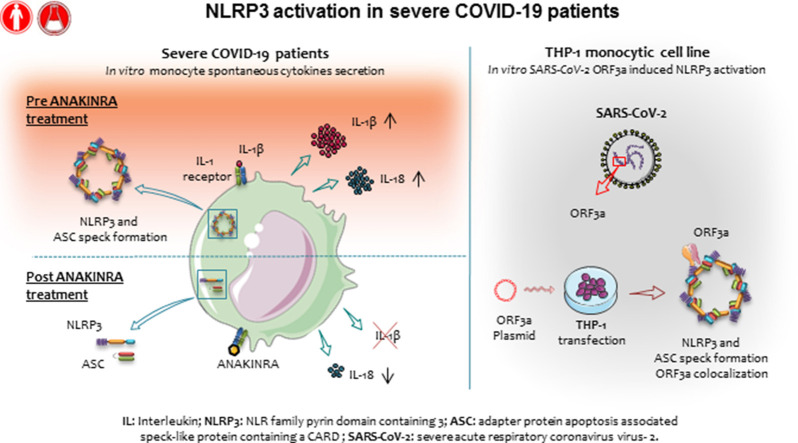
Abstract
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection may result in a severe pneumonia associated with elevation of blood inflammatory parameters, reminiscent of cytokine storm syndrome. Steroidal anti-inflammatory therapies have shown efficacy in reducing mortality in critically ill patients; however, the mechanisms by which SARS-CoV-2 triggers such an extensive inflammation remain unexplained.
Objectives
To dissect the mechanisms underlying SARS-CoV-2–associated inflammation in patients with severe coronavirus disease 2019 (COVID-19), we studied the role of IL-1β, a pivotal cytokine driving inflammatory phenotypes, whose maturation and secretion are regulated by inflammasomes.
Methods
We analyzed nod-like receptor protein 3 pathway activation by means of confocal microscopy, plasma cytokine measurement, cytokine secretion following in vitro stimulation of blood circulating monocytes, and whole-blood RNA sequencing. The role of open reading frame 3a SARS-CoV-2 protein was assessed by confocal microscopy analysis following nucleofection of a monocytic cell line.
Results
We found that circulating monocytes from patients with COVID-19 display ASC (adaptor molecule apoptotic speck like protein–containing a CARD) specks that colocalize with nod-like receptor protein 3 inflammasome and spontaneously secrete IL-1β in vitro. This spontaneous activation reverts following patient’s treatment with the IL-1 receptor antagonist anakinra. Transfection of a monocytic cell line with cDNA coding for the ORF3a SARS-CoV-2 protein resulted in ASC speck formation.
Conclusions
These results provide further evidence that IL-1β targeting could represent an effective strategy in this disease and suggest a mechanistic explanation for the strong inflammatory manifestations associated with COVID-19.
Key words: NLRP3 inflammasome, IL-1β, inflammation, SARS-CoV-2
Graphical abstract
Coronavirus disease 2019 (COVID-19) is an acute respiratory illness caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). COVID-19 severe pneumonia has been associated with systemic inflammation and elevation of parameters such as ferritin, lactic dehydrogenase, and soluble IL-2 receptor evocative of clinical inflammatory phenotypes known as macrophage activation syndrome (MAS) or hemophagocytic lymphohistiocytosis and cytokine storm syndromes.1 MAS is a life-threatening condition seen in pediatric patients affected by systemic-onset juvenile idiopathic arthritis, which is also observed in previously healthy children and known as secondary hemophagocytic lymphohistiocytosis, often triggered by viral infection.2 Anticytokine therapies targeting IL-1β (canakinumab) or IL-1 receptor antagonist (anakinra) have demonstrated the central role of this molecule in MAS and systemic hemophagocytic lymphohistiocytosis, markedly improving the outcome.3 Recently, several reports have highlighted the role of innate immune cells, particularly monocytes, and of monocyte-derived cytokines such as IL-1β, in COVID-19,4, 5, 6 and showed a correlation between the degree of involvement of innate immunity and the disease severity and outcome.7 Stimulation of PBMCs from patients with severe COVID-19 has shown an increased secretion of IL-1β compared with PBMCs from patients with severe H1N1 influenza,8 confirming gene expression data extracted by whole blood9 or single-cell transcriptomic.4 In support of a key role of IL-1β in patients with severe COVID-19, we and others have recently reported the potential efficacy of blocking IL-1β by using anti–IL-1 drugs in patients with severe COVID-19.10, 11, 12, 13
In monocytic cells from healthy subjects, IL-1β is translated as an inactive precursor, pro–IL-1β, which is processed and secreted as bioactive IL-1β on activation of the nod-like receptor protein 3 (NLRP3) inflammasome. NLRP3, activated by inflammatory signals, oligomerizes and recruits pro–caspase-1 and the adaptor molecule apoptotic speck-like protein–containing a CARD (ASC), resulting in activation of caspase-1, processing of pro–IL-1β, and secretion of the mature cytokine. NLRP3 inflammasome also mediates processing and secretion of IL-18, a leading cytokine in MAS.14 IL-1β production, processing, and secretion require a first signal, named signal 1, which induces transcription of pro–IL-1β and inflammasome genes, and a second signal, named signal 2, which triggers the assembly of the NLRP3 inflammasome. In human monocytes, signal 1 is sufficient to trigger the cascade of events leading to inflammasome activation and IL-1β secretion, low but sustained in time.15 Signal 2, although unnecessary, strongly speeds up and enhances these processes. Recently, NLRP3 and absent in melanoma 2 protein inflammasome activation and ASC speck formation in peripheral blood cells16 and CD14+ cells from autoptic lung tissue of patients with COVID-19 have been reported.16 , 17 The involvement of viral proteins N18 and open reading frame 3a (ORF3a)19 in NLRP3 activation has also been demonstrated in vitro.
Methods
Study design and participants
The study was approved by our regional institutional review board (Immunocovid-19 project). Samples were collected on informed consent from SARS-CoV-2–infected patients with a positive nasopharyngeal swab and a clinical presentation including dyspnea associated with fever, systemic inflammation, rapidly worsening respiratory distress, and pathognomonic lung abnormalities on chest computed tomography. In patients treated with anakinra, samples were collected before treatment (day 0) and at 3 and 7 days of treatment.
Patient and public involvement
Patient and public were not involved in the design, or conduct, or reporting, or dissemination plans of the research.
Isolation and stimulation of cells
PBMCs from peripheral blood were isolated by gradient centrifugation (FicollHystpaque). Then, monocytes were selected by adhesion, incubating them for 1 hour in RPMI without FCS. Monocytes were cultured in RPMI 1640 (Thermo Fisher Scientific, Waltham, Mass), supplemented with 5% FCS and in the presence or absence of LPS (100 ng/mL; Sigma Aldrich, St Louis, Mo), and MCC950 (10 μM; Sigma Aldrich). Human monocytic cell line THP1 was cultured in RPMI-1640 medium. Monocytes were differentiated in macrophages using phorbol 12-myristate 13-acetate 100 nM for 24 hours.
