timely manner as a result of well-defined self-regulatory mechanisms that are triggered by cytokines and mediators induced in the early phase of inflammation. In cirrhosis, however, these regulatory circuits are inefficient, leading to decompensation and functional decline.
It has long been recognised that decompensated liver diseases express patterns of immune activation with increased white blood cell count and C-reactive protein levels.1 Less than 1% of patients with acutely decompensated cirrhosis do not have any evidence of systemic inflammation.83 Elevated levels of circulating pro- and anti-inflammatory cytokines correlate with disease severity.1,84,85 TNFα, IL-6, IL-8, IL-10 and IL-1ra are key cytokines, as they are not only increased in ACLF but also progress through the different ACLF severity grades.84 High degree inflammation also identifies patients who are at risk of developing ACLF after decompensation.5 Therefore, data strongly suggest that inflammation represents a central pathomechanism mediating the transition from compensated cirrhosis to decompensation and further organ injury (ACLF).
Danger molecule signalling
Triggers of systemic inflammation in decompensated cirrhosis are derived from two major sources. First, increased gut permeability, changes in the composition of the gut microbiome and PH result in increased delivery of PAMPs to the liver via the portal circulation.86–91 Examples of common PAMPs include LPS, flagellin, bacterial or viral RNA/DNA or fungi. Compositional changes of gut microbiota and bacterial translocation have been linked to the development of cirrhosis-related complications, marking the transition from compensation to decompensation.92 Second, the underlying liver disease itself, whether it is alcohol use, viral hepatitis, metabolic syndrome or others, often causes constant hepatocyte and tissue damage resulting in the release of DAMPs.84 Necrotic hepatocytes may release markers of cell death, such as keratin 18 (K18) and cleaved K18. The magnitude is closely linked to disease progression and severity of inflammation. A decreasing cleaved K18:K18 ratio in ACLF indicates a predominance of non-apoptotic forms of cell death.93
Inflammasomes are intracellular pattern recognition receptors that sense mostly injured cells.94 It is generally accepted that inflammasome activation requires two signals, one of which is TLR4/LPS-mediated activation,95 which prompts NF-kB activation and nuclear translocation and triggers rapid pro-inflammatory cytokine induction (TNFα, monocyte chemoattractant protein-1, IL-6 and pro-IL-1β ).95–97 TNFα is also able to directly activate both apoptotic and necroptotic pathways.93,98 TLR4 is expressed on the plasma membrane and induces an inflammatory response following recognition of LPS.99 The relevance of this pathway is highlighted in preclinical rat and mouse models for ACLF, in which upregulated liver TRL4 expression and signalling was involved in organ sensitisation and subsequent endotoxin-driven tissue injury, which may precede acute decompensation and development of ACLF. TLR4 signalling inhibition and reduced receptor expression in the liver was found to protect against organ injury.40,100
The secondary signal for inflammasome activation is typically a DAMP.94 The inflammasome is a multiprotein complex that consists of DAMP sensors (NLRP1, NLRP2, NLRP3, NLRP4, NLRP6) and adaptor protein ASC (apoptosis-associated speck like protein containing a caspase recruitment domain). The inflammasome senses extracellular ATP, cholesterol or uric acid crystals, Ca2+ influx, HMGB-1 (high mobility group box protein 1), and K+ efflux that indicate cell stress or death.94 Binding of DAMPs triggers inflammasome assembly and activation of pro-caspase-1 to activated caspase-1 that cleaves pro-IL-1β and pro-IL-18 to a mature and bioactive IL-1β and/or IL-18.94 IL-1β amplifies inflammation and chemokine production and stimulates the recruitment of immune cells that propagate an immune response to the local infection and injury. In the liver, this results in infiltration of neutrophils and pro-inflammatory monocytes/macrophages, as well as the activation of Kupffer cells.95 Both PAMPs and DAMPs stimulate inflammatory responses that contribute to progressive recruitment of immune cells to the liver, which propagates local and systemic inflammation. Alongside dysfunctional immune regulation, this persistent inflammatory response can worsen tissue damage and lead to decompensation101–103 (Fig 2).
Fig. 2. Inflammatory processes of severe liver disease.
The underlying liver disease and the consequent effects on the gut microbiome are two major triggers of inflammation in a decompensated liver. Increased gut permeability linked to changes in the gut microbiome and portal hypertension result in high levels of PAMPs in the blood. High levels of LPS and other PAMPs from the leaky gut are then delivered to the liver via the portal vein. PAMPs and DAMPs in the liver that in turn are sensed by TLRs trigger inflammasome activation in KCs, hepatocytes and/or monocyte-derived pro-inflammatory macrophages. The persistent influx of PAMPs and DAMPs from the leaky gut and underlying disease results in a sustained inflammatory response that compromises the anti-inflammatory response. Additionally, the tissue microenvironment of the disease state (indicated by the red arrows) drives macrophage polarisation toward the M1 pro-inflammatory phenotype. While decreased polarisation of M2 macrophages results in insufficient anti-inflammatory cytokines and phagocytosis of activated neutrophils, processes that regulate inflammation in the healthy state. AIM2, absent in melanoma 2 protein; DAMPs, damaged-associated molecular patterns; HMGB1, high mobility group box protein 1; IFN, interferon; IL, interleukin; IRF, interferon regulatory factor; KC, Kupffer cell; LPS, lipopolysaccharide; M1, pro-inflammatory monocyte-derived macrophage; M2, anti-inflammatory monocyte-derived macrophage; MCP-1, monocyte chemotractant protein 1; NASH, non-alcoholic steatohepatitis; NET, neutrophil extracellular trap; NLR, NOD-like receptor; PAMPs, pathogen-associated molecular patterns; TGF-β, transforming growth factor-β; TLR, Toll-like receptor; TNFa, tumour necrosis factor-α.
Neutrophil and monocyte response in liver disease
Neutrophils are among the first immune cells that infiltrate the liver in response to damaged and dying hepatocytes. They are granulocytes that function to remove dead and infected cells by phagocytosis, releasing cytotoxic granules and/or forming neutrophil extracellular traps that capture pathogenic molecules. Monocytes represent another type of innate immune cells. They are also recruited from the circulation to the liver in response to immune activation and can rapidly differentiate into macrophages in the liver environment.104 In addition to monocyte-derived macrophages that infiltrate the liver from the portal vein, resident macrophages, called Kupffer cells, are also present in the liver.105,106 In advanced liver disease, innate immune functions are compromised resulting in an inadequate immune response to infection and injury.107
Neutrophils, monocytes and macrophages express TLRs and inflammasomes and detection of PAMPs and DAMPs in the local tissue environment initiates an immune response. In the early stages of cirrhosis and during the transition from compensation to decompensation, there is a strong innate immune response. Neutrophils secret large quantities of pro-inflammatory cytokines (TNFα, IL-1b, IL-6 etc.) and express activation markers, such as CD11b and CD62L.108 Macrophages play a major role in the progression of advanced liver disease109 and, depending on the tissue environment, macrophages change their activation state and phenotypes to become pro-inflammatory (also referred to as M1-like) or anti-inflammatory, “repair” macrophages (referred to as M2-like phenotype).110,111 Inflammatory macrophages persistently release inflammatory cytokines, such as TNFα, IL-6, and IL1β, which propagate liver damage rather than adopting a regulatory phenotype conducive to tissue repair.112,113
This early activation of innate immunity is in line with the observation that early and persistent immune activation promotes disease progression to ACLF in patients with acute decompensation 5 and increasing neutrophil and monocyte to lymphocyte ratios predict death in patients with acute decompensation and ACLF.114
If the innate immune system is persistently stimulated, especially during disease progression and with disease duration, neutrophils and monocytes/macrophages switch into a tolerogenic and non-responsive phenotype. Immune paralysis has been primarily observed in sepsis but also develops in patients with acute decompensation and ACLF.115 Upon constant IL-8 exposure, neutrophils reduce their CXCR1/2 expression which is essential for migration.116 Several neutrophil signalling defects, involving activation of AKT, mitogen-activated protein kinase (MAPK) and NADPH oxidase 2 cause a deficient response to invading pathogens.117 In the same manner, circulating monocytes (CD14+) reduce their expression of metabolic pathways and become non-responsive to pathogens.118 Therefore, initial immune activation and cytokine release favours the differentiation of immune incompetent myeloid cells, thereby increasing the risk of secondary infections and subsequent further disease progression as a typical feature of ACLF. In contrast, liver-resident macrophages (Kupffer cells) are continuously activated by DAMP release from dying hepatocytes in advanced disease stages, wherein they lose their tolerogenic profile and start secreting pro-inflammatory cytokines.119
Dysfunctional albumin exaggerates inflammation
Besides its oncotic power, albumin has other specific and relevant functions, including binding, transport and detoxification of endogenous and exogenous molecules; antioxidant activity; modulation of immune and inflammatory responses; and contributions to endothelial stabilisation and vascular integrity.120 Serum albumin abnormalities in cirrhosis are not only quantitative, but also qualitative. Indeed, in the pro-inflammatory and pro-oxidant milieu of advanced cirrhosis, the albumin molecule undergoes structural and, therefore, functional changes.121–125 Reversible and irreversible oxidation of the cysteine-34 residue prevail, but several other molecular abnormalities coexist, including glycosylation, N- and C-terminal truncations and homodimerisation.84,121–126 These abnormalities worsen in parallel with the severity of cirrhosis with a maximal abundance seen in ACLF, are associated with the occurrence of complications, and predict patient survival more closely than total serum albumin concentrations.84,123–127 Thus, patients with advanced cirrhosis not only present reduced serum albumin levels, but also, to an even greater extent, a reduction in the serum abundance of “effective albumin” – the native, functionally intact isoform of the molecule.120 This weakens the body’s defence against the systemic spread of offending molecules as PAMPs and DAMPs, sustained inflammation and oxidation, and endothelial and microvascular dysfunction. Albumin molecular alterations not only reflect inflammatory and oxidative damage, but also seem to exert a pro-inflammatory activity. Both human non-mercaptan-albumin (HNA)-1 and −2 are increased in patients with severe alcoholic hepatitis compared to heathy controls and those with stable alcohol-related cirrhosis. These oxidised isoforms activate neutrophils and induce a respiratory burst in vitro by triggering genes involved in neutrophil activation.127 HNA-1 and HNA-2 are also significantly increased in patients with decompensated cirrhosis and ACLF compared to heathy volunteers.128 Ex vivo HNA-1, but not HNA-2, induces an intense in vitro inflammatory response from peripheral blood mononuclear cells (114). Overall, these results suggest that oxidised albumin can be included among the factors that promote systemic inflammation in decompensated cirrhosis and ACLF and may provide clues to guide albumin administration in these settings.129
Pathomechanistic interplay leading to tissue injury and organ dysfunction
There are several ways in which inflammation triggers disease progression and modulates disease-related pathways. DAMP and PAMP-related immune cell activation enhances endothelial and (micro)vascular dysfunction to initiate the transition from compensation to decompensation and tissue injury. Cytokines, especially TNFα, can induce tissue injury directly by activating cell death pathways. Furthermore, altered cellular metabolic function, with high energy consumption in immune cells and decreased supply in peripheral organs, may be linked to mitochondrial dysfunction and oxidative stress. These mechanisms in concert may modulate organ function in general while individual disease mechanisms cause a rapid decline in organ function.
