Abstract
Patient: Male, 54-year-old Final
Diagnosis: Cardiomyopathy
Symptoms: Exertional dyspnea • pedal edema
Medication: —
Clinical Procedure: Coronary angiography • echocardiography
Specialty: Cardiology
Objective:
Unknown etiology
Background:
Khat (Catha edulis) is a plant cultivated in Ethiopia, East African, and the Arabian Peninsula. Long-term khat consumption has been associated with increased rates of periodontal diseases, esophagitis, psychosis, and cardiovascular issues such as cerebrovascular accidents, myocardial ischemia, and ischemic cardiomyopathy (CM). We report a case of khat-induced non-ischemic CM in a patient with no other known cardiovascular risk factors and highlight a cardiovascular effect of chronic khat consumption.
Case Report:
A 54-year-old Yemeni man with no known medical history but a chronic khat chewer presented with worsening exertional dyspnea for 6 months and associated pedal edema. Laboratory studies were remarkable for elevated B-type natriuretic peptide (BNP). Electrocardiogram (EKG) revealed normal sinus rhythm with non-specific T wave inversions (TWI) in V5-V6. A computed tomography (CT) scan of the chest showed bilateral pleural effusions with interlobular septal thickening. Transthoracic echocardiogram (TTE) showed a left ventricular ejection fraction (LVEF) of 16–20% and global CM. Coronary angiography revealed normal coronaries.
Conclusions:
Chronic khat consumption is being recognized as a dangerous habit with serious health consequences and its association with ischemic CM is well documented. The findings of ischemic cardiac changes of acute coronary syndrome in a patient with normal coronary arteries raises the possibility that khat toxicity was associated with coronary artery spasm due to its amphetamine-like stimulatory effects. Although further research is required to substantiate this relationship, it is imperative that khat consumption be considered a risk factor when assessing for CM.
Keywords: Cardiomyopathies; Catha; Heart Failure, Systolic
Background
The fresh leaves of the khat plant have an aromatic odor and a slightly sweet taste and are chewed daily by people living in some societies in Yemen and other East African countries [1]. In these places, chewing khat is an acceptable practice and is popular in social gatherings such as funerals and wedding parties [2]. Khat is cultivated as a small tree, and is mostly grown for cash in Ethiopia, East Africa, and the southern Arabian Peninsula [3]. There are 3 significant alkaloids present in khat leaves, namely cathinone, norpseudoephedrine (cathine), and norephedrine [4]. The alkaloid cathinone, released on chewing its leaves, is a stimulant and is the principal constituent responsible for the excitement, loss of appetite, and euphoria effect [5,6].
The consequences of khat ingestion are seen in multiple organ systems, including cardiovascular, neurologic, respiratory, endocrine, and digestive systems [1]. Its sympathomimetic/vasomotor effects on the myocardium and coronary vessels are mediated via beta-1-adrenoreceptors, which explains the resulting tachycardia, palpitations, and hypertension [1].
Despite its adverse effects and its classification by the World Health Organization as a drug of abuse, about 20 million people worldwide are believed to be using khat [7,8]. These effects were previously rarely seen in Westernized nations but are becoming more prevalent due to advancements in overnight delivery systems and widespread immigration of khat chewers, leading to its globalization [7]. Even so, many khat chewers in the Western world reside in marginalized communities [9].
Literature reviews show that the many reported cardiovascular events associated with khat intake and myocardial ischemia are dose-related. Al-Motarreb et al pointed out that khat chewers had an approximately 39-fold increased risk of a myocardial infarction (MI) compared to non-khat chewers [10]. In a Persian Gulf acute coronary syndrome registry analysis, khat chewing was an independent risk factor for in-hospital mortality, recurrent ischemia, and heart failure [11].