Secretion of cytokines
In vitro secretion of cytokines by monocyte was quantified by ELISA. Data were normalized by seeded monocytes per well. The number of plated monocytes was calculated on the basis of hospital cell blood count and plated PBMCs and finally related to 1 × 106 cells. All cytokine dosages were normalized to this value. The BD CBA assays for Human Soluble Protein (BD Biosciences 558264; Franklin Lakes, NJ) was used to assess cytokine profile in blood plasma. All data were analyzed using the FCAP Array software (Soft Flow Ltd, Pécs, Hungary).
RNA and real-time PCR
Blood samples were collected in Paxgene Blood RNA. cDNA was retrotranscribed using Superscript VILO cDNA Synthesis Kit (Invitrogen, Carlsbad, Calif). Selected IFN-stimulated gene expression was quantified Briefly, IFN-stimulated gene (IFI27, IFI44L, IFIT1, ISG15, RSAD2, SIGLEC1) expression was quantified by real-time PCR using gene-specific primers and probes (custom designed by TibMolBiol, Genova, Italy) using HPRT and G6PD as reference genes, as previously described with modifications.20 ORF3a was detected in monocyte isolated from patient’s PBMCs and THP-1 by quantitative PCR (CGGATGGCTTATTGTTGGCG, Forward, TGAACACCCTTGGAGAGTGC, Reverse).
DNA transfection
PCMV6-AC-rfp-ORF3a and PCMV6-AC-rfp were purchased from Origene (Rockville, Md). 106 THP-1 cells were transfected with nucleofector device II (Amaxa, Nordrhein-Westfalen, Germany) and nucleofector kit V (Lonza, Basel, Switzerland) using U-001 program with 5 μg of plasmid DNA (% of transfected cells: 24.20 ± 4.384 for PCMV6-AC-rfp-ORF3a and 39.40 ± 0.848 for PCMV6-AC-rfp) and 20 μg of plasmid DNA, respectively (% of transfected cells: 33.66 ± 3.951 for PCMV6-AC-rfp-ORF3a and 50.05 ± 1.484 for PCMV6-AC-rfp). Transfection efficiency and cell viability were monitored by fluorescence-activated cell sorting.
Imaging
THP-1 macrophages cells were cultured on glass coverslip and transfected as described. After 16 hours, cells were stained with anti-ASC (TMS1) (Hu) mAb-Alexa Fluor 647 conjugate (Voden, Monza e Brianza, Lombardia, Italy) and anti-NLRP3 (Rabbit, Life Technologies, Carlsbad, Calif) overnight and acquired using a Leica TCS-SP8 confocal microscope; Z-sectioning images were acquired with a z-slice thickness of about 0.15 μm.
Detection of ASC speck by fluorescence-activated cell sorting
Monocytes were isolated from PBMCs by magnetic selection using CD14 MicroBeads (MiltenyiBiotec, Bergisch Gladbach, North Rhine-Westphalia, Germany) according to manufacturer’s instructions. Cells were resuspended at 1 × 106/mL in RPMI1640 supplemented with 5% FCS, left untreated or primed with 100 ng/mL LPS (Sigma) for 3 hours, and stimulated with 5 mM ATP (Sigma Aldrich) for another 30 minutes. Cells were fixed and permeabilized using BD Cytofix/Cytoperm reagents (BD Biosciences) and incubated overnight with anti-ASC (TMS1) phycoerythrin (BioLegend, San Diego, Calif) at 4°C. Samples were analyzed using the FACSCanto A (BD Biosciences) flow cytometer and Kaluza Software 2.0 (Beckman Coulter, Brea, Calif). ASC speck formation was detected as reduction in the ASC fluorescent pulse width with a concomitant increase in the ASC pulse area, as described.21
FLICA-Caspase-1 staining
Caspase-1 activation was evaluated on frozen PBMCs by flow cytometry. On thawing, cells were allowed to recover for 1 hour at 37°C in RPMI 5% FBS. Cells were labeled for 1 hour with FLICA 660-YVAD-FMK caspasi-1 inhibitor reagent (ImmunoChemistry Technologies, LLC, Davis, Calif) according to manufacturer’s instructions. Cells were then stained with CD14 Pe-Cy7 (eBioscience, San Diego, Calif), fixed and permeabilized using BD Cytofix/Cytoperm reagents, and incubated overnight with anti-ASC (TMS1) PE at 4°C. Active caspasi-1 was analyzed on gated CD14+ASC+ monocytes or on gated CD14+ASC speck-forming cells.
RNA-sequencing data processing
Poly-A–enriched libraries were generated with the Illumina TruSeq mRNA Stranded kit. Library quality control was performed using the Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, Calif). Indexed libraries were sequenced with HiSeq 3000 instrument to generate approximately 30 M 150-bp paired-end reads per sample. Reads were trimmed for low-quality ends with TrimGalore, and transcript abundance was estimated with Kallisto.22 Kallisto counts were normalized by the regularized log transformation using the DeSeq223 R package (Bioconductor software). Data are accessible at gene expression omnibus database, accession code GSE163317.
Bioinformatic analysis for RNA sequencing
Analysis was performed by gene get enrichment analysis (GSEA) implemented in the cluster Profiler R package24 and GSEA prerank method based on log-fold change score25 (Hallmark (H) gene set collection26 , 27). Day 7 and day 0 were used to estimate log-fold change. Curated collection was retrieved from the Molecular Signature Database (SigDB) v7.2 database.26 , 27 Custom collection (see Online Repository file 1 in this article’s Online Repository at www.jacionline.org) was created, retrieving the genes from the literature.28, 29, 30 Normalized enrichment score (NES) and nominal P value (Nom P value) were calculated by GSEA.24 , 25 Gene sets between 5 and 5000 genes were retained for analysis. P value lower than .05 and false discovery rate (FDR) q value lower than 0.15 were considered significant. Functional enrichment results were visualized using dot plots implemented in the clusterProfiler R package (Bioconductor software).24 NES and −log10(q-value) were encoded in color palette, ranging from red (positive) to blue (negative), and dot size, respectively.
Statistical analysis
Statistical analysis was performed with GraphPad Prism (5.0, La Jolla, Calif). Mann-Whitney test was used to compare samples, and results were reported as median with interquartile range.
All data relevant to the study are included in the article or uploaded as Online Repository information or accessible in open access repository.
Data source are provided in Table E1 in this article’s Online Repository at www.jacionline.org. RNA-seq data are accessible at gene expression omnibus database, accession GSE163317.
There was no patient and public involvement.