The role of metabolic alterations and mitochondrial dysfunction – molecular biology of tissue injury and ACLF development in decompensated cirrhosis
The metabolic landscape in decompensated cirrhosis
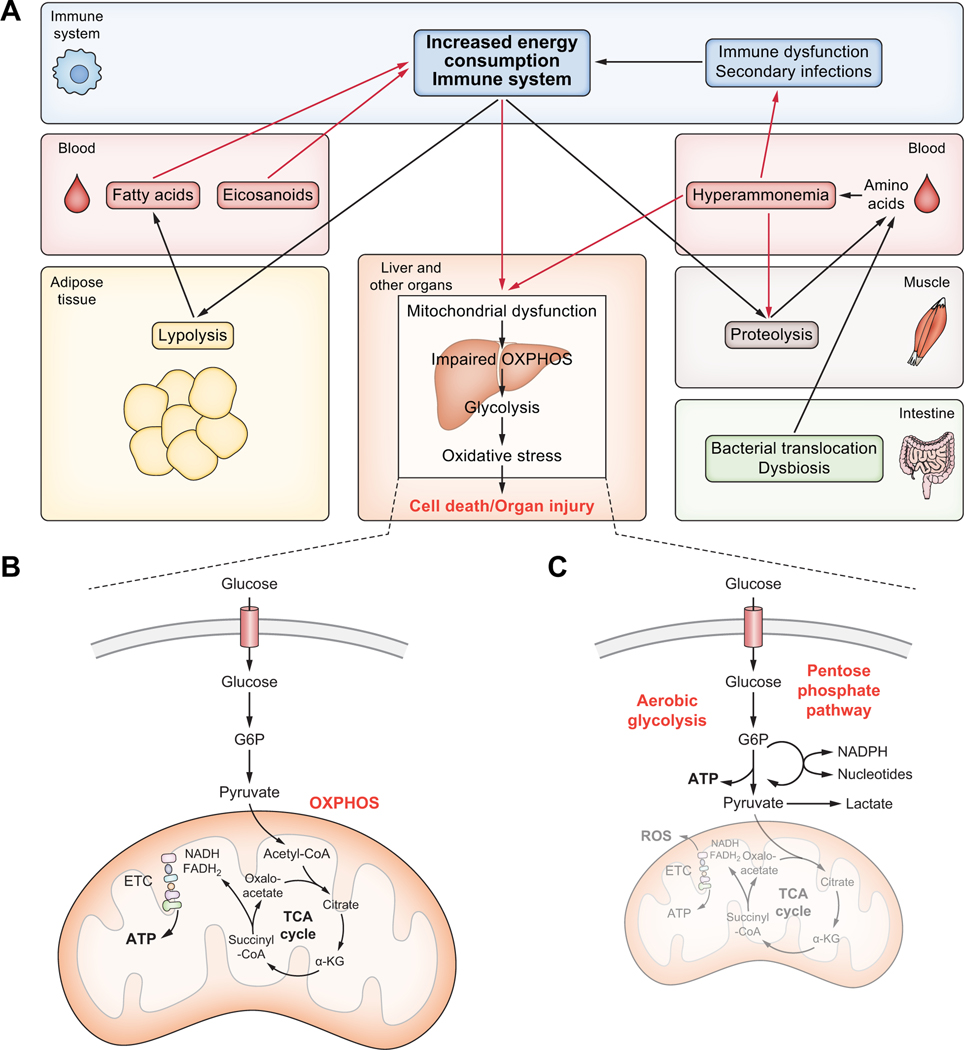
Recent advances indicating that a prevailing metabolic alteration is a hallmark of the intricate road to decompensated cirrhosis have challenged our understanding of the pathophysiological basis of advanced liver disease. This metabolic alteration is characterised by reduced oxidative glucose metabolism in the mitochondria accompanied by increased extramitochondrial glucose utilisation through glycolysis.130 Under normal conditions, mammalian cells obtain the vast majority of energy from mitochondrial oxidative phosphorylation (OXPHOS), which combines electron transport with cell respiration and ATP synthesis131 (Fig. 3). However, upon inflammatory conditions and cellular injury, mitochondria become dysfunctional and cells shift from producing ATP by OXPHOS to aerobic glycolysis131–133 (Fig. 3). Aerobic glycolysis (also known as the Warburg effect) ultimately produces lactate and generates two ATP molecules per glucose molecule, making it less efficient than OXPHOS, which generates about 36 ATPs per glucose molecule.131–133 Another disadvantage of glycolysis is that it depends on glucose as a sole fuel source, whereas mitochondrial OXPHOS has more metabolic flexibility and can use fatty acids and amino acids as carbon sources.133 Patients with decompensated cirrhosis also exhibit increased blood levels of intermediates of the pentose phosphate pathway, which branches from glycolysis at the first committed step of glucose metabolism, suggesting that cytosolic glucose metabolism through alternative routes to glycolysis is also common in these patients130 (Fig. 3). The presence of increased plasma levels of acylcarnitines, which are bona fide markers of incomplete mitochondrial fatty acid oxidation, in patients with decompensated cirrhosis130 further supports the view that mitochondrial collapse (meaning a decrease in ATP production through OXPHOS) is a prevalent feature of decompensated cirrhosis.
Fig. 3. Metabolic alterations in decompensated cirrhosis.
(A) In decompensated cirrhosis and ACLF, systemic inflammation increases energy consumption and mitochondria become dysfunctional. ATP production in peripheral organs declines in favour of less effective glycolysis and ROS production leading to cell and tissue injury. In order to cope with increased energy demands lipolysis and proteolysis are accelerated, increasing circulating lipids and amino acids. Lipids act as immune stimulants further aggravating systemic inflammation. Glutaminase is central in generating ammonia from amino acids that arise from proteolysis and intestinal translocation. Hyperammonaemia leads to mitochondrial dysfunction and tissue injury, aggravates skeletal muscle loss, and impairs neutrophil function, facilitating secondary infections which aggravate systemic inflammation. (B) Under normal conditions immune cells obtain most of their energy (about 36 ATPs per molecule of glucose) from the citric acid cycle (TCA or Krebs cycle) and OXPHOS, which take place in the matrix and the inner membrane, respectively, of the mitochondria. The TCA cycle produces NADH and FADH2, which initiate OXPHOS for ATP synthesis through the ETC. (C) Once activated, immune cells dampen OXPHOS and shift ATP production to aerobic glycolysis. The metabolic reprogramming of activated immune cells also implies the induction of the pentose phosphate pathway, which branches from glycolysis at the first committed step and use nutrients to generate nucleotides and NADPH. Aerobic glycolysis, which ultimately produces lactate, is less efficient than OXPHOS in energy production and only generates 2 ATPs per glucose molecule. Impaired OXPHOS in activated immune cells might also result in the leaking of electrons at the ETC, leading to enhanced production of ROS. α-KG, α-ketoglutarate; ETC, electron transport chain; G6P, glucose-6-phosphate; OXPHOS, oxidative phosphorylation; ROS, reactive oxygen species; TCA, tricarboxylic acid.
The prevailing metabolic alteration in decompensated cirrhosis is also characterised by intense proteolysis and lipolysis, releasing large amounts of amino acids and fatty acids, respectively, as well as severe amino acid catabolism.130,134 It is important to note that levels of blood metabolites dramatically increased in decompensated cirrhosis and patients who developed ACLF.130 Similar catabolic processes are observed in other critical illnesses associated with systemic inflammation and multiorgan failure, such as sepsis or trauma,135 suggesting that the systemic hyperinflammatory state present in patients with decompensated cirrhosis is the cause of metabolic alterations, whose intensity correlates with plasma levels of inflammatory markers.130,136 This is consistent with the stimulatory role of inflammatory cytokines on the hypothalamic-pituitary-adrenal axis and subsequent production of glucocorticoids, which induce skeletal muscle catabolism and release of proteinogenic amino acids in blood during severe acute stress and inflammatory conditions.137,138 In addition, under these conditions, the sympathetic nervous system is activated to stimulate lipolysis in the adipose tissue, resulting in the release of fatty acids.135 The purpose of this coordinated neurohumoral response is to provide nutrients to the energy-consuming anabolic metabolism of the cells engaged in the immune response.135 It has been estimated that the overall cost of this hyperinflammatory state reaches up to 10,000 calories/day for a septic patient.135
Systemic inflammation, impaired OXPHOS, oxidative stress and cell death are linked to organ dysfunction
Since systemic inflammation is energetically expensive, requiring reallocation of stored nutrients to fuel immune activation, it competes for energy with other maintenance programmes, including those ensuring proper functioning of peripheral organs. To cope with this, an energetic trade-off between immune activation and organ function homeostasis may lead to peripheral organ hypo metabolism and organ dysfunction in decompensated cirrhosis. In addition, systemic inflammation impairs both ATP production through OXPHOS and the translocation of fatty acids into the mitochondria, which is required for fatty acid β-oxidation.135,137 Therefore, in ACLF, systemic inflammation directly disrupts mitochondrial metabolism, precluding appropriate mitochondrial energy production in peripheral organs, leading to altered physiological and biochemical functioning of the organ139 (Fig. 3).