Documented reports of khat-induced CM are mainly ischemic in nature [6,10,11]. We report a case of new-onset non-ischemic heart failure with reduced ejection fraction (HFrEF) in a middle-aged Yemeni immigrant residing in Brooklyn, USU, without pertinent cardiovascular risk factors. This report aims to highlight a possible association of khat consumption and non-ischemic CM.
Case Report
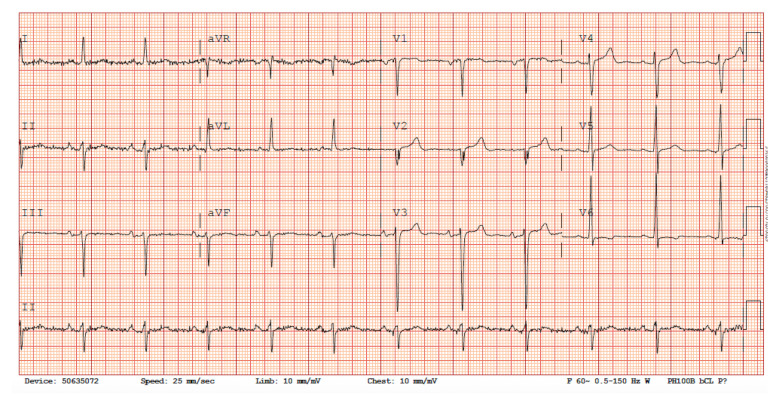
A 54-year-old Yemeni man with no past medical history presented to the emergency department with worsening shortness of breath ongoing for a period of 6 months. His dyspnea was initially only on exertion but slowly progressed to dyspnea at rest. He endorsed bilateral lower-extremity swelling and claudication. He denied any chest pain, palpitations, dizziness, syncopal episode, fever, nausea, or vomiting. He was a life-long non-cigarette smoker and did not use alcohol. However, the patient mentioned that he chewed and smoked miraa, also known as khat, for pleasure approximately 6 hours daily and had been doing so for 40 years. According to the patient, this leaf is synonymous with his culture and is used to bond with his friends and family and its use is very common at cultural festivities back home in Yemen. There was no significant family history of cardiac disease or sudden cardiac death and the patient was not on any medications. Physical examination revealed a middle-aged man in respiratory distress who was alert and oriented to time, place, and person. He was afebrile, breathing at a rate of 26 breaths per minute on 4 liters/min of oxygen through a nasal cannula, with a heart rate of 96 and a blood pressure of 110/70 mmHg. Cardiopulmonary examination revealed jugular venous distention with bilateral rales on auscultation along with a grade 2 holosystolic murmur in the mitral and tricuspid areas. The patient also had 2+ bilateral pitting edema of the lower extremities up to the mid-shins. Laboratory studies on presentation revealed an unremarkable complete blood count and comprehensive metabolic panel along with a normal thyroid function test. However, the patient did have an elevated BNP of 2103 pg/ml and an HbA1c of 7.6 (4–6%). EKG revealed normal sinus rhythm with non-specific TWI in V5–V6 with no acute ischemic changes (Figure 1). A COVID test was negative. A chest X-ray (CXR) revealed enlarged cardiac silhouette and pulmonary venous congestion (Figure 2). CT of the chest without contrast showed bilateral pleural effusions with interlobular septal thickening. Given the overall clinical profile, laboratory work, and imaging, a diagnosis of decompensated heart failure was made and the patient was admitted to the cardiology telemetry unit for further workup and management. He was treated with IV furosemide 40 mg, after which oxygen requirements decreased to room air and he expressed feeling symptomatic relief. TTE showed a LVEF of 16–20% and global CM confirmed our suspicion of heart failure. The TTE also showed severe mitral regurgitation and moderate tricuspid regurgitation. In addition, vascular arterial and venous duplex studies of the lower extremities revealed no evidence of hemodynamically significant stenosis or venous thrombosis. The next challenge was to establish the cause of the patient’s heart failure. The patient’s telemetry rhythm strip over the course of his stay did not show any paroxysmal atrial fibrillation or premature ventricular complexes.