Results
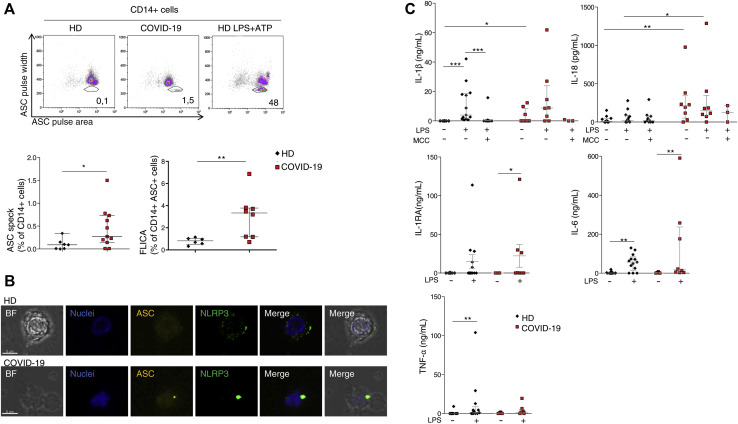
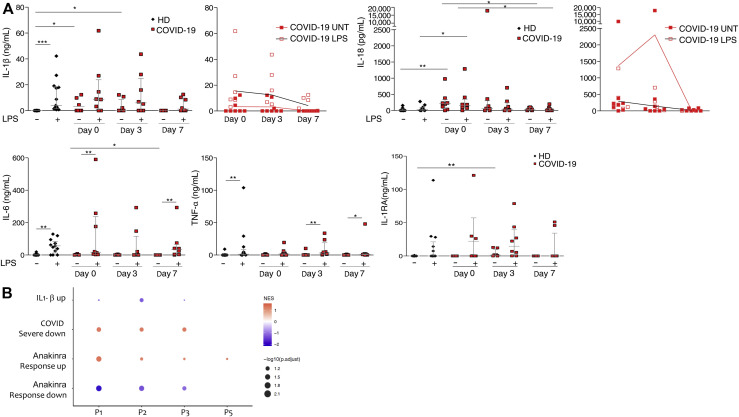
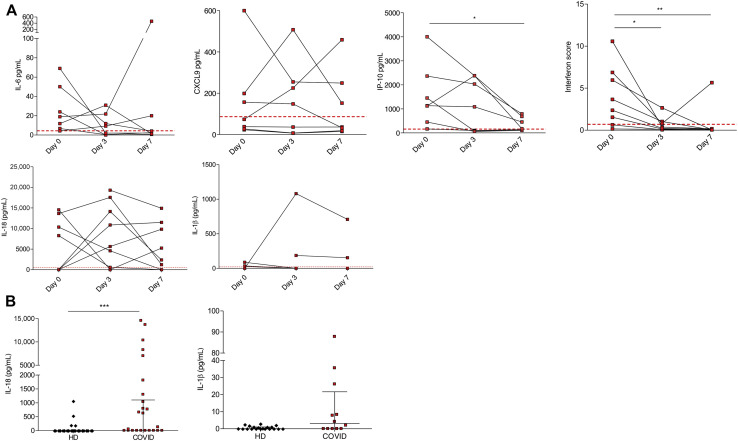
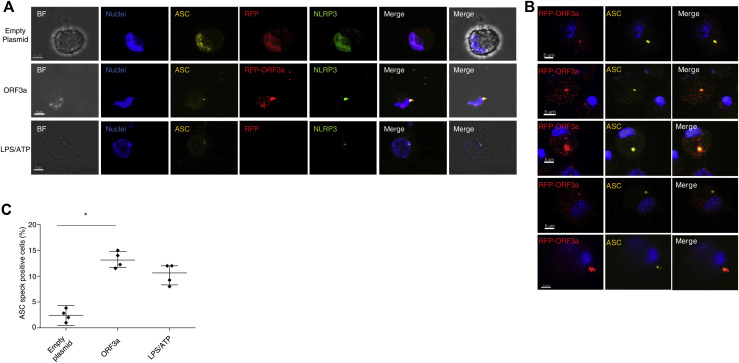
To characterize IL-1β pathway activation in COVID-19, we analyzed blood samples from patients affected by severe COVID-19 and displaying strong elevation of inflammatory markers (clinical data reported in Tables I and II ), before and after treatment with anakinra. Blood samples were collected at baseline and at day 3 and day 7 of treatment. Before treatment, RNA-sequencing analysis revealed as expected the activation of innate immune pathways linked to viral infection such as type I IFN response together with activation of inflammatory pathways involving IL-6 (see Fig E1 in this article’s Online Repository at www.jacionline.org). FACS and confocal microscopy analysis of PBMCs from patients revealed the presence of CD14+ monocytes displaying ASC specks colocalizing with NLRP3 (Fig 1 , A and B). Caspase-1 activation in ASC speck+ monocytes confirmed the involvement of NLRP3 (Fig1, A; see Fig E2 in this article’s Online Repository at www.jacionline.org). Specks are absent in CD14+ monocytes from healthy donors, supporting the activation of NLRP3 inflammasome in patients with COVID-19 but not in healthy subjects. We therefore isolated peripheral blood monocyte from patients with COVID-19 and healthy donors and cultured them in vitro for 18 hours with or without LPS, which in healthy monocytes induces pro–IL-1β expression, inflammasome activation, and secretion of both IL-1β and IL-18.15 Spontaneous secretion of IL-1β and IL-18 by unstimulated patients’ monocytes, which was partially inhibited by the NLRP3 inhibitor MCC950, was detected (Fig 1, C). These results, although consistent with the presence of ASC specks in patients’ monocytes, were quite unexpected because in our experience IL-1β and IL-18 are not secreted in vitro by unstimulated monocytes either from healthy subjects or from patients with well-established IL-1–driven genetic conditions such as cryopirin-associated periodic syndromes (CAPSs), caused by gain-of-function mutations of NLRP3.31 LPS stimulation of monocytes from patients with COVID-19 further increased the secretion of IL-1β over the levels secreted by unstimulated cells (in 6 of 8 patients, 75%). Also, IL-18 secretion was induced, although at a lesser extent (Fig 1, C). The NLRP3 blocker MCC950 inhibited secretion, indicating that secretion of both IL-1β and IL-18 is dependent on the NLRP3 inflammasome. Like in healthy monocytes, the inflammasome-independent cytokines TNF-α, IL-6, and IL-1RA were not spontaneously secreted by monocytes but induced by exposure to LPS (Fig 1, C). We previously showed that few days of treatment of patients with CAPS with anakinra result in inhibition of LPS-induced IL-1β secretion by patient’s monocytes in vitro.31 Accordingly, we observed a decrease in the spontaneous and LPS-induced IL-1β and IL-18 secretion by monocytes from patients with COVID-19 under therapy with anakinra (Fig 2 , A), more evident at 7 days from the beginning of the treatment. Secretion of TNF-α and IL-6 was not decreased in agreement with their direct induction after Toll-like receptor triggering via activation of the nuclear factor-kappa B signaling pathway independently of IL-1β receptor signaling. Other inflammatory markers did not show a significant reduction in supernatant (Fig 2, A) and in the serum (Fig 3 , A and B). Type I IFN, as measured by IFN RNA signature in peripheral blood, showed a decrease (Fig 3, A). Interestingly, when we compared through RNA sequencing the whole-blood transcriptome of patients before and after treatment with anakinra, we observed a significant downregulation of IL-1β–induced genes and upregulation of genes shown to be downregulated in patients with severe COVID-19 (Fig 2, B, and Fig E1). More importantly, following anakinra therapy, we observed the same modulation of gene sets observed in patients with CAPS treated by the same drug32 (Fig 2, B). These results were evident in patients with COVID-19 who recovered from the disease after treatment but not in the patient who died (P5). Together, these data suggest that activation of NLRP3 inflammasome and IL-1β secretion have a major role in the systemic inflammation in patients with severe COVID-19. Moreover, the modulation of IL-1β–induced or –repressed genes by IL-1 receptor blockade supports the exploitation of anakinra as an efficient therapeutic approach.33, 34, 35 The increased secretion