Impaired OXPHOS is also linked to an enhanced production of reactive oxygen species (ROS).140 ROS, such as superoxide anion and hydrogen peroxide, are derived from the reduction of oxygen in the mitochondrial respiratory chain. Excessive ROS production causes oxidative damage to DNA, proteins, and lipid membranes (Fig. 3). If the antioxidant capacity is insufficient and/or the incurred damage is not efficiently repaired, ROS ultimately leads to apoptotic cell death, tissue injury and organ dysfunction which is the pathophysiological basis for ACLF development. Paradoxically, oxidative stress leads to apoptosis by further damaging mitochondrial respiratory chain transition and increasing inner mitochondrial membrane permeability,141 damages circular histone-free mitochondrial DNA (mtDNA),142 and can also be a source of host-derived DAMPs. In fact, dying apoptotic cells actively release ATP as a “find-me” signal to alert and activate the immune system.143 Moreover, leaking ATP from dying cells is an important stimulus for the NLRP3 inflammasome and cytokine release in the so-called “cytokine storm”.144 However, fragments of mtDNA released into the circulation by damaged cells and tissues act as DAMPs propagating damage from the initial site of injury to distant organs through TLR9mediated activation in immune cells145 or TLR4-driven tissue injury.40,100
Organ crosstalk through metabolic products
Accelerated lipolysis and proteolysis in progressing decompensated cirrhosis146 culminate in the loss of adipose tissue and skeletal muscle mass (sarcopenia) and the release of lipids and amino acids into the circulation. The catabolic state is associated with poor prognosis, risk of developing cirrhosis-related complications and different types of organ failure.147–149 The link between wasting, organ failure and ACLF development suggests a potential inter-organ crosstalk where active metabolic products are not only used as nutrients for excessive energy consumption but also as mediators for tissue injuring processes (Fig. 3).
The lipidomic landscape in decompensated cirrhosis
Lipids are not only recognised as essential cellular components and sources of energy, but also as signals for inter-organ communication, cell survival and most notably for shaping immune responses.150 Indeed, lipid metabolism and immune responses are highly integrated. Altered lipid composition interferes with immune regulation leading to immune-metabolic dysregulation and uncontrolled inflammation, which is the basis for developing acute decompensation and ACLF.150 In addition, lipids interact with TLRs to trigger inflammation and several lipid derivatives are important inflammatory mediators that act as intracellular signalling molecules.151 In the context of advanced liver disease, variations in the serum levels of ceramides are associated with hepatic decompensation and survival in patients with cirrhosis,152 and low levels of sphingosine-1-phosphate are predictive of increased mortality in these patients.153 Along these lines, dysregulated formation of complex sphingolipids is characteristic of malnutrition in hospitalised patients with decompensated cirrhosis154 and circulating extracellular vesicles carrying sphingolipid cargo have diagnostic and prognostic power for decompensated alcohol-related cirrhosis155 (Fig. 3).
Consistent with the idea of lipids being involved in central pathomechanistic processes; increased fatty acid levels are a constant feature of decompensated cirrhosis.130 However, among the fatty acid repertoire, the total pool of polyunsaturated fatty acids (PUFAs) (including free as well as those esterified in triglycerides, phospholipids and cholesterol esters) is invariably reduced in patients with acute decompensation.156 This suppression is not seen when the analysis only considers the free circulating PUFAs and not the total content, which more accurately represents the actual PUFA pool in the circulation.157 Furthermore, these patients exhibit a clear imbalance between omega-6 and omega-3 PUFA families (higher arachidonic acid [AA, omega-6] to eicosapentaenoic acid [EPA, omega-3] ratio).156 This imbalance has a pathophysiological relevance since AA is the precursor of potent inflammatory lipid mediators (eicosanoids), whereas EPA is the precursor of anti-inflammatory and pro-resolving lipid mediators.158 Indeed, increased levels of leukotriene E4 (derived from AA) in parallel with reduced levels of the proresolving lipid mediator lipoxin A5 (derived from EPA) compose a specific lipid mediator signature for decompensated cirrhosis that dynamically follows the risk and severity of the disease.156 Conversely, AA-derived prostaglandin E2, which has been linked to immunosuppresion in cirrhosis,159 is moderately increased in patients with decompensated cirrhosis but is not associated with the presence or the risk of developing infections in these patients (Fig. 3).
Muscle and organ crosstalk through ammonia
Circulating amino acids and intestinal bacterial products are a source of circulating ammonia, which is elevated in decompensation and ACLF.160 Ammonia is generated directly from amino acids through phosphate-activated glutaminase, which catalyse the breakdown from glutamine to glutamate and ammonia161 and indeed, enterocytes’ glutaminase activity is increased 4-fold in cirrhosis.162 Ammonia removal from the (portovenous) blood occurs through the urea cycle located in the periportal hepatocytes, which detoxifies ammonia to urea, and glutamine synthetase (GS), located in the perivenous hepatocytes, which converts glutamate and ammonia to glutamine. Portosystemic shunts and reduced activity of detoxification pathways diminish the liver’s ammonia removal capacities, thus causing hyperammonaemia.163,164 The kidneys, another important regulator of ammonia metabolism, can switch between net ammonia excretion and production depending on plasma levels, renal function, acid base balance and renin-angiotensin-aldosterone system activation.165 In response to hyperammonaemia, the kidneys initially increase their ammonia excretion, but worsening disease severity, decreasing kidney function and prolonged disease duration reverse their function to ammonia production.165 Consequently, ammonia-regulating pathways in other organs face increasing demands. Skeletal muscles contain substantial amounts of GS and its large organ mass determines that in healthy individuals more than 50% of ammonia removal occurs through muscle GS.166 In acute liver failure, GS activity in the muscle can compensate to control ammonia levels by increasing ammonia uptake and stoichiometric release of glutamine.167 However, patients with cirrhosis and sarcopenia are prone to having high ammonia levels possibly as a consequence of reduced skeletal muscle mass.168 In line with this, after transjugular intrahepatic portosystemic shunt implantation in patients with cirrhosis, muscle GS is the predominant ammonia removing pathway, but this observation was limited to patients with normal muscle mass.169,170
In healthy individuals, ammonia plasma concentrations are tightly regulated within a range of 10–40 μmol/L to avoid ammonia exerting its detrimental effect as a general cell toxin, which alters membrane potentials and pH and generates free radicals.165,171 Consequently, alterations of cellular metabolism and protein function (post-translational modification) aggravate mitochondrial dysfunction and impair ATP generation, which culminates in enhanced tissue injury and organ dysfunction.172
The clinical relevance of hyperammonaemia and its effect on organ injury and disease progression in cirrhosis has been described on multiple levels. It has been known for some time that hyperammonaemia causes hepatic encephalopathy.173 GS in astrocytes detoxifies ammonia to glutamine that in turn causes astrocyte swelling and brain oedema.174 Its impact on brain function is further aggravated by oxidative stress. glycolysis and endothelial dysfunction leading to increased brain lactate levels and impaired cerebral perfusion.175
Circulating ammonia levels in cirrhosis also correlate with death and other organ failures,176,177 implying that the detrimental effect of hyperammonaemia goes beyond the development of hepatic encephalopathy. Indeed, hyperammonaemia initiates chronic liver injury,172 aggravates portal pressure by stellate cell contraction178 and causes structural and functional renal impairment.179,180
Further emphasis was put on the immune system, which also suffers from ammonia-induced functional alterations. Neutrophil’s phagocytic incapacity is linked to hyperammonaemia and activation of the protein kinase (p38(MAPK)) pathway.181 This observation may reveal a direct link between ammonia and susceptibility to secondary infections, which have the potential to exaggerate systemic inflammation and propel disease progression (Fig. 3).
Therefore, the current understanding points towards ammonia as a cell toxin involved in mediating tissue injury and aggravating immune dysfunction, thereby perpetuating pathomechanistic features of decompensated cirrhosis and ACLF.
The current pathophysiological paradigm and its controversies
Clinical and pathophysiological complexity is the most intriguing feature of decompensated cirrhosis, fostering our scientific engagement to explore and understand this particular disease. Linking disease phenotypes with pathomechanistic alterations provides the basis for the development of targeted treatment strategies with the final goal of tailoring therapeutic interventions to individual needs. Apart from a fundamental understanding of disease processes, this will rely on reliable biomarkers, which not only predict patient outcome, but also reliably describe the composition of activated pathways in every individual. Currently, bringing traditional and novel paradigms together allows us to identify existing controversies and, thus, areas for future research.
PH is central in initiating several cardiovascular alterations, which are involved in the transition from compensated to decompensated liver disease. Splanchnic systemic vasodilation is responsible for systemic under filling and subsequent activation of neurohumoral pathways to maintain cardiocirculatory function. In more advanced stages, especially after exposure to precipitating events, such as bacterial infections, compensatory mechanisms also fail because systemic inflammation becomes more dominant. Ascites and hypernatremia occur due to avid renal sodium and water retention. Increasing vasoconstriction and cardiomyopathy culminate in a hyperdynamic state with microvascular dysfunction and reduced organ perfusion, which is one link between cardiovascular dysfunction, disease progression to ACLF and extrahepatic organ failure. Importantly, the activated immune system plays a leading role, which not only affects various disease mechanisms but also creates the bridge between cardiovascular alterations, which mainly initiate the development of cirrhosis-related complications, and metabolic/molecular pathways, which predominantly mediate tissue injury and organ dysfunction. An overwhelming activation of the immune system can directly induce tissue injury by activating pro-inflammatory pathways but also shifts the balance of energy consumption from peripheral organs to the immune system. Consequently, parenchymal cells are forced to alter their metabolic activity to maintain their energy provision by enhancing proteolysis and lipolysis. Mitochondrial dysfunction and impaired ATP production are characteristic and together with oxidative stress may trigger cell death and subsequently organ dysfunction. PAMPs and DAMPs – released by dying cells and intestinal bacterial translocation – perpetuate systemic inflammation and maintain this process in a pathomechanistic spiral (Fig. 4).
Fig. 4. The pathophysiological paradigm of decompensated cirrhosis.
Disease progression from compensated cirrhosis to acute decompensation and acute-on-chronic liver failure is characterised by the development of cirrhosis-related complications (bacterial infections, variceal bleeding, ascites and HE) and tissue injury leading to extrahepatic organ injury. The transition from compensation to decompensation is dominated by cardiocirculatory dysfunction, whereas organ failure relates mainly back to metabolic alterations with microvascular dysfunction and reduced organ perfusion representing one potential link between both. Systemic inflammation initiated by precipitating events, as well as PAMP and DAMP release, becomes a superior modulator of disease processes but also directly induces tissue injury. Feedback mechanisms perpetuate systemic inflammation and culminate in a pathomechanistic spiral. DAMP, damage-associated molecular pattern; HE, hepatic encephalopathy; PAMP, pathogen-associated molecular pattern.