Figure 1.
Electrocardiogram. EKG showing normal sinus rhythm with non-specific TWI in leads V5–V6.
Figure 2.
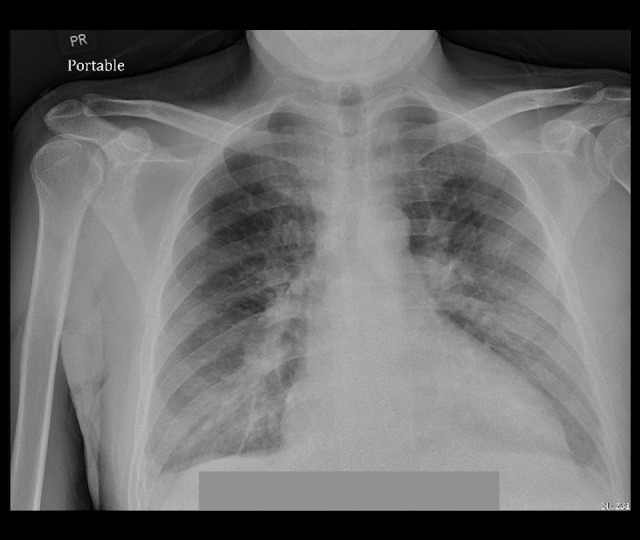
Chest X-ray. CXR showing enlarged cardiac silhouette and mild pulmonary venous congestion.
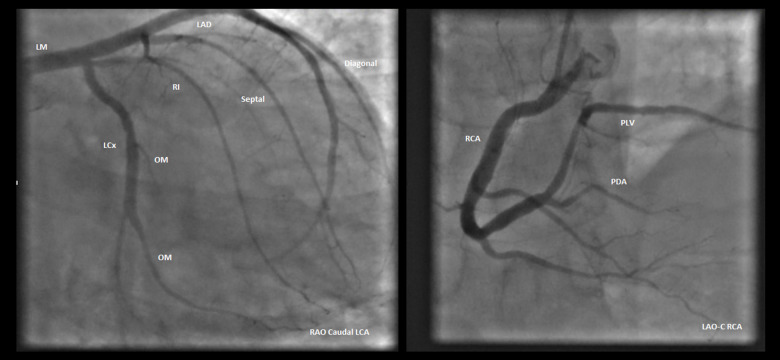
A left heart catheterization (LHC) to rule out an ischemic source of the patient’s CM revealed normal coronaries (Figure 3). The patient improved clinically through diuresis and medical optimization with heart failure medications, including diuretics, beta-blockers, and angiotensin-converting enzyme inhibitors (ACE-I). He was subsequently placed on a life vest for primary prevention of fatal cardiac arrhythmias prior to his discharge.
Figure 3.
Coronary angiography. Coronary angiography revealing normal and patent coronary arteries. LAO CAUD – left anterior oblique caudal view; LAO CRAN – left anterior oblique cranial view.
A repeat TTE was planned for 3 months after hospitalization to reevaluate his ejection fraction and mitral and tricuspid regurgitation and to make decisions regarding implantation of an automatic implantable cardioverter defibrillator (AICD) and a possible Mitraclip if warranted as an outpatient.
Discussion
This case report highlights a possible association of khat consumption with non-ischemic CM, which until now has been unreported. Chronic khat consumption-related ischemic CM is a well-documented entity believed to be due to the increased sympathomimetic activity caused by cathinone, which is the principal pharmacologically active substance in khat, with various effects on the cardiovascular system, including the release of stored nor-epinephrine, adrenaline-induced renal and coronary vasoconstriction, hypertension, and tachycardia [11,12].
Ali et al found that khat chewing was an independent risk factor of recurrent ischemia and heart failure and khat chewers had higher risk of recurrent myocardial ischemia, cardiogenic shock, and ventricular arrhythmia compared with nonkhat chewers [13].