of IL-1β by patients with COVID-19 is likely due to extrinsic and intrinsic factors. SARS-CoV-2 lung infection may induce the release in the microenvironment by viral-infected injured cells of damage-associated molecular patterns such as ATP and IL-1α and pathogen-associated molecular patterns including ssRNA and other viral proteins. These pathogen-associated molecular patterns and damage-associated molecular patterns are able to induce IL-1β production and/or processing and secretion36 and thus, contribute extrinsically to the inflammatory response in patients. In addition, considering the high frequency of the hyperinflammatory state observed in patients with COVID-19 compared with other viral respiratory lung infections,8 and the reported ability of SARS-CoV-2 to infect myeloid cells,16 , 17 we speculated that SARS-CoV-2 could intrinsically activate the NLRP3 pathway in monocytes. Studies in SARS-CoV have demonstrated that the viroprotein 3, encoded by the ORF3a viral gene, is able to induce both signal 1 and 2 in monocytes, activating IL-1β transcription through nuclear factor-κB activation and NLRP3 through TNF receptor–associated factor 3 binding.37 SARS-CoV-2 encodes the homologue protein ORF3a. We then transfected the monocytic cell line THP1 with a plasmid containing ORF3a gene tagged with red fluorescent protein. After 24 hours, we obtained confocal images of transfected cells and observed the formation of ASC specks that colocalized with ORF3a protein and NLRP3 in cells transfected with the ORF3a plasmid but not in cells transfected with the empty plasmid (Fig 4 , A-C). These data indicate that ORF3a protein of SARS-CoV-2 activates NLRP3, inducing ASC speck formation. We assessed the presence of viral ORF3a mRNA in monocytes from 3 patients with COVID-19 analyzed for IL-1β secretion in Fig 1, C: 2 of 3 tested samples expressed ORF3a. Interestingly, the 2 positive samples were from P2 and P4, who showed spontaneous secretion of IL-1β in vitro (Fig 1, C), whereas P1 monocytes, negative for ORF3a mRNA, did not secrete IL-1β spontaneously. We then assessed viral ORF3a mRNA in monocytes from other 8 patients with COVID-19 and did not detect it, suggesting that SARS-CoV-2 mRNA is only marginally present in peripheral blood patient’s monocytes.
Table I.
Patients’ clinical characteristics
| Patient ID | Age (y) | Sex | Comorbidities | Clinical | T (°C) | Sat, O2 | PaO2/FiO2 | SARS-CoV-2 nasal swab | SARS-CoV-2 serology | Day after disease onset | Days of Steroid at first sampling | Days of anakinra at first sampling | Other therapies administered | Mechanical ventilation | CPAP | FiO235%-60% (Venturi mask) | FiO224%-32% | Nasal cannula | Ambient air | CRP (0-0.5 mg/dL) | Ferritin (30-400 ng/mL) | D dimer (0-500 ng/mL) | LDH (135-200 U/L) | Lymphocytes (1.13-3.37 ×109/L) | Neutrophils (2.01-5.72 ×109/L) | Platelets (0-0.5 mg/dL) | Outcome | SARS-CoV-2 vaccine |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| COVID-01 | 55 | F | CD, HT | Fever, cough, dyspnea | 37 | 97% | 177 | POS | NA | 5 | 2 | 0 | MPred (0.5-1 mg/kg/d for 4 d, 2 d before anakinra), EXP, AZT | 0 | 0 | 1 | 0 | 0 | 0 | 3.8 | 460 | 3587 | 493 | 0.8 | 3.7 | 241 | Alive | No |
| COVID-02 | 25 | M | — | Fever, cough, dyspnea | 39 | 96% | 210 | POS | NA | 19 | 0 | 0 | MPred (2-3 mg/kg/d for 8 d with anakinra), HCQ, EXP, AZT | 0 | 0 | 1 | 0 | 0 | 0 | 19 | 1591 | — | 453 | 0.39 | 16.06 | 285 | Alive | No |
| COVID-03 | 67 | M | HT | Fever, cough, dyspnea, arthralgia | 36.8 | 94% | 226 | POS | NA | 6 | 2 | 0 | HCQ, EXP, remdesivir, AZT | 0 | 0 | 1 | 0 | 0 | 0 | 5.4 | 1443 | 1476 | 464 | 0.47 | 9.28 | 238 | Alive | No |
| COVID-04 | 59 | M | CD | Fever, anosmia, headache | 36 | 94% | 116 | POS | NA | 11 | 0 | 0 | MPred (0.5-1 mg/kg/d for 3 d), EXP, AZT | 0 | 0 | 0 | 1 | 0 | 0 | 3.97 | 1634 | 557 | 279 | 1.08 | 4.89 | 252 | Alive | No |
| COVID-05 | 72 | F | CD, HT | Cough | 39.2 | 96% | 223 | POS | NA | 11 | 0 | 0 | MPred (1-2 mg/kg/d for 7 d, with anakinra), HCQ, EXP, AZT | 0 | 0 | 1 | 0 | 0 | 0 | 15.07 | 3169 | 1148 | 420 | 0.77 | 6.2 | 166 | Death | No |
| COVID-49 | 38 | M | No | Fever, headache | 39 | 97% | 381 | POS | NA | 7 | 0 | 0 | EXP, AZT | 0 | 0 | 1 | 0 | 0 | 0 | 10.78 | 662 | 1061 | 382 | 1.45 | 1.53 | 480 | Alive | No |
| COVID-53 | 71 | M | HT, emphysema | Fever, dyspnea | 36.5 | 93% | 218 | POS | NA | 6 | 2 | 0 | MPred (1-2 mg/kg/d for 7 d), EXP, AZT | 0 | 1 | 0 | 0 | 0 | 0 | 5.88 | 621 | 728 | 376 | 0.73 | 10.25 | 341 | Alive | No |