Despite already having a clear understanding of how patients might progress from compensated cirrhosis to death, controversies in all fields still exist and require further scientific exploration. It remains unclear whether inflammation might alter PH as a driver of some complications, such as portal hypertensive bleeding. Furthermore, ATP was described as being insufficiently produced in the intracellular space and thereby leading to mitochondrial dysfunction. On the contrary, high levels of extracellular ATP released by dying cells might have a detrimental effect by activating the inflammasome. In addition, the hepatorenal syndrome has long been perceived as a purely functional alteration of renal function due to reduced perfusion but recent findings indicate that structural changes play a role. Therefore, it might be important to redefine the disease itself and its therapeutic approaches.
Beyond that, broader and more substantial questions must be addressed in order to fill the current gaps, especially to predict the development of certain complications and apply interventions early in the disease course. We need to understand how acute decompensation and ACLF are triggered when no precipitating event can be identified. We should explore the mechanisms which determine the kind of organ failure every patient develops. Finally, we must focus on describing regeneration and its link to inflammation and how modulation of both can affect disease progression.
A fundamental understanding of disease processes will be key to improving patient outcomes and quality of life in this difficult to treat population.
Supplementary Material
Key point.
ACLF is associated with a poor prognosis, limited therapeutic options and incompletely understood pathomechanisms.
Cardiovascular alterations including portal hypertension trigger the formation of portocaval shunts and varices.
Systemic under filling and arterial hypotension is compensated by vasoconstriction but might decline into a state of aggravated portal hypertension and cirrhotic cardiomyopathy, leading to a hyperdynamic state, microvascular dysfunction and reduced organ perfusion culminating in decompensation.
The immune system is dysfunctional showing a contrary co-existence of immune paralysis and immune overstimulation leading to secondary infections and inflammatory response syndrome aggravating cardiovascular alterations but also initiating tissue injury and metabolic alteration.
Mitochondrial dysfunction, impaired ATP production and oxidative stress are major mediators of cell death.
High energy consumption causes fat and muscle degradation that releases immune stimulating lipids and amino acids and serving as a source of hyperammonaemia, which may aggravate organ injury.
Acknowledgements
We would like to thank Laura A. Kehoe for her excellent English language editing.
Financial support
Cornelius Engelmann is part funded by Berlin Institute of Health (BIH) Joan Clària’s laboratory is supported by the Spanish Ministerio de Ciencia e Innovacion (PID2019-105240RB-I00) and the European Union’s Horizon 2020 research and innovation program (grant agreements N° 825694 and 847949).
Conflict of interest
Cornelius Engelmann has on-going research collaboration with Merz Pharmaceutical and Novartis. He has received speaker fees from Novartis, Gilead and Merz Pharmaceuticals. Jaime Bosch received consultancy fees from Actelion, Ambys, BioVie, BLB, BMS, Brudy, Chiasma, Exalenz, Gilead, Lipocine, Surrozen, Zydus and speaker fees from Gore. Mauro Bernardi received consultancy fees from CSL Behring GmbH, Grifols SA, Martin Pharmaceuticals and speaker fees from CSL Behring GmbH, Grifols SA, Takeda, PPTA, Octapharma AG. All other authors declared no conflict of interest.
Abbreviations
- AA
arachidonic acid
- ACLF
acute-on-chronic liver failure
- AKI
acute kidney injury
- ATN
acute tubular necrosis
- CSPH
clinically significant portal hypertension
- DAMP
damage-associated molecular patterns
- EPA
eicosapentaenoic acid
- eNOS
endothelial nitric oxide synthase
- GS
glutamine synthetase
- HNA
human non-mercaptan-albumin
- HSC
hepatic stellate cell
- HRS
hepatorenal syndrome
- HVPG
hepatic venous pressure gradient
- IL-
interleukin
- iNOS
inducible nitric oxide synthase
- LPS
lipopolysaccharide
- mtDNA
mitochondrial DNA
- NO
nitric oxide
- OXPHOS
oxidative phosphorylation
- PAMP
pathogen-associated molecular patterns
- PH
portal hypertension
- PUFA
polyunsaturated fatty acid
- ROS
reactive oxygen species
- SBP
spontaneous bacterial peritonitis
- TLR
Toll-like receptor
- TNFα
tumour necrosis factor-α
- VEGF(R)
vascular endothelial growth factor (receptor)
Footnotes
Please refer to the accompanying ICMJE disclosure forms for further details.
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhep.2021.01.002.
Transparency declaration
This article is published as part of a supplement entitled New Concepts and Perspectives in Decompensated Cirrhosis. Publication of the supplement was supported financially by CSL Behring. The sponsor had no involvement in content development, the decision to submit the manuscript or in the acceptance of the manuscript for publication.
References
- [1].Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 2013;144. 1426–1437, 1437 e1421–1429. [DOI] [PubMed] [Google Scholar]
- [2].Arroyo V, Moreau R, Jalan R. Acute-on-Chronic liver failure. N Engl J Med 2020;382:2137–2145. [DOI] [PubMed] [Google Scholar]
- [3].Gustot T, Fernandez J, Garcia E, Morando F, Caraceni P, Alessandria C, et al. Clinical Course of acute-on-chronic liver failure syndrome and effects on prognosis. Hepatology 2015;62:243–252. [DOI] [PubMed] [Google Scholar]
- [4].Engelmann C, Thomsen KL, Zakeri N, Sheikh M, Agarwal B, Jalan R, et al. Validation of CLIF-C ACLF score to define a threshold for futility of intensive care support for patients with acute-on-chronic liver failure. Crit Care 2018;22:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Trebicka J, Fernandez J, Papp M, Caraceni P, Laleman W, Gambino C, et al. The PREDICT study uncovers three clinical courses of acutely decompensated cirrhosis that have distinct pathophysiology. J Hepatol 2020;73(4):842–854. [DOI] [PubMed] [Google Scholar]
- [6].Berzigotti A. Advances and challenges in cirrhosis and portal hypertension. BMC Med 2017;15:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bernardi M, Caraceni P. Novel perspectives in the management of decompensated cirrhosis. Nat Rev Gastroenterol Hepatol 2018;15:753–764. [DOI] [PubMed] [Google Scholar]
- [8].Garcia-Tsao G, Bosch J. Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med 2010;362:823–832. [DOI] [PubMed] [Google Scholar]
- [9].Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology 2017;65:310–335. [DOI] [PubMed] [Google Scholar]
- [10].D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol 2006;44:217–231. [DOI] [PubMed] [Google Scholar]
- [11].Ripoll C, Groszmann R, Garcia-Tsao G, Grace N, Burroughs A, Planas R, et al. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology 2007;133:481–488. [DOI] [PubMed] [Google Scholar]
- [12].Bosch J, Groszmann RJ, Shah VH. Evolution in the understanding of the pathophysiological basis of portal hypertension: how changes in paradigm are leading to successful new treatments. J Hepatol 2015;62:S121–S130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bosch J, Berzigotti A, Garcia-Pagan JC, Abraldes JG. The management of portal hypertension: rational basis, available treatments and future options. J Hepatol 2008;48(Suppl 1):S68–S92. [DOI] [PubMed] [Google Scholar]
- [14].Cerini F, Vilaseca M, Lafoz E, Garcia-Irigoyen O, Garcia-Caldero H, Tripathi DM, et al. Enoxaparin reduces hepatic vascular resistance and portal pressure in cirrhotic rats. J Hepatol 2016;64:834–842. [DOI] [PubMed] [Google Scholar]
- [15].Gracia-Sancho J, Marrone G, Fernandez-Iglesias A. Hepatic microcirculation and mechanisms of portal hypertension. Nat Rev Gastroenterol Hepatol 2019;16:221–234. [DOI] [PubMed] [Google Scholar]
- [16].Gracia-Sancho J, Russo L, Garcia-Caldero H, Garcia-Pagan JC, Garcia-Cardena G, Bosch J. Endothelial expression of transcription factor Kruppel-like factor 2 and its vasoprotective target genes in the normal and cirrhotic rat liver. Gut 2011;60:517–524. [DOI] [PubMed] [Google Scholar]
- [17].Navasa M, Chesta J, Bosch J, Rodes J. Reduction of portal pressure by isosorbide-5-mononitrate in patients with cirrhosis. Effects on splanchnic and systemic hemodynamics and liver function. Gastroenterology 1989;96:1110–1118. [DOI] [PubMed] [Google Scholar]
- [18].Bellis L, Berzigotti A, Abraldes JG, Moitinho E, Garcia-Pagan JC, Bosch J, et al. Low doses of isosorbide mononitrate attenuate the postprandial increase in portal pressure in patients with cirrhosis. Hepatology 2003;37:378–384. [DOI] [PubMed] [Google Scholar]
- [19].Albillos A, Lledo JL, Rossi I, Perez-Paramo M, Tabuenca MJ, Banares R, et al. Continuous prazosin administration in cirrhotic patients: effects on portal hemodynamics and on liver and renal function. Gastroenterology 1995;109:1257–1265. [DOI] [PubMed] [Google Scholar]
- [20].Bosch J, Gracia-Sancho J, Abraldes JG. Cirrhosis as new indication for statins. Gut 2020;69:953–962. [DOI] [PubMed] [Google Scholar]