On presentation, our patient had no known cardiovascular risk factors. Surprisingly, his HbA1c was elevated at 7.5 mg/dl, although his blood glucose level was never above 200 mg/dl during hospitalization. His cardiac catheterization revealed normal coronaries, negating the likelihood of ischemic CM from coronary artery disease (CAD). He denied any previous history of acute chest pain or angina symptoms and his ECG remained unchanged compared to prior outpatient ECGs, with no evidence of active or previous myocardial ischemia or infarction. In the absence of any identifiable CAD, his severe global CM was believed to be non-ischemic in origin.
Unlike ischemic CM, where depressed ventricular contractility and function are secondary to ischemia and infarction, non-ischemic CM is a structural and functional dysfunction of the myocardium in the absence of CAD [14,15]. Its causes are variable and include genetic, toxic, metabolic, infectious, and idiopathic etiologies.
Our patient was a chronic khat chewer and smoker, a lifetime cigarette non-smoker, and did not consume alcohol. His family history was not suggestive of any genetic cardiac disease. Other than a deranged HbA1C, his laboratory investigations did not suggest other metabolic or endocrine disorders. We postulate that chronic khat ingestion was responsible for the global CM observed in our patient. The exact mechanism by which khat causes non-ischemic CM is unknown. CM has been linked to sympathomimetic agents, in particular amphetamine. Cathinone, having identical properties to amphetamine, may have the same effects on the heart muscle and may also produce dilated CM, particularly in young patients with an inherited predisposition [16]. Regular khat chewing has been associated with elevated mean diastolic pressure as well [17]. Cocaine, a sympathomimetic agent like khat is known to cause non-ischemic CM even in the absence of CAD and has been linked to the development of both hypertrophic and dilated cardiomyopathies with chronic abuse [18,19]. Possible mechanisms for cocaine-induced non-ischemic CM include catecholamine-induced toxicity on the myocardium and necrosis from chronic myocardial stimulation, local immune reactions, and cardiac myocyte apoptosis from direct toxic effects of catecholamine [17,18]. It remains uncertain if khat-associated non-ischemic CM is a result of the above-described process.
Non-ischemic CM resulting from toxins typically improve with the removal of the offending agent, like alcohol. Our patient was counseled and encouraged to discontinue the use of khat and will be followed up closely in the outpatient setting.
Conclusions
Chronic khat consumption is being recognized as a dangerous habit with serious health consequences and its association with ischemic CM is well documented. However, the present report is of a case of non-ischemic CM with khat use. Although further research is required to substantiate this relationship, it is imperative that khat consumption be considered a risk factor when assessing for CM, especially among people from the Horn of Africa and the Arabian Peninsula.
Footnotes
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
- 1.Wabe NT. Chemistry, pharmacology, and toxicology of khat (catha edulis forsk): A review. Addict Health. 2011;3(3–4):137–49. [PMC free article] [PubMed] [Google Scholar]
- 2.Oyugi A, Korir B, Kibet J, Ngari S. The possible abuse of catha edulis and its associated health and socio-economic impacts. Progress in Chemical and Biochemical Research. 1999;4(2):234–53. [Google Scholar]
- 3.Sheikh KA, El-Setouhy M, Yagoub U, et al. Khat chewing and health related quality of life: Cross-sectional study in Jazan region, Kingdom of Saudi Arabia. Health Qual Life Outcomes. 2014;12:44. doi: 10.1186/1477-7525-12-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schorno X, Steinegger E. ZNS-aktive phenylpropylamine von Catha edulis FORSK. (Celastraceae) kenyanischer herkunft. Experientia. 1979;35:572–74. [Google Scholar]