| COVID-54 | 59 | F | No | Fever, dyspnea, cough, dysgeusia | 38 | 93% | 118 | POS | NA | 9 | 1 | 0 | MPred (1-2 mg/kg/d for 7 d), EXP, AZT | 0 | 1 | 0 | 0 | 0 | 0 | 8.01 | 900 | 817 | 303 | 0.7 | 3.46 | 173 | Alive | No |
| COVID-66 | 63 | M | No | Fever, dyspnea | 36 | 92% | 82 | POS | NA | 10 | 3 | 0 | MPred (1-2 mg/kg/d for 10 d), EXP, remdesivir | 1 | 0 | 0 | 0 | 0 | 0 | 18.28 | 1894 | 1348 | 421 | 0.37 | 13.26 | 263 | Alive | No |
| COVID-67 | 76 | M | HT, CD, HPT | Fever, dyspnea | 36.5 | 88% | 170 | POS | NA | 14 | 7 | 0 | MPred (1-2 mg/kg/d for 7 d), EXP, AZT | 0 | 1 | 0 | 0 | 0 | 0 | 10.78 | 1428 | 629 | 366 | 0.43 | 17.96 | 362 | Alive | No |
| COVID-72 | 59 | M | HT | Fever, dyspnea | 36.7 | 96% | 65 | POS | NA | 15 | 5 | 0 | MPred (1-2 mg/kg/d for 7 d), EXP, AZT | 0 | 1 | 0 | 0 | 0 | 0 | 5.45 | 1810 | 416 | 256 | 0.59 | 9.37 | 356 | Alive | No |
| COVID-74 | 90 | M | HT, CD | Fever, dyspnea | 36.5 | 95% | 220 | POS | NA | 6 | 2 | 0 | MPred (1-2 mg/kg/d for 7 d), EXP, AZT, remdesivir | 0 | 0 | 1 | 0 | 0 | 0 | 5.68 | 1318 | 2402 | 238 | 0.98 | 10.47 | 212 | Alive | No |
| COVID-75 | 74 | M | HT | Fever, dyspnea | 36 | 94% | 121 | POS | NA | 18 | 7 | 0 | MPred (1-2 mg/kg/d for 7 d), EXP, AZT | 0 | 0 | 1 | 0 | 0 | 0 | 7.86 | 1683 | 3689 | 333 | 0.8 | 9.96 | 301 | Alive | No |
| COVID-78 | 66 | F | No | Nausea, hyporexia, diarrhea, fever | 36.4 | 98% | 342 | POS | NA | 7 | 4 | 0 | MPred (2 mg/kg/d for 4 d), EXP | 0 | 0 | 0 | 0 | 4 | 0 | 6.1 | 264 | 558.2 | 235 | 1.05 | 1.033 | 457 | Alive | No |
| COVID-81 | 76 | M | CD, T2D | Dyspnea | 36.6 | 98% | 417 | POS | NA | 12 | 0 | 0 | AZT, apixaban | 0 | 0 | 0 | 1 | 0 | 0 | 2.24 | 361 | — | 133 | 1.27 | 9.91 | 441 | Alive | No |
| COVID-82 | 57 | F | Psychosis | Fever, dyspnea | NA | 95% | 327 | POS | NA | 9 | 1 | 0 | MPred (1-2 mg/kg/d for 7 d), EXP, AZT | 0 | 0 | 1 | 0 | 0 | 0 | 9.79 | 384 | 807 | 311 | 0.38 | 6.11 | 272 | Alive | No |
| COVID-84 | 71 | F | HT, HPT, cholecystectomy | Fever | 35 | 93% | 259 | POS | NA | 2 | 2 | 0 | MPred (1-1.5 mg/kg/d) for 2 d), remdesivir, EXP | 0 | 0 | 2 | 0 | 0 | 0 | 39.4 | 77 | 1028 | 277 | 0.73 | 72.7 | 183 | Alive | No |
| COVID-85 | 72 | M | CD, CKD, T2D, hepatopathy | Delirium, bilateral edema | 36 | 98% | 303 | POS | NA | 2 | 2 | 0 | Dexamethasone (6 mg/d for 2 d), remdesivir, AZT | 0 | 0 | 0 | 2 | 0 | 3 | 4.5 | 323 | 547.3 | 225 | 0.46 | 4.87 | 150 | Alive | No |
| COVID-86 | 68 | M | T2D, HT, CKD | Dyspnea, fever | 36 | 98% | 276 | POS | NA | 3 | 1 | 0 | MPred (0.5-1 mg/kg/d for 1 d), levofloxacin, EXP 4000/d, remdesivir started in the following days | 0 | 0 | 2 | 0 | 0 | 0 | 53.3 | 715 | 757.l1 | 200 | 1 | 11.3 | 470 | Alive | No |
| BB4569209 | 42 | M | No | Severe pneumonia | 38 | 93% | 142 | POS | NA | 11 | 5 | 0 | Remdesivir, steroids, EXP | 0 | 1 | 0 | 0 | 0 | 0 | 5.00 | 1078 | 342 | 250 | 0.79 | 5.41 | 251 | Alive | No |
| BB8873982 | 61 | M | No | Severe pneumonia | 38 | 78% | 121 | POS | NA | 8 | 1 | 0 | Anakinra, steroids, insulin, EXP | 0 | 1 | 0 | 0 | 0 | 0 | 20.18 | 4614 | 959 | 595 | 0.65 | 10.85 | 247 | Alive | Yes |
| COVID-C5 | 57 | M | No | Severe pneumonia | 38 | 89% | 78 | POS | NA | 10 | 6 | 0 | Steroids, EXP, anakinra, | 0 | 1 | 0 | 0 | 0 | 0 | 7.04 | 883 | NA | NA | 0.89 | 8.57 | 401 | Alive | No |
| COVID-C2 | 43 | M | No | Severe pneumonia | 38 | 92% | 301 | POS | NA | 9 | 6 | 0 | Remdesivir, steroids, EXP | 0 | 0 | 0 | 1 | 1 | 0 | 7.29 | 698 | 657 | 273 | 1.7 | 6.08 | 221 | Alive | No |
| BB4988699 | 69 | F | No | Severe pneumonia | 39 | 80% | 73 | POS | IgG and IgM POS | 4 | 1 | 0 | Fentanyl, anakinra, steroids, insulin, EXP | 0 | 1 | 0 | 0 | 0 | 0 | 18.83 | 727 | 885 | 492 | 0.33 | 1.54 | 156 | Alive | No |
| BB6716896 | 64 | M | HT, obesity | Severe pneumonia | 36 | 60% | 280 | POS | IgG and IgM POS | 5 | 1 | 0 | Anakinra, steroids, insulin, EXP | 0 | 1 | 0 | 0 | 0 | 0 | 22.35 | 1612 | 1291 | 726 | 1.32 | 7.9 | 152 | Death | No |
| BB5535728 | 51 | M | No | Severe pneumonia | 38 | 90% | 211 | POS | NEG | 6 | 1 | 0 | Anakinra, steroids, EXP, AZT | 0 | 0 | 1 | 0 | 0 | 0 | 9.53 | 722 | 653 | 475 | 0.57 | 9.55 | 282 | Alive | No |
| BB3726697 | 88 | M | HT | Severe pneumonia | 37 | 90% | 68 | POS | NEG | 4 | 1 | 0 | Anakinra, steroids, EXP, AZT | 0 | 0 | 1 | 0 | 0 | 0 | 14.35 | 507 | 23232 | 702 | 0.44 | 17.04 | 186 | Death | No |
AZT, Azithromycin; CD, cardiovascular disease; CKD, chronic kidney disease; EXP, enoxaparin; F, female; HCQ, hydroxychloroquine; HPT, hypothyroidism; HT, hypertension; M, male; MPred, methylprednisolone; NA, not available; NEG, negative; POS, positive; T2D, type 2 diabetes.