- [21].Schwabl P, Brusilovskaya K, Supper P, Bauer D, Konigshofer P, Riedl F, et al. The soluble guanylate cyclase stimulator riociguat reduces fibrogenesis and portal pressure in cirrhotic rats. Sci Rep 2018;8:9372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Trebicka J, Hennenberg M, Laleman W, Shelest N, Biecker E, Schepke M, et al. Atorvastatin lowers portal pressure in cirrhotic rats by inhibition of RhoA/Rho-kinase and activation of endothelial nitric oxide synthase. Hepatology 2007;46:242–253. [DOI] [PubMed] [Google Scholar]
- [23].Marrone G, Maeso-Diaz R, Garcia-Cardena G, Abraldes JG, Garcia-Pagan JC, Bosch J, et al. KLF2 exerts antifibrotic and vasoprotective effects in cirrhotic rat livers: behind the molecular mechanisms of statins. Gut 2015;64:1434–1443. [DOI] [PubMed] [Google Scholar]
- [24].Abraldes JG, Villanueva C, Aracil C, Turnes J, Hernandez-Guerra M, Genesca J, et al. Addition of simvastatin to standard therapy for the prevention of variceal rebleeding does not reduce rebleeding but increases survival in patients with cirrhosis. Gastroenterology 2016;150:1160–1170. e1163. [DOI] [PubMed] [Google Scholar]
- [25].Tripathi DM, Vilaseca M, Lafoz E, Garcia-Caldero H, Viegas Haute G, Fernandez-Iglesias A, et al. Simvastatin prevents progression of acute on chronic liver failure in rats with cirrhosis and portal hypertension. Gastroenterology 2018;155:1564–1577. [DOI] [PubMed] [Google Scholar]
- [26].Guixe-Muntet S, Ortega-Ribera M, Wang C, Selicean S, Andreu I, Kechagia JZ, et al. Nuclear deformation mediates liver cell mechano-sensing in cirrhosis. JHEP Rep 2020;2:100145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lautt WW, Legare DJ, Ezzat WR. Quantitation of the hepatic arterial buffer response to graded changes in portal blood flow. Gastroenterology 1990;98:1024–1028. [DOI] [PubMed] [Google Scholar]
- [28].Nair H, Berzigotti A, Bosch J. Emerging therapies for portal hypertension in cirrhosis. Expert Opin Emerg Drugs 2016;21:167–181. [DOI] [PubMed] [Google Scholar]
- [29].Wiest R, Groszmann RJ. The paradox of nitric oxide in cirrhosis and portal hypertension: too much, not enough. Hepatology 2002;35:478–491. [DOI] [PubMed] [Google Scholar]
- [30].Pizcueta P, Pique JM, Fernandez M, Bosch J, Rodes J, Whittle BJ, et al. Modulation of the hyperdynamic circulation of cirrhotic rats by nitric oxide inhibition. Gastroenterology 1992;103:1909–1915. [DOI] [PubMed] [Google Scholar]
- [31].Villanueva C, Albillos A, Genesca J, Abraldes JG, Calleja JL, Aracil C, et al. Development of hyperdynamic circulation and response to beta-blockers in compensated cirrhosis with portal hypertension. Hepatology 2016;63:197–206. [DOI] [PubMed] [Google Scholar]
- [32].Fernandez M, Mejias M, Garcia-Pras E, Mendez R, Garcia-Pagan JC, Bosch J. Reversal of portal hypertension and hyperdynamic splanchnic circulation by combined vascular endothelial growth factor and platelet-derived growth factor blockade in rats. Hepatology 2007;46:1208–1217. [DOI] [PubMed] [Google Scholar]
- [33].Fernandez M, Vizzutti F, Garcia-Pagan JC, Rodes J, Bosch J. Anti-VEGF receptor-2 monoclonal antibody prevents portal-systemic collateral vessel formation in portal hypertensive mice. Gastroenterology 2004;126:886–894. [DOI] [PubMed] [Google Scholar]
- [34].Fernandez M. Molecular pathophysiology of portal hypertension. Hepatology 2015;61:1406–1415. [DOI] [PubMed] [Google Scholar]
- [35].Mejias M, Coch L, Berzigotti A, Garcia-Pras E, Gallego J, Bosch J, et al. Antiangiogenic and antifibrogenic activity of pigment epithelium-derived factor (PEDF) in bile duct-ligated portal hypertensive rats. Gut 2015;64:657–666. [DOI] [PubMed] [Google Scholar]
- [36].Coch L, Mejias M, Berzigotti A, Garcia-Pras E, Gallego J, Bosch J, et al. Disruption of negative feedback loop between vasohibin-1 and vascular endothelial growth factor decreases portal pressure, angiogenesis, and fibrosis in cirrhotic rats. Hepatology 2014;60:633–647. [DOI] [PubMed] [Google Scholar]
- [37].Heishi T, Hosaka T, Suzuki Y, Miyashita H, Oike Y, Takahashi T, et al. Endogenous angiogenesis inhibitor vasohibin1 exhibits broad-spectrum antilymphangiogenic activity and suppresses lymph node metastasis. Am J Pathol 2010;176:1950–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Vespasiani-Gentilucci U, Rombouts K. Boosting pigment epithelial-derived factor: a promising approach for the treatment of early portal hypertension. Gut 2015;64:523–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Jalan R, Saliba F, Pavesi M, Amoros A, Moreau R, Gines P, et al. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J Hepatol 2014;61:1038–1047. [DOI] [PubMed] [Google Scholar]
- [40].Engelmann C, Sheikh M, Sharma S, Kondo T, Loeffler-Wirth H, Zheng YB, et al. Toll-like receptor 4 is a therapeutic target for prevention and treatment of liver failure. J Hepatol 2020;73(1):102–112. [DOI] [PubMed] [Google Scholar]
- [41].Bellot P, Garcia-Pagan JC, Frances R, Abraldes JG, Navasa M, Perez-Mateo M, et al. Bacterial DNA translocation is associated with systemic circulatory abnormalities and intrahepatic endothelial dysfunction in patients with cirrhosis. Hepatology 2010;52:2044–2052. [DOI] [PubMed] [Google Scholar]
- [42].Gonzalez-Navajas JM, Bellot P, Frances R, Zapater P, Munoz C, Garcia-Pagan JC, et al. Presence of bacterial-DNA in cirrhosis identifies a subgroup of patients with marked inflammatory response not related to endotoxin. J Hepatol 2008;48:61–67. [DOI] [PubMed] [Google Scholar]
- [43].Fernandez J, Navasa M, Garcia-Pagan JC, Jimenez W, Bosch J, et al. Effect of intravenous albumin on systemic and hepatic hemodynamics and vasoactive neurohormonal systems in patients with cirrhosis and spontaneous bacterial peritonitis. J Hepatol 2004;41:384–390. [DOI] [PubMed] [Google Scholar]
- [44].Mendoza YP, Rodrigues SG, Bosch J, Berzigotti A. Effect of poorly absorbable antibiotics on hepatic venous pressure gradient in cirrhosis: a systematic review and meta-analysis. Dig Liver Dis 2020;52:958–965. [DOI] [PubMed] [Google Scholar]
- [45].Turco L, Garcia-Tsao G, Magnani I, Bianchini M, Costetti M, Caporali C, et al. Cardiopulmonary hemodynamics and C-reactive protein as prognostic indicators in compensated and decompensated cirrhosis. J Hepatol 2018;68:949–958. [DOI] [PubMed] [Google Scholar]
- [46].Berzigotti A, Garcia-Tsao G, Bosch J, Grace ND, Burroughs AK, Morillas R, et al. Obesity is an independent risk factor for clinical decompensation in patients with cirrhosis. Hepatology 2011;54:555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Berzigotti A, Albillos A, Villanueva C, Genesca J, Ardevol A, Augustin S, et al. Effects of an intensive lifestyle intervention program on portal hypertension in patients with cirrhosis and obesity: the SportDiet study. Hepatology 2017;65:1293–1305. [DOI] [PubMed] [Google Scholar]
- [48].Schrier RW, Arroyo V, Bernardi M, Epstein M, Henriksen JH, Rodes J. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology 1988;8:1151–1157. [DOI] [PubMed] [Google Scholar]
- [49].Iwakiri Y, Groszmann RJ. The hyperdynamic circulation of chronic liver diseases: from the patient to the molecule. Hepatology 2006;43:S121–S131. [DOI] [PubMed] [Google Scholar]
- [50].Caraceni P, Dazzani F, Salizzoni E, Domenicali M, Zambruni A, Trevisani F, et al. Muscle circulation contributes to hyperdynamic circulatory syndrome in advanced cirrhosis. J Hepatol 2008;48:559–566. [DOI] [PubMed] [Google Scholar]
- [51].Rodriguez-Roisin R, Krowka MJ. Hepatopulmonary syndrome–a liver-induced lung vascular disorder. N Engl J Med 2008;358:2378–2387. [DOI] [PubMed] [Google Scholar]
- [52].Arroyo V, Bernardi M, Epstein M, Henriksen JH, Schrier RW, Rodes J. Pathophysiology of ascites and functional renal failure in cirrhosis. J Hepatol 1988;6:239–257. [DOI] [PubMed] [Google Scholar]
- [53].Nazar A, Guevara M, Sitges M, Terra C, Sola E, Guigou C, et al. LEFT ventricular function assessed by echocardiography in cirrhosis: relationship to systemic hemodynamics and renal dysfunction. J Hepatol 2013;58:51–57. [DOI] [PubMed] [Google Scholar]
- [54].Angeli P, Garcia-Tsao G, Nadim MK, Parikh CR. News in pathophysiology, definition and classification of hepatorenal syndrome: a step beyond the International Club of Ascites (ICA) consensus document. J Hepatol 2019;71:811–822. [DOI] [PubMed] [Google Scholar]
- [55].Ruiz-del-Arbol L, Monescillo A, Arocena C, Valer P, Gines P, Moreira V, et al. Circulatory function and hepatorenal syndrome in cirrhosis. Hepatology 2005;42:439–447. [DOI] [PubMed] [Google Scholar]
- [56].Zambruni A, Trevisani F, Caraceni P, Bernardi M. Cardiac electrophysiological abnormalities in patients with cirrhosis. J Hepatol 2006;44:994–1002. [DOI] [PubMed] [Google Scholar]
- [57].Davies T, Wythe S, O’Beirne J, Martin D, Gilbert-Kawai E. Review article: the role of the microcirculation in liver cirrhosis. Aliment Pharmacol Ther 2017;46:825–835. [DOI] [PubMed] [Google Scholar]
- [58].Sheikh MY, Javed U, Singh J, Choudhury J, Deen O, Dhah K, et al. Bedside sublingual video imaging of microcirculation in assessing bacterial infection in cirrhosis. Dig Dis Sci 2009;54:2706–2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Thomson SJ, Cowan ML, Forton DM, Clark SJ, Musa S, Grounds M, et al. A study of muscle tissue oxygenation and peripheral microcirculatory dysfunction in cirrhosis using near infrared spectroscopy. Liver Int 2010;30:463–471. [DOI] [PubMed] [Google Scholar]