- 5.Nutt D, King LA, Saulsbury W, Blakemore C. Development of a rational scale to assess the harm of drugs of potential misuse. Lancet. 2007;369(9566):1047–53. doi: 10.1016/S0140-6736(07)60464-4. [DOI] [PubMed] [Google Scholar]
- 6.Saha S, Dollery C. Severe ischaemic cardiomyopathy associated with khat chewing. J R Soc Med. 2006;99(6):316–18. doi: 10.1258/jrsm.99.6.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mega TA, Dabe NE. Khat (catha edulis) as a risk factor for cardiovascular disorders: Systematic review and meta-analysis. Open Cardiovasc Med J. 2017;11:146–55. doi: 10.2174/1874192401711010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Mugahed L. Khat chewing in Yemen: Turning over a new leaf. Bull World Health Organ. 2008;86(10):741–42. doi: 10.2471/BLT.08.011008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Young JT, Butt J, Hersi A, et al. Khat dependence, use patterns, and health consequences in Australia: An exploratory study. J Stud Alcohol Drugs. 2016;77(2):343–48. doi: 10.15288/jsad.2016.77.343. [DOI] [PubMed] [Google Scholar]
- 10.Al-Motarreb A, Briancon S, Al-Jaber N, et al. Khat chewing is a risk factor for acute myocardial infarction: A case-control study. Br J Clin Pharmacol. 2005;59(5):574–81. doi: 10.1111/j.1365-2125.2005.02358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ali WM, Al Habib KF, Al-Motarreb A, et al. Acute coronary syndrome and khat herbal amphetamine use: An observational report. Circulation. 2011;124(24):2681–89. doi: 10.1161/CIRCULATIONAHA.111.039768. [DOI] [PubMed] [Google Scholar]
- 12.Al-Hashem FH, Dallak MA, Nwoye LO, et al. Acute exposure to catha edulis depresses contractility and induces myocardial infarction in spontaneously contracting, isolated rabbit’s heart. Saudi J Biol Sci. 2012;19(1):93–101. doi: 10.1016/j.sjbs.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ali WM, Zubaid M, Al-Motarreb A, et al. Association of khat chewing with increased risk of stroke and death in patients presenting with acute coronary syndrome. Mayo Clin Proc. 2010;85(11):974–80. doi: 10.4065/mcp.2010.0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu AH. Management of patients with non-ischaemic cardiomyopathy. Heart. 2007;93:403–8. doi: 10.1136/hrt.2005.085761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bozkurt B, Colvin M, Cook J, et al. Current diagnostic and treatment strategies for specific dilated cardiomyopathies: A scientific statement from the American Heart Association. [published erratum appears in Circulation. 2016;134(23): e652] Circulation. 2016;134(23):e579–e646. doi: 10.1161/CIR.0000000000000455. [DOI] [PubMed] [Google Scholar]
- 16.Al-Motarreb A, Al-Habori M, Broadley KJ. Khat chewing, cardiovascular diseases and other internal medical problems: The current situation and directions for future research. J Ethnopharmacol. 2010;132(3):540–48. doi: 10.1016/j.jep.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 17.Getahun W, Gedif T, Tesfaye F. Regular khat (catha edulis) chewing is associated with elevated diastolic blood pressure among adults in Butajira, Ethiopia: A comparative study. BMC Public Health. 2010;10:390. doi: 10.1186/1471-2458-10-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Restrepo CS, Rojas CA, Martinez S, et al. Cardiovascular complications of cocaine: imaging findings. Emerg Radiol. 2009;16(1):11–19. doi: 10.1007/s10140-008-0762-x. [DOI] [PubMed] [Google Scholar]
- 19.Wiener RS, Lockhart JT, Schwartz RG. Dilated cardiomyopathy and cocaine abuse. Report of two cases. Am J Med. 1986;81(4):699–701. doi: 10.1016/0002-9343(86)90559-0. [DOI] [PubMed] [Google Scholar]
- 20.Kim ST, Park T. Acute and chronic effects of cocaine on cardiovascular health. Int J Mol Sci. 2019;20(3):584. doi: 10.3390/ijms20030584. [DOI] [PMC free article] [PubMed] [Google Scholar]