Table II.
Anakinra-treated patients’ inflammatory parameters
| Patient ID | Treatement days | CRP (0-0.5 mg/dL) | Ferritin (30-400 ng/mL) | LDH(135-200 U/L) |
|---|---|---|---|---|
| COVID-01 | Day 0 | 5.9 | 2760 | 443 |
| Day 3 | 0.35 | 308 | 254 | |
| Day 7 | NA | 294 | 226 | |
| COVID-02 | Day 0 | 5.62 | 2948 | 392 |
| Day 3 | 1.45 | 1053 | 265 | |
| Day 7 | 0.14 | NA | 248 | |
| COVID-03 | Day 0 | 16.5 | 1346 | 322 |
| Day 3 | 1.22 | 1417 | 413 | |
| Day 7 | 0.92 | NA | 339 | |
| COVID-04 | Day 0 | 3.8 | 460 | 493 |
| Day 3 | 0.68 | 504 | 205 | |
| Day 7 | 0.19 | 405 | 186 | |
| COVID-05 | Day 0 | 12.18 | 1637 | 296 |
| Day 3 | 7.29 | 552 | 382 | |
| Day 7 | 2.42 | 1352 | 379 | |
| COVID-49 | Day 0 | 10.78 | 662 | 382 |
| Day 3 | 1.07 | NA | 425 | |
| Day 7 | NA | NA | 320 | |
| COVID-53 | Day 0 | 5.88 | 621 | 376 |
| Day 3 | 0.67 | NA | 335 | |
| Day 7 | 0.34 | NA | 316 | |
| COVID-54 | Day 0 | 8.01 | 900 | 303 |
| Day 3 | 2.31 | NA | 269 | |
| Day 7 | 0.73 | 1079 | NA |
CRP, C-Reactive protein; LDH, lactic dehydrogenase; NA, not applicable/available.
Fig 1.
NLRP3 and caspase-1 activation, and IL-1β and IL-18 spontaneous secretion, by monocytes of patients with COVID-19. A, Above: ASC speck detection in monocytes from patients with severe COVID-19 by FACS. Representative dot plots of CD14+ monocytes from HDs (left), patients with COVID-19 (center), and HDs treated with LPS + ATP, used as a positive control. ASC specks are detected gating as ASC pulse width vs ASC pulse area. Percentages of ASC speck + cells are shown. Below: left, quantification of ASC speck+ CD14+ monocytes as identified by FACS; right, percentage of cells positive for active caspase-1 quantified by FACS, using FLICA-Caspase-1. B, Representative confocal images of NLRP3 and ASC speck formation in monocytes from HDs and patients with COVID-19. Images are a result of a 3-dimensional reconstruction of Z stack. Cells were stained with anti-ASC antibody (yellow), NLRP3 (green), and DAPI (blue). Scale bar is 5 μm. C,In vitro inflammatory cytokine secretion by monocytes as measured in culture supernatant after 18 hours with or without stimulation with the indicated stimuli. BF, Brightfield; DAPI, 4'-6-diamidino-2-phenylindole, dihydrochloride; FACS, fluorescence-activated cell sorting; FLICA, fluorochrome-labeled inhibitors of caspases; HD, healthy donor; IQR, interquartile range; MCC, MCC950 NLRP3 inhibitor. Data are expressed as median ± IQR. ∗P < .05, ∗∗P < .01 as assessed by Mann-Whitney t test.
Fig 2.
Patients’ treatment with anakinra inhibits NLRP3-dependent cytokine secretion by monocytes. A,In vitro inflammatory cytokine secretion by monocytes as measured in culture supernatant after 18 hours with or without stimulation with the indicated stimuli before (day 0) and after 3 and 7 days of treatment in patients affected by severe COVID-19. B, Dot plot visualizes significantly enriched gene sets of 4 patients with COVID-19. GSEA was used to perform gene set enrichment. The analysis was based on a custom collection. Gene set names and patient identifiers are reported on the left and bottom sides, respectively. Log-fold change was used as a score for running GSEA on prerank. The gene expression profiles of each patient at day 7 and day 0 were instrumental to estimate the log-fold change of all genes. NES is a measure of gene set enrichment that accounts for the size of the gene set. The NES value of each gene set in the collection was encoded in the dot color. Colors ranged from red (positive NES) to blue (negative NES). The probability of false-positive enrichment increases with the number of gene sets tested. To account for multiple hypothesis testing, GSEA computes a q value for each gene set. The −log10 of the q value was encoded in the dot size. Legend is shown in the middle right. A gene set with nominal P value lower than .05 and FDR q value lower than 0.15 was considered significantly enriched. IQR, Interquartile range; MCC, MCC950 NLRP3 inhibitor. Data are expressed as median ± IQR. ∗P < .05, ∗∗P < .01 as assessed by Mann-Whitney t test.
Fig 3.
Inflammatory activation in patients with COVID-19. A, Concentration of the inflammatory cytokine IL-6, IL-18, and IL-1β, and chemokines CXCL9 and IP-10, measured by BD CBA assays and ELISA kit for Human Soluble Protein and peripheral blood type I interferon score measured by quantitative PCR in patients with COVID-19 before and after treatment with anakinra. Dashed lines represent the mean + 2 SD of HD control values. B, Serum secretion of IL-18 and IL-1β at day 0 in patients affected by severe COVID-19. CXCL9, CXC motif chemokine ligand 9; HD, healthy control; IP-10, CXC motif chemokine ligand 10. ∗P < .05, ∗∗P < .01 as assessed by Mann-Whitney t test.
Fig 4.
ORF3a-dependent ASC speck formation in THP1-cell–derived macrophages. A, Representative confocal images of ASC speck formation (in yellow) in the different experimental conditions (empty plasmid, ORF3a, LPS/ATP). Images are a result of a 3-D reconstruction of Z stack. Cells were stained with anti-ASC antibody (yellow), anti-NLRP3 (green), and DAPI (blue). Transfected cells PCMV6-AC-rfp-ORF3a and PCMV6-AC-rfp are in red. Scale bar is 5 μm. B, Representative confocal images of ASC specks (yellow) that colocalized with ORF3a protein (red). Images are a result of a 3-D reconstruction of Z stack. Cells were stained with anti-ASC antibody (yellow) and DAPI (blue). Transfected cells PCMV6-AC-rfp-ORF3a are in red. Scale bar is 5 μm. C, Percentage of THP1-macrophage cells forming ASC specks quantified from n = 100 cells per condition in quadruplicate (n = 4). THP1 macrophage cells were stimulated with LPS (100 ng/mL) for 3 hours followed by ATP (5 mM) for 30 minutes or transfected with PCMV6-AC-rfp-ORF3a and PCMV6-AC-rfp. 3-D, Three-dimensional; DAPI, 4'-6-diamidino-2-phenylindole, dihydrochloride; IQR, interquartile range. Data are expressed as median ± IQR (Fig 4, B and C). ∗P < .05 and ∗∗P < .01 as assessed by Mann-Whitney test.