- [60].Seino Y, Ohki K, Nakamura T, Tsukamoto H, Takano T, Aramaki T, et al. Pathophysiological characteristics of cutaneous microcirculation in patients with liver cirrhosis: relationships to cardiovascular hemodynamics and plasma neurohormonal factors. Microvasc Res 1993;46:206–215. [DOI] [PubMed] [Google Scholar]
- [61].Berzigotti A, Erice E, Gilabert R, Reverter E, Abraldes JG, Garcia-Pagan JC, et al. Cardiovascular risk factors and systemic endothelial function in patients with cirrhosis. Am J Gastroenterol 2013;108:75–82. [DOI] [PubMed] [Google Scholar]
- [62].Sakr Y, Dubois MJ, De Backer D, Creteur J, Vincent JL. Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit Care Med 2004;32:1825–1831. [DOI] [PubMed] [Google Scholar]
- [63].De Backer D, Donadello K, Sakr Y, Ospina-Tascon G, Salgado D, Scolletta S, et al. Microcirculatory alterations in patients with severe sepsis: impact of time of assessment and relationship with outcome. Crit Care Med 2013;41:791–799. [DOI] [PubMed] [Google Scholar]
- [64].Brito-Azevedo A, Perez RM, Maranhao PA, Coelho HS, Fernandes ESM, Castiglione RC, et al. Organ dysfunction in cirrhosis: a mechanism involving the microcirculation. Eur J Gastroenterol Hepatol 2019;31:618–625. [DOI] [PubMed] [Google Scholar]
- [65].Wiest R, Cadelina G, Milstien S, McCuskey RS, Garcia-Tsao G, Groszmann RJ. Bacterial translocation up-regulates GTP-cyclohydrolase I in mesenteric vasculature of cirrhotic rats. Hepatology 2003;38:1508–1515. [DOI] [PubMed] [Google Scholar]
- [66].Tazi KA, Moreau R, Herve P, Dauvergne A, Cazals-Hatem D, Bert F, et al. Norfloxacin reduces aortic NO synthases and pro-inflammatory cytokine up-regulation in cirrhotic rats: role of Akt signaling. Gastroenterology 2005;129:303–314. [DOI] [PubMed] [Google Scholar]
- [67].Pozzi M, Carugo S, Boari G, Pecci V, de Ceglia S, Maggiolini S, et al. Evidence of functional and structural cardiac abnormalities in cirrhotic patients with and without ascites. Hepatology 1997;26:1131–1137. [DOI] [PubMed] [Google Scholar]
- [68].Bortoluzzi A, Ceolotto G, Gola E, Sticca A, Bova S, Morando F, et al. Positive cardiac inotropic effect of albumin infusion in rodents with cirrhosis and ascites: molecular mechanisms. Hepatology 2013;57:266–276. [DOI] [PubMed] [Google Scholar]
- [69].Praktiknjo M, Monteiro S, Grandt J, Kimer N, Madsen JL, Werge MP, et al. Cardiodynamic state is associated with systemic inflammation and fatal acute-on-chronic liver failure. Liver Int 2020;40:1457–1466. [DOI] [PubMed] [Google Scholar]
- [70].Yotti R, Ripoll C, Benito Y, Catalina MV, Elizaga J, Rincon D, et al. Left ventricular systolic function is associated with sympathetic nervous activity and markers of inflammation in cirrhosis. Hepatology 2017;65:2019–2030. [DOI] [PubMed] [Google Scholar]
- [71].Tellez L, Ibanez-Samaniego L, Perez Del Villar C, Yotti R, Martinez J, Carrion L, et al. Non-selective beta-blockers impair global circulatory homeostasis and renal function in cirrhotic patients with refractory ascites. J Hepatol 2020;73:1404–1414. [DOI] [PubMed] [Google Scholar]
- [72].Gines P, Berl T, Bernardi M, Bichet DG, Hamon G, Jimenez W, et al. Hyponatremia in cirrhosis: from pathogenesis to treatment. Hepatology 1998;28:851–864. [DOI] [PubMed] [Google Scholar]
- [73].Peter T. All About Albumin: Biochemistry, Genetics and Medical Applications. Academic Press Inc; 1996. [Google Scholar]
- [74].Salerno F, Borroni G, Moser P, Badalamenti S, Cassara L, Maggi A, et al. Survival and prognostic factors of cirrhotic patients with ascites: a study of 134 outpatients. Am J Gastroenterol 1993;88:514–519. [PubMed] [Google Scholar]
- [75].Henriksen JH, Stage JG, Schlichting P, Winkler K. Intraperitoneal pressure: ascitic fluid and splanchnic vascular pressures, and their role in prevention and formation of ascites. Scand J Clin Lab Invest 1980;40:493–501. [DOI] [PubMed] [Google Scholar]
- [76].Haussinger D, Schliess F. Pathogenetic mechanisms of hepatic encephalopathy. Gut 2008;57:1156–1165. [DOI] [PubMed] [Google Scholar]
- [77].Jimenez W, Rodes J. Impaired responsiveness to endogenous vasoconstrictors and endothelium-derived vasoactive factors in cirrhosis. Gastroenterology 1994;107:1201–1203. [DOI] [PubMed] [Google Scholar]
- [78].Thenappan T, Goel A, Marsboom G, Fang YH, Toth PT, Zhang HJ, et al. A central role for CD68(+) macrophages in hepatopulmonary syndrome. Reversal by macrophage depletion. Am J Respir Crit Care Med 2011;183:1080–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Gomez H, Ince C, De Backer D, Pickkers P, Payen D, Hotchkiss J, et al. A unified theory of sepsis-induced acute kidney injury: inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock 2014;41:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Shah N, Mohamed FE, Jover-Cobos M, Macnaughtan J, Davies N, Moreau R, et al. Increased renal expression and urinary excretion of TLR4 in acute kidney injury associated with cirrhosis. Liver Int 2013;33:398–409. [DOI] [PubMed] [Google Scholar]
- [81].Shah N, Dhar D, El Zahraa Mohammed F, Habtesion A, Davies NA, Jover-Cobos M, et al. Prevention of acute kidney injury in a rodent model of cirrhosis following selective gut decontamination is associated with reduced renal TLR4 expression. J Hepatol 2012;56:1047–1053. [DOI] [PubMed] [Google Scholar]
- [82].Trawale JM, Paradis V, Rautou PE, Francoz C, Escolano S, Sallee M, et al. The spectrum of renal lesions in patients with cirrhosis: a clinicopathological study. Liver Int 2010;30:725–732. [DOI] [PubMed] [Google Scholar]
- [83].Arroyo V, Angeli P, Moreau R, Jalan R, Claria J, Trebicka J, et al. The systemic inflammation hypothesis: towards a new paradigm of acute decompensation and multiorgan failure in cirrhosis. J Hepatol 2020. 10.1016/j.jhep.2020.11.048. In press. [DOI] [PubMed]
- [84].Claria J, Stauber RE, Coenraad MJ, Moreau R, Jalan R, Pavesi M, et al. Systemic inflammation in decompensated cirrhosis: characterization and role in acute-on-chronic liver failure. Hepatology 2016;64:1249–1264. [DOI] [PubMed] [Google Scholar]
- [85].Tilg H, Wilmer A, Vogel W, Herold M, Nolchen B, Judmaier G, et al. Serum levels of cytokines in chronic liver diseases. Gastroenterology 1992;103:264–274. [DOI] [PubMed] [Google Scholar]
- [86].Betrapally NS, Gillevet PM, Bajaj JS. Gut microbiome and liver disease. Transl Res 2017;179:49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Dubinkina VB, Tyakht AV, Odintsova VY, Yarygin KS, Kovarsky BA, Pavlenko AV, et al. Links of gut microbiota composition with alcohol dependence syndrome and alcoholic liver disease. Microbiome 2017;5:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Lachar J, Bajaj JS. Changes in the microbiome in cirrhosis and relationship to complications: hepatic encephalopathy, spontaneous bacterial peritonitis, and sepsis. Semin Liver Dis 2016;36:327–330. [DOI] [PubMed] [Google Scholar]
- [89].Shao L, Ling Z, Chen D, Liu Y, Yang F, Li L. Disorganized gut microbiome contributed to liver cirrhosis progression: a meta-omics-based study. Front Microbiol 2018;9:3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Ye J, Lv L, Wu W, Li Y, Shi D, Fang D, et al. Butyrate protects mice against methionine-choline-deficient diet-induced non-alcoholic steatohepatitis by improving gut barrier function, attenuating inflammation and reducing endotoxin levels. Front Microbiol 2018;9:1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Qin N, Yang F, Li A, Prifti E, Chen Y, Shao L, et al. Alterations of the human gut microbiome in liver cirrhosis. Nature 2014;513:59–64. [DOI] [PubMed] [Google Scholar]
- [92].Albillos A, de Gottardi A, Rescigno M. The gut-liver axis in liver disease: pathophysiological basis for therapy. J Hepatol 2020;72:558–577. [DOI] [PubMed] [Google Scholar]
- [93].Macdonald S, Andreola F, Bachtiger P, Amoros A, Pavesi M, Mookerjee R, et al. Cell death markers in patients with cirrhosis and acute decompensation. Hepatology 2018;67:989–1002. [DOI] [PubMed] [Google Scholar]
- [94].Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol 2016;16:407–420. [DOI] [PubMed] [Google Scholar]
- [95].Szabo G, Petrasek J. Inflammasome activation and function in liver disease. Nat Rev Gastroenterol Hepatol 2015;12:387–400. [DOI] [PubMed] [Google Scholar]
- [96].Wree A, Eguchi A, McGeough MD, Pena CA, Johnson CD, Canbay A, et al. NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation, and fibrosis in mice. Hepatology 2014;59:898–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Wree A, McGeough MD, Inzaugarat ME, Eguchi A, Schuster S, Johnson CD, et al. NLRP3 inflammasome driven liver injury and fibrosis: roles of IL-17 and TNF in mice. Hepatology 2018;67:736–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Adebayo D, Morabito V, Andreola F, Pieri G, Luong TV, Dhillon A, et al. Mechanism of cell death in acute-on-chronic liver failure: a clinicopathologic-biomarker study. Liver Int 2015;35:2564–2574. [DOI] [PubMed] [Google Scholar]