Discussion
In summary, our study presents 2 main findings: (1) circulating monocytes from patients with SARS-CoV-2 severe infection present activation of the NLRP3-IL-1β/IL-18 pathway as shown by detection of ASC specks and spontaneous in vitro secretion of IL-1β and IL-18 and (2) NLRP3 inflammasome, with consequent ASC speck formation, is directly activated by the ORF3a SARS-CoV-2 viral protein, which might be present in circulating monocytes from patients with COVID-19.
IL-1β cytokine is one of the major players in the systemic inflammatory response seen in sepsis caused by bacterial infections, where LPS induces pro–IL-1β production, NLRP3 activation, and IL-1β processing and secretion.38 Similarly, it has a predominant role also in the hyperinflammatory state observed in MAS, where treatment with anti–IL-1 drugs has dramatically improved patients’ outcome.3 An important role in MAS is also played by IL-18, whose secretion is also increased by monocytes from patients with COVID-19. Although COVID-19 is not an inflammatory syndrome per se, it is frequently associated with an inflammatory state more similar to bacterial-induced systemic inflammatory response and MAS than the one observed in response to other viral respiratory infections.8
It remains to be understood when and how is IL-1β secreted by monocytes from patients with COVID-19. We previously showed that 2 different routes of secretion are activated depending on the strength of monocyte activation, a vesicular pathway for weak stimuli and a gasdermin D–dependent pathway for strong stimuli, leading to a dramatic inflammatory response.39 Notably, in patients with CAPS also, a weak stimulus is sufficient to trigger gasdermin D–mediated massive IL-1β secretion. It is tempting to speculate that in COVID-19–infected monocytes ORF3a and other viral proteins18 trigger NLRP3 inflammasome activation, leading to an abnormal IL-1β secretion with consequent severe inflammation. The concurrent presence in the infected lungs of other pathogen-associated molecular patterns and damage-associated molecular patterns may amplify the inflammatory response and promote the so-called cytokine storm. Our findings further support the importance of targeting IL-1β activity with anti–IL-1 drugs in severe COVID-19.
Key messages.
-
•
Monocytes from SARS-CoV-2–infected individuals present NLRP3 inflammasome activation and spontaneous secretion of IL-1β. This activation is blocked by administration of the IL-1R blocker anakinra.
-
•
SARS-CoV-2 protein ORF3a activates NLRP3 inflammasome inducing ASC-speck formation in patients’ monocytes.
-
•
Drugs targeting the NLRP3 IL1-β pathway might have a therapeutic role in the treatment of patients with severe COVID-19 with evidence of inflammation.
Acknowledgments
We thank the patients and their families for their willingness to participate in our study, the service of Immunohematology and Transfusion Medicine of Gaslini Institute for the collection of biological samples, and the Laboratory Core Facility of Gaslini Institute for support for confocal analysis. IRCCS G Gaslini is member of the European Reference Network for Rare Immunodeficiency, Autoinflammatory and Autoimmune Diseases (project ID no. 739543). This work was developed within the framework of the DINOGMI Department of Excellence of MIUR 2018-2022. We thank www.servier.com for the available images.
Footnotes
S.V. received financial support from AMRI (Associazione Malattie Reumatiche Infantili).
Disclosure of potential conflict of interest: M. Gattorno reports consultancies and speaker’s fee from Novartis and SOBI. S. Volpi, A. Bertoni, F. Penco, P. Bocca, I. Prigione, A. Corcione, and F. Schena report speaker fee from SOBI. The rest of the authors declare that they have no relevant conflicts of interest.
Supplementary data
References
- 1.Henderson L.A., Canna S.W., Schulert G.S., Volpi S., Lee P.Y., Kernan K.F., et al. On the alert for cytokine storm: immunopathology in COVID-19. Arthritis Rheumatol. 2020;72:1059–1063. doi: 10.1002/art.41285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bracaglia C., Prencipe G., De Benedetti F. Macrophage activation syndrome: different mechanisms leading to a one clinical syndrome. Pediatr Rheumatol Online J. 2017;15:5. doi: 10.1186/s12969-016-0130-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehta P., Cron R.Q., Hartwell J., Manson J.J., Tattersall R.S. Silencing the cytokine storm: the use of intravenous anakinra in haemophagocytic lymphohistiocytosis or macrophage activation syndrome. Lancet Rheumatol. 2020;2:e358–e367. doi: 10.1016/S2665-9913(20)30096-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schulte-Schrepping J., Reusch N., Paclik D., Bassler K., Schlickeiser S., Zhang B.W., et al. Severe COVID-19 is marked by a dysregulated myeloid cell compartment. Cell. 2020;182:1419–1440.e23. doi: 10.1016/j.cell.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mann E.R., Menon M., Knight S.B., Konkel J.E., Jagger C., Shaw T.N., et al. Longitudinal immune profiling reveals key myeloid signatures associated with COVID-19. Sci Immunol. 2020;5 doi: 10.1126/sciimmunol.abd6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silvin A., Chapuis N., Dunsmore G., Goubet A.G., Dubuisson A., Derosa L., et al. Elevated calprotectin and abnormal myeloid cell subsets discriminate severe from mild COVID-19. Cell. 2020;182:1401–1418.e18. doi: 10.1016/j.cell.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lucas C., Wong P., Klein J., Castro T.B.R., Silva J., Sundaram M., et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature. 2020;584:463–469. doi: 10.1038/s41586-020-2588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giamarellos-Bourboulis E.J., Netea M.G., Rovina N., Akinosoglou K., Antoniadou A., Antonakos N., et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27:992–1000.e3. doi: 10.1016/j.chom.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ong E.Z.Y., Chan Y.F.Z., Leong W.Y., Lee N.M.Y., Kalimuddin S., Mohideen S.M.H., et al. A dynamic immune response shapes COVID-19 progression. Cell Host Microbe. 2020;27:879–882.e2. doi: 10.1016/j.chom.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pontali E., Volpi S., Antonucci G., Castellaneta M., Buzzi D., Tricerri F., et al. Safety and efficacy of early high-dose IV anakinra in severe COVID-19 lung disease. J Allergy Clin Immunol. 2020;146:213–215. doi: 10.1016/j.jaci.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cavalli G., De Luca G., Campochiaro C., Della-Torre E., Ripa M., Canetti D., et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. 2020;2:e325–e331. doi: 10.1016/S2665-9913(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kyriazopoulou E., Poulakou G., Milionis H., Metallidis S., Adamis G., Tsiakos K., et al. Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: a double-blind, randomized controlled phase 3 trial. Nat Med. 2021;27:1752–1760. doi: 10.1038/s41591-021-01499-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kyriazopoulou E., Huet T., Cavalli G., Gori A., Kyprianou M., Pickkers P., et al. Effect of anakinra on mortality in patients with COVID-19: a systematic review and patient-level meta-analysis. Lancet Rheumatol. 2021;3:e690–e697. doi: 10.1016/S2665-9913(21)00216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiss E.S., Girard-Guyonvarc’h C., Holzinger D., de Jesus A.A., Tariq Z., Picarsic J., et al. Interleukin-18 diagnostically distinguishes and pathogenically promotes human and murine macrophage activation syndrome. Blood. 2018;131:1442–1455. doi: 10.1182/blood-2017-12-820852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piccini A., Carta S., Tassi S., Lasiglié D., Fossati G., Rubartelli A. ATP is released by monocytes stimulated with pathogen-sensing receptor ligands and induces IL-1beta and IL-18 secretion in an autocrine way. Proc Natl Acad Sci U S A. 2008;105:8067–8072. doi: 10.1073/pnas.0709684105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Junqueira C., Crespo A., Ranjbar S., de Lacerda L.B., Lewandrowski M., Ingber J., et al. FcgammaR-mediated SARS-CoV-2 infection of monocytes activates inflammation. Nature. 2022;606:576–584. doi: 10.1038/s41586-022-04702-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodrigues T.S., de Sa K.S.G., Ishimoto A.Y., Becerra A., Oliveira S., Almeida L., et al. Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients. J Exp Med. 2021;218 doi: 10.1084/jem.20201707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pan P., Shen M., Yu Z., Ge W., Chen K., Tian M., et al. SARS-CoV-2 N protein promotes NLRP3 inflammasome activation to induce hyperinflammation. Nat Commun. 2021;12:4664. doi: 10.1038/s41467-021-25015-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu H., Akinyemi I.A., Chitre S.A., Loeb J.C., Lednicky J.A., McIntosh M.T., et al. SARS-CoV-2 viroporin encoded by ORF3a triggers the NLRP3 inflammatory pathway. Virology. 2022;568:13–22. doi: 10.1016/j.virol.2022.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Volpi S., Insalaco A., Caorsi R., Santori E., Messia V., Sacco O., et al. Efficacy and adverse events during Janus kinase inhibitor treatment of SAVI syndrome. J Clin Immunol. 2019;39:476–485. doi: 10.1007/s10875-019-00645-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sester D.P., Thygesen S.J., Sagulenko V., Vajjhala P.R., Cridland J.A., Vitak N., et al. A novel flow cytometric method to assess inflammasome formation. J Immunol. 2015;194:455–462. doi: 10.4049/jimmunol.1401110. [DOI] [PubMed] [Google Scholar]
- 22.Bray N.L., Pimentel H., Melsted P., Pachter L. Near-optimal probabilistic RNA-seq quantification. Nat Biotechnol. 2016;34:525–527. doi: 10.1038/nbt.3519. [DOI] [PubMed] [Google Scholar]
- 23.Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu G., Wang L.G., Han Y., He Q.Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16:284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Subramanian A., Tamayo P., Mootha V.K., Mukherjee S., Ebert B.L., Gillette M.A., et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liberzon A., Subramanian A., Pinchback R., Thorvaldsdottir H., Tamayo P., Mesirov J.P. Molecular signatures database (MSigDB) 3.0. Bioinformatics. 2011;27:1739–1740. doi: 10.1093/bioinformatics/btr260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liberzon A., Birger C., Thorvaldsdottir H., Ghandi M., Mesirov J.P., Tamayo P. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 2015;1:417–425. doi: 10.1016/j.cels.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Balow J.E., Jr., Ryan J.G., Chae J.J., Booty M.G., Bulua A., Stone D., et al. Microarray-based gene expression profiling in patients with cryopyrin-associated periodic syndromes defines a disease-related signature and IL-1-responsive transcripts. Ann Rheum Dis. 2013;72:1064–1070. doi: 10.1136/annrheumdis-2012-202082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang H., Ai J.W., Yang W., Zhou X., He F., Xie S., et al. Metatranscriptomic characterization of COVID-19 identified a host transcriptional classifier associated with immune signaling. Clin Infect Dis. 2021;73:376–385. doi: 10.1093/cid/ciaa663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rossi M., Sharkey A.M., Vigano P., Fiore G., Furlong R., Florio P., et al. Identification of genes regulated by interleukin-1beta in human endometrial stromal cells. Reproduction. 2005;130:721–729. doi: 10.1530/rep.1.00688. [DOI] [PubMed] [Google Scholar]
- 31.Gattorno M., Tassi S., Carta S., Delfino L., Ferlito F., Pelagatti M.A., et al. Pattern of interleukin-1beta secretion in response to lipopolysaccharide and ATP before and after interleukin-1 blockade in patients with CIAS1 mutations. Arthritis Rheum. 2007;56:3138–3148. doi: 10.1002/art.22842. [DOI] [PubMed] [Google Scholar]
- 32.Canna S.W., de Jesus A.A., Gouni S., Brooks S.R., Marrero B., Liu Y., et al. An activating NLRC4 inflammasome mutation causes autoinflammation with recurrent macrophage activation syndrome. Nat Genet. 2014;46:1140–1146. doi: 10.1038/ng.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toda Y., Tsukada J., Misago M., Kominato Y., Auron P.E., Tanaka Y. Autocrine induction of the human pro-IL-1beta gene promoter by IL-1beta in monocytes. J Immunol. 2002;168:1984–1991. doi: 10.4049/jimmunol.168.4.1984. [DOI] [PubMed] [Google Scholar]
- 34.Dinarello C.A., Ikejima T., Warner S.J., Orencole S.F., Lonnemann G., Cannon J.G., et al. Interleukin 1 induces interleukin 1, I: induction of circulating interleukin 1 in rabbits in vivo and in human mononuclear cells in vitro. J Immunol. 1987;139:1902–1910. [PubMed] [Google Scholar]
- 35.Warner S.J., Auger K.R., Libby P. Interleukin 1 induces interleukin 1, II: recombinant human interleukin 1 induces interleukin 1 production by adult human vascular endothelial cells. J Immunol. 1987;139:1911–1917. [PubMed] [Google Scholar]
- 36.Swanson K.V., Deng M., Ting J.P.Y. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019;19:477–489. doi: 10.1038/s41577-019-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siu K.L., Yuen K.S., Castano-Rodriguez C., Ye Z.W., Yeung M.L., Fung S.Y., et al. Severe acute respiratory syndrome coronavirus ORF3a protein activates the NLRP3 inflammasome by promoting TRAF3-dependent ubiquitination of ASC. Faseb J. 2019;33:8865–8877. doi: 10.1096/fj.201802418R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balza E., Piccioli P., Carta S., Lavieri R., Gattorno M., Semino C., et al. Proton pump inhibitors protect mice from acute systemic inflammation and induce long-term cross-tolerance. Cell Death Dis. 2016;7 doi: 10.1038/cddis.2016.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Semino C., Carta S., Gattorno M., Sitia R., Rubartelli A. Progressive waves of IL-1β release by primary human monocytes via sequential activation of vesicular and gasdermin D-mediated secretory pathways. Cell Death Dis. 2018;9:1088. doi: 10.1038/s41419-018-1121-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.