- [99].Li W, Deng M, Loughran PA, Yang M, Lin M, Yang C, et al. LPS induces active HMGB1 release from hepatocytes into exosomes through the coordinated activities of TLR4 and caspase-11/GSDMD signaling. Front Immunol 2020;11:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Engelmann C, Adebayo D, Oria M, De Chiara F, Novelli S, Habtesion A, et al. Recombinant alkaline phosphatase prevents acute on chronic liver failure. Sci Rep 2020;10:389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Monteiro S, Grandt J, Uschner FE, Kimer N, Madsen JL, Schierwagen R, et al. Differential inflammasome activation predisposes to acute-onchronic liver failure in human and experimental cirrhosis with and without previous decompensation. Gut 2021;70(2):379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Albillos A, de la Hera A, Gonzalez M, Moya JL, Calleja JL, Monserrat J, et al. Increased lipopolysaccharide binding protein in cirrhotic patients with marked immune and hemodynamic derangement. Hepatology 2003;37:208–217. [DOI] [PubMed] [Google Scholar]
- [103].Praktiknjo M, Schierwagen R, Monteiro S, Ortiz C, Uschner FE, Jansen C, et al. Hepatic inflammasome activation as origin of Interleukin-1alpha and Interleukin-1beta in liver cirrhosis. Gut 2020. 10.1136/gutjnl-2020-322621. [DOI] [PMC free article] [PubMed]
- [104].Heymann F, Tacke F. Immunology in the liver–from homeostasis to disease. Nat Rev Gastroenterol Hepatol 2016;13:88–110. [DOI] [PubMed] [Google Scholar]
- [105].Inoue T, Ito Y, Nishizawa N, Eshima K, Kojo K, Otaka F, et al. RAMP1 in Kupffer cells is a critical regulator in immune-mediated hepatitis. PLoS One 2018;13:e0200432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Zeng TS, Liu FM, Zhou J, Pan SX, Xia WF, Chen LL. Depletion of Kupffer cells attenuates systemic insulin resistance, inflammation and improves liver autophagy in high-fat diet fed mice. Endocr J 2015;62:615–626. [DOI] [PubMed] [Google Scholar]
- [107].Bukong TN, Cho Y, Iracheta-Vellve A, Saha B, Lowe P, Adejumo A, et al. Abnormal neutrophil traps and impaired efferocytosis contribute to liver injury and sepsis severity after binge alcohol use. J Hepatol 2018;69:1145–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Rosenbloom AJ, Pinsky MR, Bryant JL, Shin A, Tran T, Whiteside T. Leukocyte activation in the peripheral blood of patients with cirrhosis of the liver and SIRS. Correlation with serum interleukin-6 levels and organ dysfunction. JAMA 1995;274:58–65. [PubMed] [Google Scholar]
- [109].Tacke F. Targeting hepatic macrophages to treat liver diseases. J Hepatol 2017;66:1300–1312. [DOI] [PubMed] [Google Scholar]
- [110].Li C, Xu MM, Wang K, Adler AJ, Vella AT, Zhou B. Macrophage polarization and meta-inflammation. Transl Res 2018;191:29–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Sun YY, Li XF, Meng XM, Huang C, Zhang L, Li J. Macrophage phenotype in liver injury and repair. Scand J Immunol 2017;85:166–174. [DOI] [PubMed] [Google Scholar]
- [112].Liu K, Zhao E, Ilyas G, Lalazar G, Lin Y, Haseeb M, et al. Impaired macrophage autophagy increases the immune response in obese mice by promoting proinflammatory macrophage polarization. Autophagy 2015;11:271–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Rahman N, Pervin M, Kuramochi M, Karim MR, Izawa T, Kuwamura M, et al. M1/M2-macrophage polarization-based hepatotoxicity in d-galactosamine-induced acute liver injury in rats. Toxicol Pathol 2018;46:764–776. [DOI] [PubMed] [Google Scholar]
- [114].Bernsmeier C, Cavazza A, Fatourou EM, Theocharidou E, Akintimehin A, Baumgartner B, et al. Leucocyte ratios are biomarkers of mortality in patients with acute decompensation of cirrhosis and acute-on-chronic liver failure. Aliment Pharmacol Ther 2020;52:855–865. [DOI] [PubMed] [Google Scholar]
- [115].Alves-Filho JC, Spiller F, Cunha FQ. Neutrophil paralysis in sepsis. Shock 2010;34(Suppl 1):15–21. [DOI] [PubMed] [Google Scholar]
- [116].Xu R, Bao C, Huang H, Lin F, Yuan Y, Wang S, et al. Low expression of CXCR1/2 on neutrophils predicts poor survival in patients with hepatitis B virus-related acute-on-chronic liver failure. Sci Rep 2016;6:38714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Rolas L, Makhezer N, Hadjoudj S, El-Benna J, Djerdjouri B, Elkrief L, et al. Inhibition of mammalian target of rapamycin aggravates the respiratory burst defect of neutrophils from decompensated patients with cirrhosis. Hepatology 2013;57:1163–1171. [DOI] [PubMed] [Google Scholar]
- [118].Weichselbaum L, Azouz A, Smolen KK, Das J, Splittgerber M, Lepida A, et al. Epigenetic basis for monocyte dysfunction in patients with severe alcoholic hepatitis. J Hepatol 2020;73:303–314. [DOI] [PubMed] [Google Scholar]
- [119].Kolios G, Valatas V, Kouroumalis E. Role of Kupffer cells in the pathogenesis of liver disease. World J Gastroenterol 2006;12:7413–7420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Garcia-Martinez R, Caraceni P, Bernardi M, Gines P, Arroyo V, Jalan R. Albumin: pathophysiologic basis of its role in the treatment of cirrhosis and its complications. Hepatology 2013;58:1836–1846. [DOI] [PubMed] [Google Scholar]
- [121].Oettl K, Stadlbauer V, Petter F, Greilberger J, Putz-Bankuti C, Hallstrom S, et al. Oxidative damage of albumin in advanced liver disease. Biochim Biophys Acta 2008;1782:469–473. [DOI] [PubMed] [Google Scholar]
- [122].Jalan R, Schnurr K, Mookerjee RP, Sen S, Cheshire L, Hodges S, et al. Alterations in the functional capacity of albumin in patients with decompensated cirrhosis is associated with increased mortality. Hepatology 2009;50:555–564. [DOI] [PubMed] [Google Scholar]
- [123].Oettl K, Birner-Gruenberger R, Spindelboeck W, Stueger HP, Dorn L, Stadlbauer V, et al. Oxidative albumin damage in chronic liver failure: relation to albumin binding capacity, liver dysfunction and survival. J Hepatol 2013;59:978–983. [DOI] [PubMed] [Google Scholar]
- [124].Domenicali M, Baldassarre M, Giannone FA, Naldi M, Mastroroberto M, Biselli M, et al. Posttranscriptional changes of serum albumin: clinical and prognostic significance in hospitalized patients with cirrhosis. Hepatology 2014;60:1851–1860. [DOI] [PubMed] [Google Scholar]
- [125].Baldassarre M, Domenicali M, Naldi M, Laggetta M, Giannone FA, Biselli M, et al. Albumin homodimers in patients with cirrhosis: clinical and prognostic relevance of a novel identified structural alteration of the molecule. Sci Rep 2016;6:35987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Giannone FA, Domenicali M, Baldassarre M, Bartoletti M, Naldi M, Laggetta M, et al. Ischaemia-modified albumin: a marker of bacterial infection in hospitalized patients with cirrhosis. Liver Int 2015;35:2425–2432. [DOI] [PubMed] [Google Scholar]
- [127].Das S, Maras JS, Hussain MS, Sharma S, David P, Sukriti S, et al. Hyperoxidized albumin modulates neutrophils to induce oxidative stress and inflammation in severe alcoholic hepatitis. Hepatology 2017;65:631–646. [DOI] [PubMed] [Google Scholar]
- [128].Alcaraz-Quiles J, Casulleras M, Oettl K, Titos E, Flores-Costa R, DuranGuell M, et al. Oxidized albumin triggers a cytokine storm in leukocytes through P38 mitogen-activated protein kinase: role in systemic inflammation in decompensated cirrhosis. Hepatology 2018;68:1937–1952. [DOI] [PubMed] [Google Scholar]
- [129].Bernardi M, Angeli P, Claria J, Moreau R, Gines P, Jalan R, et al. Albumin in decompensated cirrhosis: new concepts and perspectives. Gut 2020;69:1127–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Moreau R, Claria J, Aguilar F, Fenaille F, Lozano JJ, Junot C, et al. Blood metabolomics uncovers inflammation-associated mitochondrial dysfunction as a potential mechanism underlying ACLF. J Hepatol 2020;72:688–701. [DOI] [PubMed] [Google Scholar]
- [131].Lodish HB A, Zipursky SL, Matsudaira P, Baltimore D, Darnell J. Electron Transport and Oxidative Phosphorylation in Molecular Cell Biology. 4th edition. New York: W. H. Freeman; 2000. [Google Scholar]
- [132].Wang A, Luan HH, Medzhitov R. An evolutionary perspective on immunometabolism. Science 2019;363. [DOI] [PMC free article] [PubMed]
- [133].Mills EL, Kelly B, O’Neill LAJ. Mitochondria are the powerhouses of immunity. Nat Immunol 2017;18:488–498. [DOI] [PubMed] [Google Scholar]
- [134].Zaccherini GA F, Caracen i P, Clària J, Lozano JJ, Fenaille F. Assessing the role of amino acids in systemic inflammatory responses and organ failures in patients with ACLF. J Hepatol 2020. 10.1016/j.jhep.2020.11.035. In press. [DOI] [PubMed]
- [135].Van Wyngene L, Vandewalle J, Libert C. Reprogramming of basic metabolic pathways in microbial sepsis: therapeutic targets at last? EMBO Mol Med 2018;10. [DOI] [PMC free article] [PubMed]
- [136].Bajaj JS, Reddy KR, O’Leary JG, Vargas HE, Lai JC, Kamath PS, et al. Serum levels of metabolites produced by intestinal microbes and lipid moieties independently associated with acute on chronic liver failure and death in patients with cirrhosis. Gastroenterology 2020;159(5):1715–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Ganeshan K, Nikkanen J, Man K, Leong YA, Sogawa Y, Maschek JA, et al. Energetic trade-offs and hypometabolic states promote disease tolerance. Cell 2019;177:399–413. e312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Kelly B, Pearce EL. Amino assets: how amino acids support immunity. Cell Metab 2020;32:154–175. [DOI] [PubMed] [Google Scholar]
- [139].Nishikawa T, Bellance N, Damm A, Bing H, Zhu Z, Handa K, et al. A switch in the source of ATP production and a loss in capacity to perform glycolysis are hallmarks of hepatocyte failure in advance liver disease. J Hepatol 2014;60:1203–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Chan DC. Mitochondria: dynamic organelles in disease, aging, and development. Cell 2006;125:1241–1252. [DOI] [PubMed] [Google Scholar]
- [141].Brenner C, Galluzzi L, Kepp O, Kroemer G. Decoding cell death signals in liver inflammation. J Hepatol 2013;59:583–594. [DOI] [PubMed] [Google Scholar]
- [142].Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev 2014;94:909–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Idzko M, Ferrari D, Eltzschig HK. Nucleotide signalling during inflammation. Nature 2014;509:310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Schroder K, Tschopp J. The inflammasomes. Cell 2010;140:821–832. [DOI] [PubMed] [Google Scholar]
- [145].Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 2010;464:104–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Chang WK, Chao YC, Tang HS, Lang HF, Hsu CT. Effects of extra-carbohydrate supplementation in the late evening on energy expenditure and substrate oxidation in patients with liver cirrhosis. JPEN J Parenter Enteral Nutr 1997;21:96–99. [DOI] [PubMed] [Google Scholar]
- [147].Engelmann C, Schob S, Nonnenmacher I, Werlich L, Aehling N, Ullrich S, et al. Loss of paraspinal muscle mass is a gender-specific consequence of cirrhosis that predicts complications and death. Aliment Pharmacol Ther 2018;48:1271–1281. [DOI] [PubMed] [Google Scholar]
- [148].Ebadi M, Tandon P, Moctezuma-Velazquez C, Ghosh S, Baracos VE, Mazurak VC, et al. Low subcutaneous adiposity associates with higher mortality in female patients with cirrhosis. J Hepatol 2018;69:608–616. [DOI] [PubMed] [Google Scholar]
- [149].Praktiknjo M, Clees C, Pigliacelli A, Fischer S, Jansen C, Lehmann J, et al. Sarcopenia is associated with development of acute-on-chronic liver failure in decompensated liver cirrhosis receiving transjugular intrahepatic portosystemic shunt. Clin Transl Gastroenterol 2019;10:e00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Phillips R, Ursell T, Wiggins P, Sens P. Emerging roles for lipids in shaping membrane-protein function. Nature 2009;459:379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Koberlin MS, Snijder B, Heinz LX, Baumann CL, Fauster A, Vladimer GI, et al. A conserved circular network of coregulated lipids modulates innate immune responses. Cell 2015;162:170–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Grammatikos G, Ferreiros N, Waidmann O, Bon D, Schroeter S, Koch A, et al. Serum sphingolipid variations associate with hepatic decompensation and survival in patients with cirrhosis. PLoS One 2015;10: e0138130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Becker S, Kinny-Koster B, Bartels M, Scholz M, Seehofer D, Berg T, et al. Low sphingosine-1-phosphate plasma levels are predictive for increased mortality in patients with liver cirrhosis. PLoS One 2017;12:e0174424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Rachakonda V, Argemi J, Borhani AA, Bataller R, Tevar A, Behari J. Reduced serum sphingolipids constitute a molecular signature of malnutrition in hospitalized patients with decompensated cirrhosis. Clin Transl Gastroenterol 2019;10:e00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Sehrawat TS, Arab JP, Liu M, Amrollahi P, Wan M, Fan J, et al. Circulating extracellular vesicles carrying sphingolipid cargo for the diagnosis and dynamic risk profiling of alcoholic hepatitis. Hepatology 2020. 10.1002/hep.31256. In press. [DOI] [PMC free article] [PubMed]
- [156].Lopez-Vicario C, Checa A, Urdangarin A, Aguilar F, Alcaraz-Quiles J, Caraceni P, et al. Targeted lipidomics reveals extensive changes in circulating lipid mediators in patients with acutely decompensated cirrhosis. J Hepatol 2020;73(4):817–828. [DOI] [PubMed] [Google Scholar]
- [157].Schwarzkopf KM, Queck A, Thomas D, Angioni C, Cai C, Freygang Y, et al. Omega-3 and −6 fatty acid plasma levels are not associated with liver cirrhosis-associated systemic inflammation. PLoS One 2019;14: e0211537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Spite M, Claria J, Serhan CN. Resolvins, specialized proresolving lipid mediators, and their potential roles in metabolic diseases. Cell Metab 2014;19:21–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].O’Brien AJ, Fullerton JN, Massey KA, Auld G, Sewell G, James S, et al. Immunosuppression in acutely decompensated cirrhosis is mediated by prostaglandin E2. Nat Med 2014;20:518–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Sawhney R, Holland-Fischer P, Rosselli M, Mookerjee RP, Agarwal B, Jalan R. Role of ammonia, inflammation, and cerebral oxygenation in brain dysfunction of acute-on-chronic liver failure patients. Liver Transpl 2016;22:732–742. [DOI] [PubMed] [Google Scholar]
- [161].Weber FL Jr, Friedman DW, Fresard KM. Ammonia production from intraluminal amino acids in canine jejunum. Am J Physiol 1988;254:G264–G268. [DOI] [PubMed] [Google Scholar]
- [162].Romero-Gomez M, Ramos-Guerrero R, Grande L, de Teran LC, Corpas R, Camacho I, et al. Intestinal glutaminase activity is increased in liver cirrhosis and correlates with minimal hepatic encephalopathy. J Hepatol 2004;41:49–54. [DOI] [PubMed] [Google Scholar]
- [163].Kaiser S, Gerok W, Haussinger D. Ammonia and glutamine metabolism in human liver slices: new aspects on the pathogenesis of hyperammonaemia in chronic liver disease. Eur J Clin Invest 1988;18:535–542. [DOI] [PubMed] [Google Scholar]
- [164].Rudman D, DiFulco TJ, Galambos JT, Smith 3rd RB, Salam AA, Warren WD. Maximal rates of excretion and synthesis of urea in normal and cirrhotic subjects. J Clin Invest 1973;52:2241–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Wright G, Noiret L, Olde Damink SW, Jalan R. Interorgan ammonia metabolism in liver failure: the basis of current and future therapies. Liver Int 2011;31:163–175. [DOI] [PubMed] [Google Scholar]
- [166].Lockwood AH, McDonald JM, Reiman RE, Gelbard AS, Laughlin JS, Duffy TE, et al. The dynamics of ammonia metabolism in man. Effects of liver disease and hyperammonemia. J Clin Invest 1979;63:449–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Clemmesen JO, Kondrup J, Ott P. Splanchnic and leg exchange of amino acids and ammonia in acute liver failure. Gastroenterology 2000;118:1131–1139. [DOI] [PubMed] [Google Scholar]
- [168].Merli M, Giusto M, Lucidi C, Giannelli V, Pentassuglio I, Di Gregorio V, et al. Muscle depletion increases the risk of overt and minimal hepatic encephalopathy: results of a prospective study. Metab Brain Dis 2013;28:281–284. [DOI] [PubMed] [Google Scholar]
- [169].Olde Damink SW, Jalan R, Deutz NE, Redhead DN, Dejong CH, Hynd P, et al. The kidney plays a major role in the hyperammonemia seen after simulated or actual GI bleeding in patients with cirrhosis. Hepatology 2003;37:1277–1285. [DOI] [PubMed] [Google Scholar]
- [170].Olde Damink SW, Deutz NE, Dejong CH, Soeters PB, Jalan R. Interorgan ammonia metabolism in liver failure. Neurochem Int 2002;41:177–188. [DOI] [PubMed] [Google Scholar]
- [171].Rose C, Kresse W, Kettenmann H. Acute insult of ammonia leads to calcium-dependent glutamate release from cultured astrocytes, an effect of pH. J Biol Chem 2005;280:20937–20944. [DOI] [PubMed] [Google Scholar]
- [172].Dasarathy S, Mookerjee RP, Rackayova V, Rangroo Thrane V, Vairappan B, Ott P, et al. Ammonia toxicity: from head to toe? Metab Brain Dis 2017;32:529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Poh Z, Chang PE. A current review of the diagnostic and treatment strategies of hepatic encephalopathy. Int J Hepatol 2012;2012:480309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Joshi D, O’Grady J, Patel A, Shawcross D, Connor S, Deasy N, et al. Cerebral oedema is rare in acute-on-chronic liver failure patients presenting with high-grade hepatic encephalopathy. Liver Int 2014;34:362–366. [DOI] [PubMed] [Google Scholar]
- [175].Ott P, Clemmesen O, Larsen FS. Cerebral metabolic disturbances in the brain during acute liver failure: from hyperammonemia to energy failure and proteolysis. Neurochem Int 2005;47:13–18. [DOI] [PubMed] [Google Scholar]
- [176].Shalimar Sheikh MF, Mookerjee RP, Agarwal B, Acharya SK, Jalan R. Prognostic role of ammonia in patients with cirrhosis. Hepatology 2019;70:982–994. [DOI] [PubMed] [Google Scholar]
- [177].Jacoby KJ, Singh P, Prekker ME, Leatherman JW. Characteristics and outcomes of critically ill patients with severe hyperammonemia. J Crit Care 2020;56:177–181. [DOI] [PubMed] [Google Scholar]
- [178].Jalan R, De Chiara F, Balasubramaniyan V, Andreola F, Khetan V, Malago M, et al. Ammonia produces pathological changes in human hepatic stellate cells and is a target for therapy of portal hypertension. J Hepatol 2016;64:823–833. [DOI] [PubMed] [Google Scholar]
- [179].Nath KA, Hostetter MK, Hostetter TH. Increased ammoniagenesis as a determinant of progressive renal injury. Am J Kidney Dis 1991;17:654–657. [DOI] [PubMed] [Google Scholar]
- [180].Dan H, Peng RX, Ao Y, Liu YH. Segment-specific proximal tubule injury in tripterygium glycosides intoxicated rats. J Biochem Mol Toxicol 2008;22:422–428. [DOI] [PubMed] [Google Scholar]
- [181].Shawcross DL, Wright GA, Stadlbauer V, Hodges SJ, Davies NA, Wheeler-Jones C, et al. Ammonia impairs neutrophil phagocytic function in liver disease. Hepatology 2008;48:1202–1212. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.